1. Introduction
Pyogenic spondylodiscitis is an important clinical problem with an increasing number of cases worldwide in recent years. Spondylodiscitis accounts for 2–7% of all musculoskeletal infections [
1]. It is characterized by marked heterogeneity, which limits its scientific evaluation and has resulted in a lack of treatment recommendations [
2]. Spondylodiscitis is usually a mono-bacterial infection, and >50% of cases in Europe are caused by Staphylococcus aureus, followed by gram-negative pathogens such as Escherichia coli (11–25%) and Mycobacterium tuberculosis [
2]. The most frequent symptom is intricate and constant back pain in approximately 85% of cases, whereas signs of infection with fever in less than half of patients [
3].
Surgical intervention for spondylodiscitis comprises a multidisciplinary approach and multiple-stage surgery that includes antibiotics, open/percutaneous biopsy, and open/minimally invasive debridement surgery.
Tantalum (Ta) trabecular metal (TM) components have been increasingly used to reconstruct major bone defects in orthopedic, dental and spine surgeries since the 1940s [
4]
. With a high value coefficient of friction in biomaterials, the tantalum trabecular metal cage has the benefit of implant stabilization [
5,
6]
.
With a similar three-dimensional appearance of the human cancellous bony structure and low modulus of elasticity, the trabecular metal cage can mimic subchondral and cancellous bones with better load transfer and attenuate the phenomenon of stress shielding [
4]. Osteoblast progenitor proliferation and bone mineralization can also be stimulated by tantalum material [
7]. Thus, the tantalum trabecular metal cage expedited osteointegration, bone remodeling and vascularization.
It has several potential advantages over conventional implant materials, such as its uniformity and structural continuity, strength, low stiffness, high porosity, and high coefficient of friction [
8]. Many studies have also shown that tantalum trabecular metal enhances the host defense mechanism by increasing leukocyte chemotaxis, phagocytosis, and the bacterial killing rate [9−11].
An increasing number of researchs have demonstrated that performing instrumented spine surgery for spinal infections is a secure procedure [12−14]. Recent studies have shown the safety and effectiveness of early spinal instrumentation for pyogenic spondylodiscitis patients [
15,
16]. Thus, we considered debridement and reconstruction in a single stage with a posterior or combined posterolateral approach with a tantalum trabecular metal cage for spondylodiscitis patients with vertebral instability or deformity.
2. Materials and Methods
2.1. Patients
This was a retrospective study of spondylodiscitis patients who underwent surgery between January 2018 and March 2021 in our tertiary academic teaching hospital. We examined clinical records, radiological images, and surgical notes acquired for all patients who underwent surgical procedures for spondylodiscitis with vertebral body lysis and a propensity for deformity in our institution. Fifty-seven patients met the inclusion criteria. (35 male, 22 female). The mean age at the time of treatment was 67 years ± 14.08 years (range 25–92 years). Demographic data are presented in
Table 1.
Thirty-one patients underwent reconstructive surgery with a tantalum trabecular metal cage [ZimmerR]. For comparison, we also included twenty-six patients who underwent surgery without tantalum trabecular metal cages in the same period. We examined patient demographic data, including age and sex, as well as symptoms, laboratory results, neurological examination, radiographic images, operative reports, postoperative complications, and data from clinical and radiological follow-up. We evaluated the CRP data during therapy. In all patients, blood tests for CRP levels were obtained 3 days before surgery as baseline data. Further blood chemistry controls in all patients were performed after surgery. As blood tests were not performed on the same day for all patients, we assessed values on Days 0-3, 4-7, 8-14, 15-21, and 22-28 to obtain comparable results.
The selection of the approach hinged on the level and complexity of spondylodiscitis. Our preference was a single-stage approach in all instances. In cases of cervical spondylodiscitis, we opted for an anterior approach, while for lumbar cases, a posterior approach for lumbar cases was chosen; in some intricate cases, a posterolateral approach was favored.
The outcomes of CT-guided needle biopsies were documented when they were performed. Referring antibiotic therapy depended on the decision of the infectious disease specialists. Initial therapy necessitated the administrated of broad-spectrum intravenous antibiotics. Following the availability of comprehensive microbiology data, specific antibiotic therapy was employed upon identificaion of the pathogen. Transition to oral antibiotics was contingent upon a satisfactory clinical and laboratory response.
Exclusion criteria encompassed patients presenting evidence of complicated bacterial infection such as endocarditis, pulmonary, or urinary tract infection, as well as substantial non-orthopedic complications like cardiac decompensation or sudden-onset renal decompensation. These factors could potentially impact CRP values and exacerbate confounding bias. The study exclusively included patients who had been discharged from rehabilitation and outpatient treatment.
2.2. Statistical analyses
Statistical analysis was conducted utilizing IBM SPSS Statistics Version 24.0 (SPSS Inc., IBM Corp., Armonk, NY, USA). Variables that followed a normal distribution were presented as the mean and standard deviation. The Mann‒Whitney U test and the chi-square test were employed and statistical significance was attributed to p value < 0.05.
Redarding CRP data, the spread of metric variables among independent groups was described using the Shapiro‒Wilk test, evaluating whether the random sample survey exhibited a normal distribution. If the assumption of normal distribution was not rejected (p value ≤ 0.1), a t-test was employed for comparisons. In cases where the assumption of normal distribution was rejected, the Mann‒Whitney U test was utilized. Fisher’s exact test was applied to examine the relationship between two categorical variables. Given the exploratory nature of this study, all p values are reported descriptively.
3. Results
Out of 57 patients, 31 (54%) patients were treated with single-stage debridement and reconstruction with trabecular metal cages, and 26 (45.6%) patients received spine surgery without trabecular metal cages. Demographic data are shown in
Table 1. In the TM cage group, the mean age was 68 years (range 25–92 years), and there were 18 male and 13 female patients. In the non-TM cage group, the mean age was 65 years (range 41-85 years). The mean age and age range of the TM group were older and wider than those of the non-TM cage group. The percentage of the spine location was similar between the two groups. The level of CRP data was lower in the TM cage group at three different times (before surgery, postoperation Days 0-3, postoperation Days 22-28).
The periods of inpatient stay after the operation were shorter in the Non-TM cage group (TM cage group vs. Non-Tm cage group: 47 vs. 41 days). A second intervention for debridement, interbody fusion, vertebral body replacement, cage or screw removal was performed in 11 (19.3%) cases within one month after the first intervention. A second intervention was performed significantly more frequently in the non-TM cage group (34.6% vs. 6.5%; P = 0.0155) three months after the first surgery. The kyphotic angle correction was similar between the two groups, and the correction angle of the TM cage group was slightly smaller than that of the non-TM cage group (-6.27 degrees vs. -6.35 degrees; P = 0.7595; in our study definition, kyphosis was positive). There was no obvious statistical significance between the two groups. There was no revision surgery for implant failure in either group.
3.1. Microbiological findings
The bacterial culture results of 57 patients are listed in
Table 2.
We can see the culture report of the TM cage group as follows. The most etiological agent in both groups was Staphylococcus aureus: 7 cases in the TM cage group and 4 cases in the non-TM cage group. There were two cases in the TM cage group and 3 cases in the non-TM cage group without bacterial culture evidence. More antibiotic-resistant bacterial culture was reported in the TM cage group. Two of them were methicillin-resistant Staphylococcus aureus, one was oxacillin-resistant Staphylococcus aureus, one was methicillin-resistant Staphylococcus epidermidis, and one was extended-spectrum β-lactamase-producing Escherichia coli.
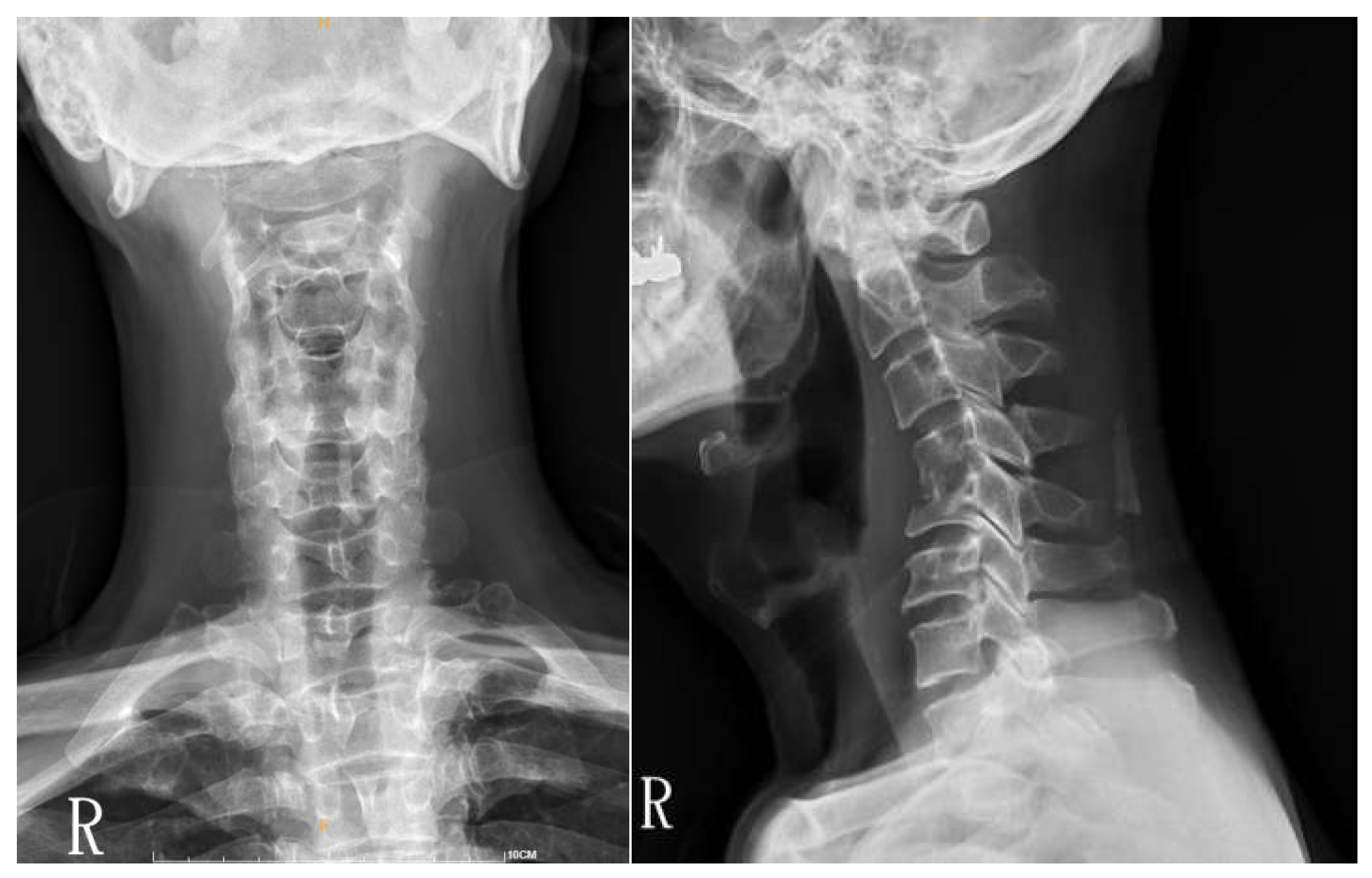
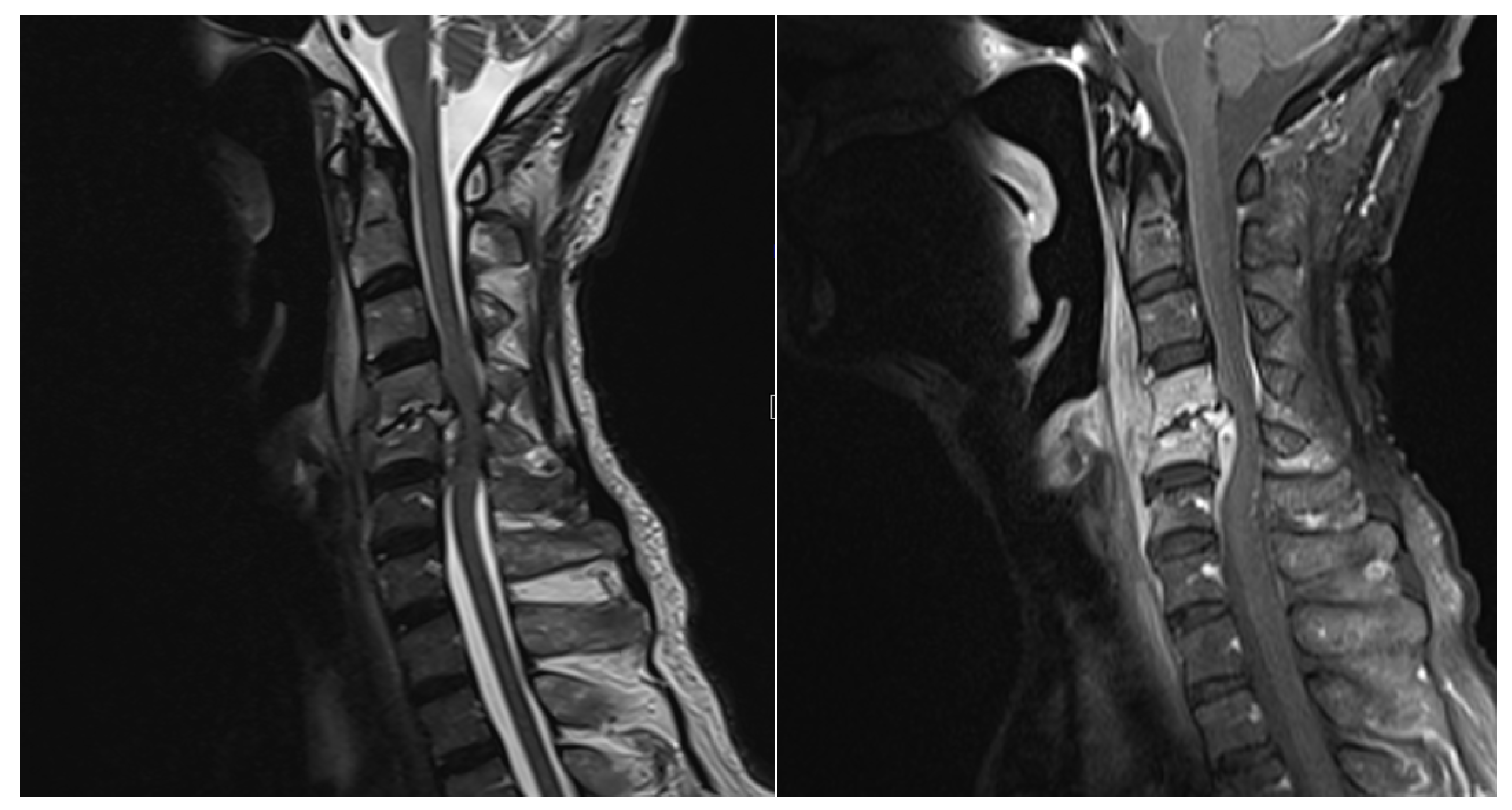
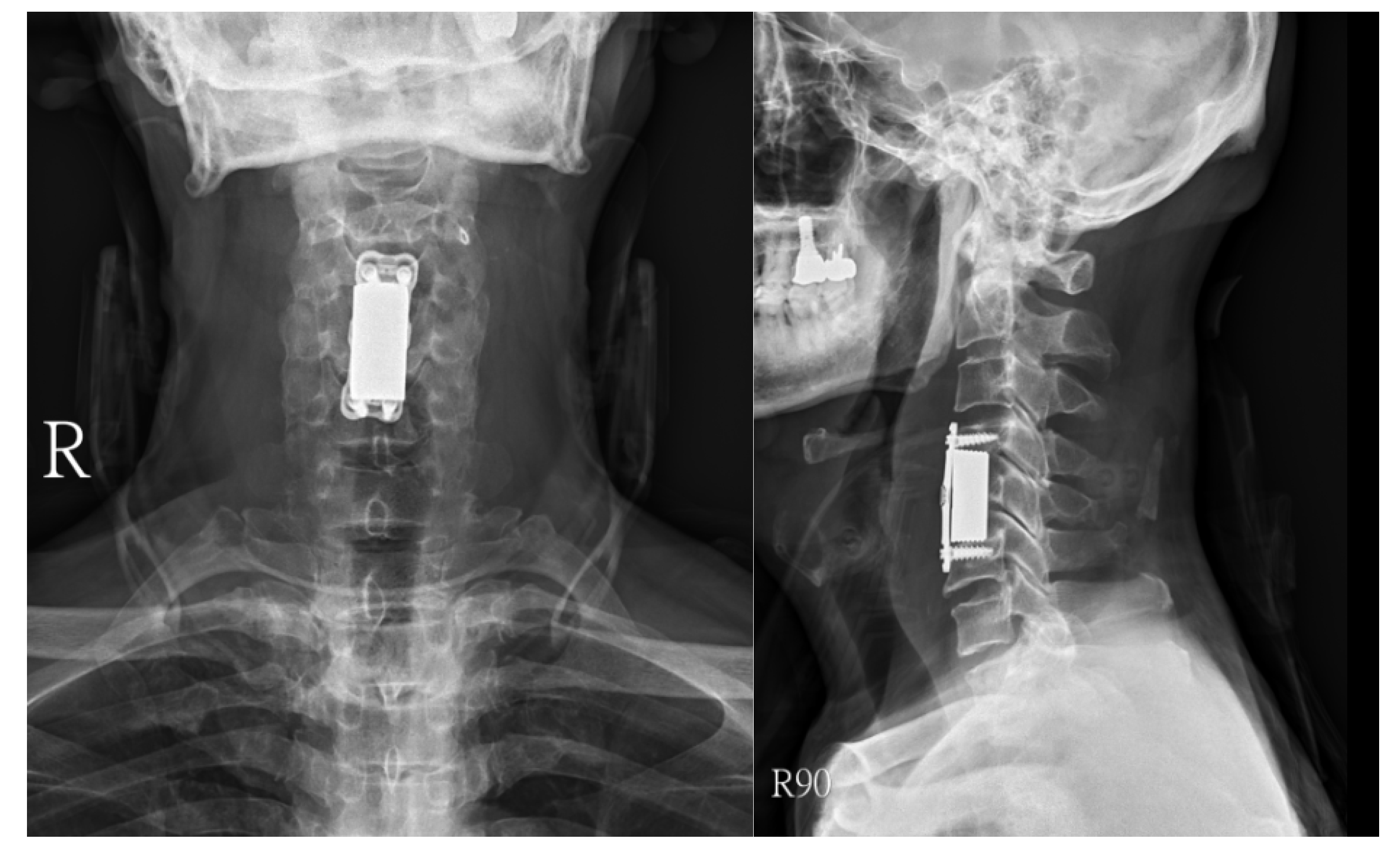
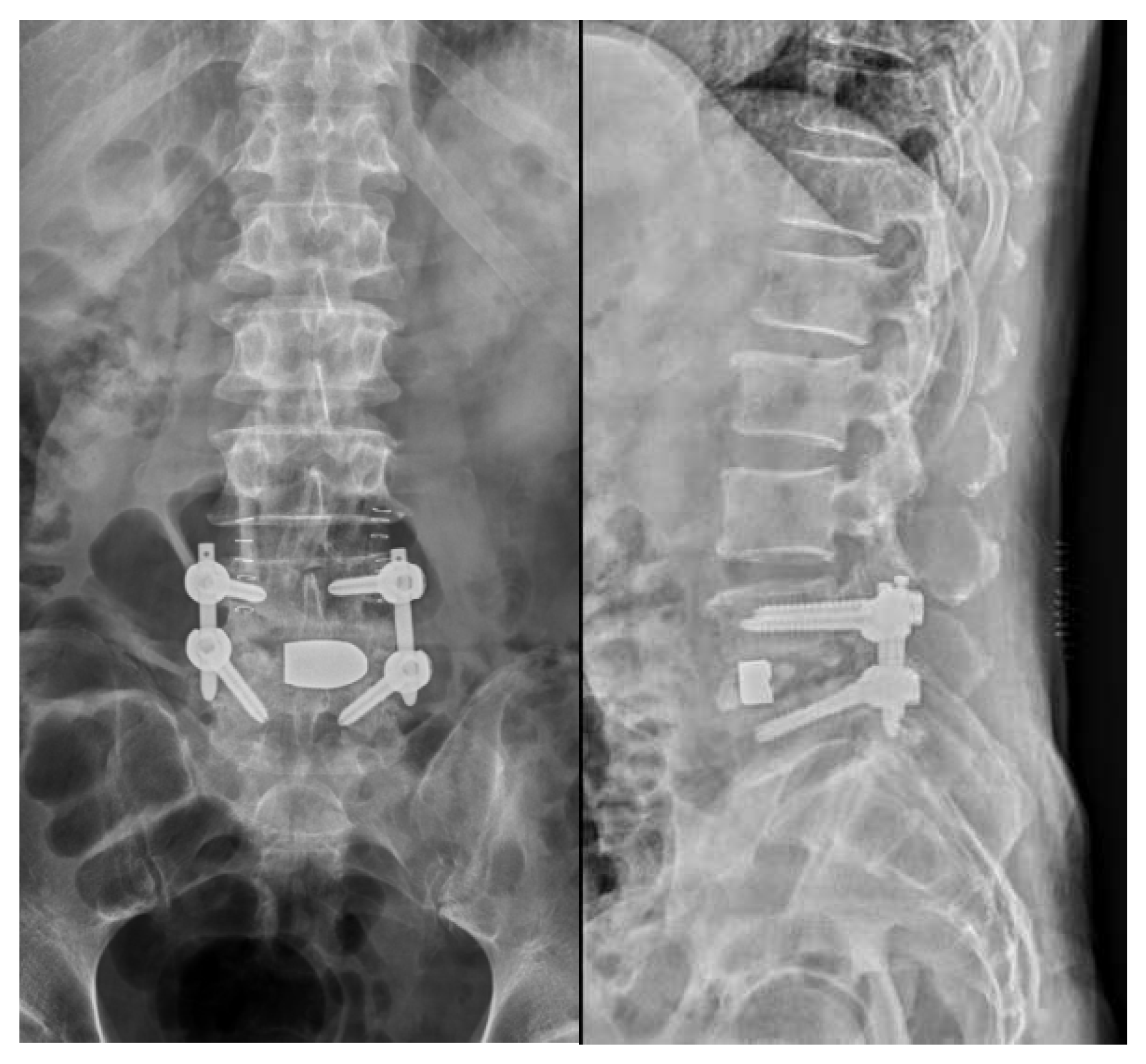
Surgical approaches were selected based on the spinal level and the extent of paraspinal muscle involvement. A posterior approach was favored in 16 thoracic or lumbar cases, while in 4 cervical cases, an anterior approach was utilized (
Figure 1,
Figure 2 and
Figure 3). For 10 cases in the thoracic or lumbar region, posterior fixation was performed either with or without a combined oblique approach (
Figure 4 and
Figure 5). Additionally, in 1 case involving the lumbar region, a combination of an anterior approach and posterior fixation was utilized.
All 31 cases were reconstructed with a Zimmer Biomet Trabecular Metal™ cage, and thoracolumbar spine cases were all combined with posterior pedicular screw fixation. An orthopedic brace or cervical collar was used for 8–12 weeks in all cases. At the latest follow-up, spinal deformity had been averted, and preexisting spinal deformities were partially rectified in all instances. Among the cases, five patients initially exhibited neurological deficits that ameliorated post-surgery. In the remaining 26 patients, spinal pain demonstrated greater responsiveness to analgesic treatment after vertebral stabilization, resulting in improved back pain or radicular pain during the follow-up period. Two patients experienced progression of spondylodiscitis necessitating intervention subsequent to the initial surgery. The follow-up duration ranged from 5–33 months with an average of 15 months. Notably, none of the patients developed new-onset neurological deficits after surgical procedure.
All patients commenced mobilization on the second day following surgery, after removal of the paravertebral drainage devices and confirmation of proper screw positioning via postoperative X-ray. Depending on their clinical status, patients were promptly transferred to either an infectious disease unit or a rehabilitation unit. Nutritional support was assessed on an individual basis for each case. As per recommendaions from infectious disease specialists, all patients underwent a six-weeks course of systemic antibiotic therapy. The risk factors in this cohort study included elderly, debilitated patients with systemic blood infection, individuals with a history of previous cardiac surgery, those with decompensated diabetes mellitus, pulmonary tuberculosis, and cases involving previous spine surgery (25 instances).
3.2. CRP kinetics
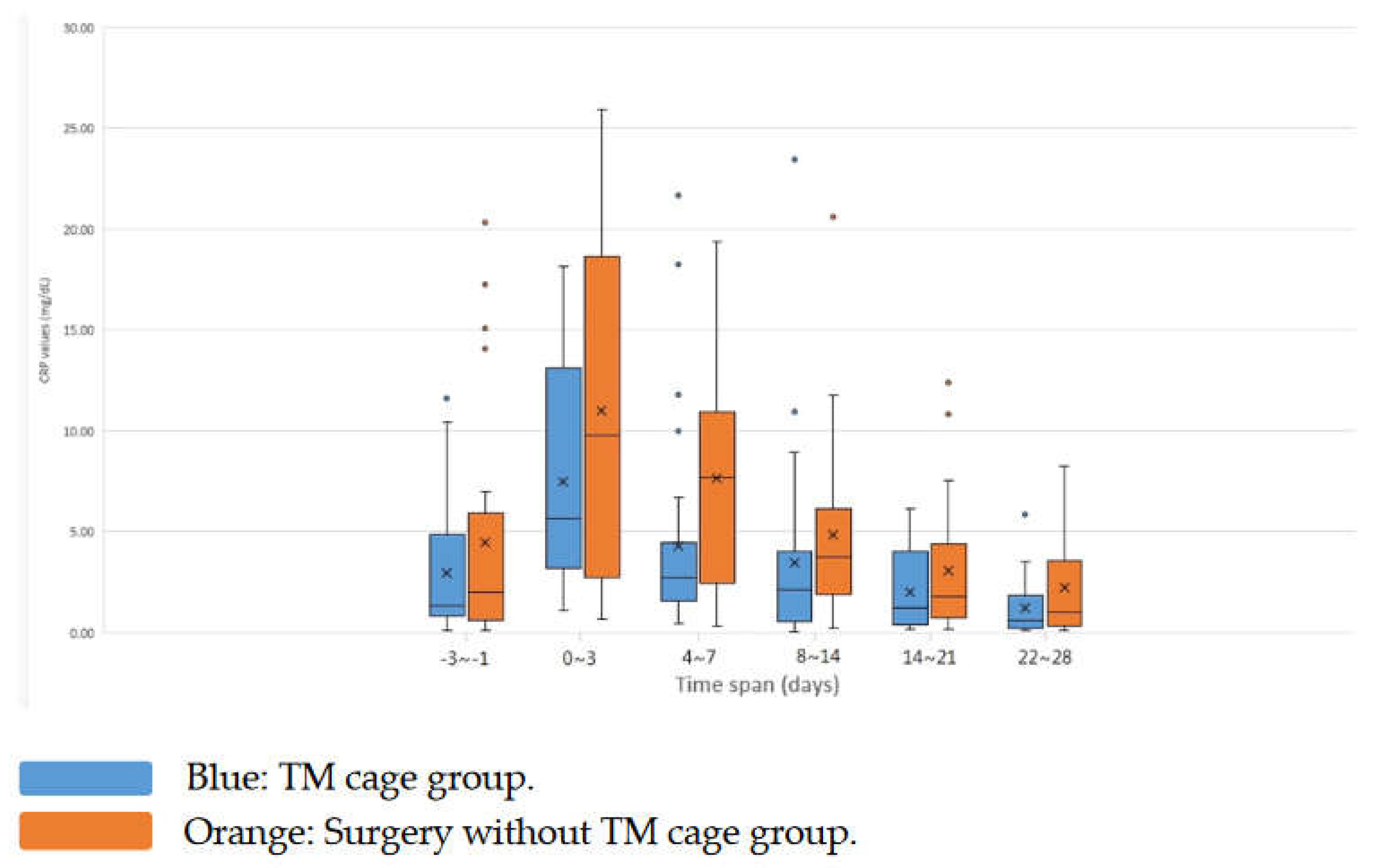
Among these two groups, the C-reactive protein (CRP) level were notably elevated before and after surgery in the non-TM cage/debridement group. The CRP data and trends of the two groups are shown in
Table 3 and
Figure 6.
The average preoperative CRP value in surgically treated patients was (TM cage group: non-TM cage group = 2.96± 3.24 [range 0.11-11.62] vs. 4.46± 5.85 [range 0.11-20.31]; P = 0.6899). The mean values increased nearly twofold-fold three days after surgery compared to baseline in the two groups (TM cage group: non-TM cage group = 7.47±5.07 [range 1.09-18.17]:11.02±8.21 [range 0.69-25.92]; P = 0.2129). The peak CRP values were attained three days post-surgery. The CRP level decreased with time after surgery in the two groups. On Days 4-7, the mean CRP values were statistically significant between the two groups (TM cage group: non-TM cage group = 4.28 ± 5.01 [range 0.42-21.69] vs. 7.65± 5.99 [range 0.35-19.37]; P = 0.0313). Further decline was then markedly lower on Days 8-14 (TM cage group: non-TM cage group = 3.44 ± 4.61 [range 0.05-23.45] vs. 4.84± 4.62 [range 0.23-20.63]; P = 0.0739). The CRP level declined slower on Days 15-21 (TM cage group: non-TM cage group = 2.01 ± 1.94 [range 0.15-6.14] vs. 3.07± 3.48 [range 0.17-12.38]; P = 0.3139). On Days 22-28, the CRP data of the two groups were not statistically significant between the two groups (TM cage group: non-TM cage group = 1.22 ± 1.34 [range 0.1-5.85] vs. 2.25±2.54 [range 0.0985.29]; P = 0.2996). Only the CRP data from Days 4-7 showed a significant difference (P<0.05). For the CRP data from Days 8-14, borderline significance was noted (P = 0.0739). The trends of the CRP level decline over time were noted from the box plot. The CRP levels of the non-TM cage group were relatively higher than those of the TM cage group before and after surgery.
4. Discussion
Spondylodiscitis can manifest as either a primary infection within intervertebral disk or as a secondary infection spread through the bloodstream. Factors that predispose individuals to spondylodiscitis encompass surgical and dental procedures, intravenous drug abuse, advanced age, compromised immune system, malignancy and chronic steroid usage [
2]. Surgical interventions are typically considered for patients who show inadequate response to antibiotic therapy or those with conditions such as instability, spinal deformity, epidural abscess, or neurologic deficits. The periods of inpatient stay after the operation were shorter in the non-TM cage group (TM cage group vs. non-TM cage group: 47 vs. 41 days). Due to the treatment strategy, many TM cage group patients were discharged after complete antibiotic therapy (at least 6 weeks). Most non-TM cage group patients had more antibiotic therapy days before surgery, and they may have less postoperative antibiotic therapy days. The complexity and severity were different between the two groups, and some cases in the non-TM cage group had limited infection with simple surgical decompression.
4.1. CRP kinetics between the two groups
CRP values remain stable for patients within a narrow normal range and are minimally affected by medication or common comorbidities, with the exception of liver failure [
17]. Routine serum CRP measurements at different times, including 3 days prior to surgery, postoperative Days 2-3 and postoperative Days 4-7, are recommended [
18]. Referring to
Table 3 and the box plot in
Figure 6, it is evident that there is an elevation in postoperative CRP levels, with a peak occurring three days after the intervention in both groups. This observation highlights a substantial iatrogenic-induced inflammatory response. The CRP level of this operative peak was separate and was irrelevant to prognosis. Peak values exhibited notable variability among patients, while patients who underwent same procedure displayed a consistent trend in CRP levels. Mok et al. and Kunakornsawat et al. suggested that in different types of spinal procedures, postoperative CRP trends are a strong resemblance to different groups of operation, magnitude, or region [18−20]. In our study, we also found an iatrogenic surge in CRP levels for the surgical management of bacterial-infected spondylodiscitis cases. In previous studies on infectious spine surgery, the postoperative peak CRP values are a reference value for assessing intervention success [17,18,21−23]. In our study, the CRP values dropped significantly in the TM cage group compared with the non-TM cage group on postoperative Days 4-7. The mean CRP values remained low and gradually decreased until postoperative Days 22-28. Higher preoperative CRP values are also an unfavorable outcome for spondylodiscitis patients [
24].
In line with other retrospective studies, our data derive from a heterogeneous cohort of patients encompassing diverse ages and comorbidities. These underlying health conditions could potentially introduce variables that complicate individual CRP values and impact the outcomes of our findings.
We attempted to isolate bacteria in all patients for better infection control. Our approach involved utilizing venous blood culture, as well as computed tomography (CT)-guided needle aspiration from the infection site before initiating antibiotics treatment, and surgical swab culture. It’s noteworthly that over 90% of the patients yielded positive culture results. The positive rate of bacterial culture in previous studies was approximately 65%-76% [
25].
4.2. Treatment strategy
Historically, treatment for spondylodiscitis primarily comprised conservative approaches involving extended periods of immobilization. Managing delayed deformities stemming from this condition proved to be considerably challenging. Utilizing instrumentation in patients with spinal infections presents a quandary, as while such instrumentation is essential for reconstruction purposes, there is a need to balance it against the risk of potentially exacerbating bacterial growth due to the presence of foreign bodies. Numerous studies have applied many different materials for spondylodiscitis patients and achieved satisfactory graft fusion, such as freeze-dried allografts [
26]. Multiple independent studies have provided evidence that utilizing instrumented spine surgery for spinal infections is a secure and achievable choice [
1,
12,
14]. With the advancement of material technology and engineering, the activation of leukocytes on the surface of TM material leads to the creation of a microenvironment that could potentially bolster local host defense mechanisms [
10,
27]. A single-stage approach was uniformly employed for all cases. With the exception of the long-term deformity case, a singular approach proved effective in ensuring vertebral stability and preventing deformity. The utilization of more intricate two-stage approaches, involving both anterior and posterior procedures, was avoided due to the fragile health condition of the patients. A more extensive follow-up period and larger sample sizes are imperative to comprehensively assess the efficacy of this strategy.
5. Conclusions
This study has limitations due to its limited number of patients and retrospective review. Early debridement and stabilization allow early mobilization of the patient and prevent subsequent spinal deformity. A single-stage surgical procedure that integrates two distinct approaches is capable of preserving vertebral stability. In cases of spondylodiscitis characterized by osteolysis and instability, it is recommended to opt for a single-stage approach involving debridement and reconstruction using a trabecular metal cage. This approach facilitates abscess drainage, enables swift mobilization, mitigates the risk of deformity, and minimally interferes with the effectiveness of antibiotics treatment.
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lener S, Hartmann S, Barbagallo GMV, Certo F, Thome C, Tschugg A. Management of spinal infection: a review of the literature. Acta Neurochir (Wien). 2018;160(3):487-496. [CrossRef]
- Herren C, Jung N, Pishnamaz M, Breuninger M, Siewe J, Sobottke R. Spondylodiscitis: Diagnosis and Treatment Options. Dtsch Arztebl Int. 2017;114(51-52):875-882. [CrossRef]
- Gouliouris T, Aliyu SH, Brown NM. Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother. 2010;65 Suppl 3:iii11-24.
- Hanc M, Fokter SK, Vogrin M, Molicnik A, Recnik G. Porous tantalum in spinal surgery: an overview. Eur J Orthop Surg Traumatol. 2016;26(1):1-7. [CrossRef]
- Paganias CG, Tsakotos GA, Koutsostathis SD, Macheras GA. Osseous integration in porous tantalum implants. Indian J Orthop. 2012;46(5):505-513. [CrossRef]
- Levine BR, Sporer S, Poggie RA, Della Valle CJ, Jacobs JJ. Experimental and clinical performance of porous tantalum in orthopedic surgery. Biomaterials. 2006;27(27):4671-4681. [CrossRef]
- Sagomonyants KB, Hakim-Zargar M, Jhaveri A, Aronow MS, Gronowicz G. Porous tantalum stimulates the proliferation and osteogenesis of osteoblasts from elderly female patients. J Orthop Res. 2011;29(4):609-616. [CrossRef]
- Cohen R. A porous tantalum trabecular metal: basic science. Am J Orthop (Belle Mead NJ). 2002;31(4):216-217.
- Bencharit S, Byrd WC, Altarawneh S, et al. Development and applications of porous tantalum trabecular metal-enhanced titanium dental implants. Clin Implant Dent Relat Res. 2014;16(6):817-826. [CrossRef]
- Schildhauer TA, Peter E, Muhr G, Koller M. Activation of human leukocytes on tantalum trabecular metal in comparison to commonly used orthopedic metal implant materials. J Biomed Mater Res A. 2009;88(2):332-341. [CrossRef]
- Komnos G, Banios K, Kolonia K, et al. Do trabecular metal and cancellous titanium implants reduce the risk of late haematogenous infection? An experimental study in rabbits. Hip Int. 2021;31(6):766-773. [CrossRef]
- Bydon M, De la Garza-Ramos R, Macki M, et al. Spinal instrumentation in patients with primary spinal infections does not lead to greater recurrent infection rates: an analysis of 118 cases. World Neurosurg. 2014;82(6):e807-814. [CrossRef]
- Hassan K, Elmorshidy E. Anterior versus posterior approach in surgical treatment of tuberculous spondylodiscitis of thoracic and lumbar spine. Eur Spine J. 2016;25(4):1056-1063. [CrossRef]
- Shetty AP, Aiyer SN, Kanna RM, Maheswaran A, Rajasekaran S. Pyogenic lumbar spondylodiscitis treated with transforaminal lumbar interbody fusion: safety and outcomes. Int Orthop. 2016;40(6):1163-1170. [CrossRef]
- Kim J, Lee JH, Kim SW, Oh JK, Kim YW, Kim TH. Outcomes of additional instrumentation in elderly patients with pyogenic vertebral osteomyelitis and previous spinal instrumentation. Spine J. 2019;19(9):1498-1511. [CrossRef]
- Lee JH, Kim J, Kim TH. Clinical Outcomes in Older Patients Aged over 75 Years Who Underwent Early Surgical Treatment for Pyogenic Vertebral Osteomyelitis. J Clin Med. 2021;10(22). [CrossRef]
- Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111(12):1805-1812. [CrossRef]
- van Gerven C, Eid K, Kruger T, et al. Serum C-reactive protein and WBC count in conservatively and operatively managed bacterial spondylodiscitis. J Orthop Surg (Hong Kong). 2021;29(1):2309499020968296.
- Mok JM, Pekmezci M, Piper SL, et al. Use of C-reactive protein after spinal surgery: comparison with erythrocyte sedimentation rate as predictor of early postoperative infectious complications. Spine (Phila Pa 1976). 2008;33(4):415-421.
- Kunakornsawat S, Tungsiripat R, Putthiwara D, et al. Postoperative Kinetics of C-Reactive Protein and Erythrocyte Sediment Rate in One-, Two-, and Multilevel Posterior Spinal Decompressions and Instrumentations. Global Spine J. 2017;7(5):448-451. [CrossRef]
- Meyer B, Schaller K, Rohde V, Hassler W. The C-reactive protein for detection of early infections after lumbar microdiscectomy. Acta Neurochir (Wien). 1995;136(3-4):145-150. [CrossRef]
- Kragsbjerg P, Holmberg H, Vikerfors T. Serum concentrations of interleukin-6, tumour necrosis factor-alpha, and C-reactive protein in patients undergoing major operations. Eur J Surg. 1995;161(1):17-22.
- Khan MH, Smith PN, Rao N, Donaldson WF. Serum C-reactive protein levels correlate with clinical response in patients treated with antibiotics for wound infections after spinal surgery. Spine J. 2006;6(3):311-315. [CrossRef]
- Pojskic M, Carl B, Schmockel V, Vollger B, Nimsky C, Sabeta B. Neurosurgical Management and Outcome Parameters in 237 Patients with Spondylodiscitis. Brain Sci. 2021;11(8). [CrossRef]
- Tani Y, Saito T, Taniguchi S, et al. A New Treatment Algorithm That Incorporates Minimally Invasive Surgery for Pyogenic Spondylodiscitis in the Thoracic and Lumbar Spines: The Results of Its Clinical Application to a Series of 34 Patients. Medicina (Kaunas). 2022;58(4). [CrossRef]
- Martinez-Gutierrez O, Pena-Martinez V, Camacho-Ortiz A, et al. Spondylodiscitis treated with freeze-dried bone allograft alone or combined with autograft: A randomized and blinded trial. J Orthop Surg (Hong Kong). 2021;29(2):23094990211019101. [CrossRef]
- Malhotra R, Dhawan B, Garg B, Shankar V, Nag TC. A Comparison of Bacterial Adhesion and Biofilm Formation on Commonly Used Orthopaedic Metal Implant Materials: An In vitro Study. Indian J Orthop. 2019;53(1):148-153. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).