Submitted:
02 September 2023
Posted:
06 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
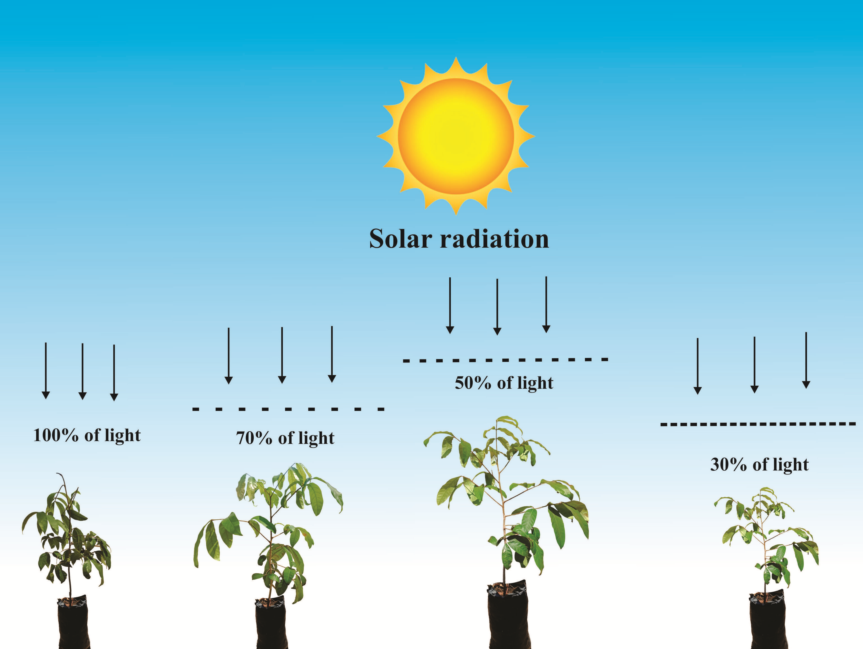
2.1. Experiment location and installation
2.2. Assessment of physiological responses
2.3. Growth parameters
2.4. Statistical analysis
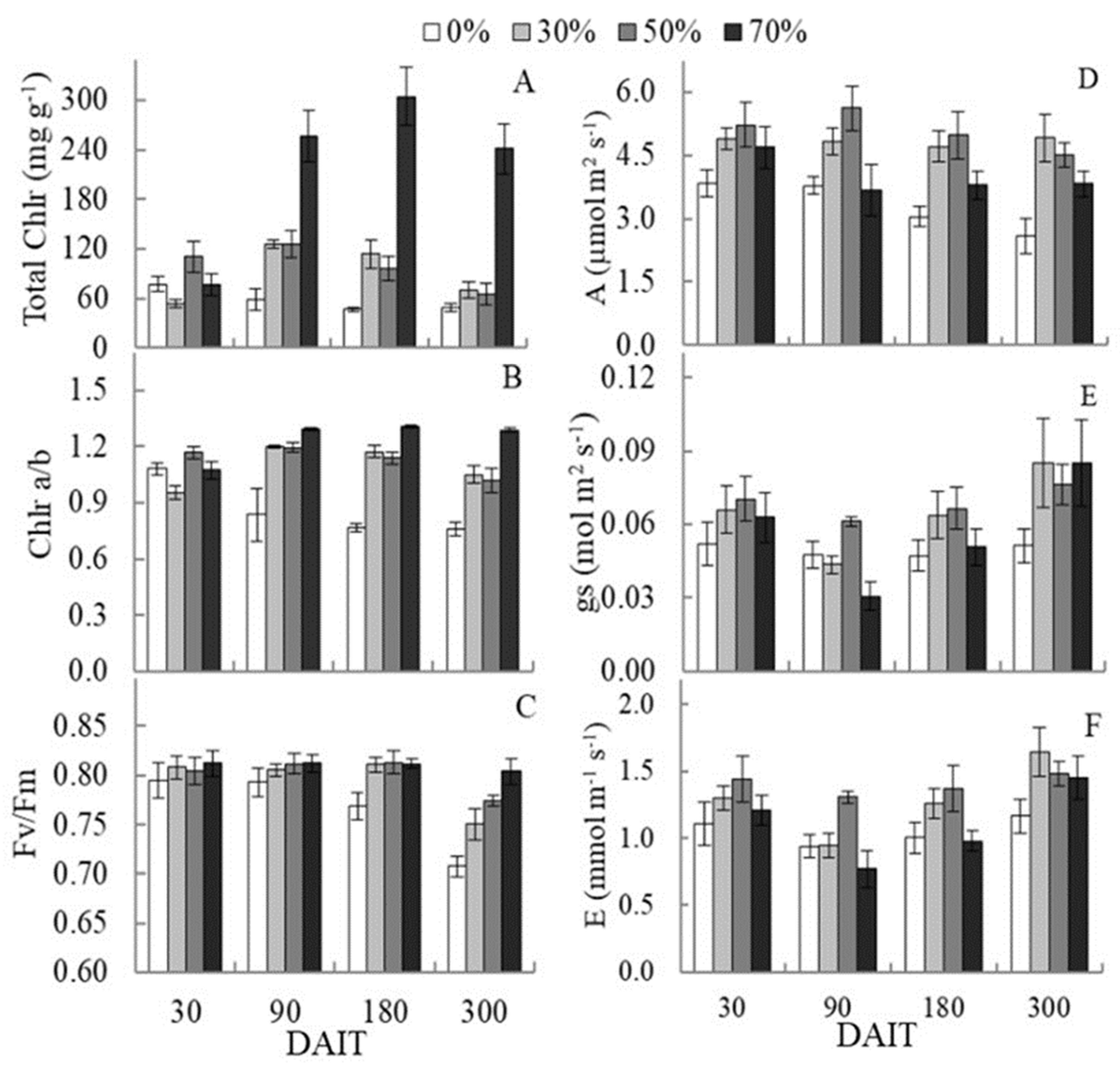
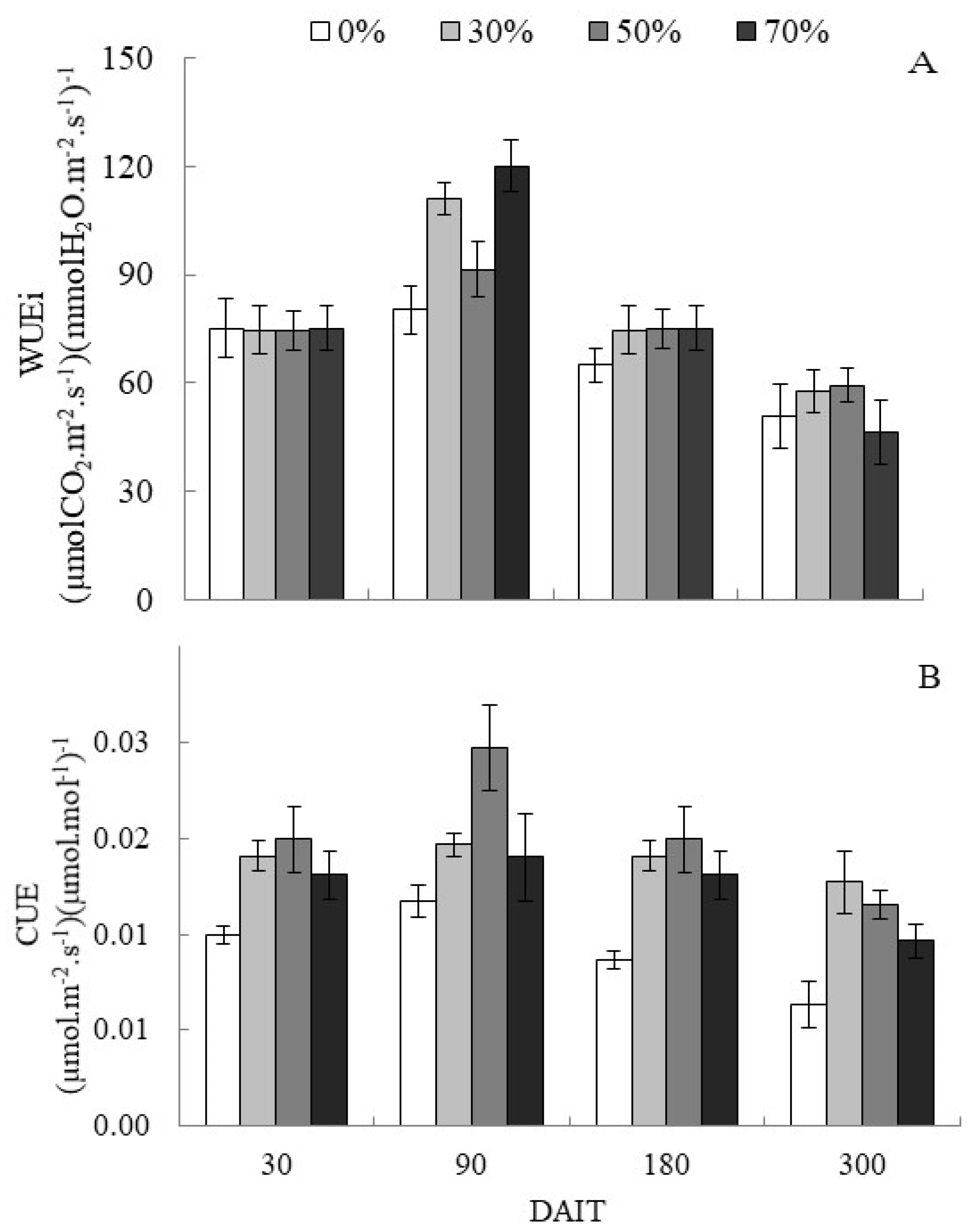
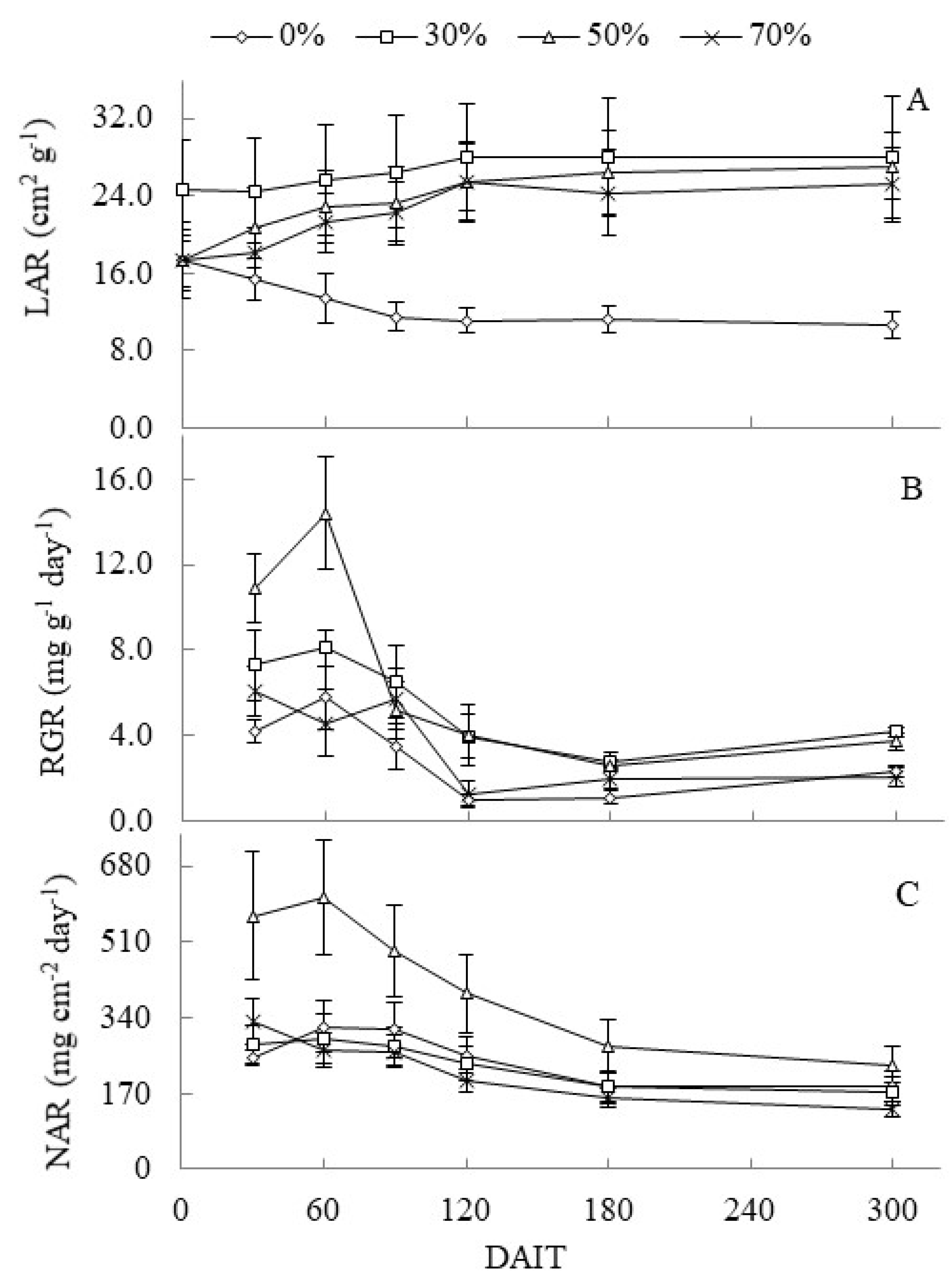
3. Results and Discussion
3. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Houghton, R. A.; Baccini, A.; Walker, W. S. Where is the residual terrestrial carbon sink?. Global change biology, 2018, 24(8), 3277-3279. [CrossRef]
- Field, C. B.; Behrenfeld, M. J.; Randerson, J. T; Falkowski, P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science, 1998, 281(5374), 237-240. [CrossRef]
- Huntingford, C.; Zelazowski, P.; Galbraith, D.; Mercado, L. M.; Sitch, S.; Fisher, R., ... & Malhi, Y. Simulated resilience of tropical rainforests to CO 2-induced climate change. Nature Geoscience, 2013, 6(4), 268. [CrossRef]
- Levine, N. M.; Zhang, K.; Longo, M.; Baccini, A.; Phillips, O. L; Lewis, S. L.; ... & Feldpausch, T. R. Ecosystem heterogeneity determines the ecological resilience of the Amazon to climate change. Proceedings of the National Academy of Sciences, 2016, 113(3), 793-797. [CrossRef]
- Ahlström, A.; Canadell, J. G.; Schurgers, G.; Wu, M; Berry, J. A; Guan, K; &Jackson, R. B. Hydrologic resilience and Amazon productivity. Nature communications, 2017, 8(1), 387. [CrossRef]
- Mitchard, E. T. A. The tropical forest carbon cycle and climate change. Nature, 2018, v. 559, n. 7715, p. 527-534. [CrossRef]
- Boulton, C.A.; Lenton, T.M.; Boers, N. Pronounced loss of Amazon rainforest resilience since the early 2000s. Nat. Clim. Chang, 2022, 12, 271–278. [CrossRef]
- Dalanhol, S. J.; Nogueira, A. C.; Gaiad, S.; Kratz, D. Efeito de micorrizas e da fertilização no crescimento de mudas de Campomanesia Xanthocarpa (Mart.) O. Berg., produzidas em diferentes substratos. Ciência Florestal, 2017, v. 27, n. 3, p. 931-945. [CrossRef]
- Camargo, M. A. B.; Marenco, R. A. Growth, leaf and stomatal traits of crabwood (Carapa guianensis Aubl.) in central Amazonia. Revista Árvore, 2012, v.36, n. 1, p. 7-16. [CrossRef]
- Klimas, C. A.; Kainer, K. A.; De Oliveira Wadt, L. H. The economic value of sustainable seed and timber harvests of multi-use species: An example using Carapa guianensis. Forest Ecology and Management, 2012, v. 268, p. 81-91. [CrossRef]
- Mota, L. H. De S.; Scalon, S. De P. Q.; Heinz, R. Sombreamento na emergência de plântulas e no crescimento inicial de Dipteryx alata Vog. Ciência Florestal, 2012, v. 22, n. 3, p. 423-431. [CrossRef]
- Magalhães, N. S.; Marenco, R. A.; Camargo, M. A. B. Do soil fertilization and forest canopy foliage affect the growth and photosynthesis of Amazonian saplings?. Scientia Agricola, 2014, v. 71, n. 1, p. 58-65. [CrossRef]
- Dutra, T. R.; Massad, M. D.; Santana, R. C. Parâmetros fisiológicos de mudas de copaíba sob diferentes substratos e condições de sombreamento. Ciência Rural, 2012, v. 42, n.7, p. 1212-1218. [CrossRef]
- Colebrook, E. H.; Thomas, S. G.; Phillips, A. L.; Hedden, P. The role of gibberellin signalling in plant responses to abiotic stress. Journal of experimental biology, 2014, v. 217, n. 1, p. 67-75. [CrossRef]
- Park, Y.; Runkle, E. S. Far-red radiation promotes growth of seedlings by increasing leaf expansion and whole-plant net assimilation. Environmental and Experimental Botany, 2017, v. 136, p. 41-49. [CrossRef]
- Souza Pinto, J. R.; Dombroski, J. L. D.; De Freitas, R. M. O. Crescimento e índices fisiológicos de Tabebuia aurea (Manso) Benth. & Hook., sob sombreamento no semiárido. FLORESTA, 2016, v. 46, n. 4, p. 465-472. [CrossRef]
- Fiorucci, A. S.; Fankhauser, C. Plant strategies for enhancing access to sunlight. Current Biology, 2017, v. 27, n. 17, p. R931-R940. [CrossRef]
- Felsemburgh, C. A.; Santos, K. J. S. Dos; Camargo, P. B. De; Carmo, J. B. Do; Tribuzy, E. S. Respostas ecofisiológicas de Aniba parviflora ao sombreamento artificial. Pesquisa florestal brasileira, 2016, v. 36, n.87, p. 201-210.
- Tribuzy, A.S.; Tabaldi, L.A.; Tribuzy, E.S.; Soares, J.C.R.; Finger, C.A.G. Crescimento e acúmulo de biomassa de Luehea divaricate Mart. & Zucc. Submetida ao sombreamento. Revista Ibero-Americana de Ciências Ambientais, 2022, v. 13, n. 3, 250-259. [CrossRef]
- Pinho, E. K. C.; Lopes, A. N. K.; Costa, A. C.; Silva, A. B. V.; Vilar, F. C. M.; Reis, R. G. E. Substratos e tamanhos de recipiente na produção de mudas de baruzeiro (Dipteryx alata Vog.). Ciência Agrícola, 2018, v. 16, n. 1, p. 11-19. [CrossRef]
- Alvares, C. A.; Stape, J. L.; Sentelhas, P. C.; Gonçalves, J. L. De M.; Sparovic, G. Köppen’s climate classification map for Brazil. Meteorologische, 2013, v. 22, n.6, p. 711–728. [CrossRef]
- Aquino, A. C. M. D. S.; Silva, M. H. M. D.; Rocha, A. K. S.; Castro, A. A.; Azevedo, G. F. C. Estudo da influência de diferentes tempos e métodos de cocção na estabilidade dos teores de clorofila e ácido ascórbico em brócolis (Brassica oleraceae). Scientia Plena, 2011, v. 7, n.1, p. 1-6.
- Lenhard, N. R.; Paiva Neto, V. B. de; Scalon, S. de P. Q.; Alvarenga, A. A. de. Crescimento de mudas de pau-ferro sob diferentes níveis de sombreamento. Pesquisa Agropecuária Tropical, 2013, v. 43, n.2, p. 178-186. [CrossRef]
- Souza, J. P.; Melo, N. M. J.; Halfeld, A. D.; Reis, J. N. Shading effects on leaf life span and functional traits in the widespread species Enterolobium contortisiliquum (Vell.) Morong. Acta Scientiarum. Biological Sciences, 2017, v. 39, n. 1, p. 113-122. [CrossRef]
- Gonçalves, J. F. De C.; Silva, C. E. M. da; Justino G. C.; Nina Jr A. da R. Efeito do ambiente de luz no crescimento de plantas jovens de mogno (Swietenia macrophylla King). Scientia Forestalis, 2012, v. 40, n. 95, p. 337-344.
- SATO, R.; ITO, H.; TANAKA, A. Chlorophyll b degradation by chlorophyll b reductase under high-light conditions. Photosynthesis research, 2015, v. 126, n. 2, p. 249-259. [CrossRef]
- Casierra-Posada, F.; Ávila-León, O. F. Shade tolerance of marigold plants (Calendula officinalis). Revista UDCA Actualidad & Divulgación Científica, 2015, v. 18, n. 1, p. 119-126. [CrossRef]
- Li, L.; Aro, E. M..; Millar, A. H. Mechanisms of photodamage and protein turnover in photoinhibition. Trends in plant science, 2018, v. 23, n. 8, p. 667-676. [CrossRef]
- Moura-Silva, C. E.; Andrade, P. C. R.; Dos Santos, W. N. Influence of luminosity on the initial growth of Carapa guianensis, Clitoria fairchildiana and Inga edulis tree seedlings. Científica, 2017, v. 45, n. 4, p. 422-429. [CrossRef]
- Marenco, R. A.; Vera, S. A. A.; Gouvêa, P. R. Dos S.; Camargo, M. A. B.; Oliveira, M. F. De; Santos, J. K. Da S. Fisiologia de espécies florestais da Amazônia: Fotossíntese, respiração e relações hídricas. Ceres, 2014, v. 61, n. 7, p. 786-799. [CrossRef]
- Tian, Y.; Yuan, H.; Xie, J.; Zheng, Y. Shade tolerance and suitability of tree species for planting in rubber plantations. Southern Forests: A Journal of Forest Science, 2016, v. 78, n. 1, p. 11-18. [CrossRef]
- Solovchenko, A.; Solovchenko, O.; Khozin-Goldberg, I.; Didi-Cohen, S.; Pal, D.; Cohen, Z.; Boussiba, S. Probing the effects of high-light stress on pigment and lipid metabolism in nitrogen-starving microalgae by measuring chlorophyll fluorescence transients: Studies with a Δ5 desaturase mutant of Parietochloris incisa (Chlorophyta, Trebouxiophyceae). Algal Research, 2013, v. 2, n. 3, p. 175-182. [CrossRef]
- Matthews, J. S.; Vialet-Chabrand, S. R.; Lawson, T. Diurnal variation in gas exchange: The balance between carbon fixation and water loss. Plant Physiology, 2017, v. 174, n. 2, p. 614-623. [CrossRef]
- Aragão, D. de S.; Lunz, A. M. P.; Oliveira, L. C. de; Raposo, A.; Fermino Jr., P. C. P. Efeito do sombreamento na anatomia foliar de plantas jovens de andiroba (Carapa guianensis Aubl.). Revista Árvore, Viçosa, 2014, v. 38, n. 4, p. 631-639. [CrossRef]
- Lima, R. B. de A.; Silva, J. A. A. da; Marangon, L. C.; Ferreira, R. L. C.; Silva, R. K. S. Da. Sucessão ecológica de um trecho de floresta ombrófila densa de terras baixas, Carauari, Amazonas. Pesquisa Florestal Brasileira, 2011, v. 31, n. 67, p. 161.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



