1. Introduction
Acute kidney disease (AKD) is a sequel of acute kidney injury (AKI) [
1]. Current paediatric studies report AKD as an independent risk factor for progression to chronic kidney disease (CKD) [
2,
3,
4]. The operational framework period of AKI that persists greater than 7 days provides time for intervention but with the cost of time dependent nephron loss. AKD development and recovery are currently assessed using filtration markers, such as serum creatinine [
1]. A step towards improving the outcomes of AKI is early identification of the individual risk factors, proper management and rapid intervention. The use of kinetic estimated glomerular filtration rate (KeGFR) has been used in acute settings as part of a multidimensional approach for AKI prediction [
5,
6,
7,
8]. Although, 10 years have passed since Chen introduced the KeGFR when the plasma creatinine is changing acutely [
9], only a few studies addressed the utility of this tool in the clinical paediatric settings [
5,
8,
10], mostly in the intensive care units (ICU). KeGFR is the adequate tool in assessing the risk of AKI in children as the formula derives from the initial serum creatinine level, the distribution volume, the creatinine production rate and the given time differences of the two serum creatinine values [
5]. Based on current data we performed a retrospective study to assess the utility of KeGFR in predicting AKD and MAKE30 in paediatric AKI settings.
2. Material and methods
A retrospective observational study was conducted in a tertiary care teaching and research hospital from Western Romania, from 1
st of July 2014 through 31
st December 2022. All patients under 18 years of age were screened based on the change in serum creatinine (SCr) through The Laboratory Information System and Hospital Information System. SCr measurement was performed using the Jaffe Abbott method. AKI was defined and staged using the 2012 Kidney Disease Improving Global Outcomes (KDIGO) SCr criteria [
11]. AKD was defined and staged according to the 2017 ADQI consensus statement [
12] in the 8
th day of persistent AKI based on the SCr level. Chronic kidney disease (CKD) was defined according to KDIGO CKD guideline from 2012 [
13]. AKI and AKD had been diagnosed based on the increase in SCr at a given time during hospitalization. Severe AKI was considered KDIGO stages 2 and 3. Severe AKD were all patients with stage 2 and 3 AKD. Exclusion criteria were: a single measurement of SCr, end stage kidney disease, patients who did not have their height and weight recorded or the lack of two consecutive SCr measurements. Demographics, clinical characteristics, and outcomes were retrieved from data at the time of admission (day one).
Estimated glomerular filtration rate (eGFR) was calculated using a Schwartz formula [
14] and KeGFR was calculated using Chen’s formula [
9],
Baseline SCr was defined as the lowest SCr in the 3 months prior to AKI diagnosis, or the minimum inpatient SCr value during follow-up. The mean SCr was calculated from two consecutive SCr measurements. The Δ SCr was the difference between the two SCr values, and Δt (hours) was the interval in hours between two SCr measurements (maximum of 24 hours). The Max ΔSCr/day was defined as the change (increase) in SCr that occurred in a state of no kidney function dependent of volume of distribution (VD). VD of creatinine is close to total body water and it was estimated using the equation of Morgenstern et al recalculation of Mellits and Cheek [
15] based on height and sex as follows:
Table 1.
Total body water formula adjusted for age and sex.
Table 1.
Total body water formula adjusted for age and sex.
| Infants from 0 to 3 months |
0.887 x (Weight)0.83
|
| Children 3 months to 13 years |
0.0846 x 0.95 [if female]x (Height x Weight)0.65
|
| Children over 13 years |
0.0758 x 0.84 [if female] x (Height x Weight)0.69
|
After adjusting all data for age and sex, KeGFR was calculated twice, using two separate SCr set of values. KeGFR
1 was calculated using the SCr levels in days 1 and 2 of identified AKI and KeGFR
2 was calculated from two SCr values in days 6 and 7, before progression to AKD .The cut-off values of KeGFR where chosen based on KDIGO CKD classification [
13] as stage 1 (KeGFR > 90ml/min/1.73sm), stage 2 (KeGFR between 60 - 90ml/min/1.73sm), stage 3 (KeGFR between 30 - 60ml/min/1.73sm), stage 4 (KeGFR between 15 -3 0ml/min/1.73sm) and stage 5 (KeGFR < 15ml/min/1.73sm).
Outcomes of AKI were evaluated by MAKE30 (major adverse kidney events within 30 days). MAKE was defined as: death, necessity of RRT or the presence of AKD at day 30 after AKI documentation. The study was approved by the Hospital’s Medical Ethics Committee in accordance with the Ethics Code of the World Medical Association and informed consent was waived.
Statistical analysis
Data is presented as median and percentage for non-normally distributed continuous variables and categorical ones respectively. All continuous variables were tested for normality using Shapiro - Wilk test. For normally distributed variables, we used independent t-test or ANOVA. For non-normally distributed continuous variables, the median and interquartile ranges (IQR) were reported, and groups were compared using the Wilcoxon Signed Ranks test. Discrete variables were analysed with the Chi-square test. Odds ratio (OR) and 95% confidence interval (95%CI) were calculated. In order to assess the independent factors that predict the risk of AKD and MAKE30 in our cohort, we employed a backward multivariable logistic regression models. An Akaike information criteria (AIC) was used in order to determine the best model. Receiver-operating characteristic curves (ROCs) were plotted to determine the prognostic values of KeGFR, and area under the curve (AUC) values were generated to determine the predictive values of KeGFR1 and KeGFR2. The following values were used to describe AUC-ROCs: 0.6-0.69 poor, 0.7-0.79 fair and 0.8-0.89 good. In this study, a p-value of 0.05 was considered the threshold for statistical significance. Data was analysed using MedCalc Statistical Software version 22.009.
3. Results
The final cohort consisted of 803 patients with AKI that met the inclusion criteria. We divided the cases into two groups: the AKD group (219 patients) and non-AKD group (585 patients). Baseline characteristics are presented in
Table 2.
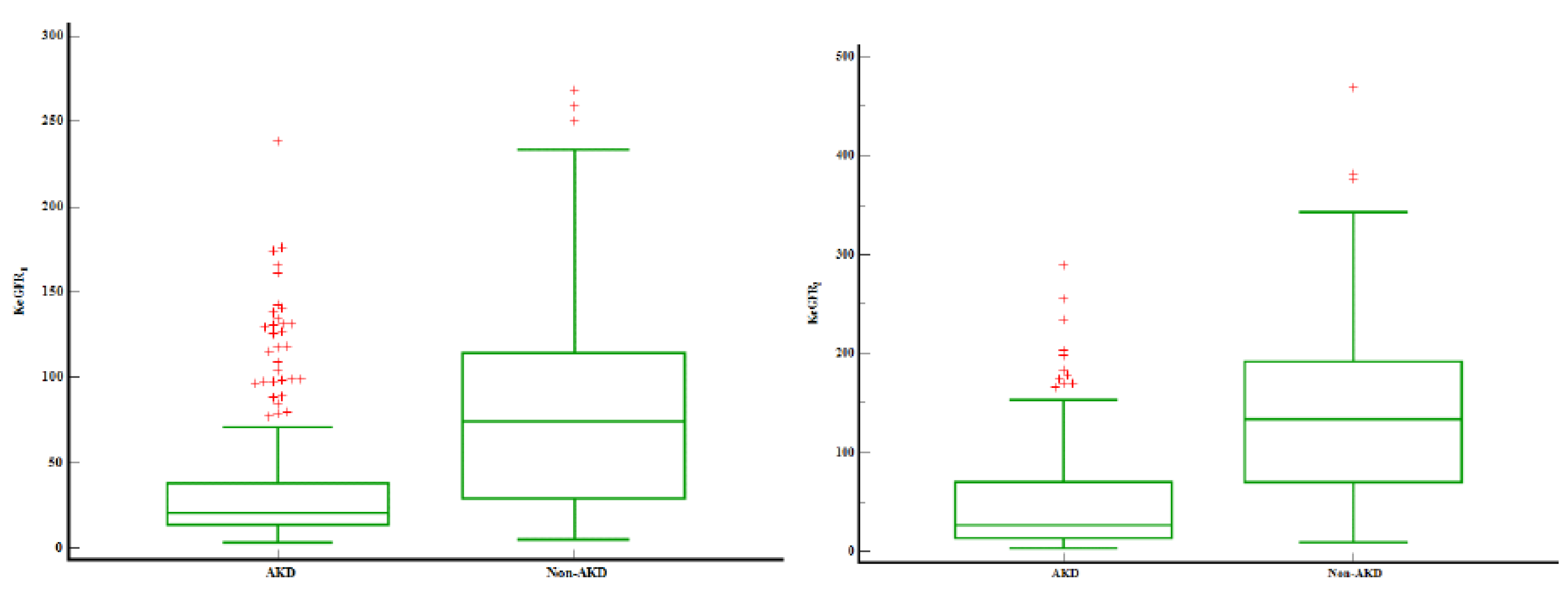
There were no differences between sexes. Patients that progressed to AKD were younger, 3 days versus 365 days (p<0.0001). The prevalence of severe AKI was higher in the AKD group (88.5%) as compared to no AKD. Patients that developed AKD maintained the same trend, with severe AKD in 72.8% of the patients (stage 2 and 3 AKD). Prerenal AKI was the main cause of AKI. Patients with intrinsic AKI were more prone to develop AKD as compared to other AKI aetiologies’. Intrinsic AKI could be traced in 28% AKD patients and only in 15.6% of the non-AKD ones. As expected baseline SCr was higher and eGFR lower in the AKD group (p<0.0001). KeGFR
1 and KeGFR
2 were 3.57 times respectively 4.99 times lower in the AKD group –
Figure 1. Out of 803 patients, only 16 required RRT (2%), with the highest rate of RRT in the AKD group (12 patients). Non-AKD patients expressed lower rates of pre-existent CKD. The overall mortality was 6.6% (53 patients) without significant statistical differences between AKD and non-AKD groups.
We identified a strong correlation between KeGFR
1 staging and AKI severity and causes –
Table 3 (p<0.0001). KeGFR was classified in KeGFR stages as described in methods. In the first 24 hours of identified AKI almost half the cases (45%) were stages 1 and 2 (KeGFR>60ml/min/1.73sm). Regarding to AKI stages, AKI stage 1 had the highest KeGFR (stages 1 and 2) as compared to severe AKI (stages 2 and 3). As severity of AKI increased, the KeGFR stages increased. In patients that progressed to AKD (217 patients out of 803), severe AKD was also associated with higher KeGFR stages.
After evaluating KeGFR in the first 24 hours of identified AKI, we performed the second analysis of KeGFR
2 on day 7 using SCr levels from days 6 and 7 in
Table 4. KeGFR
2 was calculated for 310 patients out of whom 151 children developed AKD (48.7%). The KeGFR
2 distribution was similar to KeGFR
1, with half the patients having a KeGFR>60ml/min/1.73sm (stages 1 and 2). This analysis once again confirms that AKI severity is associated with higher stages of KeGFR
2. Also, AKD severity is associated with decreased levels of KeGFR
2. In the subgroup of patients with KeGFR2 we evaluated the distribution of AKI causes. As previously mentioned, prerenal cause of AKI was the leading one in all KeGFR groups. The results from KeGFR
1 and KeGFR
2 showed that more than 60% of the patients with intrinsic AKI had a KeGFR > 60ml/min/1.73sm.
We performed a logistic regression model with AKD dependent variable and KeGFR
1 stages as independent ones (
Supplemental Table S1). The unadjusted model proved to be a good one (Nagelkerke R
2=0.27) with an AUC of 0.763. KeGFR
1 stage 3, 4 and 5 increased the risk of AKD development by 2.76, 5.33 and 27.23 times. After adjusting the regression model for sex, AKI stage and AKI causes, the model improved (Nagelkerke R
2=0.32, AUC=0.801) with KeGFR
1 increasing the incidence of AKD by 3.07, 6.56 and 28.07 times in stages 3, 4 and 5 respectively.
The logistic regression models with AKD dependent variable and KeGFR
2 stages as independent ones proved to be a good fit in both unadjusted and adjusted (Nagerkerke R
2 0.43 and 0.44, AUC=0.809 and 0.827 respectively). In unadjusted model, KeGFR
2 stage 2, 3, 4 and 5 increased the risk of AKD by 2.65, 3.03, 28.72 and 70.09 times and in the adjusted one by 2.79, 3.58, 32.75 and 80.14 respectively (
Supplemental Table S2).
MAKE30 of the entire cohort revealed 111 events (13.8%): 53 deaths, 11 patients required RRT and 47 children were with AKD. Logistic regression with MAKE30 as dependent variable and KeGFR
1 stages proved to be a good fit in both unadjusted and adjusted models (Nagerkerke R
2=0.18 and 0.23, AUC=0.675 and 0.764 respectively). Only stage 5 KeGFR
1 increased the risk of MAKE30 by 10.42 fold in unadjusted model and by 7.77 fold in the adjusted one (
Supplemental Table S1). KeGFR
2 stages 3, 4 and 5 increased the risk of MAKE30 by 2.8, 4.48 and 43.17 in unadjusted model and by 4.23, 5.89 and 69.42 in the adjusted one (Nagelkerke R
2 0.37 and 0.43, AUC=0.809 and 0.837 respectively) (
Supplemental Table S2).
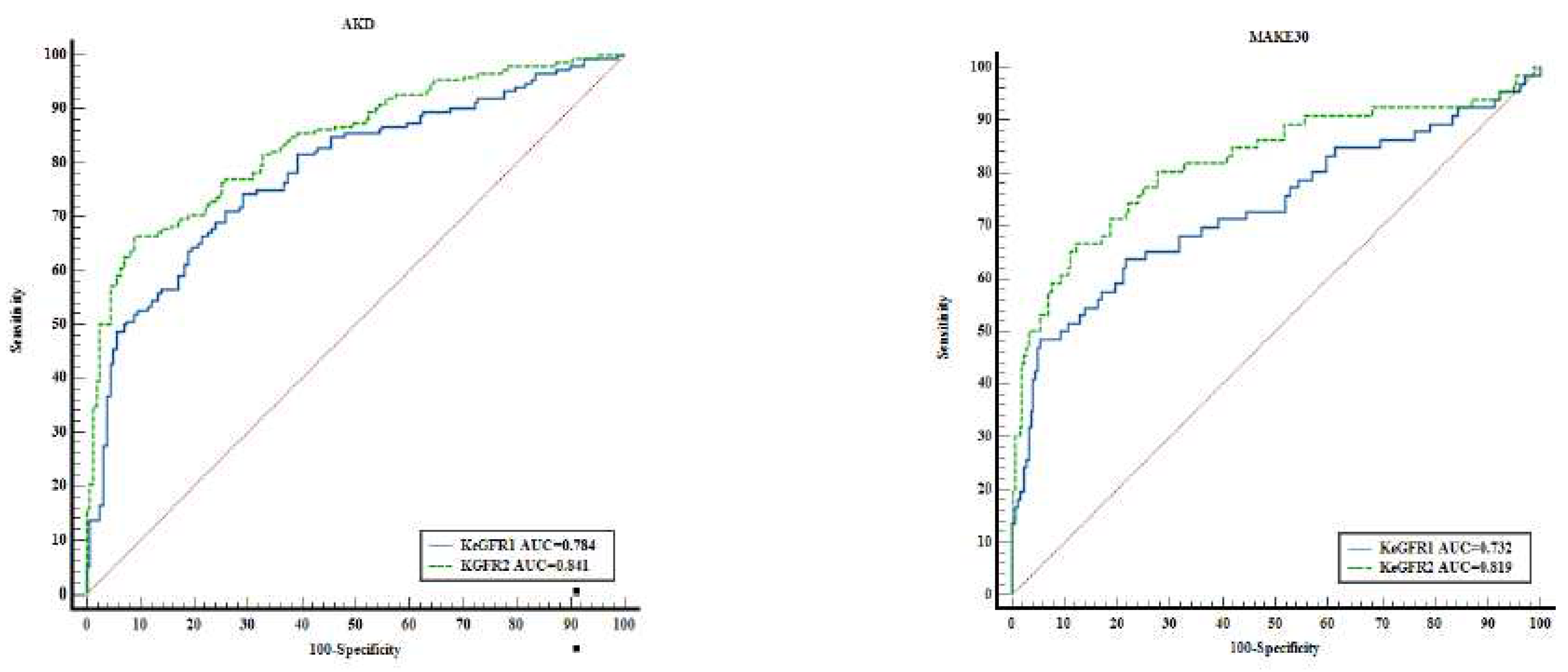
The KeGFR calculation in the first 24 hours of documented AKI as well as 24 hours prior to AKD, predicted AKD development and MAKE30 –
Figure 2. KeGFR
1 and KeGFR
2 predicted AKD with AUC values between 0.777 (95%CI 0.747-0.806) and 0.841 (95%CI 0.795-0.88), p<0.001. KeGFR
2 had the best performance in predicting AKD with 66.45% and specificity 91.14% with associated criterion ≤40.41ml.min/1.73sm as well as in predicting MAKE30 with an AUC of 0.819 (95%CI 0.772-0.861) with a sensitivity of 66.67% and specificity 87.7% with associated criterion ≤25.69ml/min/1.73sm, p<0.001. To mention the ROC-AUC of KeGFR
2 included 310 patients. KeGFR
1 had a fair performance in predicting AKD an AUC of 0.777 with a sensitivity of 76.15% and specificity of 68.55% with associated criterion ≤39.18ml/min/1.73sm. KeGFR
1 included all patients (n=803). KeGFR
1 had an AUC of 0.7 (95%CI 0.667-0.731) with an associated criterion of ≤21.28ml/min/1.73sm (p<0.001) and a 55.86% sensitivity and 81.65% specificity in predicting MAKE 30.
4. Discussions
In this study, KeGFR was analysed for the first time as a predictor of AKD in children. To best of our knowledge, there are no studies that used KeGFR for AKD prediction. KeGFR has been used in predicting AKI in high risk populations [
5,
6,
7,
8,
10,
16,
17,
18] with a high sensitivity and specificity. Until now, KeGFR was used as a predictor of AKI, thus it was calculated before AKI occurrence. The retrospective nature of our study allowed us to calculate KeGFR in the first 24 hours of identified AKI as well as 24 hours prior to AKD using the patient’s baseline SCr measurements. Our results showed that 1 in 4 children progressed to AKD (27.14%) thus the importance of predicting AKD. Children from the AKD group were younger, with higher SCr levels from day 1 to day 7 of identified AKI reflected by lower KeGFR during the documented AKI episode. Our study showed that half the patients had a KeGFR<60ml/min/1.73sm, reflecting AKI severity and AKI duration, consistent with previously reported paediatric data [
5,
8]. Besides reflecting AKI severity, reduced KeGFR levels were associated with AKD severity. We proved the direct link between lower KeGFR and the risk of AKD development. As expected, KeGFR
2 had a higher prediction rate of AKD compared to KeGFR
1. For example, when a patient with KeGFR
1 stage 5 (<15ml/min/1.73sm) has a 28 times higher risk of progressing to AKD one could use KeGFR
2 in dynamic for predicting the severity of AKD as well for the risk of MAKE. KeGFR
2 is a better predictor for AKD and MAKE as a result of prolonged kidney injury. More than 70% of the patients with AKD had a KeGFR
2 below 60ml/min/1.73sm, reflecting the transition from AKI state to AKD. The risk of AKD and MAKE30 starts from KeGFR
2 stage 3 (<60ml/min/1.73sm) as opposed to stage 5 KeGFR
1 (<15ml/min/1.73sm). With these results, we highlight the utility of repeated kinetic measurements.
In clinical settings, several markers proved to be effective in predicting AKI, including KeGFR. For instance, Dewitte et al [
19] showed that KeGFR had a better performance in predicting AKI compared to plasma neutrophil gelatinase-associated lipocalin (NGAL), renal resistive index and product between tissue inhibitor of metalloproteinase-2 and urine insulin-like growth factor-binding protein 7. Hekmat el al [
20] found KeGFR is a better AKI predictor compared to other creatinine based formulas with a strong correlation between KeGFR and NGAL. While it is counterproductive to use specific and expensive biomarkers of kidney injury in clinical practice, with this study we underline the utility of KeGFR as a cheap and handy tool.
Current studies failed to find a significant relationship between reduced KeGFR and 30 day mortality [
7,
21], while Dewitte showed a good prediction of MAKE using KeGFR based model [
19], in the ICU settings. Our results showed there is a link between reduced KeGFR and high risk of MAKE30 development. KeGFR
2 model proved to be superior compared to KeGFR
1 proving the utility of KeGFR calculation once again.
The strength of this study is represented by the high number of AKI cases from a mixt paediatric population, critically ill and non-critically ill, including neonates. Our Electronic Data System allowed us to identify the baseline SCr, being the first study to include baseline SCr and baseline eGFR in kinetic estimation formula in children, and to include all patients with consecutive SCr measurements in the first 24 hours of identified AKI and the 24 hours prior to AKD. In addition, we were able to adjust the formula of KeGFR to the maximum daily creatinine production using the equation of Morgenstern et al recalculation of Mellits and Cheek based on height and sex. The strongest point was the use of this tool in order to predict AKD and MAKE30. The limitations were the lack of urine output criteria, single centred and retrospective design.
Kinetic modelling of eGFR can be used as a complementary tool in AKI settings. There is an imperative need of prospective studies in the paediatric population in order to validate our results.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Table S1. Logistic regression with AKD and MAKE30 dependant variable with KeGFR
1 stages;
Table S2. Logistic regression with AKD and MAKE30 dependant variable with KeGFR
2 stages.
Author Contributions
F.C. and M.G. contributed equally to this article; Conceptualization, F.C., M.G.; Methodology, F.C., M.G., R.S.; Software L.C..; Validation, A.S; Formal Analysis, F.C, M.G, L.C; Investigation, F.C., M.G.; Resources, F.C, M.G., R.S.; Data Curation, L.C.; Writing – Original Draft Preparation, F.C., M.G.; Writing – Review & Editing, R.S, A.S.; Visualization, R.S; Supervision, A.S.; Project Administration, F.C., M.G.; Funding Acquisition, R.S.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of “Louis Turcanu” Clinical Emergency Hospital for Children from Timisoara, Romania (protocol code 6055, date of approval: 24 march 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data availability statement
The data collected for this study will be available for others, at request directly to the corresponding author. The data that will be available is represented by deidentified participant data. The inform consent form and statistical analysis plan will be available at request. The data will be available with publication. The data will be available at request at the e–mail address chisavulazar@gmail.com. The data will be shared after direct request and after approval of the proposal by all the authors.
Acknowledgments
The authors would like to thank ‘Louis Turcanu’ Hospital’s Medical Ethics Committee and the Laboratory Information System for the invaluable advice and support during statistical analysis. Current use of KeGFR proved to be useful in non-steady states. We demonstrate the potential utility of KeGFR in predicting AKD and MAKE30.
References
- Chawla L., Bellomo R., Bihorac A. et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol 2017;13:241–257. [CrossRef]
- Patel M, Heipertz A, Joyce E, et al. Acute kidney disease predicts chronic kidney disease in pediatric non-kidney solid organ transplant patients. Pediatr Transplant 2022;26(6):e14172. [CrossRef]
- Patel M, Hornik C, Diamantidis C, Selewski DT, Gbadegesin R. Patient level factors increase risk of acute kidney disease in hospitalized children with acute kidney injury. Pediatr Nephrol 2023;38:3465–3474. [CrossRef]
- Murdeshwar A, Krishnamurthy S, Parameswaran N, et al. Etiology and outcomes of acute kidney disease in children: a cohort study. Clin Exp Nephrol 2023;27(6):548-556. [CrossRef]
- Dasgupta MN, Montez-Rath ME, Hollander SA, Sutherland SM. Using kinetic eGFR to identify acute kidney injury risk in children undergoing cardiac transplantation. Pediatr Research 2021;90(3):632-636. [CrossRef]
- Seelhammer TG, Maile MD, Heung M, Haft JW, Jewell ES, Engoren M. Kinetic estimated glomerular filtration rate and acute kidney injury in cardiac surgery patients. J Crit Care 2016;31(1):249-54. [CrossRef]
- de Oliveira Marques F, Oliveira SA, de Lima E Souza PF, et al. Kinetic estimated glomerular filtration rate in critically ill patients: beyond the acute kidney injury severity classification system. Crit Care 2017;21(1):280. [CrossRef]
- Menon S, Basu RK, Barhight MF, Goldstein SL, Gist KM. Utility of Kinetic GFR for Predicting Severe Persistent AKI in Critically Ill Children and Young Adults. Kidney360 2021; 2(5):p869-872. [CrossRef]
- Chen S. Retooling the Creatinine Clearance Equation to Estimate Kinetic GFR when the Plasma Creatinine Is Changing Acutely. Journal of the American Society of Nephrology 2013;24(6):p 877-888. [CrossRef]
- Latha AV, Rameshkumar R, Bhowmick R, Rehman T. Kinetic Estimated Glomerular Filtration Rate and Severity of Acute Kidney Injury in Critically Ill Children. The Indian Journal of Pediatrics 2020;87:995–1000. [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney inter, Suppl 2012;2: 1–138.
- Chawla LS, Bellomo R, Bihorac A, et al. Acute Disease Quality Initiative Workgroup 16.. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol 2017;13(4):241-257. [CrossRef]
- KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney inter Suppl 2013;3:5–14.
- Schwartz GJ, Haycock GB, Edelmann CM Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 1976;58(2):259-63. [CrossRef]
- Morgenstern B, Mahoney D, Warady B: Estimating total body water in children on the basis of height and weight: A reevaluation of the formulas of Mellits and Cheek. J Am Soc Nephrol 2002;13:1884–1888.
- Libório AB, Macedo E, de Queiroz RE, et al. Kidney Disease Improving Global Outcomes or creatinine kinetics criteria in acute kidney injury: a proof of concept study. Nephrol Dial Transplant 2013;28(11):2779-87. [CrossRef]
- Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol 2009;20(3):672-9. [CrossRef]
- Kwong YD, Chen S, Bouajram R, et al. The value of kinetic glomerular filtration rate estimation on medication dosing in acute kidney injury. PLoS ONE 2019;14(11):e0225601. [CrossRef]
- Dewitte A, Joannès-Boyau O, Sidobre C, et al. Kinetic eGFR and Novel AKI Biomarkers to Predict Renal Recovery. Clin J Am Soc Nephrol 2015;10(11):1900-10. [CrossRef]
- Hekmat R, Eshraghi H, Esmailpour M, Hassankhani GG. Kinetic Glomerular Filtration Rate Estimation Compared With Other Formulas for Evaluating Acute Kidney Injury Stage Early After Kidney Donation. Exp Clin Transplant 2017;15(Suppl 1):104-109. [CrossRef]
- O'Sullivan ED, Doyle A. The clinical utility of kinetic glomerular filtration rate. Clin Kidney J 2017;10(2):202-208. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).