1. Introduction
Colletotrichum lindemuthianum (Sacc. & Magnus) Briosi & Cavara is a highly destructive pathogen that causes anthracnose (ANT) in common beans. In conditions of low temperature and high humidity, ANT can result in up to 100% yield losses. Furthermore, the pathogenic variability of
C. lindemuthianum and the emergence of new races have resulted in the reduction or total loss of yield of previously resistant cultivars [
1]. Angular leaf spot (ALS) is another disease that impacts common beans globally, caused by
Pseudocercospora griseola (Sacc.) Crous & U. Braun. This disease can lead to yield losses of up to 70% [2-4]. Adopting genetically resistant cultivars provides a cost-effective, user-friendly, and environmentally conscious strategy for managing
C. lindemuthianum and
P. griseola infections in common beans [
4]. Consequently, identifying and molecularly characterizing resistance genes is crucial for enhancing resistance efficacy and durability [5-8].
The
Co and
Phg loci associated with ANT and ALS, respectively, are often found in disease-resistance clusters on various chromosomes. Although several independent genes confer resistance to C. lindemuthianum, most resistance genes found in Andean cultivars have been mapped to the common bean chromosome Pv01. The
Co-1,
Co-12,
Co-13,
Co-14,
Co-15 and
Co-1HY alleles of the
Co-1 genes are present in the cultivars Michigan Dark Red Kidney (MDRK), Kaboon, Perry Marrow, AND 277, Widusa and Hongyundou, respectively [9-12]. Other resistance genes were mapped to the end of Pv01:
Co-x in Jalo EEP558,
Co-AC in Amendoim Cavalo [13, 14]. Moreover, the
CoPv01CDRK/PhgPv01CDRK gene in CDRK, which confers resistance to
C. lindemuthianum races 73, 2047, and 3481, as well as race 63-39 of
P. griseola, was recently fine-mapped to Pv01 [
15].
A single dominant resistance gene primarily confers resistance to the ALS pathogen; however, recent studies have also identified quantitative resistance loci (QRLs) [16-17]. To date, five resistance loci have been mapped, including three independent loci, Phg-1, Phg-2, and Phg-3, located on chromosomes Pv01, Pv08, and Pv04, respectively [9, 18-19]. Additionally, two major QRLs, Phg-4, and Phg-5, have been found on Pv04 and Pv10 [16-17, 20-21].
Candidate genes for ANT resistance loci have been analyzed thorough gene expression analysis to infer functionality in resistant cultivars [11, 22-23]. Generally, examining the expression of the candidate and disease-resistance genes can reveal their roles and interactions, thereby contributing to our understanding of how these genes collaborate in effective resistance responses. Chen [
11] evaluated the expression analysis of four candidate genes at the
Co-1HY allele in the Hongyundou cultivar inoculated with
C. lindemuthianum race 81. The authors observed significant induction of all genes at an early stage. However, expression levels decreased at 24 hours post inoculation (hpi) and beyond. In the susceptible cultivar, high expression was only observed at 120 hpi, suggesting that delayed gene expression might facilitate pathogen penetration and proliferation, ultimately leading to disease development.
To elucidate the precise timing and magnitude of expression related to resistance against
C. lindemuthianum race 73 Mahiya-Farooq [
22] analysed the expression of four candidate genes within the
Co-1 locus using near-isogenic lines that differ in the presence of the
Co-12 resistance allele. They observed that the
Phvul.001G243800 gene exhibited substantially higher expression levels, nearly 144-fold, in the resistant near-isogenic line. The molecular basis of the ANT resistance
Co-x locus was established by sequencing a 58-kb target region in the Jalo EEP558 cultivar. The
KTR2/3 gene was identified as an additional gene within a CRINKLY4 kinase cluster between the candidate genes
Phvul.001G243600 and
Phvul.001G243700. Gene expression analysis demonstrated that
KTR2/3 was upregulated in Jalo EEP558 at 24 hours post-inoculation in plants inoculated with strain 100 of
C. lindemuthianum [13, 23].
Phaseolus vulgaris CDRK from the breeding program at the University of California Davis is a landrace collected around Sacramento, CA, USA [
24]. CDRK is resistant to Andean races 2, 39, 55 and Mesoamerican races 9, 64, 65, 73, 89, 1545, 2047, and 3481 of
C. lindemuthianum [
15]. Through fine mapping, a previous study delimited
CoPv01CDRK/
PhgPv01CDRK in a genomic region of 33 Kb on chromosome Pv01 and detected five candidate genes:
Phvul.001G246000 (ATP-dependent RNA helicase),
Phvul.001G246100 (cation-dependent mannose-6-phosphate receptor),
Phvul.001G246200 (protein trichome birefringence-like 33),
Phvul.001G246300 (abscisic acid (ABA) receptor PYL5), and
Phvul.001G246400 (SNF2 domain-containing protein class 1-related). Additionally, the candidate genes
Phvul.001G245300 and
Phvul.001G246800, encode putative leucine-rich repeat protein kinases, are close to the
CoPv01CDRK/
PhgPv01CDRK loci [
15].
In summary, protein expression studies regarding the ANT resistance locus have identified the candidate genes
Phvul.001G243800 for
Co-12, KTR2/3 for
Co-x and
Phvul.001G243600 and
Phvul.001G243700 for
Co-1HY which showed high expression levels under inoculation conditions [11, 13, 22-23]. All of these loci have been mapped onto Pv01. However, allelism test revealed that
CoPv01CDRK/PhgPv01CDRK is not allelic to
Co-1 [
15]. Moreover, the physical distances between
CoPv01CDRK/PhgPv01CDRK and
Co-1,
Co-x,
Co-1HY are 211 kb, 193 kb, and 181 kb, respectively. Given these considerations, the objective of this study was to identify specific candidate gene in the CDRK cultivar effective against
C. lindemuthianum race 73 and
P. griseola race 63-39 using gene expression analysis employing quantitative real-time PCR.
3. Discussion
This study discusses the cellular mechanisms employed by plants to combat unfavorable conditions caused by biotic factors. These responses are complex networks that involve changes in gene expression, regulation of metabolic processes, reinforcement of the plant cell wall, and hormone signaling pathways. In particular, the majority of resistance genes identified encode NBS-LRR proteins, which consist of an amino-terminal signaling domain, a nucleotide-binding site (NBS), and carboxy-terminal leucine-rich repeats (LRRs). These NBS-LRR proteins are capable of recognizing pathogen effectors through protein-protein interactions, subsequently triggering effector-triggered immunity [25-26]. However, it is noteworthy that the common bean chromosome Pv01 exhibits a low abundance of NBS-LRR genes. Instead, this chromosome harbors genes that encode other proteins involved in the resistance response, such as kinases functioning as pattern-recognition receptors (PRRs) that recognize pathogen-associated molecular patterns (PAMPs) and initiate PAMP-triggered immunity (PTI).
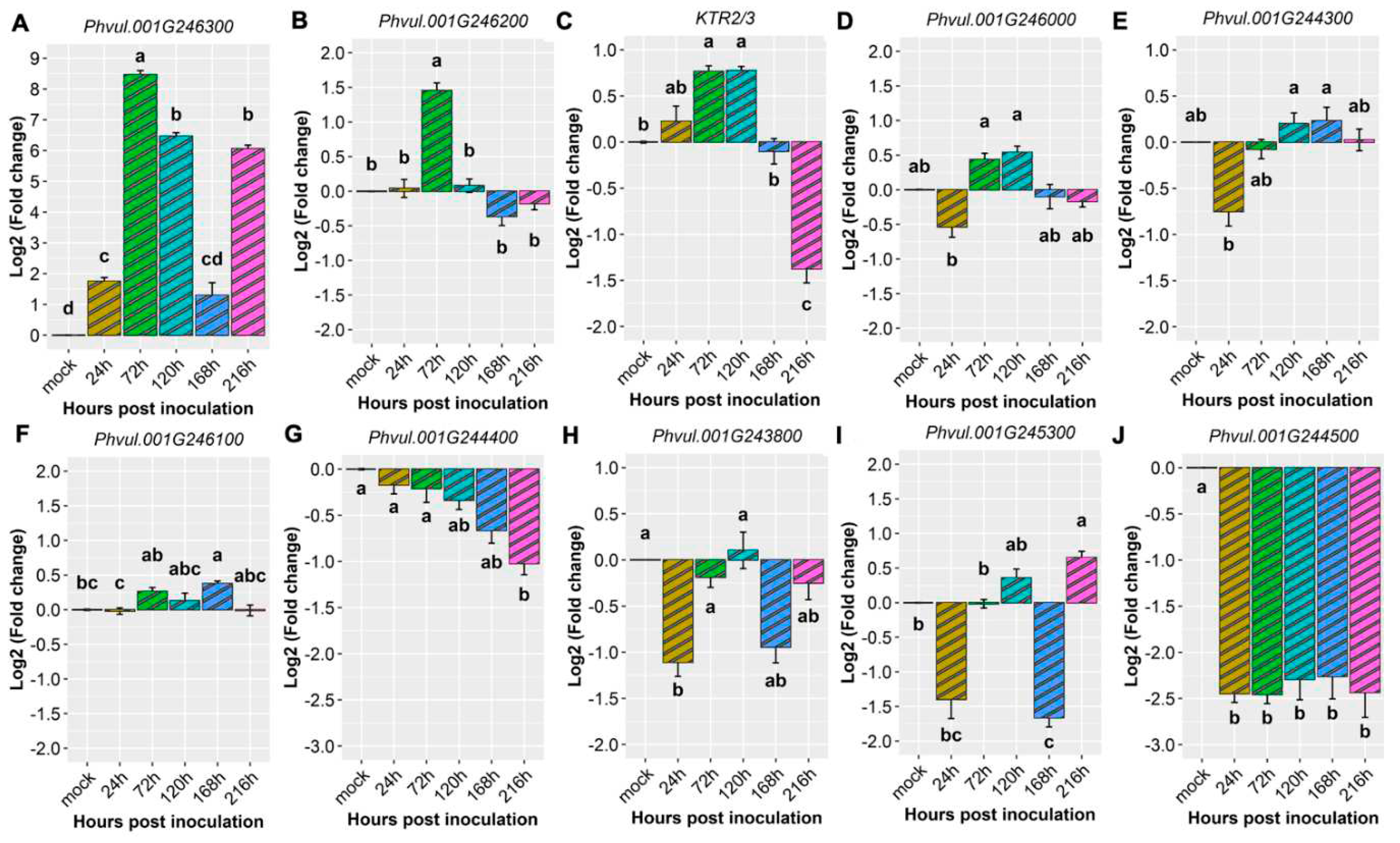
In this research we assessed the expression levels of candidate genes near the
CoPv01CDRK/PhgPv01CDRK loci and plant defense genes in CDRK cultivar. Through this analysis, we identified genes that consistently manifested high expression in resistant plants under pathogen inoculation. We observed that the candidate gene
Phvul.001G246300 as a potential candidate for the
CoPv01CDRK/PhgPv01CDRK resistance loci in CDRK plants inoculated with
C. lindemuthianum race 73, as it demonstrated the highest relative expression among the candidate genes. Among the tested candidate genes, the expression of
Phvul.001G246300 demonstrated a significant 7.8-fold increase at 24, 48, 72, 96, and 120 hpi of race 73 of
C. lindemuthianum. Moreover, it exhibited an 8.5-fold change at 72 hpi and over a 6-fold change at 120 and 216 hpi with race 63-39 of
P. griseola, indicating its heightened responsiveness to both pathogens compared to the other candidate genes examined. This particular gene encodes an abscisic acid (ABA) receptor, PYL5 protein, which is known to play a crucial role in mediating the crosstalk between ABA and jasmonic acid (JA) responses [
27]. ABA has been implicated in plant defense against pathogens and shows synergistic interactions with the ethylene (ET) signaling pathway. The upregulation of the PYR receptor during biotic stress suggests its involvement in perceiving ABA and initiating downstream signaling mediated by kinases [
28].
The Phvul.001G245300 gene ranked second in terms of induction among the candidate genes for CoPv01CDRK/PhgPv01CDRK loci in plants inoculated with race 73 of C. lindemuthianum. However, its expression was only half that of Phvul.001G246300, and when the plants were inoculated with race 63-39 of P. griseola, this gene showed a less pronounced response, with higher expression observed only at 216 hpi. Hypothetically, this gene encodes a protein belonging to the protein kinase superfamily, which is predominantly composed of catalytic domains of serine/threonine-specific and tyrosine-specific protein kinases. Furthermore, the protein contains a Leucine-Rich Repeat (LRR) domain. Proteins containing LRRs, including tyrosine kinase receptors, are involved in diverse biological processes such as signal transduction, cell adhesion, DNA repair, recombination, transcription, RNA processing, apoptosis, disease resistance, and immune responses [29, 30].
The
Phvul.001G246200 gene ranked third among the upregulated genes, mainly between 24 and 72 hpi with race 73 of
C. lindemuthianum, but its induction was three times lower than that of
Phvul.001G246300. When plants were inoculated with race 63-39 of
P. griseola, this gene exhibited a modest upregulation at 72 hpi, five times less than
Phvul.001G246300. In Arabidopsis thaliana, a homologous gene plays a crucial role in xylan acetylation and the proper deposition of secondary walls [
31]. Acetylation of wall polymers is important for cell wall strength and disease resistance, as evidenced by several Arabidopsis mutants and overexpression lines [31, 32]. Therefore, the
Phvul.001G246200 gene may contribute to the resistance response in the CDRK cultivar by modifying cell wall strength, thereby impeding pathogen infection and/or colonization of plant tissues during the biotrophic life stage.
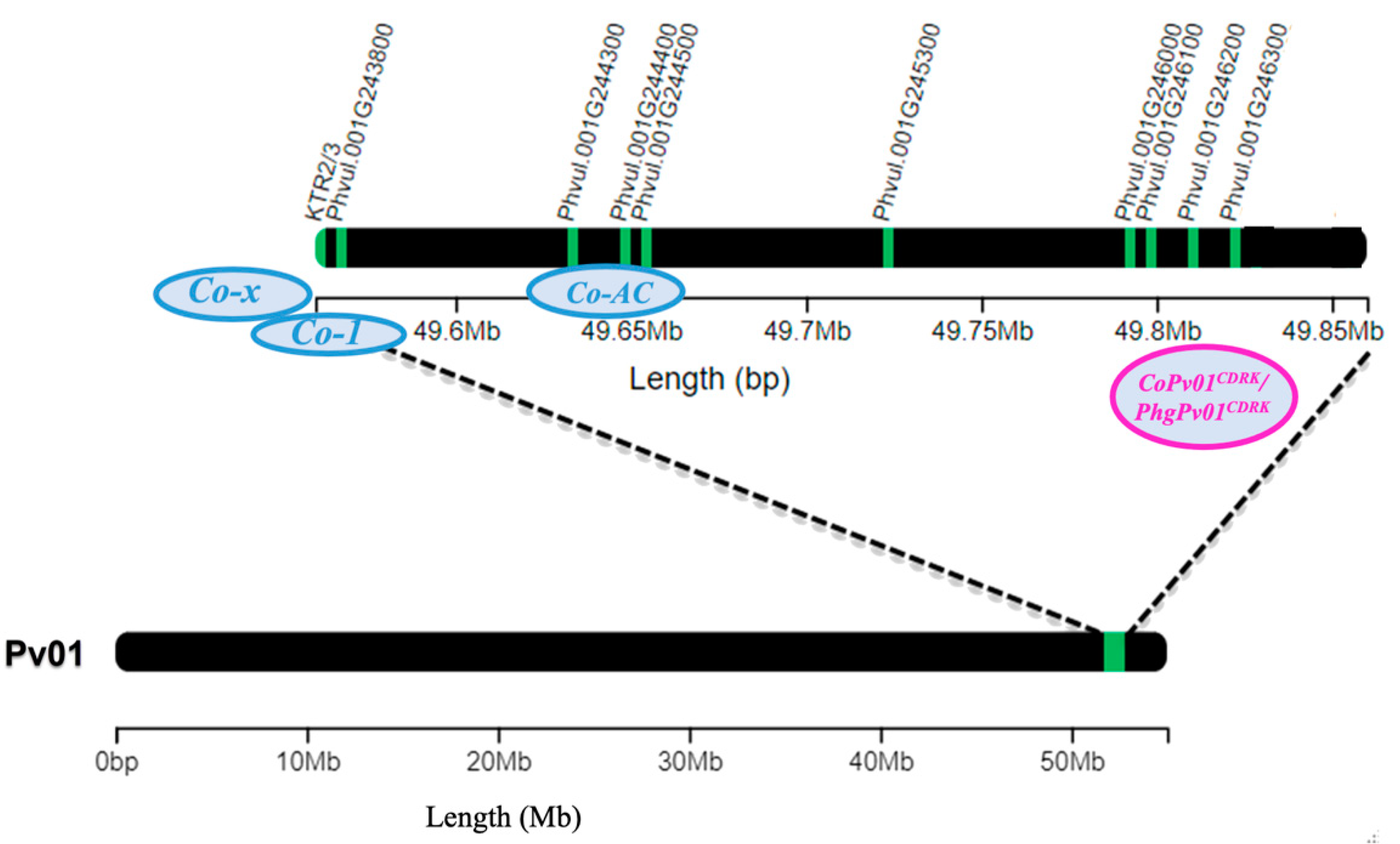
The majority of the ANT resistance genes identified in Andean cultivars are located in a resistance cluster on the common bean chromosome Pv01, including
Co-1,
Co-12,
Co-13,
Co-14,
Co-15,
Co-1HY,
Co-1X,
Co-x,
Co-AC, and
CoPv01CDRK/PhgPv01CDRK (
Figure 10). Interestingly, the resistance gene from the Jalo EEP558 cultivar,
Co-x, conferring resistance against race 3993 of
C. lindemuthianum, is located in close proximity to
CoPv01CDRK/PhgPv01CDRK loci. The protein KTR2/3 has been identified as the controlling factor for resistance of
Co-x in Jalo EEP558 cultivar [
23]. In our study with the CDRK cultivar, we observed that the expression of the
KTR2/3 gene was 2.6 times lower than that of
Phvul.001G246300 at 72 hpi in response to race 73 of
C. lindemuthianum. Furthermore, in response to race 63-39 of
P. griseola, the
KTR2/3 gene exhibited a remarkably lower expression level, approximately 10.6 times lower, compared to
Phvul.001G246300. Taking together, the physical position where
CoPv01CDRK/PhgPv01CDRK and
Co-x were mapped, and different candidate genes significantly upregulated after inoculation in each cultivar, KTR2/3 for Jalo EEP558 and
Phvul.001G246300 for CDRK its possible to conclude that different genetic resistance are involved in each cultivar against the same pathogen.
Using near-isogenic lines with differing resistance alleles, expression analysis of candidate genes for the
Co-12 allele against
C. lindemuthianum race 73 resistance revealed high levels of
Phvul.001G243800 in the resistant NIL [
22]. Furthermore, based on transcriptional analysis, it was observed that
Phvul.001G243700, located near the
Co-1 locus, was differentially upregulated in the resistant NIL at 72 and 96 hpi after race 73 inoculation [
33]. In the present study, significant differences in the expression of the
Phvul.001G246300 and
Phvul.001G243800 genes in response to race 73 of
C. lindemuthianum and race 63-39 of
P. griseola were observed in CDRK cultivar. At 72 hpi with race 73 of
C. lindemuthianum we observed a notable expression of the
Phvul.001G246300 gene, which was approximately three times higher compared to
Phvul.001G243800. Remarkably, within the context of
P. griseola race 63-39, we observed that
Phvul.001G243800 gene was repressed, on the other hand, the expression of
Phvul.001G246300 gene exhibited a substantial increase of more than 8-fold.
Overall, our findings underscore the contrasting expression patterns of the Phvul.001G246300 and Phvul.001G243800 genes within the CDRK cultivar, shedding explanation on their potential roles in the defense mechanisms against C. lindemuthianum race 73 and P. griseola race 63-39. It is worth noting that the Co-1 and CoPv01CDRK/PhgPv01CDRK loci were accurately mapped to separate regions towards the terminal end of the common bean chromosome Pv01, positioned 211 kb apart. Consequently, it is of paramount importance to emphasize that the CDRK cultivar harbors a distinct and independent gene from the Co-1 locus.
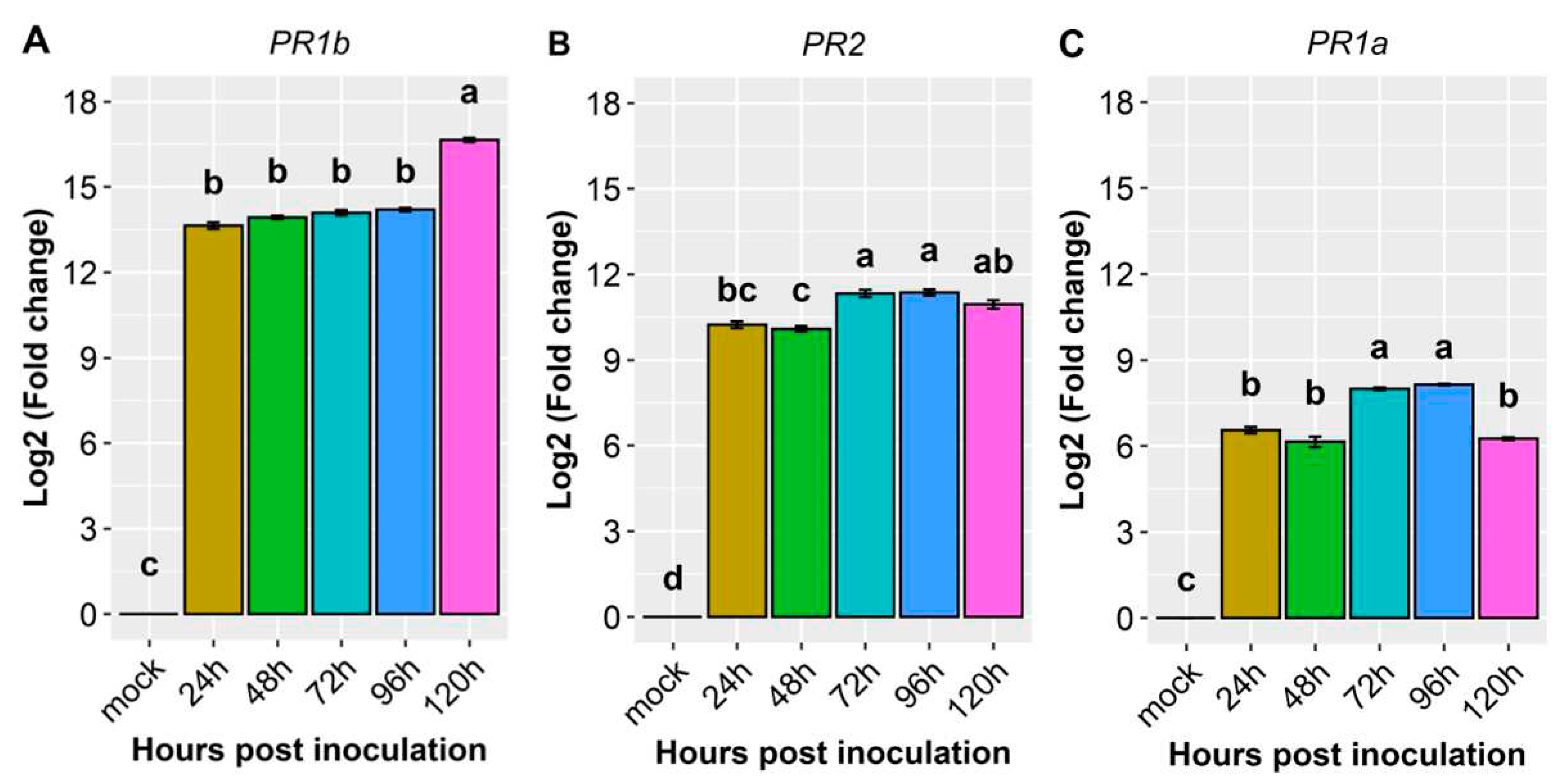
The pathogenesis-related defense genes
PR1a,
PR1b, and
PR2 exhibited significant responsiveness to the pathogen. Notably,
PR1b displayed the highest level of responsiveness to pathogen race 73, with a pronounced increase in expression at 120 hpi, reaching up to 16.7-fold higher than the control. Both
PR2 and
PR1a displayed elevated expression levels, exhibiting 11-fold and 8-fold increases, respectively, compared to the mock. Notably,
PR2 maintained consistently high expression levels from 24 hpi to 120 hpi in response to the pathogen. Mahiya-Farooq et al. [
22] reported early expression of plant defense genes in the resistant NIL, with
PR1b and
PR2 showing accumulation at 24 hpi of
C. lindemuthianum race 73. Similarly, Shams et al. [
34] observed higher expression of
PR2 in the Naz resistant bean cultivar upon inoculation with
C. lindemuthianum race 2. Although to a lesser extent, these pathogenesis-related defense genes were also upregulated in plants inoculated with race 63-39 of
P. griseola.
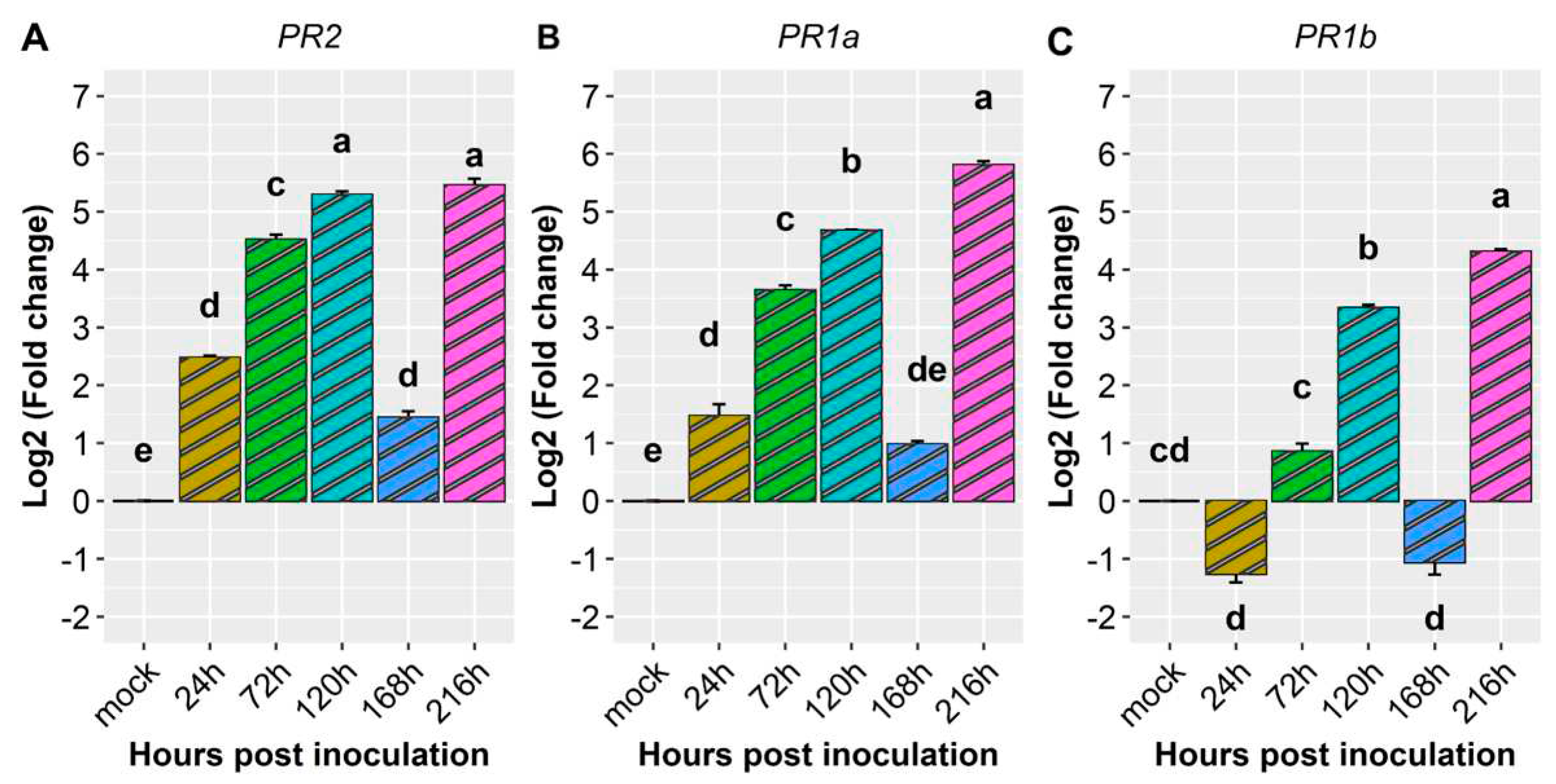
PR1a and
PR1b showed higher induction at 216 hpi, with nearly a 6-fold increase and over a 4-fold increase in gene expression, respectively. The
PR2 gene exhibited a more than 5-fold increase at 120 and 216 hpi. Interestingly, at 168 hpi, all the genes displayed reduced or no responsiveness to the pathogen.
These results reveal that the ANT and ALS resistance genes in the CDRK cultivar are controlled independently from those previously identified at the Co-1 locus. This indicates that the robust resistance against ANT and ALS in CDRK is manifested through the heightened response of the candidate gene Phvul.001G246300 to the respective pathogens. These findings point out the complex nature of plant-pathogen interactions, emphasizing the significance of comprehending gene expression mechanisms. Our findings might contribute to an enhanced comprehension of novel and efficient strategies for the development of cultivars resistant to angular leaf spot and ANT.
4. Materials and Methods
4.1. Plant material and growth conditions
Two experiments were conducted using a completely randomized design. In Experiment I, seedlings from both the resistant (R) CDRK and the susceptible (S) Yolano cultivars were inoculated with race 73 of C. lindemuthianum. The isoline derived from the CDRK × Yolano cross, namely CY 19, was used as a positive control for disease scoring to ensure that the fungus was virulent on them and that the absence of disease on the resistant genotypes was not attributed to the fungus lacking virulence. Additionally, the CY 70 isoline was used as a resistant control. The relative expression levels of 13 specific genes only in CDRK cultivar were evaluated at multiple time points: 24, 48, 72, 96, and 120 hours post inoculation (hpi), as well as in the mock. In Experiment II, a parallel approach was adopted, involving the same genotypes as in Experiment I. However, the genotypes were subjected to inoculation with race 63-39 of P. griseola. Similarly, the relative expression of the same 13 genes only in the CDRK cultivar were evaluated at 24, 72, 120, 168, and 216 hpi, in addition to the mock condition. Each experimental condition was replicated across three separate biological replicates (plants). Within each biological replicate, the assessment was further reinforced by performing three technical replicates of quantitative polymerase chain reaction (qPCR) reactions for each experiment.Top of Form
The experiments were conducted at the Núcleo de Pesquisa Aplicada à Agricultura (Nupagri) at the Universidade Estadual de Maringá (UEM) in Maringá, Paraná, Brazil (latitude 23° 26’8” S, longitude 51° 53’42”). Briefly, seeds were planted in plastic trays filled with a commercial substrate, MecPlant (MEC PREC—Ind. Com Ltda, Telemaco Borba, Brazil), that had been previously sterilized and fertilized. The seedlings were grown in greenhouses under natural light at a temperature of 25 °C until the first trifoliate leaf growth stage [
35].
4.2. Pathogenesis assays
Monosporic cultures of
C. lindemuthianum and
P. griseola were prepared according to the methodologies described by Mathur et al. [
36] and Sanglard et al. [
37], respectively. Inocula of the ANT pathogen races were produced by incubating them on a young green common bean pod medium [
38] at 22°C for 14 days. The inoculum for the ALS pathogens was multiplied in Petri dishes with tomato medium [37, 39] containing 1.61% agar (m/v), 0.25% calcium carbonate (m/v), 61.94% distilled water (v/v), and 36.2% V8
® vegetable juice [Campbell’s company soup (v/v)] and maintained in a bio-oxygen demand incubator at 24°C for 18 days. The concentration of fungal spores for the ANT pathogen was adjusted to 1.2 × 10
6 [
38]. For the ALS pathogen, it was adjusted to 1.2 × 10
4 conidia ml
−1 [
40] using a hemacytometer (1/400 mm
2, Hausser Scientific). Fourteen-day-old seedlings were inoculated with each race of the pathogen on the underside of their leaves. The inoculation process was carried out by using a manual pressurized pump sprayer for spraying. For the mock treatment, which served as the negative control, the seedlings were sprayed with only distilled water and Tween 20
® (0.01%). After inoculation, the plants were maintained in a mist chamber at >95% relative humidity, at a temperature of 20 ± 2°C, with 12 hours of daily light (680 lux) for 72. Following the inoculation process, the seedlings were moved to benches and placed under the same conditions as before, except for a high-humidity environment. This environment was maintained until the end of the experiment when all the samples were collected. To evaluate the severity of ANT and ALS symptoms, we used disease severity scales ranging from 1 to 9, as proposed by Inglis et al. [
41] and Pastor-Corrales et al. [
35]. Based on these scales, plants with disease reaction scores between 1 and 3 were classified as resistant, while those with scores ranging from 4 to 9 were classified as susceptible.
4.3. Total RNA extraction and cDNA synthesis
Leaf samples were collected from CDRK plants before inoculation (mock) and during incompatible reactions with race 73 of
C. lindemuthianum, as well as with race 63-39 of
P. griseola. Sampling was performed at specific time points critical for pathogens development: 24, 48, 72, 96, and 120 hpi for
C. lindemuthianum, and 24, 72, 120, 168, and 216 hpi for
P. griseola. Samples were obtained from three biological replicates for each pathogen. To ensure RNA integrity, the leaf samples were promptly frozen in liquid nitrogen for subsequent extraction [
42].
Total RNA was extracted from 100 mg frozen and purified using GeneJet Plant RNA Purification Kit (Thermo Fisher Scientific, Waltham, EUA) following the manufacturer’s instructions. The integrity of the total RNA was assessed by electrophoresis on a 1% m/v agarose gel, run for 80 minutes at 80 volts, at 5°C, and in the absence of light. For the assessment of both the quality and quantity of total RNA, a spectrophotometer (FEMTO 700STM) was employed to measure absorbance at specific wavelengths: 230 nm, 240 nm, 260 nm, and 280 nm. The criteria for RNA purity were determined based on the following absorbance ratios: A
260/A
230 ranging from 1.9 to 2.4, A
260/A
240 of at least 1.4, and A
260/A
280 between 1.8 and 2.2. To compute the concentration of total RNA, the formula [RNA] (ng µL-1) = A
260nm × 40 × 100, as outlined by Farrell [
43], was utilized. Total RNA samples that met the purity and integrity criteria were treated with DNase I
TM (Invitrogen™, Waltham, USA) to eliminate any possible genomic DNA contamination. The purification reaction involved 1 µg of total RNA, following the manufacturer's instructions.
To synthesize cDNA, the 'Superscript® IV First-Strand Synthesis System' kit (Invitrogen™, Waltham, USA) was used according to the manufacturer's instructions. The cDNA synthesis reaction was prepared with a total volume of 20 µL, containing the following constituents: 1 µg of total RNA, primer-oligo d(T) at a concentration of 2.5 µM, dNTP mix with each nucleotide at 0.5 mM, First-Strand Buffer at 1X concentration, DL-dithiothreitol at 5 mM, ribonuclease inhibitor with a concentration of 2 U µL-1, MMLV-RT at 10 U µL-1, and RNase-free water. The procedure began by combining total RNA, primer-oligo d(T), dNTP mix, and RNase-free water to reach a cumulative volume of 13 µL. The samples were incubated in a thermocycler (Applied Biosystems® Veriti® 96-Well Fast Thermal Cycler) at 65 °C for 5 minutes, followed by 4 °C for 1 minute. Then, the First-Strand Buffer, DL-dithiothreitol, ribonuclease inhibitor, and 1 MMLV-RT were added to the reaction. The samples were incubated at 55 °C for 10 minutes for cDNA synthesis activation, followed by 80 °C for 10 minutes to inactivate the reaction. To remove residual RNA after cDNA synthesis, 1 µL of Escherichia coli RNase H was added, and the samples were incubated at 37 °C for 20 minutes. The cDNA synthesis product (20 µL) was diluted 1:100 for qPCR analysis. To assess the cDNA synthesis efficiency, positive control was included, in which HeLa-S3 RNA (10 ng) was used instead of total RNA.
To verify the quality of the cDNA synthesis, a PCR reaction was performed on the positive control and a negative control (containing water instead of cDNA) using the following reaction mix: 5 µL PCR buffer (10 X), 2 µL MgCl (50 mM), 1 µL dNTP Mix (10 mM), 1 µL sense primer (10 µM), 1 µL antisense primer (10 µM), 2 µL of cDNA for the positive control, and 2 µL of ultrapure H2O for the negative control, 0.2 µL of Taq PlatinumTM DNA polymerase (Invitrogen™, Waltham, USA), and 37.8 µL of ultrapure H2O. The PCR reaction was performed for 35 cycles, with an initial denaturation at 94 °C for 2 minutes, denaturation at 94 °C for 15 seconds, annealing at 55 °C for 30 seconds, and synthesis at 68 °C for 1 minute. The PCR products were analyzed by electrophoresis on a 1.5% m/v agarose gel. The positive control showed a single band of approximately 353 bp, while no product was observed in the negative control, confirming the efficiency of the cDNA synthesis.
4.4. Target genes and primer design
Ten candidate genes were selected for expression analysis in CDRK plants inoculated with race 73 of
C. lindemuthianum and race 63-39 of
P. griseola. The genes evaluated in the
CoPv01CDRK/
PhgPv01CDRK resistance loci were
Phvul.001G246000,
Phvul.001G246100,
Phvul.001G246200, and
Phvul.001G246300. Additionally, the gene
Phvul.001G245300 located near this locus was also assessed [
15]. The genes proposed for the
Co-AC locus, namely
Phvul.001G244300,
Phvul.001G244400, and
Phvul.001G244500 [
14], were also tested. The gene
Phvul.001G243800 was evaluated as it was induced in the near isogenic line T9576R, which carries the
Co-12 resistance allele, when inoculated with race 73 of
C. lindemuthianum [
22]. The
KTR2/3 gene, a candidate gene for
Co-x in Jalo EEP558, which was induced in response to isolate 100 of
C. lindemuthianum, was also included [
23]. Finally, three known plant defense genes, namely
Phvul.003G109100 (
PR1a),
Phvul.006G196900 (
PR1b), and
Phvul.009G256400 (
PR2) were evaluated [16, 22, 42]. To normalize gene expression levels, the reference genes
Phvul.008G011000 (actin -
ACT) and
Phvul.001G133200 (insulin-degrading enzyme -
IDE) were used as reference genes [
44].
To design primers for qPCR, the coding sequences (CDS) and DNA sequences of the target genes were downloaded from the common bean (
P. vulgaris L.) genome available at Phytozome 12 [
45]. The 'Primer-BLAST web tool' [
46] was used to design primers that met the following specifications for efficient qPCR: primer size 18-24 bp, melting temperature between 59-61°C, amplicon size between 80-160 bp, and whenever possible, at least one intron on the corresponding genomic DNA sequence was included between the primer pair. The primers for the
KTR2/3 gene were obtained from Richard et al. [
23].
To ensure the specificity and efficiency of the primers, dimers and secondary structures were checked using Gene Runner software (version 6.5.52), the 'Multiple Prime Analyzer' web tool (Thermo Fisher Scientific:
https://bit.ly/34kZpnP), and 'The Sequence Manipulation Suite' web tool [
47]. The amplicon secondary structure was also verified using the "The Mfold Web Server" platform [
48] with coding sequences downloaded from Phytozome 12. All procedures used for primer design and
in silico validation followed literature recommendations [49, 50]. The primer sequences for each candidate gene evaluated are listed in
Table 3.
4.5. Quantitative PCR (qPCR) and data analysis
The determination of PCR efficiency for each primer involved establishing a standard curve through a fivefold serial dilution, utilizing the cDNA pool as the template. This process incorporated three replicates at every dilution point, following the methodologies outlined by Svec et al. [
51] and Rasmussen [
52]. The amplification efficiency was computed employing the equation E = [10
(-1/slope)] - 1 [
52], utilizing the slope values derived from linear regression analysis. This analysis encompassed the log
10-transformed cDNA concentrations on the x-axis and corresponding Cq values on the y-axis. The calculated amplification efficiency for each primer pair ranged from 0.92 to 1.09, while maintaining a coefficient of determination (R
2) for the linear regression of at least 0.97 (
Table 3).
The cDNA quantification reactions were conducted in the StepOnePlus™ real-time PCR system (Applied Biosystems™; StepOnePlus™ Real-Time PCR Systems) using 96-well microplates [MicroAmp™ Fast 96 -well Reaction Plate (0.1 mL)] sealed with MicroAmp™ Optical Adhesive Film. The total reaction volume was 10 µL, consisting of 3.4 µL of cDNA, 1.6 µL of forward and reverse primer mix (800 nM), and 5 µL of PowerUp™ SYBR™ Green Master Mix (Applied Biosystems™). The thermocycling conditions included 50°C for 2 minutes, 95°C for 2 minutes, 40 cycles of 15 seconds at 95°C, and 30 seconds at 60°C.
After completion of the cDNA quantitation reaction, a dissociation curve was performed to verify target specificity using the manufacturer's standard continuous melt curve setup, and only samples exhibiting specificity based on the dissociation curve were used. Cq values were obtained using StepOnePlus™ Software v2.3 (Applied Biosystems™), with the baseline determined automatically and the threshold was determined manually in the exponential phase of amplification (0.7707 for all cDNA quantification reactions).
The genes
Phvul.001G133200 (
ACT) and
Phvul.008G011000 (
IDE) were used as reference genes [
44], with the arithmetic mean of Cq values (quantification cycle) [
53] calculated in each experimental condition evaluated. Relative expression was determined based on Cq values normalized with the reference genes, using the 2
-ΔΔCT method [54, 55]. Mean Cq values were calculated for each gene at each experimental condition based on three biological repetitions and three replicates (n=3x3).
The relative expression of candidate genes to the
CoPv01CDRK/
PhgPv01CDRK loci and known disease resistance genes were investigated in response to race 73 of
C. lindemuthianum at 24, 48, 72, 96, and 120 hpi, as well as in response to race 63-39 of
P. griseola at 24, 72, 120, 168, and 216 hpi in CDRK cultivar. The calibrator condition for each gene was the relative expression in the mock (control, without pathogen). To analyze the data and show results, logarithmic base 2 transformation was performed before statistical analysis. The expression levels among experimental conditions were compared using the Alexander-Govern test with a significance level of 5%. Pairwise comparisons of relative expression mean among time points for each gene were assessed, and the significance level was adjusted using Bonferroni correction (p≤0.05). These statistical analyses were performed using the 'oneway-test' [
56] and 'companion' R packages. All data wrangling and statistical analysis were performed using R software (version 4.0.3) (R Core Team), with plots generated using the package ggplot2 [
57] and R base. Error bars represent the standard deviation of the means from three biological and three technical replicates (3x3). Heatmaps were generated with mean Cq values using the ‘heatmaply’ R package, and the dendrogram was based on the Euclidean distance measure and the average linkage function [
58] among the relative expression of the genes.
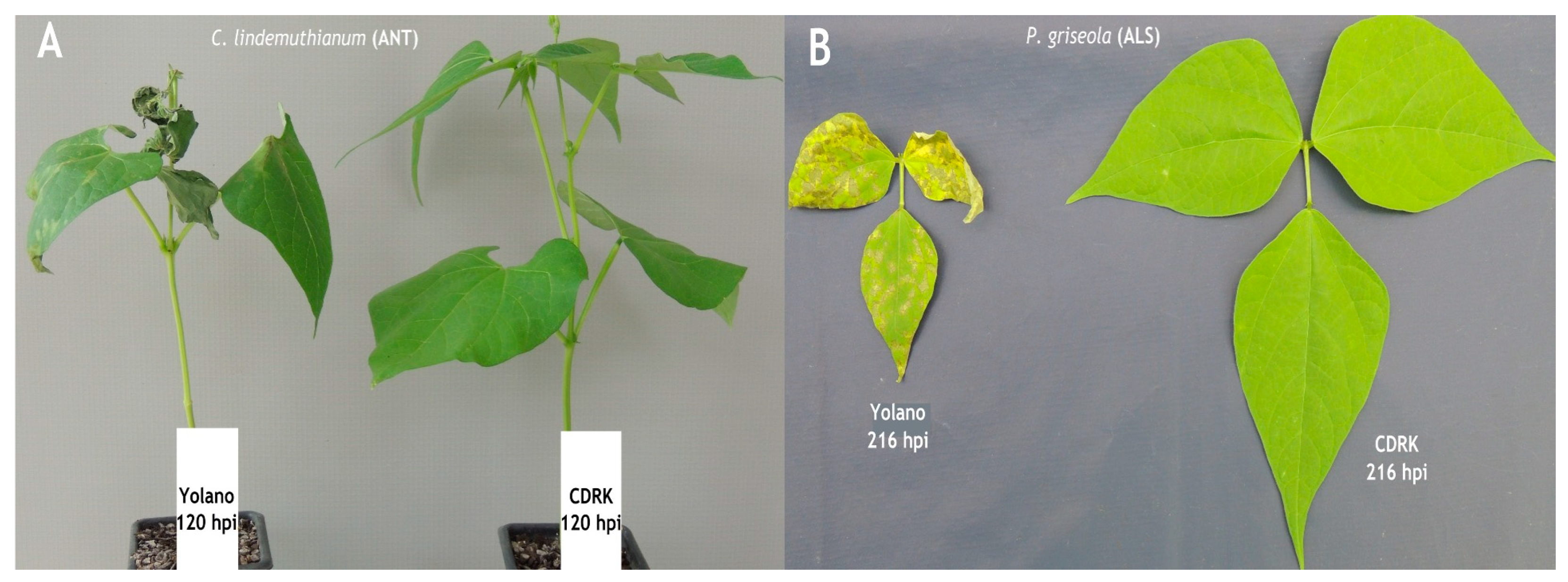
Figure 1.
(A) Disease reaction of susceptible Yolano cultivar and no reaction in resistant California Dark Red Kidney cultivar at 120 h post inoculation with C. lindemuthianum race 73. (B) Disease reaction of susceptible Yolano cultivar and no reaction in resistant California Dark Red Kidney cultivar at 216 h post inoculation with P. griseola race 63-39.
Figure 1.
(A) Disease reaction of susceptible Yolano cultivar and no reaction in resistant California Dark Red Kidney cultivar at 120 h post inoculation with C. lindemuthianum race 73. (B) Disease reaction of susceptible Yolano cultivar and no reaction in resistant California Dark Red Kidney cultivar at 216 h post inoculation with P. griseola race 63-39.
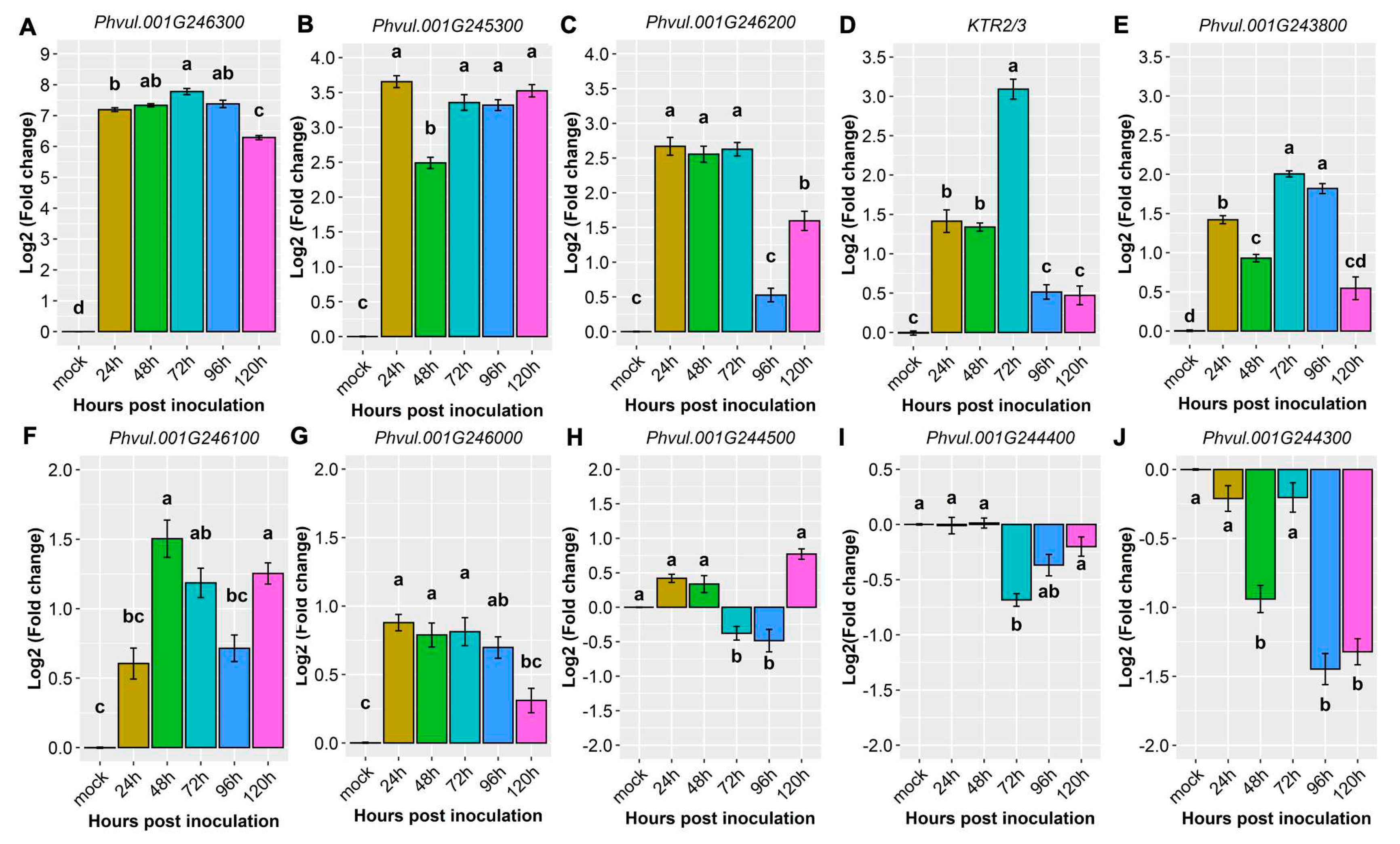
Figure 2.
Relative expression of candidate genes: (A) Phvul.001G246300; (B) Phvul.001G245300; (C) Phvul.001G246200; (D) KTR2/3; (E) Phvul.001G243800; (F) Phvul.001G246000; (G) Phvul.001G246100; (H) Phvul.001G244500; (I) Phvul.001G244400; (J) Phvul.001G244300 in California Dark Red Kidney at 24, 48, 72, 96, and 120 h post inoculation with race 73 of C. lindemuthianum and mock. The results are presented as logarithmic base 2 of the fold change of gene expression. Error bars represent the standard deviation of the mean from three biological replicates and three technical replicates.
Figure 2.
Relative expression of candidate genes: (A) Phvul.001G246300; (B) Phvul.001G245300; (C) Phvul.001G246200; (D) KTR2/3; (E) Phvul.001G243800; (F) Phvul.001G246000; (G) Phvul.001G246100; (H) Phvul.001G244500; (I) Phvul.001G244400; (J) Phvul.001G244300 in California Dark Red Kidney at 24, 48, 72, 96, and 120 h post inoculation with race 73 of C. lindemuthianum and mock. The results are presented as logarithmic base 2 of the fold change of gene expression. Error bars represent the standard deviation of the mean from three biological replicates and three technical replicates.
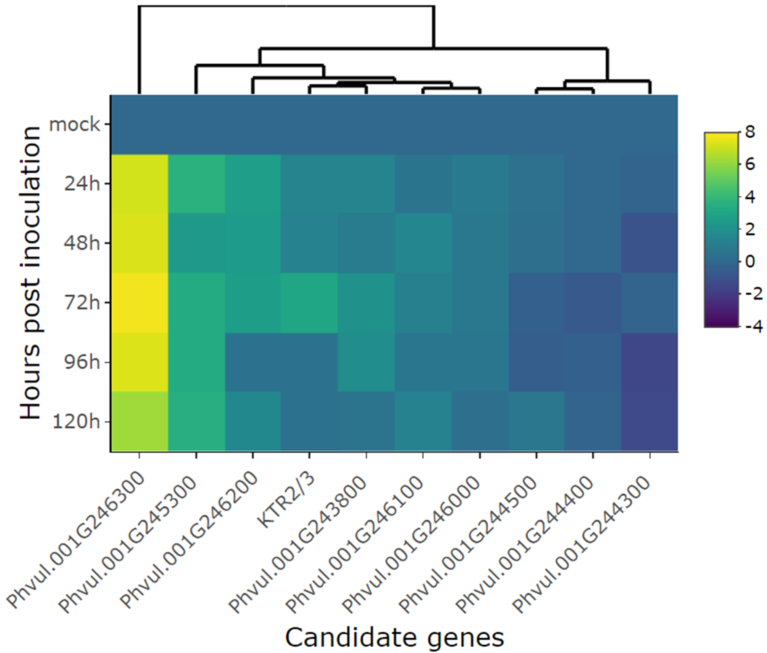
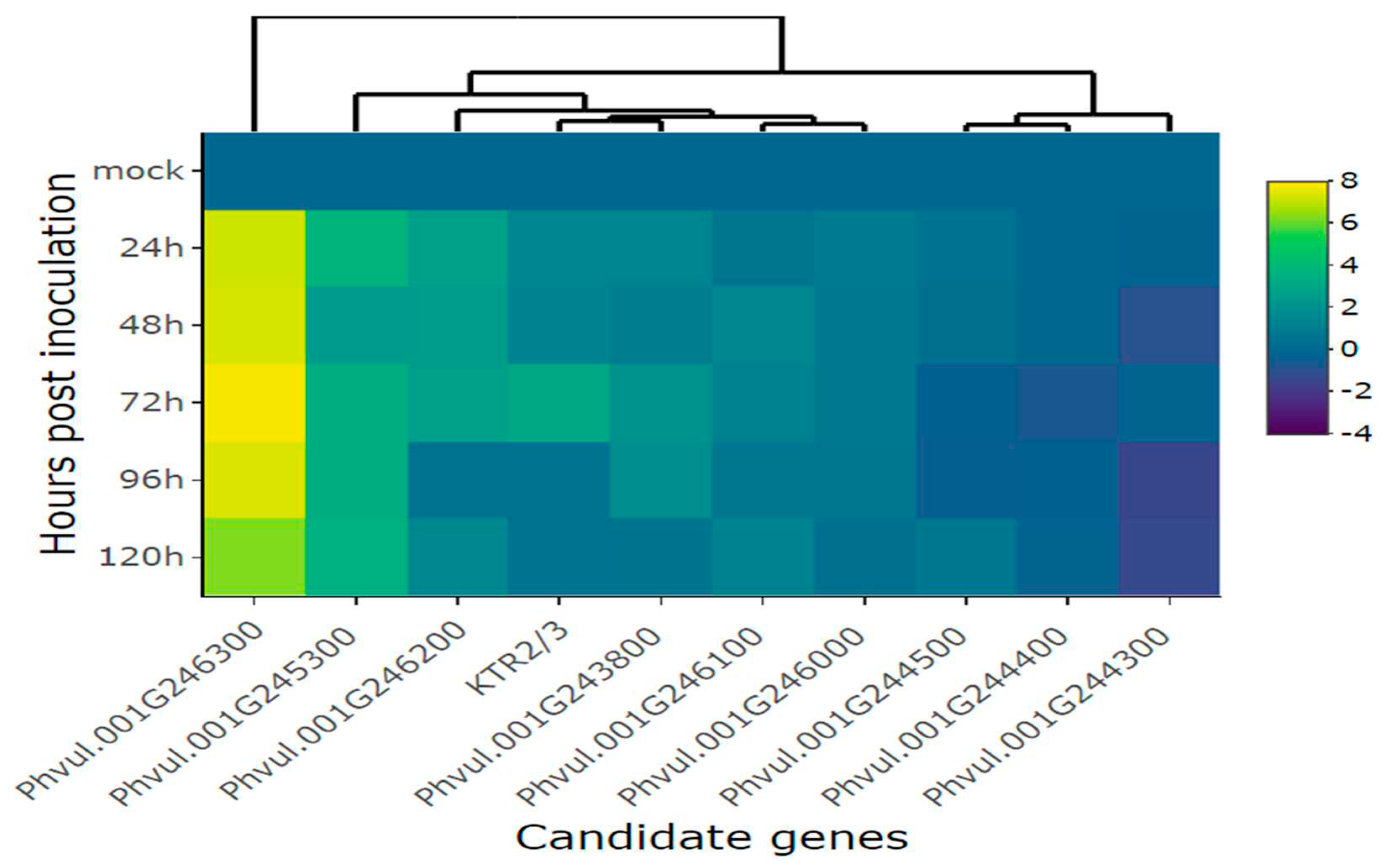
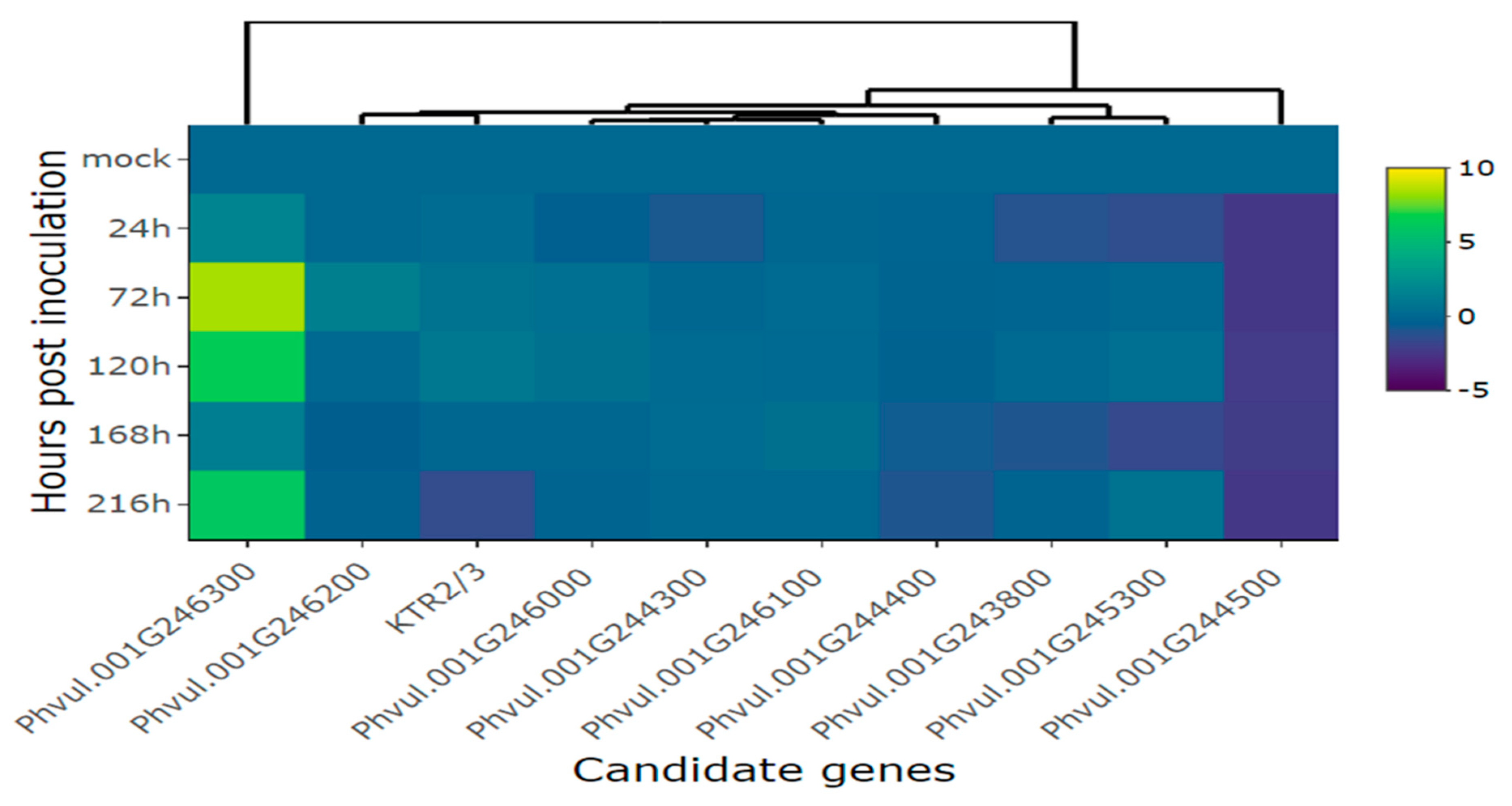
Figure 3.
Heatmap of relative expression of candidate genes for the CoPv01CDRK/PhgPv01CDRK and genes proximal to this loci in California Dark Red Kidney at 24, 48, 72, 96, and 120 h post inoculation with race 73 of C. lindemuthianum and mock. The genes evaluated were Phvul.001G246300, Phvul.001G245300, Phvul.001G246200, KTR2/3, Phvul.001G243800, Phvul.001G246100, Phvul.001G246000, Phvul.001G244500, Phvul.001G244400, Phvul.001G244300. Yellow shading indicates higher expression and dark blue shading lower expression than that of reference genes.
Figure 3.
Heatmap of relative expression of candidate genes for the CoPv01CDRK/PhgPv01CDRK and genes proximal to this loci in California Dark Red Kidney at 24, 48, 72, 96, and 120 h post inoculation with race 73 of C. lindemuthianum and mock. The genes evaluated were Phvul.001G246300, Phvul.001G245300, Phvul.001G246200, KTR2/3, Phvul.001G243800, Phvul.001G246100, Phvul.001G246000, Phvul.001G244500, Phvul.001G244400, Phvul.001G244300. Yellow shading indicates higher expression and dark blue shading lower expression than that of reference genes.
Figure 4.
Relative expression of plant defense genes: (A) Phvul.006G196900 (PR1b); (B) Phvul.009G256400 (PR2); (C) Phvul.003G109100 (PR1a) in the common bean cultivar California Dark Red Kidney at 24, 48, 72, 96, and 120 h post inoculation with race 73 of C. lindemuthianum and mock. The results are presented as logarithmic base 2 of the fold change of gene expression. Error bars represent the standard deviation of the mean from three biological replicates and three technical replicates.
Figure 4.
Relative expression of plant defense genes: (A) Phvul.006G196900 (PR1b); (B) Phvul.009G256400 (PR2); (C) Phvul.003G109100 (PR1a) in the common bean cultivar California Dark Red Kidney at 24, 48, 72, 96, and 120 h post inoculation with race 73 of C. lindemuthianum and mock. The results are presented as logarithmic base 2 of the fold change of gene expression. Error bars represent the standard deviation of the mean from three biological replicates and three technical replicates.
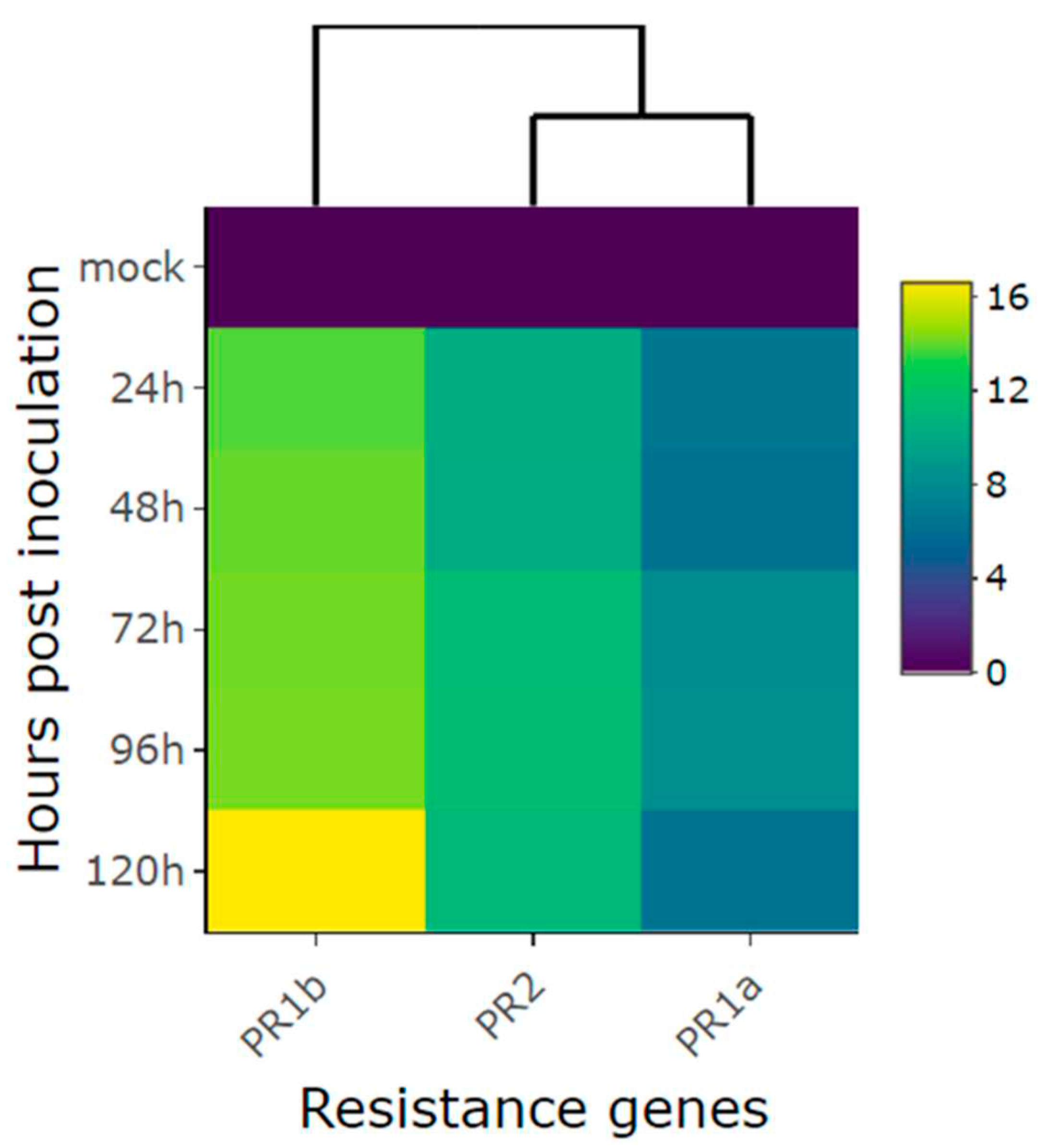
Figure 5.
Heatmap of relative expression of plant defense genes Phvul.006G196900 (PR1b), Phvul.009G256400 (PR2), and Phvul.003G109100 (PR1a) in the common bean cultivar California Dark Red Kidney at 24, 48, 72, 96, and 120 h post inoculation with race 73 of C. lindemuthianum and mock. Yellow shading indicates higher expression and dark blue has lower expression than that of reference genes.
Figure 5.
Heatmap of relative expression of plant defense genes Phvul.006G196900 (PR1b), Phvul.009G256400 (PR2), and Phvul.003G109100 (PR1a) in the common bean cultivar California Dark Red Kidney at 24, 48, 72, 96, and 120 h post inoculation with race 73 of C. lindemuthianum and mock. Yellow shading indicates higher expression and dark blue has lower expression than that of reference genes.
Figure 6.
Relative expression of candidate genes: (A) Phvul.001G246300; (B) Phvul.001G246200; (C) KTR2/3; (D) Phvul.001G246000; (E) Phvul.001G244300; (F) Phvul.001G246100; (G) Phvul.001G244400; (H) Phvul.001G243800; (I) Phvul.001G245300; (J) Phvul.001G244500 in California Dark Red Kidney at 24, 72, 120, 168 and 216 h post inoculation with race 63-39 of P. griseola and mock. The results are presented as logarithmic base 2 of the fold change of gene expression. Error bars represent the standard deviation of the mean from three biological replicates and three technical replicates.
Figure 6.
Relative expression of candidate genes: (A) Phvul.001G246300; (B) Phvul.001G246200; (C) KTR2/3; (D) Phvul.001G246000; (E) Phvul.001G244300; (F) Phvul.001G246100; (G) Phvul.001G244400; (H) Phvul.001G243800; (I) Phvul.001G245300; (J) Phvul.001G244500 in California Dark Red Kidney at 24, 72, 120, 168 and 216 h post inoculation with race 63-39 of P. griseola and mock. The results are presented as logarithmic base 2 of the fold change of gene expression. Error bars represent the standard deviation of the mean from three biological replicates and three technical replicates.
Figure 7.
Heatmap of relative expression of candidate genes for the CoPv01CDRK/PhgPv01CDRK and genes proximal to this loci in California Dark Red Kidney at 24, 72, 120, 168 and 216 h post inoculation with race 63-39 of P. griseola and mock. The genes evaluated were Phvul.001G246300, Phvul.001G246200, KTR2/3, Phvul.001G246000, Phvul.001G244300, Phvul.001G246100, Phvul.001G244400, Phvul.001G243800, Phvul.001G245300 and Phvul.001G244500. Yellow shading indicates higher expression and dark blue shading lower expression than that of reference genes.
Figure 7.
Heatmap of relative expression of candidate genes for the CoPv01CDRK/PhgPv01CDRK and genes proximal to this loci in California Dark Red Kidney at 24, 72, 120, 168 and 216 h post inoculation with race 63-39 of P. griseola and mock. The genes evaluated were Phvul.001G246300, Phvul.001G246200, KTR2/3, Phvul.001G246000, Phvul.001G244300, Phvul.001G246100, Phvul.001G244400, Phvul.001G243800, Phvul.001G245300 and Phvul.001G244500. Yellow shading indicates higher expression and dark blue shading lower expression than that of reference genes.
Figure 8.
Relative expression of plant defense genes: (A) Phvul.009G256400 (PR2); (B) Phvul.003G109100 (PR1a); (C) Phvul.006G196900 (PR1b) in the common bean cultivar California Dark Red Kidney at 24, 72, 120, 168 and 216 h post inoculation with race 63-39 of P. griseola and mock. The results are presented as logarithmic base 2 of the fold change of gene expression. Error bars represent the standard deviation of the mean from three biological replicates and three technical replicates.
Figure 8.
Relative expression of plant defense genes: (A) Phvul.009G256400 (PR2); (B) Phvul.003G109100 (PR1a); (C) Phvul.006G196900 (PR1b) in the common bean cultivar California Dark Red Kidney at 24, 72, 120, 168 and 216 h post inoculation with race 63-39 of P. griseola and mock. The results are presented as logarithmic base 2 of the fold change of gene expression. Error bars represent the standard deviation of the mean from three biological replicates and three technical replicates.
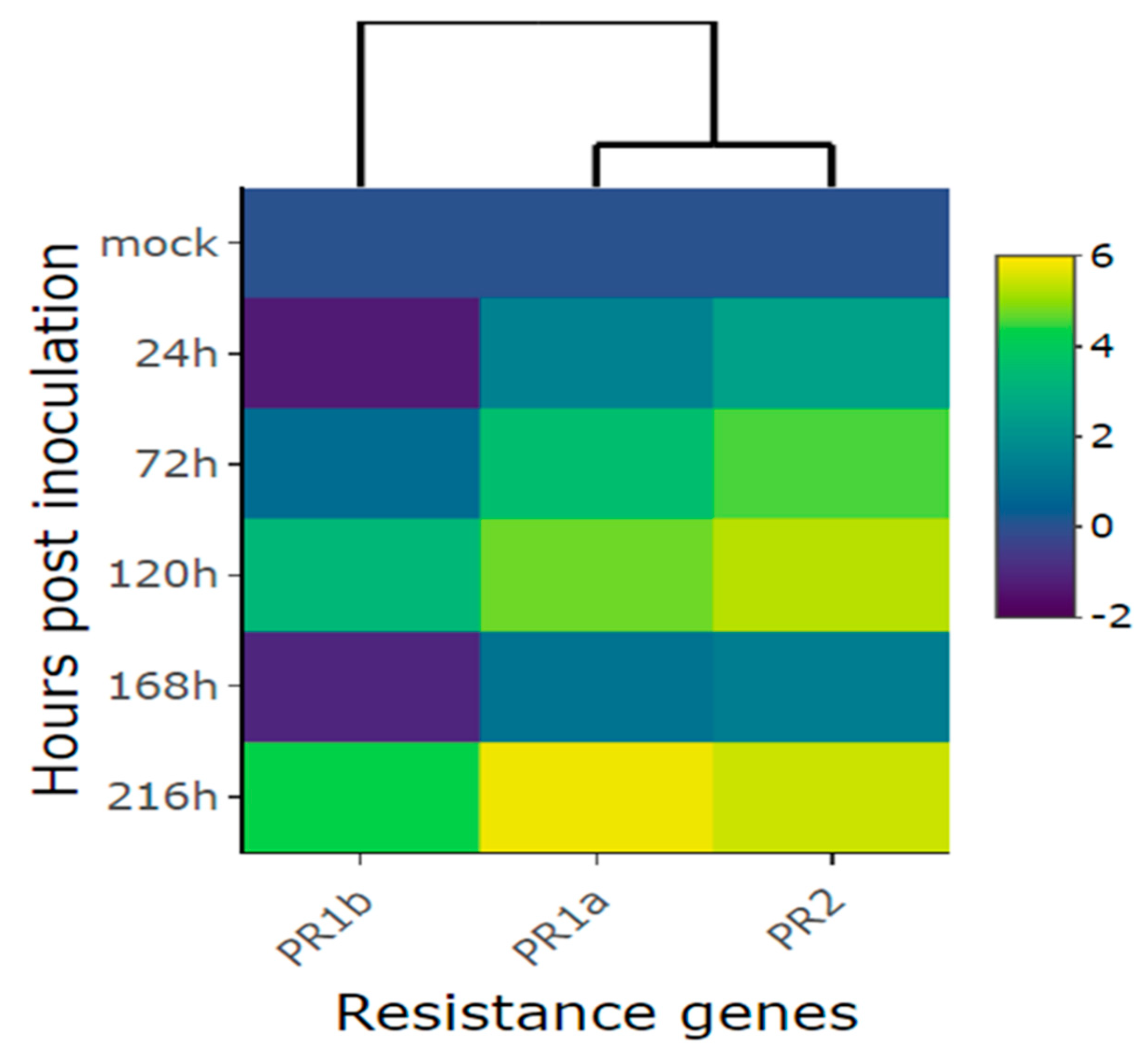
Figure 9.
Heatmap of relative expression of plant defense genes Phvul.003G109100, Phvul.006G196900, Phvul.009G256400 in the common bean cultivar California Dark Red Kidney at 24, 72, 120, 168 and 216 h post inoculation with race 63-39 of P. griseola and mock. Yellow shading indicates higher expression and dark blue has lower expression than that of reference genes.
Figure 9.
Heatmap of relative expression of plant defense genes Phvul.003G109100, Phvul.006G196900, Phvul.009G256400 in the common bean cultivar California Dark Red Kidney at 24, 72, 120, 168 and 216 h post inoculation with race 63-39 of P. griseola and mock. Yellow shading indicates higher expression and dark blue has lower expression than that of reference genes.
Figure 10.
Common bean chromosome Pv01 containing candidate genes for anthracnose resistance genes Co-x (KTR2/3), Co-1 (Phvul.001G243800), Co-AC (Phvul.001G244300, Phvul.001G244400, and Phvul.001G244500), and CoPv01CDRK/PhgPv01CDRK (Phvul.001G245300, Phvul.001G246000, Phvul.001G246100, Phvul.001G246200 and Phvul.001G246300).
Figure 10.
Common bean chromosome Pv01 containing candidate genes for anthracnose resistance genes Co-x (KTR2/3), Co-1 (Phvul.001G243800), Co-AC (Phvul.001G244300, Phvul.001G244400, and Phvul.001G244500), and CoPv01CDRK/PhgPv01CDRK (Phvul.001G245300, Phvul.001G246000, Phvul.001G246100, Phvul.001G246200 and Phvul.001G246300).
Table 1.
Gene model, previously mapped genes and functional annotation using Phytozome.
Table 1.
Gene model, previously mapped genes and functional annotation using Phytozome.
| Gene model |
Gene |
Functional annotation on Phytozome |
| Phvul.001G243800 |
Co-1 |
Serine/Threonine-protein kinase-like protein CCR3- related |
| KTR2/3 |
Co-x |
Serine/Threonine-protein kinase-like protein CCR3- related |
| Phvul.001G244300 |
Co-AC |
Clathrin Heavy Chain (CLTC) involved in plant defense signaling |
| Phvul.001G244400 |
Co-AC |
Unknown function |
| Phvul.001G244500 |
Co-AC |
Helix-loop-helix DNA-binding domain with possible transcription function |
| Phvul.001G245300 |
CoPv01CDRK/ PhgPv01CDRK |
Protein tyrosine kinase (pkinase_tyr) //leucine-rich repeat n-terminal domain (lrrnt_2) |
| Phvul.001G246000 |
CoPv01CDRK/ PhgPv01CDRK |
ATP-dependent RNA helicase ddx55/spb4 [ec:3.6.4.13] (ddx55, spb4) |
| Phvul.001G246100 |
CoPv01CDRK/ PhgPv01CDRK |
Cation-dependent mannose-6-phosphate receptor |
| Phvul.001G246200 |
CoPv01CDRK/ PhgPv01CDRK |
Protein trichome birefringence-like 33 |
| Phvul.001G246300 |
CoPv01CDRK/ PhgPv01CDRK |
Abscisic acid receptor pyl5 |
| Phvul.003G109100 |
PR1a |
Pathogenesis-related protein Bet v I family |
| Phvul.006G196900 |
PR1b |
Pathogenesis-related protein 1 |
| Phvul.009G256400 |
PR2 |
Pathogenesis-related protein 2 |
Table 2.
Summary table of mean relative gene expression [Log2(fold change)] of CoPv01CDRK/PhgPv01CDRK candidate genes and pathogenesis-related genes in response to ANT and ALS in CDRK cultivar.
Table 2.
Summary table of mean relative gene expression [Log2(fold change)] of CoPv01CDRK/PhgPv01CDRK candidate genes and pathogenesis-related genes in response to ANT and ALS in CDRK cultivar.
| Gene |
Gene model |
C. lindemuthianum race 73 (ANT) |
P. griseola race 63-39 (ALS) |
| 24 hpi |
48 hpi |
72 hpi |
96 hpi |
120 hpi |
24 hpi |
72 hpi |
120 hpi |
168 hpi |
216 hpi |
| Co-x |
KTR2/3 |
1.4 |
1.3 |
3.1 |
0.5 |
0.5 |
0.2 |
0.8 |
0.8 |
-0.1 |
-1.4 |
| Co-1 |
Phvul.001G243800 |
1.4 |
0.9 |
2.0 |
1.8 |
0.6 |
-1.1 |
-0.2 |
0.1 |
-0.9 |
-0.3 |
| Co-AC |
Phvul.001G244300 |
-0.2 |
-0.9 |
-0.2 |
-1.4 |
-1.3 |
-0.8 |
-0.1 |
0.2 |
0.2 |
0.0 |
| Phvul.001G244400 |
0.0 |
0.0 |
-0.7 |
-0.4 |
-0.2 |
-0.2 |
-0.2 |
-0.3 |
-0.7 |
-1.0 |
| Phvul.001G244500 |
0.4 |
0.3 |
-0.4 |
-0.5 |
0.8 |
-2.4 |
-2.5 |
-2.3 |
-2.3 |
-2.4 |
| CoPv01CDRK /PhgPv01CDRK |
Phvul.001G245300 |
3.7 |
2.5 |
3.4 |
3.3 |
3.5 |
-1.4 |
0.0 |
0.4 |
-1.7 |
0.7 |
| Phvul.001G246000 |
0.9 |
0.8 |
0.8 |
0.7 |
0.3 |
-0.5 |
0.4 |
0.5 |
-0.1 |
-0.2 |
| Phvul.001G246100 |
0.6 |
1.5 |
1.2 |
0.7 |
1.3 |
0.0 |
0.3 |
0.1 |
0.4 |
0.0 |
| Phvul.001G246200 |
2.7 |
2.6 |
2.6 |
0.5 |
1.6 |
0.0 |
1.5 |
0.1 |
-0.4 |
-0.2 |
| Phvul.001G246300 |
7.2 |
7.3 |
7.8 |
7.4 |
6.3 |
1.7 |
8.5 |
6.5 |
1.3 |
6.1 |
| Pathogenesis-related genes |
PR1a |
6.6 |
6.2 |
8.0 |
8.1 |
6.3 |
1.5 |
3.6 |
4.7 |
1.0 |
5.8 |
| PR1b |
13.6 |
13.9 |
14.1 |
14.2 |
16.7 |
-1.3 |
0.9 |
3.3 |
-1.1 |
4.3 |
| PR2 |
10.2 |
10.1 |
11.3 |
11.4 |
11.0 |
2.5 |
4.5 |
5.3 |
1.4 |
5.5 |
Table 3.
Target genes, primers used, qPCR product size (amplicon), primer melting temperature (Tm), amplification efficiency (E) and coefficient of determination of linear regression (R²).
Table 3.
Target genes, primers used, qPCR product size (amplicon), primer melting temperature (Tm), amplification efficiency (E) and coefficient of determination of linear regression (R²).
| Gene Model a |
Reference |
Primers Forward (F) and Reverse (R) (5`-3`) |
Amplicon(bp) |
Tm(°C) |
Eb |
R² c |
| Phvul.001G133200 |
IDE |
F: AAGCAGGTATCTTGGCCATCTC |
126 |
60.16 |
1.04 |
0.99 |
| R: AAAGCAAACTCCAAGCTCCAATC |
59.99 |
| Phvul.008G011000 |
ACT |
F: GAAGTTCTCTTCCAACCATCC |
154 |
59.67 |
1 |
0.98 |
| R: TTTCCT TGCTCATTCTGTCCG |
58.38 |
| Phvul.001G243800 |
Co-1 |
F: CCTCAAGGTGGGGCTTTTGAG |
118 |
61.16 |
1.04 |
0.99 |
| R: TCACCGAGAAACTCCCATTGC |
60.61 |
| KTR2/3 |
Co-x |
F: ATGCACAGGGGAATGGGATG |
279 |
60.11 |
1.04 |
0.98 |
| R: GCCATAGCGAGTGAGAGTGCG |
63.42 |
| Phvul.001G244300 |
Co-AC |
F: GAAACGTCTCCGCAGAATAGTG |
150 |
59.4 |
1.03 |
0.99 |
| R: GTCTTGTGTTGTTCCTTGGAGTTG |
60.44 |
| Phvul.001G244400 |
Co-AC |
F: TACAGCAAGAGAGCGGTTAAAGG |
121 |
60.62 |
1.01 |
0.99 |
| R: CCCTTTGTCACTTTGTTTTGAAGC |
59.67 |
| Phvul.001G244500 |
Co-AC |
F: CAATGCACAGCTCGCAACTC |
141 |
60.45 |
1.09 |
0.97 |
| R: GGAACTGTGAAAGCTCTGCTAAC |
59.81 |
| Phvul.001G245300 |
CoPv01CDRK/ PhgPv01CDRK |
F: TCTGCTGGAAGGGTGGTAGTC |
93 |
61.17 |
1.07 |
0.98 |
| R: GGACGTTATGTGAACAAGGTTTGC |
61.08 |
| Phvul.001G246000 |
CoPv01CDRK/ PhgPv01CDRK |
F: ATGAAGCGGATGGATGTCTTG |
132 |
58.43 |
1.01 |
0.97 |
| R: TCTACGAAGCTTAGGCAATTGAG |
58.57 |
| Phvul.001G246100 |
CoPv01CDRK/ PhgPv01CDRK |
F: CACGGTATCCTCAGCGAAGAC |
119 |
60.53 |
1.05 |
0.99 |
| R: CAGCAGTCAGCACATACTGGAG |
60.99 |
| Phvul.001G246200 |
CoPv01CDRK/ PhgPv01CDRK |
F: GAAGGAGGCTGTGACGTGTTC |
150 |
61.2 |
1.04 |
0.99 |
| R: CCATCGCCACCGTTGATACTC |
61.13 |
| Phvul.001G246300 |
CoPv01CDRK/ PhgPv01CDRK |
F: CTTCTTCCCTTCACTTCGATACC |
87 |
58.57 |
1.09 |
0.99 |
| R: GTTGAGAGTGTTTGTGGCAGT |
58.98 |
| Phvul.003G109100 |
PR1a |
F: GTCCTAACGGAGGATCACTCA |
148 |
58.62 |
1.06 |
0.99 |
| R: CAGGGATTGGCCAGAAGGTAT |
59.5 |
| Phvul.006G196900 |
PR1b |
F: GGTTTGCCTATGATCCCAATGC |
115 |
59.96 |
1.03 |
0.99 |
| R: TGTTGTGAGCGTTGAGGAAGTC |
61.06 |
| Phvul.009G256400 |
PR2 |
F: CAGAGGTTCTCATTTGCTGCTTTC |
98 |
60.62 |
1.07 |
0.99 |
| R: ATGCCATAACACACCCCGATTTG |
61.75 |