Submitted:
01 September 2023
Posted:
04 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Specimen and solution preparation
2.3. Pressure impregnation and heat treatment
2.4. Specimen characterization
3. Results and discussion
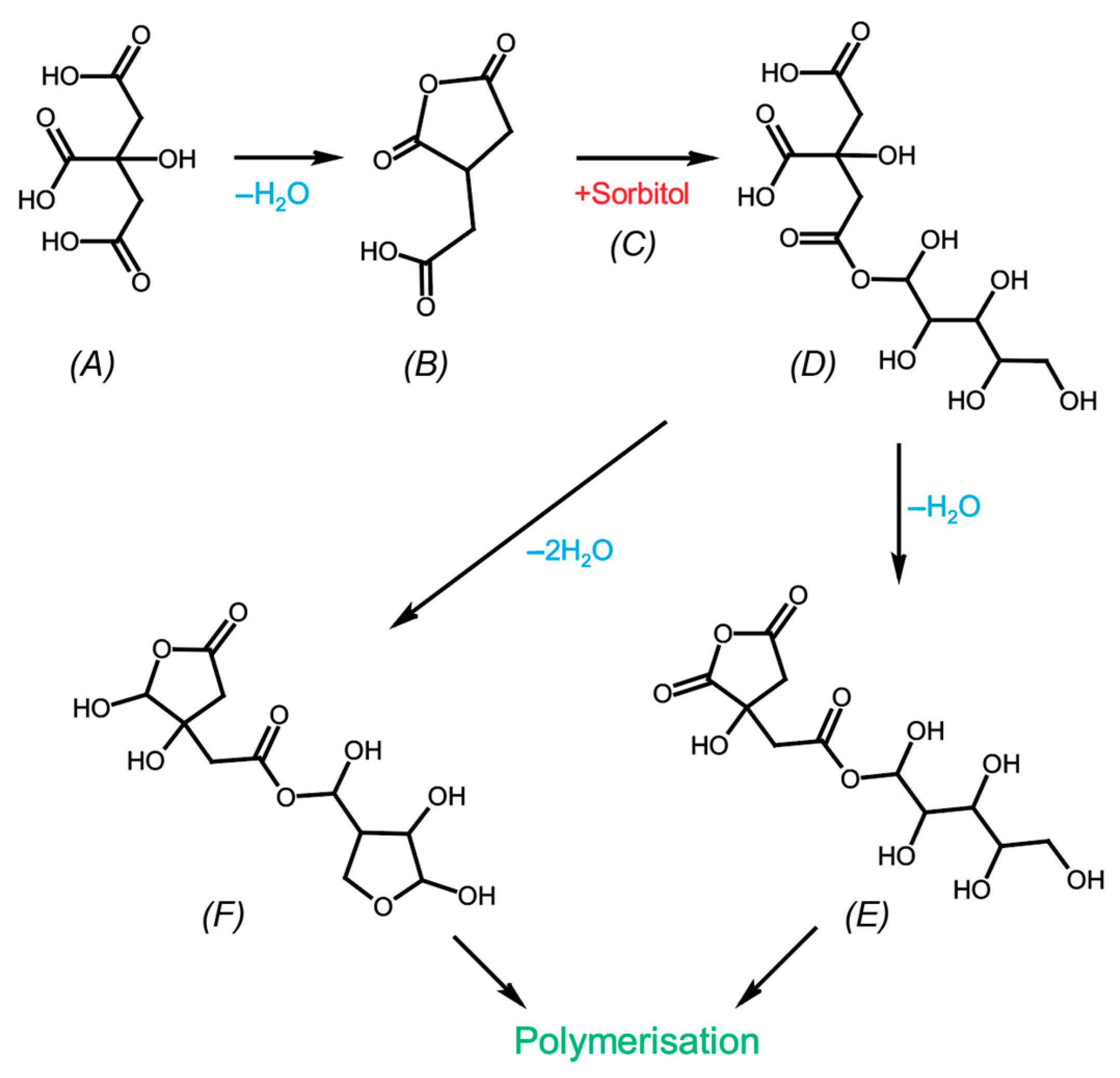
3.1. Impregnation and heat treatment
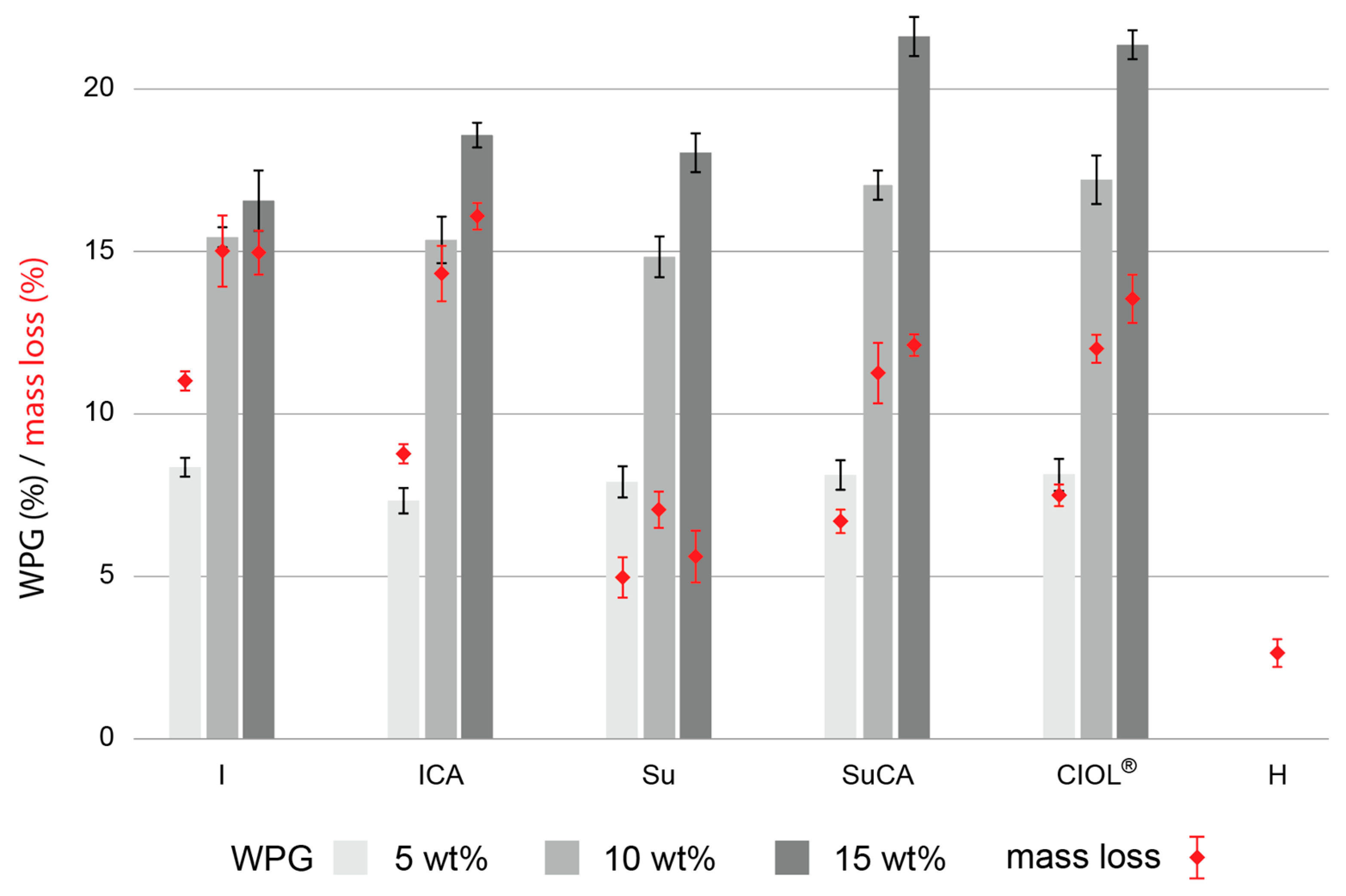
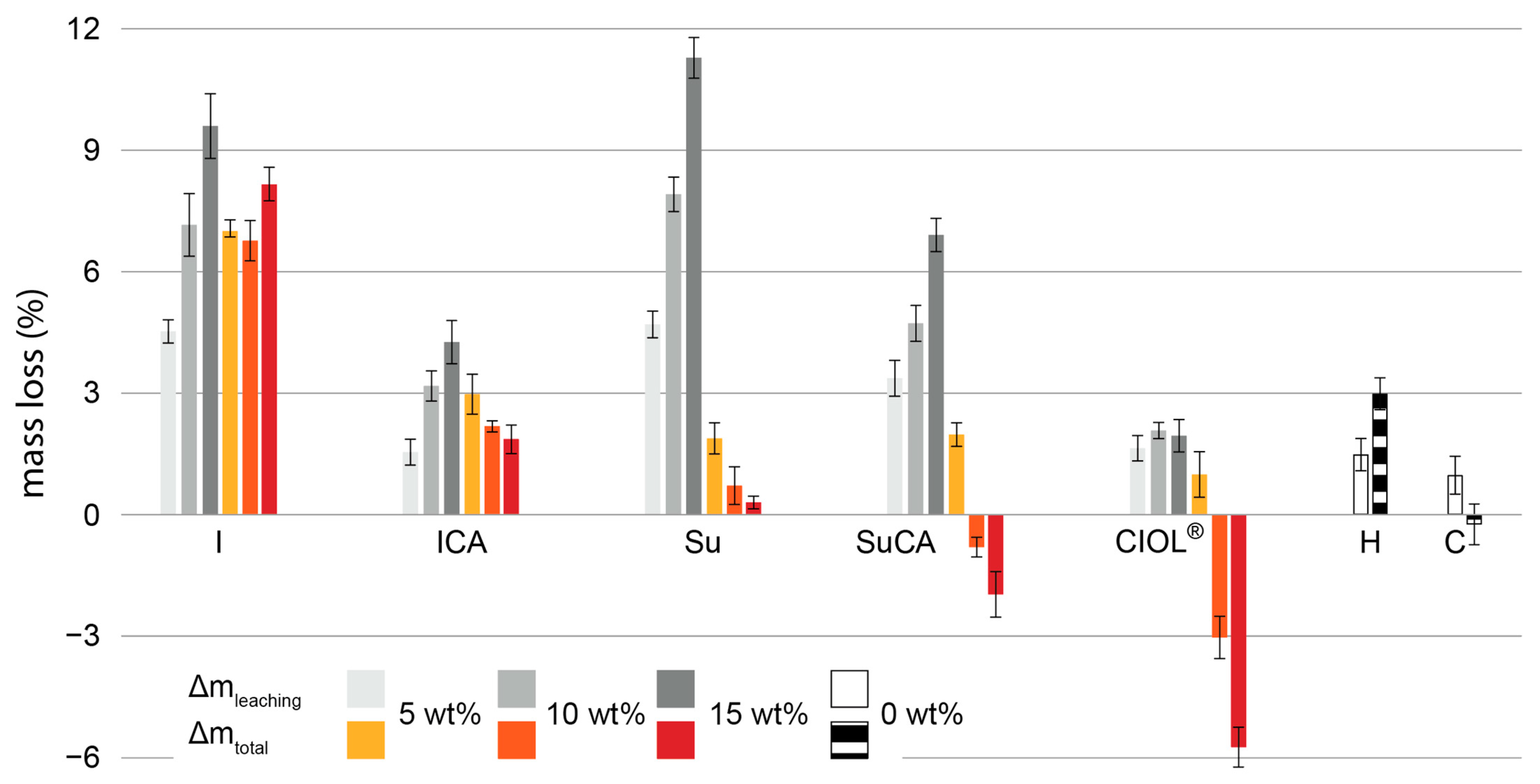
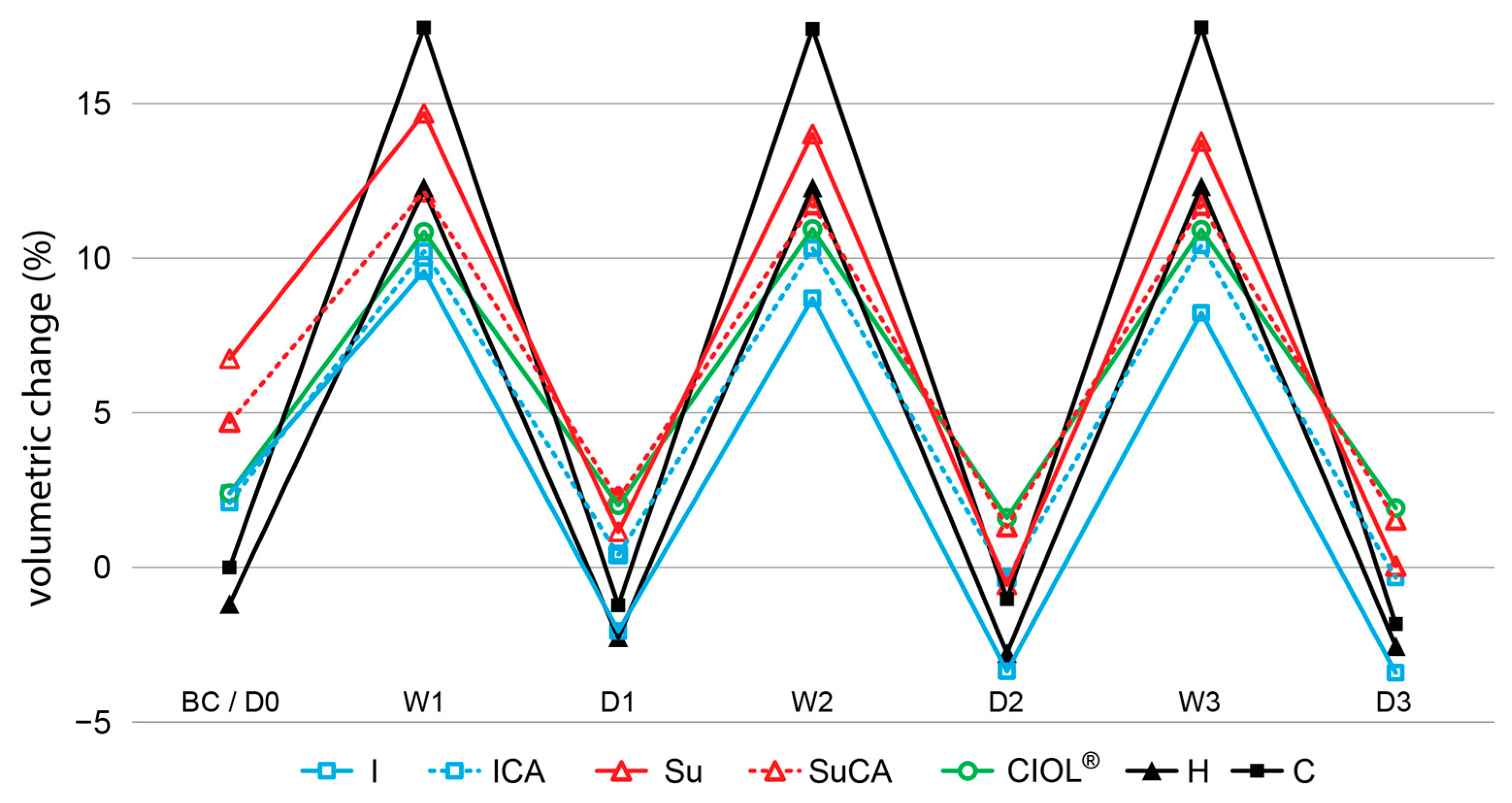
3.2. Anti-swelling efficiency and water stability
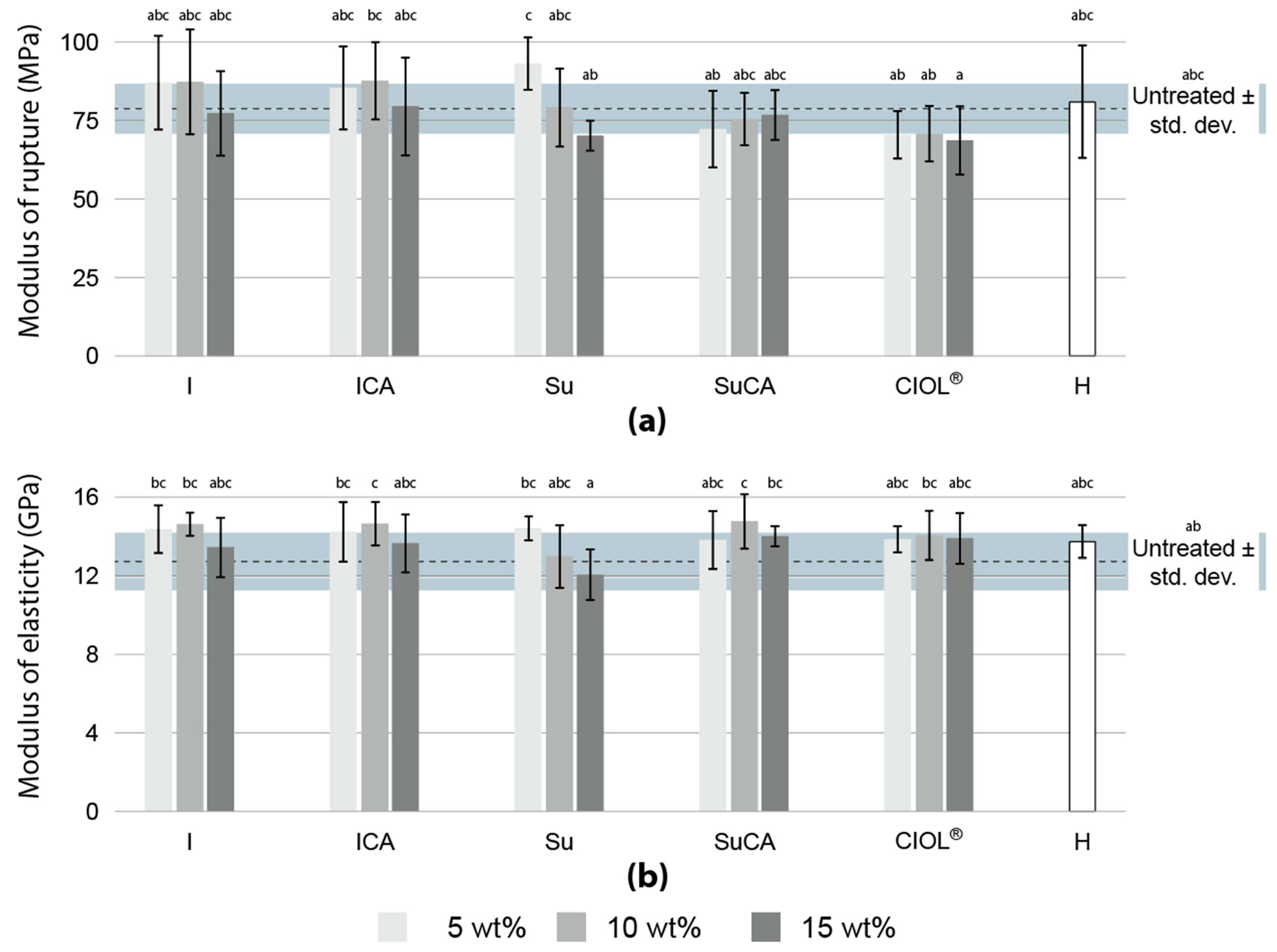
3.3. Bending performance
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hill, C.A.S. Wood Modification: Chemical, Thermal and Other Processes, 1st ed.; Wiley: Hoboken, NJ, USA, 2006; ISBN 978-0-470-02172-9. [Google Scholar]
- Sandberg, D.; Kutnar, A.; Karlsson, O.; Jones, D. Wood Modification Technologies: Principles, Sustainability, and the Need for Innovation, 1st ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2021; ISBN 978-0-367-76782-2. [Google Scholar]
- Esteves, B.M.; Pereira, H.M. Wood Modification by Heat Treatment: A Review. BioRes 2008, 4, 370–404. [Google Scholar] [CrossRef]
- Klauditz, W.; Stegmann, G. Beiträge zur Kenntnis des Ablaufes, und der Wirkung thermischer Reaktionen bei der Bildung von Holzwerkstoffen. Holz Als Roh- Und Werkstoff 1955, 13, 434–440. [Google Scholar] [CrossRef]
- Kollmann, F.; Fengel, D. Änderungen der chemischen Zusammensetzung von Holz durch thermische Behandlung. Holz Als Roh- Und Werkstoff 1965, 23, 461. [Google Scholar] [CrossRef]
- Gérardin, P. New Alternatives for Wood Preservation Based on Thermal and Chemical Modification of Wood—A Review. Ann. For. Sci. 2016, 73, 559–570. [Google Scholar] [CrossRef]
- Mai, C.; Militz, H. Wood Modification. In Springer Handbook of Wood Science and Technology; Niemz, P., Teischinger, A., Sandberg, D., Eds.; Springer Handbooks Series; Springer International Publishing: Cham, Switzerland, 2023; pp. 873–910. ISBN 978-3-030-81314-7. [Google Scholar]
- Tjeerdsma, B.F.; Militz, H. Chemical Changes in Hydrothermal Treated Wood: FTIR Analysis of Combined Hydrothermal and Dry Heat-Treated Wood. Holz Roh Werkst 2005, 63, 102–111. [Google Scholar] [CrossRef]
- Dirol, D.; Guyonnet, R. Durability by Rectification Process. In Proceedings of the International Research Group Wood Pre, Section 4-Processes, No IRG/WP 93-40015, Orlando, FL, USA, 16–21 May 1993. [Google Scholar]
- Tjeerdsma, B.F.; Boonstra, M.; Pizzi, A.; Tekely, P.; Militz, H. Characterisation of Thermally Modified Wood: Molecular Reasons for Wood Performance Improvement. Holz Als Roh- Und Werkstoff 1998, 56, 149–153. [Google Scholar] [CrossRef]
- Weiland, J.J.; Guyonnet, R. Study of Chemical Modifications and Fungi Degradation of Thermally Modified Wood Using DRIFT Spectroscopy. Holz Als Roh- Und Werkstoff 2003, 61, 216–220. [Google Scholar] [CrossRef]
- Boonstra, M.J.; Van Acker, J.; Tjeerdsma, B.F.; Kegel, E.V. Strength Properties of Thermally Modified Softwoods and Its Relation to Polymeric Structural Wood Constituents. Ann. For. Sci. 2007, 64, 679–690. [Google Scholar] [CrossRef]
- Kim, G.; Yun, K.; Kim, J. Effect of Heat Treatment on the Decay Resistance and the Bending Properties of Radiata Pine Sapwood. Mater. Org. 1998, 32, 101–108. [Google Scholar]
- Ohmae, K.; Minato, K.; Norimoto, M. The Analysis of Dimensional Changes Due to Chemical Treatments and Water Soaking for Hinoki (Chamaecyparis Obtusa) Wood. Holzforschung 2002, 56, 98–102. [Google Scholar] [CrossRef]
- Verma, P.; Junga, U.; Militz, H.; Mai, C. Protection Mechanisms of DMDHEU Treated Wood against White and Brown Rot Fungi. Holzforschung 2009, 63, 371–378. [Google Scholar] [CrossRef]
- Show, P.L.; Oladele, K.O.; Siew, Q.Y.; Aziz Zakry, F.A.; Lan, J.C.-W.; Ling, T.C. Overview of Citric Acid Production from Aspergillus niger. Front. Life Sci. 2015, 8, 271–283. [Google Scholar] [CrossRef]
- Yang, C.Q. FT-IR Spectroscopy Study of the Ester Crosslinking Mechanism of Cotton Cellulose. Text. Res. J. 1991, 61, 433–440. [Google Scholar] [CrossRef]
- Bischof Vukusic, S.; Katovic, D.; Schramm, C.; Trajkovic, J.; Sefc, B. Polycarboxylic Acids as Non-Formaldehyde Anti-Swelling Agents for Wood. Holzforschung 2006, 60, 439–444. [Google Scholar] [CrossRef]
- Zoldners, J.; Kiseleva, T. Modification of Hemicelluloses with Polycarboxylic Acids. Holzforschung 2013, 67, 567–571. [Google Scholar] [CrossRef]
- Fang, G.; Li, J.; Xu, X. The Intermediate of Crosslinking Reaction between Wood and Polycarboxylic Acid. Sci. Silvae Sin. 2000, 36, 51–54. [Google Scholar] [CrossRef]
- Katović, D.; Trajković, J.; Bischof Vukusic, S.; Bogoslav, S. Alternative Agents and Methods for Chemical Modification of Wood. Drv. Ind. 2004, 55, 175–180. [Google Scholar]
- Xie, Y.; Krause, A.; Militz, H.; Turkulin, H.; Richter, K.; Mai, C. Effect of Treatments with 1,3-Dimethylol-4,5-Dihydroxy-Ethyleneurea (DMDHEU) on the Tensile Properties of Wood. Holzforschung 2007, 61, 43–50. [Google Scholar] [CrossRef]
- Lee, S.H.; Md Tahir, P.; Lum, W.C.; Tan, L.P.; Bawon, P.; Park, B.-D.; Osman Al Edrus, S.S.; Abdullah, U.H. A Review on Citric Acid as Green Modifying Agent and Binder for Wood. Polymers 2020, 12, 1692. [Google Scholar] [CrossRef]
- Feng, X.; Xiao, Z.; Sui, S.; Wang, Q.; Xie, Y. Esterification of Wood with Citric Acid: The Catalytic Effects of Sodium Hypophosphite (SHP). Holzforschung 2014, 68, 427–433. [Google Scholar] [CrossRef]
- L’Hostis, C.; Thévenon, M.-F.; Fredon, E.; Gérardin, P. Improvement of Beech Wood Properties by in situ Formation of Polyesters of Citric and Tartaric Acid in Combination with Glycerol. Holzforschung 2018, 72, 291–299. [Google Scholar] [CrossRef]
- Umemura, K.; Sugihara, O.; Kawai, S. Investigation of a New Natural Adhesive Composed of Citric Acid and Sucrose for Particleboard. J. Wood Sci. 2013, 59, 203–208. [Google Scholar] [CrossRef]
- Essoua, G.G.; Blanchet, P.; Landry, V.; Beauregard, R. Pine Wood Treated with a Citric Acid and Glycerol Mixture: Biomaterial Performance Improved by a Bio-Byproduct. BioResources 2016, 11, 3049–3072. [Google Scholar] [CrossRef]
- He, X.; Xiao, Z.; Feng, X.; Sui, S.; Wang, Q.; Xie, Y. Modification of Poplar Wood with Glucose Crosslinked with Citric Acid and 1,3-Dimethylol-4,5-dihydroxy ethyleneurea. Holzforschung 2016, 70, 47–53. [Google Scholar] [CrossRef]
- Berube, M.-A.; Schorr, D.; Ball, R.J.; Landry, V.; Blanchet, P. Determination of in situ Esterification Parameters of Citric Acid-Glycerol Based Polymers for Wood Impregnation. J. Polym. Environ. 2018, 26, 970–979. [Google Scholar] [CrossRef]
- Larnøy, E.; Karaca, A.; Gobakken, L.R.; Hill, C.A.S. Polyesterification of Wood Using Sorbitol and Citric Acid under Aqueous Conditions. Int. Wood Prod. J. 2018, 9, 66–73. [Google Scholar] [CrossRef]
- Guo, W.; Xiao, Z.; Wentzel, M.; Emmerich, L.; Xie, Y.; Militz, H. Modification of Scots Pine with Activated Glucose and Citric Acid: Physical and Mechanical Properties. BioRes 2019, 14, 3445–3458. [Google Scholar] [CrossRef]
- Centolella, A.P.; Razor, B.G. Polyesters of Citric Acid and Sorbitol 1972. U.S. Patent No. 3,661,955, 9 May 1972. [Google Scholar]
- Doll, K.M.; Shogren, R.L.; Willett, J.L.; Swift, G. Solvent-Free Polymerization of Citric Acid And D-Sorbitol. J. Polym. Sci. A Polym. Chem. 2006, 44, 4259–4267. [Google Scholar] [CrossRef]
- Mubarok, M.; Militz, H.; Dumarçay, S.; Gérardin, P. Beech Wood Modification Based on in situ Esterification with Sorbitol and Citric Acid. Wood Sci. Technol. 2020, 54, 479–502. [Google Scholar] [CrossRef]
- Beck, G. Leachability and Decay Resistance of Wood Polyesterified with Sorbitol and Citric Acid. Forests 2020, 11, 650. [Google Scholar] [CrossRef]
- Morais, A.R.C.; Pinto, J.V.; Nunes, D.; Roseiro, L.B.; Oliveira, M.C.; Fortunato, E.; Bogel-Łukasik, R. Imidazole: Prospect Solvent for Lignocellulosic Biomass Fractionation and Delignification. ACS Sustain. Chem. Eng. 2016, 4, 1643–1652. [Google Scholar] [CrossRef]
- Grylewicz, A.; Spychaj, T.; Zdanowicz, M. Thermoplastic Starch/Wood Biocomposites Processed with Deep Eutectic Solvents. Compos. Part A Appl. Sci. Manuf. 2019, 121, 517–524. [Google Scholar] [CrossRef]
- Del Menezzi, C.; Amirou, S.; Pizzi, A.; Xi, X.; Delmotte, L. Reactions with Wood Carbohydrates and Lignin of Citric Acid as a Bond Promoter of Wood Veneer Panels. Polymers 2018, 10, 833. [Google Scholar] [CrossRef]
- Patil, M.; Rajput, S. Succinimides: Synthesis, Reaction and Biological Activity. Int. J. Pharm. Pharm. Sci. 2014, 6, 8–14. [Google Scholar]
- Li, X.; Zhang, Z.; Suo, S.; Qiao, Y.; Du, B.; Deng, H.; Hao, Z.; Luo, H. Microstructure of Silver Coating of Cyanide-Free Brush Plating Based on Multicomponent Coordination System. IOP Conf. Ser. Mater. Sci. Eng. 2019, 688, 033047. [Google Scholar] [CrossRef]
- Larnøy, E.; Biørnstad, J.; Treu, A. Advances in CIOL-Protected Wood—The Road Towards Commercialization. In Proceedings of the 17th annual meeting of the Northern European Network for Wood Science and Engineering, Kaunas, Lithuania, 14–15 October 2021; pp. 89–90. [Google Scholar]
- Treu, A.; Larnøy, E.; Bjørnstad, J. CIOL®-Protection of Wood—An Update. In Proceedings of the IRG Annual Meeting, IRG/WP 22-40932, Bled, Slovenia, 29 May–2 June 2022. [Google Scholar]
- Kim, I.; Karlsson, O.; Jones, D.; Mantanis, G.; Sandberg, D. Dimensional Stabilisation of Scots Pine (Pinus sylvestris L.) Sapwood by Reaction with Maleic Anhydride and Sodium Hypophosphite. Eur. J. Wood Prod. 2021, 79, 589–596. [Google Scholar] [CrossRef]
- Stamm, A.J. Wood and Cellulose Science; Ronald Press: New York, NY, USA, 1964. [Google Scholar]
- Altgen, M.; Willems, W.; Militz, H. Wood Degradation Affected by Process Conditions during Thermal Modification of European Beech in a High-Pressure Reactor System. Eur. J. Wood Prod. 2016, 74, 653–662. [Google Scholar] [CrossRef]
- CEN. EN 408: 2010 + A1: 2012; Timber Structures. Structural Timber and Glued Laminated Timber. Determination of Some Physical and Mechanical Properties. European Committee for Standardization: Brussels, Belgium, 2010.
- Umemura, K.; Ueda, T.; Kawai, S. Characterization of Wood-Based Molding Bonded with Citric Acid. J. Wood Sci. 2012, 58, 38–45. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Dahryn Trivedi, A.B.; Gunin Saikia, G.N. Physical and Structural Characterization of Biofield Treated Imidazole Derivatives. Nat. Prod. Chem. Res. 2015, 3, 1000187. [Google Scholar] [CrossRef]
- Tudorachi, N.; Chiriac, A.P. TGA/FTIR/MS Study on Thermal Decomposition of Poly(succinimide) and Sodium Poly(aspartate). Polym. Test. 2011, 30, 397–407. [Google Scholar] [CrossRef]
- Alén, R.; Kotilainen, R.; Zaman, A. Thermochemical Behavior of Norway Spruce (Picea abies) at 180–225 °C. Wood Sci. Technol. 2002, 36, 163–171. [Google Scholar] [CrossRef]
- Wentzel, M.; Altgen, M.; Militz, H. Analyzing Reversible Changes in Hygroscopicity of Thermally Modified Eucalypt Wood from Open and Closed Reactor Systems. Wood Sci. Technol. 2018, 52, 889–907. [Google Scholar] [CrossRef]
- Wang, D.; Fu, F.; Lin, L. Molecular-Level Characterization of Changes in the Mechanical Properties of Wood in Response to Thermal Treatment. Cellulose 2022, 29, 3131–3142. [Google Scholar] [CrossRef]

| TreatmentID | Total concentration of solution (wt%) | Imidazole (wt%) | Succinimide (wt%) | Citric acid (wt%) | Sorbitol (wt%) | Heat treatment temperature (°C) |
|---|---|---|---|---|---|---|
| I5 | 5 | 5.0 | - | - | - | 220 |
| I10 | 10 | 10.0 | - | - | - | 220 |
| I15 | 15 | 15.0 | - | - | - | 220 |
| ICA5 | 5 | 2.8 | - | 2.2 | - | 220 |
| ICA10 | 10 | 5.6 | - | 4.4 | - | 220 |
| ICA15 | 15 | 8.4 | - | 6.6 | - | 220 |
| Su5 | 5 | - | 5.0 | - | - | 220 |
| Su10 | 10 | - | 10.0 | - | - | 220 |
| Su15 | 15 | - | 15.0 | - | - | 220 |
| SuCA5 | 5 | - | 2.8 | 2.2 | - | 220 |
| SuCA10 | 10 | - | 5.6 | 4.4 | - | 220 |
| SuCA15 | 15 | - | 8.4 | 6.6 | - | 220 |
| CIOL®51 | 5 | - | - | 3.8 | 1.2 | 220 |
| CIOL®101 | 10 | - | - | 7.6 | 2.4 | 220 |
| CIOL®151 | 15 | - | - | 11.4 | 3.6 | 220 |
| H | - | - | - | - | - | 220 |
| C | - | - | - | - | - | - |
| Imidazole | Imidazole + citric acid | Succinimide | Succinimide + citric acid | Citric acid + sorbitol | H | C | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (wt%) | 5 | 10 | 15 | 5 | 10 | 15 | 5 | 10 | 15 | 5 | 10 | 15 | 5 | 10 | 15 | - | - |
| EMC | 4.5 | 5.2 | 4.6 | 5.2 | 5.3 | 5.0 | 6.3 | 6.1 | 5.6 | 5.3 | 6.1 | 4.8 | 5.5 | 6.3 | 5.0 | 7.0 | 11.0 |
| ASE 1st cycle | 43.7 | 53.8 | 59.8 | 38.0 | 44.1 | 54.4 | 36.3 | 47.2 | 57.4 | 45.6 | 50.6 | 59.2 | 40.8 | 47.9 | 52.7 | 22.4 | 0 |
| ASE 2nd cycle | 34.5 | 35.2 | 37.1 | 32.7 | 37.0 | 43.4 | 22.7 | 24.2 | 27.2 | 34.2 | 40.5 | 46.5 | 36.4 | 43.4 | 49.9 | 14.7 | - 8.1 |
| ASE 3rd cycle | 29.6 | 33.4 | 31.3 | 29.2 | 34.7 | 38.2 | 19.0 | 21.0 | 17.4 | 31.6 | 37.9 | 41.2 | 35.0 | 41.5 | 47.5 | 11.1 | - 7.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





