1. Introduction
The onset of chronic, debilitating symptoms following SARS-CoV-2 vaccination is thought to constitute a novel disease entity, for which the term
post-acute COVID-19 vaccination syndrome (PACVS) has recently been suggested [
1]. The symptoms reported by PACVS-affected persons start shortly after SARS-CoV-2 vaccination, continue in episodes over several months, and severely compromise quality of life. A systematic survey of the clinical features of PACVS has yet to be carried out. However, published case reports [
1] indicate that PACVS differs from the usual adverse effects of SARS-CoV-2 vaccination [
2,
3,
4,
5]. The symptoms most frequently reported in the context of PACVS comprise in varied composition impaired well-being (exhaustion, malaise, chronic fatigue), cardiovascular disturbances (orthostatic intolerance, tachycardia, palpitations), peripheral neuropathy (dysesthesia, hypesthesia), central nervous system dysfunction (lack of concentration, brain fog, cognitive deficits, sleep disorders), muscular dysfunction (myalgia, weakness, fibrillations), and gastro-intestinal afflictions (nausea, strong weight changes). In summary, PACVS presents a phenotype of acquired autonomous dysfunction, which overlaps with various established multisystemic dis-autonomy syndromes such as myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) [
6,
7], postural tachycardia syndrome (POTS) [
8], fibromyalgia/chronic pain syndrome [
9], small fiber neuropathy (SFN) [
10] and mast cell activation syndrome (MCAS) [
11]. Interestingly, symptoms similarly conforming to ME/CFS and POTS have been observed following vaccinations against human papilloma virus [
12,
13,
14,
15,
16] and hepatitis B virus [
17].
ME/CFS and POTS occurring un-related to vaccination is frequently associated with alterations of humoral auto-immunity against receptors and transmitters involved in autonomous regulation [
18,
19,
20,
21]. Increases in circulating levels of these antibodies are linked to incidence, duration and severity of ME/CFS [
22] and POTS [
23,
24,
25]. IgG-directed therapy has been successful in ameliorating symptoms [
26,
27]. Increases in circulating receptor antibodies were also observed in severe COVID-19 [
28,
29,
30,
31,
32], which similarly exhibits ME/CFS-like symptoms [
33] amenable to IgG-directed therapy [
34].
Taken together, the above considerations prompt the hypothesis that antibodies against autonomous regulation elements could play a role in PACVS and possibly serve as therapeutic targets or diagnostic markers. To address this hypothesis, we have here investigated the impact of SARS-CoV-2 vaccination on receptor antibodies known to be involved in POTS [
20,
23,
24], ME/CFS [
18,
22,
25] and immune homeostasis [
35]. Circulating levels of these antibodies were measured before and six months after vaccination in normal healthy individuals not affected by PACVS. Normal post-vaccination levels were compared with corresponding levels of a matched cohort presumed to be affected by PACVS because exhibiting persistent symptoms of chronic severe autonomous dysfunction [
6,
7,
8,
9,
10,
11] following SARS-CoV-2 vaccination.
2. Materials and Methods
2.1. Study participants
Study participants exhibiting PACVS following SARS-CoV-2 vaccination (N = 159 females, N = 32 males, mean/median age = 40/39 years) were recruited from self-help groups via on-line questionaire. Participants were diagnosed with ME/CFS, POTS, or related/overlapping syndromes (fibromyalgia/chronic pain syndrome, SFN and MCAS) and exhibited at least three symptoms conforming to these syndromes [
6,
7,
8,
9,
10,
11] (details:
Table S2). A comparable list of symptoms has recently been observed in chronic sequelae of COVID-19 [
36]. Participants were only included if above diagnoses or symptoms were confirmed by a physician/in a hospital and had persisted for five months or more following vaccination. Vaccination regimen pre-ceding PACVS encompassed one (47 cases), two (96 cases) or three cycles (48 cases) of vaccination with Spikevax, Moderna (32 cases) or Comirnaty, Pfizer/BioNTech (159 cases). In 17 cases the mRNA-vaccination entailing PACVS was pre-ceded by one vaccination cycle with a vector-based vaccine (details:
Table S1). Exclusion criteria encompassed (i) occurrence of the above symptoms after other vaccinations (including non-mRNA-SARS-CoV-2 vaccinations) and/or after acute SARS-CoV-2 infection, (ii) pre-vaccination histories of ME/CFS, POTS or other potentially confounding diseases or syndromes, (iii) confounding pre-medications (details:
Table S2). Of 1500 individuals applying for study participation, 1309 were excluded (
Figure S3).
2.2. Controls
Healthy controls (N = 89, N = 18 males, mean/median age = 39/49 years) matched for gender and chronological age (p < 0.001, U-test) were recruited from a surveillance study of healthy hospital employees subjected to initial dual vaccination with mRNA-vaccine (Spikevax, Moderna) [
37]. Paired serum samples were obtained 48 h before first vaccination and six months after second vaccination. Control candidates were excluded when reporting disease symptoms or exhibiting serological evidence of inflammation, cardiac dysfunction or inter-current SARS-CoV-2-infection during the surveillance period of six months after the second vaccination (details:
Table S2,
Figure S3).
2.3. Validation of SARS-CoV-2 Vaccination and -Infection
SARS-CoV-2-vaccination response of controls and PACVS-affected study subjects was confirmed in all cases by sero-reactivity against SARS-CoV-2 spike S1 protein (SAB). Completed SARS-CoV-2 infections were identified by sero-reactivity against SARS-CoV-2 nucleocapsid protein (NAB) [
37]. Controls were excluded if NAB-reactive or reporting SARS-CoV-2 infections and/or COVID-19 re-convalescence in their case history. Study participants were excluded if suffering from florid (PCR-positive) SARS-CoV-2 infection.
2.4. Ethics
Clinical trial protocols were approved by the local ethics board of Heinrich-Heine University Düsseldorf (study numbers 2022-1948 and 2020-1259). The investigation conforms with the principles outlined in the World’s Medical Association Declaration of Helsinki. Before inclusion, all participants have given written informed consent.
2.5. Laboratory Measurements
Serum was collected by antecubital vein puncture, processed by accredited procedures and stored for up to 6 months at -20 °C. Antibodies against AT1R (Angiotensin II type 1 receptor), ETAR (Endothelin-1 type A receptor), IL-1-Rb (Interleukin-1 receptor type 2), α1-adr-R (Alpha-1 adrenergic receptor), α2a-adr-R (Alpha-2A adrenergic receptor), α2b-adr-R (Alpha-2B adrenergic receptor), α2c-adr-R (Alpha-2C adrenergic receptor), β1-adr-R (Beta-1 adrenergic receptor), β2-adr-R (Beta-2 adrenergic receptor), M1R–M5R (muscarinic acetylcholine receptor M1–M5), MASR (MAS 1 receptor), ACE-II (Angiotensin-converting enzyme 2) were measured in serum with commercially available immuno-assays (CellTrend GmbH, Luckenwalde, Germany) according to the instructions of the manufacturer. The assays were calibrated with polyclonal standard sera, yielding quantitative values for receptor-specific IgG expressed in arbitrary units/ml. panIg-antibodies against SARS CoV-2 spike S1 protein (SAB) and nucleocapsid protein (NAB) were determined as previously described [
37]. All other laboratory test were performed by accredited routine laboratory diagnostic procedures. Unless stated otherwise, reference values followed recommendations of the international federation of Clinical Chemistry (IFCC).
2.6. Statistical Methods
IBM SPSS Statistics 28 software (IBM Corp. Released 2021. IBM SPSS Statistics for Windows, Version 28.0. Armonk, New York, USA: IBM Corp.) and Graph Pad Prism 9 (Graph Pad Software, Inc., San Diego, California, USA, Graph Pad Prism 9 for Windows or Apple Macintosh, released 2020) were used for analysis. Normal distribution was tested according to Shapiro-Wilk and Q-Q-graphs. Non-normally distributed data was descriptively analyzed by mean/median values, interquartile-range, 95% or 99% confidence intervals and boxplots. Correlations were analyzed by Spearman correlation performed using 95% confidence intervals. Differences of controls before and after vaccination were analyzed by the t-test for paired samples (two-tailed). Differences between study subjects and controls were analyzed by the Mann-Whitney-U test (two-tailed). Correlations were assumed to be good at Spearman’s R ≥ 0.7. For all tests statistical significance was assumed at p < 0.001. Missing data were managed by listwise deletion
3. Results
3.1. Impact of SARS-CoV-2 vaccination on receptor-antibodies in healthy controls
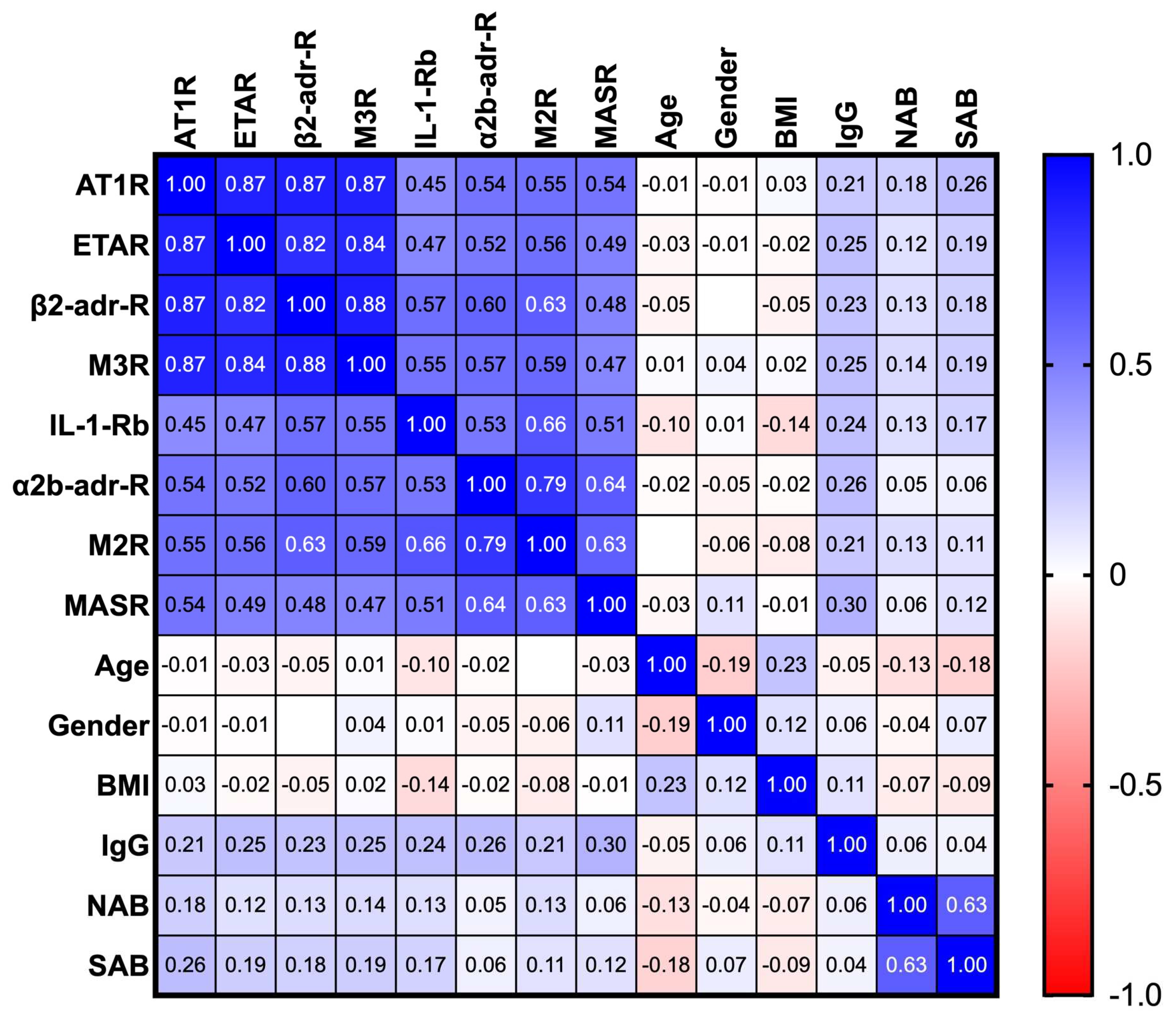
Control sera were collected during first-time vaccination with two cycles of the mRNA- vaccine Spikevax (Moderna). Samples were obtained 48 h before first vaccination and six months after second vaccination from 89 healthy individuals not reporting adverse vaccination-reactions that persisted for more than two weeks after complete vaccination and not suffering from potentially confounding diseases. In normal pairs of vaccination-naïve and post-vaccination sera the circulating levels of IgG specific for various receptors (expressed as U/ml) were to some extent covariant with each other. Circulating levels of receptor antibodies were neither before nor after vaccination correlated or co-variant with chronological age, gender, total IgG, rain natriuretic pro-peptide (pBNP) or interleukin 6 (IL-6), excluding these factors as potential analytic confounders (
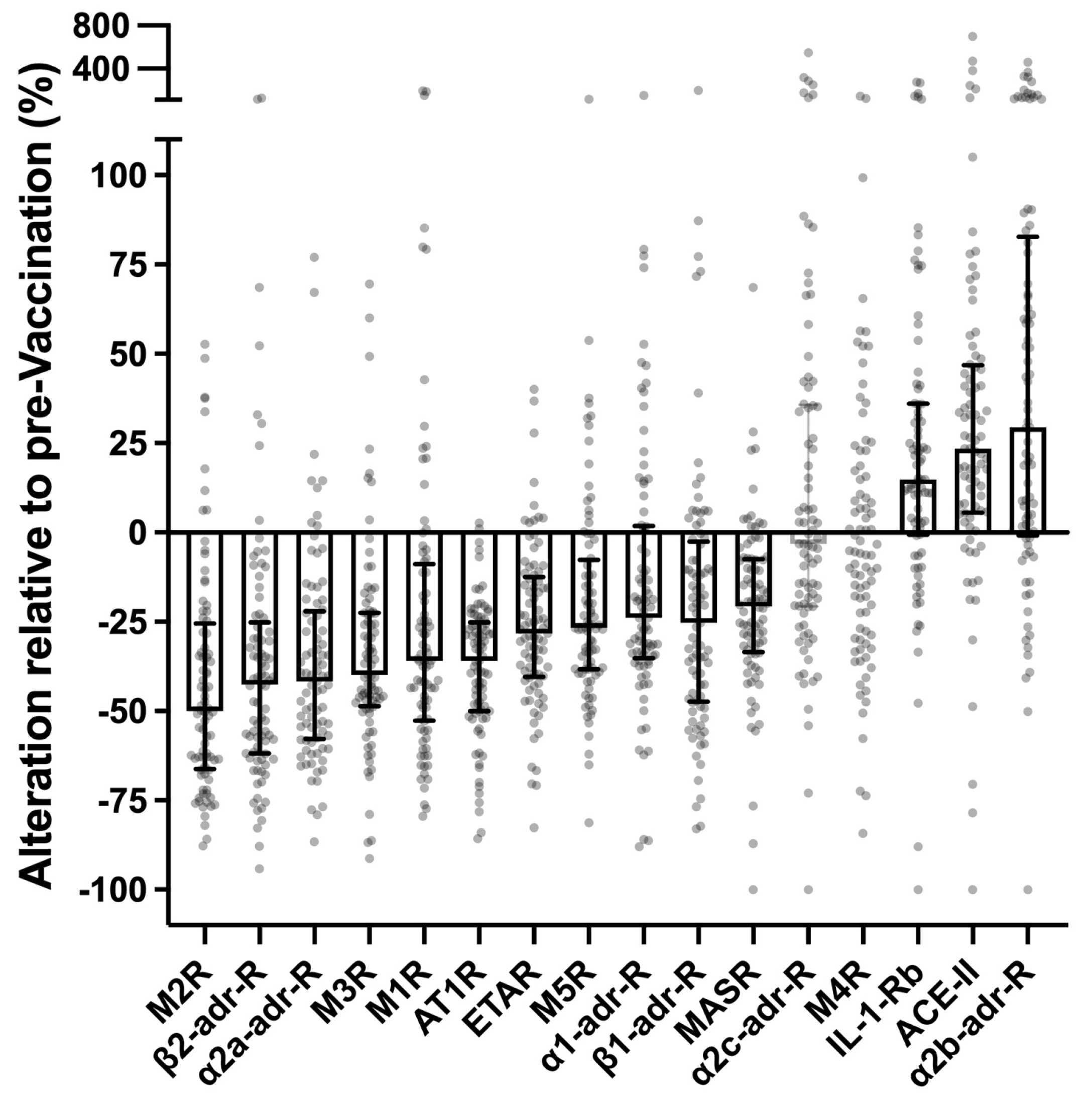
Figure S1). Almost all potentially disease-relevant receptor-antibodies differed markedly between pre- and post-vaccination sera (
Figure 1): In post-vaccination sera the levels of antibodies against AT1R, ETAR, M1R, M2R, M3R, M5R, α1-adr-R, α2a-adr-R, β1-adr-R, β2-adr-R and MASR were markedly lower (in median by 25–50%), while the levels of antibodies against IL-1-Rb, ACE-II and α2b-adr-R were markedly higher (in the median by 15–25%). Only two of the analyzed receptor antibodies (α2c-adr-R and M4R) were unaffected by vaccination. Vaccination-responses of circulating receptor antibodies were highly significant (p < 0.0001, paired t-test) and persisted for at least six months after the last vaccination-shot. It should be emphasized, that the marked impact of SARS-CoV-2-vaccination on circulating levels of certain receptor antibodies was observed in healthy individuals not exhibiting any long-termed disease symptoms following vaccination. These alterations can therefore be considered a normal (non-pathological and non-pathognomonic) reaction or adaptation of humoral receptor-autoimmunity to vaccinations with SARS-CoV-2 mRNA-vaccines.
3.2. GPCR-antibodies in post-vaccination controls and PACVS-affected subjects
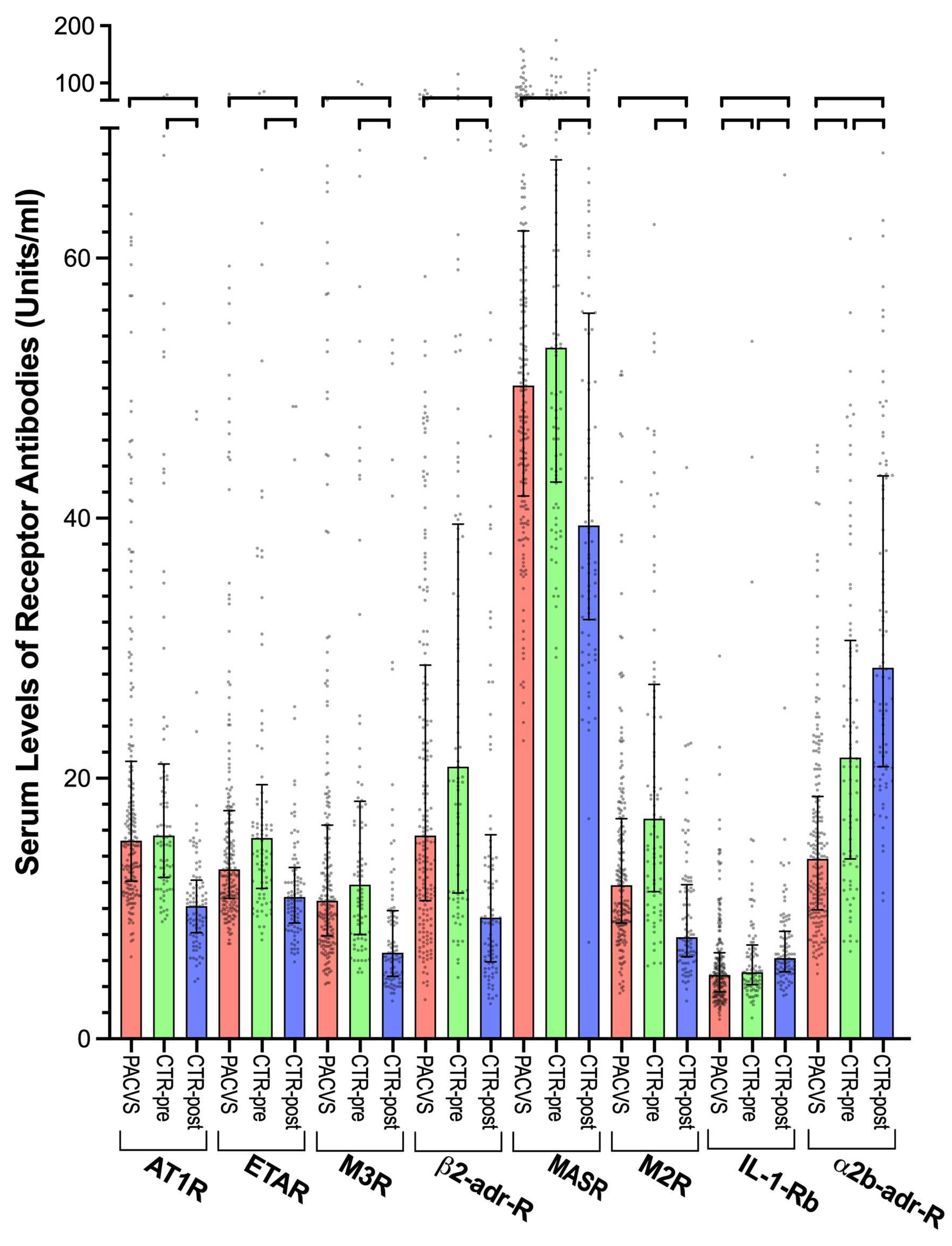
A subset of eight of the analyzed receptor antibodies differed significantly (p < 0.0001) between post-vaccination sera (6 months after the last vaccination) of the control cohort and post-vaccination sera (> 5 months after the last vaccination) of PACVS-afflicted persons (
Figure 2, compare red with blue columns). Six of these antibodies (AT1R, ETAR, M2R, M3R, β2-adr-R, MASR) were significantly (p < 0.0001) higher in PACVS- subjects than in post-vaccination controls. Coincidentally, these six receptor antibodies exhibited in controls vaccination-associated decreases (
Figure 1). Consequently, in the PACVS-subjects, the serum levels of these antibodies were higher than in post-vaccination controls (
Figure 2, compare red with blue columns) while similar to pre-vaccination controls (
Figure 2, compare red and green columns). In contrast, antibodies against IL-1-Rb and α2b-adr-R were significantly (p < 0.0001) lower in PACVS subjects than in controls (both pre- and post-vaccination), while exhibiting vaccination-associated increases in controls (
Figure 1). It should be noted that the above PACVS-associated alterations of circulating receptor antibodies were not associated with any particular vaccination regimen listed in
Table S1.
The 95% confidence intervals of the eight receptor antibodies that were different in PACVS did not overlap between PACVS-subjects and post-vaccination controls (
Figure 2 and
Table 1), suggesting that these receptor antibodies might provide biomarkers allowing serological discrimination of PACVS from the normal post-vaccination state. This assumption was tested by analysis of receiver-operator-characteristics (ROC). All eight candidate receptor antibodies exhibited significant areas under the ROC curve (
Table 2 and
Figure S2). The sensitivities for discriminating PACVS-subjects from post-vaccination controls at 95% specificity (based on the confidence limits of the post-vaccination controls) ranged from 40-90% (
Table 2), which indicates that not all of these receptor antibodies had a similar pre-dictive power. Moreover, cross-correlation analysis of the above receptor antibodies (
Figure 3) revealed two clusters of significant covariance (Spearman’s R ≥ 0.7, p < 0.0001), the one consisting of AT1R, ETAR, M3R, β2-adr-R and the other consisting of α2b-adr-R and M2R. Of note, PACVS-relevant receptor antibodies were not correlated (Spearman’s R < 0.7, p > 0.1) with total IgG, COVID-serology (SAB, NAB), gender, age, or body mass index (BMI), excluding these factors as confounders (
Figure 3). Optimal discrimination of PACVS subjects from post-vaccination controls was obtained based on increases in AT1R, and MASR and decreases of IL-1-Rb and α2b-adr-R relative to the 95% confidence limits of healthy post-vaccination controls. Under these conditions, AT1R and α2b-adr-R exhibited good sensitivities (90.1 and 89.5%, resp.) and MASR and IL-1-Rb exhibited moderate sensitivities (71.8 and 66.5%, resp.) for PACVS (
Table 2).
3.3. Discrimination of PACVS from post-vaccination controls by interleukins
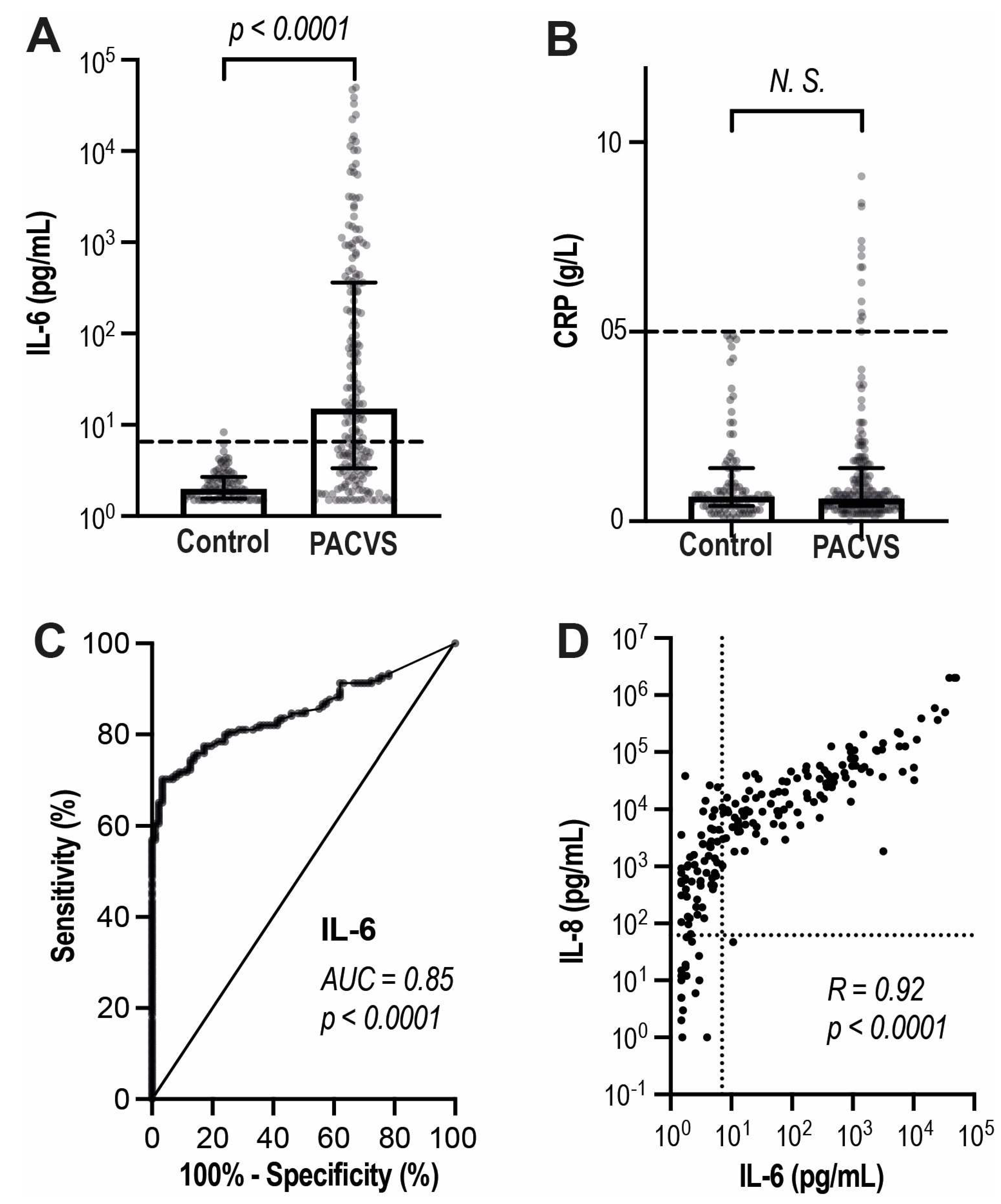
We compared PACVS subjects and post-vaccination controls by a basic panel of potentially relevant laboratory markers encompassing total IgG (IgG), SARS-CoV-2 serology (SAB, NAB), cardiac markers (proBNP, Troponin T) and inflammation markers (IL-6 and C-reactive protein, CRP). Among these parameters, only IL-6 imposed as a potentially discriminative biomarker of PACVS (
Table 2 and
Figure 4). IL-6 was increased above reference level in most PACVS-subjects and significantly (p < 0.0001) higher than in post-vaccination controls (
Figure 4A). ROC-curves indicated a reasonable discrimination of PACVS-subjects from post-vaccination controls by IL-6 (AUC = 0.85,
Figure 4C). Interestingly, CRP was similar in PACVS- subjects and controls
Figure 4B, while in PACVS, the increased levels of IL-6 were linearly correlated with even more pronounced increases in interleukin 8 (IL-8) (
Figure 4D). IL-6 and IL-8 where thus indicated as further biomarkers of PACVS.
3.4. Exclusion of SARS-CoV-2 infection/COVID-19 reconvalescence as confounder of PACVS
Persons suffering from florid SARS-CoV-2 infection were excluded from the study. However, a subgroup of included PACVS-afflicted subjects (76/191) exhibited NAB-reactivity. 52 of these reported SARS-CoV-2 infection or COVID-19 re-convalescence in their case histories. The other 24 NAB-positive participants appeared to have undergone SARS-CoV-2 infection without noting. In contrast, post-vaccination controls were selected for absence of NAB-reactivity and no report of SARS-CoV-2 infection during the monitoring period. To exclude NAB-reactivity as possible confounder of PACVS diagnostic biomarker, we compared the candidate biomarkers of PACVS (listed in
Table 2) between NAB-positive (N = 76) and NAB-negative (N = 115) PACVS subjects. All candidate PACVS-biomarkers exhibited slightly higher values in NAB-positive than in NAB-negative PACVS-subjects (
Table 3, first three columns from the left). Most of these differences were small (median effect size < 10%) and insignificant (p > 0.5, U-test). Only AT1R and M3R exhibited more pronounced (median effect sizes 12.8 and 20.2%, resp.) and marginally significant (p ≤ 0.05, U-test) increases in NAB-positive as compared to NAB-negative PACVS-subjects. However, corresponding differences of AT1R and M3R between NAB-negative PACVS-subjects and NAB-negative post-vaccination controls were much greater (median effect sizes > 40%) and of higher significance (p < 0.0001) (
Table 3, compare columns 4 and 5). Thus, we assume that the confounding effect of SARS-CoV-2 infections on PACVS-diagnostic is very minor and negligible.
4. Discussion
4.1. Salient findings
We present a set of observations potentially relevant for the understanding and diagnosis of PACVS, a dysautonomia syndrome associated with, and possibly triggered by, SARS-CoV-2 mRNA-vaccination [
1]:
In healthy persons not affected by PACVS, the repertoire of receptor antibodies involved in cardiovascular regulation and immune homeostasis undergoes a long-termed adjustment following SARS-CoV-2 mRNA-vaccination.
Above adjustment seems blunted, absent or even inversed in persons who present the clinical phenotype of PACVS after SARS-CoV-2 mRNA-vaccination.
PACVS-afflicted persons can be distinguished from individuals subjected to SARS-CoV-2 mRNA-vaccination without developing PACVS by serum levels of IL-6/IL-8 and of antibodies against AT1R and α2b-adr-R.
4.2. Limitations
Our study is restricted to SARS-CoV-2 mRNA-vaccines, for which we had an appropriate control cohort. Whether our findings apply to chronic sequelae following other types of SARS-CoV-2- vaccination remains to be investigated.
The clinical PACVS-phenotype here studied is based on a long list of symptoms. It is heterogeneous and possibly encompasses more than one clinical entity. Moreover, selection of studied PACVS cases is biased by the exclusion of 71 applicants with potentially confounding co-morbidities or medications who could nevertheless suffer for PACVS.
The PACVS-cohort was recruited five or more months after vaccination. Matching pre-vaccination serum from these same persons could not be obtained. Consequently, vaccination-associated serological alterations of the PACVS-cohort could not be determined intra-dividually, but had to be judged by comparison with a matched post-vaccination control cohort.
Receptor antibodies were determined by IgG-binding to the native receptors. We and others have previously demonstrated that such antibodies can modulate receptor function in several ways [
38], but functional properties of receptor antibodies were not directly assessed in this study.
4.3. The physiological response of receptor antibodies to SARS-CoV-2 mRNA-vaccination
In persons not affected by PACVS, only two of 16 tested receptor antibody species remained unaltered following SARS-CoV-2 mRNA-vaccination, whereas 11 were decreased and three were increased for a prolonged period of time. This robust and durable response was prevalent in a healthy cohort. Therefore, it probably represents a physiological vaccination-response of the receptor antibody repertoire comprising two distinct features:
Downregulation of a cluster of receptor antibodies targeting the renin-angiotensin-aldosterone system and other components of cardiovascular regulation. Incidentally, some of these receptor antibodies are frequently increased in POTS [
20,
23,
24], ME/CFS [
18,
22,
25], severe COVID-19 [
28,
29,
30,
31,
32], chronic heart failure [
39,
40], and allograft rejection [
41]. The most distinctive candidate of this cluster is the AT1R antibody.
Upregulation of two receptor antibodies. One of these, the IL-1-Rb antibody, is thought to play a role in immune homeostasis [
35] and rheumatic diseases [
42]. The other one, the α2b-adr-R antibody, has no obvious disease-association. The receptor thereby targeted plays a role in central blood pressure adaptation [
43].
4.4. Putative pathogenic role of blunted receptor antibody adaptation in PACVS
Receptor antibody levels in serum of PACVS-affected persons were dissimilar from the post-vaccination state and similar to the pre-vaccination state of persons not suffering from PACVS. Thus, PACVS seems associated with lack or attenuation of the physiological adjustment of the receptor antibody repertoire following SARS-CoV-2 mRNA vaccination. This conclusion could not be corroborated by irrefutable evidence, because vaccination-naïve serum could not be obtained post festum from the PACVS-affected persons.
Many of the receptor antibodies down-regulated in the healthy persons and elevated in the PACVS subjects have previously been implicated as disease markers, risk factors, pathogens or even therapy targets in POTS [
20,
23,
24], ME/CFS [
18,
22,
25], severe COVID-19 [
28,
29,
30,
31,
32], chronic heart failure [
39,
40], allograft rejection [
41], rheumatic diseases [
42] and various other syndromes and diseases [
44]. It is plausible to assume that vaccination-associated downregulation of these receptor antibodies possibly protects from the above diseases and syndromes, while lack or attenuation thereof mimics them, at least in part. Conversely, the IL-1Rb-antibody decreased in the PACVS subjects targets a receptor involved in cytokine release [
35,
42]. Upregulation of this antibody could therefore play a role in the limitation of inflammatory responses to SARS-CoV-2 mRNA vaccination. Lack thereof could contribute to persistence of increases in IL-6, which distinguish the PACVS cohort from the normal post-vaccination state. Interestingly, PACVS-associated upregulation of IL-6 is correlated to an even more pronounced upregulation of IL-8, which has also been observed in post-COVID-19 ME/CFS [
33]. Vaccination-associated alterations of the α2b-adr-R antibody and PACVS-associated alterations thereof have to our knowledge no disease relevance, although α-adrenergic receptor antibodies have been implied in severe COVID-19 [
31].
In summary, these considerations give rise to the attractive speculation that PACVS could result from un-ability to respond to SARS-CoV-2 mRNA vaccination with protective adjustments of the receptor antibody repertoire entailing phenotypic mimicry of syndromes associated with corresponding aberrations of receptor antibodies, e.g., POTS, ME/CFS, and certain rheumatic diseases [
42]. It should be noted, that PACVS as presented by the participants this studied appears distinct from various acute autoimmune-phenomena casuistically reported in the context of SARS-CoV-2 vaccination [
5].
4.5. The blood marker signature of PACVS
Irrespectively of the putative pathogenetic role of receptor antibodies in PACVS, a combination of two index receptor antibodies (AT1R and α2b-adr-R) in conjunction with IL-6 allows discrimination of PACVS from the normal post-vaccination state with a cumulative sensitivity and specificity of up to 90%. However, increases in IL-6 [
45], IL-8 [
33] and AT1R antibodies [
28] have also been observed in long COVID-19 and post-COVID-19 ME/CFS. Thus, further studies will be required to find out, whether the suggested blood marker signature similarly distinguishes PACVS from vaccination-unrelated forms of potentially confounding diseases such as long-COVID-19 and ME/CSF, which were excluded from this study. It is conceivable that the discriminative power of PACVS diagnostic can be improved by adding further independent blood markers identified in this study, most notably antibodies against MASR and IL-1-Rb.
5. Conclusions
The fraction of vaccinated persons suffering from PACVS is unknown. Current estimates assume an incidence of 0.02% amounting to 40,000 affected persons in Germany alone. These patients are currently not treated appropriately for several reasons: (i) The number of unreported cases is high, because diagnostic criteria are not established. It is not even generally accepted that the syndrome exists. (ii) The number of false-positive cases is high, because PACVS is similar to various diseases and syndromes unrelated to vaccination. Moreover, sequelae of undetected SARS-CoV-2 infections could be erroneously blamed on SARS-CoV-2 vaccination. Due to these factors, PACVS is currently not/rarely diagnosed in terms of a somatic disease. Instead, PACVS cases tend to be classified as psychosomatic or discarded as irrelevant or imaginary.
Our study may help to improve this unsatisfactory situation in two ways: We provide evidence of PACVS as a somatic disease by linking a clinical phenotype with specific pathognomonic alterations of serological markers. Thereby we suggest diagnostic criteria for an objective discrimination of PACVS from the healthy post-vaccination condition. These criteria may not be sufficiently specific for separating PACVS from all confounding diseases, or for the diagnosis of PACVS in clinical health care. However, the proposed laboratory diagnostic can act as a stringent rule-out criterion allowing future PACVS-studies to focus on the probable cases.
Great care was taken to exclude possible confounders from this study (
Figure S3). However, inclusion as defined by the symptoms listed in
Table S2 was less focused. As a consequence, the PACVS phenotype emerging from the present study is heterogeneous and probably encompasses more than one clinical entity. We believe that one objective of future studies should be to draw a clearer and more differentiated clinical picture of PACVS and to use the suggested biomarker signature for patient stratification in a prospective study setting.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure S1: Co-variance of Receptor Antibodies in Serum of healthy Volunteers (n = 89);Figure S2: ROC curves of Receptor Antibodies discriminating PACVS Subjects from post-Vaccination Controls; Figure S3: Flow charts of inclusion of study participants and controls; Table S1: Vaccination History of Participants; Table S2: Meta Data and in-/exclusion criteria of controls and PACVS subjects.
Author Contributions
Conceptualization, F.B. and J.R.; methodology, A.S., F.B., J.R.; validation, K.J.L., F.B., J.R., S.G.M. and M.P.; formal analysis, K.J.L., M.P., S.G.M.; investigation, A.S., A.S.K., F.B., J.R.; resources, F.B., J.R.; data curation, A.S., J.R., F.B., M.S., H.H., K.S.F., A.K.M., S.B., M.P., S.M.; writing—original draft preparation, F.B.; writing—review and editing, A.S., F.B., J.R., K.S.B., H.H., K.J.L., M.U., M.P., S.G.M.; visualization, A.S., F.B.; supervision, F.B., J.R.; project administration, A.S.K., K.S.B., F.B., J.R., funding acquisition, F.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. Measurement of receptor antibodies was provided free of charge by Cell Trend GmbH, Luckenwalde, Germany.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the local Ethics Committee of the Medical Faculty of the Heinrich-Heine University Düsseldorf (study numbers 2022-1948 and 2020-1259).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy concerns of the study participants.
Acknowledgments
Technical assistance is gratefully acknowledged to Alexander Weinstein, Susanne Herrmann, Petra Meirowski and Rosemarie Lott. Support of on-line acquisition and data management is gratefully acknowledged to Aiman Louah, Sascha Grehl and Michael Hoffkamp).
Conflicts of Interest
Harald Heidecke and Kai Schulze-Forster are co-owners and chief executive officers of CellTrend, Luckenwalde, Germany. All authors have no other conflict of interest to declare.
Abbreviations
| α1-adr-R-AB |
Alpha-1 adrenergic receptor antibody |
| α2a-adr-R-AB |
Alpha-2A adrenergic receptor antibody |
| α2b-adr-R-AB |
Alpha-2B adrenergic receptor antibody |
| α2c-adr-R-AB |
Alpha-2C adrenergic receptor antibody |
| ACE-II-AB |
Angiotensin-converting enzyme 2 antibody |
| AT1R-AB |
Angiotensin II type 1 receptor antibody |
| β1-adr-R-AB |
Beta-1 adrenergic receptor antibody |
| β2-adr-R-AB |
Beta-2 adrenergic receptor antibody |
| CRP |
C-reactive protein |
| ETAR-AB |
Endothelin-1 type A receptor antibody |
| IL-1-Rb-AB |
Interleukin-1 receptor type 2 antibody |
| IL-6/-8 |
Interleukin 6/8 |
| M1R-AB |
muscarinic acetylcholine receptor M1 |
| M2R-AB |
muscarinic acetylcholine receptor M2 |
| M3R-AB |
muscarinic acetylcholine receptor M3 |
| M4R-AB |
muscarinic acetylcholine receptor M4 |
| M5R-AB |
muscarinic acetylcholine receptor M5 |
| MASR-AB |
MAS 1 receptor antibody |
| MCAS |
Mastcell activation syndrome |
| ME/CFS |
Myalgic encephalomyelitis/chronic fatigue syndrome |
| NAB |
panIg-reactivity against SARS-CoV-1 nucleocapsid protein |
| pBNP |
pro-brain natriuretic peptide |
| PEM |
Post exertional malaise |
| POTS |
Postural tachycardia syndrome |
| PACVS |
Post-acute COVID-19 vaccination syndrome |
| ROC |
Receiver-operator characteristics |
| SAB |
panIg-reactivity against SARS-CoV-1 spike S1 protein |
| SFN |
Small fiber neuropathy |
References
- Scholkmann, F.; May, C.-A. COVID-19, post-acute COVID-19 syndrome (PACS, “long COVID”) and post-COVID-19 vaccination syndrome (PCVS, “post-COVIDvacsyndrome”):Similarities and differences. Pathology - Research and Prcatice 2023. [Google Scholar] [CrossRef] [PubMed]
- Almas, T.; Rehman, S.; Mansour, E.; Khedro, T.; Alansari, A.; Malik, J.; Alshareef, N.; Nagarajan, V.R.; Al-Awaid, A.H.; Alsufyani, R.; et al. Epidemiology, clinical ramifications, and cellular pathogenesis of COVID-19 mRNA-vaccination-induced adverse cardiovascular outcomes: A state-of-the-heart review. Biomed Pharmacother 2022, 149, 112843. [Google Scholar] [CrossRef]
- Finsterer, J. Neurological side effects of SARS-CoV-2 vaccinations. Acta Neurol Scand 2022, 145, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Pan, J.; Zhang, C.; Sun, X. Cardiovascular Complications of COVID-19 Vaccines. Front Cardiovasc Med 2022, 9, 840929. [Google Scholar] [CrossRef] [PubMed]
- Jara, L.J.; Vera-Lastra, O.; Mahroum, N.; Pineda, C.; Shoenfeld, Y. Autoimmune post-COVID vaccine syndromes: does the spectrum of autoimmune/inflammatory syndrome expand? Clin Rheumatol 2022, 41, 1603–1609. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.J.; Son, C.G. Review of case definitions for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J Transl Med 2020, 18, 289. [Google Scholar] [CrossRef]
- Carruthers, B.M.; van de Sande, M.I.; De Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T.; Staines, D.; Powles, A.C.; Speight, N.; Vallings, R.; et al. Myalgic encephalomyelitis: International Consensus Criteria. J Intern Med 2011, 270, 327–338. [Google Scholar] [CrossRef]
- Fedorowski, A. Postural orthostatic tachycardia syndrome: clinical presentation, aetiology and management. J Intern Med 2019, 285, 352–366. [Google Scholar] [CrossRef]
- Bair, M.J.; Krebs, E.E. Fibromyalgia. Ann Intern Med 2020, 172, ITC33–ITC48. [Google Scholar] [CrossRef]
- Tavee, J.; Zhou, L. Small fiber neuropathy: A burning problem. Cleve Clin J Med 2009, 76, 297–305. [Google Scholar] [CrossRef]
- Valent, P.; Hartmann, K.; Bonadonna, P.; Niedoszytko, M.; Triggiani, M.; Arock, M.; Brockow, K. Mast Cell Activation Syndromes: Collegium Internationale Allergologicum Update 2022. Int Arch Allergy Immunol 2022, 183, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Blitshteyn, S.; Brook, J. Postural tachycardia syndrome (POTS) with anti-NMDA receptor antibodies after human papillomavirus vaccination. Immunol Res 2017, 65, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Arana, J.; Mba-Jonas, A.; Jankosky, C.; Lewis, P.; Moro, P.L.; Shimabukuro, T.T.; Cano, M. Reports of Postural Orthostatic Tachycardia Syndrome After Human Papillomavirus Vaccination in the Vaccine Adverse Event Reporting System. J Adolesc Health 2017, 61, 577–582. [Google Scholar] [CrossRef]
- Martínez-Lavín, M. Hypothesis: Human papillomavirus vaccination syndrome—small fiber neuropathy and dysautonomia could be its underlying pathogenesis. Clinical Rheumatology 2015, 34, 1165–1169. [Google Scholar] [CrossRef] [PubMed]
- Hineno, A.; Ikeda, S.I. A Long-Term Observation on the Possible Adverse Effects in Japanese Adolescent Girls after Human Papillomavirus Vaccination. Vaccines (Basel) 2021, 9. [Google Scholar] [CrossRef]
- Afrin, L.B.; Dempsey, T.T.; Weinstock, L.B. Post-HPV-Vaccination Mast Cell Activation Syndrome: Possible Vaccine-Triggered Escalation of Undiagnosed Pre-Existing Mast Cell Disease? Vaccines (Basel) 2022, 10. [Google Scholar] [CrossRef]
- Agmon-Levin, N.; Zafrir, Y.; Kivity, S.; Balofsky, A.; Amital, H.; Shoenfeld, Y. Chronic fatigue syndrome and fibromyalgia following immunization with the hepatitis B vaccine: another angle of the ‘autoimmune (auto-inflammatory) syndrome induced by adjuvants’ (ASIA). Immunologic Research 2014, 60, 376–383. [Google Scholar] [CrossRef]
- Loebel, M.; Grabowski, P.; Heidecke, H.; Bauer, S.; Hanitsch, L.G.; Wittke, K.; Meisel, C.; Reinke, P.; Volk, H.D.; Fluge, O.; et al. Antibodies to beta adrenergic and muscarinic cholinergic receptors in patients with Chronic Fatigue Syndrome. Brain, behavior, and immunity 2016, 52, 32–39. [Google Scholar] [CrossRef]
- Li, H.; Yu, X.; Liles, C.; Khan, M.; Vanderlinde-Wood, M.; Galloway, A.; Zillner, C.; Benbrook, A.; Reim, S.; Collier, D.; et al. Autoimmune basis for postural tachycardia syndrome. J Am Heart Assoc 2014, 3, e000755. [Google Scholar] [CrossRef]
- Li, H.; Zhang, G.; Forsythe, E.; Okamoto, L.E.; Yu, X. Implications of Antimuscarinic Autoantibodies in Postural Tachycardia Syndrome. J Cardiovasc Transl Res 2022, 15, 438–440. [Google Scholar] [CrossRef]
- Kharraziha, I.; Axelsson, J.; Ricci, F.; Di Martino, G.; Persson, M.; Sutton, R.; Fedorowski, A.; Hamrefors, V. Serum Activity Against G Protein-Coupled Receptors and Severity of Orthostatic Symptoms in Postural Orthostatic Tachycardia Syndrome. J Am Heart Assoc 2020, 9, e015989. [Google Scholar] [CrossRef] [PubMed]
- Freitag, H.; Szklarski, M.; Lorenz, S.; Sotzny, F.; Bauer, S.; Philippe, A.; Kedor, C.; Grabowski, P.; Lange, T.; Riemekasten, G.; et al. Autoantibodies to Vasoregulative G-Protein-Coupled Receptors Correlate with Symptom Severity, Autonomic Dysfunction and Disability in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J Clin Med 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Gunning, W.T., 3rd; Kvale, H.; Kramer, P.M.; Karabin, B.L.; Grubb, B.P. Postural Orthostatic Tachycardia Syndrome Is Associated With Elevated G-Protein Coupled Receptor Autoantibodies. J Am Heart Assoc 2019, 8, e013602. [Google Scholar] [CrossRef] [PubMed]
- Gunning, W.T., 3rd; Stepkowski, S.M.; Kramer, P.M.; Karabin, B.L.; Grubb, B.P. Inflammatory Biomarkers in Postural Orthostatic Tachycardia Syndrome with Elevated G-Protein-Coupled Receptor Autoantibodies. J Clin Med 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Szklarski, M.; Freitag, H.; Lorenz, S.; Becker, S.C.; Sotzny, F.; Bauer, S.; Hartwig, J.; Heidecke, H.; Wittke, K.; Kedor, C.; et al. Delineating the Association Between Soluble CD26 and Autoantibodies Against G-Protein Coupled Receptors, Immunological and Cardiovascular Parameters Identifies Distinct Patterns in Post-Infectious vs. Non-Infection-Triggered Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Front Immunol 2021, 12, 644548. [Google Scholar] [CrossRef] [PubMed]
- Scheibenbogen, C.; Loebel, M.; Freitag, H.; Krueger, A.; Bauer, S.; Antelmann, M.; Doehner, W.; Scherbakov, N.; Heidecke, H.; Reinke, P.; et al. Immunoadsorption to remove ss2 adrenergic receptor antibodies in Chronic Fatigue Syndrome CFS/ME. PLoS One 2018, 13, e0193672. [Google Scholar] [CrossRef]
- Scheibenbogen, C.; Sotzny, F.; Hartwig, J.; Bauer, S.; Freitag, H.; Wittke, K.; Doehner, W.; Scherbakov, N.; Loebel, M.; Grabowski, P. Tolerability and Efficacy of s.c. IgG Self-Treatment in ME/CFS Patients with IgG/IgG Subclass Deficiency: A Proof-of-Concept Study. J Clin Med 2021, 10. [Google Scholar] [CrossRef]
- Rodriguez-Perez, A.I.; Labandeira, C.M.; Pedrosa, M.A.; Valenzuela, R.; Suarez-Quintanilla, J.A.; Cortes-Ayaso, M.; Mayan-Conesa, P.; Labandeira-Garcia, J.L. Autoantibodies against ACE2 and angiotensin type-1 receptors increase severity of COVID-19. J Autoimmun 2021, 122, 102683. [Google Scholar] [CrossRef]
- Miedema, J.; Schreurs, M.; van der Sar-van der Brugge, S.; Paats, M.; Baart, S.; Bakker, M.; Hoek, R.; Dik, W.A.; Endeman, H.; Van Der Velden, V.; et al. Antibodies Against Angiotensin II Receptor Type 1 and Endothelin A Receptor Are Associated With an Unfavorable COVID19 Disease Course. Front Immunol 2021, 12, 684142. [Google Scholar] [CrossRef]
- Jiang, Y.; Duffy, F.; Hadlock, J.; Raappana, A.; Styrchak, S.; Beck, I.; Mast, F.D.; Miller, L.R.; Chour, W.; Houck, J.; et al. Angiotensin II receptor I auto-antibodies following SARS-CoV-2 infection. PLoS One 2021, 16, e0259902. [Google Scholar] [CrossRef]
- Cabral-Marques, O.; Halpert, G.; Schimke, L.F.; Ostrinski, Y.; Vojdani, A.; Baiocchi, G.C.; Freire, P.P.; Filgueiras, I.S.; Zyskind, I.; Lattin, M.T.; et al. Autoantibodies targeting GPCRs and RAS-related molecules associate with COVID-19 severity. Nat Commun 2022, 13, 1220. [Google Scholar] [CrossRef] [PubMed]
- Wallukat, G.; Hohberger, B.; Wenzel, K.; Furst, J.; Schulze-Rothe, S.; Wallukat, A.; Honicke, A.S.; Muller, J. Functional autoantibodies against G-protein coupled receptors in patients with persistent Long-COVID-19 symptoms. J Transl Autoimmun 2021, 4, 100100. [Google Scholar] [CrossRef] [PubMed]
- Kedor, C.; Freitag, H.; Meyer-Arndt, L.; Wittke, K.; Hanitsch, L.G.; Zoller, T.; Steinbeis, F.; Haffke, M.; Rudolf, G.; Heidecker, B.; et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat Commun 2022, 13, 5104. [Google Scholar] [CrossRef]
- Bornstein, S.R.; Voit-Bak, K.; Donate, T.; Rodionov, R.N.; Gainetdinov, R.R.; Tselmin, S.; Kanczkowski, W.; Muller, G.M.; Achleitner, M.; Wang, J.; et al. Chronic post-COVID-19 syndrome and chronic fatigue syndrome: Is there a role for extracorporeal apheresis? Mol Psychiatry 2021. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Marques, O.; Marques, A.; Giil, L.M.; De Vito, R.; Rademacher, J.; Gunther, J.; Lange, T.; Humrich, J.Y.; Klapa, S.; Schinke, S.; et al. GPCR-specific autoantibody signatures are associated with physiological and pathological immune homeostasis. Nat Commun 2018, 9, 5224. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Kuechler, A.S.; Weinhold, S.; Boege, F.; Adams, O.; Muller, L.; Babor, F.; Bennstein, S.B.; Pham, T.U.; Hejazi, M.; Reusing, S.B.; et al. A Diagnostic Strategy for Gauging Individual Humoral Ex Vivo Immune Responsiveness Following COVID-19 Vaccination. Vaccines (Basel) 2022, 10. [Google Scholar] [CrossRef]
- Bornholz, B.; Weidtkamp-Peters, S.; Schmitmeier, S.; Seidel, C.A.; Herda, L.R.; Felix, S.B.; Lemoine, H.; Hescheler, J.; Nguemo, F.; Schafer, C.; et al. Impact of human autoantibodies on beta1-adrenergic receptor conformation, activity, and internalization. Cardiovascular research 2013, 97, 472–480. [Google Scholar] [CrossRef]
- Boivin-Jahns, V.; Jahns, R. GPCR-autoantibodies in chronic heart failure. Front Biosci (Landmark Ed) 2018, 23, 2065–2081. [Google Scholar] [CrossRef]
- Wallukat, G.; Schimke, I. Agonistic autoantibodies directed against G-protein-coupled receptors and their relationship to cardiovascular diseases. Semin Immunopathol 2014, 36, 351–363. [Google Scholar] [CrossRef]
- Dragun, D.; Muller, D.N.; Brasen, J.H.; Fritsche, L.; Nieminen-Kelha, M.; Dechend, R.; Kintscher, U.; Rudolph, B.; Hoebeke, J.; Eckert, D.; et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med 2005, 352, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Marques, O.; Riemekasten, G. Functional autoantibodies targeting G protein-coupled receptors in rheumatic diseases. Nat Rev Rheumatol 2017, 13, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Kable, J.W.; Murrin, L.C.; Bylund, D.B. In vivo gene modification elucidates subtype-specific functions of α2-adrenergic receptors. Journal of Pharmacology and Experimental Therapeutics 2000, 293, 1–7. [Google Scholar]
- Meyer, C.; Heidecke, H. Antibodies Against GPCR. Front Biosci (Landmark Ed) 2018, 23, 2177–2194. [Google Scholar] [PubMed]
- Yin, J.X.; Agbana, Y.L.; Sun, Z.S.; Fei, S.W.; Zhao, H.Q.; Zhou, X.N.; Chen, J.H.; Kassegne, K. Increased interleukin-6 is associated with long COVID-19: a systematic review and meta-analysis. Infect Dis Poverty 2023, 12, 43. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).