Submitted:
05 October 2023
Posted:
09 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
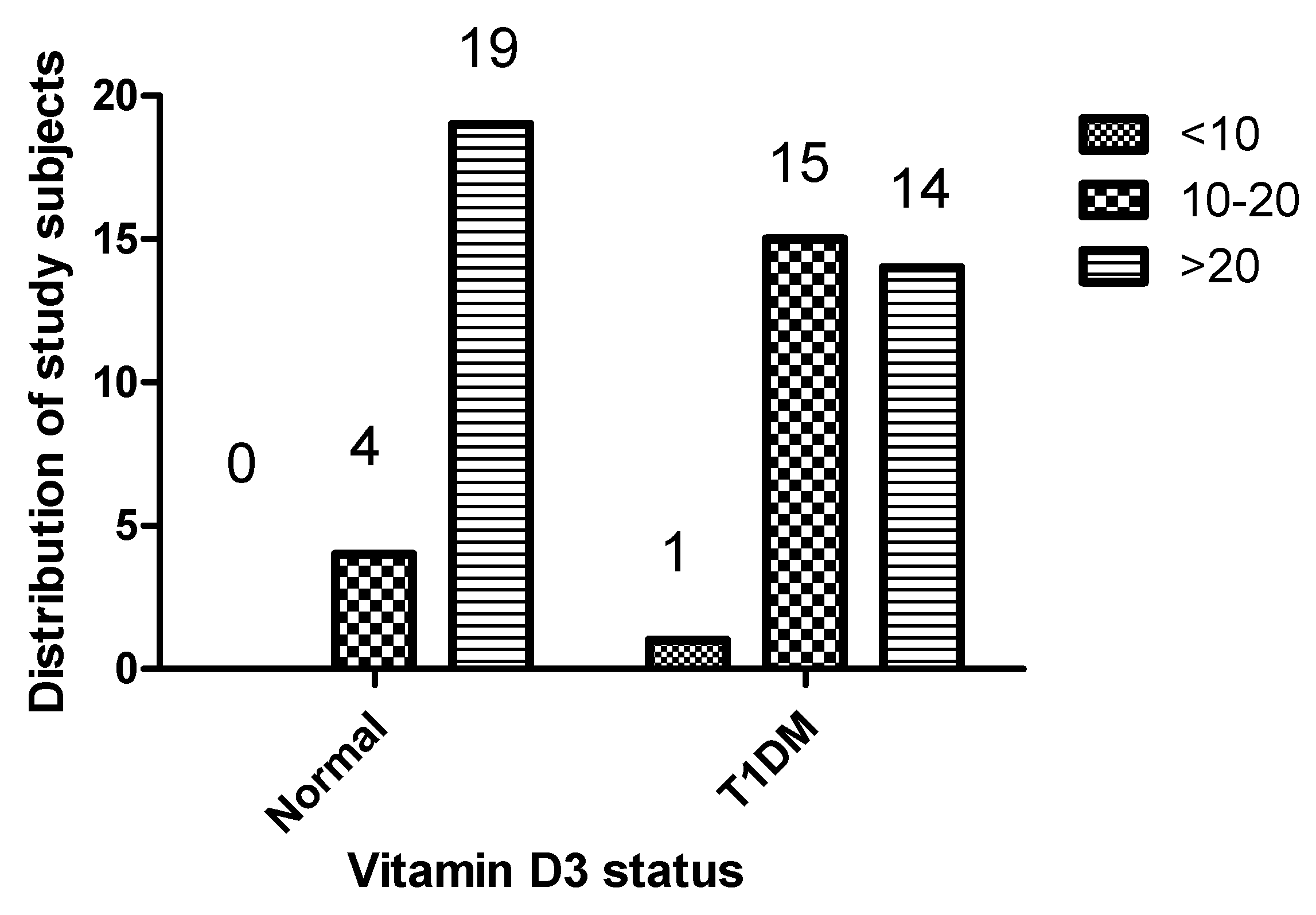
2.1. Comparison of Demographic Characteristics and 1,25(OH)2D3 Levels of Patients with Type 1 Diabetes and Healthy Controls
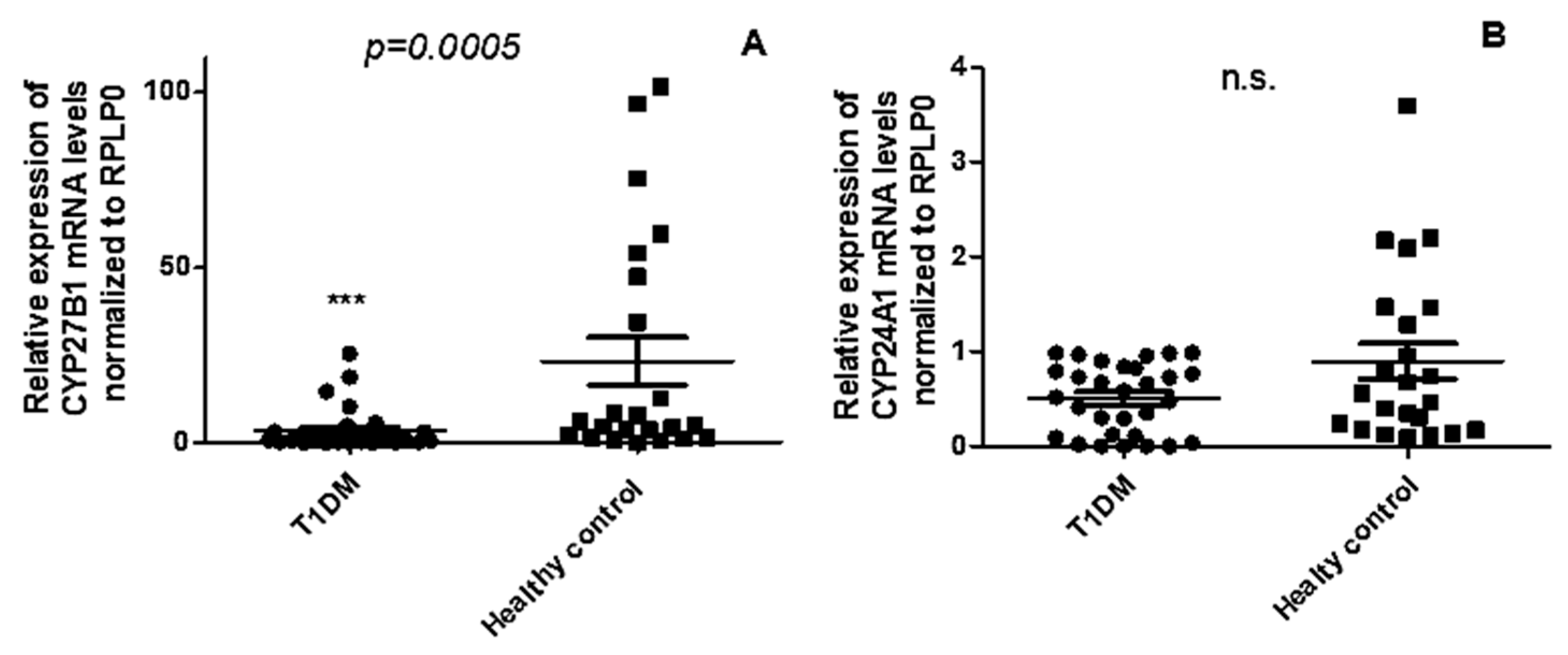
2.2. CYP27B1 mRNA Expression is Downregulated in PBMCs from T1DM Patients Compared to Healthy Controls, While CYP24A1 Remains Unchanged
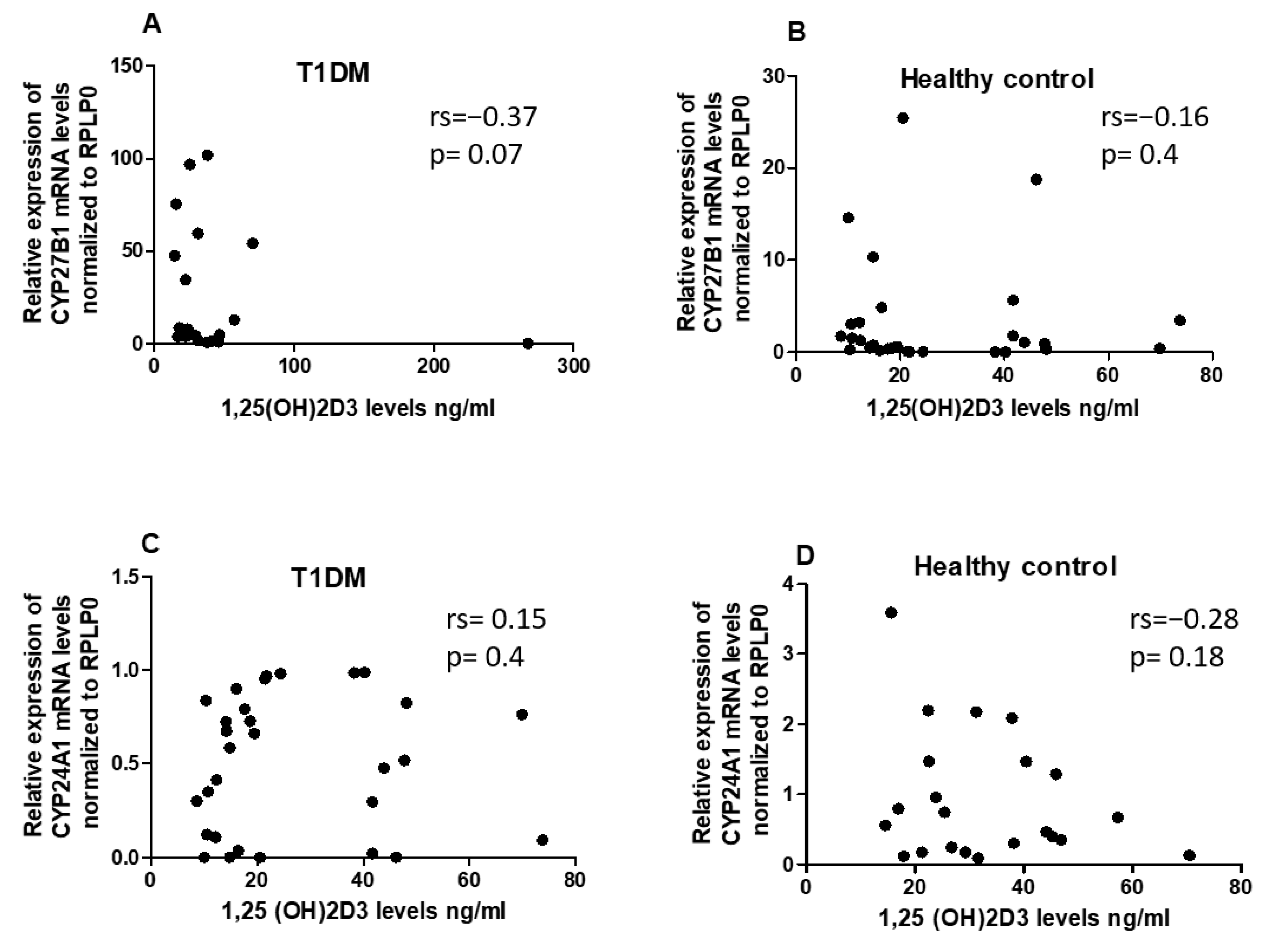
2.3. No Correlation Observed Between 1,25(OH)2D3 serum levels and Expression Levels of CYP24A1 and CYP27B1 mRNA
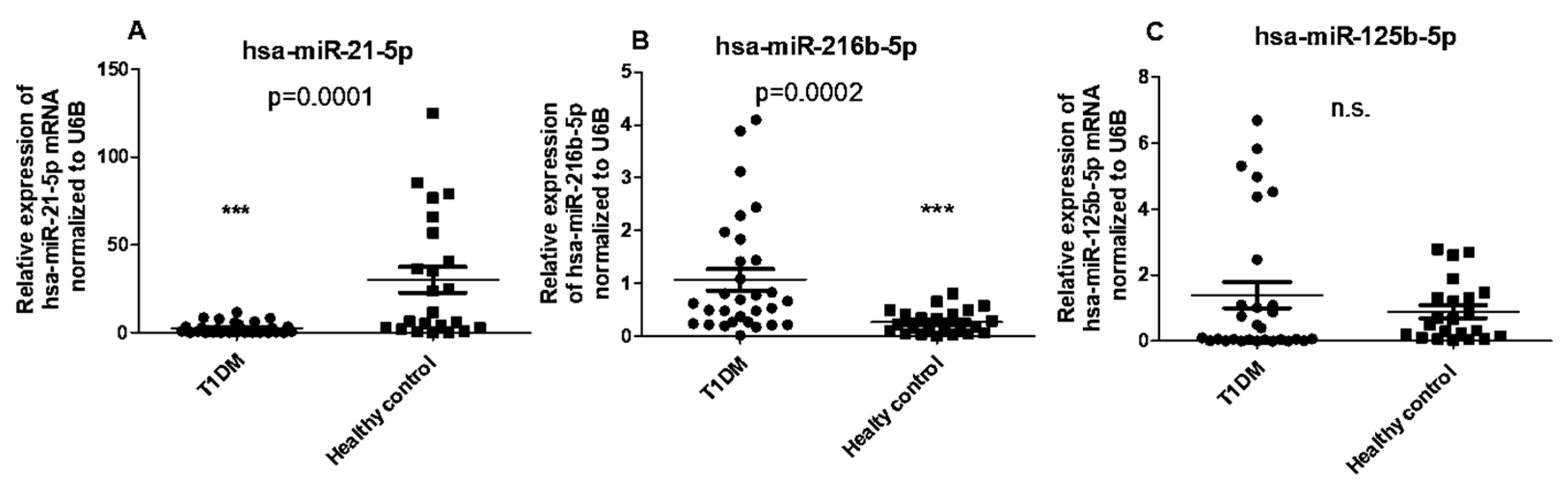
2.4. Circulating Levels of has-miR-21-5p and has-miR-216b-5p, but not has-miR-125b-5p, are Differentially Expressed in PBMCs of T1DM Patients Compared with Healthy Controls
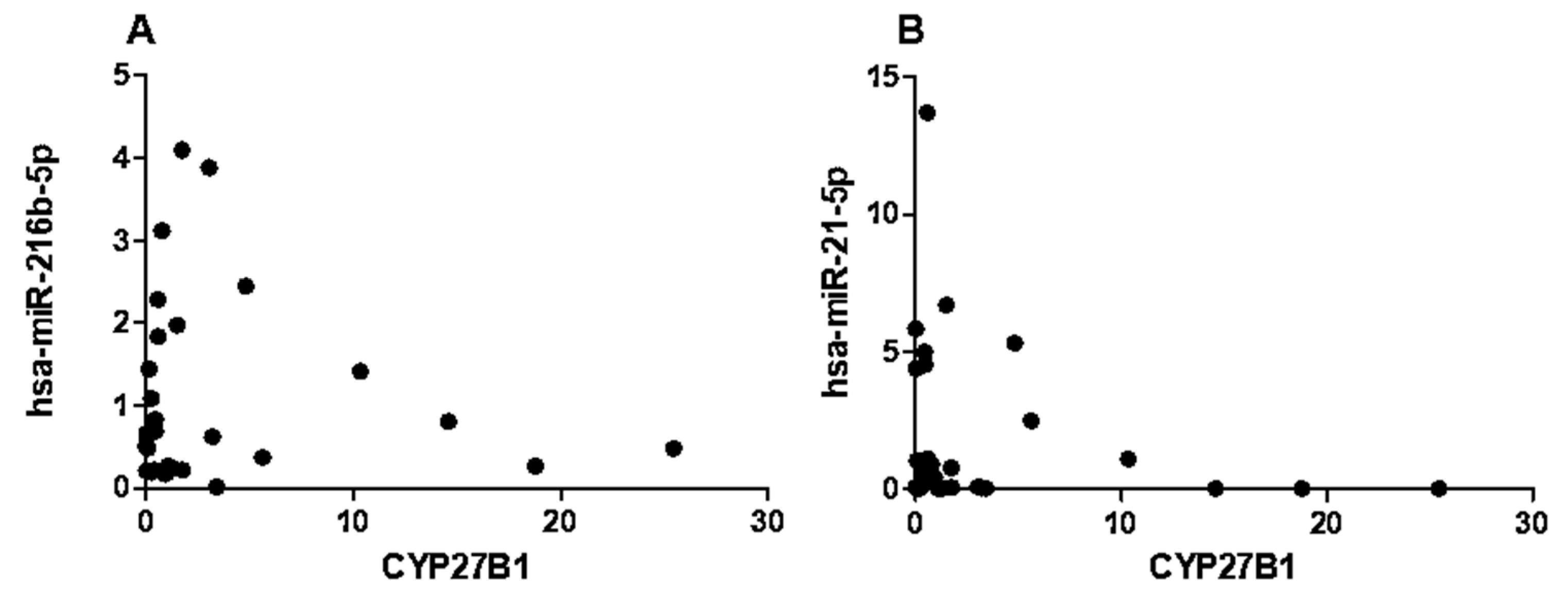
2.5. CYP27B1 Not Correlated with hsa-miR-216b-5p and hsa-miR-21-5p in T1DM
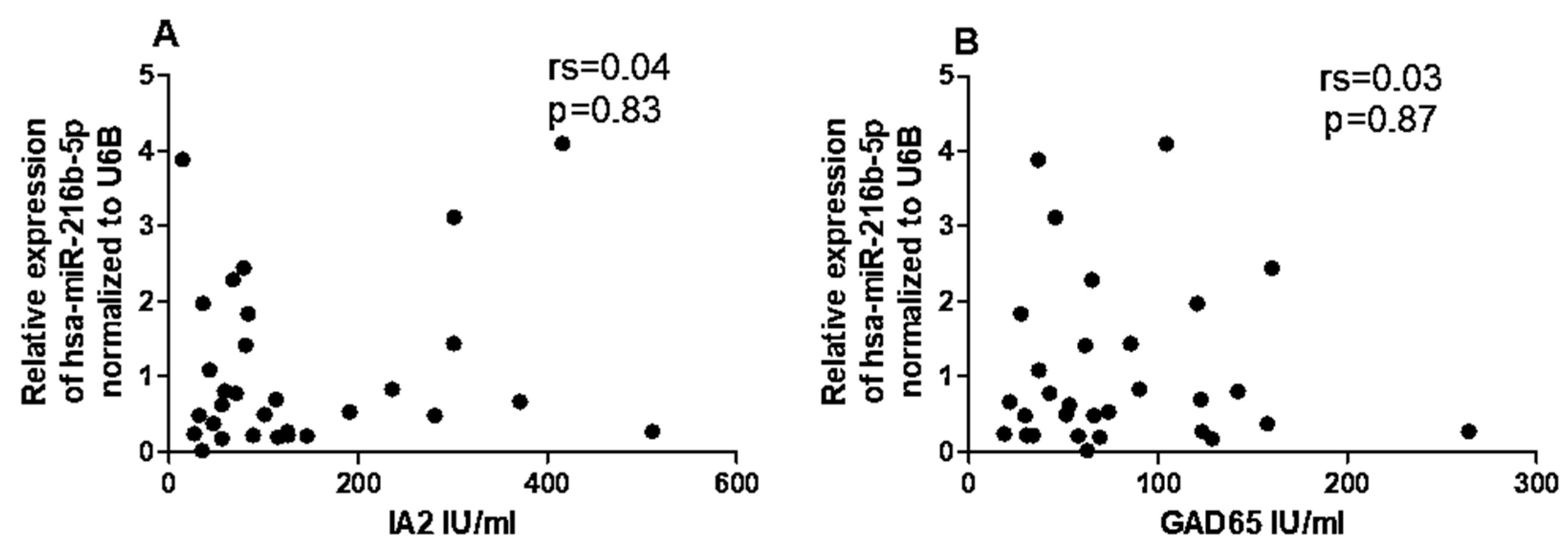
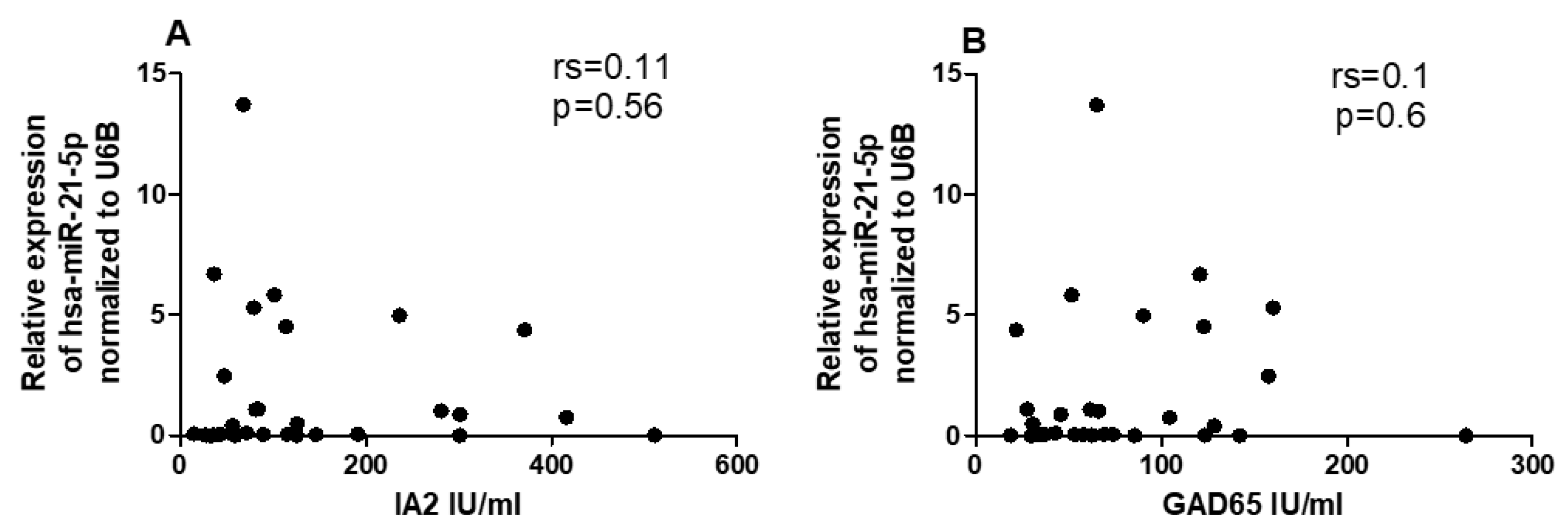
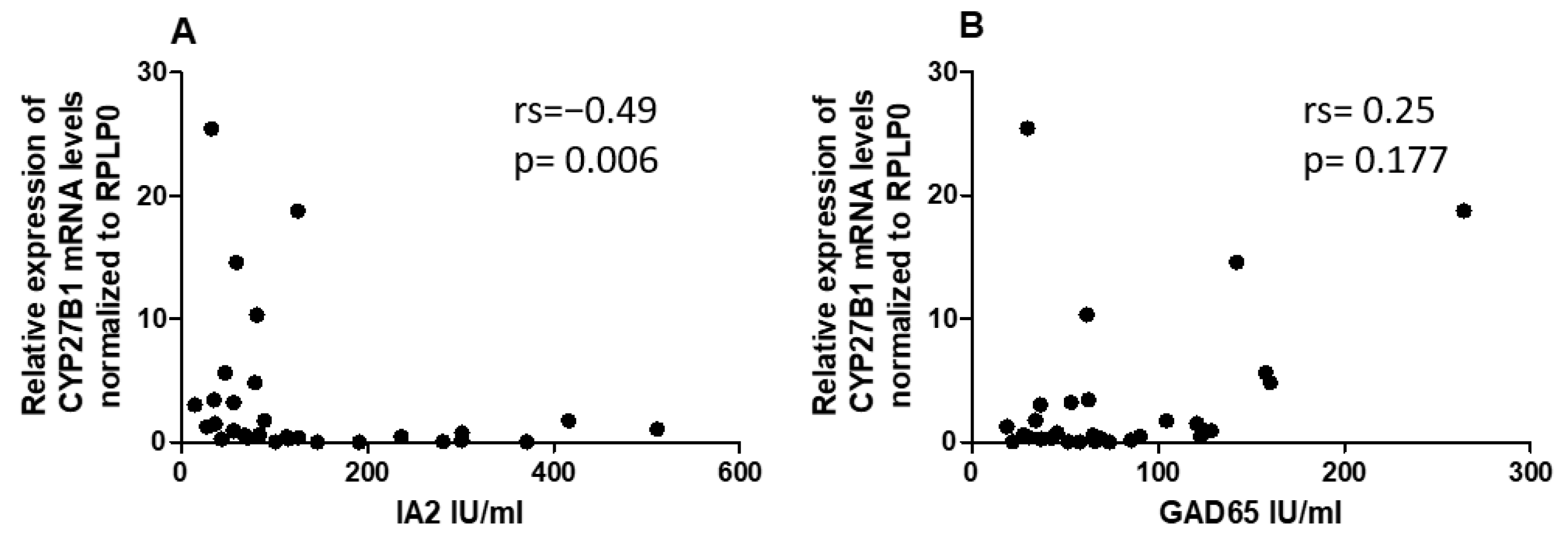
2.6. Correlation Between miRNAs and CYP27B1 with Islet Autoantibodies
3. Discussion
4. Materials and Methods
4.1. Ethics and Consent
4.2. Study Design, Recruitment of T1DM Patients, Healthy Control, Inclusion and Exclusion Criteria
4.3. Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
4.4. Total RNA and miRNA Extraction from PBMCs
4.5. Reverse Transcription and Quantitative Real-Time PCR
4.6. Serum Sample Collection and 1,25(OH)2D3 Level Measurement
4.7. Serological Analysis
4.8. Target Gene Identification and Bioinformatic Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
References
- Yoon, J.-W.; Jun, H.-S. Autoimmune destruction of pancreatic beta cells. Am. J. Ther. 2005, 12, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Pozzilli, P.; Manfrini, S.; Crino, A.; Picardi, A.; Leomanni, C.; Cherubini, V.; Valente, L.; Khazrai, M.; Visalli, N. Low levels of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 in patients with newly diagnosed type 1 diabetes. Horm. Metab. Res. = Horm. und Stoffwechselforsch. = Horm. Metab. 2005, 37, 680–683. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, A. High Prevalence of Vitamin D Deficiency Among Saudi Children And Adolescents With Type 1 Diabetes In Albaha Region, Saudi Arabia; 2017; Vol. 12;
- Tashiro, K.; Abe, T.; Oue, N.; Yasui, W.; Ryoji, M. Characterization of vitamin D-mediated induction of the CYP 24 transcription. Mol. Cell. Endocrinol. 2004, 226, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Rak, K.; Bronkowska, M. Immunomodulatory Effect of Vitamin D and Its Potential Role in the Prevention and Treatment of Type 1 Diabetes Mellitus-A Narrative Review. Molecules 2018, 24. [Google Scholar] [CrossRef] [PubMed]
- Lin, R. Crosstalk between Vitamin D Metabolism, VDR Signalling, and Innate Immunity. Biomed Res. Int. 2016, 2016, 1375858. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Xing, W.; Xie, L. Regulatory Roles of MicroRNAs in Diabetes. Int. J. Mol. Sci. 2016, 17, 1729. [Google Scholar] [CrossRef]
- Komagata, S.; Nakajima, M.; Takagi, S.; Mohri, T.; Taniya, T.; Yokoi, T. Human CYP24 Catalyzing the Inactivation of Calcitriol Is Post-Transcriptionally Regulated by miR-125b. Mol. Pharmacol. 2009. [Google Scholar] [CrossRef]
- Essa, S.; Denzer, N.; Mahlknecht, U.; Klein, R.; Collnot, E.M.; Tilgen, W.; Reichrath, J. VDR microRNA expression and epigenetic silencing of vitamin D signaling in melanoma cells. J. Steroid Biochem. Mol. Biol. 2010, 121, 110–113. [Google Scholar] [CrossRef]
- Mohri, T.; Nakajima, M.; Takagi, S.; Komagata, S.; Yokoi, T. MicroRNA regulates human vitamin D receptor. Int. J. cancer 2009, 125, 1328–1333. [Google Scholar] [CrossRef]
- Sebastiani, G.; Grieco, F.A.; Spagnuolo, I.; Galleri, L.; Cataldo, D.; Dotta, F. Increased expression of microRNA miR-326 in type 1 diabetic patients with ongoing islet autoimmunity. Diabetes. Metab. Res. Rev. 2011, 27, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Adorini, L.; Penna, G. Control of autoimmune diseases by the vitamin D endocrine system. Nat. Clin. Pract. Rheumatol. 2008, 4, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Gager, G.M.; Eyileten, C.; Postuła, M.; Nowak, A.; Gąsecka, A.; Jilma, B.; Siller-Matula, J.M. Expression Patterns of MiR-125a and MiR-223 and Their Association with Diabetes Mellitus and Survival in Patients with Non-ST-Segment Elevation Acute Coronary Syndrome. Biomedicines 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.T.; Wheelwright, M.; Teles, R.; Komisopoulou, E.; Edfeldt, K.; Ferguson, B.; Mehta, M.D.; Vazirnia, A.; Rea, T.H.; Sarno, E.N.; et al. MicroRNA-21 targets the vitamin D-dependent antimicrobial pathway in leprosy. Nat. Med. 2012, 18, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, Q.; Zhao, H.; Bishop, J.O.; Zhou, G.; Olson, L.K.; Moore, A. miR-216a-targeting theranostic nanoparticles promote proliferation of insulin-secreting cells in type 1 diabetes animal model. Sci. Rep. 2020, 10, 5302. [Google Scholar] [CrossRef] [PubMed]
- El-Samahy, M.H.; Adly, A.A.; Elhenawy, Y.I.; Ismail, E.A.; Pessar, S.A.; Mowafy, M.E.-S.; Saad, M.S.; Mohammed, H.H. Urinary miRNA-377 and miRNA-216a as biomarkers of nephropathy and subclinical atherosclerotic risk in pediatric patients with type 1 diabetes. J. Diabetes Complications 2018, 32, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Margaritis, K.; Margioula-Siarkou, G.; Giza, S.; Kotanidou, E.P.; Tsinopoulou, V.R.; Christoforidis, A.; Galli-Tsinopoulou, A. Micro-RNA Implications in Type-1 Diabetes Mellitus: A Review of Literature. Int. J. Mol. Sci. 2021, 22. [Google Scholar] [CrossRef]
- Scherm, M.G.; Daniel, C. miRNA-Mediated Immune Regulation in Islet Autoimmunity and Type 1 Diabetes. Front. Endocrinol. (Lausanne). 2020, 11, 606322. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Z.; Zhou, Z. miRNAs: novel regulators of autoimmunity-mediated pancreatic β-cell destruction in type 1 diabetes. Cell. &Amp; Mol. Immunol. 2017, 14, 488. [Google Scholar]
- Salas-Pérez, F.; Codner, E.; Valencia, E.; Pizarro, C.; Carrasco, E.; Pérez-Bravo, F. MicroRNAs miR-21a and miR-93 are down regulated in peripheral blood mononuclear cells (PBMCs) from patients with type 1 diabetes. Immunobiology 2013, 218, 733–737. [Google Scholar] [CrossRef]
- Sebastiani, G.; Grieco, F.A.; Spagnuolo, I.; Galleri, L.; Cataldo, D.; Dotta, F. Increased expression of microRNA miR-326 in type 1 diabetic patients with ongoing islet autoimmunity. Diabetes. Metab. Res. Rev. 2011, 27, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Azhir, Z.; Dehghanian, F.; Hojati, Z. Increased expression of microRNAs, miR-20a and miR-326 in PBMCs of patients with type 1 diabetes. Mol. Biol. Rep. 2018, 45, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Song, L.-J.; Qin, X.-Y. Advances in the cellular immunological pathogenesis of type 1 diabetes. J. Cell. Mol. Med. 2014, 18, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Ritterhouse, L.L.; Crowe, S.R.; Niewold, T.B.; Merrill, J.T.; Roberts, V.C.; Dedeke, A.B.; Neas, B.R.; Thompson, L.F.; Guthridge, J.M.; James, J.A. B lymphocyte stimulator levels in systemic lupus erythematosus: higher circulating levels in African American patients and increased production after influenza vaccination in patients with low baseline levels. Arthritis Rheum. 2011, 63, 3931–3941. [Google Scholar] [CrossRef]
- Piantoni, S.; Andreoli, L.; Scarsi, M.; Zanola, A.; Dall’Ara, F.; Pizzorni, C.; Cutolo, M.; Airò, P.; Tincani, A. Phenotype modifications of T-cells and their shift toward a Th2 response in patients with systemic lupus erythematosus supplemented with different monthly regimens of vitamin D. Lupus 2015, 24, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Chakhtoura, M.; Azar, S.T. The role of vitamin d deficiency in the incidence, progression, and complications of type 1 diabetes mellitus. Int. J. Endocrinol. 2013, 2013, 148673. [Google Scholar] [CrossRef]
- Bener, A.; Alsaied, A.; Al-Ali, M.; Al-Kubaisi, A.; Basha, B.; Abraham, A.; Guiter, G.; Mian, M. High prevalence of vitamin D deficiency in type 1 diabetes mellitus and healthy children. Acta Diabetol. 2009, 46, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Lopez, E.; Brück, P.; Jansen, T.; Pfeilschifter, J.M.; Radeke, H.H.; Badenhoop, K. CYP2R1-, CYP27B1- and CYP24-mRNA expression in German type 1 diabetes patients. J. Steroid Biochem. Mol. Biol. 2007, 103, 807–810. [Google Scholar] [CrossRef]
- Singh, R.; Yadav, V.; kumar, S.; Saini, N. MicroRNA-195 inhibits proliferation, invasion and metastasis in breast cancer cells by targeting FASN, HMGCR, ACACA and CYP27B1. Sci. Rep. 2015, 5, 17454. [Google Scholar] [CrossRef]
- Cheung, R.; Pizza, G.; Chabosseau, P.; Rolando, D.; Tomas, A.; Burgoyne, T.; Wu, Z.; Salowka, A.; Thapa, A.; Macklin, A.; et al. Glucose-Dependent miR-125b Is a Negative Regulator of β-Cell Function. Diabetes 2022, 71, 1525–1545. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, H.; Pan, X.; Wu, W.; Wang, H.; Yan, L.; Zhang, M.; Liu, X.; Xia, S.; Shao, Q. miR-34a and miR-125b are upregulated in peripheral blood mononuclear cells from patients with type 2 diabetes mellitus. Exp Ther Med 2017, 14, 5589–5596. [Google Scholar] [CrossRef] [PubMed]
- Garo, L.P.; Murugaiyan, G. Contribution of MicroRNAs to autoimmune diseases. Cell. Mol. Life Sci. 2016, 73, 2041–2051. [Google Scholar] [CrossRef] [PubMed]
- Assmann, T.S.; Recamonde-Mendoza, M.; De Souza, B.M.; Crispim, D. MicroRNA expression profiles and type 1 diabetes mellitus: systematic review and bioinformatic analysis. Endocr. Connect. 2017, 6, 773–790. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Martin, A.; Adams, B.D.; Lai, M.; Shepherd, J.; Salvador-Bernaldez, M.; Salvador, J.M.; Lu, J.; Nemazee, D.; Xiao, C. The microRNA miR-148a functions as a critical regulator of B cell tolerance and autoimmunity. Nat. Immunol. 2016, 17, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Abuhatzira, L.; Xu, H.; Tahhan, G.; Boulougoura, A.; Schäffer, A.A.; Notkins, A.L. Multiple microRNAs within the 14q32 cluster target the mRNAs of major type 1 diabetes autoantigens IA-2, IA-2β, and GAD65. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2015, 29, 4374–4383. [Google Scholar] [CrossRef] [PubMed]
- Ongagna, J.C.; Pinget, M.; Belcourt, A. Vitamin D-binding protein gene polymorphism association with IA-2 autoantibodies in type 1 diabetes. Clin. Biochem. 2005, 38, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Januszewski, A.S.; Cho, Y.H.; Joglekar, M.V.; Farr, R.J.; Scott, E.S.; Wong, W.K.M.; Carroll, L.M.; Loh, Y.W.; Benitez-Aguirre, P.Z.; Keech, A.C.; et al. Insulin micro-secretion in Type 1 diabetes and related microRNA profiles. Sci. Rep. 2021, 11, 11727. [Google Scholar] [CrossRef]
- Grieco, G.E.; Cataldo, D.; Ceccarelli, E.; Nigi, L.; Catalano, G.; Brusco, N.; Mancarella, F.; Ventriglia, G.; Fondelli, C.; Guarino, E.; et al. Serum Levels of miR-148a and miR-21-5p Are Increased in Type 1 Diabetic Patients and Correlated with Markers of Bone Strength and Metabolism. Non-coding RNA 2018, 4. [Google Scholar] [CrossRef]
- Morales-Sánchez, P.; Lambert, C.; Ares-Blanco, J.; Suárez-Gutiérrez, L.; Villa-Fernández, E.; Garcia, A.V.; García-Villarino, M.; Tejedor, J.R.; Fraga, M.F.; Torre, E.M.; et al. Circulating miRNA expression in long-standing type 1 diabetes mellitus. Sci. Rep. 2023, 13, 8611. [Google Scholar] [CrossRef]
| Variable | Control group, n = 23 | T1DM group, n = 30 | P-value |
|---|---|---|---|
| HbA1c (%) | 5.126± 0.4158 | 8.957 ±1.353 | <0.0001 |
| IA2 (IU/ml) | 0 | 140.4 (14.45–511.0) | - |
| GAD65 (IU/ml) | 2.62 (0.4–4.7) | 79.55 (18.77–264.3) | - |
| Age (years) | 9.296 ±3.006 | 10.71 ± 6.072 | 0.3098 |
| Sex: male/female | 11/12 | 15/15 | - |
| BMI | 21.86 ±3.250 | 22.31±4.248 | 0.6775 |
| 1,25(OH)2D3 (ng/ml) | 43.18±50.89 | 24.37±17.14 | 0.0045 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





