Submitted:
02 September 2023
Posted:
05 September 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Methods
Study design and site
Study variables
Data analysis
Results
Discussion
Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yun, J.; Li, Y.; Xu, C.-T.; Pan, B.-R. Epidemiology and Rb1 gene of retinoblastoma. International Journal of Ophthalmology 2011, 4, 103. [Google Scholar]
- Aerts, I.; Lumbroso-Le Rouic, L.; Gauthier-Villars, M.; Brisse, H.; Doz, F.; Desjardins, L. Retinoblastoma. Orphanet journal of rare diseases 2006, 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Deng, Y.-P. Retinoblastoma: concerning its initiation and treatment. International journal of ophthalmology 2013, 6, 397. [Google Scholar] [PubMed]
- Epee, E.; Moukouri, E.; Koki, G.; Pondy, A.; Mbassi, K. Clinical features and prognosis of retinoblastoma at the University Teaching Hospital of Yaounde-Cameroon. HEALTH SCIENCES AND DISEASE 2014, 15. [Google Scholar]
- Jain, M.; Rojanaporn, D.; Chawla, B.; Sundar, G.; Gopal, L.; Khetan, V. Retinoblastoma in Asia. Eye 2019, 33, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Dimaras, H.; Kimani, K.; Dimba, E.A.; Gronsdahl, P.; White, A.; Chan, H.S.; Gallie, B.L. Retinoblastoma. The Lancet 2012, 379, 1436–1446. [Google Scholar] [CrossRef]
- Wabinga, H.R.; Nambooze, S.; Amulen, P.M.; Okello, C.; Mbus, L.; Parkin, D.M. Trends in the incidence of cancer in Kampala, Uganda 1991–2010. International journal of cancer 2014, 135, 432–439. [Google Scholar] [CrossRef]
- Waddell, K.M.; Kagame, K.; Ndamira, A.; Twinamasiko, A.; Picton, S.V.; Simmons, I.G.; Revill, P.; Johnston, W.T.; Newton, R. Improving survival of retinoblastoma in Uganda. British Journal of Ophthalmology 2015, 99, 937–942. [Google Scholar] [CrossRef]
- MacCarthy, A.; Draper, G.; Steliarova-Foucher, E.; Kingston, J. Retinoblastoma incidence and survival in European children (1978–1997). Report from the Automated Childhood Cancer Information System project. European Journal of Cancer 2006, 42, 2092–2102. [Google Scholar] [CrossRef]
- Houston, S.K.; Murray, T.G.; Wolfe, S.Q.; Fernandes, C.E. Current update on retinoblastoma. International ophthalmology clinics 2011, 51, 77. [Google Scholar] [CrossRef]
- Waddell, K.M.; Kagame, K.; Ndamira, A.; Twinamasiko, A.; Picton, S.V.; Simmons, I.G.; Johnston, W.T.; Newton, R. Clinical features and survival among children with retinoblastoma in Uganda. British Journal of Ophthalmology 2015, 99, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Nyawira, G.; Kahaki, K.; Kariuki-Wanyoike, M. Survival among retinoblastoma patients at the Kenyatta National Hospital, Kenya. 2013.
- Kazadi Lukusa, A.; Aloni, M.N.; Kadima-Tshimanga, B.; Mvitu-Muaka, M.; Gini Ehungu, J.L.; Ngiyulu, R.; Ekulu Mfutu, P.; Budiongo Nzazi, A. Retinoblastoma in the democratic republic of congo: 20-year review from a tertiary hospital in kinshasa. Journal of cancer epidemiology 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Chawla, B.; Hasan, F.; Azad, R.; Seth, R.; Upadhyay, A.D.; Pathy, S.; Pandey, R. Clinical presentation and survival of retinoblastoma in Indian children. British Journal of Ophthalmology 2016, 100, 172–178. [Google Scholar] [CrossRef]
- Yousef, Y.A.; Hajja, Y.; Nawaiseh, I.; Mehyar, M.; Sultan, I.; Deebajah, R.; Rawashdeh, K.; Khurma, S.; Jaradat, I.; Al-Hussaini, M. A histopathologic analysis of 50 eyes primarily enucleated for retinoblastoma in a tertiary cancer center in Jordan. Turk Patoloji Derg 2014, 30, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Adhi, M.I.; Kashif, S.; Muhammed, K.; Siyal, N. Clinical pattern of Retinoblastoma in Pakistani population: Review of 403 eyes in 295 patients. JPMA. The Journal of the Pakistan Medical Association 2018, 68, 376–380. [Google Scholar] [PubMed]
- Musa, K.O.; Aribaba, O.T.; Oluleye, T.S.; Olowoyeye, A.O.; Akinsete, A.M. Challenges of Retinoblastoma management at a Nigerian tertiary eyecare facility. 2017.
- Reddy, S.; Anusya, S. Clinical presentation of retinoblastoma in Malaysia: a review of 64 patients. International Journal of Ophthalmology 2010, 3, 64. [Google Scholar] [PubMed]
- Gichigo, N.; Kariuki-Wanyoike, M.; Kimani, K. Clinico-surgical histopathological findings of retinoblastoma cases treated at Kenyatta National Hospital. East African Medical Journal 2011, 88, 423–429. [Google Scholar]
- Khan, A.A.; Bukhari, M.H.; Mehboob, R. Association of retinoblastoma with clinical and histopathological risk factors. 2013.
- Lukamba, R.M.; Yao, J.-J.A.; Kabesha, T.A.; Budiongo, A.N.; Monga, B.B.; Mwembo, A.T.; Bey, P.; Chenge, G.B.; Desjardins, L.; Luboya, O.N. Retinoblastoma in sub-Saharan Africa: case studies of the Republic of Côte d’Ivoire and the Democratic Republic of the Congo. Journal of global oncology 2018, 4, 1–8. [Google Scholar] [CrossRef]
- El Zomor, H.; Nour, R.; Alieldin, A.; Taha, H.; Montasr, M.M.; Moussa, E.; El Nadi, E.; Ezzat, S.; Alfaar, A.S. Clinical presentation of intraocular retinoblastoma; 5-year hospital-based registry in Egypt. Journal of the Egyptian National Cancer Institute 2015, 27, 195–203. [Google Scholar] [CrossRef]
- Kashyap, S.; Meel, R.; Pushker, N.; Sen, S.; Bakhshi, S.; Sreenivas, V.; Sethi, S.; Chawla, B.; Ghose, S. Clinical predictors of high risk histopathology in retinoblastoma. Pediatric blood & cancer 2012, 58, 356–361. [Google Scholar]
- Suryawanshi, P.; Ramadwar, M.; Dikshit, R.; Kane, S.V.; Kurkure, P.; Banavali, S.; Viswanathan, S. A study of pathologic risk factors in postchemoreduced, enucleated specimens of advanced retinoblastomas in a developing country. Archives of pathology & laboratory medicine 2011, 135, 1017–1023. [Google Scholar]
- Biswas, J.; Das, D.; Krishnakumar, S.; Shanmugam, M.P. Histopathologic analysis of 232 eyes with retinoblastoma conducted in an Indian tertiary-care ophthalmic center. Slack Incorporated Thorofare, NJ: 2003; Vol. 40, pp 265-267.
- Shields, C.L.; Shields, J.A. Recent developments in the management of retinoblastoma. Journal of Pediatric Ophthalmology & Strabismus 1999, 36, 8–9. [Google Scholar]
- Eagle Jr, R.C. High-risk features and tumor differentiation in retinoblastoma: a retrospective histopathologic study. Archives of pathology & laboratory medicine 2009, 133, 1203–1209. [Google Scholar]
- Shields, C.L.; Shields, J.A.; Baez, K.; Cater, J.R.; de Potter, P. Optic nerve invasion of retinoblastoma. Metastatic potential and clinical risk factors. Cancer 1994, 73, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Vemuganti, G.K.; Reddy, V.A.P.; Honavar, S.G. Histopathologic risk factors in retinoblastoma in India. Archives of pathology & laboratory medicine 2009, 133, 1210–1214. [Google Scholar]
- Cuenca, A.; Giron, F.; Castro, D.; Fandino, A.; Guitter, M.; de Dávila, M.T.; Chantada, G. Microscopic scleral invasion in retinoblastoma: clinicopathological features and outcome. Archives of ophthalmology 2009, 127, 1006–1010. [Google Scholar] [CrossRef]
- Ali, M.J.; Honavar, S.G.; Reddy, V.A. Orbital retinoblastoma: Present status and future challenges–A review. Saudi Journal of Ophthalmology 2011, 25, 159–167. [Google Scholar] [CrossRef]
- Honavar, S.G.; Manjandavida, F.P.; Reddy, V.A.P. Orbital retinoblastoma: An update. Indian Journal of Ophthalmology 2017, 65, 435. [Google Scholar] [CrossRef]
- Levy, J.; Frenkel, S.; Baras, M.; Neufeld, M.; Pe’er, J. Calcification in retinoblastoma: histopathologic findings and statistical analysis of 302 cases. British journal of ophthalmology 2011, 95, 1145–1150. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, H.; Hsu, W.; Lee, S.; Cheng, C. Retinoblastoma in Taiwan: incidence and survival characteristics from 1979 to 2003. Eye 2010, 24, 318–322. [Google Scholar] [CrossRef]
- Bowman, R.; Mafwiri, M.; Luthert, P.; Luande, J.; Wood, M. Outcome of retinoblastoma in east Africa. Pediatric blood & cancer 2008, 50, 160–162. [Google Scholar]
- Saw, S.-M.; Tan, N.; Lee, S.-B.; Eong, K.-G.A.; Chia, K.-S. Incidence and survival characteristics of retinoblastoma in Singapore from 1968-1995. Slack Incorporated Thorofare, NJ: 2000; Vol. 37, pp 87-93.
- Cook, M.B.; McGlynn, K.A.; Devesa, S.S.; Freedman, N.D.; Anderson, W.F. Sex disparities in cancer mortality and survival. Cancer epidemiology, biomarkers & prevention 2011, 20, 1629–1637. [Google Scholar]
- Tulla, M.; Berthold, F.; Graf, N.; Rutkowski, S.; Von Schweinitz, D.; Spix, C.; Kaatsch, P. Incidence, trends, and survival of children with embryonal tumors. Pediatrics 2015, 136, e623–e632. [Google Scholar] [CrossRef] [PubMed]
- Dorak, M.T.; Karpuzoglu, E. Gender differences in cancer susceptibility: an inadequately addressed issue. Frontiers in genetics 2012, 3, 268. [Google Scholar] [CrossRef] [PubMed]
- Abramson, D.H.; Beaverson, K.; Sangani, P.; Vora, R.A.; Lee, T.C.; Hochberg, H.M.; Kirszrot, J.; Ranjithan, M. Screening for retinoblastoma: presenting signs as prognosticators of patient and ocular survival. Pediatrics 2003, 112, 1248–1255. [Google Scholar] [CrossRef]
- Kopelman, J.E.; McLean, I.W.; Rosenberg, S.H. Multivariate analysis of risk factors for metastasis in retinoblastoma treated by enucleation. Ophthalmology 1987, 94, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Finger, P.T.; Harbour, J.W.; Karcioglu, Z.A. Risk factors for metastasis in retinoblastoma. Survey of ophthalmology 2002, 47, 1–16. [Google Scholar] [CrossRef]
- Chong, E.-M.; Coffee, R.E.; Chintagumpala, M.; Hurwitz, R.L.; Hurwitz, M.Y.; Chévez-Barrios, P. Extensively necrotic retinoblastoma is associated with high-risk prognostic factors. Archives of pathology & laboratory medicine 2006, 130, 1669–1672. [Google Scholar]
- Nawaiseh, I.; Al-Hussaini, M.; Alhamwi, A.; Meyar, M.; Sultan, I.; Alrawashdeh, K.; Jaradat, I.; Yousef, Y.A. The impact of growth patterns of retinoblastoma (Endophytic, Exophytic, and Mixed Patterns). Turk Patoloji Derg 2015, 31, 45–50. [Google Scholar] [CrossRef]
| Characteristic | n(%) |
|---|---|
| Age in months, median (IQR) | 31 (18-39) |
| Age categories (months) <12 12-59 ≥60 |
10 (12.8) 58 (78.4) 10 (12.8) |
| Gender Male Female |
43 (55.1) 35 (44.9) |
| Region Western Eastern Central Northern International |
26 (33.3) 21 (26.9) 22 (28.2) 5 (6.4) 4 (5.1) |
| Laterality Unilateral Bilateral |
55 (70.5) 23 (29.5) |
| Chemoreduction therapy Given Not given |
63 (80.8) 15 (19.2) |
| Characteristic | n(%) |
|---|---|
| Choroidal invasion Absent Focal Massive |
55 (70.5) 13 (16.7) 10(12.8) |
| Optic nerve invasion Absent Prelaminar Intra-laminar Retrolaminar Surgical margin/cut end |
48 (61.5) 10 (12.8) 4 (5.1) 3 (3.9) 13 (16.7) |
| Scleral invasion Absent Partial thickness Full thickness |
72 (92.3) 1 (1.3) 5 (6.4) |
| Orbital extension | 13 (16.7) |
| Iris | 13 (16.7) |
| Trabecular meshwork | 13 (16.7) |
| Growth pattern Endophytic Exophytic Mixed |
56 (71.8) 14 (17.9) 8 (10.3) |
| Lens Invasion | 1 (1.3) |
| Ciliary body invasion | 13 (16.7) |
| Corneal epithelium invasion | 2(2.6) |
| Conjunctival invasion | 1 (1.3) |
| Vascular invasion | 2(2.6) |
| Flexner-Wintersteiner rosettes None Mild Moderate Many |
51 (65.4) 12 (15.4) 7 (9.0) 8 (10.2) |
| Homer-Wright rosettes None Present |
73 (93.6) 5 (6.4) |
| Necrosis None Mild Moderate Massive |
22 (28.2) 18 (23.1) 12 (15.4) 26 (33.3) |
| Calcification None Mild Moderate Massive |
46 (59.0) 13 (16.6) 12 (15.4) 7 (9.0) |
| Status at 2 years | n (%) | Proportion (CI) |
|---|---|---|
| Dead | 30 (38.5) | 38 (28- 50) |
| Alive | 48 (61.5) | 62 (50- 72) |
| Characteristic | Dead n (%) |
Alive n (%) |
Unadjusted (95% CI) | RR p value |
|---|---|---|---|---|
| Gender Female Male |
7 (20) 23 (53.5) |
28 (80) 20 (46.5) |
1.7 (1.20- 2.47) 1.0 |
0.003 |
| Region Eastern Central Southern International Western |
11 (52.4) 7 (61.8) 0 (0) 2 (50) 10 (38.5) |
10 (47.6) 15 (38.2) 5 (100) 2 (50) 16 (61.5) |
0.8 (0.45- 1.33) 1.1 (0.73- 1.68) 1.7 (1.26- 2.30) 0.8 (0.29- 2.27) 1.0 |
0.354 0.630 0.001 0.692 |
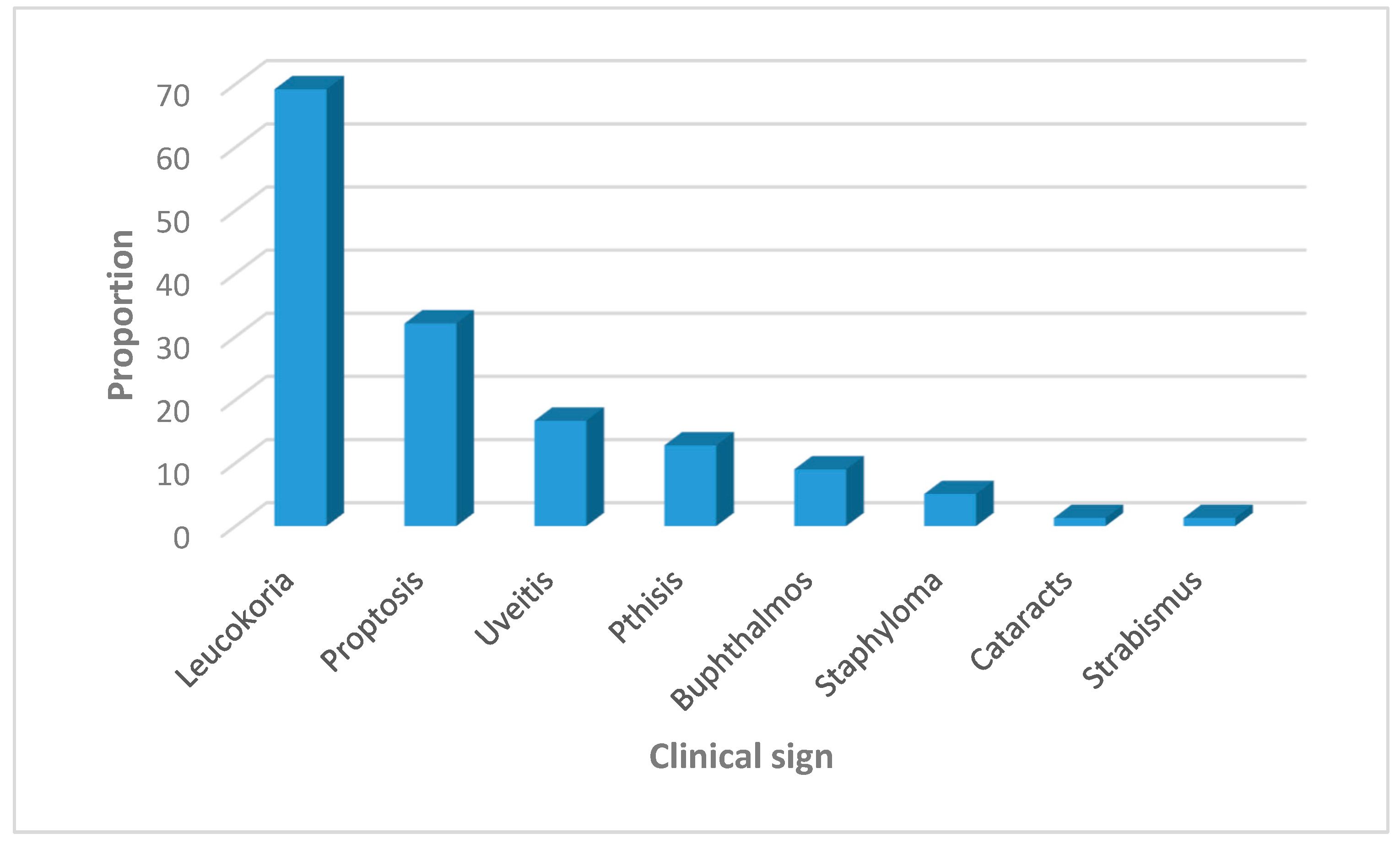
| Leukocoria Present Absent |
15 (27.8) 15 (62.5) |
39 (72.2) 9 (37.5) |
1.9 (1.12- 3.31) 1.0 |
0.018 |
| Proptosis Present Absent |
20 (80) 10 (18.9) |
5 (20) 43 (81.1) |
4.1 (1.83- 8.98) 1.0 |
0.001 |
| Cataract Present Absent |
0 (0) 30 (38) |
1 (100) 47 (61) |
1.7(1.43- 2.05) 1.0 |
0.000 |
| Choroidal invasion Absent Focal Massive |
17 (30.9) 4 (30.8) 9 (90) |
38 (69.1) 9 (69.2) 1 (10) |
6.9 (1.07- 44.72) 6.9 (1.04- 46.03) 1.0 |
0.043 0.045 |
| Optic nerve invasion Absent Prelaminar Intralaminar retrolaminar Surgical margin/ cut end |
14 (29.2) 2 (20) 0 (0) 2 (66.7) 12 (92.3) |
34 (70.8) 8 (80) 4 (100) 1 (33.3) 1 (7.7) |
9.2 (1.39- 61.06) 10.4 (1.4- 70.12) 13.6 (2.07- 89.4) 4.3 (0.37- 51.29) 1.0 |
0.021 0.016 0.007 0.245 |
| Orbital extension Absent Present |
18 (27.7) 12 (92.3) |
47 (72.3) 1 (7.7) |
9.4 (1.42- 62.16) 1.0 |
0.020 |
| Necrosis None Mild Moderate Severe |
3 (13.6) 8 (44.4) 3 (25) 16 (61.5) |
19 (83.4) 10 (55.6) 9 (75) 10 (38.5) |
2.3 (1.34- 2.75) 1.4 (0.76- 2.73) 1.95 (1.08- 3.50) 1.0 |
0.002 0.259 0.025 |
| Variable | Adjusted RR (95% CI) | P value |
|---|---|---|
| Gender Female Male |
1.4 (1.07- 1.70) 1.0 |
0.009 |
| Leukocoria Present Absent |
1.1 (1.10- 1.11) 1.0 |
0.000 |
| Optic nerve (ON) invasion Absent Prelaminar ON invasion Intralaminar ON Retrolaminar ON Surgical margin ON |
6.0 (0.85- 42.3) 7.0 (0.99- 49.27) 7.6 (1.08- 53.98) 2.4 (0.18- 31.04) 1.0 |
0.072 0.052 0.042 0.504 |
| Orbital extension Absent Present |
7.0 (1.00- 49.25) 1.0 |
0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





