Submitted:
04 September 2023
Posted:
06 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Glucocorticoids and the Eye
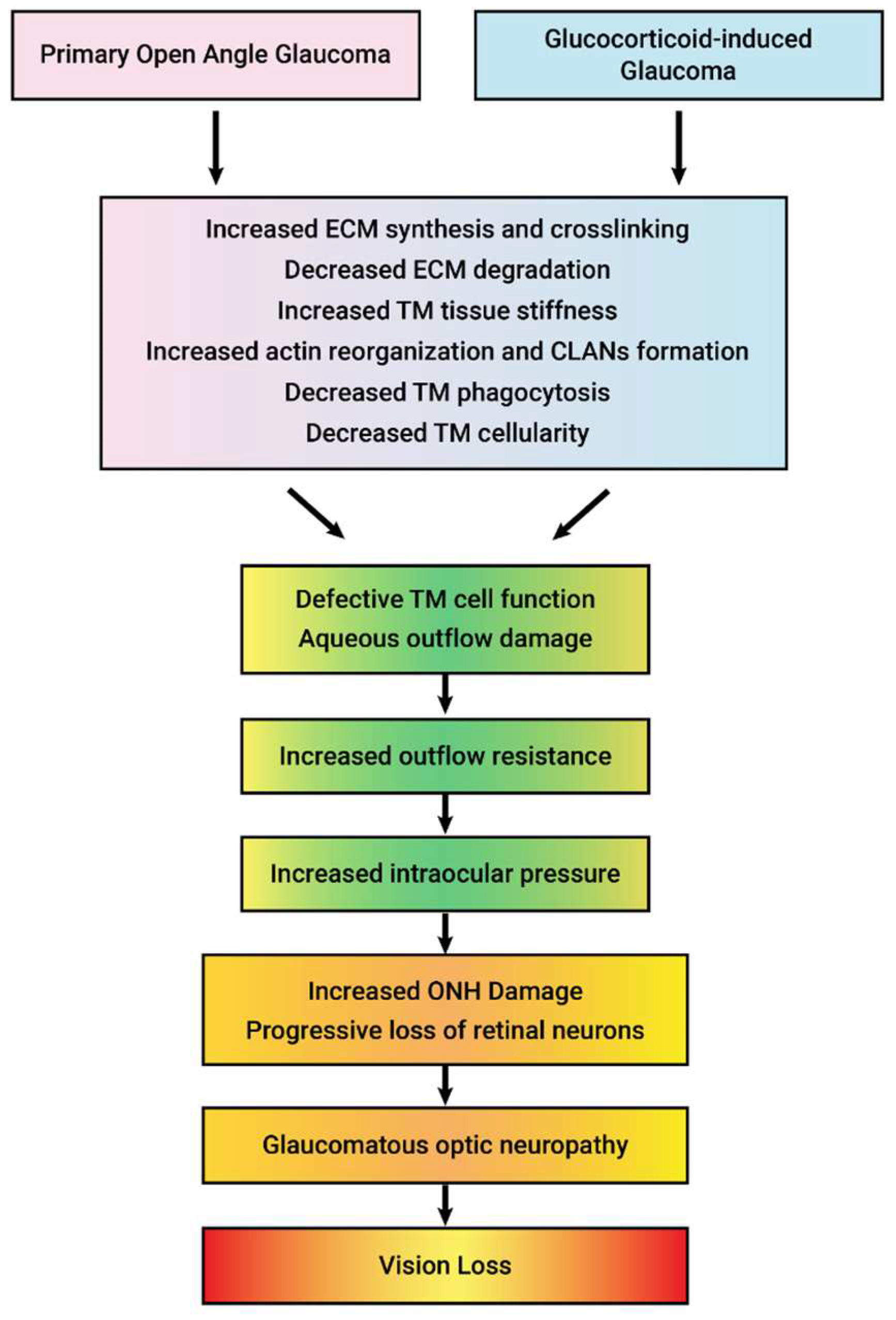
3. Glucocorticoid-Induced Ocular Hypertension and Glucocorticoid-Induced Glaucoma

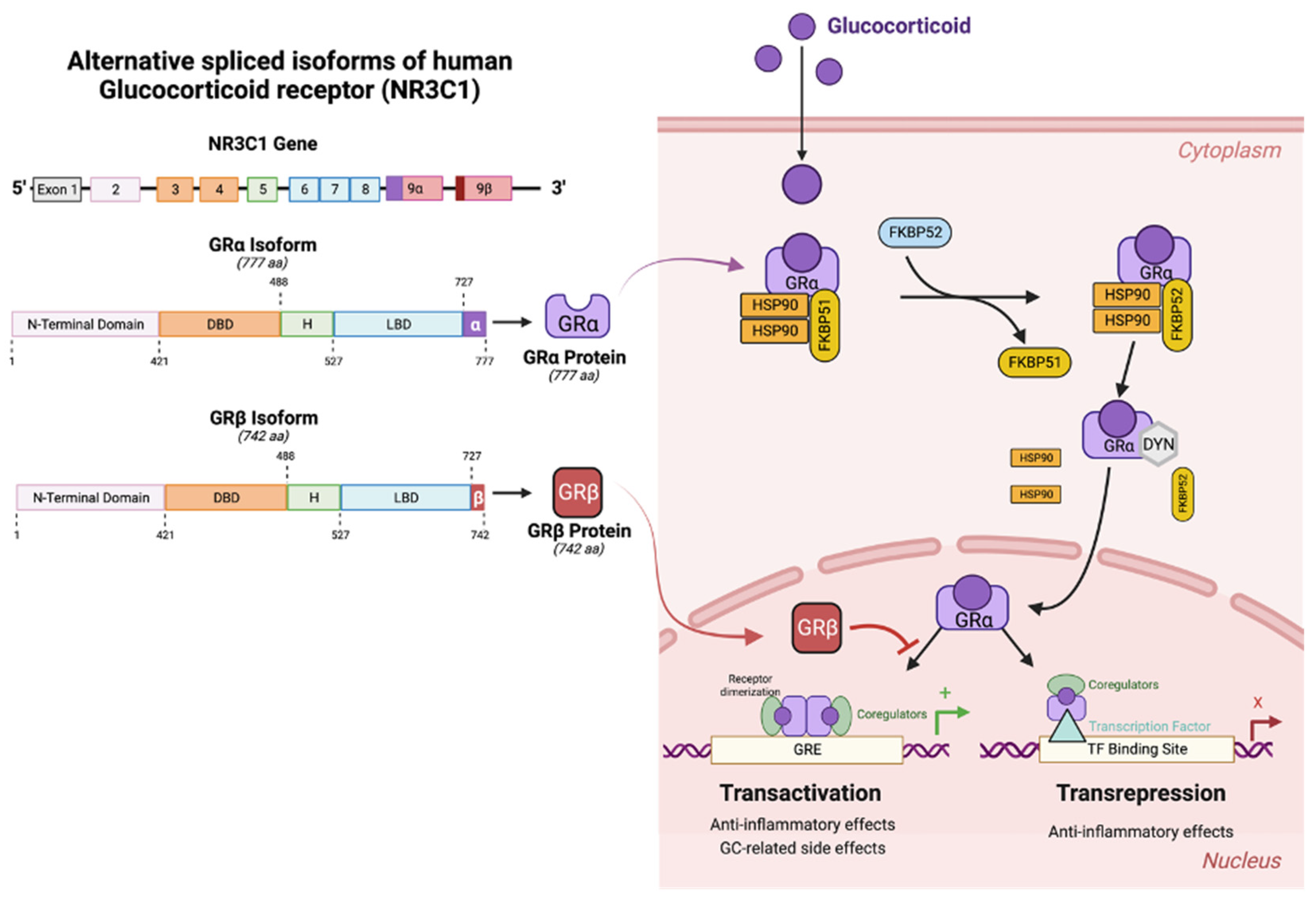
3. Glucocorticoid Receptors (GRs)
5. Steroid Responders: Prevalence and Associated Risk Factors
6. Properties of GCs That Lead to OHT
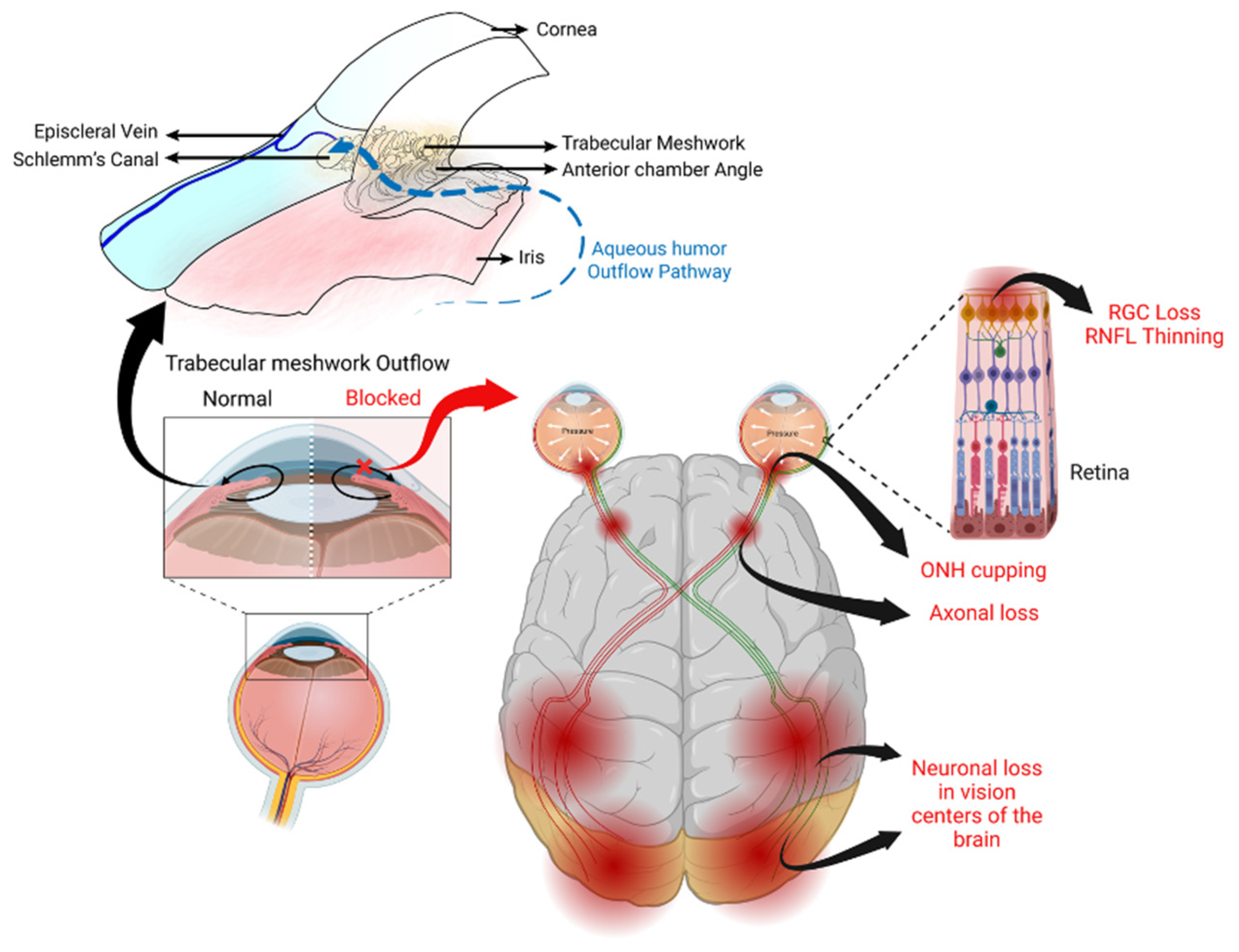
7. Mechanism of GC-Induced IOP Elevation
8. Role of Endogenous Cortisol and Cortisol Metabolism in POAG
9. Models of Glucocorticoid-Induced Ocular Hypertension
10. Role of GRb in Regulating DEX-OHT
11. Conclusions and Future Directions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Tham, Y.C.; et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Shih, V.; et al. Clinical and Economic Burden of Glaucoma by Disease Severity: A United States Claims-Based Analysis. Ophthalmol Glaucoma 2021, 4, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.O.; et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. 2002, 120, 714–720; discussion 829–30. [Google Scholar] [CrossRef] [PubMed]
- The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration.The AGIS Investigators. Am J Ophthalmol 2000, 130, 429–440. [CrossRef] [PubMed]
- Tielsch, J.M.; Sommer, A.; Katz, J.; Royall, R.M.; A Quigley, H.; Javitt, J. Racial variations in the prevalence of primary open-angle glaucoma. The Baltimore Eye Survey. JAMA 1991, 266, 369–74. [Google Scholar] [CrossRef] [PubMed]
- Varma, R.; Ying-Lai, M.; A Francis, B.; Nguyen, B.B.-T.; Deneen, J.; Wilson, M.; Azen, S.P.; Los Angeles Latino Eye Study Group. Prevalence of open-angle glaucoma and ocular hypertension in Latinos: The Los Angeles Latino Eye Study. Ophthalmology 2004, 111, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Becker, B.; Chevrette, L. Topical Corticosteroid Testing in Glaucoma Siblings. Arch. Ophthalmol. 1966, 76, 484–487. [Google Scholar] [CrossRef]
- Becker, B.; Hahn, K.A. Topical Corticosteroids and Heredity in Primary Open-Angle Glaucoma*. Arch. Ophthalmol. 1964, 57, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.M.; Priddy, T.; Judd, J.; Gordon, M.O.; Kass, M.A.; Kolker, A.E.; Becker, B. Intraocular Pressure Response to Topical Dexamethasone as a Predictor for the Development of Primary Open-Angle Glaucoma. Am. J. Ophthalmol. 1988, 106, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, Y.; Horie, T. The Prognosis of Corticosteroid-Responsive Individuals. Arch. Ophthalmol. 1981, 99, 819–823. [Google Scholar] [CrossRef]
- Bartlett, J.D.; Woolley, T.W.; Adams, C.M. Identification of high ocular pressure reesponders to topical corticosteroids. J Ocular Pharm 1993, 9, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Garudadri, C.; Senthil, S.; Khanna, R.C.; Sannapaneni, K.; Rao, H.B.L. Prevalence and Risk Factors for Primary Glaucomas in Adult Urban and Rural Populations in the Andhra Pradesh Eye Disease Study. Ophthalmology 2010, 117, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.Y.; et al. Association Between PM(2.5) Exposure Level and Primary Open-Angle Glaucoma in Taiwanese Adults: A Nested Case-Control Study. Int J Environ Res Public Health 2021, 18. [Google Scholar]
- Gupta, S.K.; Agarwal, R.; Agarwal, P.; Saxena, R.; Agrawal, S.S. Current concepts in the pathophysiology of glaucoma. Indian J. Ophthalmol. 2009, 57, 257–266. [Google Scholar] [CrossRef]
- Yorio, T.; Krishnamoorthy, R.; Prasanna, G. Endothelin: is it a contributor to glaucoma pathophysiology? J Glaucoma 2002, 11, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Lommatzsch, C.; Rothaus, K.; Schopmeyer, L.; Feldmann, M.; Bauer, D.; Grisanti, S.; Heinz, C.; Kasper, M. Elevated endothelin-1 levels as risk factor for an impaired ocular blood flow measured by OCT-A in glaucoma. Sci. Rep. 2022, 12, 11801. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.M.; Crawley, L.; Pahlitzsch, M.; Javaid, F.; Cordeiro, M.F. Glaucoma: the retina and beyond. Acta Neuropathol. 2016, 132, 807–826. [Google Scholar] [CrossRef]
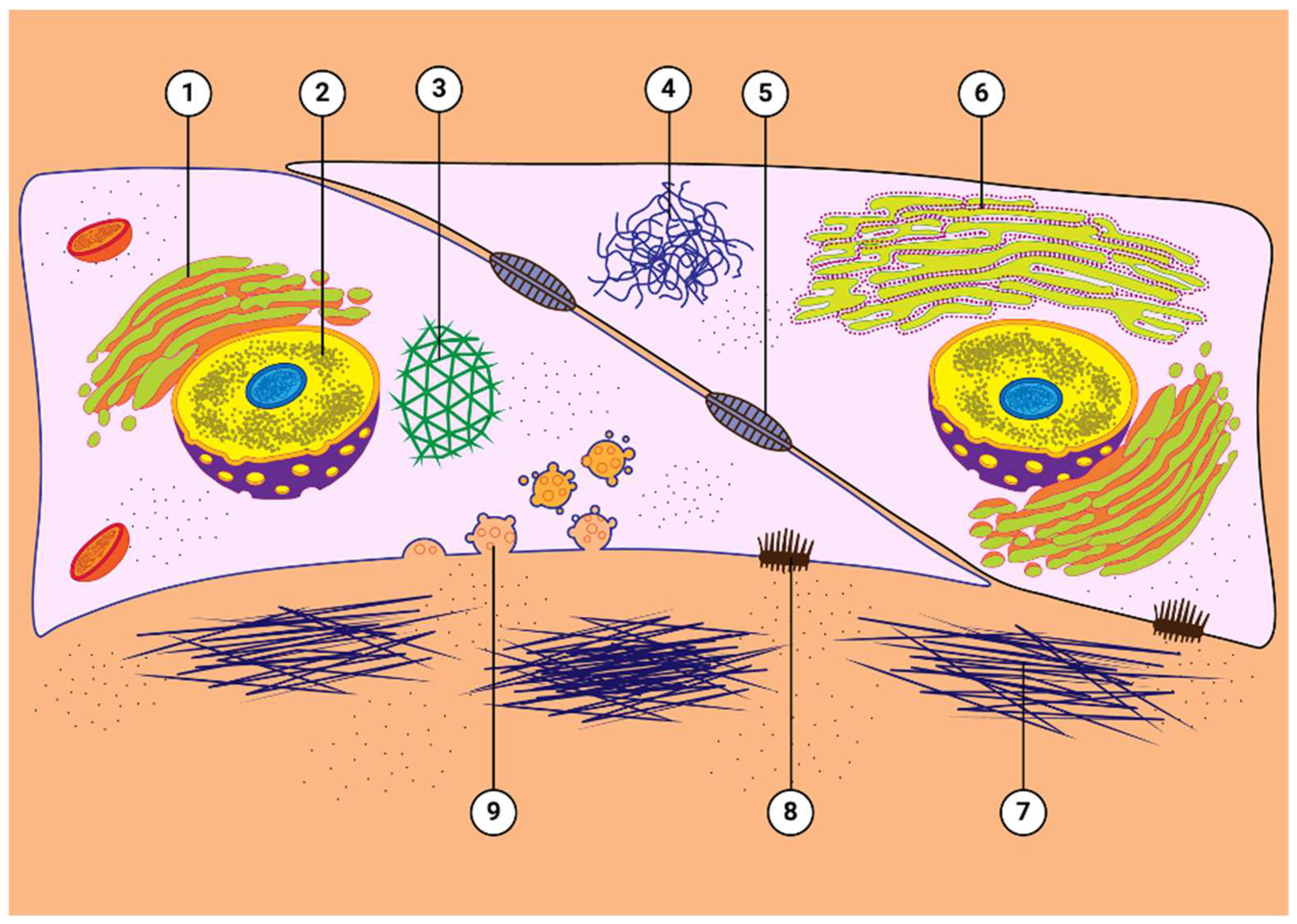
- Tektas, O.-Y.; Lütjen-Drecoll, E. Structural changes of the trabecular meshwork in different kinds of glaucoma. Exp. Eye Res. 2009, 88, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.E.; Peters, D.M. Pathogenesis of glaucoma: Extracellular matrix dysfunction in the trabecular meshwork-A review. Clin. Exp. Ophthalmol. 2022, 50, 163–182. [Google Scholar] [CrossRef]
- Clark, A.F.; Miggans, S.T.B.; Wilson, K.B.; Browder, S.B.; McCartney, M.D. Cytoskeletal Changes in Cultured Human Glaucoma Trabecular Meshwork Cells. Eur. J. Gastroenterol. Hepatol. 1995, 4, 183–188. [Google Scholar] [CrossRef]
- Clark, A.F. The Cell and Molecular Biology of Glaucoma: Biomechanical Factors in Glaucoma. Investig. Opthalmology Vis. Sci. 2012, 53, 2473–2475. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Guy, J.; Anderson, D.R. Blockade of rapid axonal transport. Effect of intraocular pressure elevation in primate optic nerve. Arch Ophthalmol 1979, 97, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; et al. Primary open-angle glaucoma. Nat Rev Dis Primers 2016, 2, 16067. [Google Scholar] [CrossRef] [PubMed]
- Bollinger, K.; Kim, J.; Lowder, C.Y.; Kaiser, P.K.; Smith, S.D. Intraocular Pressure Outcome of Patients with Fluocinolone Acetonide Intravitreal Implant for Noninfectious Uveitis. Ophthalmology 2011, 118, 1927–1931. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.M.; Tanna, A.P. Glaucoma. Med Clin North Am 2021, 105, 493–510. [Google Scholar] [CrossRef] [PubMed]
- Deploey, N.; et al. The Biologist’s Guide to the Glucocorticoid Receptor’s Structure. Cells 2023, 12. [Google Scholar] [CrossRef]
- Armaly, M.F. Statistical attributes of the steroid hypertensive response in the clinically normal eye. Investig. Ophthalmol. 1965, 4, 187–195. [Google Scholar]
- Becker, B. Intraocular pressure response to topical steroids. Invest Ophthalmol 1965, 4, 198–205. [Google Scholar] [PubMed]
- Clark, A.F. Basic sciences in clinical glaucoma: steroids, ocular hypertension, and glaucoma. J. Glaucoma 1995, 4, 354–369. [Google Scholar] [CrossRef]
- Marcus, M.W.; et al. Corticosteroids and open-angle glaucoma in the elderly: a population-based cohort study. Drugs Aging 2012, 29, 963–970. [Google Scholar] [CrossRef]
- Gupta, S.; Shah, P.; Grewal, S.; Chaurasia, A.K.; Gupta, V. Steroid-induced glaucoma and childhood blindness. Br. J. Ophthalmol. 2015, 99, 1454–1456. [Google Scholar] [CrossRef] [PubMed]
- Cantrill, H.L.; Palmberg, P.F.; Zink, H.A.; Waltman, S.R.; Podos, S.M.; Becker, B. Comparison of in Vitro Potency of Corticosteroids with Ability to Raise Intraocular Pressure. Arch. Ophthalmol. 1975, 79, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Wordinger, R.J.; Clarka, A.F. Effects of glucocorticoids on the trabecular meshwork: towards a better understanding of glaucoma. Prog. Retin. Eye Res. 1999, 18, 629–667. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.F.; Wordinger, R.J. The role of steroids in outflow resistance. Exp. Eye Res. 2009, 88, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.F.; Wilson, K.; McCartney, M.D.; Miggans, S.T.; Kunkle, M.; Howe, W. Glucocorticoid-induced formation of cross-linked actin networks in cultured human trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 1994, 35, 281–294. [Google Scholar]
- Clark, A.F.; Brotchie, D.; Read, A.T.; Hellberg, P.; English-Wright, S.; Pang, I.-H.; Ethier, C.R.; Grierson, I. Dexamethasone alters F-actin architecture and promotes cross-linked actin network formation in human trabecular meshwork tissue. Cell Motil. Cytoskelet. 2004, 60, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Hoare, M.-J.; Grierson, I.; Brotchie, D.; Pollock, N.; Cracknell, K.; Clark, A.F. Cross-Linked Actin Networks (CLANs) in the Trabecular Meshwork of the Normal and Glaucomatous Human Eye In Situ. Investig. Opthalmology Vis. Sci. 2009, 50, 1255–1263. [Google Scholar] [CrossRef]
- Zhang, X.; et al. Dexamethasone inhibition of trabecular meshwork cell phagocytosis and its modulation by glucocorticoid receptor beta. Exp Eye Res 2007, 84, 275–284. [Google Scholar] [CrossRef]
- Kasimov, E.M.; Aghaeva, F.A. Cortisol levels in plasma and aqueous humour of patients with steroid induced and other glaucomas. Vestnik oftal’mologii 2017, 133, 39–45. [Google Scholar] [CrossRef]
- McCarty, G.R.; Schwartz, B. Reduced plasma cortisol binding to albumin in ocular hypertension and primary open-angle glaucoma. Curr. Eye Res. 1999, 18, 467–476. [Google Scholar] [CrossRef]
- Janousková, K.; Tĕsínský, P.; Topolcan, O. [Hormonal changes in open-angle glaucoma. II. Levels of cortisol and somatotropin in serum]. Ceskoslovenska Oftalmol. 1993, 49, 22–29. [Google Scholar]
- Schwartz, B.; McCarty, G.; Rosner, B. Increased Plasma Free Cortisol in Ocular Hypertension and Open Angle Glaucoma. Arch. Ophthalmol. 1987, 105, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Rozsíval, P.; Hampl, R.; Obenberger, J.; Stárka, L.; Řehák, S. Aqueous humour and plasma cortisol levels in glaucoma and cataract patients. Curr. Eye Res. 1981, 1, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, B.; Rabin, P.A.; Wysocki, A.B.; Martin, J.M. Decreased Plasma Cortisol in Response to Intramuscular ACTH in Ocular Hypertensives and Primary Open-angle Glaucomas. Eur. J. Gastroenterol. Hepatol. 2007, 16, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, B.; Wysocki, A.; Qi, Y. Decreased Response of Plasma Cortisol to Intravenous Metyrapone in Ocular Hypertension and Primary Open-Angle Glaucoma. Eur. J. Gastroenterol. Hepatol. 2005, 14, 474–481. [Google Scholar] [CrossRef]
- Stokes, J.; Walker, B.R.; Campbell, J.C.; Seckl, J.R.; O’brien, C.; Andrew, R. Altered Peripheral Sensitivity to Glucocorticoids in Primary Open-Angle Glaucoma. Investig. Opthalmology Vis. Sci. 2003, 44, 5163–5167. [Google Scholar] [CrossRef]
- Kass, M.A.; Krupin, T.; Becker, B. Plasma Cortisol Suppression Test Used to Predict the Development of Primary Open-Angle Glaucoma. Am. J. Ophthalmol. 1976, 82, 496–497. [Google Scholar] [CrossRef]
- Bigger, J.F.; Palmberg, P.F.; Zink, H.A. In Vitro Corticosteroid: Correlation Response With Primary Open-Angle Glaucoma and Ocular Corticosteroid Sensitivity. Am. J. Ophthalmol. 1975, 79, 92–97. [Google Scholar] [CrossRef]
- Weinstein, B.I.; Munnangi, P.; Gordon, G.G.; Southren, A.L. Defects in cortisol-metabolizing enzymes in primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 1985, 26, 890–893. [Google Scholar]
- Southren, A.L.; Gordon, G.G.; Munnangi, P.R.; Vittek, J.; Schwartz, J.; Monder, C.; Dunn, M.W.; I Weinstein, B. Altered cortisol metabolism in cells cultured from trabecular meshwork specimens obtained from patients with primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 1983, 24, 1413–1417. [Google Scholar]
- Polansky, J.; Palmberg, P.; Matulich, D.; Lan, N.; Hajek, S.; Hajek, A.; Becker, B.; Baxter, J. Cellular sensitivity to glucocorticoids in patients with POAG. Steroid receptors and responses in cultured skin fibroblasts. Ophthalmol. Vis. Sci. 1985, 26, 805–809. [Google Scholar]
- Mygind, N.; Thomsen, J. Diurnal Variation of Nasal Protein Concentration. Acta Oto-Laryngologica 1976, 82, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.F.; Wilson, K.; De Kater, A.W.; Allingham, R.R.; McCartney, M.D. Dexamethasone-induced ocular hypertension in perfusion-cultured human eyes. Investig. Ophthalmol. Vis. Sci. 1995, 36, 478–89. [Google Scholar]
- Awan, M.A.; Agarwal, P.K.; Watson, D.G.; McGhee, C.N.J.; Dutton, G.N. Penetration of topical and subconjunctival corticosteroids into human aqueous humour and its therapeutic significance. Br. J. Ophthalmol. 2009, 93, 708–713. [Google Scholar] [CrossRef]
- Fingert, J.; Clark, A.F.; Craig, J.; Alward, W.L.; Snibson, G.R.; McLaughlin, M.; Tuttle, L.; A Mackey, D.; Sheffield, V.; Stone, E.M. Evaluation of the myocilin (MYOC) glaucoma gene in monkey and human steroid-induced ocular hypertension. Investig. Ophthalmol. Vis. Sci. 2001, 42, 145–152. [Google Scholar]
- Zhou, L.; Li, Y.; Yue, B.Y. Glucocorticoid effects on extracellular matrix proteins and integrins in bovine trabecular meshwork cells in relation to glaucoma. Int. J. Mol. Med. 1998, 1, 339–385. [Google Scholar] [CrossRef] [PubMed]
- Tane, N.; Dhar, S.; Roy, S.; Pinheiro, A.; Ohira, A.; Roy, S. Effect of excess synthesis of extracellular matrix components by trabecular meshwork cells: Possible consequence on aqueous outflow. Exp. Eye Res. 2007, 84, 832–842. [Google Scholar] [CrossRef]
- O’Reilly, S.; Pollock, N.; Currie, L.; Paraoan, L.; Clark, A.F.; Grierson, I. Inducers of Cross-Linked Actin Networks in Trabecular Meshwork Cells. Investig. Opthalmology Vis. Sci. 2011, 52, 7316–7324. [Google Scholar] [CrossRef]
- Wade, N.; Grierson, I.; O’Reilly, S.; Hoare, M.; Cracknell, K.; Paraoan, L.; Brotchie, D.; Clark, A. Cross-linked actin networks (CLANs) in bovine trabecular meshwork cells. Exp. Eye Res. 2009, 89, 648–659. [Google Scholar] [CrossRef]
- Danias, J.; Gerometta, R.; Ge, Y.; Ren, L.; Panagis, L.; Mittag, T.W.; Candia, O.A.; Podos, S.M. Gene Expression Changes in Steroid-Induced IOP Elevation in Bovine Trabecular Meshwork. Investig. Opthalmology Vis. Sci. 2011, 52, 8636–8645. [Google Scholar] [CrossRef]
- Liesenborghs, I.; Eijssen, L.M.T.; Kutmon, M.; Gorgels, T.G.M.F.; Evelo, C.T.; Beckers, H.J.M.; Webers, C.A.B.; Schouten, J.S.A.G. The Molecular Processes in the Trabecular Meshwork After Exposure to Corticosteroids and in Corticosteroid-Induced Ocular Hypertension. Investig. Opthalmology Vis. Sci. 2020, 61, 24–24. [Google Scholar] [CrossRef] [PubMed]
- Gerometta, R.; Podos, S.M.; Candia, O.A.; Wu, B.; Malgor, L.A.; Mittag, T.; Danias, J. Steroid-Induced Ocular Hypertension in Normal Cattle. Arch. Ophthalmol. 2004, 122, 1492–1497. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Tovar-Vidales, T.; Yorio, T.; Wordinger, R.J.; Clark, A.F. Perfusion-Cultured Bovine Anterior Segments as an Ex Vivo Model for Studying Glucocorticoid-Induced Ocular Hypertension and Glaucoma. Investig. Opthalmology Vis. Sci. 2011, 52, 8068–8075. [Google Scholar] [CrossRef] [PubMed]
- Gerometta, R.; Podos, S.M.; Danias, J.; Candia, O.A. Steroid-Induced Ocular Hypertension in Normal Sheep. Investig. Opthalmology Vis. Sci. 2009, 50, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Candia, O.A.; Gerometta, R.; Millar, J.C.; Podos, S.M. Suppression of Corticosteroid-Induced Ocular Hypertension in Sheep by Anecortave. Arch. Ophthalmol. 2010, 128, 338–343. [Google Scholar] [CrossRef]
- Gerometta, R.; Kumar, S.; Shah, S.; Alvarez, L.; Candia, O.; Danias, J. Reduction of Steroid-Induced Intraocular Pressure Elevation in Sheep by Tissue Plasminogen Activator. Investig. Opthalmology Vis. Sci. 2013, 54, 7903–7909. [Google Scholar] [CrossRef]
- Borrás, T.; Buie, L.K.; Spiga, M.G. Inducible scAAV2.GRE.MMP1 lowers IOP long-term in a large animal model for steroid-induced glaucoma gene therapy. Gene Ther. 2016, 23, 438–449. [Google Scholar] [CrossRef]
- Gosling, A.A.; Kiland, J.A.; Rutkowski, L.E.; Hoefs, A.; Ellinwood, N.M.; McLellan, G.J. Effects of topical corticosteroid administration on intraocular pressure in normal and glaucomatous cats. Veter- Ophthalmol. 2016, 19, 69–76. [Google Scholar] [CrossRef]
- McLean, N.J.; Ward, D.A.; Hendrix, D.V.H.; Vaughn, R.K. Effects of one-week versus one-day preoperative treatment with topical 1% prednisolone acetate in dogs undergoing phacoemulsification. J. Am. Veter- Med Assoc. 2012, 240, 563–569. [Google Scholar] [CrossRef]
- Bhattacherjee, P.; Paterson, C.A.; Spellman, J.M.; Graff, G.; Yanni, J.M. Pharmacological validation of a feline model of steroid-induced ocular hypertension. Arch. Ophthalmol. 1999, 117, 361–364. [Google Scholar] [CrossRef]
- Gelatt, K.N.; Mackay, E.O. The Ocular Hypertensive Effects of Topical 0.1% Dexamethasone in Beagles with Inherited Glaucoma. J. Ocul. Pharmacol. Ther. 1998, 14, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Zhan, G.-L.; Miranda, O.C.; Bito, L.Z. Steroid glaucoma: Corticosteroid-induced ocular hypertension in cats. Exp. Eye Res. 1992, 54, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Bhattacherjee, P.; Mukhopadhyay, P.; Paterson, C.A.; Graff, G.; Gamache, D.A.; Yanni, J.M. AL-2512, a Novel Corticosteroid: Preclinical Assessment of Anti-Inflammatory and Ocular Hypertensive Effects. J. Ocul. Pharmacol. Ther. 2003, 19, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Lorenzetti, O.J. Effects of corticosteroids on ocular dynamics in rabbits. J. Pharmacol. Exp. Ther. 1970, 175. [Google Scholar]
- Knepper, P.A.; Collins, J.A.; Frederick, R. Effects of dexamethasone, progesterone, and testosterone on IOP and GAGs in the rabbit eye. Investig. Ophthalmol. Vis. Sci. 1985, 26, 1093–1100. [Google Scholar]
- Bonomi, L.; Perfetti, S.; Noya, E.; Bellucci, R.; Tomazzoli, L. Experimental corticosteroid ocular hypertension in the rabbit. Graefe’s Arch. Clin. Exp. Ophthalmol. 1978, 209, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-G.; Bao, F.-J.; Joda, A.; Fu, X.-A.; Zhou, S.; Wang, J.; Hu, X.-L.; Wang, Q.-M.; Elsheikh, A. Influence of glucocorticosteroids on the biomechanical properties of in-vivo rabbit cornea. J. Mech. Behav. Biomed. Mater. 2014, 29, 350–359. [Google Scholar] [CrossRef]
- Sturdivant, J.; Williams, S.S.; Ina, M.; Weksler, M.; McDougal, A.; Clancy, D.; Delong, M.A.; Girouard, N.; Zaretskaia, M.; Brennan, K.; et al. Discovery and Preclinical Development of Novel Intraocular Pressure-Lowering Rho Kinase Inhibitor: Corticosteroid Conjugates. J. Ocul. Pharmacol. Ther. 2023, 39, 117–127. [Google Scholar] [CrossRef]
- Shafiee, A.; Bucolo, C.; Budzynski, E.; Ward, K.W.; López, F.J. In Vivo Ocular Efficacy Profile of Mapracorat, a Novel Selective Glucocorticoid Receptor Agonist, in Rabbit Models of Ocular Disease. Investig. Opthalmology Vis. Sci. 2011, 52, 1422–1430. [Google Scholar] [CrossRef]
- Iqbal, Z.; et al. Relationship between the concentration of copper and iron in the aqueous humour and intraocular pressure in rabbits treated with topical steroids. Clin Exp Ophthalmol 2002, 30, 28–35. [Google Scholar]
- Southren, A.L.; L’Hommedieu, D.; Gordon, G.G.; I Weinstein, B. Intraocular hypotensive effect of a topically applied cortisol metabolite: 3 alpha, 5 beta-tetrahydrocortisol. Investig. Ophthalmol. Vis. Sci. 1987, 28, 901–903. [Google Scholar]
- Moore, C.G.; Milne, S.T.; Morrison, J.C. Noninvasive measurement of rat intraocular pressure with the Tono-Pen. Investig. Ophthalmol. Vis. Sci. 1993, 34, 363–369. [Google Scholar]
- Wang, W.-H.; Millar, J.C.; Pang, I.-H.; Wax, M.B.; Clark, A.F. Noninvasive Measurement of Rodent Intraocular Pressure with a Rebound Tonometer. Investig. Opthalmology Vis. Sci. 2005, 46, 4617–4621. [Google Scholar] [CrossRef] [PubMed]
- Miyara, N.; Shinzato, M.; Yamashiro, Y.; Iwamatsu, A.; Kariya, K.-I.; Sawaguchi, S. Proteomic analysis of rat retina in a steroid-induced ocular hypertension model: Potential vulnerability to oxidative stress. Jpn. J. Ophthalmol. 2008, 52, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Shinzato, M.; Yamashiro, Y.; Miyara, N.; Iwamatsu, A.; Takeuchi, K.; Umikawa, M.; Bayarjargal, M.; Kariya, K.-I.; Sawaguchi, S. Proteomic Analysis of the Trabecular Meshwork of Rats in a Steroid-Induced Ocular Hypertension Model: Downregulation of Type I Collagen C-Propeptides. Ophthalmic Res. 2007, 39, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Sawaguchi, K.; Nakamura, Y.; Nakamura, Y.; Sakai, H.; Sawaguchi, S. Myocilin Gene Expression in the Trabecular Meshwork of Rats in a Steroid-Induced Ocular Hypertension Model. Ophthalmic Res. 2005, 37, 235–242. [Google Scholar] [CrossRef]
- Razali, N.; Agarwal, R.; Agarwal, P.; Kumar, S.; Tripathy, M.; Vasudevan, S.; Crowston, J.G.; Ismail, N.M. Role of adenosine receptors in resveratrol-induced intraocular pressure lowering in rats with steroid-induced ocular hypertension. Clin. Exp. Ophthalmol. 2014, 43, 54–66. [Google Scholar] [CrossRef]
- Razali, N.; Agarwal, R.; Agarwal, P.; Kapitonova, M.Y.; Kutty, M.K.; Smirnov, A.; Bakar, N.S.; Ismail, N.M. Anterior and posterior segment changes in rat eyes with chronic steroid administration and their responsiveness to antiglaucoma drugs. Eur. J. Pharmacol. 2015, 749, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Horng, C.-T.; Yang, Y.-L.; Chen, C.-C.; Huang, Y.-S.; Chen, C.; Chen, F.-A. Intraocular pressure-lowering effect of Cordyceps cicadae mycelia extract in a glaucoma rat model. Int. J. Med Sci. 2021, 18, 1007–1014. [Google Scholar] [CrossRef]
- Rodrigo, M.; Garcia-Herranz, D.; Aragón-Navas, A.; Subias, M.; Martinez-Rincón, T.; Mendez-Martínez, S.; Cardiel, M.; García-Feijoo, J.; Ruberte, J.; Herrero-Vanrell, R.; et al. Long-term corticosteroid-induced chronic glaucoma model produced by intracameral injection of dexamethasone-loaded PLGA microspheres. Drug Deliv. 2021, 28, 2427–2446. [Google Scholar] [CrossRef]
- Sato, K.; Nishiguchi, K.M.; Maruyama, K.; Moritoh, S.; Fujita, K.; Ikuta, Y.; Kasai, H.; Nakazawa, T. Topical ocular dexamethasone decreases intraocular pressure and body weight in rats. J. Negat. Results Biomed. 2016, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, N.A.; McKnight, B.; Corcoran, K.N.; Rodriguez, L.A.; Rice, D.S. Increased Intraocular Pressure in Mice Treated with Dexamethasone. Investig. Opthalmology Vis. Sci. 2010, 51, 6496–503. [Google Scholar] [CrossRef] [PubMed]
- Overby, D.R.; Bertrand, J.; Tektas, O.-Y.; Boussommier-Calleja, A.; Schicht, M.; Ethier, C.R.; Woodward, D.F.; Stamer, W.D.; Lütjen-Drecoll, E. Ultrastructural Changes Associated With Dexamethasone-Induced Ocular Hypertension in Mice. Investig. Opthalmology Vis. Sci. 2014, 55, 4922–4933. [Google Scholar] [CrossRef] [PubMed]
- Zode, G.S.; Sharma, A.B.; Lin, X.; Searby, C.C.; Bugge, K.; Kim, G.H.; Clark, A.F.; Sheffield, V.C. Ocular-specific ER stress reduction rescues glaucoma in murine glucocorticoid-induced glaucoma. J. Clin. Investig. 2014, 124, 1956–1965. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.C.; et al. Glucocorticoid receptor GRbeta regulates glucocorticoid-induced ocular hypertension in mice. Sci Rep 2018, 8, 862. [Google Scholar] [CrossRef]
- Patel, G.C.; Millar, J.C.; Clark, A.F. Glucocorticoid Receptor Transactivation Is Required for Glucocorticoid-Induced Ocular Hypertension and Glaucoma. Investig. Opthalmology Vis. Sci. 2019, 60, 1967–1978. [Google Scholar] [CrossRef]
- Patel, G.C.; et al. Dexamethasone-Induced Ocular Hypertension in Mice: Effects of Myocilin and Route of Administration. Am J Pathol 2017.
- Maddineni, P.; Kasetti, R.B.; Patel, P.D.; Millar, J.C.; Kiehlbauch, C.; Clark, A.F.; Zode, G.S. CNS axonal degeneration and transport deficits at the optic nerve head precede structural and functional loss of retinal ganglion cells in a mouse model of glaucoma. Mol. Neurodegener. 2020, 15, 48. [Google Scholar] [CrossRef]
- Wang, K.; Li, G.; Read, A.T.; Navarro, I.; Mitra, A.K.; Stamer, W.D.; Sulchek, T.; Ethier, C.R. The relationship between outflow resistance and trabecular meshwork stiffness in mice. Sci. Rep. 2018, 8, 5848. [Google Scholar] [CrossRef]
- Zhang, X.; Clark, A.F.; Yorio, T. Regulation of glucocorticoid responsiveness in glaucomatous trabecular meshwork cells by glucocorticoid receptor-beta. Invest Ophthalmol Vis Sci 2005, 46, 4607–4616. [Google Scholar] [CrossRef]
- Jain, A.; Wordinger, R.J.; Yorio, T.; Clark, A.F. Spliceosome Protein (SRp) Regulation of Glucocorticoid Receptor Isoforms and Glucocorticoid Response in Human Trabecular Meshwork Cells. Investig. Opthalmology Vis. Sci. 2012, 53, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Leibowitz, H.M.; Bartlett, J.D.; Rich, R.; McQuirter, H.; Stewart, R.; Assil, K. Intraocular Pressure-Raising Potential of 1.0% Rimexolone in Patients Responding to Corticosteroids. Arch. Ophthalmol. 1996, 114, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Hochhaus, G.; Moellmann, H.W. Binding affinities of rimexolone (ORG 6216), flunisolide and their putative metabolites for the glucocorticoid receptor of human synovial tissue. Inflamm. Res. 1990, 30, 377–380. [Google Scholar] [CrossRef] [PubMed]
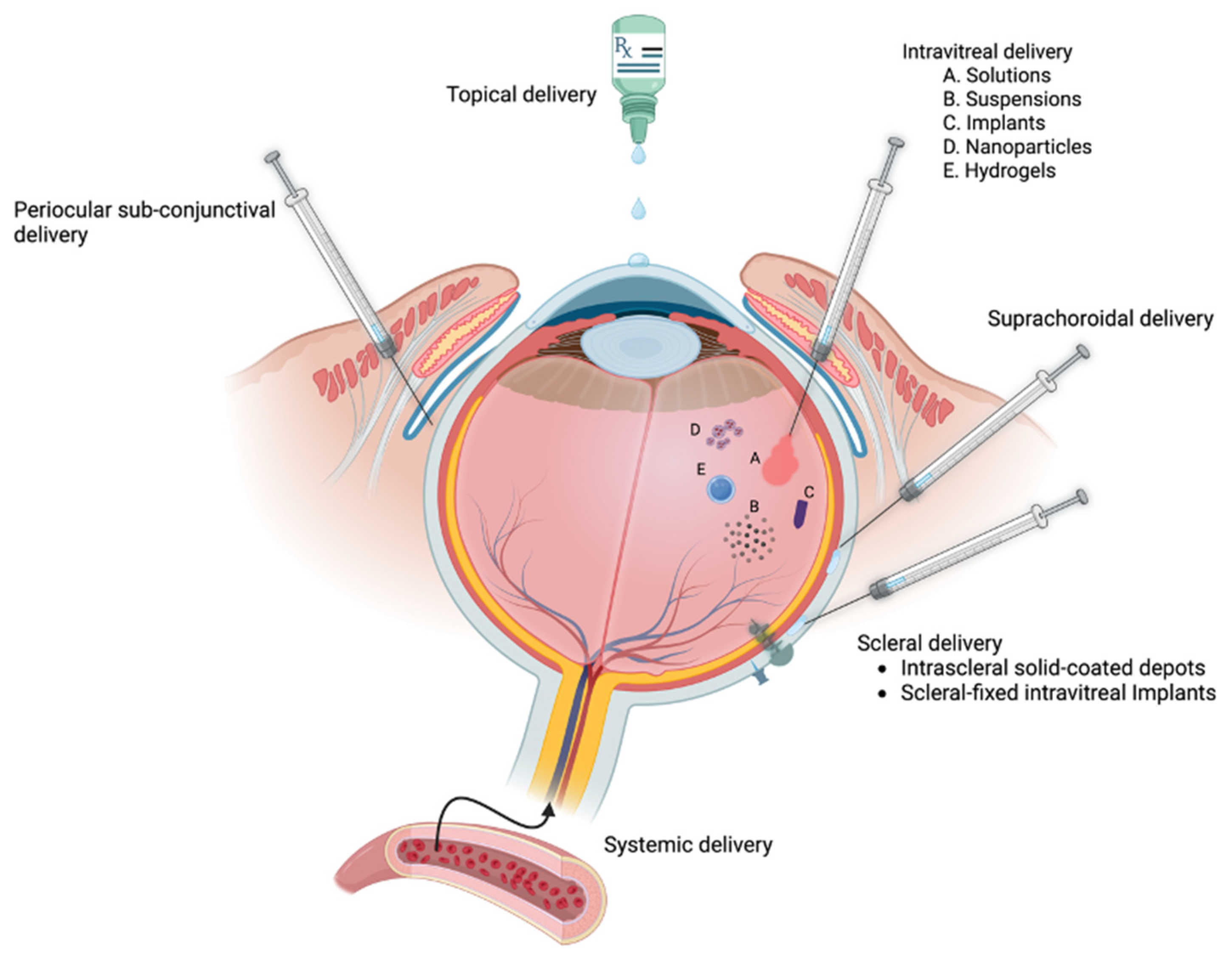
| • Note: GCs unmatched for anti-inflammatory/immunosuppressive activities |
| • Post-operative inflammation |
| • Macular edema (diabetic retinopathy and AMD) |
| • Allergic and hypersensitivity reactions |
| • Uveitis (uveal tract inflammation) |
| • Retinitis |
| • Scleritis and episcleritis |
| • Giant-cell arteritis |
| • Trauma |
| • Herpes zoster ophthalmicus |
| Generic Name | Drug Name | Concentration | Dosing |
|---|---|---|---|
| Prednisolone-21-acetate | Prednisol Pred Mild Pred Forte |
Suspension (0.12%, 0.125%, 1%) | Topical ocular |
| Prednisolone sodium phosphate | Solution (0.125% and 1%) | Topical ocular | |
| Dexamethasone | Maxidex | Suspension (1mg/mL) | Topical ocular |
| Dexycu | Suspension (517ug/5uL) | Posterior chamber injection | |
| Ozurdex | 0.7mg/implant | Ivt implant | |
| Dextenza | 0.4mg/insert | Tear duct insert | |
| Dexamethasone sodium phosphate | Ocu-Dex | Solution (1 mg/mL) | Topical ocular |
| Loteprednol etabonate |
Lotemax (0.5%) Lotemax SM 0.38% gel) Eysuvis (0.25%) Inveltys (1%) Alrex (0.2%) |
eye suspension (5mg/mL) eye gel (3.8 and 5 mg/g) eye ointment (5mg/g) |
Topical ocular |
| Fluoromethalone | FML | Suspension (0.1%) | Topical ocular |
| Fluor-Op | |||
| FML Forte | Suspension (0.25%) | Topical ocular | |
| FML S.O.P. | Ointment (0.1%) | Topical ocular | |
| Fluoromethalone acetate | Flarex | Suspension (1mg/mL) | Topical ocular |
| Yutiq | Implant (0.18 mg) | Ivt implant | |
| Rimexolone | Vexol | Suspension (1%) | Topical ocular |
| difluprednate | Durezol | Ophthalmic emulsion (0.5mg/mL) | Topical ocular |
| Fluocinolone acetonide | Retisert | 0.59 g/implant | Ivt implant |
| Illuvien | 0.19mg/implant | Ivt implant | |
| Yutiq | 0.18mg/implant | Ivt implant | |
| Triamcinolone acetonide | Xipere | 40 mg/mL | Superchoroidal injectable suspension |
| Trivaris | 80 mg/mL | Injectable suspension | |
| Triesence | 40 mg/mL | Injectable suspension |
| Glucocorticoid | IC50 (nM) | * Potency | IOP Rise (mmHg) |
|---|---|---|---|
| Loteprednol | 1.9 | 105.8 | 4.1 |
| Rimexolone | 6.2 | 32.4 | 6.2 |
| Dexamethasone | 8.4 | 23.8 | 22.0 |
| Fluoromethalone | 9.7 | 20.8 | 6.1 |
| Prednisolone | 87 | 2.3 | 10.0 |
| Medrysone | 117 | 1.7 | 1.0 |
| Cortisol | 201 | 1.0 | 3.2 |
| Species | Glucocorticoid | Route |
|---|---|---|
| Human perfused anterior segments | Dexamethasone | In perfusion medium |
| Nonhuman primate | Dexamethasone | Topical ocular |
| Cow – in vivo | Prednisolone | Topical ocular |
| Cow - ex vivo | Dexamethasone | In perfusion medium |
| Sheep | Prednisolone | Topical ocular |
| Rabbits | Dexamethasone Betamethasone Prednisolone-Ac Cortisone Triamcinolone Fluorometholone Loteprednol-Etabonate Rimexolone |
Topical ocular Subconj injection Topical ocular Subconj injection Subconj injection Topical ocular Topical ocular Topical ocular |
| Cats | Dexamethasone Prednisolone-Ac Fluorometholone Loteprednol-Etabonate Rimexolone |
Topical ocular |
| Rats | Dexamethasone Betamethasone |
Topical ocular |
| Mice | Dexamethasone | Systemic |
| Dexamethasone Acetate | Periocular injection |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





