Submitted:
04 September 2023
Posted:
06 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Cyclodextrins
| Physicochemical Properties | α-CD | β-CD | γ-CD |
|---|---|---|---|
| Chemical formula | |||
| Glucose units | 6 | 7 | 8 |
| Molecular weight (Da) | 972 | 1135 | 1297 |
| Internal diameter (nm) | 0.47-0.53 | 0.60-0.65 | 0.75-0.83 |
| Outer diameter (nm) | 1.46 | 1.54 | 1.75 |
| Height of torus (nm) | 0.79 | 0.79 | 0.79 |
| Internal volume () | 0.174 | 0.262 | 0.427 |
| Solubility in water at 25°C (mg/mL) | 145 | 18.5 | 232 |
| Internal water molecules | 6-8 | 11-12 | 13-17 |
3. Solubility and toxicological considerations
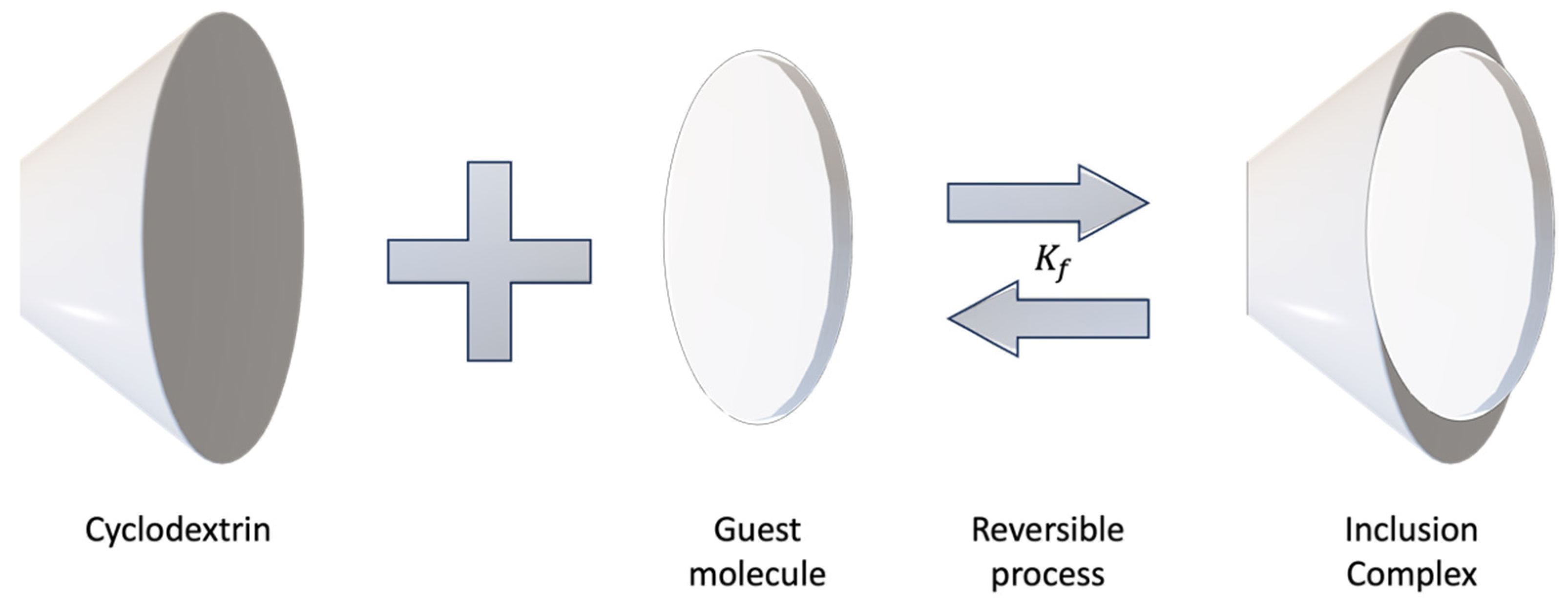
4. Formation of inclusion complexes
4.1. Inclusion complex formation techniques
4.1.1. Precipitation method
4.1.2. Physical mixture
4.2. Characterization techniques
4.3. Effect on the guest properties
4.3.1. Protection
4.3.2. Taste modifications
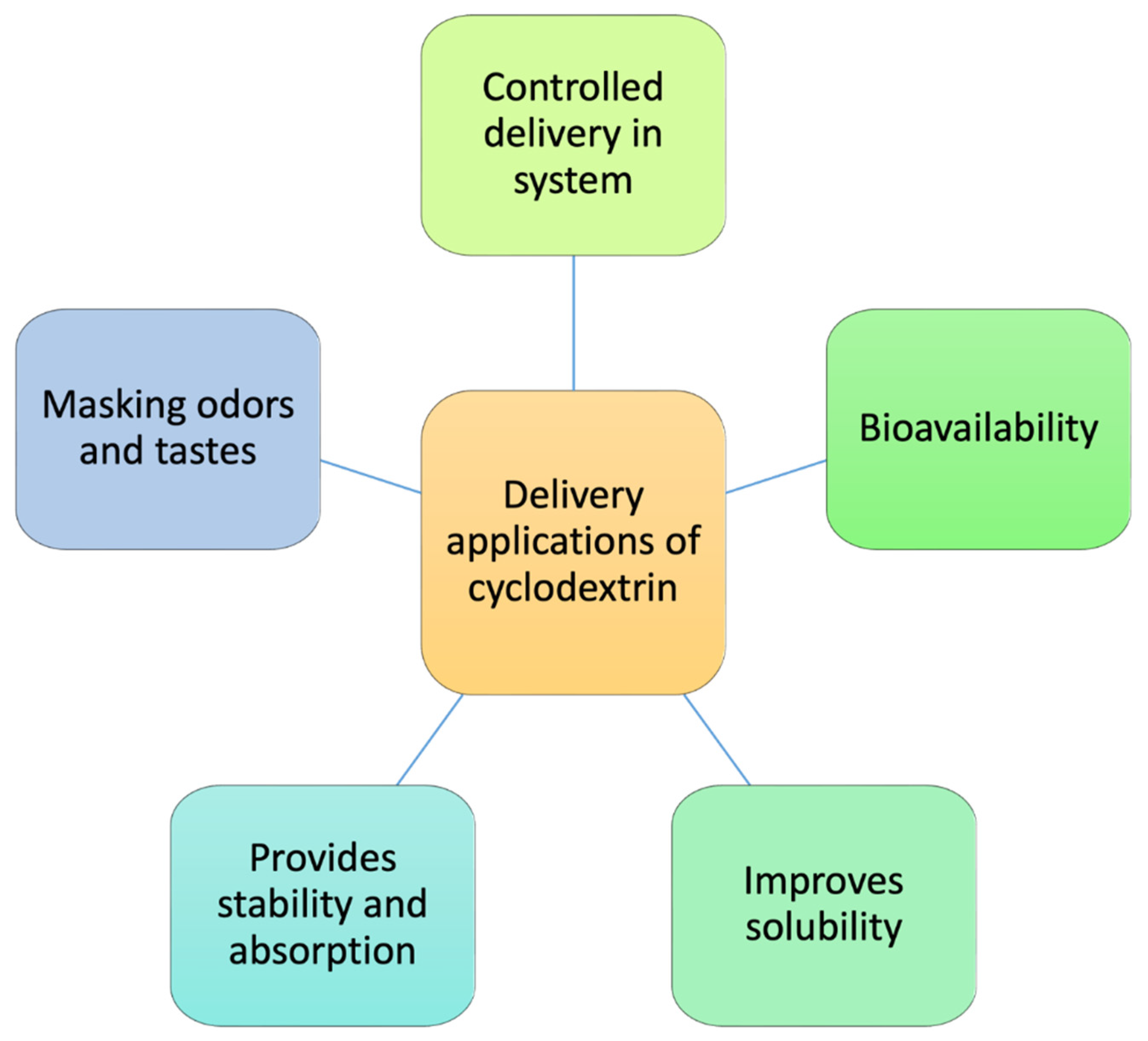
5. Cyclodextrins in food packaging
5.1. Active packaging
| Packaging | Enhanced properties | References |
|---|---|---|
| PLA/β-CD:AITC | • Increases solubility • Increases absorption • Increases releases rate |
[73] |
| LLDPE/β-CD:D-limonene | • Prevents the loss of the volatile compounds • Antibacterial and antifungal activities • Protects from oxidation |
[74] |
| PLA/β-CD-thymol | • Prolongs shelf-life one week • Microbial inhibition • Decreases in the weight loss • Reduces changes in color |
[120] |
| CGRE:β-CD | • Increases polyphenol content • Protect against temperature |
[113] |
| SEO:β-CD | • Microbial inhibition | [75] |
| CA:β-CD | • Increases solubility • Antioxidant function • Thermal stability |
[118] |
| Curcumin:β-CD | • Reduces microbial counts • Inhibit the lipid oxidase activity • Extents storability • Improves color |
[100] |
| CEO:β-CD | • Increases antioxidant activivity • Increases antibacterial activivity |
[101] |
| BITC:β-CD | • Inhibition of S. aureus and E. coli growth • Improves shelf-life • Improves the stability and controlled release • Flavor masking |
[117] |
| Chicken lipid:β-CD | • Thermal and oxidative stability • Stability in the fatty components |
[121] |
| Vitamin:γ-CD | • Enhanced the bioavailability of vitamin D3 and E | [110] |
| SA:β-CD/NPsBA:β-CD/NPs | • Loading efficiency • Prolonged and sustained release profile |
[111] |
| Naringenin:β-CD/CQDs | • Antioxidant properties • Improves encampsulation efficiency |
[68] |
| Lutein:β-CD | • Improves stability and bioavailability | [67] |
| Quercetin:γ-CD | • Improves solubility • Enhanced the free radical scavenging ability |
[112] |
| CNC/zein:catechin:β-CD | • Inhibits oxidation • Prolongs shelf-life |
[69] |
5.2. Intelligent Packaging
5.2.1. Leak indicators
5.2.2. Freshness indicators
5.2.4. Spoilage indicators
5.2.5. Electrochemical sensors
| Packaging | Sensor | Indicator | References |
|---|---|---|---|
| Chitosan/montmorillonite:β-CD | Oxygen | Colorimetric (changing the color from colorless to blue) | [136] |
| CNF/CP:β-CD | pH | Colorimetric (changing the color from coral to gold) | [144] |
| PVA/β-CD/acylated roselle anthocyanin | Freshness | Colorimetric | [137] |
| Chitosan/PVA/curcumin:β-CD | Freshness | Colorimetric | [138] |
| Rhodamine 6G-adamantamine and β-CD | Shelf-life | Fluorescence | [154] |
| GCE/GNDPC:β-CD | Electrochemical | Recovery (MP) | [147] |
| Chitosan/cation guar gum/MWCNTs/β-CD/GCE | Electrochemical | Recovery (amaranth) | [151] |
| MWCNTs/β-CD/GCE | Electrochemical | Recovery (capsaicin) | [97] |
| MWCNTs/β-CD/GCE | Electrochemical | Quantification (vitamins) | [153] |
| β-CD:AuNPs | Spoilage | Colorimetric | [145] |
| rGO/GCE/β-CD | Electrochemical | Quantification (capsaicin) | [149] |
| AuNPs:β-CD/arginine | Electrochemical | Quantification (colorant) | [148] |
6. Conclusion and future trends
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Accorsi, R.; Manzini, R. (Eds.) Modeling inclusive food supply chains toward sustainable ecosystem planning. In Sustainable Food Supply Chains; Academic Press: Dordrecht, The Netherlands, 2019; pp. 1–21. [Google Scholar]
- Haji, M.; Kerbache, L.; Al-Ansari, T. Food Quality, Drug Safety, and Increasing Public Health Measures in Supply Chain Management. Processes 2022, 10, 1715. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, M.; Fang, Z. Perspectives on Novel Technologies of Processing and Monitoring the Safety and Quality of Prepared Food Products. Foods 2023, 12, 3052. [Google Scholar] [CrossRef] [PubMed]
- Han, J. W.; Ruiz-Garcia, L.; Qian, J. P.; Yang, X. T. (2018). Food Packaging: A Comprehensive Review and Future Trends. In Comprehensive Reviews in Food Science and Food Safety (Vol. 17, Issue 4, pp. 860–877). Blackwell Publishing Inc.
- Alam, A.U.; Rathi, P.; Beshai, H.; Sarabha, G.K.; Deen, M.J. Fruit Quality Monitoring with Smart Packaging. Sensors 2021, 21, 1509. [Google Scholar] [CrossRef] [PubMed]
- Stramarkou, M.; Boukouvalas, C.; Koskinakis, S.E.; Serifi, O.; Bekiris, V.; Tsamis, C.; Krokida, M. Life Cycle Assessment and Preliminary Cost Evaluation of a Smart Packaging System. Sustainability 2022, 14, 7080. [Google Scholar] [CrossRef]
- Da Hu, Q.; Tang, G.P.; Chu, P.K. Cyclodextrin-based host-guest supramolecular nanoparticles for delivery: From design to applications. Acc. Chem. Res. 2014, 47, 2017–2025. [Google Scholar]
- Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; López-Nicolás, J.M. Applications of cyclodextrins in food science. A review. Trends Food Sci. Technol. 2020, 104, 132–143. [Google Scholar] [CrossRef]
- Astray, G.; Mejuto, J.C.; Simal-Gandara, J. Latest developments in the application of cyclodextrin host-guest complexes in beverage technology processes. Food Hydrocoll. 2020, 106, 105882. [Google Scholar] [CrossRef]
- Liu, H.-N.; Jiang, X.-X.; Naeem, A.; Chen, F.-C.; Wang, L.; Liu, Y.-X.; Li, Z.; Ming, L.-S. Fabrication and Characterization of β-Cyclodextrin/Mosla Chinensis Essential Oil Inclusion Complexes: Experimental Design and Molecular Modeling. Molecules 2023, 28, 37. [Google Scholar] [CrossRef]
- Bortolus, P.; Grabner, G.; Köhler, G.; Monti, S. Photochemistry of cyclodextrin host-guest complexes. Coord. Chem. Rev. 1993, 125, 261–268. [Google Scholar] [CrossRef]
- Cram, D.J.; Cram, J.M. Host-Guest Chemistry. Science 1974, 183, 803–809. [Google Scholar] [CrossRef]
- Lehn, J.M. Supramolecular chemistry: Receptors, catalysts, and carriers. Science 1985, 227, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Aiassa, V.; Garnero, C.; Zoppi, A.; Longhi, M.R. Cyclodextrins and Their Derivatives as Drug Stability Modifiers. Pharmaceuticals 2023, 16, 1074. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, A.H.; Szeleszczuk, Ł. A Review of Applications of Solid-State Nuclear Magnetic Resonance (ssNMR) for the Analysis of Cyclodextrin-Including Systems. Int. J. Mol. Sci. 2023, 24, 3648. [Google Scholar] [CrossRef] [PubMed]
- Stella, V.J.; Rajewski, R.A. Sulfobutylether-β-cyclodextrin. Int. J. Pharm. 2020, 583, 119396. [Google Scholar] [CrossRef] [PubMed]
- González Pereira, A.; Carpena, M.; García Oliveira, P.; Mejuto, J.C.; Prieto, M.A.; Simal Gandara, J. Principales aplicaciones de las ciclodextrinas en la industria alimentaria como los compuestos de elección para formar complejos huésped-invitado. Int. J. Mol. Sci. 2021, 22, 1339. [Google Scholar]
- Zhou, J.; Jia, J.; He, J.; Li, J.; Cai, J. Cyclodextrin Inclusion Complexes and Their Application in Food Safety Analysis: Recent Developments and Future Prospects. Foods 2022, 11, 3871. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Munoz-Shuguli, C.; Vidal, C.P.; Cantero-Lopez, P.; Lopez-Polo, J. Encapsulation of plant extract compounds using cyclodextrin inclusion complexes, liposomes, electrospinning and their combinations for food purposes. Trends Food Sci. Technol. 2021, 108, 177–186. [Google Scholar] [CrossRef]
- Chizhov, A.O.; Tsvetkov, Y.E.; Nifantiev, N.E. Gas-Phase Fragmentation of Cyclic Oligosaccharides in Tandem Mass Spectrometry. Molecules 2019, 24, 2226. [Google Scholar] [CrossRef]
- Szente, L.; Szejtli, J. Cyclodextrins as food ingredients. Trends Food Sci. Technol. 2004, 15, 137–142. [Google Scholar] [CrossRef]
- van der Veen, B.A.; Uitdehaag, J.C.M.; Penninga, D.; van Alebeek, G.; Smith, L.M.; Dijkstra, B.W.; Dijkhuizen, L. Rational design of cyclodextrin glycosyltransferase from Bacillus circulans strain 251 to increase alpha-cyclodextrin production. J. Mol. Biol. 2000, 296, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Messner, M.; Kurkov, S.V.; Flavia-Piera, R.; Brewster, M.E.; Loftsson, T. Self-assembly of cyclodextrins: The effect of the guest molecule. Int. J. Pharm. 2011, 408, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Luo, Z.; Xiao, Z. Preparation, Characterization, and Thermal Stability of β-Cyclodextrin/Soybean Lecithin Inclusion Complex. Carbohydr. Polym. 2014, 101, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Braithwaite, M.C.; Kumar, P.; Choonara, Y.E.; du Toit, L.C.; Tomar, L.K.; Tyagi, C.; Pillay, V. A novel multi-tiered experimental approach unfolding the mechanisms behind cyclodextrin-vitamin inclusion complexes for enhanced vitamin solubility and stability. Int. J. Pharm. 2017, 532, 90–104. [Google Scholar] [CrossRef]
- Szente, L.; Fenyvesi, É. Cyclodextrin-Enabled Polymer Composites for Packaging. Molecules 2018, 23, 1556. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, S.; Gu, Z.; Chen, J.; Wu, J. Alpha-cyclodextrin: Enzymatic production and food applications. Trends Food Sci. Technol. 2014, 35, 151–160. [Google Scholar] [CrossRef]
- Samuelsen, L.; Holm, R.; Schönbeck, C. Simultaneous Determination of Cyclodextrin Stability Constants as a Function of pH and Temperature—A Tool for Drug Formulation and Process Design. J. Drug Deliv. Sci. Technol. 2021, 65, 102675. [Google Scholar] [CrossRef]
- Wei, X.; Yu, C.-Y.; Wei, H. Application of Cyclodextrin for Cancer Immunotherapy. Molecules 2023, 28, 5610. [Google Scholar] [CrossRef]
- Murrieta-Rico, F.N.; Yocupicio-Gaxiola, R.I.; Antúnez-Garcia, J.; Reyes-Serrato, A.; Sánchez, P.; Petranovskii, V. Textile Functionalization Using LTA and FAU Zeolitic Materials. Polymers 2022, 15, 99. [Google Scholar] [CrossRef]
- Sadjadi, S.; Koohestani, F. Composite of β-cyclodextrin and bentonite clay: A promising support for Pd immobilization and developing a catalyst for hydrogenation of nitroarenes under mild reaction condition. J. Phys. Chem. Solids 2021, 151, 109894. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Pharmaceutical Applications of Cyclodextrins: Basic Science and Product Development. J. Pharm. Pharmacol. 2010, 62, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Păduraru, D.N.; Niculescu, A.G.; Bolocan, A.; Andronic, O.; Grumezescu, A.M.; Bîrlă, R. An Updated Overview of Cyclodextrin-Based Drug Delivery Systems for Cancer Therapy. Pharmaceutics 2022, 14, 1748. [Google Scholar] [CrossRef]
- Crini, G.; Fourmentin, S.; Fenyvesi, É.; Torri, G.; Fourmentin, M.; Morin-Crini, N. Fundamentals and applications of cyclodextrins. In Cyclodextrin Fundamentals, Reactivity and Analysis; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–55. [Google Scholar]
- Řezanka, M. Synthesis of cyclodextrin derivatives. In Cyclodextrin Fundamentals, Reactivity and Analysis; Springer: Berlin/Heidelberg, Germany, 2018; pp. 57–103. [Google Scholar]
- Leclercq, L. Interactions between cyclodextrins and cellular components: Towards greener medical applications? Beilstein J. Org. Chem. 2016, 12, 2644–2662. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Y.N.; Gao, X.L.; Fu, J.J.; Hu, L.D. Application of cyclodextrin in food industry. Crit. Rev. Food Sci. Nutr. 2022, 62, 2627–2640. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Rehman, Q.; Akash, M.S.H.; Imran, I.; Rehman, K. Stability of Pharmaceutical Products. In Drug Stability and Chemical Kinetics; Akash, M.S.H., Rehman, K., Eds.; Springer: Singapore, 2020; pp. 147–154. ISBN 978-981-15-6426-0. [Google Scholar]
- Loftsson, T. Introduction. In Drug Stability for Pharmaceutical Scientists; Loftsson, T., Ed.; Academic Press: New York, NY, USA, 2014; pp. 1–3. ISBN 9780124115484. [Google Scholar]
- Przybyla, M.A.; Yilmaz, G.; Becer, C.R. Natural cyclodextrins and their derivatives for polymer synthesis. Polym. Chem. 2020, 11, 7582–7602. [Google Scholar] [CrossRef]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in pharmaceutical formulations II: Solubilization, binding constant, and complexation efficiency. Drug Discov. Today 2016, 21, 363–368. [Google Scholar] [CrossRef]
- Lachowicz, M.; Stańczak, A.; Kołodziejczyk, M. Characteristic of Cyclodextrins: Their Role and Use in the Pharmaceutical Technology. Curr. Drug Targets 2020, 21, 1495–1510. [Google Scholar] [CrossRef]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of cyclodextrins and drug/cyclodextrin complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef]
- De Marco, I.; Franco, P. Effect of the Carrier on the Coprecipitation of Curcumin through Supercritical-Assisted Atomization. ChemEngineering 2021, 5, 59. [Google Scholar] [CrossRef]
- Linde, G.A.; Laverde, A.; Colauto, N.B. Changes to Taste Perception in the Food Industry: Use of Cyclodextrins. In Handbook of Behavior, Food and Nutrition; Preedy, V.R., Watson, R.R., Martin, C.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 99–118. [Google Scholar]
- Blanch, G.P.; Ruiz del Castillo, M.L.; del Mar Caja, M.; Pérez-Méndez, M.; Sánchez-Cortés, S. Stabilization of all-trans-lycopene from tomato by encapsulation using cyclodextrins. Food Chem. 2007, 105, 1335–1341. [Google Scholar] [CrossRef]
- Andreu-Sevilla, A.J.; López-Nicolás, J.M.; Carbonell-Barrachina, Á.A.; García-Carmona, F. Comparative Effect of the Addition of α-, β-, or γ-Cyclodextrin on Main Sensory and Physico-Chemical Parameters. J. Food Sci. 2011, 76, S347–S353. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, C.; Buera, P.; Mazzobre, F. Novel trends in cyclodextrins encapsulation. Applications in food science. Curr. Opin. Food Sci. 2017, 16, 106–113. [Google Scholar] [CrossRef]
- Irie, T.; Uekama, K. Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J. Pharm. Sci. 1997, 86, 147–162. [Google Scholar] [PubMed]
- Krawczyk, K.; Silvestri, D.; Nguyen, N.H.; Ševců, A.; Łukowiec, D.; Padil, V.V.; Řezanka, M.; Černík, M.; Dionysiou, D.D.; Wacławek, S. Enhanced degradation of sulfamethoxazole by a modified nano zero-valent iron with a β-cyclodextrin polymer: Mechanism and toxicity evaluation. Sci. Total Environ. 2022, 817, 152888. [Google Scholar] [CrossRef]
- Jumah, M.N.B.; Eid, M.H.; AL-Huqail, A.A.; Mohammad, M.A.; Bin-Murdhi, N.S.; Abu-Taweel, G.M.; Altoom, N.; Allam, A.A.; AbuKhadra, M.R. Enhanced remediation of As (V) and Hg (II) ions from aqueous environments using β-cyclodextrin/MCM-48 composite: Batch and column studies. J. Water Process. Eng. 2021, 42, 102118. [Google Scholar] [CrossRef]
- Paramita, V.; Novia, S.F.; Ariyanto, H.D.; Pramudono, B.; Yoshii, H.; Kusumayanti, H.; Amalia, R. The influence of α-cyclodextrin on the stability and fatty acids of medium-chain triglycerides high oil load nanoemulsion. Mater. Today Proc. 2022, 63, S312–S317. [Google Scholar] [CrossRef]
- Cid-Samamed, A.; Rakmai, J.; Mejuto, J.C.; Simal-Gandara, J.; Astray, G. Cyclodextrins inclusion complex: Preparation methods, analytical techniques and food industry applications. Food Chem. 2022, 384, 132467. [Google Scholar] [CrossRef]
- Astray, G.; Gonzalez-Barreiro, C.; Mejuto, J.C.; Rial-Otero, R.; Simal-Gandara, J. A review on the use of cyclodextrins in foods. Food Hydrocoll. 2009, 23, 1631–1640. [Google Scholar] [CrossRef]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Leblanc, J.; et al. Re-evaluation of β-cyclodextrin (E 459) as a food additive. EFSA J. 2016, 14, e04628. [Google Scholar]
- Munro, I.C.; Newberne, P.M.; Young, V.R.; Bär, A. Safety assessment of γ-cyclodextrin. Regul. Toxicol. Pharmacol. 2004, 39, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Mura, P. Analytical Techniques for Characterization of Cyclodextrin Complexes in Aqueous Solution: A Review. J. Pharm. Biomed. Anal. 2014, 101, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Abukhadra, M.R.; Refay, N.M.; Sharaf, M.F.; El-Meligy, M.A.; Awwad, E.M. Synthesis of chitosan/MCM-48 and β-cyclodextrin/MCM-48 composites as bio-adsorbents for environmental removal of Cd2+ ions; kinetic and equilibrium studies. React. Funct. Polym. 2020, 154, 104675. [Google Scholar] [CrossRef]
- Chen, L.; Dong, Q.; Shi, Q.; Du, Y.; Zeng, Q.; Zhao, Y.; Wang, J.J. Novel 2,3-Dialdehyde Cellulose-Based Films with Photodynamic Inactivation Potency by Incorporating the β-Cyclodextrin/Curcumin Inclusion Complex. Biomacromolecules 2021, 22, 2790–2801. [Google Scholar] [CrossRef] [PubMed]
- Muankaew, C.; Loftsson, T. Cyclodextrin-based formulations: A non-invasive platform for targeted drug delivery. Basic Clin. Pharmacol. Toxicol. 2018, 122, 46–55. [Google Scholar] [CrossRef]
- Novac, M.; Musuc, A.M.; Ozon, E.A.; Sarbu, I.; Mitu, M.A.; Rusu, A.; Petrescu, S.; Atkinson, I.; Gheorghe, D.; Lupuliasa, D. Design and Evaluation of Orally Dispersible Tablets Containing Amlodipine Inclusion Complexes in Hydroxypropyl-β-cyclodextrin and Methyl-β-cyclodextrin. Materials 2022, 15, 5217. [Google Scholar] [CrossRef]
- Hernandez-Sanchez, P.; Lopez-Miranda, S.; Guardiola, L.; Serrano-Martinez, A.; Gabaldon, J.A.; Nunez-Delicado, E. Optimization of a method for preparing solid complexes of essential clove oil with beta-cyclodextrins. J. Sci. Food Agric. 2017, 97, 420–426. [Google Scholar] [CrossRef]
- Jiang, L.W.; Yang, J.D.; Wang, Q.; Ren, L.; Zhou, J. Physicochemical properties of catechin/beta-cyclodextrin inclusion complex obtained via co-precipitation. CyTA-J. Food 2019, 17, 544–551. [Google Scholar] [CrossRef]
- Shi, Z.; Kong, G.; Wang, F.; Gao, H.; Wei, A.; Ren, S.; Yan, X. Improvement in the stability and bioavailability of pumpkin lutein using β-cyclodextrin microcapsules. Food Sci Nutr 2023, 11, 3067–3074. [Google Scholar] [CrossRef]
- Liang, Y.; Hou, D.; Ni, Z.; Cao, M.; Cai, L. Preparation, characterization of naringenin, β-cyclodextrin and carbon quantum dot antioxidant nanocomposites. Food Chem 2022, 375, 131646. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Han, Y.; Meng, X.; Xiao, Y.; Zhang, H. Cellulose Nanocrystals Reinforced Zein/Catechin/β-Cyclodextrin Inclusion Complex Nanoparticles Nanocomposite Film for Active Food Packaging. Polymers 2021, 13, 2759. [Google Scholar] [CrossRef] [PubMed]
- Salazar Sandoval, S.; Bruna, T.; Maldonado-Bravo, F.; Bolaños, K.; Adasme-Reyes, S.; Riveros, A.; Caro, N.; Yutronic, N.; Silva, N.; Kogan, M.J.; et al. β-Cyclodextrin Nanosponges Inclusion Compounds Associated with Silver Nanoparticles to Increase the Antimicrobial Activity of Quercetin. Materials 2023, 16, 3538. [Google Scholar] [CrossRef] [PubMed]
- Farouk, A.; Sharaf, S.; Refaie, R.; Abd El-Hady, M.M. Highly Durable Antibacterial Properties of Cellulosic Fabric via β-Cyclodextrin/Essential Oils Inclusion Complex. Polymers 2022, 14, 4899. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, L.; Peng, L.; Du, J.; Lan, M.; Cheng, Y.; Ma, L.; Zhang, Y. Dual encapsulation of β-carotene by β-cyclodextrin and chitosan for 3D printing application. Food Chem. 2022, 378, 132088. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Shugulí, C.; Rodríguez-Mercado, F.; Benbettaieb, N.; Guarda, A.; Galotto, M.J.; Debeaufort, F. Development and Evaluation of the Properties of Active Films for High-Fat Fruit and Vegetable Packaging. Molecules 2023, 28, 3045. [Google Scholar] [CrossRef]
- Dobrzyńska-Mizera, M.; Knitter, M.; Piss, M.; Del Barone, C.; Mallardo, S.; Santagata, G.; Di Lorenzo, M.L. Thermal and Morphological Analysis of Linear Low-Density Polyethylene Composites Containing d-limonene/β-cyclodextrin for Active Food Packaging. Molecules 2023, 28, 1220. [Google Scholar] [CrossRef]
- Christaki, S.; Kelesidou, R.; Pargana, V.; Tzimopoulou, E.; Hatzikamari, M.; Mourtzinos, I. Inclusion Complexes of β-Cyclodextrin with Salvia officinalis Bioactive Compounds and Their Antibacterial Activities. Plants 2023, 12, 2518. [Google Scholar] [CrossRef]
- Li, W.; Liu, X.; Yang, Q.; Zhang, N.; Du, Y.; Zhu, H. Preparation and characterization of inclusion complex of benzyl isothiocyanate extracted from papaya seed with β-cyclodextrin. Food Chem. 2015, 184, 99–104. [Google Scholar] [CrossRef]
- Garcia-Sotelo, D.; Silva-Espinoza, B.; Perez-Tello, M.; Olivas, I.; Alvarez-Parrilla, E.; González-Aguilar, G.A.; Ayala-Zavala, J.F. Antimicrobial activity and thermal stability of rosemary essential oil: β−cyclodextrin capsules applied in tomato juice. LWT 2019, 111, 837–845. [Google Scholar] [CrossRef]
- Liu, D.; Zhong, Y.; Pu, Y.; Li, X.; Chen, S.; Zhang, C. Preparation of pH-Responsive Films from Polyvinyl Alcohol/Agar Containing Cochineal for Monitoring the Freshness of Pork. Foods 2023, 12, 2316. [Google Scholar] [CrossRef]
- Liu, F.; Yu, Z.; Wang, B.; Chiou, B.-S. Changes in Structures and Properties of Collagen Fibers during Collagen Casing Film Manufacturing. Foods 2023, 12, 1847. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Yue, S.; Xiang, H.; Xie, M. Inclusion complexes of eucalyptus essential oil with β-cyclodextrin: Preparation, characterization and controlled release. J. Porous Mater. 2018, 25, 1577–1586. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, R.; Sun, X.; Yu, X.; Xiao, Y.; Wang, L.; Hu, W.; Zhong, T. The p-Anisaldehyde/β-cyclodextrin inclusion complexes as a sustained release agent: Characterization, storage stability, antibacterial and antioxidant activity. Food Control 2022, 132, 108561. [Google Scholar] [CrossRef]
- Santana, D.V.S.; Trindade, I.A.S.; Carvalho, Y.M.B.G.; Carvalho-Neto, A.G.; Silva, E.C.D.; Silva-Júnior, E.F.; Leite, R.F.S.; Quintans-Júnior, L.J.; Aquino, T.M.; Serafini, M.R.; et al. Analytical techniques to recognize inclusion complexes formation involving monoterpenes and cyclodextrins: A study case with (–) borneol, a food ingredient. Food Chem. 2021, 339, 127791. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Wang, F.; Wang, L.-X. Preparation and Properties of Ginger Essential Oil β-Cyclodextrin/Chitosan Inclusion Complexes. Coatings 2018, 8, 305. [Google Scholar] [CrossRef]
- Grgac, S.F.; Jablan, J.; Inić, S.; Malinar, R.; Kovaček, I.; Čorak, I. The Effect of Ultrasonic Treatment on the Binding of the Inclusion Complex β-Cyclodextrin-peppermint Oil with Cellulose Material. Materials 2022, 15, 470. [Google Scholar] [CrossRef] [PubMed]
- Torres-Alvarez, C.; Castillo, S.; Sánchez-García, E.; Aguilera González, C.; Galindo-Rodríguez, S.A.; Gabaldón-Hernández, J.A.; Báez-González, J.G. Inclusion Complexes of Concentrated Orange Oils and β-Cyclodextrin: Physicochemical and Biological Characterizations. Molecules 2020, 25, 5109. [Google Scholar] [CrossRef]
- Wu, H.; Ao, X.; Liu, J.; Zhu, J.; Bi, J.; Hou, H.; Hao, H.; Zhang, G. A Bioactive Chitosan−Based Film Enriched with Benzyl Isothiocyanate/α−Cyclodextrin Inclusion Complex and Its Application for Beef Preservation. Foods 2022, 11, 2687. [Google Scholar] [CrossRef]
- Liu, J.; Wu, H.; Ao, X.; Hao, H.; Bi, J.; Hou, H.; Zhang, G. Characterization of the Inclusion Complexes of Isothiocyanates with γ-Cyclodextrin for Improvement of Antibacterial Activities against Staphylococcus aureus. Foods 2022, 11, 60. [Google Scholar] [CrossRef]
- Xiao, Z.; Hou, W.; Kang, Y.; Niu, Y.; Kou, X. Encapsulation and sustained release properties of watermelon flavor and its characteristic aroma compounds from γ-cyclodextrin inclusion complexes. Food Hydrocolloids 2019, 97, 105202. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Y.; OuYang, Q.; Duan, B.; Wang, Z.; Meng, K.; Tan, X.; Tao, N. γ-Cyclodextrin encapsulated thymol for citrus preservation and its possible mechanism against Penicillium digitatum. Pesticide Biochemistry and Physiology 2023, 194, 105501. [Google Scholar] [CrossRef] [PubMed]
- Šimko, P.; Kolarič, L. Decrease in Aflatoxin M1 Concentration in Milk during Cholesterol Removal by Application of β-Cyclodextrin. Toxins 2022, 14, 379. [Google Scholar] [CrossRef] [PubMed]
- Kolarič, L.; Kántorová, P.; Šimko, P. β-Cyclodextrin as the Key Issue in Production of Acceptable Low-Cholesterol Dairy Products. Molecules 2022, 27, 2919. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.; Jiranek, V.; Taylor, D.K.; Wilkinson, K.L. Removal of Volatile Phenols from Wine Using Crosslinked Cyclodextrin Polymers. Molecules 2020, 25, 910. [Google Scholar] [CrossRef]
- Fliszár-Nyúl, E.; Lemli, B.; Kunsági-Máté, S.; Szente, L.; Poór, M. Interactions of Mycotoxin Alternariol with Cyclodextrins and Its Removal from Aqueous Solution by Beta-Cyclodextrin Bead Polymer. Biomolecules 2019, 9, 428. [Google Scholar] [CrossRef]
- Kiel, S.; Poverenov, E. Rechargeable films for protection of dry foods: A sustainable method for covalent grafting of β-cyclodextrin-thymol complex on PET/viscose platform. Food Chem 2023, 412, 135560. [Google Scholar] [CrossRef]
- Szejtli, J.; Szente, L. Elimination of bitter, disgusting tastes of drugs and foods by cyclodextrins. Eur. J. Pharm. Biopharm. 2005, 61, 115–125. [Google Scholar] [CrossRef]
- Wu, X.; Fu, B.; Ma, Y.; Dong, L.; Du, M.; Dong, X.; Xu, X. A Debittered Complex of Glucose-Phenylalanine Amadori Rearrangement Products with β-Cyclodextrin: Structure, Molecular Docking and Thermal Degradation Kinetic Study. Foods 2022, 11, 1309. [Google Scholar] [CrossRef]
- Gu, Q.; Lu, C.; Chen, K.; Chen, X.; Ma, P.; Wang, Z.; Xu, B. Electrochemical Determination of Capsaicinoids Content in Soy Sauce and Pot-Roast Meat Products Based on Glassy Carbon Electrode Modified with Β-Cyclodextrin/Carboxylated Multi-Wall Carbon Nanotubes. Foods 2021, 10, 1743. [Google Scholar] [CrossRef]
- Lachowicz, S.; Oszmiański, J.; Uździcka, M.; Chmielewska, J. The Influence of Yeast Strain, β-Cyclodextrin, and Storage Time on Concentrations of Phytochemical Components, Sensory Attributes, and Antioxidative Activity of Novel Red Apple Ciders. Molecules 2019, 13, 2477. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Jiang, S.; Chen, Y.; Wei, Y.; Shao, X. Comparison of Inhibitory Effects of Cinnamic Acid, β-Cyclodextrin, L-Cysteine, and Ascorbic Acid on Soluble and Membrane-Bound Polyphenol Oxidase in Peach Fruit. Foods 2023, 12, 167. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, J.; Xu, F.; Zhou, A.; Zeng, S.; Zheng, B.; Lin, S. Effective Preservation of Chilled Pork Using Photodynamic Antibacterial Film Based on Curcumin-β-Cyclodextrin Complex. Polymers 2023, 15, 1023. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, T.; Chai, X.; Duan, X.; He, D.; Yu, H.; Liu, X.; Tao, Z. Encapsulation Efficiency and Functional Stability of Cinnamon Essential Oil in Modified β-cyclodextrins: In Vitro and In Silico Evidence. Foods 2023, 12, 45. [Google Scholar] [CrossRef]
- Zhang, N.; Bi, F.; Xu, F.; Yong, H.; Bao, Y.; Jin, C.; Liu, J. Structure and functional properties of active packaging films prepared by incorporating different flavonols into chitosan based matrix. Int. J. Biol. Macromol. 2020, 165, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Beshai, H.; Sarabha, G.K.; Rathi, P.; Alam, A.U.; Deen, M.J. Freshness Monitoring of Packaged Vegetables. Appl. Sci. 2020, 10, 7937. [Google Scholar] [CrossRef]
- Bangalore, D.V.; McGlynn, W.; Scott, D.D. Effect of beta-cyclodextrin in improving the correlation between lycopene concentration and ORAC values. J. Agric. Food Chem. 2005, 53, 1878–1883. [Google Scholar] [CrossRef]
- Choi, M.-J.; Ruktanonchai, U.; Min, S.-G.; Chun, J.-Y.; Soottitantawat, A. Physical characteristics of fish oil encapsulated by β-cyclodextrin using an aggregation method or polycaprolactone using an emulsion-diffusion method. Food Chem. 2010, 119, 1694–1703. [Google Scholar] [CrossRef]
- Li, J.; Xiong, S.; Wang, F.; Regenstein, J.M.; Liu, R. Optimization of Microencapsulation of Fish Oil with Gum Arabic/Casein/Beta-Cyclodextrin Mixtures by Spray Drying. J. Food Sci. 2015, 80, C1445–C1452. [Google Scholar] [CrossRef]
- Kuswandi, B. Jumina Active and intelligent packaging, safety, and quality controls. In Fresh-Cut Fruits and Vegetables: Technologies and Mechanisms for Safety Control; Wasim Siddiqui, M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 243–294. ISBN 9780128161845. [Google Scholar]
- Wyrwa, J.; Barska, A. Innovations in the food packaging market: Active packaging. Eur. Food Res. Technol. 2017, 243, 1681–1692. [Google Scholar] [CrossRef]
- Iturralde-García, R.D.; Cinco-Moroyoqui, F.J.; Martínez-Cruz, O.; Ruiz-Cruz, S.; Wong-Corral, F.J.; Borboa-Flores, J.; Cornejo-Ramírez, Y.I.; Bernal-Mercado, A.T.; Del-Toro-Sánchez, C.L. Emerging Technologies for Prolonging Fresh-Cut Fruits’ Quality and Safety during Storage. Horticulturae 2022, 8, 731. [Google Scholar] [CrossRef]
- Nowak, J.K.; Sobkowiak, P.; Drzymała-Czyż, S.; Krzyżanowska-Jankowska, P.; Sapiejka, E.; Skorupa, W.; Pogorzelski, A.; Nowicka, A.; Wojsyk-Banaszak, I.; Kurek, S.; et al. Fat-Soluble Vitamin Supplementation Using Liposomes, Cyclodextrins, or Medium-Chain Triglycerides in Cystic Fibrosis: A Randomized Controlled Trial. Nutrients 2021, 13, 4554. [Google Scholar] [CrossRef] [PubMed]
- Goñi-Ciaurriz, L.; González-Gaitano, G.; Vélaz, I. Cyclodextrin-grafted nanoparticles as food preservative carriers. Int J Pharm 2020, 588, 119664. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, Y.; Jiang, Y.; Zhou, F.; Wu, Y.; Jiang, H.; Wang, R.; Xu, Q.; Hua, C. Encapsulating quercetin in cyclodextrin metal-organic frameworks improved its solubility and bioavailability. J. Sci. Food Agric. 2022, 102, 3887–3896. [Google Scholar] [CrossRef]
- Vhangani, L.N.; Favre, L.C.; Rolandelli, G.; Van Wyk, J.; del Pilar Buera, M. Optimising the Polyphenolic Content and Antioxidant Activity of Green Rooibos (Aspalathus linearis) Using Beta-Cyclodextrin Assisted Extraction. Molecules 2022, 27, 3556. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Xie, P.; Zhou, Y.; Guo, J. Characterization, Thermal Stability and Antimicrobial Evaluation of the Inclusion Complex of Litsea cubeba Essential Oil in Large-Ring Cyclodextrins (CD9–CD22). Foods 2023, 12, 2035. [Google Scholar] [CrossRef]
- Arrais, A.; Bona, E.; Todeschini, V.; Caramaschi, A.; Massa, N.; Roncoli, M.; Minervi, A.; Perin, E.; Gianotti, V. Thymus vulgaris Essential Oil in Beta-Cyclodextrin for Solid-State Pharmaceutical Applications. Pharmaceutics 2023, 15, 914. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, T.; Chai, X.; Duan, X.; He, D.; Yu, H.; Liu, X.; Tao, Z. Encapsulation Efficiency and Functional Stability of Cinnamon Essential Oil in Modified β-cyclodextrins: In Vitro and In Silico Evidence. Foods 2023, 12, 45. [Google Scholar] [CrossRef]
- Li, H.; Ming, X.; Wang, Z.; Li, J.; Liang, Y.; Xu, D.; Liu, Z.; Hu, L.; Mo, H. Encapsulation of Benzyl Isothiocyanate with β-Cyclodextrin Using Ultrasonication: Preparation, Characterization, and Antibacterial Assay. Foods 2022, 11, 3724. [Google Scholar] [CrossRef]
- Liu, C.; Tian, Y.; Ma, Z.; Zhou, L. Pickering Emulsion Stabilized by β-Cyclodextrin and Cinnamaldehyde/β-Cyclodextrin Composite. Foods 2023, 12, 2366. [Google Scholar] [CrossRef]
- Zhai, X.; Zou, X.; Shi, J.; Huang, X.; Sun, Z.; Li, Z.; Sun, Y.; Li, Y.; Wang, X.; Holmes, M.; et al. Amine-responsive bilayer films with improved illumination stability and electrochemical writing property for visual monitoring of meat spoilage. Sens. Actuators B Chem. 2020, 302, 127130. [Google Scholar] [CrossRef]
- Velázquez-Contreras, F.; García-Caldera, N.; Padilla de la Rosa, J.D.; Martínez-Romero, D.; Núñez-Delicado, E.; Gabaldón, J.A. Effect of PLA Active Packaging Containing Monoterpene-Cyclodextrin Complexes on Berries Preservation. Polymers 2021, 13, 1399. [Google Scholar] [CrossRef] [PubMed]
- Hădărugă, N.G.; Chirilă, C.A.; Szakal, R.N.; Gălan, I.M.; Simandi, M.D.; Bujancă, G.S.; David, I.; Riviş, A.; Stanciu, S.M.; Hădărugă, D.I. FTIR–PCA Approach on Raw and Thermally Processed Chicken Lipids Stabilized by Nano-Encapsulation in β-Cyclodextrin. Foods 2022, 11, 3632. [Google Scholar] [CrossRef] [PubMed]
- Kato, L.S.; Conte-Junior, C.A. Safety of plastic food packaging: The challenges about non-intentionally added-substances (NIAS) discovery, identification and risk assessment. Polymers 2021, 13, 2077. [Google Scholar] [CrossRef]
- Liu, D.; Cui, Z.; Shang, M.; Zhong, Y. A colorimetric film based on polyvinyl alcohol/sodium carboxymethyl cellulose incorporated with red cabbage anthocyanin for monitoring pork freshness. Food Packag. Shelf Life 2021, 28, 100641. [Google Scholar] [CrossRef]
- Drago, E.; Campardelli, R.; Pettinato, M.; Perego, P. Innovations in Smart Packaging Concepts for Food: An Extensive Review. Foods 2020, 9, 1628. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.G.; Yuhana, N.Y.; Zawawi, E.Z.E. Review of bioplastics as food packaging materials. AIMS Mater. Sci. 2021, 8, 166–184. [Google Scholar] [CrossRef]
- Gallo, M.; Ferrara, L.; Calogero, A.; Montesano, D.; Naviglio, D. Relationships between food and diseases: What to know to ensure food safety. Food Res. Int. 2020, 137, 109414. [Google Scholar] [CrossRef]
- Petrescu, D.C.; Vermeir, I.; Petrescu-Mag, R.M. Consumer Understanding of Food Quality, Healthiness, and Environmental Impact: A Cross-National Perspective. Int. J. Environ. Res. Public Health 2020, 17, 169. [Google Scholar] [CrossRef]
- Fang, Z.; Zhao, Y.; Warner, R.D.; Johnson, S.K. Active and intelligent packaging in meat industry. Trends Food Sci. Technol. 2017, 61, 60–71. [Google Scholar] [CrossRef]
- Cheng, H.; Xu, H.; McClements, D.J.; Chen, L.; Jiao, A.; Tian, Y.; Miao, M.; Jin, Z. Recent advances in intelligent food packaging materials: Principles, preparation and applications. Food Chem. 2022, 375, 131738. [Google Scholar] [CrossRef] [PubMed]
- Ghaani, M.; Cozzolino, C.A.; Castelli, G.; Farris, S. An overview of the intelligent packaging technologies in the food sector. Trends Food Sci. Technol. 2016, 51, 1–11. [Google Scholar] [CrossRef]
- Ahmed, I.; Lin, H.; Zou, L.; Li, Z.; Brody, A.L.; Qazi, I.M.; Lv, L.; Pavase, T.R.; Khan, M.U.; Khan, S.; et al. An overview of smart packaging technologies for monitoring safety and quality of meat and meat products. Packag. Technol. Sci. 2018, 31, 449–471. [Google Scholar] [CrossRef]
- Soltani Firouz, M.; Mohi-Alden, K.; Omid, M. A critical review on intelligent and active packaging in the food industry: Research and development. Food Res. Int. 2021, 141, 110113. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, M.; Lu, P.; Gao, L.; Yan, S.; Wang, S. Development of pH Indicator and Antimicrobial Cellulose Nanofibre Packaging Film Based on Purple Sweet Potato Anthocyanin and Oregano Essential Oil. Int. J. Biol. Macromol. 2020, 149, 271–280. [Google Scholar] [CrossRef]
- Chaudhary, V.; Punia Bangar, S.; Thakur, N.; Trif, M. Recent Advancements in Smart Bio-genic Packaging: Reshaping the Future of the Food Packaging Industry. Polymers 2022, 14, 829. [Google Scholar] [CrossRef]
- Müller, P.; Schmid, M. Intelligent Packaging in the Food Sector: A Brief Overview. Foods 2019, 8, 16. [Google Scholar] [CrossRef]
- Jarupatnadech, T.; Chalitangkoon, J.; Monvisade, P. Colorimetric oxygen indicator films based on β-cyclodextrin grafted chitosan/montmorillonite with redox system for intelligent food packaging. Packaging Technol. and Sci 2022, 35, 515–525. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, X.; Song, W.; Du, L.; Shi, J.; Huang, X.; Zhang, J.; Li, Z.; Liu, L. Development and Application of Visual Freshness Indicator Film with High Stability Based on Acylated Anthocyanins. Shipin Kexue/Food Science 2023, 44, 194–200. [Google Scholar]
- Lin, W.; Hong, W.; Sun, Y.; Huang, J.; Li, Z. Triple-function chitosan-based film for pork and shrimp packaging. Food Chemistry 2023, 417, 135903. [Google Scholar] [CrossRef]
- Rawdkuen, S.; Faseha, A.; Benjakul, S.; Kaewprachu, P. Application of Anthocyanin as a Color Indicator in Gelatin Films. Food Biosci. 2020, 36, 100603. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Y.; Bai, Y.; Yuan, C.; Wu, C.; Hu, Y. Intelligent Gelatin/Oxidized Chitin Nanocrystals Nanocomposite Films Containing Black Rice Bran Anthocyanins for Fish Freshness Monitorings. Int. J. Biol. Macromol. 2020, 155, 1296–1306. [Google Scholar] [CrossRef] [PubMed]
- Eze, F.N.; Jayeoye, T.J.; Singh, S. Fabrication of Intelligent pH-Sensing Films with Antioxidant Potential for Monitoring Shrimp Freshness via the Fortification of Chitosan Matrix with Broken Riceberry Phenolic Extract. Food Chem. 2022, 366, 130574. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.; Wang, X.; Bai, R.; Miao, Z.; Zhang, X.; Liu, J. Development of antioxidant and intelligent pH-sensing packaging films by incorporating purple-fleshed sweet potato extract into chitosan matrix. Food Hydrocolloids 2019, 90, 216–224. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, T.; Das, A.; Chakraborty, R. Natural colorants from plant pigments and their encapsulation: An emerging window for the food industry. LWT 2022, 153, 112527. [Google Scholar] [CrossRef]
- Bakhshizadeh, M.; Moghaddam, T.N.; Tavassoli, M.; Ayaseh, A.; Bangar, S.P. Gelatin/chitosan nanofibres containing β-cyclodextrin complex and corn poppy (Papaver rhoeas L.) for intelligent packaging. Int. J. Food Sci. Technol. 2023, 58, 2360–2368. [Google Scholar] [CrossRef]
- Wei, S.; Wang, X.; Wang, F.; Hao, X.; Li, H.; Su, Z.; Guo, Y.; Shi, X.; Liu, X.; Li, J.; Zhao, C. Colorimetric detection of Salmonella typhimurium based on hexadecyl trimethyl ammonium bromide-induced supramolecular assembly of β-cyclodextrin-capped gold nanoparticles. Analytical and Bioanalytical Chemistry 2022, 414, 6069–6076. [Google Scholar] [CrossRef]
- Mustafa, F.; Andreescu, S. Nanotechnology-based approaches for food sensing and packaging applications. RSC Adv. 2020, 10, 19309–19336. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhu, G.; Li, F.; Liu, Y.; Guo, M.; Liu, R.; Komarneni, S. 3D interconnected honeycomb-like ginkgo nut-derived porous carbon decorated with β-cyclodextrin for ultrasensitive detection of methyl parathion. Sensors and Actuators B: Chemical 2023, 380, 133309. [Google Scholar] [CrossRef]
- Ahmadi, S.; Hasanzadeh, M.; Ghasempour, Z. Sub-micro electrochemical recognition of carmoisine, sunset yellow, and tartrazine in fruit juices using P(β-CD/Arg)/CysA-AuNPs/AuE. Food Chemistry 2023, 402, 134501. [Google Scholar] [CrossRef] [PubMed]
- Yun, N.; Lu, C.; Sun, T.; Xu, B.; Song, C.; Zong, Z.; Chen, K.; Huang, G.; Chen, X.; Gu, Q. High Sensitivity Detection of Capsaicin in Red Pepper Oil Based on Reduced Graphene Oxide Enhanced by β-Cyclodextrin. Food Analytical Methods 2023, 16, 318–329. [Google Scholar] [CrossRef]
- Yao, H.; Yu, H.; Zhang, B.Y.; Chen, K.; Yi, Q.; Xie, H.; Hu, X.; Tang, T.; Cheng, Y.; Tao, X.; et al. Approximately 1 nm-sized artificial tunnels in wrinkled graphene-graphene oxide composite membranes for efficient dye/dye separation and dye desalination. Chem. Eng. J. 2022, 445, 136753. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Y.; Wang, R.; Waterhouse, G.I.N.; Xu, Z. One-pot synthesis of a novel conductive molecularly imprinted gel as the recognition element and signal amplifier for the selective electrochemical detection of amaranth in foods. Biosensors and Bioelectronics 2023, 228, 115185. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.; Campos, E.; Rodríguez-Torres, M.D.P.; Acosta-Torres, L.; Diaz-Torres, L.; Grillo, R.; Swamy, M.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Avan, A.A.; Filik, H. Simultaneous Determination of Fat-Soluble Vitamins by Using Modified Glassy Carbon Electrode. Russian Journal of Electrochemistry 2021, 57, 858–871. [Google Scholar] [CrossRef]
- Sun, W.; Fan, L.; Zhao, Y.; Dong, T.; Jin, Y.; Saldaña, M.D.A.; Sun, W. Preparation, characterization, and morphology of on/off detection indicator based on rhodamine 6G-adamantanamine loaded onto nonwoven polyethylene terephthalate. Packaging Technology and Science 2020, 33, 385–394. [Google Scholar] [CrossRef]
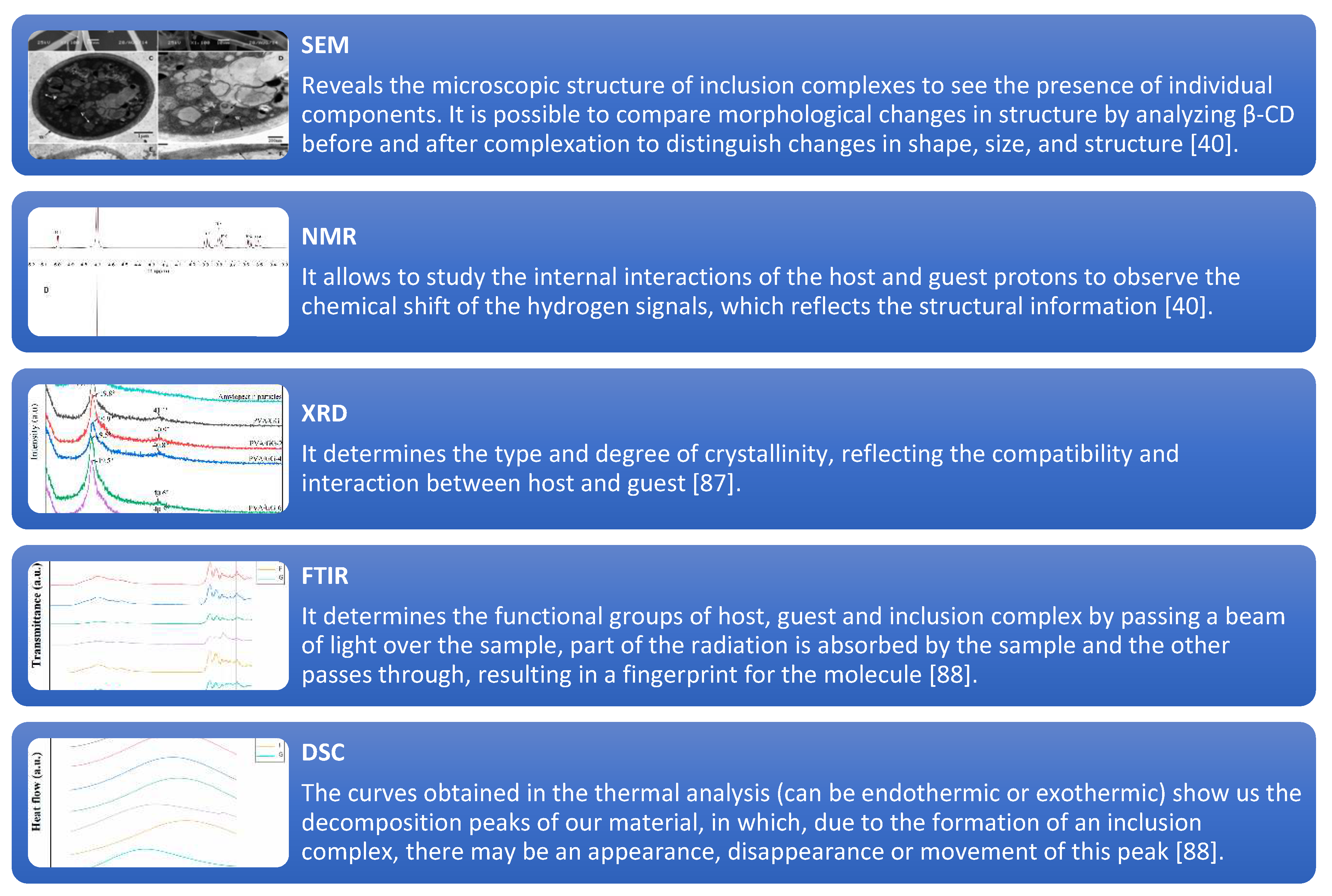
| CDs | Guest molecule | Technique | Results | References |
|---|---|---|---|---|
| β- | Curcumin | SEM, FT-IR, TGA, UV-vis spectra | SEM: pores disappeared attributed to CD. FT-IR: no new chemical bonds were broken or formed. TGA: didn’t present water loss. |
[62] |
| β- | Sage essential oil | FT-IR | FT-IR: peak attributed to SEO disappeared. | [75] |
| β- | Eucalyptus essential oil | FT-IR, DSC, TGA, SEM | FT-IR: blue-shifted. DSC & TGA: improve the stability of EEO and retard the volatilization. SEM: structure of particle has transformed, resulting in a smooth surface of the crystal structure |
[80] |
| β- | p-Anisaldehyde | SEM, XRD, TGA, FT-IR | SEM: reduction in size and rhomboid crystals in shape. TGA: protection of the inclusion structure for the volatile compounds. FT-IR: peaks disappeared. XRD: new sharp peaks. |
[81] |
| β- HP-β- |
(-) borneol | DSC & TG, FT-IR, SEM, XRD, NMR | Comparative study. Promising results demonstrated by TG analysis. | [82] |
| β- | Rosemary essential oil | FT-IR, TGA-DSC | FT-IR: signal of the oil constituents appeared in the capsule spectrum. TGA-DSC: yeast reduction |
[77] |
| β- | d-limonene | SEM, FT-IR (ATR), TGA, DSC | SEM: particles appeared homogeneously distributed. DSC: prevents the loss of the volatile essential oil. |
[74] |
| β- | Ginger essential oil | SEM, DSC, TGA, FT-IR, XRD | FT-IR: red shift. XRD: disappearance and formation of diffraction peaks and the intensities changed. TGA: thermal protection for GEO. |
[83] |
| β- | Peppermint oil | FT-IR | Confirmation of the formed structure. | [84] |
| β- | Concentrated orange oil | SEM, FT-IR | SEM: differences in shape and size. FT-IR: changes in spectra. |
[85] |
| α | Benzyl isothiocyanate (BITC) | FT-IR, XRD | FT-IR: slight wavelength shifts XRD: formation of amorphous complex. |
[86] |
| γ- | BITC, phenethyl isothiocyanate (PEITC), and 3-methylthiopropyl isothiocyanate (MTPITC) | FT-IR, TGA, XRD | TGA: elevated temperature required for the complete decomposition of ITCs. XRD: Sharp peaks appeared. |
[87] |
| γ- | Watermelon flavor | SEM, FT-IR, DSC, XRD | SEM: particles smaller than CD. FT-IR: peaks of watermelon disappeared. DSC: higher temperature for evaporation. |
[88] |
| γ- | thymol | SEM, XRD, FT-IR, NMR, DSC, TGA | TGA: improved by hydrogen bonding. XRD: characteristic diffraction peaks disappeared. SEM: smooth surface. |
[89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





