1. Introduction
Diabetes mellitus is a cluster of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both. According to the results from the International Diabetes Federation Diabetes Atlas, 9th edition, the global diabetes prevalence in 2019 is estimated to be 9.3% (463 million people), rising to 10.2% (578 million) by 2030 and 10.9% (700 million) by 2045.
Due to the disturbance of antioxidants defense system in body of diabetes patients and mainly because of alteration in antioxidant enzymes, impaired glutathione metabolism, and decreased ascorbic acid levels hyperglycemic conditions can be occurred (Akpoveso 2023). Therefore, having more antioxidants is a potential remedy for long term complications of diabetes mellitus and other similar diseases.
Coccinia grandis (Ivy gourd) is a tropical vine that belongs to the Cucurbitaceae family and is commonly found in South Asian countries. Both fruits and leaves of the Coccinia grandis vine are considered edible and the whole plant is used in Ayurvedic medicine (Nautiyal1 et al., 2018). When considering the medicinal value of Coccinia grandis, it has a higher potent hypoglycemic effect. The leaves of Coccinia grandis showed strong antioxidant activity, reducing power ability, free radical scavenging activity, and metal chelating ability (Settu 2023, Umamaheswari and Chatterjee, 2008). It has been proved that Coccinia grandis leaves can stimulate insulin secretion by in vitro experiments of diabetic rats (Tamilselvan et al., 2011; Kondhare and Lade, 2017).
The leaves of Coccinia grandis contain phytochemicals such as alkaloids, flavonoids, glycosides, saponins, sterol, and tannins as its secondary metabolites (Alamgir, Rahman and Rahman, 2014). Flavonoids contribute to suppressing the glucose level, reducing plasma cholesterol and triglycerides significantly, and increasing hepatic glucokinase activity probably by enhancing the insulin release from pancreatic islets (Al-Ishaq et al., 2019; Patel et al., 2012). Some flavonoids inhibit the aldose reductase enzyme which is involved in diabetes mellitus (Kondhare and Lade, 2017; Shukla, 2019). Saponins contribute to stimulating the release of insulin and block the formation of glucose in the bloodstream and ferulic acid stimulates insulin secretion. 1-tetrabutyl-1-5,6,7 trimethoxyiaisoquinolene is an alkaloid present in Coccinia grandis leaves. It is the most active compound in the RLAR (rat lens aldose reductase) and HRAR (human recombinant aldose reductase) (Choudhury et al., 2013; Rajbongshi Lata, 2017). The terpenoids present in Coccinia grandis leaves also contribute to antidiabetic, anti-inflammatory, antyhypernociceptive properties.
However, the quantity and the effectiveness of medicinal value of bioactive compounds and nutrients present in Coccinia grandis leaves can be altered with the processing conditions for consumption and due to the degradation of different gastrointestinal tract enzymes. To overcome these issues and to preserve bioactive compounds from environmental stresses, improve their physicochemical functionalities, and health-promoting and ant-disease activities nanoencapsulation technology can be used (Shishir et al., 2018).
Hence, this study is mainly focused on developing an herbal porridge cube incorporated with nano-encapsulated Coccinia grandis (Aka ivy gourd) leaf extract which will be one of the best choice for diabetic patients as a healthy snack in order to enhance the consumer reachability of this valuable medicine in enhanced effectivity.
2. Materials and Methods
2.1. Development of herbal porridge cube
Development of herbal rice porridge is done with wildly grown Coccidia grandis (Aka ivy gourd) leaves
Cleaned fresh
Coccinia grandis (
Figure 1) leaves were dehydrated at 48°C for 30hrs and they were ground using a domestic grinder and very fine powder (0.42µm) was obtained. “Kalu heenati” (
Oryza sativa) traditional rice and Soybeans were ground separately using a domestic grinder and sieved through 0.42 µm strainer to obtain the fine particles.
Coccinia grandis leaf powder, rice flour, soybean flour, garlic powder, coconut milk and salt were mixed and a thick paste was cut into cubes and they were stored under frozen conditions. The different treatments used are described in
Table 1.
2.2. Evaluation of porridge for sensory appeal
A sensory evaluation was done for the developed porridge to find out the most preferred recipe for porridge cube. Different attributes such as color, flavor, viscosity, aroma, mouth feel, and overall acceptability were tested using a seven-point hedonic scale ranging from 1 (dislike extremely) to 7 (like extremely).
2.3. Evaluation of post-prandial blood glucose level variation
The post-prandial blood glucose level variation was tested using 15 paid volunteers with the approval of the ethics committee, Faculty of Livestock, Fisheries, and Nutrition, Wayamba University of Sri Lanka. Adult men and women who had a BMI between 18.5 and 22.9 and showed fasting blood sugar levels below 100mg/dL were selected and subjects were excluded who were suffering from any kind of non-communicable diseases, pregnant, lactating mothers, and females with menstruation. Subjects were asked to fast for 10-12 hrs. and their fasting blood sugar levels were recorded. They were asked to consume porridge which was prepared by dissolving 1 instant herbal porridge cube (28g) in 200mL of hot water within 2-3 minutes and their blood glucose levels were measured at 15-minute intervals for 1st hour and 30-minute intervals and 2nd hour were tested using MEGA CHECK TD-4257 Blood glucose monitoring system.
2.4. Determination of Total Phenolic Content
In order to analysis of different properties of the developed porridge cube, an ethanolic extract of the porridge cube was taken. The total phenolic content was determined by applying the method of Ranilla et al. (2010) with slight modifications. An aliquot of porridge extract (1 ml) was transferred to a test tube and mixed with 96% ethanol (4 ml) to dilute it. Then, 1 mL of an aliquot from the diluted sample was transferred to a Gerber tube and mixed with 96% ethanol (1 ml) and distilled water (5 ml). Folin–Ciocalteu reagent (50% v/v; 0.5 ml) was then added to each sample followed by thorough mixing with a vortex. After keeping for 5 min at room temperature, 5% (w/v) sodium carbonate (1 ml) was added. The solution was mixed and incubated for 60 minutes at room temperature. The absorbance of the mixture was measured at 725 nm using a UV–visible spectrophotometer (Biochrom-Libra S22, UK). The total phenolic content was expressed as mg gallic acid equivalent per 1 g of dried sample (mg GAE/g).
2.5. Antioxidant activity assay by 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical inhibition assay
The antioxidant activity was analyzed by the method of (Ranilla et al. 2010) with some modifications. An aliquot (1 mL) of the porridge extract was transferred into a test tube, and then 60 lM DPPH (1.25 ml) in a 95% ethanol solution was added. The mixture was thoroughly vortexed and kept in the dark for 30 min. Absorbance at 517 nm was measured using a UV–visible spectrophotometer (Biochrom-Libra S22, UK). The absorbance reading was compared to the control, which contained distilled water instead of the extract.
2.6. Nanoencapsulation of Coccinia grandis extract
Ethanolic extract of Coccinia grandis leaves was taken according to the method described in (Zhang et al., 2016) with slight modifications. First 2% (w/v) aqueous alginate solution was prepared stirring at 60°C for 1 hour. It was allowed to cool and it was mixed with concentrated ethanolic Coccinia grandis extract (1:1) and the solution was stirred continuously for 1 hour. Then the pH of the solution was adjusted to 6 and it was injected into 10% Calcium chloride solution while stirring at ambient temperature using a 27G clinical syringe. The solution mixture with beads was centrifuged at 4000rpm for 10 minutes and the beads were safely separated and freeze-dried. Then the porridge cubes were prepared replacing the same amount of nano-encapsulated material instead of dehydrated Coccina grandis leaf powder.
2.7. In vitro digestion assay
Nano-encapsulated Coccinia grandis extract was mixed with other ingredients according to the same formula and an encapsulated Coccinia grandis porridge cube was prepared. Invitro digestion assay for both nano-encapsulated porridge and non-encapsulated porridge was done according to the method described in (Mel et al, 2020). 10g of both porridges were dissolved in 50 mL of hot water and it was homogenized for the analysis.
2.7.1. Gastric digestion
For the gastric digestion, 50 mL of the homogenized samples were taken into a volumetric flask, and simulated gastric fluid containing 50 mL NaCl (0.9%), 8 mL HCL (0.1 M), 4 mL pepsin solution (40 mg/mL) was added. The pH of each sample was adjusted to 2.0 by the addition of HCL. Then the mixture was incubated for 1 h in a shaking water bath at 37 °C and 100 rpm. Before and after gastric digestion, pH was verified (target pH 2–2.5). After gastric digestion, reactions were terminated by cooling the test tubes in ice. All aliquots taken for further testing were, filtered through a Whatman no 41 filter paper and stored at −18 °C for further analysis. As described by (Peter C, 2010) for intestinal digestion, the pH was then increased to 5.3 with 0.9 M sodium bicarbonate followed by the addition of 200 μL of bile salts glycodeoxycholate (0.04 g in 1 mL saline), taurodeoxycholate (0.025 g in 1 mL saline), taurocholate (0.04 g in 1 mL saline) and 100 μL of pancreatin (0.04 g in 500 μL saline). The pH of each sample was increased to 7.4 with 1 M NaOH. Samples were incubated in a shaking water bath (95 rpm) at 37 °C for 2 h to complete the intestinal phase of the in vitro digestion process. After the intestinal phase, 500 μL of each sample was extracted and stored at −18°C for further analysis.
2.7.2. α-amylase inhibitory assay and α-Glucosidase Inhibition assay
The α-amylase inhibitory assay and α-Glucosidase Inhibition assay were done for both porridge samples. The a-amylase inhibitory activity was determined by (JS, CS, and KH, 2000; P et al., 2011; Kim, Rioux and Turgeon, 2014), with some modifications. 500 µL of each sample was mixed with 500 µL of 0.02 M sodium phosphate buffer (pH 6.9 with 0.006 M NaCl) containing a-amylase solution (0.5 mg/ml). After incubation at 25 °C in a water bath for 10 min, 500 µL of 1% (w/v) starch solution in 0.02 M sodium phosphate buffer (pH 6.9 with 0.006 M NaCl) was added, and the mixture was re-incubated at 25 °C in a water bath for 10 minutes. The reaction was terminated with 1.0 ml of dinitrosalicyclic acid (DNSA) colour reagent. The mixture w for 7 minutes. Subsequently, 1.0 ml of 18.2% tartrate solution was added to each tube after boiling, and before cooling to room temperature. The mixture was diluted with 10 mL of distilled water. Absorbance was measured at 540 nm with a UV–visible spectrophotometer (Biochrom-Libra S22, UK). The absorbance reading was compared to the control, which contained 500 µL of buffer solution, instead of the extract.
The inhibition of a-glucosidase was determined by use of the (JS, CS and KH, 2000; P et al., 2011; Kim, Rioux and Turgeon, 2014), with some modification. 500 µL of each sample were mixed with 1 mL of a 0.1 M potassium phosphate buffer (pH 6.9) containing a-glucosidase solution (1 U/mL). After 10 minutes of incubation at 25°C in a water bath, 500 µL of a 5 mM p-nitro phenyl-a-D-glucopyranoside solution in 0.1 M potassium phosphate buffer (pH 6.9) was added, and the mixture was then re incubated at 25 °C in a water bath for 5 min. The absorbance was measured at 405 nm using a UV–visible spectrophotometer (Biochrom-Libra S22, UK), before and after the incubation period. The absorbance reading was compared to the control, which had 500 µL of buffer solution instead of the extract.
2.10. Fourier-transform infrared spectroscopy (FTIR) Analysis
The objective of performing the FTIR test is to detect either the appearance of new chemical bonds or the modification of existing bonds, which can be attributed to possible interactions between sodium alginate and the Coccinia grandis extract. For that, the nano-encapsulated Coccinia grandis leaf extract samples were sent to the Sri Lanka Institute of Nanotechnology (SLINTEC) to analyze the FTIR spectrum using Bruker Vertex80 FT-IR Spectrometer.
2.11. Statistical analysis
All the data ware analyzed using SPSS 16.0 software. Sensory evaluation data were analysed using Friedman ranking test.
3. Results
3.1. Sensorial acceptance for the porridge cube
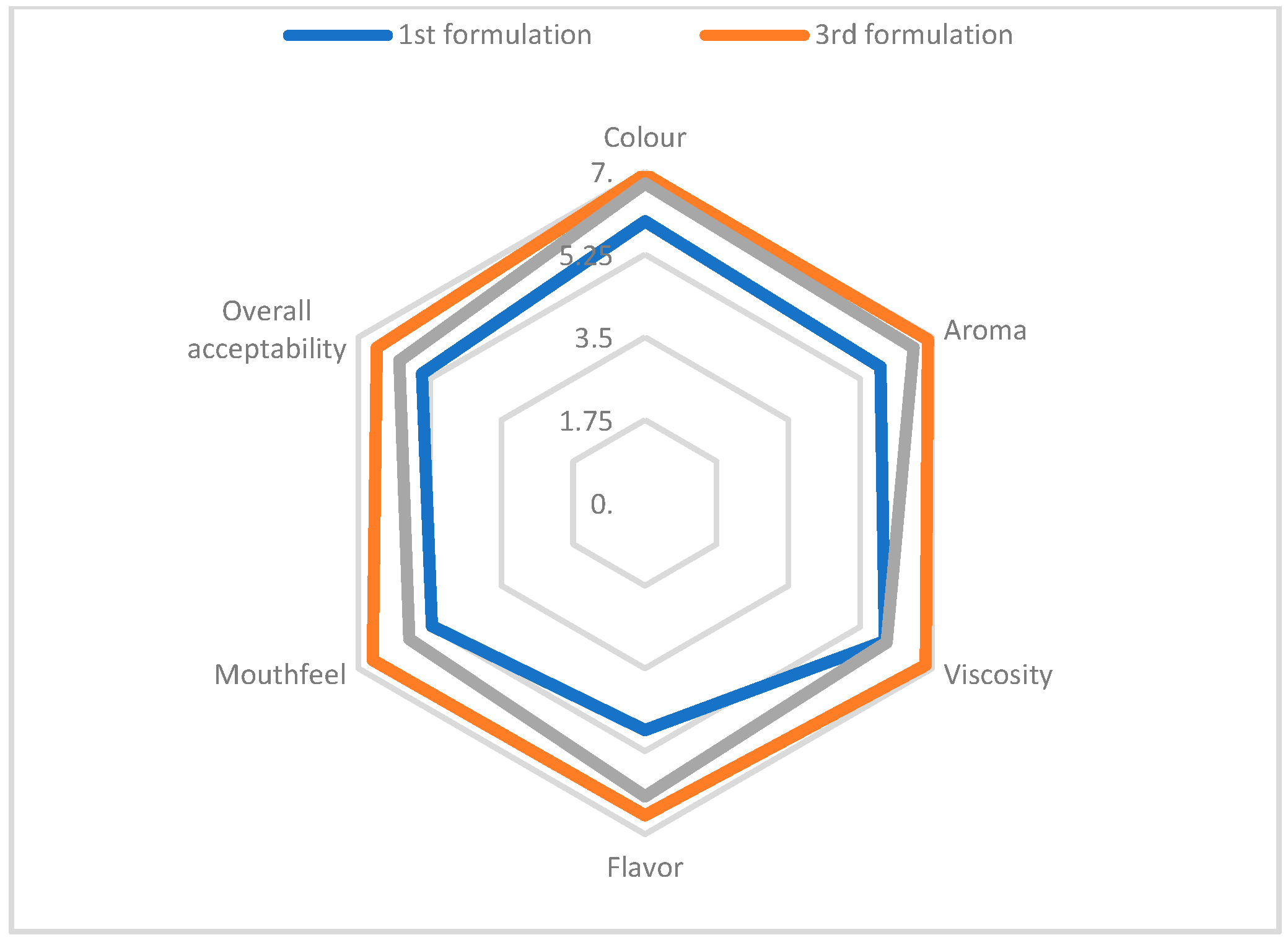
The mean score values for each parameter tested for the three formulations of the porridge cube are presented in
Table 2. The better understanding can be obtained from
Figure 2 and results showed that 3
rd formulation is the best for the porridge cube.
3.2. Evaluation of post-prandial blood glucose level variation
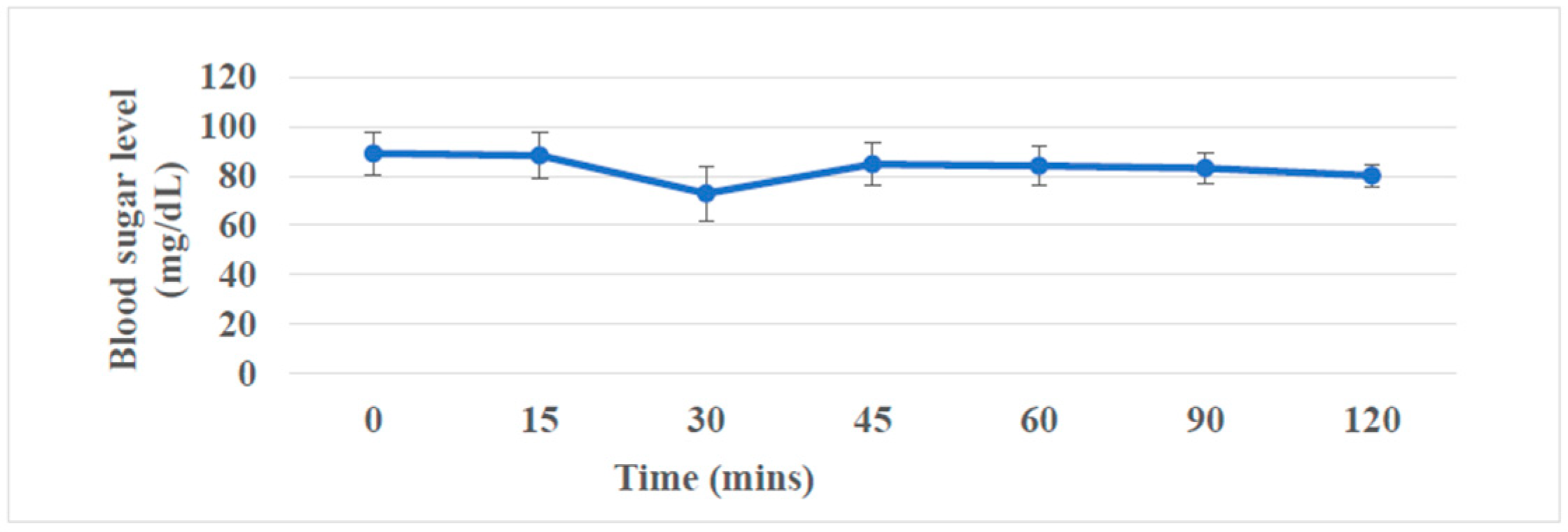
Postprandial blood sugar level variation after consuming a newly prepared instant herbal porridge cube is shown in
Figure 3. It shows a suppression in blood glucose levels compared with fasting blood glucose levels after 30 minutes of ingestion and gradually increases to some extent within two hours. The results imply that the newly developed instant herbal porridge cube is a good option for those who are looking for a snack without blood sugar elevation.
3.3. Total Phenolic Content of porridge cube extract
Ethanol extract of porridge cue is done for selected formula and showed the Coccinia grandis leaf powder incorporated herbal porridge cube contained a total phenolic content of 66.76±0.74 (mg/g GAE).
3.4. Antioxidant activity of porridge cube extract
The 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical inhibition assay and showed 27.65±6.79% DPPH free radical scavenging inhibition activity of the porridge cube extract.
3.5. In-vitro digestion
Table 3 shows the impact of gastrointestinal digestion on the antidiabetic activity of the encapsulated instant porridge cube samples and nonencapsulated instant porridge cube samples under each digestive phase. Alpha-amylase inhibition activity for the gastric phase and the intestinal phase and alpha-glucosidase inhibition activity for the intestinal phase were significantly higher in the nano-encapsulated porridge. (p<0.05)
3.6. Results of the FTIR
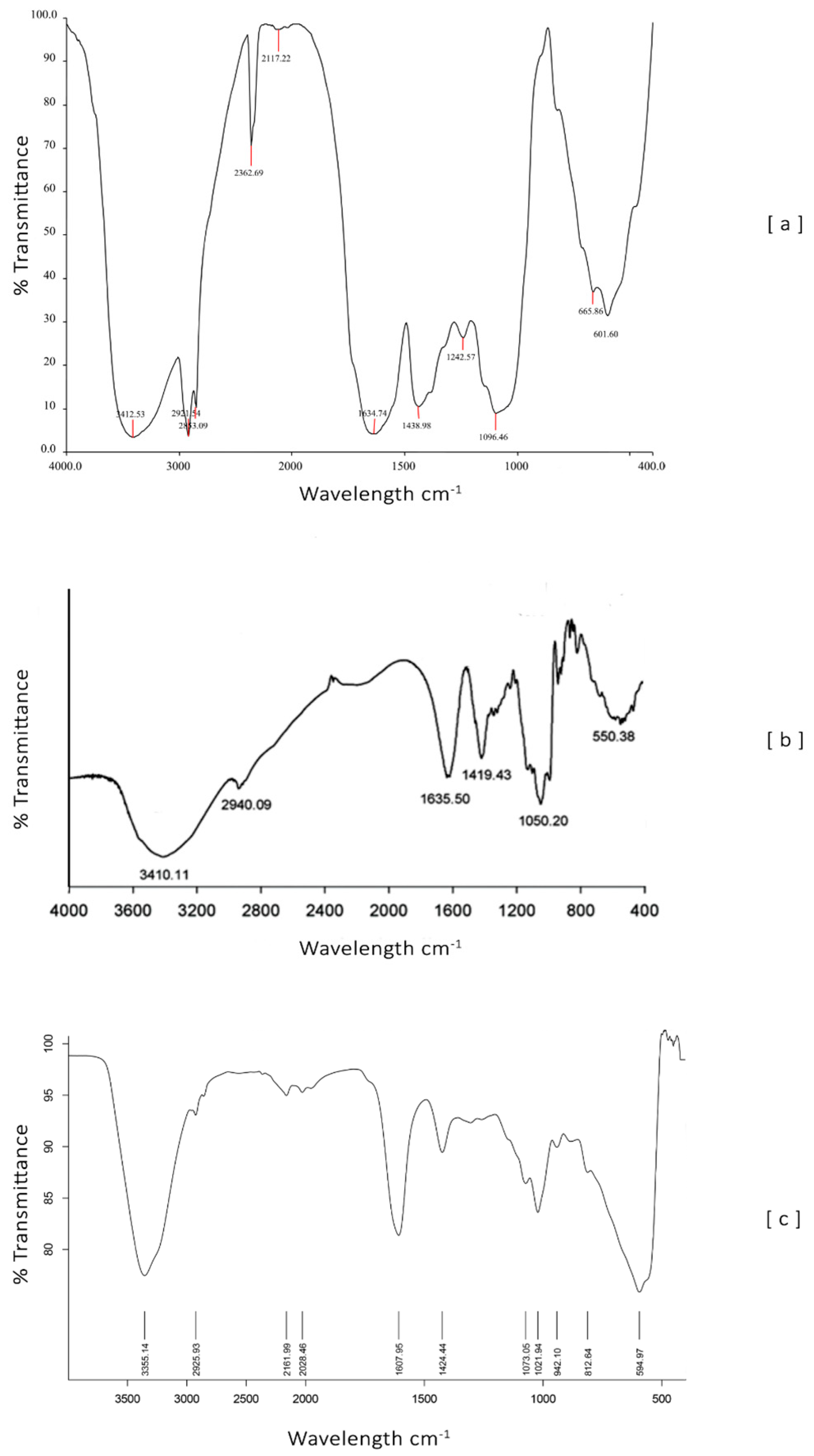
According to the FTIR spectrum of nano-encapsulated
Coccinia grandis extract with sodium alginate particles (
Figure 4), there is a characteristic peak at 3555.14/cm. This may be due to the presence of amines, alkanes, and alcohol. Peaks at approximately 2952/cm indicate the presence of alkanes, and the peaks with very low intensity at 2961.99 and 2028.46/cm are almost similar to the peaks of FTIR of leaf extract (Isothiocyanates stretch, Conjugated alkenes stretch). The sharp peaks at 1607.95 and 1424.44/cm are similar to the peaks of sodium alginates. Other peaks at 1073.05, 1021.94, 942.10, 812.64, and 594.97/cm are almost similar to the FTIR peaks of leaf extract. These results were confirmed with the characterization of nanoparticle components, which indicated that the
Coccinia grandis extract was loaded in the nanoparticles.
4. Discussion
Sensory analysis is carried out by using experienced panelists to measure sensory characteristics such as senses of sight, smell, taste, touch, and acceptability of food products. The sensory rating of porridge for color shows that the 3rd formulation (6.95) ranked at the top due to excellent appearance, followed by the 2nd formulation (6.75) while the minimum color was observed in the 3rd formulation (5.95). The mean score for flavor revealed that the 3rd formulation had the highest score (6.6) followed by the 2nd formulation (6.2), while the 1st formulation (4.8) had the lowest score. The mean score for the flavor of porridges had decreased from 6.6 to 4.8 with increasing levels of soybean flour, which could be due to the beany flavor of soybean. The mean score of viscosity decreased from 6.85 to 5.95 with an increasing level of addition of both soybean and Kaluheenati four. The highest mean score for the aroma was also shown by the 3rd formulation (6.9), followed by the 2nd (6.55), and the lowest score was recorded for the 1st formulation (5.75). The same style was followed by the mean score of mouth feel which also showed the highest mean score by the 3rd formulation (6.65) followed by the 2nd (5.75), and the lowest score was recorded for the 1st formulation (5.2). Overall acceptability was determined based on quality scores obtained from the evaluation of the color, flavor, mouthfeel, aroma, and viscosity of the porridge. According to the results, the most acceptable formulation was the 3rd formulation (6.55), followed by the 2nd formulation (6.0), and the 1st formulation showed the lowest acceptability (5.45). The decrease in overall acceptability was due to a decrease in color, flavor, aroma mouthfeel, and viscosity scores.
After consuming the developed instant herbal porridge cube, it shows a suppression in blood glucose levels compared with fasting blood glucose levels after 30 minutes of ingestion and gradually increases to some extent within two hours. It can be assumed that blood suppression of blood glucose levels was due to the antihyperglycemic effects contributed by the ingredients present in the instant herbal porridge cube and it claims that the newly developed instant herbal porridge cube is a good option for those who are looking for a snack without blood sugar elevation.
Polyphenolic compounds contain multiple hydroxyl substituents on an aromatic ring. Because of this specific structure, polyphenol compounds act as good electron and proton donors. They can scavenge free radicals and reduce oxidative stress by transferring H-atoms from their hydroxyl groups to free radicals (Namchaiw et al., 2021) The multiple hydroxyl groups that are attached to the aromatic ring manipulate the antioxidant activity as a free radical scavenging and metal ion chelating site, the greater they have hydroxyl groups, the greater they are antioxidant (Namchaiw et al., 2021). The current study showed the ethanolic extract of the developed porridge contained a polyphenolic content of 66.76±0.74 (mg/g GAE). Therefore, the presence of polyphenolic compounds in the developed product may contribute to its antioxidant activity and those polyphenolic compounds should be further investigated.
The DPPH radical scavenging assay is used to measure the electron-donating ability of the extract, and the scavenging of radicals is indicated by the extent of the decolorization of the DPPH solution. The addition of DPPH resulted in a purple discoloration in ethanol and sample solutions due to the presence of electron or hydrogen donors to become more stable molecules. Color fading reduced the absorbance value of light on the UV-Vis spectrophotometer. Thus, the lower the absorbance value is, the higher the antioxidant activity. The ethanolic extract of the developed product showed 27.65 ± 6.79% DPPH radical scavenging inhibition activity.
The results of the invitro digestion assay show how the nano-encapsulated material incorporated porridge performs against alpha-amylase and alpha-glucosidase enzymes compared to the non-nano-encapsulated porridge and how long it works as a safe delivery method for the bioactive compounds present in the developed products. Both α-amylase and α- glucosidase enzymes contribute to postprandial hyperglycemia. α-Amylase starts carbohydrate digestion in the human digestive system catalyzing the hydrolysis of the internal α-1,4-glycosidic linkages in starch, converting starch into low-molecular-weight products such as glucose, maltose, and maltotriose. α-Glucosidase catalyzes the conversion of disaccharides to monosaccharides (Telagari and Hullatti, 2015). α- Amylase inhibitors are also called starch blockers as they prevent or slow the absorption of starch into the body mainly by blocking the hydrolysis of 1,4-glycosidic linkages of starch and other oligosaccharides into maltose, maltriose, and other simple sugars. Alpha-Glucosidase catalyzes disaccharides into monosaccharides, which leads to postprandial hyperglycemia (Hossain et al., 2016). Alpha-amylase inhibition activity for the gastric phase and intestinal phase and alpha-glucosidase inhibition activity for the intestinal phase were significantly higher in the nanoencapsulated porridge (p<0.05). This may be due to the higher retention of bioactive compounds from adverse conditions in the digestive system with encapsulation. Because, inhibitors of α-amylase and α-glucosidase are present in the developed products, which are useful in the control of hyperglycemia, as they delay carbohydrate digestion, which consequently reduces the postprandial plasma glucose level.
The objective of performing the FTIR test is to detect either the appearance of new chemical bonds or the modification of existing bonds, which can be attributed to possible interactions between sodium alginate and the Coccinia grandis extract.
According to (Coates, 2006; Vinothkumar et al., 2019) the FTIR spectrum of Coccinia spp confirms the presence of amines, alkanes, and alcohol at a peak value of 3412.53/cm. The peaks at 2921.54 and 2362.69/cm represent the alkanes. The remaining peaks at 2117.22, 1634.74, 1438.98, 1242.57, 1096.46, 665.86, and 601.60/cm indicate the presence of Isothiocyanates stretch, conjugated alkenes stretch, bent carboxylic acids, Alcohols stretch, ether stretch, alkyl halides stretch and acid chlorides stretch as functional groups, respectively.
The FTIR spectrum of sodium alginate exhibits an absorption band at 3410/cm, which can be due to the hydroxyl group (–OH),1635/cm (asymmetric stretching vibration of COO groups),1419/cm (symmetric stretching vibration of COO groups), and 1050/cm (elongation of C-O groups) (Pereira et al., 2011).
5. Conclusions
As per the observation and the obtained results of this research, it can be concluded that the newly developed herbal porridge cube reveals the suitability of this novel product for usage by diabetic patients as a healthy snack. Since it is instant, it can be simply used as a quick breakfast alternative for diabetes patients. Furthermore, in order to enhance the bioavailability of the phytotherapeutics in this valuable herb, Coccinia grandis leaf extract was nano-encapsulated with sodium alginate. The in-vitro digestion analysis results show that the alpha-amylase activity inhibition activity for the gastric phase and alpha-glucosidase inhibition activity for the intestinal phase were significantly higher in the nano-encapsulated porridge (p<0.05). Therefore, consuming nano-encapsulated herbal porridges will be more beneficial for diabetic patients. However, the exact effective dosage of the porridge and suitable frequency of usage should be further investigated. Also, it needs to be further investigated the specified bioactive compounds present in Coccinia grandis leaves which contribute to the antidiabetic effect.
Author Contributions
From the initiation to the end each and every step is supervised and guided by Senior Prof. Chamila Jayasinghe, Professor of Food Science and Technology (Chair Professor), Department of Food Science and Technology, Faculty of Livestock, Fisheries and Nutrition, Wayamba University of Sri Lanka. Also, Senior Prof. Chamila planned the research project and, reviewed the research proposal and all the procedures, methods, data, and manuscript were reviewed. Research idea generation from the brain streaming session, research plan, proposal writing, performing all necessary laboratory tests and data collection, and manuscript writing were done by Vichakshi Sashenka Bamunusingha Witharana, Department of Food Science and Technology, Faculty of Livestock, Fisheries and Nutrition, Wayamba University of Sri Lanka.
Funding
This research was partially funded by the Innovation Commercialization Enhancement (ICE) Grant (2020), AHEAD, Department of Food Science and Technology, Faculty of Livestock, Fisheries and Nutrition, Wayamba University of Sri Lanka.
Acknowledgments
We acknowledge the partial funding made by the Innovation Commercialization Enhancement (ICE) Grant (2020), AHEAD, Wayamba University of Sri Lanka.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Alamgir, A.N.M.; Rahman, M.; Rahman, A. Phytochemical Characteristics, Antimitotic, Cytotoxic and Antiinflamatory Activities of Coccinia grandis (L.) J. Voigt. Journal of Pharmacognosy and Phytochemistry 2014, 3, 222–225. Available online: www.uni-ulm.de/.
- Bagri, P.; et al. Antidiabetic effect of Punica granatum flowers: Effect on hyperlipidemia, pancreatic cells lipid peroxidation and antioxidant enzymes in experimental diabetes. Food and Chemical Toxicology 2009, 47, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Chai, T.-T.; et al. Alpha-glucosidase Inhibitory and Antioxidant Potential of Antidiabetic Herb Alternanthera sessilis: Comparative Analyses of Leaf and Callus Solvent Fractions. Pharmacognosy Magazine 2016, 12, 253. [Google Scholar] [CrossRef] [PubMed]
- CHOUDHURY, S.N.; et al. No Title. Asian Journal of Chemistry 2013, 25. Available online: http://www.asianjournalofchemistry.co.in/User/ViewFreeArticle.aspx?ArticleID=25_18_32.
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. Encyclopedia of Analytical Chemistry 2006, 1–23. [Google Scholar] [CrossRef]
- Dewanjee, S.; et al. Antidiabetic activity of Diospyros peregrina fruit: Effect on hyperglycemia, hyperlipidemia and augmented oxidative stress in experimental type 2 diabetes. Food and Chemical Toxicology 2009, 47, 2679–2685. [Google Scholar] [CrossRef]
- DK, P.; et al. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pacific journal of tropical biomedicine 2012, 2, 320–330. [Google Scholar] [CrossRef]
- Hossain, M.K.; et al. Molecular Mechanisms of the Anti-Obesity and Anti-Diabetic Properties of Flavonoids. International Journal of Molecular Sciences 2016, 17. [Google Scholar] [CrossRef]
- JS, K.; CS, K.; KH, S. Inhibition of alpha-glucosidase and amylase by luteolin, a flavonoid. Bioscience, biotechnology, and biochemistry 2000, 64, 2458–2461. [Google Scholar] [CrossRef]
- Kim, K.T.; Rioux, L.E.; Turgeon, S.L. Alpha-amylase and alpha-glucosidase inhibition is differentially modulated by fucoidan obtained from Fucus vesiculosus and Ascophyllum nodosum. Phytochemistry 2014, 98, 27–33. [Google Scholar] [CrossRef]
- Kondhare, D.; Lade, H. Phytochemical profile, aldose reductase inhibitory, and antioxidant activities of Indian traditional medicinal Coccinia grandis (L.) fruit extract. 3 Biotech 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Mel, M.M.R.D.; Gunathilake, K.D.P.P.; Fernando, C.A.N. Formulation of microencapsulated rutin and evaluation of bioactivity and stability upon in vitro digestive and dialysis conditions. International Journal of Biological Macromolecules 2020. Available online: https://sci-hub.ru/https://www.sciencedirect.com/science/article/abs/pii/S0141813020332396 (accessed on 6 February 2022). [CrossRef] [PubMed]
- Nautiyal1, R.; et al. TO ITS MEDICINAL USES AND PHARMACOLOGICAL ACTIVITY. European Journal of Biomedical AND Pharmaceutical sciences 2018, 5, 181–185. Available online: https://storage.googleapis.com/journal-uploads/ejbps/article_issue/volume_5_august_issue_8/1533017567.pdf.
- Neha Mishra1, R.C. () (PDF) Development of functional biscuit from soy flour & rice bran. 2012. Available online: https://www.researchgate.net/publication/313406474_Development_of_functional_biscuit_from_soy_flour_rice_bran (accessed on 6 February 2022).
- Oke-Oghene Philomena Akpoveso, Antioxidant Phytochemicals as Potential Therapy for Diabetic Complications. 2023. [CrossRef]
- P, S.; et al. Potent α-amylase inhibitory activity of Indian Ayurvedic medicinal plants. BMC Complementary and Alternative Medicine 2011, 11, 1–10. [Google Scholar] [CrossRef]
- Patel, D.K.; et al. Natural medicines from plant source used for therapy of diabetes mellitus: An overview of its pharmacological aspects. Asian Pacific Journal of Tropical Disease 2012, 2, 239–250. [Google Scholar] [CrossRef]
- Pereira, R.; et al. Preparation and characterization of films based on alginate and aloe vera. International Journal of Polymer Analysis and Characterization 2011, 16, 449–464. [Google Scholar] [CrossRef]
- Qaisar, M.N.; et al. “Evaluation of α-glucosidase inhibitory activity of dichloromethane and methanol extracts of croton bonplandianum baill. Tropical Journal of Pharmaceutical Research 2014, 13, 1833–1836. [Google Scholar] [CrossRef]
- Qaisar, M.N.; et al. Evaluation of α-glucosidase inhibitory activity of dichloromethane and methanol extracts of croton bonplandianum baill. Tropical Journal of Pharmaceutical Research 2014, 13, 1833–1836. [Google Scholar] [CrossRef]
- Rajbongshi Lata, H.F.H.S. INHIBITORY ACTIVITIES OF ALKALOID FROM COCCINIA GRANDIS AGAINST ALDOSE REDUCTASE AND GENERATION OF ADVANCED GLYCATION ENDPRODUCTS. 2017. [CrossRef]
- S, P.S. Pharmacological Activities of Coccinia Grandis: Review. Journal of Applied Pharmaceutical Science 2013, 3, 114–119. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; et al. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends in Food Science & Technology 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Shukla, P. Physiochemical analysis and phytochemical screening of ivy gourd, Coccinia grandis (L.) Voigt leaves. Journal of Pharmacognosy and Phytochemistry 2019, 8, 1091–1094. [Google Scholar]
-
25. Sugashini Settu1 , Sathiavelu Arunachalam. Evaluation of Anti-inflammatory activity of selected medicinal plants of Cucurbitaceae family. Research Journal of Pharmacy and Technology, 2023; 16. [CrossRef]
- Tamilselvan, N.; et al. Pharmacognosy of Coccinia grandis: a review. Asian Pacific Journal of Tropical Biomedicine 2011, 1, S299–S302. [Google Scholar] [CrossRef]
- Telagari, M.; Hullatti, K. In-vitro α-amylase and α-glucosidase inhibitory activity of Adiantum caudatum Linn. and Celosia argentea Linn. extracts and fractions. Indian Journal of Pharmacology 2015, 47, 425. [Google Scholar] [CrossRef] [PubMed]
- Umamaheswari, M.; Chatterjee, T. In vitro antioxidant activities of the fractions of Coccinia grandis l. leaf extract. 2008. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).