Submitted:
01 September 2023
Posted:
05 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Taxonomy and Geographical Distribution of Cylas species
3. Description and Life cycle of Cylas species
4. Host Range and Dispersal of Cylas species
5. Damage Caused by Cylas species
6. Management and Control Strategies
7. Mechanisms of resistance of sweetpotato to Cylas spp.
8. Progress in breeding for resistance to Cylas spp.
9. The genetic and biochemical basis for sweetpotato weevil resistance
10. Marker assisted selection and Genomic selection for SPW resistance
11. Genomic resources for sweetpotato improvement
12. Genetic transformation for weevil resistance
13. Future Prospects
14. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT Food and Agricultural Organization of the United Nations. Statistical Database. Rome, FAO. 2023.
- Woolfe J.A Sweet Potato-Past and Present. In Sweet potato: an untapped food resource; Cambridge University Press: Cambridge, Great Britain, 1992.
- Anyanga, M.O.; Yada, B.; Yencho, G.C.; Ssemakula, G.N.; Alajo, A.; Farman, D.I.; Mwanga, R.O.M.; Stevenson, P.C. Segregation of Hydroxycinnamic Acid Esters Mediating Sweetpotato Weevil Resistance in Storage Roots of Sweetpotato. Front. Plant Sci. 2017, 8, 1011. https://doi.org/10.3389/fpls.2017.01011. [CrossRef]
- USDA National Nutrient Database for Standard Reference 1 Release April, 2018; United States Department of Agriculture, 2018;
- Yanggen, D; Nagujja, S The Use of Orange-Fleshed Sweetpotato to Combat Vitamin A Deficiency in Uganda. A Study of Varietal Preferences, Extension Strategies and Post-Harvest Utilization; International Potato Center (CIP): Lima (Peru). International Potato Center (CIP), 2006;
- Hotz, C.; Loechl, C.; Lubowa, A.; Tumwine, J.K.; Ndeezi, G.; Nandutu Masawi, A.; Baingana, R.; Carriquiry, A.; de Brauw, A.; Meenakshi, J.V.; et al. Introduction of β-Carotene–Rich Orange Sweet Potato in Rural Uganda Resulted in Increased Vitamin A Intakes among Children and Women and Improved Vitamin A Status among Children. The Journal of Nutrition 2012, 142, 1871–1880. https://doi.org/10.3945/jn.111.151829. [CrossRef]
- Rukarwa, R.J; Prentice, K.,; Ormachea, M.,; Kreuze, J.F.,; Tovar, J.,; Mukasa, S.B.,; Ssemakula, G.,; Mwanga, R.O.M.; Ghislain, M. Evaluation of Bioassays for Testing Bt Sweetpotato Events against Sweetpotato Weevils. Afr Crop Sci J 2013, 21, 235–244.
- Sorensen, K.A. Sweetpotato Insects: Identification, Biology and Management. In The Sweetpotato; Springer-Verlag New York Inc.: New York, 2009.
- Fuglie, K.O. Priorities for Sweetpotato Research in Developing Countries: Results of a Survey. horts 2007, 42, 1200–1206. https://doi.org/10.21273/HORTSCI.42.5.1200. [CrossRef]
- Capinera, J. Sweetpotato Weevil; Department of entomology and nematology. University of Florida, 2006;
- Stathers, T. E., Rees, D., Nyango, A., Kiozya, H., Mbilinyi, L., Jeremiah, S., Kabi, S. and Smit, N. Sweetpotato Infestation by Cylas Spp. in East Africa: II. Investigating the Role of Root Characteristics. International Journal of Pest Management 2003, 49, 141–146. [CrossRef]
- Musana, P; Okonya, J. S.; Mujica, N; Carhuapoma, P; Kroschel, J. Sweetpotato Weevil, Cylas Brunneus (Fabricius). In Pest Distribution and Risk Atlas for Africa. Potential Global and Regional Distribution and Abundance of Agricultural and Horticultural Pests and Associated Biocontrol Agents Under Current and Future Climates; International Potato Center (CIP)): eds. J. Kroschel, N. Mujica, P. Carhuapoma and M. Sporleder (Lima (Peru), 2016; pp. 63–73.
- Okonya, J. S.; Mujica, N.; Carhuapoma, P; Kroschel, J Sweetpotato Weevil, Cylas Puncticollis (Boheman 1883). In Pest Distribution and Risk Atlas for Africa. Potential Global and regional Distribution and Abundance of Agricultural and Horticultural Pests and Associated Biocontrol Agents Under Current and Future Climates; eds. J. Kroschel, N. Mujica, P. Carhuapoma and M. Sporleder (Lima (Peru), 2016; pp. 54–63.
- Ssali, R.T.; Sseruwu, G.; Yada, B.; Ssemakula, G.; Wasonga, C.; Grüneberg, W.J.; Eyzaguirre, R.; Low, J.W.; Mwanga, R.O.M. Efficiency of the Polycross and Controlled Hybridization Methods in Sweetpotato Breeding in Uganda. Journal of Agricultural Science (Toronto, Ont.) 2019, 11, 123. https://doi.org/10.5539/JAS.V11N17P123. [CrossRef]
- Stevenson, P.C.; Muyinza, H.; Hall, D.R.; Porter, E.A.; Farman, D.I.; Talwana, H.; Mwanga, R.O.M. Chemical Basis for Resistance in Sweetpotato Ipomoea Batatas to the Sweetpotato Weevil Cylas Puncticollis. Pure and Applied Chemistry 2009, 81, 141–151. https://doi.org/10.1351/PAC-CON-08-02-10. [CrossRef]
- Anyanga, M.O.; Muyinza, H.; Talwana, H.; Hall, D.R.; Farman, D.I.; Ssemakula, G.N.; Mwanga, R.O.M.; Stevenson, P.C. Resistance to the Weevils Cylas Puncticollis and Cylas Brunneus Conferred by Sweetpotato Root Surface Compounds. J. Agric. Food Chem. 2013, 61, 8141–8147. https://doi.org/10.1021/jf4024992. [CrossRef]
- Yada, B.; Alajo, A.; Ssemakula, G.N.; Brown-Guedira, G.; Otema, M.A.; Stevenson, P.C.; Mwanga, R.O.M.; Craig Yencho, G. Identification of Simple Sequence Repeat Markers for Sweetpotato Weevil Resistance. Euphytica 2017a, 213, 129. https://doi.org/10.1007/s10681-017-1917-1. [CrossRef]
- Wolfe, G.W. The Origin and Dispersal of the Pest Species of Cylas with a Key to the Pest Species Groups of the World. In: Jansson, R.K. AndRaman, K.V. (Eds.). In Sweet Potato Pest Management. A Global Perspective; Westview Press: Boulder, Colorado, 1991.
- Chalfant, R.; Jansson, R; Dakshina, R.; Schalk, J. Ecology and Management of Sweetpotato Insects. Annu. Rev. Entomol 1990, 17–180, doi:doi: 10.1146/ annurev.en.35.010190.001105. [CrossRef]
- Ames, T; Smit, N.E.J.M.; Braun, A.R; O’Sullivan, J.N.; Skoglund, L.G. Sweetpotato: Major Pests, Diseases, and Nutritional Disorders; International Potato Center (CIP): Lima, Peru., 1996.
- Nderitu, J.; Sila, M; Nyamasyo, G.; Kasina, M Effectiveness of Entomopathogenic Nematodes against Sweet Potato Weevil (Cylas Puncticollis Boheman (Coleoptera: Apionidae)] under Semi-Field Conditions in Kenya. J Entomol 2009, 6, 145–154. [CrossRef]
- Abidin, P.E. Sweetpotato Breeding for North Eastern Uganda: Farmer Varieties, Farmer-Articipatory Selection, and Stability of Performance. Ph.D. thesis, Wageningen University: Wageningen, Netherlands, 2004.
- Muyinza, H.; Talwana, H.L.; Mwanga, R.O.M.; Stevenson, P.C. Sweetpotato Weevil ( Cylas Spp.) Resistance in African Sweetpotato Germplasm. International Journal of Pest Management 2012, 58, 73–81. https://doi.org/10.1080/09670874.2012.655701. [CrossRef]
- Smit, N.E.J.M.; Matengo, L.O. Farmers’ Cultural Practices and Their Effects on Pest Control in Sweetpotato in South Nyanza, Kenya. International Journal of Pest Management 1995, 41, 2–7. https://doi.org/10.1080/09670879509371912. [CrossRef]
- Smit, N. The Effect of the Indigenous Cultural Practices of In-Ground Storage and Piecemeal Harvesting of Sweet Potato on Yield and Quality Losses Caused by Sweet Potato Weevil in Uganda. Agric Ecosyst Environ 1997, 191–200. [CrossRef]
- Smit, N. E. J. M.; Van Huis, A. Biology of the African Sweetpotato Weevil Species Cylas Puncticollis (Boheman) and C. Brunneus (Fabricius) (Coleoptera: Apionidae). Int. J. Trop. Insect Sci. 1998, 18, 93–100. [CrossRef]
- Stathers, T; Namanda, S; Mwanga, R. O. M; Khissa, G; Kapinga, R Manual for Sweetpotato Integrated Production and Pest Management Farmer Field Schools in Sub-Saharan Africa 2005.
- Smit, N.E.J.M.; Downham, M.C.A.; Laboke, P.O.; Hall, D.R.; Odongo, B. Mass-Trapping Male Cylas Spp. with Sex Pheromones: A Potential IPM Component in Sweetpotato Production in Uganda. Crop Protection 2001, 20, 643–651. https://doi.org/10.1016/S0261-2194(01)00026-6. [CrossRef]
- Talekar, N. S. Insect Factors in Breeding and Cultivation of Sweet Potato. In Sweet Potato Technology for the 21st Century. eds. W. Hill, C. Bonsai and P. A. Loretan; Tuskegee University: Tuskegee, USA, 1992.
- AVRDC Asian Vegetable Research and Development Center. Sweet Potato Breeding and Sweet Potato Entomology; 1987; pp. 163–198;
- Ebregt, E.; Struik, P.C.; Odongo, B.; Abidin, P.E. Pest Damage in Sweet Potato, Groundnut and Maize in North-Eastern Uganda with Special Reference to Damage by Millipedes (Diplopoda). J Life Sci. 2005, 53, 49–69. [CrossRef]
- Otto, N; Russel, M; Eric, C Sweetpotato Weevil. A Review of Recent Management Advances and Appraisal of Previous Research in Papua.; 2006;
- Allard, G. B. Integrated Control of Arthropod Pests of Root Crops; CAB International Institute of Biological Control: mid-term Report-November 1988-December 1989: Nairobi, Kenya, 1990;
- Hwang, J. S Integrated Control of Sweetpotato Weevil, Cylas Formicarius Fabricius, with Sex Pheromone and Insecticide 2000.
- Bassey, E. E. Field Evaluation of Yield and Resistances of Local and Improved Sweet Potato (Ipomoea Batatas (L) Lam) Accessions to Cylas Puncticollis and Meloidogyne Incognita in Southeastern Nigeria. Asian J. Agric. Sci. 2012.
- Jansson, R.K. Biological Control of Cylas Spp. In Jansson, R.K. and Raman, K.V. (eds.) Sweet Potato Pest Management: A Global Perspective.; Westview Press Inc.: Boulder, Colorado, 1991.
- Ondiaka, S.; Maniania, N.K.; Nyamasyo, G.H.N.; Nderitu, J.H. Virulence of the Entomopathogenic Fungi Beauveria Bassiana and Metarhizium Anisopliae to Sweet Potato Weevil Cylas Puncticollis and Effects on Fecundity and Egg Viability. Annals of Applied Biology 2008, 153, 41–48. https://doi.org/10.1111/j.1744-7348.2008.00236.x. [CrossRef]
- Kaya, H.K. Soil Ecology. In: Gaugler, R. and Kaya, H.K. (Eds.). In Entomopathogenic nematodes in biological control; CRC Press: Boca Raton, FL, 1990.
- Kaur, S. Molecular Approaches towards Development of Novel Bacillus Thuringiensis Biopesticides. World Journal of Microbiology and Biotechnology 2000, 16, 781–793. https://doi.org/10.1023/A:1008931207374. [CrossRef]
- Lacey, L.A; Frutos, R.; Kaya, H.K.; Vail, P. Insect Pathogens as Biological Control Agents: Do They Have a Future? Biological Control 2001, 21, 230–248. [CrossRef]
- Stathers, T.E.; Rees, D.; Kabi, S.; Mbilinyi, L.; Smit, N.; Kiozya, H.; Jeremiah, S.; Nyango, A.; Jeffries, D. Sweetpotato Infestation by Cylas Spp. in East Africa: I. Cultivar Differences in Field Infestation and the Role of Plant Factors. International Journal of Pest Management 2003, 49, 131–140. https://doi.org/10.1080/0967087021000043085. [CrossRef]
- Skoglund, L.G.; Smit, N.E. Major Diseases and Pests of Sweetpotato in Eastern Africa; International Potato Center (CIP): Peru, 1994; p. 67;
- Kreuze, K.F; Valkonen, J.P.T.; Ghislain, M. Genetic Engineering In: Loebenstein, G. and Thottappilly, G. (Eds.). In The Sweetpotato; Springer-Verlag New York Inc.: New York, 2009.
- Magira, P. Evaluation of Sweetpotato Clones from International Potato Center (CIP) for Resistance to the Sweetpotato Weevils, Cylas Puncticollis and C. Brunneus (Coleoptera: Curculionidae). MSc. Dissertation, Makerere University, 2003.
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of Phenolic Acids: Metabolites versus Parent Compounds: A Review. Food Chemistry 2015, 173, 501–513. https://doi.org/10.1016/j.foodchem.2014.10.057. [CrossRef]
- Lin, K.H; Lai, Y.C; Chang, K.Y; Chen, Y. F; Hwang, S.Y.; Lo, H.F Improving Breeding Efficiency for Quality and Yield of Sweetpotato. Bot. Stud. 2007, 283–292.
- Cervantes-Flores, J.C.; Sosinski, B.; Pecota, K.V.; Mwanga, R.O.M.; Catignani, G.L.; Truong, V.D.; Watkins, R.H.; Ulmer, M.R.; Yencho, G.C. Identification of Quantitative Trait Loci for Dry-Matter, Starch, and β-Carotene Content in Sweetpotato. Mol Breeding 2011, 28, 201–216. https://doi.org/10.1007/s11032-010-9474-5. [CrossRef]
- Grüneberg, W.J.; Mwanga, R.O.M.; Andrade, M.; Dapaah, H. Sweetpotato Breeding. In In: Andrade M, Barker I, Cole D, Dapaah H, Elliott H, Fuentes S, Gruneberg WJ, Kapinga R, Kroschel J, Labarta R, Lemaga B, Loechl C, Low J, Lynam J, Mwanga R, Ortiz O, Oswald A, Thiele G (eds). Unleashing the potential of sweetpotato in sub-Saharan Africa: current challenges and way forward Nairobi, CIP SSA; 2009.
- Mwanga, R.; Odongo, B.; Niringiye, C.; Kapinga, R.; Tumwegamire, S.; Abidin, P.; Carey, E.; Lemaga, B.; Nsumba, J.; Zhang, D. Sweetpotato Selection Releases: Lessons Learnt from Uganda. African Crop Science Journal 2010, 15. https://doi.org/10.4314/acsj.v15i1.54406. [CrossRef]
- Osaru, F.; Karungi, J.; Odama, R.; Chelangat, D.M.; Musana, P.; Otema, M.A.; Oloka, B.; Gibson, P.; Edema, R.; Ssali, R.T.; et al. Identification of the Key Morphological Sweetpotato Weevil Resistance Predictors in Ugandan Sweetpotato Genotypes Using Correlation and Path-coefficient Analysis. Crop Science 2023, csc2.20915. https://doi.org/10.1002/csc2.20915. [CrossRef]
- Oloka B.M. Genetic Linkage Map Construction and QTL Analysis of Important Pest and Agronomic Traits in Two Bi-Parental Sweetpotato SNP Mapping Populations. PhD Thesis, North Carolina State University: USA, 2019.
- Mugisa, I.; Karungi, J.; Musana, P.; Odama, R.; Alajo, A.; Chelangat, D.M.; Anyanga, M.O.; Oloka, B.M.; Gonçalves dos Santos, I.; Talwana, H.; et al. Combining Ability and Heritability Analysis of Sweetpotato Weevil Resistance, Root Yield, and Dry Matter Content in Sweetpotato. Front. Plant Sci. 2022, 13, 956936. https://doi.org/10.3389/fpls.2022.956936. [CrossRef]
- Gaffney, J.; Schussler, J.; Löffler, C.; Cai, W.; Paszkiewicz, S.; Messina, C.; Groeteke, J.; Keaschall, J.; Cooper, M. Industry-Scale Evaluation of Maize Hybrids Selected for Increased Yield in Drought-Stress Conditions of the US Corn Belt. Crop Science 2015, 55, 1608–1618. https://doi.org/10.2135/cropsci2014.09.0654. [CrossRef]
- Larkin; Lozada; Mason Genomic Selection—Considerations for Successful Implementation in Wheat Breeding Programs. Agronomy 2019, 9, 479. https://doi.org/10.3390/agronomy9090479. [CrossRef]
- Chang, K; Lo, H; Lai, Y; Yao, P; Lin, K; Hwang, S Identification of Quantitative Trait Loci Associated with Yield-Related Traits in Sweetpotato (Ipomoea Batatas). Bot. Stud. 2009, 43–50.
- Yada, B Genetic Analysis of Agronomic Traits and Resistance to Sweetpotato Weevil and Sweet Potato Virus Disease in a Bi-Parental Sweetpotato Population. PhD Thesis, North Carolina State University (NCSU): USA, 2014.
- Mwanga, R.O.M; Ghislain, M; Kreuze, J; Ssemakula, G. N; Yencho, C Exploiting the Use of Biotechnology in Sweet Potato for Improved Nutrition and Food Security: Progress and Future Outlook. In AgroBiotechnology, Biosafety and Seed Systems in Developing Countries; 2011; pp. 25–31.
- Cervantes-Flores JC; Yencho GC; Kriegner A; Pecota KV; Faulk MA; Mwanga ROM; Sosinski B Development of a Genetic Linkage Map and Identification of Homologous Linkage Groups in Sweetpotato Using Multiple-Dose AFLP Markers. Mol Breeding 2008, 511–532. [CrossRef]
- Collard, B.C.Y.; Mackill, D.J. Marker-Assisted Selection: An Approach for Precision Plant Breeding in the Twenty-First Century. Phil. Trans. R. Soc. B 2008, 363, 557–572. https://doi.org/10.1098/rstb.2007.2170. [CrossRef]
- Buteler, M.I.; Jarret, R.L.; LaBonte, D.R. Sequence Characterization of Microsatellites in Diploid and Polyploid Ipomoea: Theor Appl Genet 1999, 99, 123–132. https://doi.org/10.1007/s001220051216. [CrossRef]
- Hu, J.; Nakatani, M.; Mizuno, K.; Fujimura, T. Development and Characterization of Microsatellite Markers in Sweetpotato. Breed. Sci. 2004, 54, 177–188. https://doi.org/10.1270/jsbbs.54.177. [CrossRef]
- Zhang, D.P; Cervantes, J; Huamán, Z; Ghislain, M Assessing Genetic Diversity of Sweetpotato (Ipomoea Batatas (L) Lam) Varieties from Tropical America Using AFLP. Genet Resour Crop Evol 2000, 659–665.
- Nakayama, H.; Tanaka, M.; Takahata, Y.; Matsui, K.; Iwahori, H.; Sano, Z.; Yoshinaga, M. Development of AFLP-Derived SCAR Markers Associated with Resistance to Two Races of Southern Root-Knot Nematode in Sweetpotato. Euphytica 2012, 188, 175–185. https://doi.org/10.1007/s10681-012-0678-0. [CrossRef]
- Kriegner, A.; Cervantes, J.C.; Burg, K.; Mwanga, R.O.M.; Zhang, D. A Genetic Linkage Map of Sweetpotato [Ipomoea Batatas (L.) Lam.] Based on AFLP Markers. Molecular Breeding 2003, 11, 169–185. https://doi.org/10.1023/A:1022870917230. [CrossRef]
- Oloka, B.M.; Da Silva Pereira, G.; Amankwaah, V.A.; Mollinari, M.; Pecota, K.V.; Yada, B.; Olukolu, B.A.; Zeng, Z.-B.; Craig Yencho, G. Discovery of a Major QTL for Root-Knot Nematode (Meloidogyne Incognita) Resistance in Cultivated Sweetpotato (Ipomoea Batatas). Theor Appl Genet 2021, 134, 1945–1955. https://doi.org/10.1007/s00122-021-03797-z. [CrossRef]
- Yada, B.; Brown-Guedira, G.; Alajo, A.; Ssemakula, G.N.; Mwanga, R.O.M.; Yencho, G.C. Simple Sequence Repeat Marker Analysis of Genetic Diversity among Progeny of a Biparental Mapping Population of Sweetpotato. horts 2015, 50, 1143–1147. https://doi.org/10.21273/HORTSCI.50.8.1143. [CrossRef]
- Gemenet, D.C.; Lindqvist-Kreuze, H.; De Boeck, B.; da Silva Pereira, G.; Mollinari, M.; Zeng, Z.B.; Craig Yencho, G.; Campos, H. Sequencing Depth and Genotype Quality: Accuracy and Breeding Operation Considerations for Genomic Selection Applications in Autopolyploid Crops. Theoretical and Applied Genetics 2020, 133, 3345–3363. https://doi.org/10.1007/S00122-020-03673-2/FIGURES/7. [CrossRef]
- Mahadevaiah, C.; Appunu, C.; Aitken, K.; Suresha, G.S.; Vignesh, P.; Mahadeva Swamy, H.K.; Valarmathi, R.; Hemaprabha, G.; Alagarasan, G.; Ram, B. Genomic Selection in Sugarcane: Current Status and Future Prospects. Frontiers in Plant Science 2021, 12, 708233. https://doi.org/10.3389/FPLS.2021.708233/BIBTEX. [CrossRef]
- Gezan, S.A.; Osorio, L.F.; Verma, S.; Whitaker, V.M. An Experimental Validation of Genomic Selection in Octoploid Strawberry. Horticulture Research 2017, 4. https://doi.org/10.1038/HORTRES.2016.70/42567762/41438_2017_ARTICLE_BFHORTRES201670.PDF. [CrossRef]
- Badji, A.; Machida, L.; Kwemoi, D.B.; Kumi, F.; Okii, D.; Mwila, N.; Agbahoungba, S.; Ibanda, A.; Bararyenya, A.; Nghituwamhata, S.N.; et al. Factors Influencing Genomic Prediction Accuracies of Tropical Maize Resistance to Fall Armyworm and Weevils. Plants 2021, Vol. 10, Page 29 2020, 10, 29. https://doi.org/10.3390/PLANTS10010029. [CrossRef]
- Wu, S.; Lau, K.H.; Cao, Q.; Hamilton, J.P.; Sun, H.; Zhou, C.; Eserman, L.; Gemenet, D.C.; Olukolu, B.A.; Wang, H.; et al. Genome Sequences of Two Diploid Wild Relatives of Cultivated Sweetpotato Reveal Targets for Genetic Improvement. Nat Commun 2018, 9, 4580. https://doi.org/10.1038/s41467-018-06983-8. [CrossRef]
- Komaki K; Regima HN; Katayama K; Tamiya S Morphological and RAPD Pattern Variations in Sweetpotato and Its Closely Related Species. Breeding Sci 1998, 281–286. [CrossRef]
- Huang, J.C.; Sun, M. Genetic Diversity and Relationships of Sweetpotato and Its Wild Relatives in Ipomoea Series Batatas (Convolvulaceae) as Revealed by Inter-Simple Sequence Repeat (ISSR) and Restriction Analysis of Chloroplast DNA: Theor Appl Genet 2000, 100, 1050–1060. https://doi.org/10.1007/s001220051386. [CrossRef]
- Srisuwan, S.; Sihachakr, D.; Siljak-Yakovlev, S. The Origin and Evolution of Sweet Potato (Ipomoea Batatas Lam.) and Its Wild Relatives through the Cytogenetic Approaches. Plant Science 2006, 171, 424–433. https://doi.org/10.1016/j.plantsci.2006.05.007. [CrossRef]
- Roullier, C.; Duputié, A.; Wennekes, P.; Benoit, L.; Fernández Bringas, V.M.; Rossel, G.; Tay, D.; McKey, D.; Lebot, V. Disentangling the Origins of Cultivated Sweet Potato (Ipomoea Batatas (L.) Lam.). PLoS ONE 2013, 8, e62707. https://doi.org/10.1371/journal.pone.0062707. [CrossRef]
- Yang, J.; Moeinzadeh, M.-H.; Kuhl, H.; Helmuth, J.; Xiao, P.; Haas, S.; Liu, G.; Zheng, J.; Sun, Z.; Fan, W.; et al. Haplotype-Resolved Sweet Potato Genome Traces Back Its Hexaploidization History. Nature Plants 2017, 3, 696–703. https://doi.org/10.1038/s41477-017-0002-z. [CrossRef]
- Rosyara, U.R.; De Jong, W.S.; Douches, D.S.; Endelman, J.B. Software for Genome-Wide Association Studies in Autopolyploids and Its Application to Potato. The Plant Genome 2016, 9. https://doi.org/10.3835/plantgenome2015.08.0073. [CrossRef]
- Pham Bich Ngoc; Vu Thi Lan; Tran Thu Trang; Nguyen Hoai Thuong; Le Thu Ngoc; Chu Hoang Ha; Le Tran Binh Agrobacterium-Mediated Transformation of Cry8db Gene in Vietnam Sweet Potato Cultivar. JLS 2015, 10. https://doi.org/10.17265/1934-7391/2015.06.002. [CrossRef]
- Sefasi, A; Ssemakula, G; Ghislain, M; Prentice, K; Kiggundu, A; Mwanga, R.O.M Transient Expression of β -Glucoronidase in Recalcitrant Ugandan Sweetpotato and Putative Transformation with Two Cry Genes. Afr. Crop Sci. J. 2014, 215–227.
- Ekobu, M.; Solera, M.; Kyamanywa, S.; Mwanga, R.O.M.; Odongo, B.; Ghislain, M.; Moar, W.J. Toxicity of Seven Bacillus Thuringiensis Cry Proteins Against Cylas Puncticollis and Cylas Brunneus (Coleoptera: Brentidae) Using a Novel Artificial Diet. ec 2010, 103, 1493–1502. https://doi.org/10.1603/EC09432. [CrossRef]
- Anyanga, M.O.; Farman, D.I.; Ssemakula, G.N.; Mwanga, R.O.M.; Stevenson, P.C. Effects of Hydroxycinnamic Acid Esters on Sweetpotato Weevil Feeding and Oviposition and Interactions with Bacillus Thuringiensis Proteins. J Pest Sci 2021, 94, 783–794. https://doi.org/10.1007/s10340-020-01297-5. [CrossRef]
- Prentice, K.; Pertry, I.; Christiaens, O.; Bauters, L.; Bailey, A.; Niblett, C.; Ghislain, M.; Gheysen, G.; Smagghe, G. Transcriptome Analysis and Systemic RNAi Response in the African Sweetpotato Weevil (Cylas Puncticollis, Coleoptera, Brentidae). PLoS ONE 2015, 10, e0115336. https://doi.org/10.1371/journal.pone.0115336. [CrossRef]
- Christiaens, O.; Prentice, K.; Pertry, I.; Ghislain, M.; Bailey, A.; Niblett, C.; Gheysen, G.; Smagghe, G. RNA Interference: A Promising Biopesticide Strategy against the African Sweetpotato Weevil Cylas Brunneus. Sci Rep 2016, 6, 38836. https://doi.org/10.1038/srep38836. [CrossRef]
- Cobb, J.N.; DeClerck, G.; Greenberg, A.; Clark, R.; McCouch, S. Next-Generation Phenotyping: Requirements and Strategies for Enhancing Our Understanding of Genotype–Phenotype Relationships and Its Relevance to Crop Improvement. Theor Appl Genet 2013, 126, 867–887. https://doi.org/10.1007/s00122-013-2066-0. [CrossRef]
- Cabrera-Bosquet, L.; Crossa, J.; Von Zitzewitz, J.; Serret, M.D.; Luis Araus, J. High-Throughput Phenotyping and Genomic Selection: The Frontiers of Crop Breeding ConvergeF: High-Throughput Phenotyping and Genomic Selection. Journal of Integrative Plant Biology 2012, 54, 312–320. https://doi.org/10.1111/j.1744-7909.2012.01116.x. [CrossRef]
- Armengaud, P.; Zambaux, K.; Hills, A.; Sulpice, R.; Pattison, R.J.; Blatt, M.R.; Amtmann, A. EZ-Rhizo: Integrated Software for the Fast and Accurate Measurement of Root System Architecture. The Plant Journal 2009, 57, 945–956. https://doi.org/10.1111/j.1365-313X.2008.03739.x. [CrossRef]
- Backhaus, A.; Kuwabara, A.; Bauch, M.; Monk, N.; Sanguinetti, G.; Fleming, A. leafprocessor : A New Leaf Phenotyping Tool Using Contour Bending Energy and Shape Cluster Analysis. New Phytologist 2010, 187, 251–261. https://doi.org/10.1111/j.1469-8137.2010.03266.x. [CrossRef]
- Clark, R.T.; MacCurdy, R.B.; Jung, J.K.; Shaff, J.E.; McCouch, S.R.; Aneshansley, D.J.; Kochian, L.V. Three-Dimensional Root Phenotyping with a Novel Imaging and Software Platform. Plant Physiology 2011, 156, 455–465. https://doi.org/10.1104/pp.110.169102. [CrossRef]
- Yada, B.; Tukamuhabwa, P.; Alajo, A.; Mwanga, R.O.M. Field Evaluation of Ugandan Sweetpotato Germplasm for Yield, Dry Matter and Disease Resistance. South African Journal of Plant and Soil 2011, 28, 142–146. https://doi.org/10.1080/02571862.2011.10640026. [CrossRef]
- Allison, G.G.; Thain, S.C.; Morris, P.; Morris, C.; Hawkins, S.; Hauck, B.; Barraclough, T.; Yates, N.; Shield, I.; Bridgwater, A.V.; et al. Quantification of Hydroxycinnamic Acids and Lignin in Perennial Forage and Energy Grasses by Fourier-Transform Infrared Spectroscopy and Partial Least Squares Regression. Bioresource Technology 2009, 100, 1252–1261. https://doi.org/10.1016/j.biortech.2008.07.043. [CrossRef]
- Yencho, G.C.; Pecota, K.V.; Schultheis, J.R.; VanEsbroeck, Z.-P.; Holmes, G.J.; Little, B.E.; Thornton, A.C.; Truong, V.-D. ‘Covington’ Sweetpotato. horts 2008, 43, 1911–1914. https://doi.org/10.21273/HORTSCI.43.6.1911. [CrossRef]
- Grüneberg, W.J.; Ma, D.; Mwanga, R.O.M.; Carey, E.E.; Huamani, K.; Diaz, F.; Eyzaguirre, R.; Guaf, E.; Jusuf, M.; Karuniawan, A.; et al. Advances in Sweetpotato Breeding from 1992 to 2012. In Potato and sweetpotato in Africa: transforming the value chains for food and nutrition security; Low, J., Nyongesa, M., Quinn, S., Parker, M., Eds.; CABI: UK, 2015; pp. 3–68 ISBN 978-1-78064-420-2.
- Gurmu, F; Hussein, S; Laing, M Self-and Cross Incompatibilities in Sweetpotato and Their Implications on Breeding. Aust. J. Crop Sci 2013, 7, 2074–2078.
- Buteler, M.I; LaBonte, D.R.; Jarret, R.L.; Macchiavelli, R .E. Microsatellite-Based Paternity Analysis in Polyploidy Sweetpotato. Journal of the American Society for Horticultural Science 2002, 392–396.
- Jarret RL; Bowen N Simple Sequence Repeats (SSRs) for Sweetpotato Germplasm Characterization. Plant Genet Res Newslett 1994, 9–11.
- Grüneberg WJ, Eyzaguirre R, Espinoza J, Mwanga ROM, Andrade M, Dapaah H, Tumwegamire S, Agili S, Ndingo-Chipungu FP, Attaluri S, Kapinga R, Nguyen T, Kaiyung X, Tjintokohadi K, Carey EE, Low J (2010) Procedures for the evaluation and analysis of sweetpotato trials. International Potato Center, Lima, Peru.
- Mwanga, R.O.M; Odongo, B.; Ocitti p’Obwoya, C.; Gibson, R.W.; Smit, N.E.J.M.; Carey. E.E. Release of five sweetpotato cultivars in Uganda. HortScience 2001, 36, 385-386, https://doi.org/10.21273/HORTSCI.36.2.385. [CrossRef]
- Mwanga, R.O.M; Odongo, B.; Turyamureeba, G.; Alajo, A.; Yencho, G.C.; Gibson, R.W.; Smit, N.E.J.M.; Carey, E.E. Release of six sweetpotato cultivars (‘NASPOT 1 to NASPOT 6’) in Uganda. HortScience 2003, 38, 475-476, https://doi.org/10.21273/HORTSCI.38.3.475. [CrossRef]
- Mwanga, R.O.M; Odongo, B.; Niringiye, C.; Alajo, A.; Abidin, P.E.; Kapinga, R.; Tumwegamire, S.; Lemaga, B.; Nsumba.; S; Carey, E.E. Release of two orange-fleshed sweetpotato cultivars, ‘SPK004’ (‘Kakamega’) and ‘Ejumula’ in Uganda. HortScience 2007, 42, 1728-1730. [CrossRef]
- Mwanga, R.O.M; Odongo, B.; Niringiye, C.N.; Alajo, A.; Kigozi, B.; Makumbi, R.; Lugwana, E.; Namakula, J.; Mpembe, I.; Kapinga, R.; Lemaga, B.; Nsumba, J.; Tumwegamire, S.; Yencho. C.G. ‘NASPOT 7’, ‘NASPOT 8’, ‘NASPOT 9 O’, ‘NASPOT 10 O’, and ‘Dimbuka-Bukulula’ Sweetpotato. HortScience 2009, 44, 828-832, https://doi.org/10.21273/HORTSCI.44.3.828. [CrossRef]
- Mwanga, R.O.M; Niringiye, C.; Alajo, A.; Kigozi, B.; Namakula, J.; Mpembe, I.; Tumwegamire, S.; Gibson, R.W.; Yencho, C.G. ‘NASPOT 11’, a sweetpotato cultivar bred by a participatory plant-breeding approach in Uganda. HortScience 2011, 46, 317-321. [CrossRef]
- Mwanga, R.O.M; Kyalo, G.; Ssemakula, G. N.; Niringiye, C.; Yada, B.; Otema, M. A.; Namakula, J.; Alajo, A.; Kigozi, B.; Makumbi, R.N.; Ball, A.M. ‘NASPOT 12 O’and ‘NASPOT 13 O’ Sweetpotato. HortScience 2016,.51, 291-295, https://doi.org/10.21273/HORTSCI.51.3.291. [CrossRef]
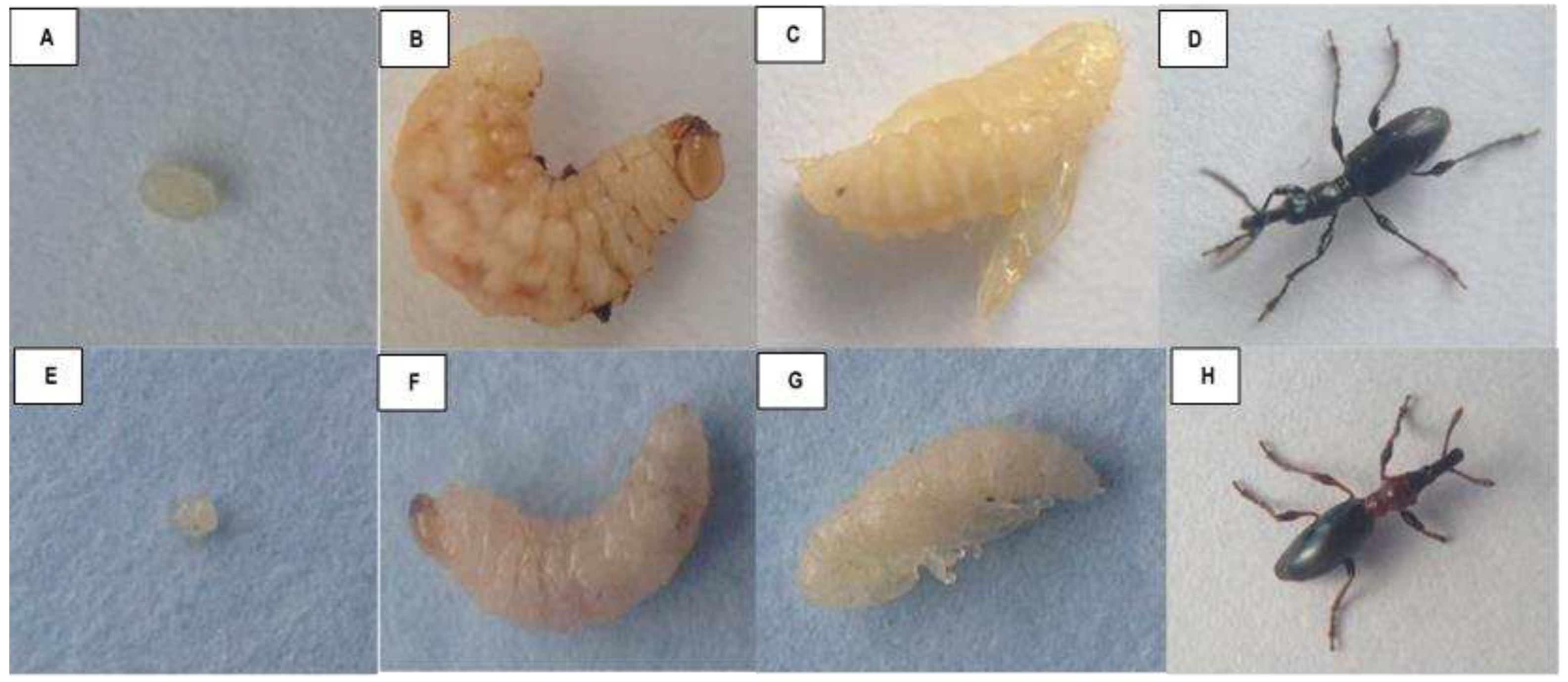

| Trait | Cylas puncticollis | Cylas brunneus |
|---|---|---|
| Morphology |
Eggs:0.45 x 0.30 mm in size Larvae:5–10 mm long Pupae:6–7 mm long; creamy-white cuticle Adults: 5–8 mm long; eyes narrowly separated |
0.7 X 0.5 mm in size 7–8 mm in length 4–5 mm long; white in colour 5–7 mm long; eyes widely separated |
| Oviposition | Females take 2–24 days before laying eggs | Females start laying eggs a day or so after becoming sexually active |
| Development | Takes place between 17.5°–35°C | Is possible between 17.5°–32°C |
| Coloration | Initially creamy white, but later change to gray and black | Turn from creamy white to brown and finally black, some are bi-colored (brown and black) |
| Host range | Has a wider host range including morning glory, cotton, sesame and maize | Has a smaller host range including morning glory and water spinach |
| Distribution | Reported in 24 African countries | Recorded in 9 African countries |
| Dispersion | Adults can fly for longer distances (up to 1000m) | Adults fly for short distances (up to 80m) |
|
Cultural practices Use of clean planting material, crop rotation [29], hilling up and mulching, removal of alternate crops and wild plants, field sanitation [27,30], use of barrier crops, intercropping, planting of new crop away from weevil-infested fields, timely or early planting and harvesting before onset of the dry season [24,31], and flooding [32] |
|
Chemical practices Insecticides as foliar sprays controls weevils to some extent [33,34]. They can reduce the populations of adult weevils, but may not adequately control immature larvae due to their cryptic nature or when they have already infested roots and vines [8]. Dipping plant material into a synthetic pesticide before planting, can delay pest infestation for several months [27]. Insecticides are expensive and at times inaccessible to growers in SSA making their use impractical and unsustainable[35] |
|
Biological Natural enemies such as ants, maggots and wasps attack weevils, but most of them seem to be ineffective at suppressing SPW populations under field conditions [25,36]. Entomopathogenic fungi such as Beauveria bassiana have successfully been used to control SPWs in combination with other control methods. They caused a reduction in feeding ability, fecundity and egg viability in C. puncticollis [37]. Entomopathogenic nematodes such as Steinernema carpocapsae and Heterorhabditis bacteriophora, have shown potential for practical biological suppression of Cylas spp but are not readily available and small-scale farmers may not have the required purchasing power [21,38]. Bacteria such as Bacillus thuringiensis Berliner (Bt), have been developed to confer inherent pest resistance against SPWs [39,40]. Bt sweetpotato events against SPWs have been tested in SSA [7]. |
|
Host Plant Resistance Involves the use of sweetpotato weevil resistant or tolerant clones [15,41]. Deep-rooting and early maturing varieties are less susceptible to infestation than shallow rooting and late maturing varieties [42].Efforts to develop SPW resistant cultivars are still ongoing in SSA Transgenic sweetpotato plants expressing the Bt cry genes are also currently in use in some countries [43]. |
| Year of release | Variety | Reaction to SPW |
| 1995 | “Bwanjule” | MR |
| “New Kawogo” | MR | |
| “Sowola” | MR | |
| “Tanzania” | S | |
| “Wagabolige” | MR | |
| “Tororo 3” | MR | |
| 1999 | NASPOT 1 | S |
| NASPOT 2 | S | |
| NASPOT 3 | MR | |
| NASPOT 4 | MR | |
| NASPOT 5 | MR | |
| NASPOT 6 | MR | |
| 2004 | “Ejumula” | S |
| SPK004 (Kakamega) | S | |
| 2007 | NASPOT 7 | S |
| NASPOT 8 | S | |
| NASPOT 9 O | S | |
| NASPOT 10 O | S | |
| “Dimbuka Bukulula” | S | |
| 2010 | NASPOT 11 | S |
| 2013 | NASPOT 12 O | S |
| NASPOT 13 O | S | |
| 2017 | NAROSPOT 1 | MR |
| NAROSPOT 2 | Low | |
| NAROSPOT 3 | Low | |
| NAROSPOT 4 | Low | |
| NAROSPOT 5 | Low | |
| 2023 | NAROSPOT 6 | MR |
| NAROSPOT 7 O | MR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





