1. Introduction
Climate change-induced temperature fluctuations pose a significant threat to crop production, particularly in the Southern Hemisphere, where strong warming is predicted across various global warming scenarios [
1,
2,
3,
4]. Recently, Rivelli et al. (2021) reported a temperature increase in the Southern Cone of South America over the past 30-50 years, highlighting the urgency of understanding crop responses to these changes [
5].
Southern Chile is a high-yield potential region for temperate crops, including rapeseed [
6,
7,
8,
9]. The increasing temperature due to climate change is a critical environmental factor affecting rapeseed, particularly during the grain filling period [
10,
11]. Although numerous studies have evaluated rapeseed responses to heat stress, most have been conducted under controlled conditions, limiting the applicability of their findings to field conditions [
12].
Heat stress during the critical reproductive stages, such as flowering and seed filling, can result in significant losses in rapeseed seed yield [
13]. High temperatures during flowering reduce pollen viability and fertilization success, leading to fewer seeds per pod [
14]. Additionally, heat stress during seed filling shortens the duration of this stage, impairing seed development and lowering seed weights [
15]. Both daytime and nighttime heat negatively impact reproductive development, with nighttime heat at flowering and seed filling substantially reducing the number of seeds per pod and overall seed yield [
16].
Advancements in high-throughput RNA sequencing (RNA-seq) have illuminated the transcriptomic adaptations of rapeseed in response to heat stress [
13]. Comparative analyses between rapeseed cultivars subjected to control and heat stress conditions have identified a conserved heat stress response mechanism, primarily mediated through the upregulation of heat shock proteins (HSPs) and downregulation of metabolic pathways [
17]. Moreover, heat-tolerant and heat-sensitive cultivars display distinct stress-responsive transcriptional profiles, offering valuable insights into potential adaptive mechanisms [
17].
Extending these insights, studies focusing on mature
B.napus seeds have identified differentially expressed genes (DEGs) involved in protein processing, secondary metabolite pathways, and specific transcription factor families such as NAC, ERF, and bHLH [
18,
19]. These findings collectively elucidate the complex transcriptional landscape that contributes to compromised reproductive success under heat stress conditions.
The trade-off between seed number and seed weight is a critical factor in plant reproduction and crop yield improvement [
20]. In rapeseed, seed weight is controlled by maternal genotype, which regulates seed size mainly through cell number [
21]. The pod wall serves as a source of nutrients for seed development, with pod wall photosynthesis contributing to seed filling and final seed weight [
21]. Transcriptome analysis of rapeseed has revealed genes involved in the interaction between seed weight (SW) and seed number (SN) [
20], and genome-wide association studies have identified genetic loci controlling these traits [
22].
Previous research on the sensitivity of grain yield to source-sink ratio reduction in southern Chile found that rapeseed is more susceptible to source reduction between the start of flowering and 15 days after [
8], which overlaps with the critical period of rapeseed [
23]. Interestingly, seed yield showed partial or full resilience during the beginning of flowering from the start to 15 days after flowering (DAF) [
8,
23,
24]. In a recent heat stress study conducted in southern Chile [
25], where air temperature increased after the beginning of flowering (from 0 to 15 DAF), seed weight of rapeseed was negatively affected, but not seed weight window. This background raises the question about the molecular mechanisms involved in the rapeseed response to both the increased temperature and source reduction during flowering and the beginning of grain filling.
The objective of this paper is to investigate the transcriptome and physiological responses of rapeseed to post-flowering temperature increases, providing valuable insights into the molecular mechanisms underlying rapeseed tolerance to heat stress. This knowledge can inform crop improvement strategies targeting yield optimization under changing environmental conditions.
2. Materials and Methods
2.1. Field Experiment, Treatments, and Crop
Management
The field experiments and conditions were described in Verdejo and Calderini [
25]. Briefly, two field experiments were set in 2019-20 and 2020-21 growing seasons at the Austral Farming Experimental Station (EEAA) of the Universidad Austral de Chile in Valdivia, Chile (39° 47’ S., 73° 14’ W.). In each experiment, two adapted spring rapeseed hybrids (Lumen and Solar from NPZ-Lembke, Germany) were assessed under two heat stress treatments: a control without manipulation and a thermal increase treatment where plots were heated by 5°C above the environmental temperature from the beginning of flowering [BBCH 61] to 15 days after flowering. The hybrids Lumen and Solar were chosen for their similar phenology and confirmed adaptation to southern Chile. Experiment 1 was arranged in a split plot design with three replicates in which the heat stress treatments were assigned to the main plots and genotypes to sub-plots. In Experiment 2, plots were arranged in a randomized block design with three replicates in which, the heat stress treatments and genotypes were fully randomized in each block. In both experiments rapeseed plants were sown at a plant density of 55 plants m-2.
2.2. Phenology and Physiological Plant Sampling
Crop development was followed twice a week in both experiments according to the BBCH phenological scale for rapeseed [
26]. In order to determine seed yield (SY), seed number (SN), thousand seed weight (TSW) and quality traits as grain oil and protein concentrations, the seed samples were harvested in one lineal meter from the central rows of each plot at maturity (BBCH 89), when the seeds inside the siliques were dark and hard. Seed yield and number was measured after oven drying the samples at 65 °C for 48 h. Seed number was counted by using a grain counter (Pfeuffer GmbH, Kitzengen, Germany). Then, SY was measured and TSW was estimated as the ratio between seed yield and SN.
Oil concentration of seeds was determined by near infrared reflectometry (NIR) (Foss Infratec 1241, Hilleroed, Denmark) and nitrogen concentration of seed by the Kjeldahl procedure [
27]. Seed protein concentration was calculated with a conversion factor of 5.8 [
28]. Seed concentrations of both oil and protein were expressed on a dry matter basis.
2.3. Seed Sampling and RNA Isolation
Seed samples for RNA-seq analysis were collected from 5 plants per plot (25 siliques per plant from the basal portion of the primary inflorescence) across treatments at two developmental stages: 7 and 14 days after flowering, corresponding to one and two weeks after initiation of the heat stress treatment, respectively. Immediately after harvest, the seed samples were flash frozen in liquid nitrogen and stored at -80°C until processing. The frozen seeds were ground into a fine powder using liquid nitrogen and a chilled mortar and pestle. Total RNA was extracted from 100 mg of each pulverized seed sample using a protocol adapted for Brassica seeds [
29] and NucleoSpin Gel and PCR clean-up columns (Macherey-Nagel, Germany) as described previously [
30].
2.4. RNA-seq analysis
RNA samples were transported to Novogene facilities located in Sacramento, CA, USA, under cryogenic conditions utilizing dry ice. Subsequent analyses for RNA quality, as well as library construction and high-throughput sequencing, were executed by Novogene’s Beijing-based laboratory. Sequencing was conducted employing a 2 × 150 bp paired-end sequencing kit on an Illumina NovaSeq 6000 platform, targeting a sequencing depth of 6 Gb per individual sample. Quality control metrics revealed an average Q20 score of 97.9% and a Q30 score of 94.0% across all samples, thereby attesting to the high fidelity of the sequencing process.
The RNA-seq reads were subjected to a comprehensive computational workflow utilizing pseudoalignment algorithms for accurate transcript quantification and downstream differential expression analysis. Specifically, the Kallisto software (version 0.46.1) was employed for the initial quantification steps [
31]. A reference transcriptome index was generated using the “kallisto index” function and rapeseed annotation version AST_PRJEB5043_v1, subsequent to which the “kallisto quant” function was executed on each sample with 100 bootstraps with default parameters. The output provided transcript abundance estimates in terms of Transcripts Per Million (TPM).
For the identification of Differentially Expressed Genes (DEGs) between distinct experimental conditions, the R-based statistical package Sleuth (version 0.30.0) was employed [
32]. A Sleuth object was instantiated via the “sleuth_prep” function, incorporating multiple variables into the model, namely genotype, heat treatment, temporal factors, and seasonal variations. Two statistical models were then fitted using the “sleuth_fit” function: a reduced model incorporating all main effects and their respective interactions, and a full model encompassing a four-way interaction term. Likelihood ratio tests (LRT) were executed between these models using the “sleuth_lrt” function, culminating in the identification of DEGs using an adjusted p-value <0.01.
2.5. Hierarchical clustering analysis
For the purpose of uncovering the latent structure in gene expression profiles, hierarchical clustering was conducted. Initially, the gene expression matrix was subjected to normalization, centering, and scaling using native R functions. The Pearson correlation distance matrix was subsequently employed for hierarchical clustering through the “hclust” function, utilizing average linkage as the agglomeration method. The Dynamic Tree Cut algorithm, implemented in the “cutreeDynamic” function from the dynamicTreeCut package [
33], was leveraged to partition the hierarchical tree into an optimal number of gene expression modules. Visualization of these modules was facilitated using the ComplexHeatmap package [
34].
Functional enrichment analysis of the gene modules was performed using the g:Profiler toolkit [
35]. The gene sets corresponding to each identified module were subjected to Gene Ontology (GO) enrichment analysis against
Brassica napus gene databases using the “gost” function implemented in the R package gprofiler2 [
36]. This enabled the elucidation of the biological pathways and molecular functions significantly associated with each gene module.
2.6. Gene Coexpression Network Analysis
To identify modules of coexpressed genes associated with seed yield and quality traits, we performed weighted gene coexpression network analysis (WGCNA) as described previously [
37]. The analysis was conducted using the R package BioNERO [
38] on normalized RNA-seq count data.
As a first step, we fitted a soft thresholding function to the count data to determine the power (
) for weighted network construction. Using the “SFT_fit function” with a signed hybrid network type, Pearson correlation, and a target R
2 of 0.9 for scale independence, a power of 8 was selected. Next, the exponentiated transformed count data was used to construct a signed coexpression network with the “exp2gcn” function of BioNERO R package [
38], specifying the previously determined soft threshold power, a Pearson correlation, and a module merging threshold of 0.8.
The resulting gene coexpression network consisted of 35,432 genes grouped into 33 modules based on topological overlap. To identify modules associated with phenotypes, we calculated module-trait correlations using the module_trait_cor function. Eight modules showed significant correlations with at least one trait related to plant growth, seed yield, or quality (|r| > 0.7 and p < 0.0001).
To visualize the module-trait relationships, a customized heatmap was generated using the ComplexHeatmap package in R [
34]. Modules were assigned to three broad categories (growth, yield, quality) and ordered accordingly. The heatmap depicts module-trait correlations, with the strength of positive correlations shown in red and negative correlations in blue. This systems-level visualization reveals major gene coexpression modules associated with different yield and quality traits.
To infer potential regulators of the yield and quality associated modules, we performed gene regulatory network analysis using the “exp2grn” function of BioNERO R package [
38]. Transcription factors were specified based on PlantTFDB 4.0 annotations [
39], and the expression data was used to predict regulatory interactions [
38]. Hub regulators with the highest connectivity in each module were identified using the “get_hubs_grn” function of BioNERO R package [
38], which implement implements three popular algorithms: GENIE3 [
40], ARACNE [
41] and CLR [
42]. The transcriptomic network analysis provides a systems-level view of potential genetic factors influencing yield and quality traits in response to heat stress.
3. Results
3.1. Effects of heat stress on seed yield and quality traits of two rapeseed genotypes
The effects of heat stress on seed yield and quality traits of two rapeseed genotypes (Lumen and Solar) were evaluated in two experiments conducted under similar climate conditions during two consecutive growing seasons. Air temperature and average solar radiation between emergence and the start of flowering recorded across the experiments were 10.9 and 11.4°C and 17.2 to 18.9 MJ m2 day -1, respectively. Crop phenology of control plants was similar between genotypes and seasons.
The heat stress treatment increased average temperature between 4.3°C and 4.8 °C from the beginning of flowering to 15 days after flowering. The seed filling period was affected by heat stress across genotypes and seasons. For instance, seed filling period was shortened by three and six days in Lumen in Exps. 1 and 2, respectively, while Solar was delayed by one day in Exp. 1 and shortened by two days in Exp. 2.
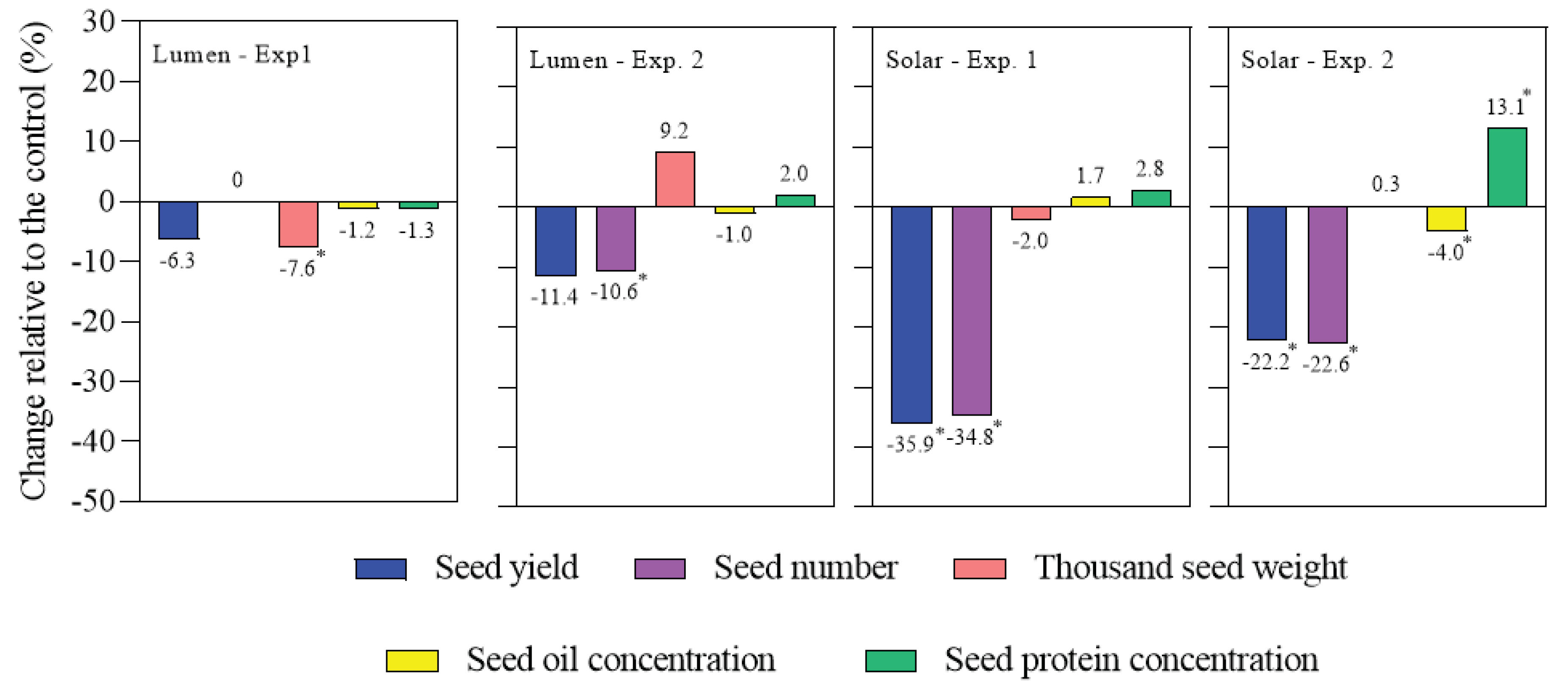
Seed yield and seed number showed a significant interaction between genotype and heat stress (p=0.03 and p=0.04, respectively), indicating that both traits were negatively affected by heat stress but to different extents depending on the genotype (
Figure 1). Lumen showed an averaged seed yield reduction of 9.3% and no significant change was found in seed number under heat stress, while Solar showed an average seed yield reduction of 28.7%, completely explained by the seed number reduction. Moreover, seed yield was negatively correlated with average temperature during the period of heat stress in Solar (R2= 0.67; p=0.05), but not in Lumen (P >0.05).
Seed weight was affected by genotype (p=0.03), with Lumen having higher seed weight than Solar. On the contrary, this trait did not show any significant response to heat stress (
Figure 1). Seed oil concentration was also influenced by genotype (p<0.001), with Lumen reaching higher values (50.5%) than Solar (48.7%). In addition, seed protein concentration was sensitive to both genotype (p<0.001) and heat stress (p=0.038). Contrary to oil, seed protein concentration was higher in Solar (17.0%) than in Lumen (15.4%) in control plants (
Figure 1), supporting a trade-off, which has been often reported between these quality traits in rapeseed. Under heat stress, protein seed concentration increased in Solar by 13.1% only during the second growing season.
3.2. Multivariate Analysis of RNA-Seq Data reveals
differentially effect of heat stress on source and sink tissues
To gain further insights into the molecular factors underlying heat stress response in rapeseed plants under field conditions, we conducted a multivariate RNA-seq analysis using pod and seed samples to distinguish the effects of stress on source and sink tissues. Our dataset incorporated samples from two independent seasons, with three biological replicates per condition during the first season and four biological replicates for the second season. In total, 112 samples were sequenced, generating 2,500 million paired-end reads and averaging 22.3 million raw reads per sample, with a mean pseudoalignment of 16.4 million reads per sample (Table S1).
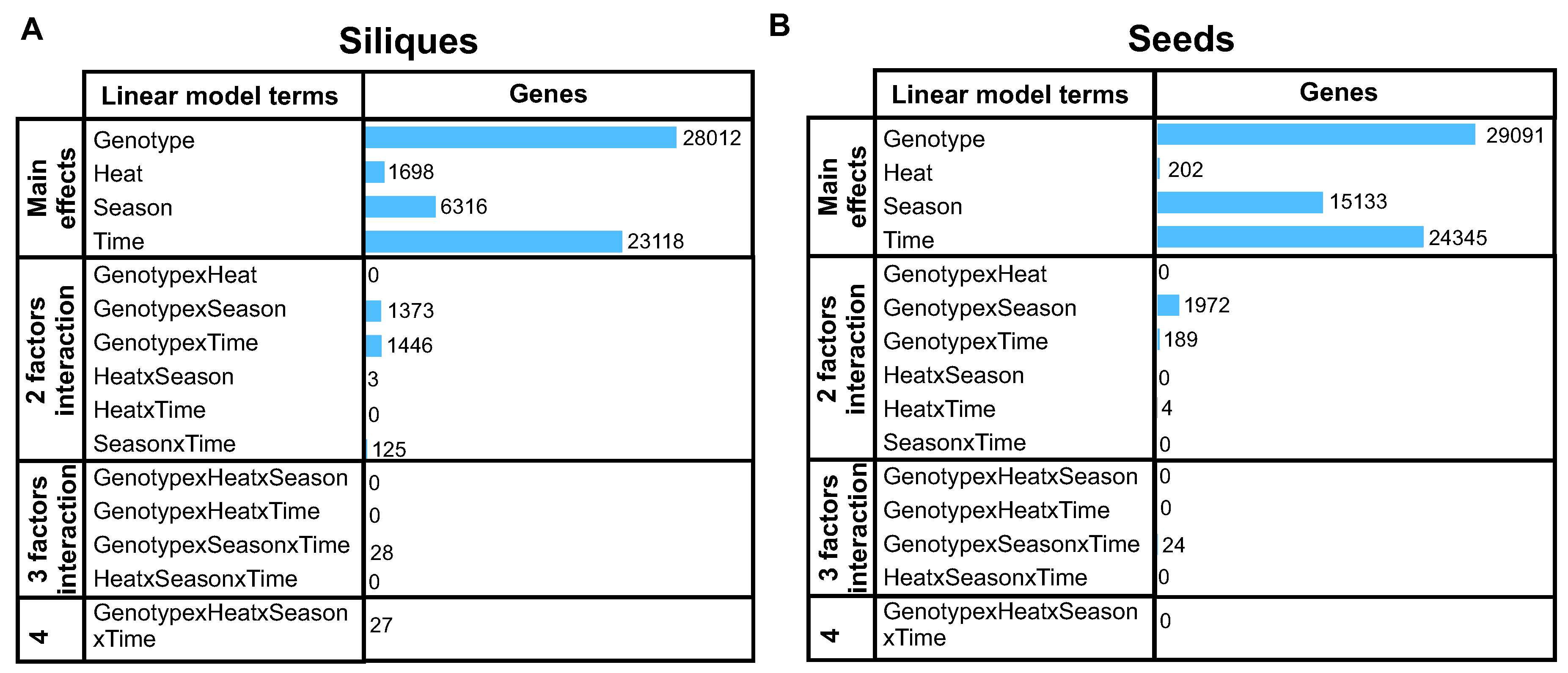
The multifactorial analysis revealed minimal to non-existent interaction between 2, 3, or 4 factors in both tissues, with most transcriptomic changes being attributable to the primary factors (
Figure 2A and
Figure 2B). Notably, genotype was the predominant factor, affecting 28,012 and 29,091 genes in siliques and seeds, respectively (
Figure 2A and
Figure 2B). Interestingly, the number of genes affected by other factors was also of similar magnitude in both tissues, except for heat treatment. We found 8.5 times more heat-regulated genes in siliques than in seeds (1698 vs. 202 genes, q-value<0.01), suggesting that source tissues are considerably more sensitive to temperature stress. Moreover, the overlap between these two sets of heat-regulated genes is only 35 genes (Figure S1), indicating a tissue-specific response to this factor.
3.3. Hierarchical Clustering Unveils Patterns of Heat-Induced Gene Expression in Siliques
To investigate how heat-responsive genes are expressed in siliques, we performed a hierarchical clustering analysis on the 1698 genes that were significantly impacted by temperature (q-value<0.01) in these maternal tissues. We used the “Dynamic Tree Cut” package in R to determine the optimal number of clusters, which was eight (
Figure 3A). These clusters showed different patterns of gene expression in response to heat treatment and between genotypes.
Clusters 1, 2, and 5 comprised genes that were downregulated by heat stress, representing around 60% of the total heat-responsive genes (947 out of 1698 genes). Most of these genes had higher expression levels in Lumen than in Solar (
Figure 3A). Cluster 1 contained genes that were more highly expressed at 7 DAF than at 14 DAF, and were enriched for biological processes related to photosynthesis and starch metabolism. Cluster 2 included genes that were associated with pigment metabolism and response to light. Cluster 5 had no significantly enriched biological process (
Figure 3B).
Clusters 3, 4, 6, 7, and 8 consisted of genes that were upregulated by heat stress, but they were smaller (between 80 and 200 genes) and less functionally enriched than the downregulated clusters. Clusters 3 and 7 had no significantly enriched biological process (
Figure 3B). Clusters 4 and 6 showed consistent differences in gene expression between genotypes, with higher levels in Solar than in Lumen. These clusters were enriched for biological processes involved in cell wall metabolism, carbohydrate metabolism and protein folding (
Figure 3B).
Cluster 8 was the most interesting cluster, as it was highly enriched for genes involved in protein folding and showed a genotype-specific temporal response to heat stress. At 7 DAF, the expression levels and heat response of these genes were similar between genotypes. However, at 14 DAF, these genes exhibited a strong decrease in expression in Lumen but not in Solar (
Figure 3B).
Taking together, these findings suggest that heat treatment primarily has a negative impact on the expression of genes associated with photosynthesis in siliques. The results also highlight distinct gene clusters that reflect different aspects of heat stress adaptation in this tissue. In particular, the biological functions associated with cluster 8 genes suggest a role for protein folding in maintaining silique development and seed quality under high temperature conditions.
3.4. Hierarchical Clustering Reveals Three Distinct Patterns of Heat-Responsive Gene Expression in Seeds
In the case of seeds, the hierarchical clustering analysis on 202 heat-responsive genes revealed three clusters with distinct expression patterns (
Figure 4A). Cluster 1 ( 81 genes) was negatively regulated by temperature, cluster 2 (63 genes) was positively regulated by temperature, and cluster 3 (58 genes) showed differential expression between the genotypes and positive response to heat treatment (
Figure 4A).
Cluster 1 and 2 also exhibited a temporal effect on their expression levels. In cluster 1, most genes had lower expression at 14 DAF than at 7 DAF or 21 DAF, regardless of the temperature. In contrast, in cluster 2, most genes had higher expression at 14 DAF than at other time points, regardless of the temperature (
Figure 4A). Gene ontology analysis indicated that cluster 1 genes were involved in “glycolipid metabolism” and “cellular response to phosphate starvation”, while cluster 2 genes were involved in “carboxylic acid metabolic process” and “sucrose biosynthesis” (
Figure 4B).
Cluster 3 was the only cluster that showed a clear genotypic difference in gene expression. However, this difference was not consistent across time or seasons (
Figure 4A). For instance, at 7 DAF in the first season, the genes in this cluster had lower expression in Lumen than in Solar, but this trend was reversed at the same time point in the second season. Moreover, gene ontology analysis revealed that this cluster was enriched in genes related to “protein folding” and “response to heat” (
Figure 4B).
3.5. Genotype-specific effects of heat stress on
photosynthesis-related genes in siliques
Our physiological analysis revealed that the Lumen genotype exhibits increased resistance to heat stress compared to Solar in terms of seed yield. To investigate the genetic basis of this differential tolerance, we used the GeneOverlap R package to identify genes that were regulated by both genotype and heat stress in each group of tissues.
We found a significant intersection of 1015 genes in siliques (pvalue=5x10-36,
Figure 5A), but not in seeds, suggesting that siliques are more sensitive to genotype-dependent heat responses. We then mapped these 1015 genes to the clusters of siliques shown in
Figure 3 and found that most of them belonged to clusters 1 and 2 (
Figure 5B), which were enriched for photosynthesis and light response genes and showed a negative response to heat stress (
Figure 3A).
Moreover, these clusters consistently exhibited higher expression levels of genes in Lumen than in Solar, indicating that Lumen maintains photosynthetic activity better than Solar under heat stress. To illustrate these differences, we selected two representative genes from each cluster:
SBPase from cluster 1, which encodes the chloroplast enzyme sedoheptulose-1,7-bisphosphatase involved in the carbon reduction of the Calvin cycle [
43], and
INAP1 from cluster 2, which encodes the catalytic subunit of the chloroplastic ferredoxin/thioredoxin reductase [
44]. In both cases, we observed significant differences between genotypes in most of the samples analyzed, showing lower mRNA levels in Solar than in Lumen (
Figure 5C and
Figure 5D).
3.6. Co-expression network analysis reveals major
modules for rapeseed yield and quality
To identify candidate genes related to rapeseed yield and quality, we performed a gene co-expression network analysis using the transcriptomes of siliques and seeds by the WGCNA method [
37] as indicated in the materials and methods section. We constructed four co-expression networks corresponding to two different times after flowering (7 and 14 days) and two group of tissues (seeds and siliques) and compared them to select the network that showed the highest number of genes correlated with the agronomic parameters of yield and seed quality.
We found that the seed network at 7 DAF had the highest number of genes significantly correlated with the main agronomic traits of yield and quality (Figure S2). The seed network at 7 DAF showed more than five times as many genes correlated with seed number and yield than the silique networks at both 7 and 14 DAF (Figure S2). These results indicate that the co-expression network of seeds at 7 DAF is the most suitable for finding genes that explain the parameters of yield and seed quality.
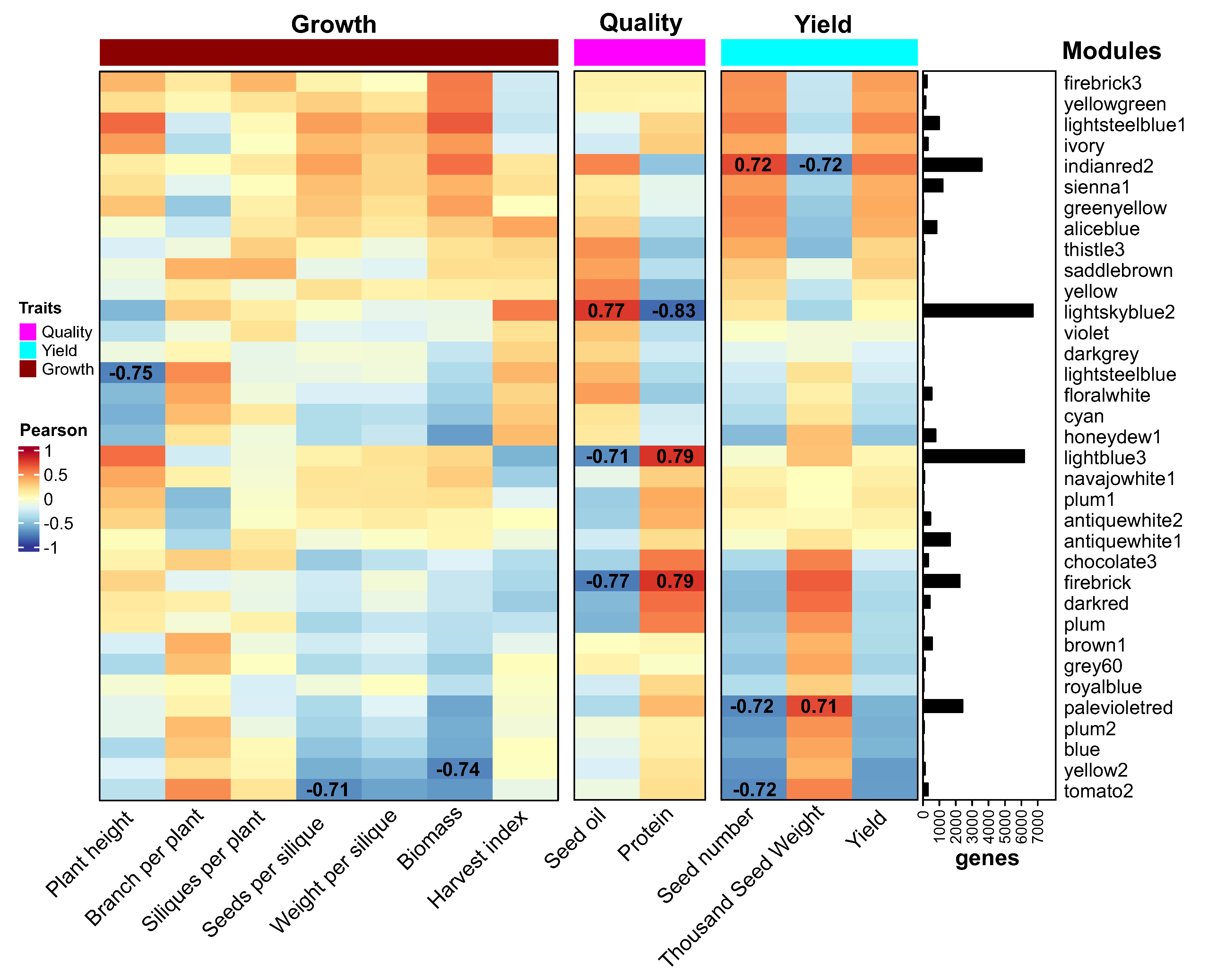
The co-expression network of seeds at 7 DAF consisted of 35432 genes distributed in 33 modules (
Figure 6A). These traits included growth and development (plant height, branch per plant, siliques per plant, seeds per silique, weight per silique, biomass and harvest index), seed quality (oil and protein content) and yield (seed number, thousand seed weight, yield). We identified eight co-expression modules with high correlation with some of these agronomic parameters (at least one absolute correlation of 0.7 or more and a p-value less than 0.0001,
Figure 6B). Interestingly, five of these eight modules were correlated with quality and yield parameters and were also the largest ones in the network.
For seed quality, we found two modules positively correlated with protein content (the lightblue3 and firebrick modules) and one module positively correlated with oil content (lightskyblue2,
Figure 6B). The overrepresentation analysis of biological functions showed that the lightblue3 and firebrick modules were enriched in genes related to protein synthesis and transport as well as photosynthesis and small molecule metabolism (Table S2). The lightskyblue2 module was enriched in genes related to glycolysis, purine metabolism, ribonucleoprotein complex biogenesis, protein folding and mRNA splicing (Table S2).
3.7. Identification of Regulatory Factors Associated with the Compensation of SW and SN flowering
In the case of yield, we found two highly correlated co-expression modules, “indianred2” and “palevioletred” with 3584 and 216 genes respectively. Interestingly, both modules showed an inverse correlation between seed number and weight, suggesting that they could be related to the trade-off between these two yield-determining factors. Given the relevance of this topic for crop performance and improvement, we analyzed these modules in more detail.
As shown in
Figure 7A, the genes in the indianred2 module showed a positive correlation with seed number and a negative correlation with weight. A biological function enrichment analysis revealed that it is especially enriched in genes associated with translation, as well as some functions related to transcription, protein ubiquitination and protein targeting (
Figure 7B). To identify the regulators that control the expression of this module, we performed a regulatory network analysis using the R package Bionero and the transcription factor annotation from PlantTFDB 4.0 [
39].
In this way, we inferred a network with 93 transcription factors and 1256 targets, being the 5 transcription factors with the highest connectivity BnaC03g43430D (PHL2), BnaC05g18750D (INO1), BnaA03g18970D (ABI4), BnaC08g31840D (MYC67) and BnaC03g57540D (SEP3) (
Figure 7C). Interestingly, functional information available in Arabidopsis indicates that 4 of these 5 factors are related to floral architecture [
45] or seed development [
46,
47,
48].
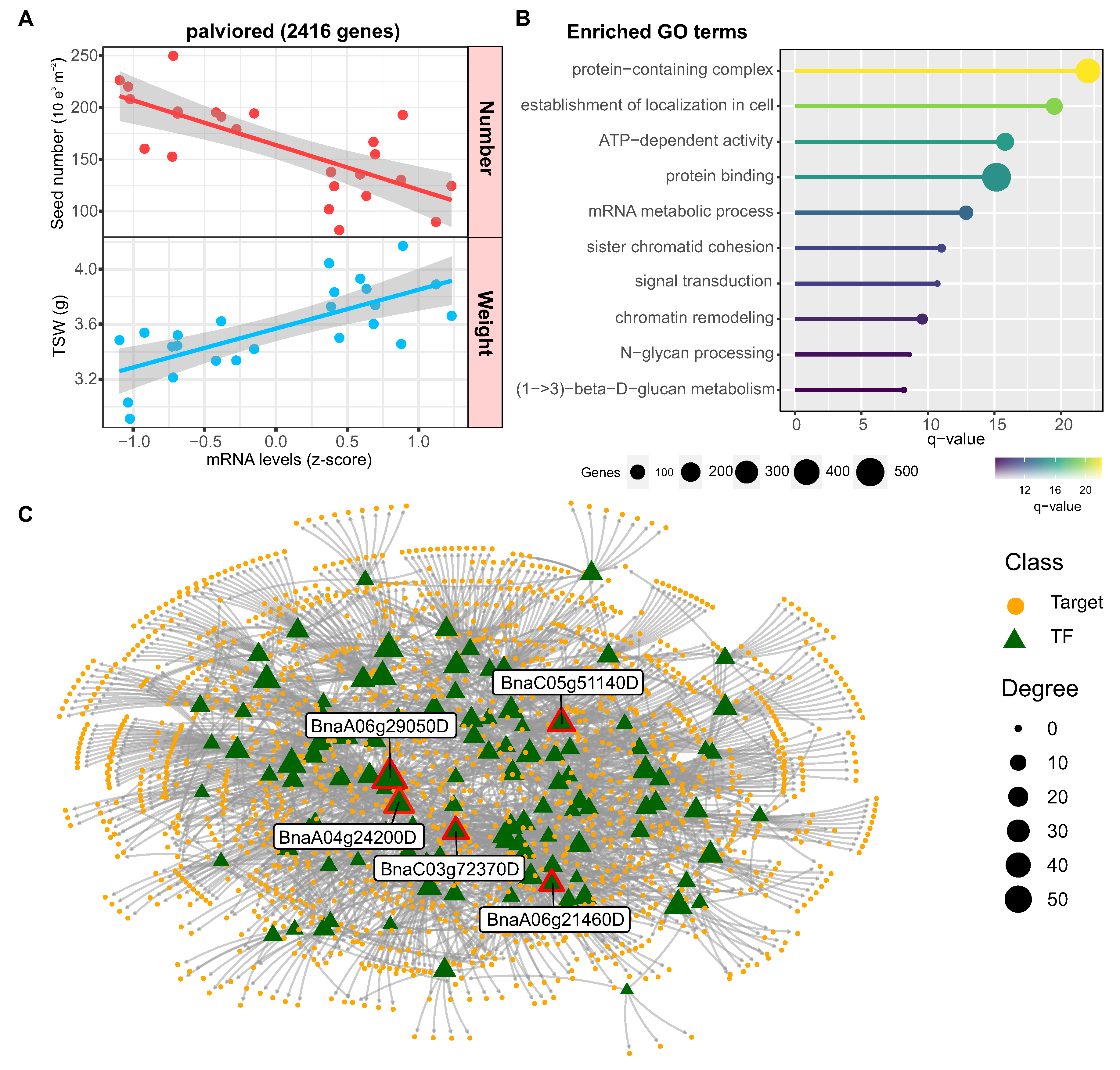
In the case of the palevioletred module, the genes showed higher expression levels in samples with lower seed number and higher weight (
Figure 8A). According to gene ontology analysis, the genes in this module are associated with biological functions related to intracellular transport, mRNA metabolism, cell signaling and chromatin remodeling (
Figure 8B). The regulatory network analysis revealed that this co-expression module is controlled by 118 TFs that potentially regulate 1483 targets. The 5 transcription factors with the highest connectivity were
BnaA06g29050D,
BnaA04g24200D (
OSX2),
BnaC05g51140D,
BnaC03g72370D (
NGA1) and
BnaA06g21460D (
ARF2) (
Figure 8C). Importantly, the transcription factor ARF2 has been shown to increase seed size due to increased cell proliferation, suggesting that the regulators identified could be important for controlling seed weight [
49].
4. Discussion
Brassica crops such as canola, mustard, and cabbage are cool-season plants that are susceptible to high temperatures, especially during reproductive development [
14,
50,
51]. Even short episodes of heat stress during flowering can significantly reduce seed and oil yields in canola by impairing pollen viability, pollen tube growth, fertilization success, and embryo development [
14,
52]. Our study found genotype-specific responses to heat stress during flowering and early seed development. The Solar genotype showed greater reductions in seed yield and number compared to Lumen under heat stress. Seed oil and protein concentrations also showed genotype-dependent responses. Lumen maintained higher seed oil content while Solar increased protein content under heat stress. These results demonstrate differential heat tolerance between genotypes that impacts yield and quality, however the underlying molecular mechanisms are unknown. This motivated a large-scale transcriptomic analysis (112 samples) which provides novel insights into the molecular mechanisms governing heat stress responses in rapeseed during the critical reproductive stages of flowering and early seed set. Leveraging field experiments and multi-level omics across source and sink tissues, we reveal both conserved and divergent strategies for thermal adaptation.
4.1. Divergent Molecular Responses to Heat Stress in Source and Sink Tissues
Our multivariate analysis of RNA-seq data revealed distinct effects of heat stress on siliques (source) versus seeds (sink). In the pod wall, heat stress triggered extensive transcriptional repression of genes regulating photosynthesis and carbon assimilation. This corroborates previous observations in model species like Arabidopsis, where high temperature inhibits RuBisCO activase [
53], electron transport, and carbon fixation [
54]. Exposure to high temperatures was found to negatively impact components of the photosynthetic machinery in
B. napus seeds, including reduced oil accumulation, lowered photosynthetic and respiration rates, decreased maximum quantum yield of photosystem II and reduced levels of light-harvesting pigments like chlorophyll [
55]. Moreover, a previous transcriptomic analysis in
B.napus seeds also revealed up-regulation of genes related to the response to high light intensity and genes associated with chloroplasts and photoinhibition in response to heat stress [
55].
In maturing seeds, the predominant heat response entailed upregulation of protein homeostasis processes like folding and stabilization. Such activation of heat shock factors, chaperones, and related pathways enables survival under protein-denaturing conditions [
56]. Intriguingly, Lumen exhibited fluctuating induction of these genes while Solar showed constitutively high expression. This hints at distinct regulatory logic where Lumen employs a reactive mechanism triggered by stress, while Solar utilizes an anticipatory strategy.
Our finding that photosynthesis-related genes were downregulated in siliques but not seeds under short heat waves suggests reproductive tissues are more sensitive than vegetative tissues to high temperature damage during flowering. This aligns with previous work showing decreased expression of sucrose transporters like OsSUT1 in rice grains exposed to heat stress, which limits photoassimilate supply and grain filling [
57]. Reduced photosynthate translocation is a common consequence of heat stress and a major cause of yield loss [
58,
59,
60].
4.2. Differential Transcriptome Rapeseed Genotype Responses to Heat Stress
We newly uncover genotype-dependent variation in the extent of photosynthetic gene repression. The heat-tolerant Lumen maintained higher expression of light reaction and Calvin cycle transcripts compared to the sensitive Solar. Sustaining pod photosynthesis likely maintains carbon supply to developing seeds under heat stress, as reflected by Lumen’s yield stability (
Figure 1). We found that an important gene of the Calvin cycle,
SBPase, was downregulated under heat stress and consistently showed lower levels in the Solar genotype. Previous research has shown the importance of SBPase activity in photosynthesis and plant growth [
61]. Specifically, transgenic plants with decreased SBPase activity have exhibited reduced rates of CO2 assimilation and carbon fixation [
62,
63]. This provides evidence that SBPase plays a key role in regulating carbon flow in the Calvin cycle. Furthermore, overexpressing SBPase has been demonstrated to increase photosynthetic capacity, carbon fixation, biomass accumulation, and starch and sucrose levels in tobacco [
64,
65]. Therefore, the downregulation of SBPase we observed under heat stress likely contributes to decreases in photosynthesis and growth in heat sensitive genotypes. Our findings suggest
SBPase could be a useful target for genetic modification to improve heat tolerance by maintaining carbon assimilation and plant productivity under high temperatures.
Our results are consistent with the study by Jedlickova et al.,2023 in which photosynthesis-related genes clustered in two co-expression modules showing higher expression in heat-sensitive cultivars DH12075 and Westar compared to heat-tolerant Topas after heat stress [
17]. The authors suggest induction of these genes may indicate heat damage to photosystems in sensitive cultivars. Genes connected to photosynthesis and light-harvesting proteins were also up-regulated by heat specifically in seeds of sensitive cultivars [
17]. The effects of heat stress on impairing photosynthesis and related gene expression likely contribute to reduced seed production and oil content in sensitive
B. napus cultivars. Identifying and selecting for thermotolerance factors that protect photosynthesis may improve yield stability.
4.3. Distinct Regulators Mediate the Seed Number Versus Weight Trade-off
Beyond heat responses, our integrated omics approach discovered novel gene modules regulating the complex physiological trade-off between seed number and weight, an area of intense agronomic interest. The identification of the indianred2 and palevioletred modules that show an inverse correlation between seed number and weight suggests there are specific genetic networks controlling this trade-off in rapeseed. To further explore these networks, we analyzed the transcription factors with the highest connectivity in each module.
In the case of indianred2, the transcription factors
INO (
INNER NO OUTER) and ABI4 (ABA INSENSITIVE 4) stand out for their relationship with seed development [
47,
66]. The Arabidopsis
INO gene encodes a YABBY family transcription factor that acts exclusively in regulating development of the ovule outer integument, with loss-of-function mutants lacking this structure [
46,
67]. We identified the ortholog of INO in
B.napus as a hub of a gene coexpression module that showed a positive correlation with seed number per silique and a negative correlation with seed weight. This correlation is consistent with the specific role of INO in promoting outer integument growth. In wild-type Arabidopsis plants, the outer integument facilitates pollen tube guidance, fertilization, and supports embryo development [
46]. Thus, loss of INO function leads to reduced seed number due to failed fertilization, while seeds that do form in heterozygous plants show increased weight and size, likely due to additional resources available from unfertilized ovules [
68]. The central role of
INO in regulating outer integument growth and its downstream effects on seed number and weight underlies its identification as a hub of the correlated gene coexpression module.
Our finding that
ABI4 expression positively correlates with seed number in B. napus is consistent with previous studies demonstrating a role for
ABI4 in promoting seed production. Specifically, Shu et al., 2016 [
47] showed that constitutive overexpression of
ABI4in
Arabidopsis thalianaresulted in plants with a 20-26% reduction in total seed yield compared to wildtype. This decrease was attributed to a significant reduction in seed number, as the 1000-grain weight was not affected by ABI4 overexpression [
47]. The reduction in seed number was likely due to the altered ABA/GA balance caused by ABI4-mediated upregulation of ABA biosynthesis genes like NCED6 and downregulation of GA catabolic genes like
GA2ox7 [
47]. The resulting increase in ABA and decrease in GA probably impaired fertilization and seed development. Taken together, these results indicate that precise control of ABI4 expression is critical for proper seed yield, as both too little and too much ABI4 can negatively impact production by reducing seed number. Thus, our observed positive correlation between ABI4 and seed number in
B. napus suggests this gene may play a similar role in regulating the ABA/GA balance and reproductive development in oilseed crop species. Further characterization of ABI4’s effects on hormone signaling and the seed setting process in
B. napus will clarify its utility as a target for improving seed yield.
Conversely, in the palevioletred module,
ARF2 (
AUXIN RESPONSE FACTOR 2) emerges as a high-connectivity transcription factor.
ARF2 is a pivotal regulator in auxin signaling and is implicated in controlling seed size through the modulation of cell expansion and endoreduplication [
49]. Our gene co-expression analysis shows a negative correlation with seed number but a positive correlation with seed weight in
Brassica napus. This result aligns with previous findings in Arabidopsis thaliana that loss-of-function mutations in ARF2 lead to increased seed number but decreased individual seed weight, while
ARF2overexpression has the opposite effect of decreasing seed number but increasing seed size [
49]. The conserved role for
ARF2 in promoting seed growth across distinct
Brassicaceae species is likely due to its activity in stimulating cell proliferation in the integuments of developing ovules, as demonstrated in Arabidopsis [
69]. The enlarged integuments generated more cells in the seed coat, allowing increased embryo growth and final seed size. Taken together, these data establish
ARF2 as a key maternal regulator of seed size in multiple Brassicaceae species, functioning to promote integument cell proliferation and limit seed number in order to increase the biomass allocated per seed. Modulating
ARF2 expression or activity provides a promising route to altering seed size and yield in oilseed crops like canola. These transcription factors may represent important nodes in the co-expression modules that modulate the trade-off between seed number and weight in rapeseed.
The co-expression modules associated with the seed number versus weight trade-off differed between this work and our previous work focused on shading treatments effects on seed yield [
20]. In the prenset study under heat stress, the indianred2 module linked to higher seed number showed enrichment for translation and protein functions, while the palevioletred module for higher seed weight was associated with transport and signaling. In contrast, in our previous work assessing light stress [
20], the turquoise module correlated with seed number was enriched for cell cycle and DNA replication genes, while the blue module for seed weight showed lipid metabolism functions. These results indicate distinct molecular processes mediate the trade-off in response to heat versus shading. The hub transcription factors also differed between studies - in this work identified floral, embryo and growth regulators as key for seed number or weight (
INO,
ABI4,
ARF2) while in Canales et al., 2021
[20] highlighted
NAC082,
NGAL2 and
IDD1. The distinct functional enrichment and hub genes likely reflect the different environmental stimuli used in each study to modify source-sink balance during seed set. The low overlap in the modules and regulators identified between the two studies indicates there are mostly specific, rather than conserved, molecular mechanisms mediating the trade-off in response to heat versus shading. Further comparative analyses to find common genetic factors influencing plasticity in seed number and weight would be valuable, but the current results suggest the underlying networks are largely distinct.
5. Conclusions
In summary, our multi-level study of rapeseed under field conditions provides new insights into heat stress responses during the critical reproductive phase. We reveal genotype-dependent variation in photosynthetic gene regulation that may confer thermotolerance through sustained carbon assimilation. Our integrated omics approach also newly identified candidate networks and transcriptional regulators governing the agronomically important trade-off between seed number and weight. These findings advance molecular understanding of heat adaptation and yield determination in oilseed crops.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org. Figure S1:UpSet plots and Gene Ontology (GO) enrichment analysis of differentially expressed genes (DEGs) in seeds and siliques. (A) UpSet plot illustrating the intersections of DEGs identified in seeds and siliques. Each vertical bar represents a unique combination of DEGs, with the number of shared genes indicated by the size of the bar. (B) GO enrichment analysis of shared DEGs between seeds and siliques. The top significantly enriched biological process, molecular function, and cellular component GO terms are displayed; Figure S2: Bar plot illustrating the number of genes significantly correlated with agronomic traits of yield and quality in seed and silique networks at different developmental stages; Table S1:Summary of RNA-seq data for heat stress response analysis in rapeseed plants. This table provides an overview of the sequencing and pseudoalignment statistics for 112 pod and seed samples; Table S2: Enriched biological processes for the co-expression modules in the seed network at 7 DAF that were highly correlated with seed quality traits.
Author Contributions
Conceptualization, J.C.; methodology, J.V; software, J.C.; validation, J.C.; formal analysis, J.C.; investigation, J.V, J.C., and D.F.C.; resources, D.F.C. and J.C.; data curation, J.V. and J.C.; writing—original draft preparation J.C; writing—review and editing, J.C, J.V, and D.F.C.; visualization, J.V and J.C.; supervision, J.C. and D.F.C.; project administration J.C., and D.F.C.; funding acquisition, J.C., and D.F.C. All authors have read and agreed to the published version of the manuscript.
Funding
J.C. and and D.F.C were supported by the National Agency for Research and Development (ANID) Chile with Program FONDECYT Regular 1230833 and FONDECYT Regular 1211040. J.C. was also supported by ANID—Millennium Science Initiative Program—ICN17-022.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The RNA-Seq datasets generated during this study are available in the NCBI Gene Expression Omnibus (GEO) repository, accession number GSE241954. All other data generated during this study are included in this published article and its Supplementary Materials.
Acknowledgments
We appreciate the technical assistance of Beatriz Shibar, Eusebio Miranda, and the staff at the Austral Farming Experimental Station (EEAA) of the Universidad Austral de Chile. Furthermore, we extend our heartfelt thanks to every member of the Plant Nutrition and Genomics Laboratory of the same University for their unwavering support and collaborative efforts throughout this study
Conflicts of Interest
The authors declare no conflict of interest.The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Cheng, W.; Sakai, H.; Yagi, K.; Hasegawa, T. Interactions of elevated [CO2] and night temperature on rice growth and yield. Agricultural and Forest Meteorology 2009, 149, 51–58. [Google Scholar] [CrossRef]
- Kim, H.R.; You, Y.H. Effects of elevated CO2 concentration and increased temperature on leaf related-physiological responses of Phytolacca insularis (native species) and Phytolacca americana (invasive species). Journal of Ecology and Environment 2010, 33, 195–204. [Google Scholar] [CrossRef]
- Ray, D.K.; West, P.C.; Clark, M.; Gerber, J.S.; Prishchepov, A.V.; Chatterjee, S. Climate change has likely already affected global food production. PLOS ONE 2019, 14, e0217148. [Google Scholar] [CrossRef] [PubMed]
- on Climate Change, I.P. Climate Change 2021 – The Physical Science Basis; Cambridge University Press, 2023. [Google Scholar] [CrossRef]
- Rivelli, G.M.; Long, M.E.F.; Abeledo, L.G.; Calderini, D.F.; Miralles, D.J.; Rondanini, D.P. Assessment of heat stress and cloudiness probabilities in post-flowering of spring wheat and canola in the Southern Cone of South America. Theoretical and Applied Climatology 2021, 145, 1485–1502. [Google Scholar] [CrossRef]
- Sandaña, P.A.; Harcha, C.I.; Calderini, D.F. Sensitivity of yield and grain nitrogen concentration of wheat lupin and pea to source reduction during grain filling. A comparative survey under high yielding conditions. Field Crops Research 2009, 114, 233–243. [Google Scholar] [CrossRef]
- Mera, M.; Lizana, X.C.; Calderini, D.F. Cropping systems in environments with high yield potential of southern Chile. In Crop Physiology; Elsevier, 2015; pp. 111–140. [Google Scholar] [CrossRef]
- Verdejo, J.; Calderini, D.F. Plasticity of seed weight in winter and spring rapeseed is higher in a narrow but different window after flowering. Field Crops Research 2020, 250, 107777. [Google Scholar] [CrossRef]
- del Pozo, A.; Jobet, C.; Matus, I.; Méndez-Espinoza, A.M.; Garriga, M.; Castillo, D.; Elazab, A. Genetic Yield Gains and Changes in Morphophysiological-Related Traits of Winter Wheat in Southern Chilean High-Yielding Environments. Frontiers in Plant Science 2022, 12. [Google Scholar] [CrossRef]
- Slafer, G.; Rawson, H. Sensitivity of Wheat Phasic Development to Major Environmental Factors: a Re-Examination of Some Assumptions Made by Physiologists and Modellers. Functional Plant Biology 1994, 21, 393. [Google Scholar] [CrossRef]
- Sadras, V.O.; Calderini, D.F. Crop Physiology Case Histories for Major Crops; Elsevier, 2021. [Google Scholar] [CrossRef]
- Secchi, M.A.; Fernandez, J.A.; Stamm, M.J.; Durrett, T.; Prasad, P.V.; Messina, C.D.; Ciampitti, I.A. Effects of heat and drought on canola (Brassica napus L.) yield oil, and protein: A meta-analysis. Field Crops Research 2023, 293, 108848. [Google Scholar] [CrossRef]
- Kourani, M.; Mohareb, F.; Rezwan, F.I.; Anastasiadi, M.; Hammond, J.P. Genetic and Physiological Responses to Heat Stress in Brassica napus. Frontiers in Plant Science 2022, 13. [Google Scholar] [CrossRef]
- Young, L.W.; Wilen, R.W.; Bonham-Smith, P.C. High temperature stress of Brassica napus during flowering reduces micro- and megagametophyte fertility induces fruit abortion, and disrupts seed production. Journal of Experimental Botany 2004, 55, 485–495. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, L.M.M.; Coquemont-Guyot, M.; Elie, N.; Morvan-Bertrand, A.; Avice, J.C.; Mollier, A.; Brunel-Muguet, S. Repeated heat stress events during the reproductive phase impact the dynamic development of seeds in Brassica napus L. Plant Science 2023, 327, 111559. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Stefanova, K.; Siddique, K.H.M.; Cowling, W.A. Transient daily heat stress during the early reproductive phase disrupts pod and seed development in Brassica napus L. Food and Energy Security 2020, 10. [Google Scholar] [CrossRef]
- Jedličková, V.; Hejret, V.; Demko, M.; Jedlička, P.; Štefková, M.; Robert, H.S. Transcriptome analysis of thermomorphogenesis in ovules and during early seed development in Brassica napus. BMC Genomics 2023, 24. [Google Scholar] [CrossRef]
- Gao, G.; Hu, J.; Zhang, X.; Zhang, F.; Li, M.; Wu, X. Transcriptome analysis reveals genes expression pattern of seed response to heat stress in Brassica napus L. Oil Crop Science 2021, 6, 87–96. [Google Scholar] [CrossRef]
- Lohani, N.; Singh, M.B.; Bhalla, P.L. RNA-Seq Highlights Molecular Events Associated With Impaired Pollen-Pistil Interactions Following Short-Term Heat Stress in Brassica napus. Frontiers in Plant Science 2021, 11. [Google Scholar] [CrossRef]
- Canales, J.; Verdejo, J.; Carrasco-Puga, G.; Castillo, F.M.; Arenas-M, A.; Calderini, D.F. Transcriptome Analysis of Seed Weight Plasticity in Brassica napus. International Journal of Molecular Sciences 2021, 22, 4449. [Google Scholar] [CrossRef]
- Li, N.; Song, D.; Peng, W.; Zhan, J.; Shi, J.; Wang, X.; Liu, G.; Wang, H. Maternal control of seed weight in rapeseed (Brassica napus L.): the causal link between the size of pod (mother source) and seed (offspring, sink). Plant Biotechnology Journal 2018, 17, 736–749. [Google Scholar] [CrossRef]
- Dong, H.; Tan, C.; Li, Y.; He, Y.; Wei, S.; Cui, Y.; Chen, Y.; Wei, D.; Fu, Y.; He, Y.; et al. Genome-Wide Association Study Reveals Both Overlapping and Independent Genetic Loci to Control Seed Weight and Silique Length in Brassica napus. Frontiers in Plant Science 2018, 9. [Google Scholar] [CrossRef]
- Kirkegaard, J.A.; Lilley, J.M.; Brill, R.D.; Ware, A.H.; Walela, C.K. The critical period for yield and quality determination in canola (Brassica napus L.). Field Crops Research 2018, 222, 180–188. [Google Scholar] [CrossRef]
- Labra, M.H.; Struik, P.C.; Evers, J.B.; Calderini, D.F. Plasticity of seed weight compensates reductions in seed number of oilseed rape in response to shading at flowering. European Journal of Agronomy 2017, 84, 113–124. [Google Scholar] [CrossRef]
- Verdejo, J.; Castro, C.; Bustos, D.; Calderini, D.F. Sensibility of seed yield, yield components and quality traits of rapeseed to increased temperature and reduced source-sink ratio during different phases of grain filling. In Proceedings of the RAFV Conference 2023 XXXIV Argentinian Meeting of Plant Physiology, Rosario, Argentina; 2023. [Google Scholar]
- Meier, U. Growth stages of mono-and dicotyledonous plants; Julius Kühn-Institut: Quedlinburg, Germany, 2018. [Google Scholar]
- Kirk, P.L. Kjeldahl Method for Total Nitrogen. Analytical Chemistry 1950, 22, 354–358. [Google Scholar] [CrossRef]
- Merrill, A.; Watt, B. Energy Value of Foods: Basis and Derivation; Energy Value of Foods: Basis and Derivation, 1973. [Google Scholar]
- Graeber, K.; Linkies, A.; Wood, A.T.; Leubner-Metzger, G. A Guideline to Family-Wide Comparative State-of-the-Art Quantitative RT-PCR Analysis Exemplified with a Brassicaceae Cross-Species Seed Germination Case Study. The Plant Cell 2011, 23, 2045–2063. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, H.; Buxdorf, K.; Shapira, I.; Ein-Gedi, S.; Zvi, M.M.B.; Fridman, E.; Moshelion, M.; Levy, M. LogSpin: a simple economical and fast method for RNA isolation from infected or healthy plants and other eukaryotic tissues. BMC Research Notes 2012, 5. [Google Scholar] [CrossRef] [PubMed]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nature Biotechnology 2016, 34, 525–527. [Google Scholar] [CrossRef]
- Pimentel, H.; Bray, N.L.; Puente, S.; Melsted, P.; Pachter, L. Differential analysis of RNA-seq incorporating quantification uncertainty. Nature Methods 2017, 14, 687–690. [Google Scholar] [CrossRef]
- Langfelder, P.; Zhang, B.; Horvath, S. Defining clusters from a hierarchical cluster tree: the Dynamic Tree Cut package for R. Bioinformatics 2007, 24, 719–720. [Google Scholar] [CrossRef]
- Gu, Z. Complex heatmap visualization. iMeta 2022, 1. [Google Scholar] [CrossRef]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. g:Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Research 2019, 47, W191–W198. [Google Scholar] [CrossRef]
- Kolberg, L.; Raudvere, U.; Kuzmin, I.; Vilo, J.; Peterson, H. gprofiler2 – an R package for gene list functional enrichment analysis and namespace conversion toolset g:Profiler. F1000Research 2020, 9, 709. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 2008, 9. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Silva, F.; Venancio, T.M. BioNERO: an all-in-one R/Bioconductor package for comprehensive and easy biological network reconstruction. Functional Integrative Genomics 2021, 22, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Tian, F.; Yang, D.C.; Meng, Y.Q.; Kong, L.; Luo, J.; Gao, G. PlantTFDB 4.0: toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Research 2016, 45, D1040–D1045. [Google Scholar] [CrossRef] [PubMed]
- Huynh-Thu, V.A.; Irrthum, A.; Wehenkel, L.; Geurts, P. Inferring Regulatory Networks from Expression Data Using Tree-Based Methods. PLoS ONE 2010, 5, e12776. [Google Scholar] [CrossRef] [PubMed]
- Margolin, A.A.; Nemenman, I.; Basso, K.; Wiggins, C.; Stolovitzky, G.; Favera, R.D.; Califano, A. ARACNE: An Algorithm for the Reconstruction of Gene Regulatory Networks in a Mammalian Cellular Context. BMC Bioinformatics 2006, 7. [Google Scholar] [CrossRef]
- Faith, J.J.; Hayete, B.; Thaden, J.T.; Mogno, I.; Wierzbowski, J.; Cottarel, G.; Kasif, S.; Collins, J.J.; Gardner, T.S. Large-Scale Mapping and Validation of Escherichia coli Transcriptional Regulation from a Compendium of Expression Profiles. PLoS Biology 2007, 5, e8. [Google Scholar] [CrossRef]
- Liu, X.; Yu, H.; Guan, Y.; Li, J.; Guo, F. Carbonylation and loss-of-function analyses of SBPase reveal its metabolic interface role in oxidative stress, carbon assimilation, and multiple aspects of growth and development in Arabidopsis. Mol Plant 2012, 5, 1082–99. [Google Scholar] [CrossRef]
- Hashida, S.; Miyagi, A.; Nishiyama, M.; Yoshida, K.; Hisabori, T.; Kawai-Yamada, M. Ferredoxin/thioredoxin system plays an important role in the chloroplastic NADP status of Arabidopsis. Plant J 2018, 95, 947–960. [Google Scholar] [CrossRef]
- Yu, H.; Murchie, E.H.; González-Carranza, Z.H.; Pyke, K.A.; Roberts, J.A. Decreased photosynthesis in the erect panicle 3 (ep3) mutant of rice is associated with reduced stomatal conductance and attenuated guard cell development. Journal of Experimental Botany 2015, 66, 1543–1552. [Google Scholar] [CrossRef]
- Villanueva, J.M.; Broadhvest, J.; Hauser, B.A.; Meister, R.J.; Schneitz, K.; Gasser, C.S. INNER NO OUTER regulates abaxial- adaxial patterning in Arabidopsis ovules. Genes Development 1999, 13, 3160–3169. [Google Scholar] [CrossRef]
- Shu, K.; Chen, Q.; Wu, Y.; Liu, R.; Zhang, H.; Wang, P.; Li, Y.; Wang, S.; Tang, S.; Liu, C.; et al. ABI4 mediates antagonistic effects of abscisic acid and gibberellins at transcript and protein levels. The Plant Journal 2016, 85, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Renard, J.; Niñoles, R.; Martínez-Almonacid, I.; Gayubas, B.; Mateos-Fernández, R.; Bissoli, G.; Bueso, E.; Serrano, R.; Gadea, J. Identification of novel seed longevity genes related to oxidative stress and seed coat by genome-wide association studies and reverse genetics. Plant Cell Environment 2020, 43, 2523–2539. [Google Scholar] [CrossRef] [PubMed]
- Schruff, M.C.; Spielman, M.; Tiwari, S.; Adams, S.; Fenby, N.; Scott, R.J. The AUXIN RESPONSE FACTOR 2 gene of Arabidopsis links auxin signalling, cell division, and the size of seeds and other organs. Development 2006, 133, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Angadi, S.V.; Cutforth, H.W.; Miller, P.R.; McConkey, B.G.; Entz, M.H.; Brandt, S.A.; Volkmar, K.M. Response of three Brassica species to high temperature stress during reproductive growth. Canadian Journal of Plant Science 2000, 80, 693–701. [Google Scholar] [CrossRef]
- Lohani, N.; Singh, M.B.; Bhalla, P.L. High temperature susceptibility of sexual reproduction in crop plants. Journal of Experimental Botany 2019, 71, 555–568. [Google Scholar] [CrossRef]
- Mácová, K.; Prabhullachandran, U.; Štefková, M.; Spyroglou, I.; Pěnčík, A.; Endlová, L.; Novák, O.; Robert, H.S. Long-Term High-Temperature Stress Impacts on Embryo and Seed Development in Brassica napus. Frontiers in Plant Science 2022, 13. [Google Scholar] [CrossRef]
- Kim, K.; Portis, A.R. Temperature Dependence of Photosynthesis in Arabidopsis Plants with Modifications in Rubisco Activase and Membrane Fluidity. Plant and Cell Physiology 2005, 46, 522–530. [Google Scholar] [CrossRef]
- Wang, L.; Ma, K.B.; Lu, Z.G.; Ren, S.X.; Jiang, H.R.; Cui, J.W.; Chen, G.; Teng, N.J.; Lam, H.M.; Jin, B. Differential physiological transcriptomic and metabolomic responses of Arabidopsis leaves under prolonged warming and heat shock. BMC Plant Biology 2020, 20. [Google Scholar] [CrossRef]
- Huang, R.; Liu, Z.; Xing, M.; Yang, Y.; Wu, X.; Liu, H.; Liang, W. Heat Stress Suppresses Brassica napus Seed Oil Accumulation by Inhibition of Photosynthesis and BnWRI1 Pathway. Plant and Cell Physiology 2019, 60, 1457–1470. [Google Scholar] [CrossRef]
- ul Haq; Khan.; Ali.; Khattak.; Gai.; Zhang.; Wei.; Gong. Heat Shock Proteins: Dynamic Biomolecules to Counter Plant Biotic and Abiotic Stresses. International Journal of Molecular Sciences 2019, 20, 5321. [CrossRef]
- Miyazaki, M.; Araki, M.; Okamura, K.; Ishibashi, Y.; Yuasa, T.; Iwaya-Inoue, M. Assimilate translocation and expression of sucrose transporter OsSUT1, contribute to high-performance ripening under heat stress in the heat-tolerant rice cultivar Genkitsukushi. Journal of Plant Physiology 2013, 170, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.E.; Meacham-Hensold, K.; Lemonnier, P.; Slattery, R.A.; Benjamin, C.; Bernacchi, C.J.; Lawson, T.; Cavanagh, A.P. The effect of increasing temperature on crop photosynthesis: from enzymes to ecosystems. Journal of Experimental Botany 2021, 72, 2822–2844. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, M.; Burritt, D.J.; Gupta, A.; Tsujimoto, H.; Tran, L.S.P. Heat stress effects on source–sink relationships and metabolome dynamics in wheat. Journal of Experimental Botany 2019, 71, 543–554. [Google Scholar] [CrossRef]
- Kumar, S.; Bhushan, B.; Wakchaure, G.C.; Dutta, R.; Jat, B.S.; Meena, K.K.; Rakshit, S.; Pathak, H. Unveiling the impact of heat stress on seed biochemical composition of major cereal crops: Implications for crop resilience and nutritional value. Plant Stress 2023, 9, 100183. [Google Scholar] [CrossRef]
- Harrison, E.P.; Olcer, H.; Lloyd, J.C.; Long, S.P.; Raines, C.A. Small decreases in SBPase cause a linear decline in the apparent RuBP regeneration rate but do not affect Rubisco carboxylation capacity. Journal of Experimental Botany 2001, 52, 1779–1784. [Google Scholar] [CrossRef]
- Harrison, E.P.; Willingham, N.M.; Lloyd, J.C.; Raines, C.A. Reduced sedoheptulose-1,7-bisphosphatase levels in transgenic tobacco lead to decreased photosynthetic capacity and altered carbohydrate accumulation. Planta 1997, 204, 27–36. [Google Scholar] [CrossRef]
- LAWSON, T.; BRYANT, B.; LEFEBVRE, S.; LLOYD, J.C.; RAINES, C.A. Decreased SBPase activity alters growth and development in transgenic tobacco plants. Plant Cell and Environment 2006, 29, 48–58. [Google Scholar] [CrossRef]
- Miyagawa, Y.; Tamoi, M.; Shigeoka, S. Overexpression of a cyanobacterial fructose-1,6-/sedoheptulose-1, 7-bisphosphatase in tobacco enhances photosynthesis and growth. Nature Biotechnology 2001, 19, 965–969. [Google Scholar] [CrossRef]
- Lefebvre, S.; Lawson, T.; Fryer, M.; Zakhleniuk, O.V.; Lloyd, J.C.; Raines, C.A. Increased Sedoheptulose-1,7-Bisphosphatase Activity in Transgenic Tobacco Plants Stimulates Photosynthesis and Growth from an Early Stage in Development. Plant Physiology 2005, 138, 451–460. [Google Scholar] [CrossRef]
- Skinner, D.J.; Dang, T.; Gasser, C.S. The Arabidopsis INNER NO OUTER (INO) gene acts exclusively and quantitatively in regulation of ovule outer integument development. Plant Direct 2023, 7. [Google Scholar] [CrossRef]
- Skinner, D.J. Regulation of Ovule Development. THE PLANT CELL ONLINE 2004, 16, S32–S45. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Chen, L.G.; Yin, G.; Yang, X.; Gao, Z.; Guo, Y.; Sun, Y.; Tang, W. Brassinosteroids regulate outer ovule integument growth in part via the control of INNER NO OUTER by BRASSINOZOLE-RESISTANT family transcription factors. Journal of Integrative Plant Biology 2020, 62, 1093–1111. [Google Scholar] [CrossRef] [PubMed]
- Adamski, N.M.; Anastasiou, E.; Eriksson, S.; O’Neill, C.M.; Lenhard, M. Local maternal control of seed size by KLUH/CYP78A5-dependent growth signaling. Proceedings of the National Academy of Sciences 2009, 106, 20115–20120. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Response of seed yield, seed number, thousand seed weight and quality traits (seed oil and protein concentrations) across experiments due to heat stress relative to the control. Asterisks indicate significant effect of heat stress treatment on the control by using Fisher’s LSD test (p < 0.05).
Figure 1.
Response of seed yield, seed number, thousand seed weight and quality traits (seed oil and protein concentrations) across experiments due to heat stress relative to the control. Asterisks indicate significant effect of heat stress treatment on the control by using Fisher’s LSD test (p < 0.05).
Figure 2.
Multivariate analysis of RNA-seq data in siliques and seeds under heat stress. The bar plots display the number of differentially expressed genes (DEGs) attributed to various factors and their interactions in (A) siliques and (B) seeds based on sleuth analysis (q<0.01). Genotype is the predominant factor affecting gene expression, while heat treatment has a more pronounced effect on siliques than seeds, indicating tissue-specific responses to temperature stress. Data were obtained from field experiments conducted in Valdivia, Chile, using two rapeseed genotypes (Lumen and Solar) subjected to control and heat stress conditions.
Figure 2.
Multivariate analysis of RNA-seq data in siliques and seeds under heat stress. The bar plots display the number of differentially expressed genes (DEGs) attributed to various factors and their interactions in (A) siliques and (B) seeds based on sleuth analysis (q<0.01). Genotype is the predominant factor affecting gene expression, while heat treatment has a more pronounced effect on siliques than seeds, indicating tissue-specific responses to temperature stress. Data were obtained from field experiments conducted in Valdivia, Chile, using two rapeseed genotypes (Lumen and Solar) subjected to control and heat stress conditions.
Figure 3.
Hierarchical clustering analysis reveals distinct patterns of heat-responsive gene expression in siliques. (A) Heatmap showing hierarchical clustering of 1698 heat-responsive genes in siliques into eight distinct clusters based on their expression profiles across samples using ComplexHeatmap [
34]. Rows represent genes and columns represent samples from two genotypes (Lumen and Solar), two heat treatments (control and heat stress), two time points (7 and 14 DAF), and two seasons. (B) Enrichment analysis of biological processes for each gene cluster. The number of genes and FDR-adjusted p-values are shown for the top enriched terms in each cluster. Clusters 1, 2, and 5 contain genes downregulated by heat stress and involved in photosynthesis, pigment metabolism, and carbohydrate metabolism. Clusters 3, 4, 6, 7, and 8 show heat-induced genes associated with various processes including protein folding and cell wall metabolism. Cluster 8 displays a genotype-specific temporal response, with decreased expression of protein folding genes in Lumen but not Solar at 14 DAF under heat stress.
Figure 3.
Hierarchical clustering analysis reveals distinct patterns of heat-responsive gene expression in siliques. (A) Heatmap showing hierarchical clustering of 1698 heat-responsive genes in siliques into eight distinct clusters based on their expression profiles across samples using ComplexHeatmap [
34]. Rows represent genes and columns represent samples from two genotypes (Lumen and Solar), two heat treatments (control and heat stress), two time points (7 and 14 DAF), and two seasons. (B) Enrichment analysis of biological processes for each gene cluster. The number of genes and FDR-adjusted p-values are shown for the top enriched terms in each cluster. Clusters 1, 2, and 5 contain genes downregulated by heat stress and involved in photosynthesis, pigment metabolism, and carbohydrate metabolism. Clusters 3, 4, 6, 7, and 8 show heat-induced genes associated with various processes including protein folding and cell wall metabolism. Cluster 8 displays a genotype-specific temporal response, with decreased expression of protein folding genes in Lumen but not Solar at 14 DAF under heat stress.
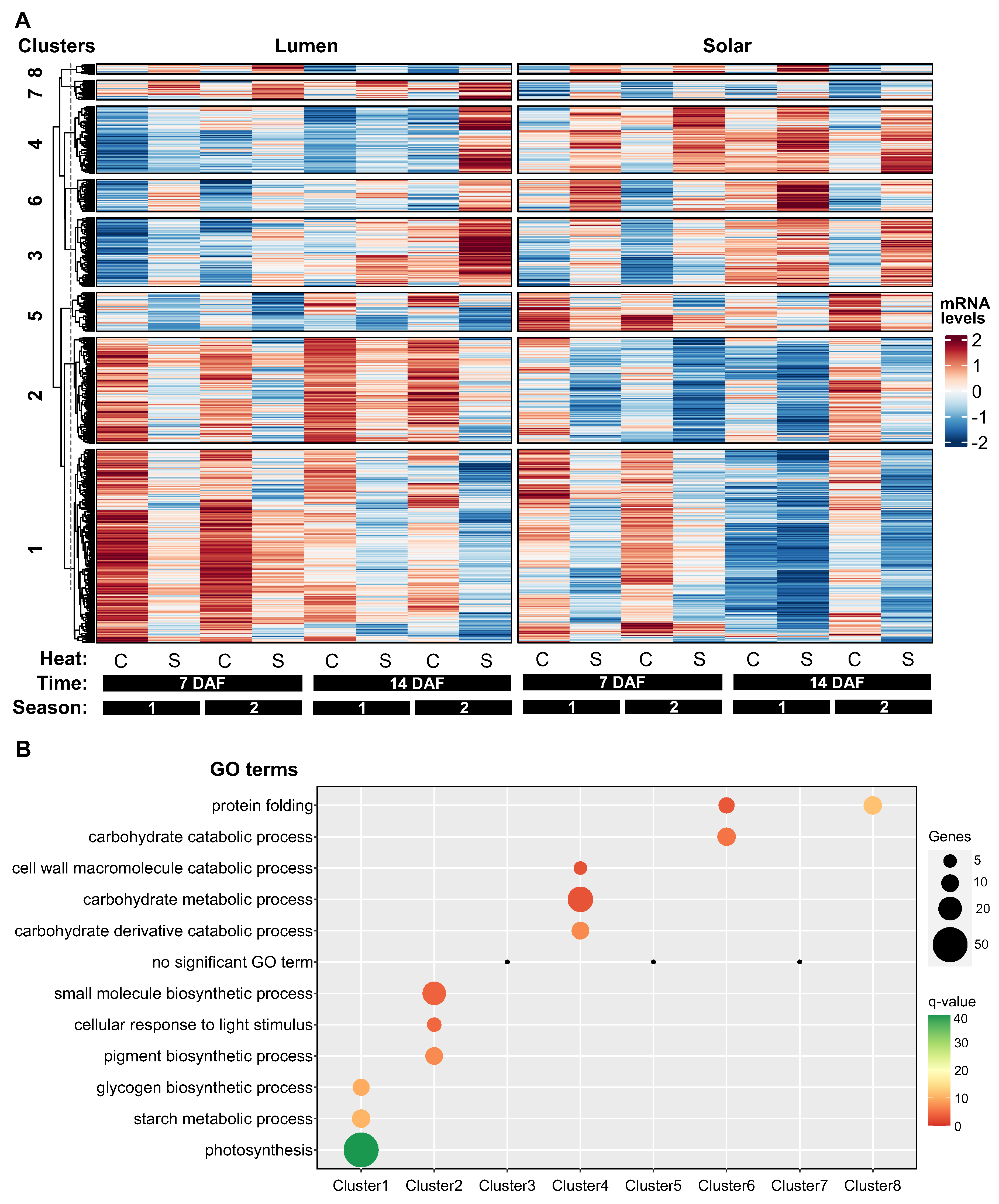
Figure 4.
Hierarchical clustering reveals three distinct patterns of heat-responsive gene expression in rapeseed seeds. Heatmap visualization of 202 differentially expressed genes (q-value <0.01) in response to heat treatment, clustered into 3 groups with distinct expression profiles. Rows represent genes and columns represent different genotype (Lumen or Solar), timepoint (7 or 14 days after flowering), season (1 or 2), and temperature treatment (control or heat stress) conditions. Normalized expression values are color-coded based on row z-score. Cluster 1 (81 genes) shows downregulation by heat stress. Cluster 2 (63 genes) exhibits upregulation by heat. Cluster 3 (58 genes) displays genotype-specific responses to heat. Gene ontology enrichment analysis indicates Cluster 1 is involved in glycolipid metabolism and phosphate starvation response, Cluster 2 in carboxylic acid metabolism and sucrose biosynthesis, and Cluster 3 in protein folding and response to heat. Data derived from RNA-seq analysis of seed samples under field conditions, as described in the Materials and Methods.
Figure 4.
Hierarchical clustering reveals three distinct patterns of heat-responsive gene expression in rapeseed seeds. Heatmap visualization of 202 differentially expressed genes (q-value <0.01) in response to heat treatment, clustered into 3 groups with distinct expression profiles. Rows represent genes and columns represent different genotype (Lumen or Solar), timepoint (7 or 14 days after flowering), season (1 or 2), and temperature treatment (control or heat stress) conditions. Normalized expression values are color-coded based on row z-score. Cluster 1 (81 genes) shows downregulation by heat stress. Cluster 2 (63 genes) exhibits upregulation by heat. Cluster 3 (58 genes) displays genotype-specific responses to heat. Gene ontology enrichment analysis indicates Cluster 1 is involved in glycolipid metabolism and phosphate starvation response, Cluster 2 in carboxylic acid metabolism and sucrose biosynthesis, and Cluster 3 in protein folding and response to heat. Data derived from RNA-seq analysis of seed samples under field conditions, as described in the Materials and Methods.
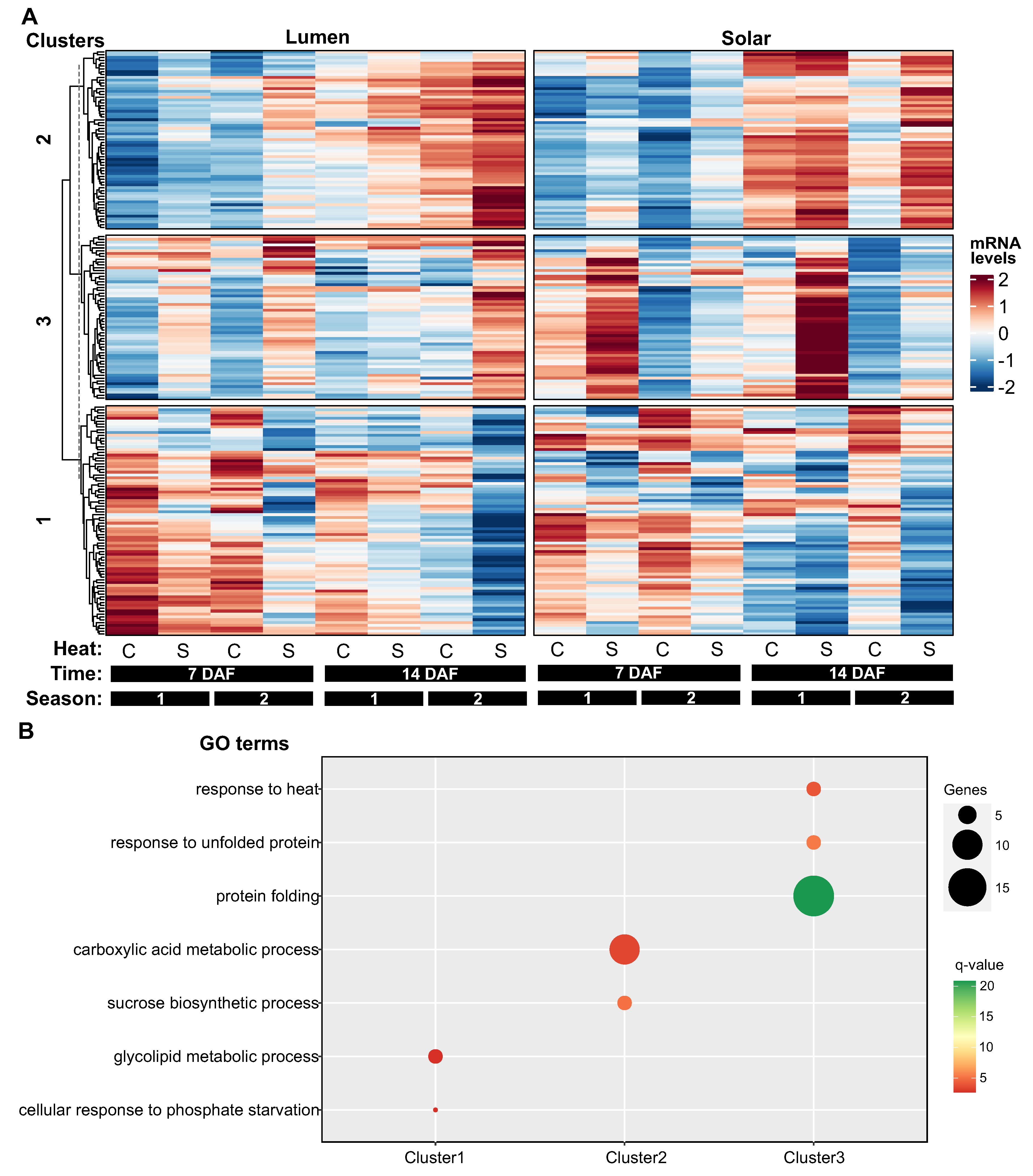
Figure 5.
Genotype-dependent effects of heat stress on photosynthesis-related genes in siliques. (A) Venn diagrams showing the number of genes significantly regulated by heat, genotype, or both factors in siliques and seeds. Only siliques showed a significant overlap between the two factors (p < 0.05, GeneOverlap test). (B) Histogram showing the distribution of the 121 genes that were regulated by heat and genotype in siliques across clusters 1 and 2, which contain photosynthesis and light response genes. (C) Expression profiles of the photosynthesis-related genes SBPase (cluster 1) and INAP1 (cluster 2) in silique samples under control and heat conditions. SBPase and INAP1 mRNA levels were consistently higher in the Lumen genotype compared to Solar under heat stress based on RNA-seq data, suggesting improved maintenance of photosynthetic gene expression in the heat tolerant Lumen variety. Bars represent means ± SE (n=3). Asterisks indicate significant differences between genotypes (NS, not significant, * P < 0.05, ** P < 0.01, *** P < 0.001).
Figure 5.
Genotype-dependent effects of heat stress on photosynthesis-related genes in siliques. (A) Venn diagrams showing the number of genes significantly regulated by heat, genotype, or both factors in siliques and seeds. Only siliques showed a significant overlap between the two factors (p < 0.05, GeneOverlap test). (B) Histogram showing the distribution of the 121 genes that were regulated by heat and genotype in siliques across clusters 1 and 2, which contain photosynthesis and light response genes. (C) Expression profiles of the photosynthesis-related genes SBPase (cluster 1) and INAP1 (cluster 2) in silique samples under control and heat conditions. SBPase and INAP1 mRNA levels were consistently higher in the Lumen genotype compared to Solar under heat stress based on RNA-seq data, suggesting improved maintenance of photosynthetic gene expression in the heat tolerant Lumen variety. Bars represent means ± SE (n=3). Asterisks indicate significant differences between genotypes (NS, not significant, * P < 0.05, ** P < 0.01, *** P < 0.001).
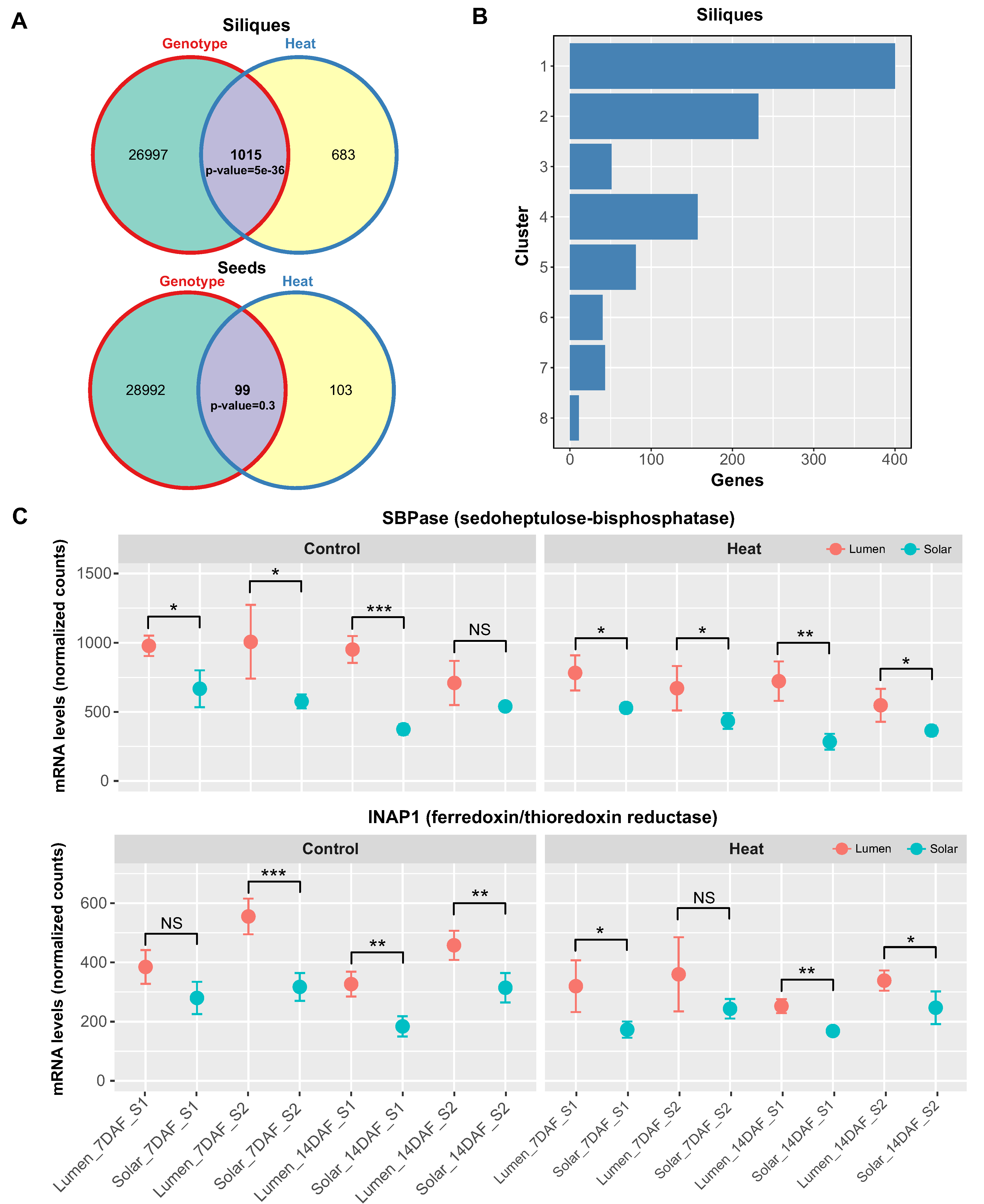
Figure 6.
Correlation heatmap of gene coexpression modules with yield and quality traits in rapeseed. Heatmap showing Pearson correlation coefficients between module eigengenes and phenotypic traits related to plant growth, seed yield, and quality. The modules were generated by weighted gene coexpression network analysis of transcriptomic data from 28 samples of seeds at 7 days after flowering. Modules are grouped into categories and ordered based on strongest correlations. Red indicates a positive correlation, blue indicates a negative correlation. The largest modules positively correlated with protein content (lightblue3, firebrick), oil content (lightskyblue2), seed number (indianred2), and seed weight (palevioletred) are highlighted. This systems-level analysis reveals major coexpression modules associated with key agronomic traits that determine yield and quality in rapeseed.
Figure 6.
Correlation heatmap of gene coexpression modules with yield and quality traits in rapeseed. Heatmap showing Pearson correlation coefficients between module eigengenes and phenotypic traits related to plant growth, seed yield, and quality. The modules were generated by weighted gene coexpression network analysis of transcriptomic data from 28 samples of seeds at 7 days after flowering. Modules are grouped into categories and ordered based on strongest correlations. Red indicates a positive correlation, blue indicates a negative correlation. The largest modules positively correlated with protein content (lightblue3, firebrick), oil content (lightskyblue2), seed number (indianred2), and seed weight (palevioletred) are highlighted. This systems-level analysis reveals major coexpression modules associated with key agronomic traits that determine yield and quality in rapeseed.
Figure 7.
Co-expression network analysis reveals regulators of seed number and weight tradeoff. (A) Correlation plot showing the relationship between average expression of indianred2 module genes and seed number/weight across samples. Each point represents the mean expression level of module genes for a sample, with red indicating seed number and blue indicating seed weight. (B) Enriched Gene Ontology terms for the indianred2 module related to translation, protein targeting, and other functions. The x-axis shows statistical significance. GO term enrichment analysis was performed using g:Profiler with a q-value cutoff of 0.05 (C) Inferred gene regulatory network for the indianred2 module. The network was constructed using GENIE3, ARACNE, and CLR algorithms on gene expression data. Nodes represent transcription factors (triangles) and target genes (circles). The 5 transcription factors with highest connectivity are labeled. Node size corresponds to degree. Data is from RNA-seq analysis of rapeseed seeds under control and heat stress conditions at 7 days after flowering, as described in the Materials and Methods.
Figure 7.
Co-expression network analysis reveals regulators of seed number and weight tradeoff. (A) Correlation plot showing the relationship between average expression of indianred2 module genes and seed number/weight across samples. Each point represents the mean expression level of module genes for a sample, with red indicating seed number and blue indicating seed weight. (B) Enriched Gene Ontology terms for the indianred2 module related to translation, protein targeting, and other functions. The x-axis shows statistical significance. GO term enrichment analysis was performed using g:Profiler with a q-value cutoff of 0.05 (C) Inferred gene regulatory network for the indianred2 module. The network was constructed using GENIE3, ARACNE, and CLR algorithms on gene expression data. Nodes represent transcription factors (triangles) and target genes (circles). The 5 transcription factors with highest connectivity are labeled. Node size corresponds to degree. Data is from RNA-seq analysis of rapeseed seeds under control and heat stress conditions at 7 days after flowering, as described in the Materials and Methods.
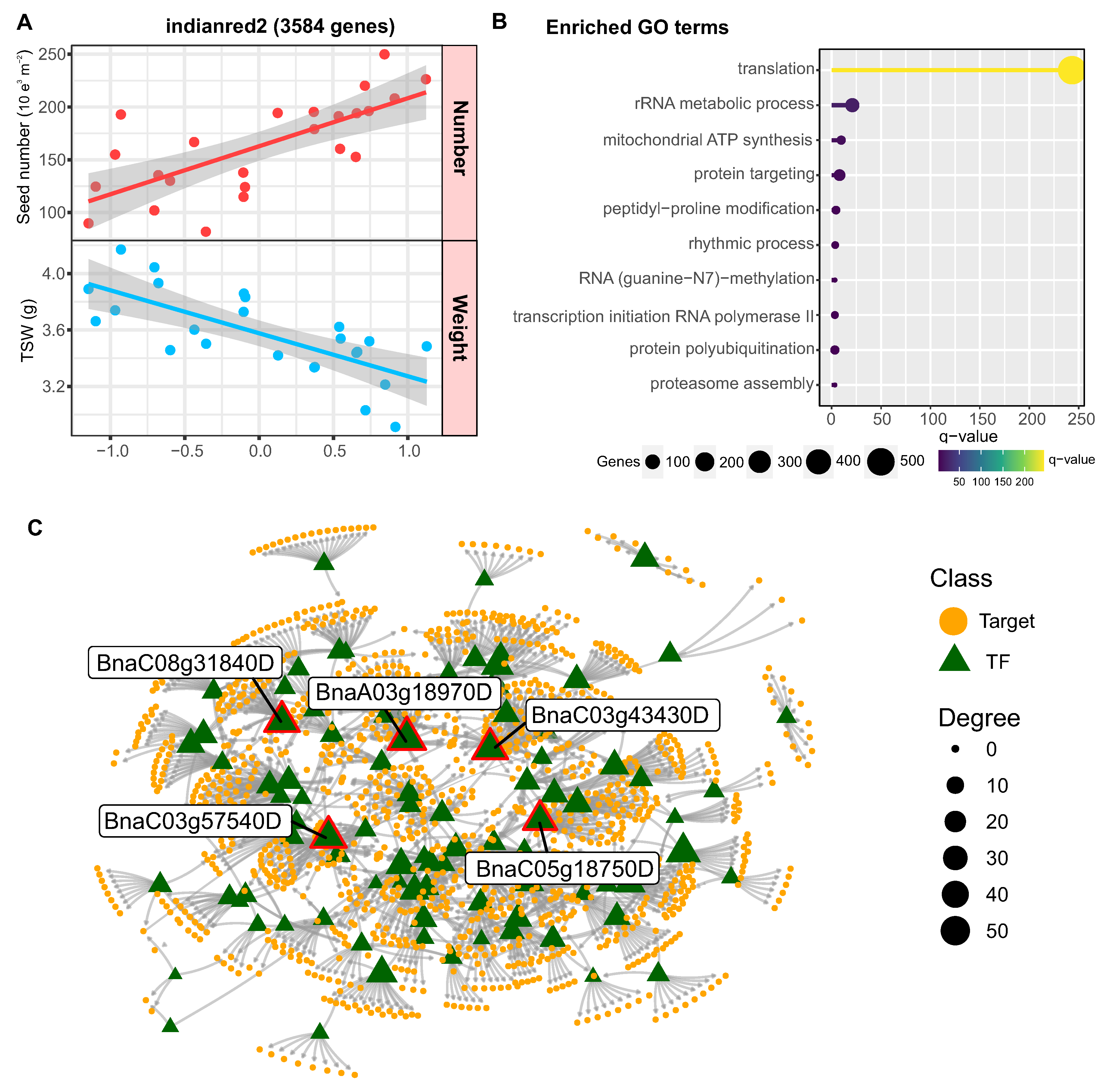
Figure 8.
Co-expression module associated with seed weight in rapeseed. (A) Scatterplot showing the negative correlation between seed number and the expression pattern of genes in the palevioletred module (r = -0.77, p < 0.0001). Each dot represents a specific sample. (B) Enriched gene ontology (GO) terms for the palevioletred module related to intracellular transport, mRNA metabolism, signaling, and chromatin remodeling. The bar plot shows the number of genes annotated to each term out of the 2416 genes in the module. GO term enrichment analysis was performed using g:Profiler with a q-value cutoff of 0.05. (C) Regulatory network showing predicted transcription factor (TF) regulators of the palevioletred module. The network was constructed using GENIE3, ARACNE, and CLR algorithms on gene expression data. The 5 transcription factors with highest connectivity are labeled. Node size corresponds to degree.
Figure 8.
Co-expression module associated with seed weight in rapeseed. (A) Scatterplot showing the negative correlation between seed number and the expression pattern of genes in the palevioletred module (r = -0.77, p < 0.0001). Each dot represents a specific sample. (B) Enriched gene ontology (GO) terms for the palevioletred module related to intracellular transport, mRNA metabolism, signaling, and chromatin remodeling. The bar plot shows the number of genes annotated to each term out of the 2416 genes in the module. GO term enrichment analysis was performed using g:Profiler with a q-value cutoff of 0.05. (C) Regulatory network showing predicted transcription factor (TF) regulators of the palevioletred module. The network was constructed using GENIE3, ARACNE, and CLR algorithms on gene expression data. The 5 transcription factors with highest connectivity are labeled. Node size corresponds to degree.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).