Submitted:
01 September 2023
Posted:
04 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
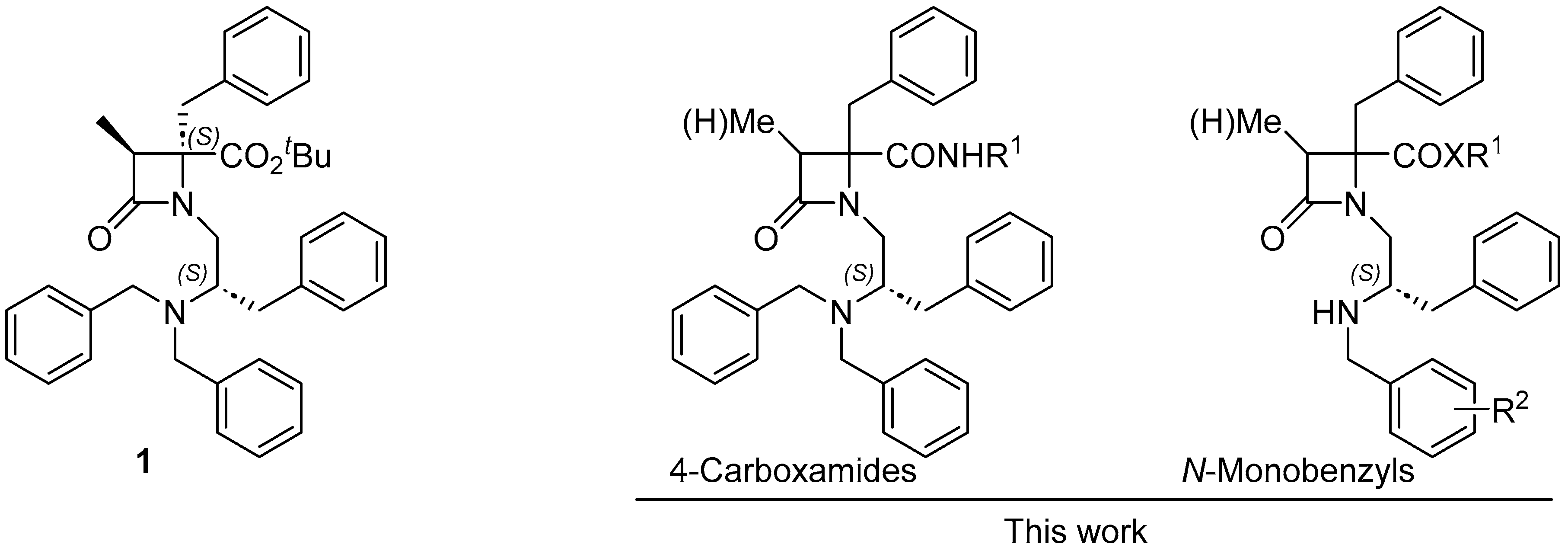
2. Results and Discussion
2.1. 1,4,4-Trisubstituted β–lactam Derivatives
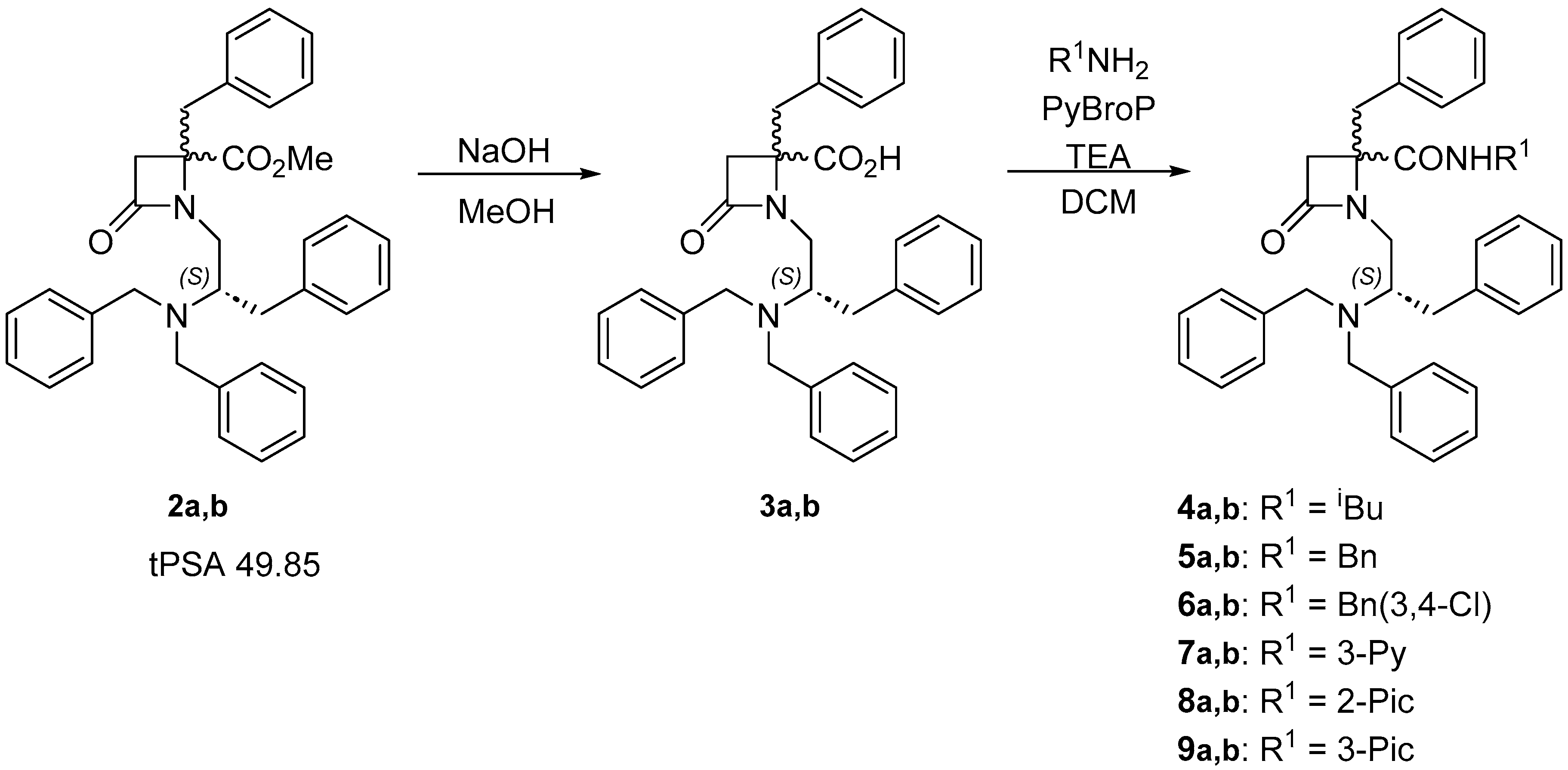
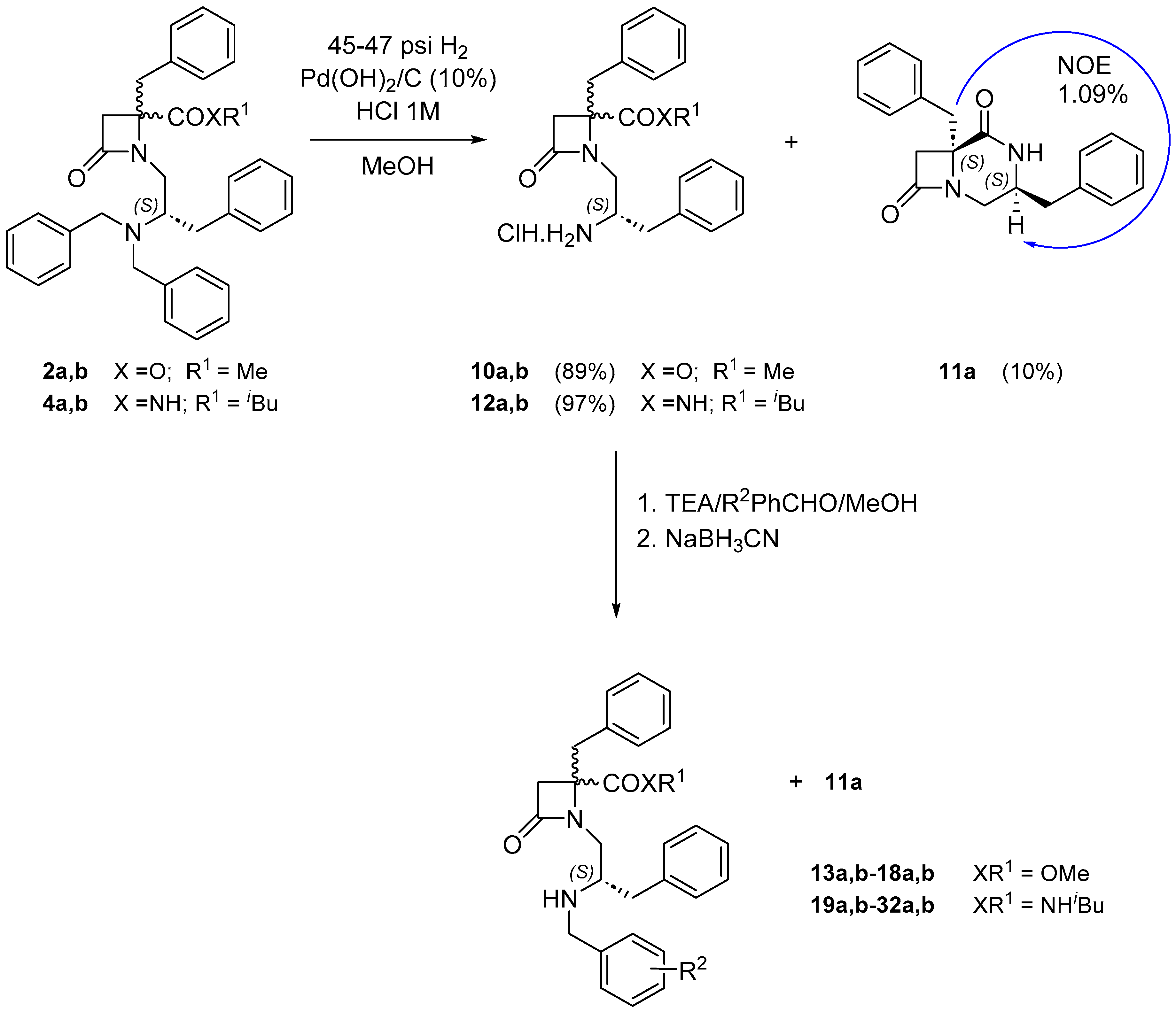
| Compound | R1 | a:b | IC50 (μM) | 95% confidential intervals |
|---|---|---|---|---|
| 2a,b | 1:1.8 | 0.15 ± 1.23 | 0.095 to 0.22 | |
| 4a,b | iBu | 1:1.6 | 0.71 ± 1.20 | 0.49 to 1.03 |
| 5a,b | Bn | 1:2.6 | 0.71 ± 1.27 | 0.43 to 1.16 |
| 6a | (3,4-Cl)Bn | ND | _ | |
| 6a,b | (3,4-Cl)Bn | 1:1.4 | ND | _ |
| 7a,b | 3-Py | 1:1.4 | 11.62 ± 1.30 | 6.73 to 20.06 |
| 8a,b | 2-Pic | 1:1.8 | 1.99 ± 1.26 | 1.24 to 3.17 |
| 9a,b | 3-Pic | 1:1.2 | 10.69 ± 1.38 | 5.52 to 20.73 |
| 1 | 1.06 ± 1.21 | 0.72 to 1.55 | ||
| AMTB | 7.3 ± 1.5 | 8.82 to 7.85 |
| Compound | XR1 | R2 | a:b | IC50 (μM) | 95% confidential intervals |
|---|---|---|---|---|---|
| 2a,b | OMe | - | 1:1.8 | 0.15 ± 1.23 | 0.095 to 0.22 |
| 4a,b | NHiBu | - | 1:1.6 | 0.71 ± 1.20 | 0.49 to 1.03 |
| 11a | - | - | - | ND | ND |
| 13a,b | OMe | H | 1:3.6 | 3.95 ± 1.20 | 2.66 to 5.85 |
| 14a,b | OMe | 3-Me | 1:3.6 | 3.58 ± 1.41 | 1.74 to 7.35 |
| 15a,b | OMe | 4-Me | 1:5.6 | 3.90 ± 1.47 | 1.74 to 8.74 |
| 16a,b | OMe | 3-Br | 1:2 | 1.10 ± 1.38 | 0.56 to 2.16 |
| 17a,b | OMe | (2-Nph)CH2 | 1:4 | 2.60 ± 1.23 | 1.69 to 3.98 |
| 18a,b | OMe | 4-OPr | 1:9.5 | 8.90 ± 1.40 | 4.37 to 18.10 |
| 19a,b | NHiBu | 3-Me | 1:1.5 | 3.97 ± 1.31 | 2.28 to 6.93 |
| 20a,b | NHiBu | 3-Ph | 1:1.1 | 2.03 ± 1.32 | 1.15 to 3.60 |
| 21a,b | NHiBu | 3-F | 1:2.6 | 5.15 ± 1.46 | 2.37 to 11.22 |
| 22a,b | NHiBu | 3-Cl | 1:2.4 | 2.57 ± 1.32 | 1.45 to 4.53 |
| 23a,b | NHiBu | 3-Br | 1:2.9 | 3.30 ± 1.36 | 1.73 to 6.29 |
| 24a,b | NHiBu | 3-I | 1:2.7 | 3.83 ± 1.28 | 2.33 to 6.29 |
| 25a,b | NHiBu | 3-OMe | 1:2.5 | 4.48 ± 1.25 | 2.83 to 7.09 |
| 26a,b | NHiBu | 3-OPh | 1:5.7 | 8.22 ± 1.30 | 4.79 to 14.08 |
| 27a,b | NHiBu | 3-OBn | 1:1.5 | 3.27 ± 1.37 | 1.72 to 6.20 |
| 28a,b | NHiBu | 3-CN | 1:1.1 | 12.10 ± 1.31 | 6.99 to 20.94 |
| 29a,b | NHiBu | 3-NO2 | 1.2:1 | 2.12 ± 1.28 | 1.28 to 3.50 |
| 30a,b | NHiBu | 4-Ph | 1:1.9 | 4.57 ± 1.22 | 3.07 to 6.82 |
| 31a,b | NHiBu | 4-F | 1.1:1 | 8.32 ± 1.30 | 4.87 to 14.22 |
| 32a,b | NHiBu | 3,4-Ph (2-Nph) | 1:1.1 | 1.81 ± 1.21 | 1.22 to 2.68 |
| 1 | 1.06 ± 1.21 | 0.72 to 1.55 | |||
| AMTB | 7.30 ± 1.50 | 8.82 to 7.85 |
2.2. 1,3,4,4-Tetrasubstituted, Enantiopure β–lactam Derivatives
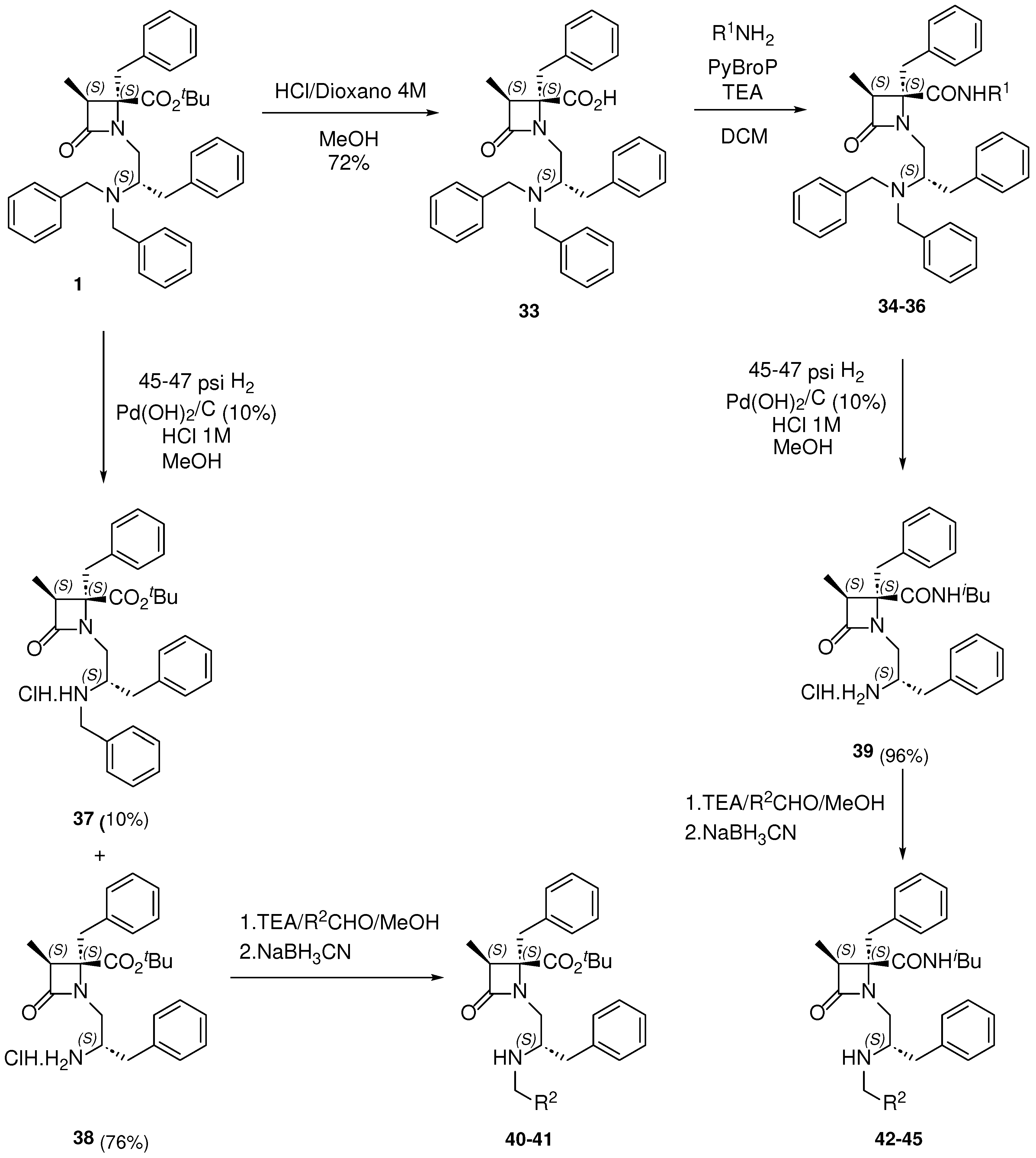
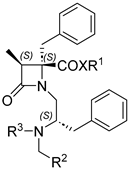 | |||||
|---|---|---|---|---|---|
| Compound | XR1 | R2 | R3 | IC50 (µM) | 95% Confidence Intervals |
| 1 | OtBu | 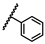 |
Bn | 1.06 ± 1.21 | 0.72 to 1.55 |
| 34 | NHiBu | 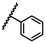 |
Bn | 0.30 ± 1.26 | 0.19 to 0.50 |
| 35 | NHBn | 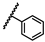 |
Bn | 0.49 ± 1.27 | 0.30 to 0.79 |
| 36 | NHCH2(2-Pic) | 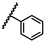 |
Bn | 0.84 ± 1.32 | 0.48 to 1.49 |
| 37 | OtBu | 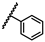 |
H | 3.06 ± 1.25 | 2.67 to 3.51 |
| 40 | OtBu | 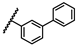 |
H | 6.36 ± 1.41 | 3.08 to 13.17 |
| 41 | OtBu | 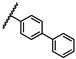 |
H | 9.26 ± 1.41 | 4.44 to 19.35 |
| 42 | NHiBu | 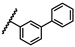 |
H | 2.19 ± 1.36 | 1.15 to 4.17 |
| 43 | NHiBu | 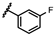 |
H | 4.03 ± 1.35 | 2.14 to 7.60 |
| 44 | NHiBu | 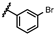 |
H | 3.27 ± 1.31 | 1.86 to 5.75 |
| 45 | NHiBu | 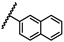 |
H | 3.56 ± 1.40 | 1.74 to 7.31 |
| AMTB | 7.3 ± 1.50 | 6.82 to 7.85 | |||
2.3. Activity in hTRPM8 Channels and Electrophysiology Assays
| Compound | Ca2+ Microfluorography Assays | Patch-Clamp Assay | ||||
|---|---|---|---|---|---|---|
|
rTRPM8 IC50 (µM) |
95% Confidence Intervals |
hTRPM8 IC50 (µM) |
95% Confidence Intervals |
rTRPM8 IC50 (µM) |
95% Confidence Intervals | |
| 1 | 1.06 ± 1.21 | 0.72 to 1.55 | 1.74 ± 1.19 | 1.23 to 2.45 | 0.60 ± 1.66 | 0.20 to 1.76 |
| 34 | 0.30 ± 1.26 | 0.19 to 0.50 | 2.58 ± 1.24 | 1.65 to 4.02 | 0.16 ± 0.82 | 0.10 to 0.271 |
| 35 | 0.49 ± 1.27 | 0.30 to 0.79 | 3.33 ± 1.28 | 2.03 to 5.48 | 0.48 ± 1.33 | 0.18 to 1.23 |
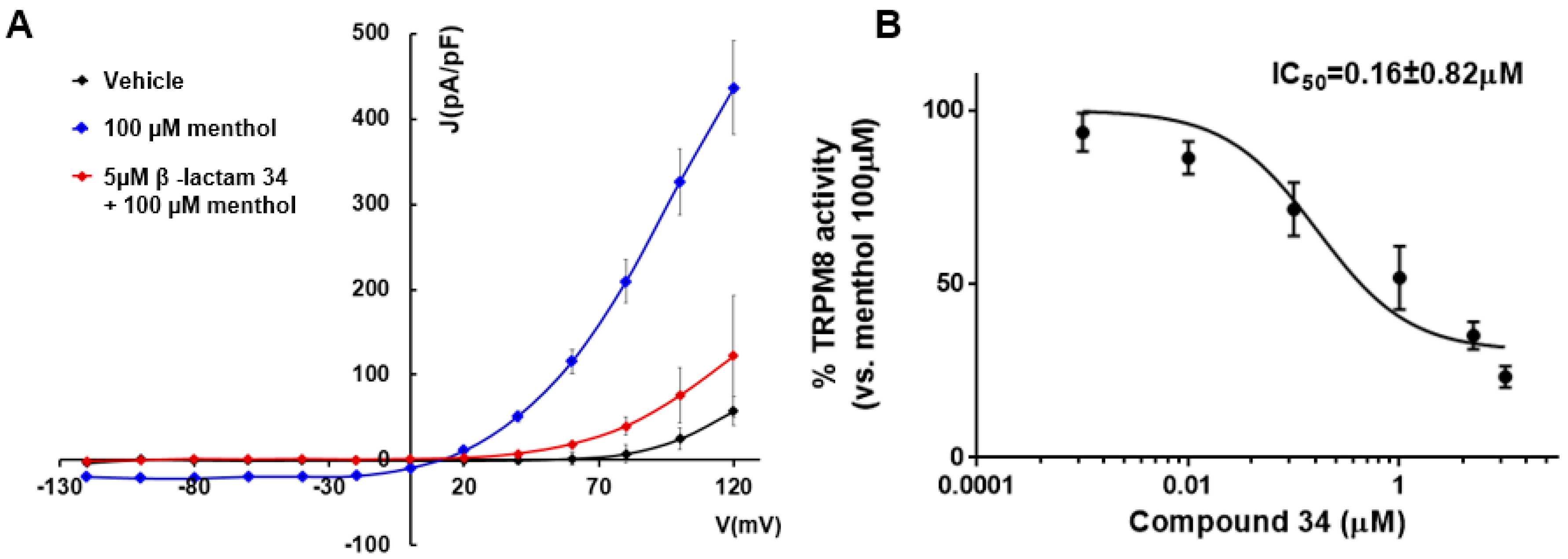
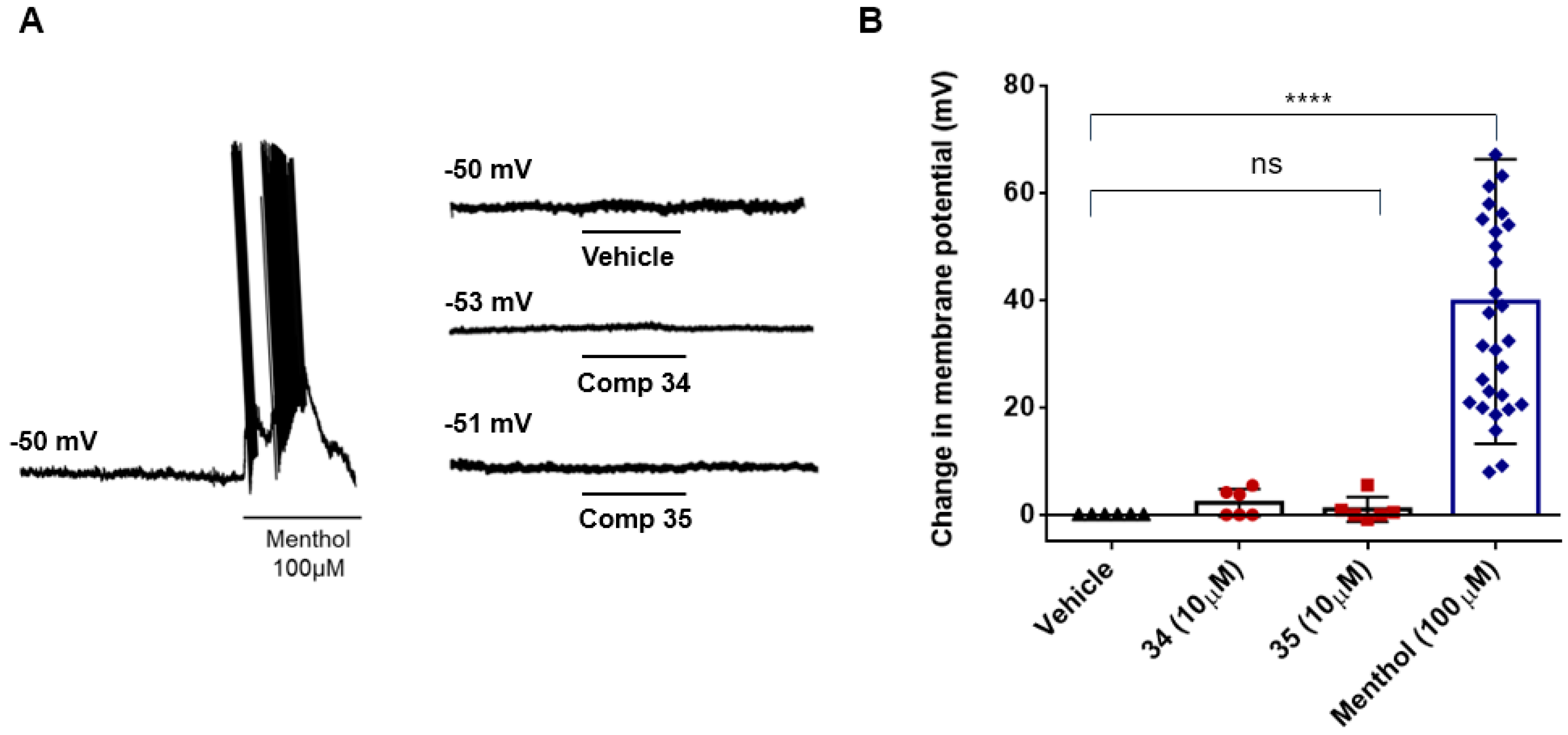
2.4. Activity in Other TRP Channels and Pain-Related Peripheral Receptors
| Compound | % Channel Inhibition at 10 μM | % Binding at 10 μM | ||||||
|---|---|---|---|---|---|---|---|---|
| hTRPV1 | hTRPV3 | hTRPA1 | TRPM3 | ASIC3 | hCGRPR | hCB2 | hM3 | |
| 1 | 19.4 ± 1.7 | −6.3 ± 5.9 | 4.2 ± 1.1 | ND | 6.0 ± 0.9 | −1.0 ± 4.3 | 7.3 ± 0.3 | −4.1 ± 3.1 |
| 34 | 8.9 ± 2.9 | 17.3 ± 5.7 | 2.0 ± 3.4 | 6.9 ± 10.7 | 2.2 ± 9.6 | −11.9 ± 5.2 | 7.0 ± 2.1 | 5.2 ± 3.2 |
2.5. Antinociceptive Activity in a Mouse Model of Chemotherapy-Induced Cold Allodynia
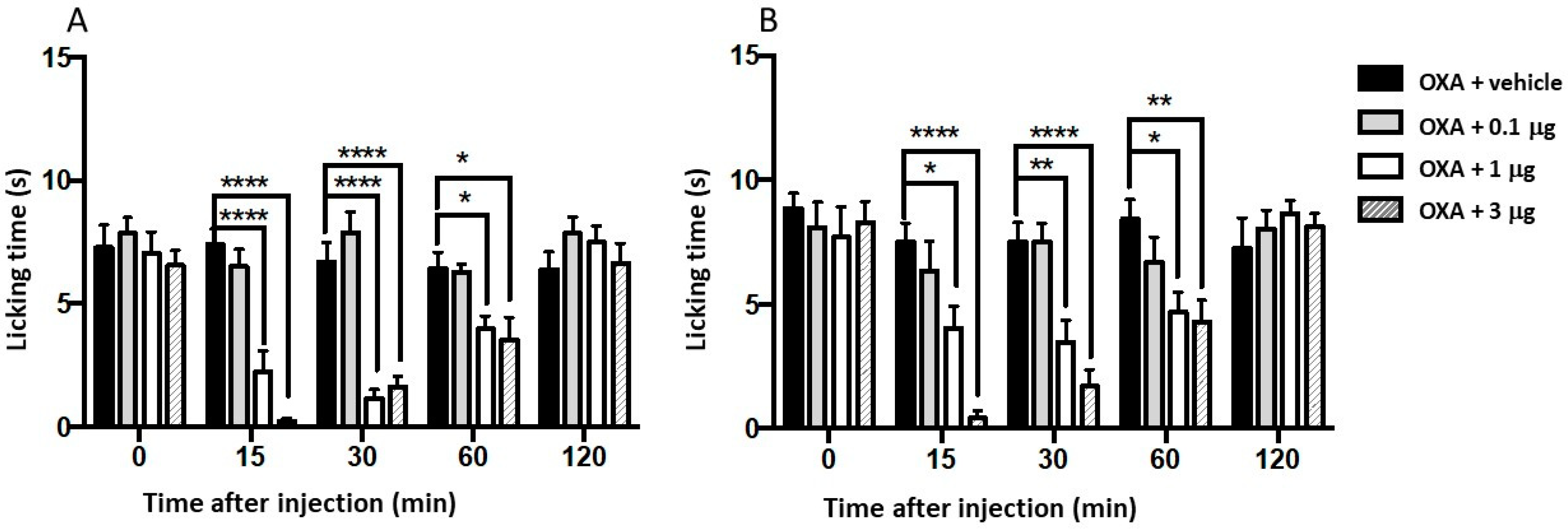
2.6. Insights into the Mode of Interaction of Selected β–lactams with the TRPM8 Channel
| Subsite | Channel Location | 34 | 35 | 37 |
|---|---|---|---|---|
| 1 | Pore, external tower | 20.0 (9.09) | 14.4 (7.82) | 28.2 (9.85) |
| 2 | Pore, high S3-S4, S6 | 13.8 (8.51) | 16.4 (10.11) | 7.4 (7.40) |
| 3 | Inner pore, S5S6, S5 loops | 29.3 (9.81) | 24.6 (9.40) | 31.8 (7.62) |
| 4 | Pore, internal mouth | 6 .0 (11.21) | 7.6 (10.68) | 13.9 (8.49) |
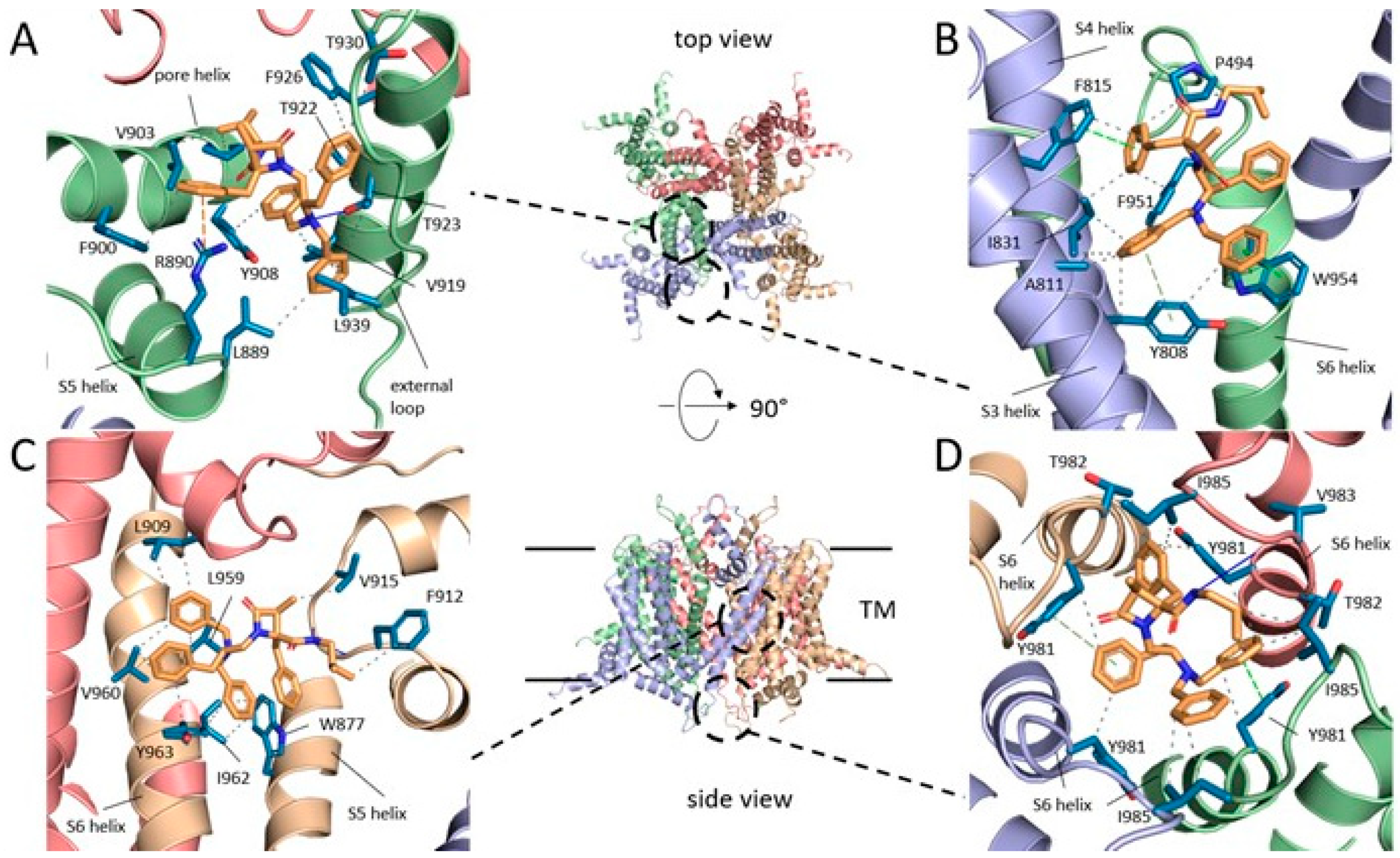
3. Materials and Methods
3.1. Synthesis
3.2. Molecular Modeling
3.3. Biological Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kashio, M.; Tominaga, M. TRP channels in thermosensation. Curr. Opin. Neurobiol. 2022, 75, 102591. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M. Temperature sensing. Igaku no Ayumi 2019, 270, 998–1003. [Google Scholar]
- Yue, L.; Xu, H. TRP channels in health and disease at a glance. J. Cell Sci. 2021, 134, jcs258372. [Google Scholar] [CrossRef]
- Guibert, C.; Ducret, T.; Savineau, J.-P. Expression and physiological roles of TRP channels in smooth muscle cells. Adv. Exp. Med. Biol. 2011, 704, 687–706. [Google Scholar] [PubMed]
- Voets, T.; Vriens, J.; Vennekens, R. Targeting TRP Channels - Valuable Alternatives to Combat Pain, Lower Urinary Tract Disorders, and Type 2 Diabetes? Trends Pharmacol. Sci. 2019, 40, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Cobos, J.C.; Zhang, X.; Motiani, R.K.; Harmon, K.E.; Trebak, M. TRPs to cardiovascular disease. In Proceedings of the TRP Channels Drug Discovery; Springer, 2012; Vol. 2; pp. 3–40. [Google Scholar]
- Nilius, B.; Owsianik, G.; Voets, T.; Peters, J.A. Transient receptor potential cation channels in disease. Physiol. Rev. 2007, 87, 165–217. [Google Scholar] [CrossRef]
- Perez de Vega, M.J.; Gomez-Monterrey, I.; Ferrer-Montiel, A.; Gonzalez-Muniz, R. Transient Receptor Potential Melastatin 8 Channel (TRPM8) Modulation: Cool Entryway for Treating Pain and Cancer. J. Med. Chem. 2016, 59, 10006–10029. [Google Scholar] [CrossRef]
- Izquierdo, C.; Martin-Martinez, M.; Gomez-Monterrey, I.; Gonzalez-Muniz, R. TRPM8 Channels: Advances in Structural Studies and Pharmacological Modulation. Int. J. Mol. Sci. 2021, 22, 8502. [Google Scholar] [CrossRef]
- Zhang, L.; Barritt, G.J. TRPM8 in prostate cancer cells: a potential diagnostic and prognostic marker with a secretory function? Endocr. Relat. Cancer 2006, 13, 27–38. [Google Scholar] [CrossRef]
- Bautista, D.M.; Siemens, J.; Glazer, J.M.; Tsuruda, P.R.; Basbaum, A.I.; Stucky, C.L.; Jordt, S.E.; Julius, D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007, 448, 204–208. [Google Scholar] [CrossRef]
- Dhaka, A.; Earley, T.J.; Watson, J.; Patapoutian, A. Visualizing cold spots: TRPM8-expressing sensory neurons and their projections. J. Neurosci. 2008, 28, 566–575. [Google Scholar] [CrossRef]
- De Caro, C.; Cristiano, C.; Avagliano, C.; Bertamino, A.; Ostacolo, C.; Campiglia, P.; Gomez-Monterrey, I.; La Rana, G.; Gualillo, O.; Calignano, A.; et al. Characterization of new TRPM8 modulators in pain perception. Int. J. Mol. Sci. 2019, 20, 5544. [Google Scholar] [CrossRef]
- Soeda, M.; Ohka, S.; Nishizawa, D.; Hasegawa, J.; Nakayama, K.; Ebata, Y.; Ikeda, K.; Soeda, M.; Fukuda, K.-I.; Ichinohe, T. Cold pain sensitivity is associated with single-nucleotide polymorphisms of PAR2/F2RL1 and TRPM8. Mol. Pain 2021, 17, 17448069211002008. [Google Scholar] [CrossRef]
- Pertusa, M.; Solorza, J.; Madrid, R. Molecular determinants of TRPM8 function: key clues for a cool modulation. Front. Pharmacol. 2023, 14, 1213337. [Google Scholar] [CrossRef] [PubMed]
- Voets, T.; Owsianik, G.; Nilius, B. TRPM8. Handb. Exp. Pharmacol. 2007, 179, 329–344. [Google Scholar]
- Almaraz, L.; Manenschijn, J.-A.; de la Pena, E.; Viana, F. TRPM8. Handb. Exp. Pharmacol. 2014, 222, 547–579. [Google Scholar]
- Liu, Y.; Mikrani, R.; He, Y.; Faran Ashraf Baig, M.M.; Abbas, M.; Naveed, M.; Tang, M.; Zhang, Q.; Li, C.; Zhou, X. TRPM8 channels: A review of distribution and clinical role. Eur. J. Pharmacol. 2020, 882, 173312. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, N.; Fujita, T. The TRPM8 channel as a potential therapeutic target for bladder hypersensitive disorders. J. Smooth Muscle Res. 2022, 58, 11–21. [Google Scholar] [CrossRef]
- Wu, B.; Su, X.; Zhang, W.; Zhang, Y.-H.; Feng, X.; Ji, Y.-H.; Tan, Z.-Y. Oxaliplatin depolarizes the IB4- dorsal root ganglion neurons to drive the development of neuropathic pain through TRPM8 in mice. Front. Mol. Neurosci. 2021, 14, 690858. [Google Scholar] [CrossRef] [PubMed]
- Aierken, A.; Xie, Y.-K.; Dong, W.; Apaer, A.; Lin, J.-J.; Zhao, Z.; Yang, S.; Xu, Z.-Z.; Yang, F. Rational Design of a Modality-Specific Inhibitor of TRPM8 Channel against Oxaliplatin-Induced Cold Allodynia. Adv. Sci. 2021, 8, 2101717. [Google Scholar] [CrossRef]
- Luyts, N.; Daniluk, J.; Freitas, A.C.N.; Bazeli, B.; Janssens, A.; Mulier, M.; Everaerts, W.; Voets, T. Inhibition of TRPM8 by the urinary tract analgesic drug phenazopyridine. Eur. J. Pharmacol. 2023, 942, 175512. [Google Scholar] [CrossRef] [PubMed]
- Fakih, D.; Baudouin, C.; Goazigo, A.R.-L.; Parsadaniantz, S.M. TRPM8: a therapeutic target for neuroinflammatory symptoms induced by severe dry eye disease. Int. J. Mol. Sci. 2020, 21, 8756. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-R.; Liu, Q.; Chen, G.-Y.; Hu, Y.; Sham, J.S.K.; Lin, M.-J. Down-Regulation of TRPM8 in Pulmonary Arteries of Pulmonary Hypertensive Rats. Cell. Physiol. Biochem. 2013, 31, 892–904. [Google Scholar] [CrossRef]
- Naumov, D.E.; Kotova, O.O.; Gassan, D.A.; Sugaylo, I.Y.; Afanas’eva, E.Y.; Sheludko, E.G.; Perelman, J.M. Effect of TRPM8 and TRPA1 Polymorphisms on COPD Predisposition and Lung Function in COPD Patients. J. Pers. Med. 2021, 11. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Q.; Hua, L.; Pan, J. Inhibition of transient receptor potential melastatin 8 alleviates airway inflammation and remodeling in a murine model of asthma with cold air stimulus. Acta Biochim. Biophys. Sin. 2018, 50, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Szallasi, A. ThermoTRP Channel Expression in Cancers: Implications for Diagnosis and Prognosis (Practical Approach by a Pathologist). Int. J. Mol. Sci. 2023, 24, 9098. [Google Scholar] [CrossRef]
- Gonzalez-Muniz, R.; Bonache, M.A.; Martin-Escura, C.; Gomez-Monterrey, I. Recent progress in TRPM8 modulation: an update. Int. J. Mol. Sci. 2019, 20, 2618. [Google Scholar] [CrossRef]
- Horne, D.B.; Biswas, K.; Brown, J.; Bartberger, M.D.; Clarine, J.; Davis, C.D.; Gore, V.K.; Harried, S.; Horner, M.; Kaller, M.R.; et al. Discovery of TRPM8 Antagonist (S)-6-(((3-Fluoro-4-(trifluoromethoxy)phenyl)(3-fluoropyridin-2-yl)methyl)carbamoyl)nicotinic Acid (AMG 333), a Clinical Candidate for the Treatment of Migraine. J. Med. Chem. 2018, 61, 8186–8201. [Google Scholar] [CrossRef]
- Andrews, M.D.; Af Forselles, K.; Beaumont, K.; Galan, S.R.G.; Glossop, P.A.; Grenie, M.; Jessiman, A.; Kenyon, A.S.; Lunn, G.; Maw, G.; et al. Discovery of a selective TRPM8 antagonist with clinical efficacy in cold-related pain. ACS Med. Chem. Lett. 2015, 6, 419–424. [Google Scholar] [CrossRef]
- Fernandez-Carvajal, A.; Gonzalez-Muniz, R.; Fernandez-Ballester, G.; Ferrer-Montiel, A. Investigational drugs in early phase clinical trials targeting thermotransient receptor potential (thermoTRP) channels. Expert Opin. Investig. Drugs 2020, 29, 1209–1222. [Google Scholar] [CrossRef]
- Gosset, J.R.; Beaumont, K.; Matsuura, T.; Winchester, W.; Attkins, N.; Glatt, S.; Lightbown, I.; Ulrich, K.; Roberts, S.; Harris, J.; et al. A cross-species translational pharmacokinetic-pharmacodynamic evaluation of core body temperature reduction by the TRPM8 blocker PF-05105679. Eur. J. Pharm. Sci. 2017, 109S, S161–S167. [Google Scholar] [CrossRef] [PubMed]
- Gavva, N.R.; Davis, C.; Lehto, S.G.; Rao, S.; Wang, W.; Zhu, D.X.D. Transient receptor potential melastatin 8 (TRPM8) channels are involved in body temperature regulation. Mol. Pain 2012, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Thapa, D.; Barrett, B.; Argunhan, F.; Brain, S.D. Influence of Cold-TRP Receptors on Cold-Influenced Behaviour. Pharmaceuticals 2022, 15, 42. [Google Scholar] [CrossRef] [PubMed]
- de la Torre-Martinez, R.; Bonache, M.A.; Llabres-Campaner, P.J.; Balsera, B.; Fernandez-Carvajal, A.; Fernandez-Ballester, G.; Ferrer-Montiel, A.; Perez de Vega, M.J.; Gonzalez-Muniz, R. Synthesis, high-throughput screening and pharmacological characterization of β-lactam derivatives as TRPM8 antagonists. Sci. Rep. 2017, 7, 10766. [Google Scholar] [CrossRef] [PubMed]
- Bonache, M.Á.; Llabrés, P.J.; Martín-Escura, C.; De la Torre-Martínez, R.; Medina-Peris, A.; Butrón, L.; Gómez-Monterrey, I.; Roa, A.M.; Fernández-Ballester, G.; Ferrer-Montiel, A.; et al. Phenylalanine-derived β-lactam trpm8 modulators. Configuration effect on the antagonist activity. Int. J. Mol. Sci. 2021, 22, 2370. [Google Scholar] [CrossRef] [PubMed]
- Bonache, M.A.; Martin-Escura, C.; de la Torre Martinez, R.; Medina, A.; Gonzalez-Rodriguez, S.; Francesch, A.; Cuevas, C.; Roa, A.M.; Fernandez-Ballester, G.; Ferrer-Montiel, A.; et al. Highly functionalized β-lactams and 2-ketopiperazines as TRPM8 antagonists with antiallodynic activity. Sci. Rep. 2020, 10, 14154. [Google Scholar] [CrossRef]
- Martin-Escura, C.; Medina-Peris, A.; Spear, L.A.; de la Torre Martinez, R.; Olivos-Ore, L.A.; Barahona, M.V.; Gonzalez-Rodriguez, S.; Fernandez-Ballester, G.; Fernandez-Carvajal, A.; Artalejo, A.R.; et al. β-Lactam TRPM8 Antagonist RGM8-51 Displays Antinociceptive Activity in Different Animal Models. Int. J. Mol. Sci. 2022, 23, 2692. [Google Scholar] [CrossRef]
- Werkheiser, J.L.; Rawls, S.M.; Cowan, A. Mu and kappa opioid receptor agonists antagonize icilin-induced wet-dog shaking in rats. Eur. J. Pharmacol. 2006, 547, 101–105. [Google Scholar] [CrossRef]
- Baell, J.B.; Holloway, G.A. New Substructure Filters for Removal of Pan Assay Interference Compounds (PAINS) from Screening Libraries and for Their Exclusion in Bioassays. J. Med. Chem. 2010, 53, 2719–2740. [Google Scholar] [CrossRef]
- Perez-Faginas, P.; O’Reilly, F.; O’Byrne, A.; Garcia-Aparicio, C.; Martin-Martinez, M.; Perez de Vega, M. J.; Garcia-Lopez, M. T.; Gonzalez-Muniz, R. Exceptional Stereoselectivity in the Synthesis of 1,3,4-Trisubstituted 4-Carboxy β-Lactam Derivatives from Amino Acids. Org. Lett. 2007, 9, 1593–1596. [Google Scholar] [CrossRef]
- Journigan, V.B.; Alarcón-Alarcón, D.; Feng, Z.; Wang, Y.; Liang, T.; Dawley, D.C.; Amin, A.R.M.R.; Montano, C.; Van Horn, W.D.; Xie, X.Q.; et al. Structural and in Vitro Functional Characterization of a Menthyl TRPM8 Antagonist Indicates Species-Dependent Regulation. ACS Med. Chem. Lett. 2021, 12, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Vangeel, L.; Benoit, M.; Miron, Y.; Miller, P.E.; De Clercq, K.; Chaltin, P.; Verfaillie, C.; Vriens, J.; Voets, T. Functional expression and pharmacological modulation of TRPM3 in human sensory neurons. Br. J. Pharmacol. 2020, 177, 2683–2695. [Google Scholar] [CrossRef] [PubMed]
- Li, W.G.; Xu, T. Le ASIC3 channels in multimodal sensory perception. ACS Chem. Neurosci. 2011, 2, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Khan, S.; Kim, Y.S. Insight into Pain Modulation: Nociceptors Sensitization and Therapeutic Targets. Curr. Drug Targets 2019, 20, 775–788. [Google Scholar] [CrossRef]
- Yang, C.; Yamaki, S.; Jung, T.; Kim, B.; Huyhn, R.; McKemy, D.D. Endogenous inflammatory mediators produced by injury activate TRPV1 and TRPA1 nociceptors to induce sexually dimorphic cold pain that is dependent on TRPM8 and GFRa3. J. Neurosci. 2023, 43, 2803–2814. [Google Scholar] [CrossRef]
- Chen, S.R.; Chen, H.; Yuan, W.X.; Wess, J.; Pan, H.L. Dynamic control of glutamatergic synaptic input in the spinal cord by muscarinic receptor subtypes defined using knockout mice. J. Biol. Chem. 2010, 285, 40427–40437. [Google Scholar] [CrossRef]
- Lee, J.H.; Go, D.; Kim, W.; Lee, G.; Bae, H.; Quan, F.S.; Kim, S.K. Involvement of spinal muscarinic and serotonergic receptors in the anti-allodynic effect of electroacupuncture in rats with oxaliplatin-induced neuropathic pain. Korean J. Physiol. Pharmacol. 2016, 20, 407–414. [Google Scholar] [CrossRef]
- Camilleri, M. Toward an effective peripheral visceral analgesic: responding to the national opioid crisis. Am. J. Physiol. 2018, 314, G637. [Google Scholar] [CrossRef]
- Beijers, A.J.M.; Jongen, J.L.M.; Vreugdenhil, G. Chemotherapy-induced neurotoxicity: The value of neuroprotective strategies. Neth. J. Med. 2012, 70, 18–25. [Google Scholar]
- Rimola, V.; Osthues, T.; Koenigs, V.; Geisslinger, G.; Sisignano, M. Oxaliplatin causes transient changes in TRPM8 channel activity. Int. J. Mol. Sci. 2021, 22, 4962. [Google Scholar] [CrossRef]
- Journigan, V.B.; Feng, Z.; Rahman, S.; Wang, Y.; Amin, A.R.M.R.; Heffner, C.E.; Bachtel, N.; Wang, S.; Gonzalez-Rodriguez, S.; Fernández-Carvajal, A.; et al. Structure-Based Design of Novel Biphenyl Amide Antagonists of Human Transient Receptor Potential Cation Channel Subfamily M Member 8 Channels with Potential Implications in the Treatment of Sensory Neuropathies. ACS Chem. Neurosci. 2020, 11, 268–290. [Google Scholar] [CrossRef] [PubMed]
- Bertamino, A.; Ostacolo, C.; Medina, A.; Di Sarno, V.; Lauro, G.; Ciaglia, T.; Vestuto, V.; Pepe, G.; Basilicata, M.G.; Musella, S.; et al. Exploration of TRPM8 Binding Sites by β-Carboline-Based Antagonists and Their In Vitro Characterization and In Vivo Analgesic Activities. J. Med. Chem. 2020, 63, 9672–9694. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Wu, M.; Zubcevic, L.; Borschel, W.F.; Lander, G.C.; Lee, S.-Y. Structure of the cold- and menthol-sensing ion channel TRPM8. Science. 2018, 359, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Yin, Y.; Wu, M.; Zubcevic, L.; Borschel, W.F.; Lander, G.C.; Lee, S. Structure of the cold- and menthol-sensing ion channel. Science. 2017, 359, 237–241. [Google Scholar] [CrossRef]
- Diver, M.M.; Cheng, Y.; Julius, D. Structural insights into TRPM8 inhibition and desensitization. Science. 2019, 365, 1434–1440. [Google Scholar] [CrossRef]
- Yin, Y.; Le, S.C.; Hsu, A.L.; Borgnia, M.J.; Yang, H.; Lee, S.-Y. Structural basis of cooling agent and lipid sensing by the cold-activated TRPM8 channel. Science. 2019, 363, eaav9334. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock and AutoDockTools: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Duan, Y.; Wu, C.; Chowdhury, S.; Lee, M.C.; Xiong, G.; Zhang, W.; Yang, R.; Cieplak, P.; Luo, R.; Lee, T.; et al. A point-charge force field for molecular mechanics simulations of proteins based on condensed-phase quantum mechanical calculations. J. Comput. Chem. 2003, 24, 1999–2012. [Google Scholar] [CrossRef]
- Ozvoldik, K.; Stockner, T.; Rammner, B.; Krieger, E. Assembly of Biomolecular Gigastructures and Visualization with the Vulkan Graphics API. J. Chem. Inf. Model. 2021, 61, 5293–5303. [Google Scholar] [CrossRef]
- Salentin, S.; Schreiber, S.; Haupt, V.J.; Adasme, M.F.; Schroeder, M. PLIP: fully automated protein-ligand interaction profiler. Nucleic Acids Res. 2015, 43, W443. [Google Scholar] [CrossRef]
- Vriens, J.; Owsianik, G.; Hofmann, T.; Philipp, S.E.; Stab, J.; Chen, X.-D.; Benoit, M.; Xue, F.-Q.; Janssens, A.; Kerselaers, S.; et al. TRPM3 Is a Nociceptor Channel Involved in the Detection of Noxious Heat. Neuron 2011, 70, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Muniz, R.; Perez Ma. Jesus, de V.; Bonache de Marcos, M.A.; Ferrer Montiel, A.; Fernandez Carvajal, A.; De la Torr, e. R. Heterocyclic compounds as TRPM8 channel antagonists and uses thereof. WO 2017005950 2017, WO 2017005950.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





