Submitted:
04 September 2023
Posted:
06 September 2023
You are already at the latest version
Abstract
Keywords:
1. Definition and epidemiology of myocarditis
2. Etiological factors and pathophysiological mechanisms of myocarditis
2.1. Infectious causes of myocarditis
2.2. Eosinophilic myocarditis
2.3. Giant cell myocarditis
2.4. Myocarditis associated with immune checkpoint inhibitors
2.5. Myocarditis associated with systemic diseases
3. Division of myocarditis
3.1. Acute myocarditis
3.2. Subacute myocarditis
3.3. Chronic myocarditis
3.4. Chronic inflammatory cardiomyopathy
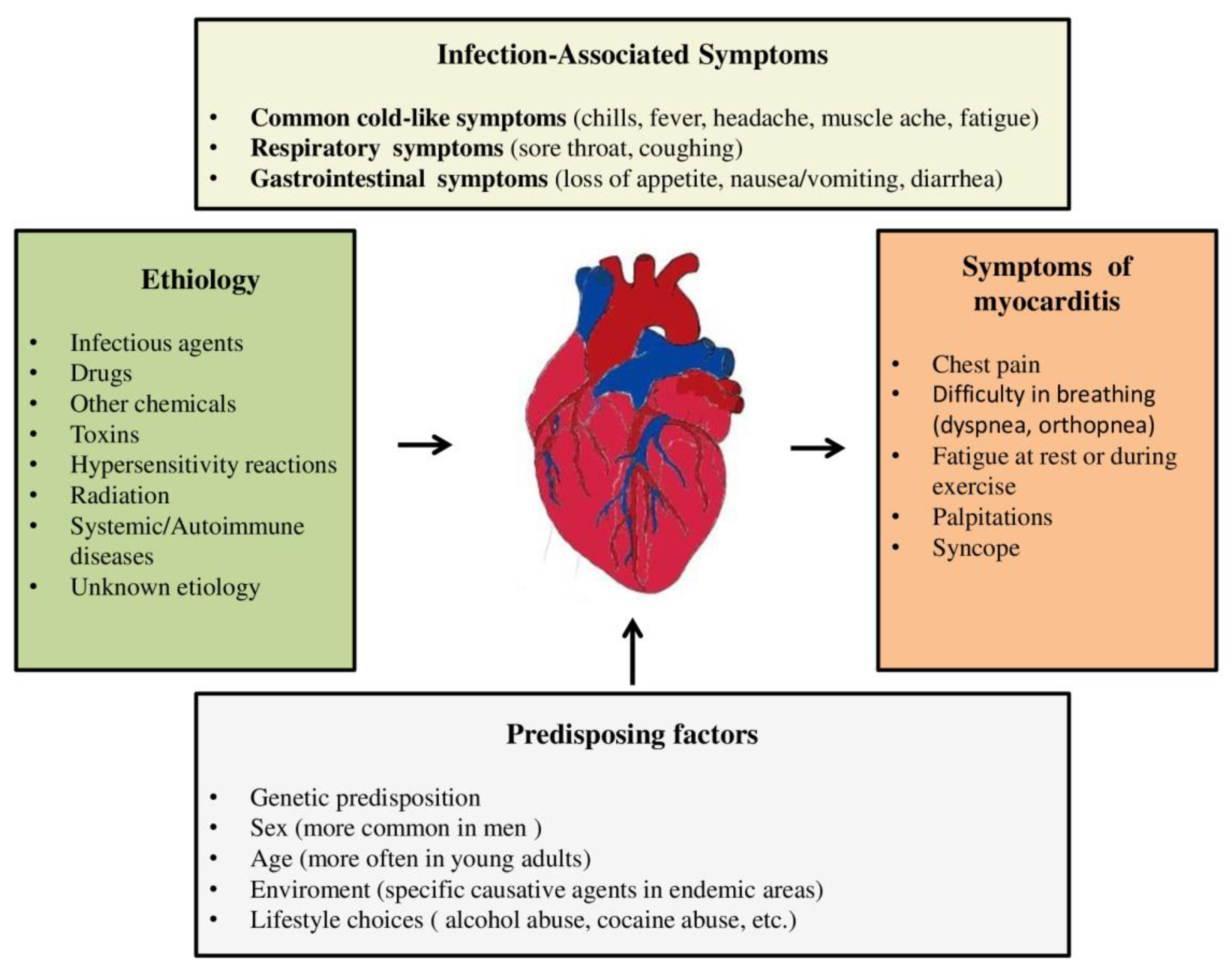
4. CLINICAL PICTURE
- Acute (non-fulminant) myocarditis – about 65% of patients have this form of myocarditis [70]. This phenotype includes asymptomatic cases, cases in which some degree of cardiac damage may occur with partial or complete regression [33]. These patients have a clinical, electrocardiographic picture and biohumoral syndrome similar to acute myocardial infarction (AMI). In rare cases, death occurs [71].
- Fulminant myocarditis (FM) – there are about 8.6% of all patients with myocarditis [72]. It is characterized by an acute onset of the disease, which is characterized by rapid deterioration and usually death [33]. Usually, patients present with symptoms and signs of HF (up to pulmonary edema) and not infrequently up to cardiogenic shock, and the clinical course is accompanied by malignant arrhythmias. In these patients, inotropic stimulation or mechanical circulatory support is usually required [39,73]. Patients with FM compared to patients with a non-fulminant form of this disease have a higher early mortality ((28.0% vs. 1.8%, p=0.0001) and late mortality during 7 years of follow-up (47.7% vs. 10.4%, p<0.0001) respectively [71]. Earlier studies that monitored the prognosis of patients with FM showed conflicting results regarding the long-term prognosis [74,75,76]. Ammirati E. et al also showed a correlation between the histological subtype of FM and patient prognosis. Giant cell FM is associated with a significantly worse patient prognosis compared to eosinophilic and lymphocytic subtypes of myocarditis [71].
- Chronic persistent myocarditis (7% of cases) – it is characterized by a mild onset of the disease, usually without cardiac decompensation [33].
4.1. Symptoms related to the previous infectious agent
4.2. Chest pain
4.3. Symptoms and signs of heart failure
4.4. Arrhythmias
4.5. Syncope
4.6. Myocarditis in children
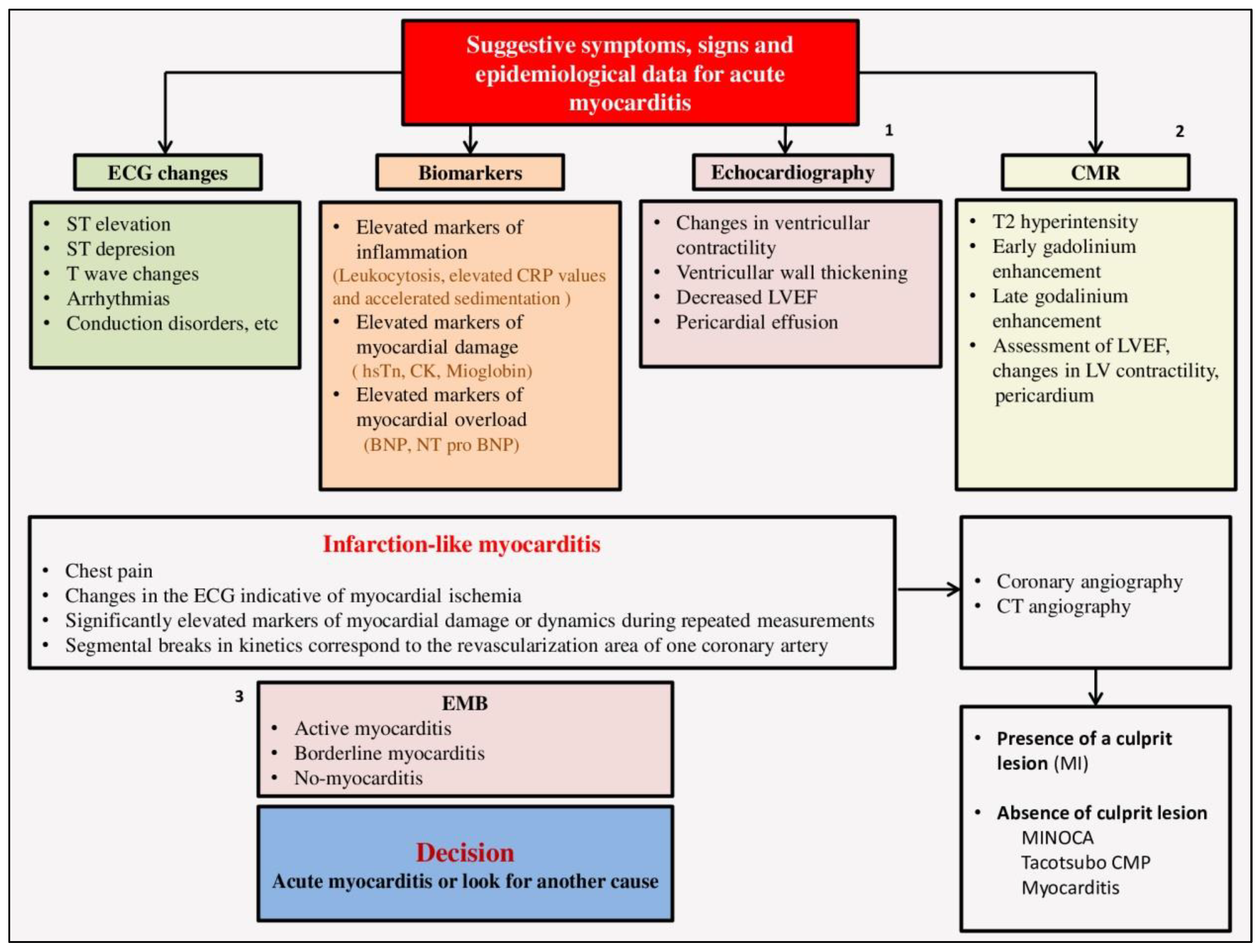
5. DIAGNOSIS OF MYOCARDITIS
5.1. ECG in myocarditis
5.2. Biomarkers
- Markers of inflammation: high-sensitivity C-reactive protein (hs-CRP), elevated leukocyte count and accelerated sedimentation.
- b.
- Markers of myocardial damage
- c.
- Dysfunction markers
- d.
- Anti cardiac antibodies
- e.
- Micro RNA
- f.
- Viral antibodies
5.3. Echocardiography
5.4. Cardiac magnetic resonance (CMR)
5.5. FDG-PET Myocarditis
5.6. Endomyocardial biopsy (EMB)
6. Differential diagnosis
7. Treatment
- A.)
- Classical treatment
- B.)
- Immunomodulatory therapy
- C.)
- Immunosuppressive therapy
8. Prognosis
Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ammirati, E.; Frigerio, M.; Adler, E.D.; Basso, C.; Birnie, D.H.; Brambatti, M.; Friedrich, M.G.; Klingel, K.; Lehtonen, J.; Moslehi, J.J.; et al. Management of Acute Myocarditis and Chronic Inflammatory Cardiomyopathy: An Expert Consensus Document. Circ Heart Fail. 2020, 13, e007405. [Google Scholar] [CrossRef]
- Ikegami, Y.; Tase, C. ; Acute Myocarditis in Emergency Medicine. JACC 2012, 59, 779–792. [Google Scholar]
- Golpour, A.; Patriki, D.; Hanson, P.J.; McManus, B.; Heidecker, B. Epidemiological Impact of Myocarditis. J Clin Med. 2021, 10, 603. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Kontogeorgos, S.; Thunström, E.; Zverkova, S.T.; Kroon, C.; Bollano, E.; Schaufelberger, M.; Rosengren, A. Trends in myocarditis incidence, complications and mortality in Sweden from 2000 to 2014. Sci Rep. 2022, 12, 1810. [Google Scholar] [CrossRef] [PubMed]
- Basso, C.; Calabrese, F.; Corrado, D.; Thiene, G. Postmortem diagnosis in sudden cardiac death victims: macroscopic, microscopic and molecular findings. Cardiovasc Res. 2001, 50, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Doerer, J.J.; Haas, T.S.; Tierney, D.M.; Mueller, F.O. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980-2006. Circulation. 2009, 119, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Jouriles, N.J; Chapter 80. In: Marx, J.A.; Hockberger, R.S.; Walls, R.M.; editors. Rosen’s emergency medicine: concepts and clinical practice. 7th ed. Philadelphia: Mosby; 2009. p. 1064-1068.
- Maron, B.J.; Towbin, J.A.; Thiene, G.; Antzelevitch, C.; Corrado, D.; Arnett, D.; Moss, A.J.; Seidman, C.E.; Young, J.B.; American Heart Association; Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006, 113, 1807–1816. [Google Scholar]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef]
- Altay, S. COVID-19 myocarditis cardiac magnetic resonance findings in symptomatic patients. Acta Radiol. 2022, 63, 1475–1480. [Google Scholar] [CrossRef]
- Voleti, N.; Reddy, S.P.; Ssentongo, P. Myocarditis in SARS-CoV-2 infection vs. COVID-19 vaccination: A systematic review and meta-analysis. Front Cardiovasc Med. 2022, 9, 951314. [Google Scholar] [CrossRef]
- Fairweather, D.; Beetler, D.J.; Musigk, N.; Heidecker, B.; Lyle, M.A.; Cooper, L.T., Jr.; Bruno, K.A. Sex and gender differences in myocarditis and dilated cardiomyopathy: An update. Front Cardiovasc Med. 2023, 10, 1129348. [Google Scholar] [CrossRef]
- Kytö, V.; Sipilä, J.; Rautava, P. The effects of gender and age on occurrence of clinically suspected myocarditis in adulthood. Heart. 2013, 99, 1681–1684. [Google Scholar] [CrossRef]
- Laufer-Perl, M.; Havakuk, O.; Shacham, Y.; Steinvil, A.; Letourneau-Shesaf, S.; Chorin, E.; Keren, G.; Arbel, Y. Sex-based differences in prevalence and clinical presentation among pericarditis and myopericarditis patients. Am J Emerg Med. 2017, 35, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Lynge, T.H.; Nielsen, T.S.; Gregers Winkel, B.; Tfelt-Hansen, J.; Banner, J. Sudden cardiac death caused by myocarditis in persons aged 1-49 years: a nationwide study of 14 294 deaths in Denmark. Forensic Sci Res. 2019, 4, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Durani, Y.; Egan, M.; Baffa, J.; Selbst, S.M.; Nagar, A.L. Pediatric myocarditis: presenting clinical characteristics. Am J Emerg Med. 2009, 27, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Arola, A.; Pikkarainen, E.; Sipilä, J.O.; Pykäri, J.; Rautava, P.; Kytö, V. Occurrence and Features of Childhood Myocarditis: A Nationwide Study in Finland. J Am Heart Assoc. 2017, 6, e005306. [Google Scholar] [CrossRef] [PubMed]
- Tschöpe, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.P.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hübner, N.; et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. 2021, 18, 169–193. [Google Scholar] [CrossRef] [PubMed]
- Lévêque, N.; Garcia, M.; Bouin, A.; Nguyen, J.H.C.; Tran, G.P.; Andreoletti, L.; Semler, B.L. Functional Consequences of RNA 5'-Terminal Deletions on Coxsackievirus B3 RNA Replication and Ribonucleoprotein Complex Formation. J Virol. 2017, 91, e00423–17. [Google Scholar] [CrossRef] [PubMed]
- Strabelli, T.M.; Siciliano, R.F.; Vidal Campos, S.; Bianchi Castelli, J.; Bacal, F.; Bocchi, E.A.; Uip, D.E. Toxoplasma gondii Myocarditis after Adult Heart Transplantation: Successful Prophylaxis with Pyrimethamine. J Trop Med. 2012, 2012, 853562. [Google Scholar] [CrossRef]
- Mylvaganam, R.; Glaser, A.; Moss, N.; Rana, M. A case of late-onset cytomegalovirus myocarditis in an orthotopic heart transplant recipient; case report and review of the literature. Diagn Microbiol Infect Dis. 2018, 91, 153–155. [Google Scholar] [CrossRef]
- Massilamany, C.; Huber, S.A.; Cunningham, M.W.; Reddy, J. Relevance of molecular mimicry in the mediation of infectious myocarditis. J Cardiovasc Transl Res. 2014, 7, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Pannucci, P.; Jefferson, S.R.; Hampshire, J.; Cooper, S.L.; Hill, S.J.; Woolard, J. COVID-19-Induced Myocarditis: Pathophysiological Roles of ACE2 and Toll-like Receptors. Int J Mol Sci. 2023, 24, 5374. [Google Scholar] [CrossRef] [PubMed]
- Lasica, R.; Djukanovic, L.; Mrdovic, I.; Savic, L.; Ristic, A.; Zdravkovic, M.; Simic, D.; Krljanac, G.; Popovic, D.; Simeunovic, D.; Rajic, D.; Asanin, M. Acute Coronary Syndrome in the COVID-19 Era-Differences and Dilemmas Compared to the Pre-COVID-19 Era. J Clin Med. 2022, 11, 3024. [Google Scholar] [CrossRef] [PubMed]
- Dennert, R.; Crijns, H.J.; Heymans, S. Acute viral myocarditis. Eur Heart J. 2008, 29, 2073–2082. [Google Scholar] [CrossRef]
- Andréoletti, L.; Lévêque, N.; Boulagnon, C.; Brasselet, C.; Fornes, P. Viral causes of human myocarditis. Arch Cardiovasc Dis. 2009, 102, 559–568. [Google Scholar] [CrossRef]
- Seitz, A.; Martínez Pereyra, V.; Hubert, A.; Klingel, K.; Bekeredjian, R.; Sechtem, U.; Ong, P. Epicardial and microvascular coronary artery spasm in biopsy-proven viral myocarditis. Int J Cardiol. 2022, 360, 1–4. [Google Scholar] [CrossRef]
- Keramari, S.; Poutoglidis, A.; Chatzis, S.; Keramaris, M.; Savopoulos, C.; Kaiafa, G. Parvovirus B19-Associated Myocarditis: A Literature Review of Pediatric Cases. Cureus. 2022, 14, e21726. [Google Scholar] [CrossRef]
- Schultheiss, H.P.; Baumeier, C.; Aleshcheva, G.; Bock, C.T.; Escher, F. Viral Myocarditis-From Pathophysiology to Treatment. J Clin Med. 2021, 10, 5240. [Google Scholar] [CrossRef] [PubMed]
- Palecek, T.; Ganame, J.; Di Salvo, G. Myocardial Diseases: Current Views on Etiopathogenesis, Diagnostic Modalities, and Therapeutic Options. Biomed Res Int. 2016, 2016, 1720405. [Google Scholar] [CrossRef]
- Saad, H.A.B.; Ntusi, N. HIV-Associated Cardiovascular Disease. Advances in HIV and AIDS Control. IntechOpen; 2018. [CrossRef]
- Freiberg, M.S.; Chang, C.H.; Skanderson, M.; Patterson, O.V.; DuVall, S.L.; Brandt, C.A.; So-Armah, K.A.; Vasan, R.S.; Oursler, K.A.; Gottdiener, J.; et al. Association Between HIV Infection and the Risk of Heart Failure with Reduced Ejection Fraction and Preserved Ejection Fraction in the Antiretroviral Therapy Era: Results from the Veterans Aging Cohort Study. JAMA Cardiol. 2017, 2, 536–546. [Google Scholar] [CrossRef]
- Rroku, A.; Kottwitz, J.; Heidecker, B. Update on myocarditis - what we know so far and where we may be heading. Eur Heart J Acute Cardiovasc Care. 2020, 2048872620910109. [Google Scholar] [CrossRef] [PubMed]
- Lumsden, R.H.; Bloomfield, G.S. The Causes of HIV-Associated Cardiomyopathy: A Tale of Two Worlds. Biomed Res Int. 2016, 2016, 8196560. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, G.S.; Alenezi, F.; Barasa, F.A.; Lumsden, R.; Mayosi, B.M.; Velazquez, E.J. Human Immunodeficiency Virus and Heart Failure in Low- and Middle-Income Countries. JACC Heart Fail. 2015, 3, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Feinstein, M. Epidemiology, pathophysiology, and prevention of heart failure in people with HIV. Prog Cardiovasc Dis. 2020, 63, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Fishbein, M.C.; Siegel, R.J. Cardiac manifestations of acquired immune deficiency syndrome: a 1991 update. Am Heart J. 1991, 122, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Caforio, A.L.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013, 34, 2636–2648. [Google Scholar]
- Nagai, T.; Inomata, T.; Kohno, T.; Sato, T.; Tada, A.; Kubo, T.; Nakamura, K.; Oyama-Manabe, N.; Ikeda, Y.; Fujino, T.; et al. Japanese Circulation Society Joint Working Group. JCS 2023 Guideline on the Diagnosis and Treatment of Myocarditis. Circ J. 2023, 87, 674–754. [Google Scholar] [CrossRef]
- Tefferi, A.; Gotlib, J.; Pardanani, A. Hypereosinophilic syndrome and clonal eosinophilia: point-of-care diagnostic algorithm and treatment update. Mayo Clin Proc. 2010, 85, 158–164. [Google Scholar] [CrossRef]
- Ogbogu, P.U.; Bochner, B.S.; Butterfield, J.H.; Gleich, G.J.; Huss-Marp, J.; Kahn, J.E.; Leiferman, K.M.; Nutman, T.B.; Pfab, F.; Ring, J. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol. 2009, 124, 1319–1325e3. [Google Scholar] [CrossRef]
- Datta, T.; Solomon, A.J. Clozapine-induced myocarditis. Oxf Med Case Reports. 2018, 2018, omx080. [Google Scholar] [CrossRef]
- Garty, B.Z.; Offer, I.; Livni, E.; Danon, Y.L. Erythema multiforme and hypersensitivity myocarditis caused by ampicillin. Ann Pharmacother. 1994, 28, 730–731. [Google Scholar] [CrossRef] [PubMed]
- Çetin, M.; Mis, M.D.; Karaman, K.; Yavuz, Í.H.; Geylan, H.; Tunçdemir, P.; Demir, F. Carbamazepine-induced DRESS syndrome leading to reversible myocarditis in a child. Cent Eur J Immunol. 2019, 44, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Shaughnessy, K.K.; Bouchard, S.M.; Mohr, M.R.; Herre, J.M.; Salkey, K.S. Minocycline-induced drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome with persistent myocarditis. J Am Acad Dermatol. 2010, 62, 315–318. [Google Scholar] [CrossRef]
- Bryde, R.E.; Ray, J.C.; Sacco, K.A.; Shapiro, B.; Cooper, L. Eosinophillic Myocarditis Secondary to Metastatic Melanoma. Radiol Cardiothorac Imaging. 2019, 1, e190076. [Google Scholar] [CrossRef]
- Epelboin, L.; Jauréguiberry, S.; Estève, J.B.; Danis, M.; Komajda, M.; Bricaire, F.; Caumes, E. Myocarditis during acute schistosomiasis in two travelers. Am J Trop Med Hyg. 2010, 82, 365–367. [Google Scholar] [CrossRef]
- Pakbaz, M.; Pakbaz, M. Cardiac Involvement in Eosinophilic Granulomatosis with Polyangiitis: A Meta-Analysis of 62 Case Reports. J Tehran Heart Cent. 2020, 15, 18–26. [Google Scholar] [CrossRef]
- Xu, J.; Brooks, E.G. Giant Cell Myocarditis: A Brief Review. Arch Pathol Lab Med. 2016, 140, 1429–1434. [Google Scholar] [CrossRef]
- Hu, Y.; Ren, J.; Dong, X.; Zhang, D.; Qu, Y.; Yang, C.; Sun, Y.; Li, J.; Luo, F.; Wang, W.; et al. Fulminant Giant Cell Myocarditis vs. Lymphocytic Myocarditis: A Comparison of Their Clinical Characteristics, Treatments, and Outcomes. Front Cardiovasc Med. 2021, 8, 770549. [Google Scholar] [CrossRef]
- Gadela, N.V.; Krishnan, A.M.; Mukarram, O.; Sthalekar, N. Giant cell myocarditis. Proc (Bayl Univ Med Cent). 2021, 34, 401–402. [Google Scholar] [CrossRef]
- Ghaly, M.; Schiliro, D.; Stepczynski, J. Giant Cell Myocarditis: A Time Sensitive Distant Diagnosis. Cureus. 2020, 12, e6712. [Google Scholar] [CrossRef]
- Al-Kindi, S.G.; Oliveira, G.H. Reporting of immune checkpoint inhibitor-associated myocarditis. Lancet. 2018, 392, 382–383. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Wang, Q.; Meng, D.; Li, K.; Zhang, Y. Immune checkpoint inhibitors-related myocarditis in patients with cancer: an analysis of international spontaneous reporting systems. BMC Cancer. 2021, 21, 38. [Google Scholar] [CrossRef] [PubMed]
- Vasbinder, A.; Chen, Y.; Procureur, A.; Gradone, A.; Azam, T.U.; Perry, D.; Shadid, H.; Anderson, E.; Catalan, T.; Blakely, P.; et al. Biomarker Trends, Incidence, and Outcomes of Immune Checkpoint Inhibitor-Induced Myocarditis. JACC CardioOncol. 2022, 4, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Perel-Winkler, A.; Bokhari, S.; Perez-Recio, T.; Zartoshti, A.; Askanase, A.; Geraldino-Pardilla, L. Myocarditis in systemic lupus erythematosus diagnosed by 18F-fluorodeoxyglucose positron emission tomography. Lupus Sci Med. 2018, 5, e000265. [Google Scholar] [CrossRef]
- Aretz, H.T. Myocarditis: the Dallas criteria. Hum Pathol. 1987, 18, 619–624. [Google Scholar] [CrossRef]
- Brociek, E.; Tymińska, A.; Giordani, A.S.; Caforio, A.L.P.; Wojnicz, R.; Grabowski, M.; Ozierański, K. Myocarditis: Etiology, Pathogenesis, and Their Implications in Clinical Practice. Biology 2023, 12, 874. [Google Scholar] [CrossRef]
- Pandey, S.; Rajasurya, V. Nonviral Myocarditis. Treasure Island (FL): StatPearls Publishing; 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK536928/.
- Nguyen, L.S.; Cooper, L.T.; Kerneis, M.; Funck-Brentano, C.; Silvain, J.; Brechot, N.; Hekimian, G.; Ammirati, E.; Ben M'Barek, B.; Redheuil, A.; et al. Systematic analysis of drug-associated myocarditis reported in the World Health Organization pharmacovigilance database. Nat Commun. 2022, 13, 25. [Google Scholar] [CrossRef]
- Kounis, N.G.; Koniari, I.; Mplani, V.; Plotas, P.; Velissaris, D. Hypersensitivity Myocarditis and the Pathogenetic Conundrum of COVID-19 Vaccine-Related Myocarditis. Cardiology 2022, 147, 413–415. [Google Scholar] [CrossRef]
- Bracamonte-Baran, W.; Čiháková, D. Cardiac Autoimmunity: Myocarditis. Adv Exp Med Biol. 2017, 1003, 187–221. [Google Scholar]
- Park, Y.; Ahn, S.G.; Ko, A.; Ra, S.H.; Cha, J.; Jee, Y.G.; Lee, J.H. Hypersensitivity myocarditis confirmed by cardiac magnetic resonance imaging and endomyocardial biopsy. Korean J Intern Med. 2014, 29, 236–240. [Google Scholar] [CrossRef]
- JCS Joint Working Group. Guidelines for diagnosis and treatment of myocarditis (JCS 2009): digest version. Circ J. 2011, 75, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, E.; Moslehi, J.J. Diagnosis and Treatment of Acute Myocarditis: A Review. JAMA 2023, 329, 1098–1113. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, E.; Buono, A.; Moroni, F.; Gigli, L.; Power, J.R.; Ciabatti, M.; Garascia, A.; Adler, E.D.; Pieroni, M. State-of-the-Art of Endomyocardial Biopsy on Acute Myocarditis and Chronic Inflammatory Cardiomyopathy. Curr Cardiol Rep. 2022, 24, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Lampejo, T.; Durkin, S.M.; Bhatt, N.; Guttmann, O. Acute myocarditis: aetiology, diagnosis and management. Clin Med 2021, 21, e505–e510. [Google Scholar] [CrossRef] [PubMed]
- Daniels, C.J.; Rajpal, S.; Greenshields, J.T.; Rosenthal, G.L.; Chung, E.H.; Terrin, M.; Jeudy, J.; Mattson, S.E.; Law, I.H.; Borchers, J.; et al. Big Ten COVID-19 Cardiac Registry Investigators. Prevalence of Clinical and Subclinical Myocarditis in Competitive Athletes with Recent SARS-CoV-2 Infection: Results from the Big Ten COVID-19 Cardiac Registry. JAMA Cardiol. 2021, 6, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Caforio, A.L.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013, 34, 2636–2648. [Google Scholar]
- Al-Akchar, M.; Shams, P.; Kiel, J. Acute Myocarditis. Treasure Island (FL): StatPearls Publishing; 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441847/.
- Ammirati, E.; Veronese, G.; Brambatti, M.; Merlo, M.; Cipriani, M.; Potena, L.; Sormani, P.; Aoki, T.; Sugimura, K.; Sawamura, A.; et al. Fulminant Versus Acute Nonfulminant Myocarditis in Patients with Left Ventricular Systolic Dysfunction. J Am Coll Cardiol. 2019, 74, 299–311. [Google Scholar] [CrossRef]
- Ammirati, E.; Cipriani, M.; Moro, C.; Raineri, C.; Pini, D.; Sormani, P.; Mantovani, R.; Varrenti, M.; Pedrotti, P.; Conca, C.; et al. Registro Lombardo delle Miocarditi. Clinical presentation and outcome in a contemporary cohort of patients with acute myocarditis: multicenter lombardy registry. Circulation. 2018, 138, 1088–1099. [Google Scholar] [CrossRef]
- Kociol, R.D.; Cooper, L.T.; Fang, J.C.; Moslehi, J.J.; Pang, P.S.; Sabe, M.A.; Shah, R.V.; Sims, D.B.; Thiene, G.; Vardeny, O. American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology. Recognition and Initial Management of Fulminant Myocarditis: A Scientific Statement from the American Heart Association. Circulation. 2020, 141, e69–e92. [Google Scholar] [CrossRef]
- McCarthy, R.E., 3rd; Boehmer, J.P.; Hruban, R.H.; Hutchins, G.M.; Kasper, E.K.; Hare, J.M.; Baughman, K.L. Long-term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. N Engl J Med. 2000, 342, 690–695. [Google Scholar] [CrossRef]
- Anzini, M.; Merlo, M.; Sabbadini, G.; Barbati, G.; Finocchiaro, G.; Pinamonti, B.; Salvi, A.; Perkan, A.; Di Lenarda, A.; Bussani, R.; et al. Long-term evolution and prognostic stratification of biopsy-proven active myocarditis. Circulation. 2013, 128, 2384–2394. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, E.; Cipriani, M.; Lilliu, M.; Sormani, P.; Varrenti, M.; Raineri, C.; Petrella, D.; Garascia, A.; Pedrotti, P.; Roghi, A.; et al. Survival and Left Ventricular Function Changes in Fulminant Versus Nonfulminant Acute Myocarditis. Circulation. 2017, 136, 529–545. [Google Scholar] [CrossRef] [PubMed]
- Jeserich, M.; Konstantinides, S.; Olschewski, M.; Pavlik, G.; Bode, C.; Geibel, A. Diagnosis of early myocarditis after respiratory or gastrointestinal tract viral infection: insights from cardiovascular magnetic resonance. Clin Res Cardiol. 2010, 99, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Vohra, S.; Yadav, A.; Sharma, P.; Khan, S.; Jaiswal, V. Acute myocarditis masquerading as anterior wall myocardial infarction: A case report. Ann Med Surg 2022, 84, 104884. [Google Scholar] [CrossRef] [PubMed]
- White, J.A.; Hansen, R.; Abdelhaleem, A.; Mikami, Y.; Peng, M.; Rivest, S.; Satriano, A.; Dykstra, S.; Flewitt, J.; Heydari, B.; et al. Natural History of Myocardial Injury and Chamber Remodeling in Acute Myocarditis. Circ Cardiovasc Imaging. 2019, 12, e008614. [Google Scholar] [CrossRef] [PubMed]
- Gräni, C.; Eichhorn, C.; Bière, L.; Murthy, V.L.; Agarwal, V.; Kaneko, K.; Cuddy, S.; Aghayev, A.; Steigner, M.; Blankstein, R.; et al. Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J Am Coll Cardiol. 2017, 70, 1964–1976. [Google Scholar] [CrossRef] [PubMed]
- Younis, A.; Matetzky, S.; Mulla, W.; Masalha, E.; Afel, Y.; Chernomordik, F.; Fardman, A.; Goitein, O.; Ben-Zekry, S.; Peled, Y.; et al. Epidemiology characteristics and outcome of patients with clinically diagnosed acute myocarditis. Am J Med. 2020, 133, 492–499. [Google Scholar] [CrossRef]
- Adamopoulos, S.; Miliopoulos, D.; Karavidas, A.; Nikolaou, M.; Lazaros, G.; Gkouziouta, A.; Manginas, A.; Sevastos, G.; Karvounis, H.; Karamitsos, T.D.; et al. HEllenic Registry on Myocarditis SyndromES on behalf of Hellenic Heart Failure Association: The HERMES-HF Registry. ESC Heart Fail. 2020, 7, 3676–3684. [Google Scholar] [CrossRef]
- Dries, D.J. Chest Pain. Air Med J. 2016, 35, 107–110. [Google Scholar] [CrossRef]
- Aquaro, G.D.; Perfetti, M.; Camastra, G.; Monti, L.; Dellegrottaglie, S.; Moro, C.; Pepe, A.; Todiere, G.; Lanzillo, C.; Scatteia, A.; et al. Cardiac Magnetic Resonance Working Group of the Italian Society of Cardiology. Cardiac MR With Late Gadolinium Enhancement in Acute Myocarditis with Preserved Systolic Function: ITAMY Study. J Am Coll Cardiol. 2017, 70, 1977–1987. [Google Scholar] [CrossRef]
- Cannata, A.; Bhatti, P.; Roy, R.; Al-Agil, M.; Daniel, A.; Ferone, E.; Jordan, A.; Cassimon, B.; Bradwell, S.; Khawaja, A.; et al. Prognostic relevance of demographic factors in cardiac magnetic resonance-proven acute myocarditis: A cohort study. Front Cardiovasc Med. 2022, 9, 1037837. [Google Scholar] [CrossRef] [PubMed]
- Angelini, A.; Calzolari, V.; Calabrese, F.; Boffa, G.M.; Maddalena, F.; Chioin, R.; Thiene, G. Myocarditis mimicking acute myocardial infarction: role of endomyocardial biopsy in the differential diagnosis. Heart. 2000, 84, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, M.H.; Espinosa, R.E.; Nishimura, R.A.; Sinak, L.J.; Hayes, S.N.; Melduni, R.M.; Oh, J.K. Pericardial disease: diagnosis and management. Mayo Clin Proc. 2010, 85, 572–593. [Google Scholar] [CrossRef] [PubMed]
- Aota, H.; Suzuki, H.; Godo, S.; Kuniyoshi, S.; Fujishima, F.; Tahakashi, J.; Yasuda, S. A teenage boy with acute myocarditis and reversible microvascular angina: A case report. J Cardiol Cases. 2023, 27, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; Marques, P.; Martins, S.; Bordalo, E.; Sá, A.L.; Nóbrega, J.; Duarte, J.; Almeida, A.G.; Gabriel, H.M.; Correia, M.J.; Diogo, A.N. Coronary artery vasospasm and acute myocarditis: a rare association. Rev Port Cardiol. 2010, 29, 1879–1888. [Google Scholar] [PubMed]
- McCully, R.B.; Cooper, L.T.; Schreiter, S. Coronary artery spasm in lymphocytic myocarditis: a rare cause of acute myocardial infarction. Heart. 2005, 91, 202. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Pabani, U.K.; Gul, A.; Muhammad, S.A.; Yousif, Y.; Abumedian, M.; Elmahdi, O.; Gupta, A. COVID-19 Vaccine-Induced Myocarditis: A Systemic Review and Literature Search. Cureus. 2022, 14, e27408. [Google Scholar] [CrossRef]
- Bonanni, M.; Angelini, G.; Leo, L.A.; Schlossbauer, S.A.; Bergamaschi, L.; Landi, A.; Sangiorgi, G.M.; Forleo, C.; Pasotti, E.; Pedrazzini, G.; et al. Multimodality Imaging Evaluation to Detect Subtle Right Ventricular Involvement in Patients with Acute Myocarditis and Preserved Left Ventricular Ejection Fraction. J Clin Med. 2023, 12, 4308. [Google Scholar] [CrossRef]
- Duncan, B.W.; Bohn, D.J.; Atz, A.M.; French, J.W.; Laussen, P.C.; Wessel, D.L. Mechanical circulatory support for the treatment of children with acute fulminant myocarditis. J Thorac Cardiovasc Surg. 2001, 122, 440–448. [Google Scholar] [CrossRef]
- Pages, O.N.; Aubert, S.; Combes, A.; Luyt, C.E.; Pavie, A.; Léger, P.; Gandjbakhch, I.; Leprince, P. Paracorporeal pulsatile biventricular assist device versus extracorporal membrane oxygenation-extracorporal life support in adult fulminant myocarditis. J Thorac Cardiovasc Surg. 2009, 137, 194–197. [Google Scholar] [CrossRef]
- Peretto, G.; Sala, S.; Rizzo, S.; De Luca, G.; Campochiaro, C.; Sartorelli, S.; Benedetti, G.; Palmisano, A.; Esposito, A.; Tresoldi, M.; et al. Arrhythmias in myocarditis: State of the art. Heart Rhythm. 2019, 16, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Janga, C.; Deshmukh, A.J. Arrhythmias Associated with Inflammatory Cardiomyopathies. Curr Treat Options Cardiovasc Med. 2020, 22, 76. [Google Scholar] [CrossRef] [PubMed]
- Kragholm, K.H.; Lindgren, F.L.; Zaremba, T.; Freeman, P.; Andersen, N.H.; Riahi, S.; Pareek, M.; Køber, L.; Torp-Pedersen, C.; Søgaard, P.; et al. Mortality and ventricular arrhythmia after acute myocarditis: a nationwide registry-based follow-up study. Open Heart. 2021, 8, e001806. [Google Scholar] [CrossRef] [PubMed]
- Sozzi, F.B.; Gherbesi, E.; Faggiano, A.; Gnan, E.; Maruccio, A.; Schiavone, M.; Iacuzio, L.; Carugo, S. Viral Myocarditis: Classification, Diagnosis, and Clinical Implications. Front Cardiovasc Med. 2022, 9, 908663. [Google Scholar] [CrossRef] [PubMed]
- Law, Y.M.; Lal, A.K; Chen, S.; Čiháková, D.; Cooper, L.T.; Deshpande, S.; Godown, J.; Grosse-Wortmann, L.; Robinson, J.D.; Towbin, J.A. American Heart Association Pediatric Heart Failure and Transplantation Committee of the Council on Lifelong Congenital Heart Disease and Heart Health in the Young and Stroke Council. Diagnosis and Management of Myocarditis in Children: A Scientific Statement from the American Heart Association. Circulation 2021, 144, e123–e135. [Google Scholar] [PubMed]
- Subahi, A.; Akintoye, E.; Yassin, A.S.; Abubakar, H.; Adegbala, O.; Mishra, T.; Abdelrahman, M.; Shokr, M.; Afonso, L. Impact of atrial fibrillation on patients hospitalized for acute myocarditis: Insights from a nationally-representative United States cohort. Clin Cardiol. 2019, 42, 26–31. [Google Scholar] [CrossRef]
- Rosier, L.; Zouaghi, A.; Barré, V.; Martins, R.; Probst, V.; Marijon, E.; Sadoul, N.; Chauveau, S.; Da Costa, A.; Badoz, M.; et al. High Risk of Sustained Ventricular Arrhythmia Recurrence After Acute Myocarditis. J Clin Med. 2020, 9, 848. [Google Scholar] [CrossRef]
- Okura, Y.; Dec, G.W.; Hare, J.M.; Kodama, M.; Berry, G.J.; Tazelaar, H.D.; Bailey, K.R.; Cooper, L.T. A clinical and histopathologic comparison of cardiac sarcoidosis and idiopathic giant cell myocarditis. J Am Coll Cardiol. 2003, 41, 322–329. [Google Scholar] [CrossRef]
- Kandolin, R.; Lehtonen, J.; Kupari, M. Cardiac sarcoidosis and giant cell myocarditis as causes of atrioventricular block in young and middle-aged adults. Circ Arrhythm Electrophysiol. 2011, 4, 303–309. [Google Scholar] [CrossRef]
- Wan, D.; Baranchuk, A. Lyme carditis and atrioventricular block. CMAJ. 2018, 190, E622. [Google Scholar] [CrossRef]
- Alamri, A.S.; Khayat, L.T.; Alzahrani, A.J.; Kurdi, L.K.; Alkhameesi, N.F.; Bahaidarah, S.A. Clinical Presentation of Myocarditis in the Pediatric Age Group and Predictors of Poor Early and Late Outcomes: Academic Hospital Experience. Cureus. 2022, 14, e31643. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Tsuda, E.; Miyazaki, A.; Ishibashi-Ueda, H.; Yamada, O. Clinical characteristics and long-term outcome of acute myocarditis in children. Heart Vessels. 2013, 28, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013, 128, 1810–1852. [Google Scholar] [CrossRef] [PubMed]
- Károlyi, M.; Kolossváry, M.; Weber, L.; Matziris, I.; Polacin, M.; Sokolska, J.M.; Gotschy, A.; Alkadhi, H.; Manka, R. Association between ECG parameters and late gadolinium enhancement along the course of myocarditis. Int J Cardiovasc Imaging. 2023, 39, 1169–1178. [Google Scholar] [CrossRef]
- Cooper, L.T.Jr. Myocarditis. N Engl J Med. 2009, 360, 1526–1538. [Google Scholar] [CrossRef]
- Nakashima, H.; Honda, Y.; Katayama, T. Serial electrocardiographic findings in acute myocarditis. Intern Med. 1994, 33, 659–666. [Google Scholar] [CrossRef]
- Ogunbayo, G.O.; Elayi, S.C.; Ha, L.D.; Olorunfemi, O.; Elbadawi, A.; Saheed, D.; Sorrell, V.L. Outcomes of Heart Block in Myocarditis: A Review of 31,760 Patients. Heart Lung Circ. 2019, 28, 272–276. [Google Scholar] [CrossRef]
- Stulova, M.A.; Konstantinova, E.V. Ventricular extrasystole as manifestation of viral myocarditis and myopericarditis in young patients. Ter Arkh. 2007, 79, 28–34. [Google Scholar]
- Baksi, A.J.; Kanaganayagam, G.S.; Prasad, S.K. Arrhythmias in viral myocarditis and pericarditis. Card Electrophysiol Clin. 2015, 7, 269–281. [Google Scholar] [CrossRef]
- Chen, J.; Chen, S.; Li, Z.; Zhou, P.; Huang, W.; Wang, H.; Shi, J.; Ni, Y.; Lin, L.; Lei, Y. Role of electrocardiograms in assessment of severity and analysis of the characteristics of ST elevation in acute myocarditis: A two-centre study. Exp Ther Med. 2020, 20, 20. [Google Scholar] [CrossRef]
- Buttà, C.; Zappia, L.; Laterra, G.; Roberto, M. Diagnostic and prognostic role of electrocardiogram in acute myocarditis: A comprehensive review. Ann Noninvasive Electrocardiol. 2020, 25, e12726. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.C.; Brady, W.J.; Pollack, M. Electrocardiographic manifestations: acute myopericarditis. J Emerg Med. 1999, 17, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.; Birnbaum, Y. ST-segment elevation: Distinguishing ST elevation myocardial infarction from ST elevation secondary to nonischemic etiologies. World J Cardiol. 2014, 6, 1067–1079. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, Y.; Perez Riera, A.R.; Nikus, K. PR depression with multi lead ST elevation and ST depression in aVR: Is it always acute pericarditis? J Electrocardiol. 2019, 54, 13–17. [Google Scholar] [CrossRef]
- Sarda, L.; Colin, P.; Boccara, F.; Daou, D.; Lebtahi, R.; Faraggi, M.; Nguyen, C.; Cohen, A.; Slama, M.S.; Steg, P.G.; Le Guludec, D. Myocarditis in patients with clinical presentation of myocardial infarction and normal coronary angiograms. J Am Coll Cardiol. 2001, 37, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Dai, Q.; Wu, H.; Chen, J.; Zhang, J.; Wei, Z. The diagnostic capability of electrocardiography on the cardiogenic shock in the patients with acute myocarditis. BMC Cardiovasc Disord. 2020, 20, 502. [Google Scholar] [CrossRef]
- Di Bella, G.; Florian, A.; Oreto, L.; Napolitano, C.; Todaro, M.C.; Donato, R.; Calamelli, S.; Camastra, G.S.; Zito, C.; Carerj, S.; et al. Electrocardiographic findings and myocardial damage in acute myocarditis detected by cardiac magnetic resonance. Clin Res Cardiol. 2012, 101, 617–624. [Google Scholar] [CrossRef]
- De Lazzari, M.; Zorzi, A.; Baritussio, A.; Siciliano, M.; Migliore, F.; Susana, A.; Giorgi, B.; Lacognata, C.; Iliceto, S.; Perazzolo Marra, M.; Corrado, D. Relationship between T-wave inversion and transmural myocardial edema as evidenced by cardiac magnetic resonance in patients with clinically suspected acute myocarditis: clinical and prognostic implications. J Electrocardiol. 2016, 49, 587–595. [Google Scholar] [CrossRef]
- Ahluwalia, M.; O'Quinn, R.; Ky, B.; Callans, D.; Kucharczuk, J.; Carver, J.R. Persistent PR segment change in malignant pericardial disease. Cardiooncology. 2016, 2, 6. [Google Scholar] [CrossRef]
- Madias, J.E. Transient attenuation of the amplitude of the QRS complexes in the diagnosis of Takotsubo syndrome. Eur Heart J Acute Cardiovasc Care. 2014, 3, 28–36. [Google Scholar] [CrossRef]
- Ukena, C.; Mahfoud, F.; Kindermann, I.; Kandolf, R.; Kindermann, M.; Böhm, M. Prognostic electrocardiographic parameters in patients with suspected myocarditis. Eur J Heart Fail. 2011, 13, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Dai, Q.; Wu, H.; Chen, J.; Zhang, J.; Wei, Z. The diagnostic capability of electrocardiography on the cardiogenic shock in the patients with acute myocarditis. BMC Cardiovasc Disord. 2020, 20, 502. [Google Scholar] [CrossRef] [PubMed]
- Brambatti, M.; Matassini, M.V.; Adler, E.D.; Klingel, K.; Camici, P.G.; Ammirati, E. Eosinophilic Myocarditis: Characteristics, Treatment, and Outcomes. J Am Coll Cardiol. 2017, 70, 2363–2375. [Google Scholar] [CrossRef] [PubMed]
- Viwe, M.; Nery, P.; Birnie, D.H. Management of ventricular tachycardia in patients with cardiac sarcoidosis. Heart Rhythm O2. 2021, 2, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Morgera, T.; Di Lenarda, A.; Dreas, L.; Pinamonti, B.; Humar, F.; Bussani, R.; Silvestri, F.; Chersevani, D.; Camerini, F. Electrocardiography of myocarditis revisited: clinical and prognostic significance of electrocardiographic changes. Am Heart J. 1992, 124, 455–467. [Google Scholar] [CrossRef]
- Bhattacharya, I.S.; Dweck, M.; Francis, M. Lyme carditis: a reversible cause of complete atrioventricular block. J R Coll Physicians Edinb. 2010, 40, 121–122. [Google Scholar] [CrossRef]
- Kandolin, R.; Lehtonen, J.; Salmenkivi, K.; Räisänen-Sokolowski, A.; Lommi, J.; Kupari, M. Diagnosis, treatment, and outcome of giant-cell myocarditis in the era of combined immunosuppression. Circ Heart Fail. 2013, 6, 15–22. [Google Scholar] [CrossRef]
- Sekhri, V.; Sanal, S.; Delorenzo, L.J.; Aronow, W.S.; Maguire, G.P. Cardiac sarcoidosis: a comprehensive review. Arch Med Sc 2011, 7, 546–554. [Google Scholar] [CrossRef]
- Kaczmarek, K.A.; Szwabe, K.; Urbanek, I.; Ptaszynski, P.; Strzelecki, A.; Wranicz, J.K.; Cygankiewicz, I. Prevalence of Lyme Carditis in Patients with Atrioventricular Blocks. Int J Environ Res Public Health. 2022, 19, 14893. [Google Scholar] [CrossRef]
- Suresh, A.; Martens, P.; Tang, W.H.W. Biomarkers for Myocarditis and Inflammatory Cardiomyopathy. Curr Heart Fail Rep. 2022, 19, 346–355. [Google Scholar] [CrossRef]
- Amioka, N.; Nakamura, K.; Kimura, T.; Ohta-Ogo, K.; Tanaka, T.; Toji, T.; Akagi, S.; Nakagawa, K.; Toh, N.; Yoshida, M.; et al. Pathological and clinical effects of interleukin-6 on human myocarditis. J Cardiol. 2021, 78, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Fuse, K.; Kodama, M.; Okura, Y.; Ito, M.; Hirono, S.; Kato, K.; Hanawa, H.; Aizawa, Y. Predictors of disease course in patients with acute myocarditis. Circulation. 2000, 102, 2829–2835. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, X.; Shen, J.; Jiang, B.; Hu, D.; Zhao, M. Heparin-Binding Protein: A Novel Biomarker Linking Four Different Cardiovascular Diseases. Cardiol Res Pract. 2020, 15, 9575373. [Google Scholar] [CrossRef] [PubMed]
- Müller, I.; Vogl, T.; Kühl, U.; Krannich, A.; Banks, A.; Trippel, T.; Noutsias, M.; Maisel, A.S.; van Linthout, S.; Tschöpe, C. Serum alarmin S100A8/S100A9 levels and its potential role as biomarker in myocarditis. ESC Heart Fail. 2020, 7, 1442–1451. [Google Scholar] [CrossRef] [PubMed]
- Coronado, M.J.; Bruno, K.A.; Blauwet, L.A.; Tschöpe, C.; Cunningham, M.W.; Pankuweit, S.; van Linthout, S.; Jeon, E.S.; McNamara, D.M.; Krejčí, J.; et al. Elevated Sera sST2 Is Associated with Heart Failure in Men ≤50 Years Old With Myocarditis. J Am Heart Assoc. 2019, 8, e008968. [Google Scholar] [CrossRef]
- Mirna, M.; Schmutzler, L.; Topf, A.; Hoppe, U.C.; Lichtenauer, M. Neutrophil-to-lymphocyte ratio and monocyte-to-lymphocyte ratio predict length of hospital stay in myocarditis. Sci Rep. 2021, 11, 18101. [Google Scholar] [CrossRef]
- Kanda, T.; Kobayashi, I.; Suzuki, T.; Murata, K.; Radio, S.J.; McManus, B.M. Elevation of ALT to AST ratio in patients with enteroviral myocarditis. J Med. 1995, 26, 153–162. [Google Scholar]
- Omran, F.; Kyrou, I.; Osman, F.; Lim, V.G.; Randeva, H.S.; Chatha, K. Cardiovascular Biomarkers: Lessons of the Past and Prospects for the Future. Int J Mol Sci. 2022, 23, 5680. [Google Scholar] [CrossRef]
- Jeremias, A.; Gibson, C.M. Narrative review: alternative causes for elevated cardiac troponin levels when acute coronary syndromes are excluded. Ann Intern Med. 2005, 142, 786–791. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Z.; Chen, K.; Cui, G.; Chen, C.; Wang, L.; Jiang, J. The absolute and relative changes in high-sensitivity cardiac troponin I are associated with the in-hospital mortality of patients with fulminant myocarditis. BMC Cardiovasc Disord. 2021, 21, 571. [Google Scholar] [CrossRef]
- Lehmann, L.H.; Heckmann, M.B.; Bailly, G.; Finke, D.; Procureur, A.; Power, J.R.; Stein, F.; Bretagne, M.; Ederhy, S.; Fenioux, C.; et al. Cardiomuscular Biomarkers in the Diagnosis and Prognostication of Immune Checkpoint Inhibitor Myocarditis. Circulation. 2023, 148, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Sara, B.; Monteiro, J.; Carvalho, P.; Ribeiro Carvalho, C.; Chemba, J.; Ferreira, C.; Moreira, J.I. Are high NT-proBNP levels more related to inflammation than to left ventricular systolic dysfunction in acute myocarditis? Eur Heart J Acute Cardiovasc Care. 2021, 10, 189. [Google Scholar] [CrossRef]
- Ukena, C.; Kindermann, M.; Mahfoud, F.; Geisel, J.; Lepper, P.M.; Kandolf, R.; Böhm, M.; Kindermann, I. Diagnostic and prognostic validity of different biomarkers in patients with suspected myocarditis. Clin Res Cardiol. 2014, 103, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Heuser, J.S.; Cunningham, L.C.; Kosanke, S.D.; Cunningham, M.W. Mimicry and antibody-mediated cell signaling in autoimmune myocarditis. J Immunol. 2006, 177, 8234–8240. [Google Scholar] [CrossRef]
- Tymińska, A.; Ozierański, K.; Skwarek, A.; Kapłon-Cieślicka, A.; Baritussio, A.; Grabowski, M.; Marcolongo, R.; Caforio, A.L. Personalized Management of Myocarditis and Inflammatory Cardiomyopathy in Clinical Practice. J Pers Med. 2022, 12, 183. [Google Scholar] [CrossRef] [PubMed]
- Blagova, O.; Varionchik, N.; Zaidenov, V.; Savina, P.; Sarkisova, N. Anti-heart antibodies levels and their correlation with clinical symptoms and outcomes in patients with confirmed or suspected diagnosis COVID-19. Eur J Immunol. 2021, 51, 893–902. [Google Scholar] [CrossRef]
- Caforio, A.L.; Tona, F.; Bottaro, S.; Vinci, A.; Dequal, G.; Daliento, L.; Thiene, G.; Iliceto, S. Clinical implications of anti-heart autoantibodies in myocarditis and dilated cardiomyopathy. Autoimmunity 2008, 41, 35–45. [Google Scholar] [CrossRef]
- Goldberg, L.; Tirosh-Wagner, T.; Vardi, A.; Abbas, H.; Pillar, N.; Shomron, N.; Nevo-Caspi, Y.; Paret, G. Circulating MicroRNAs: a Potential Biomarker for Cardiac Damage, Inflammatory Response, and Left Ventricular Function Recovery in Pediatric Viral Myocarditis. J Cardiovasc Transl Res. 2018, 11, 319–328. [Google Scholar] [CrossRef]
- Besler, C.; Urban, D.; Watzka, S.; Lang, D.; Rommel, K.P.; Kandolf, R.; Klingel, K.; Thiele, H.; Linke, A.; Schuler, G.; et al. Endomyocardial miR-133a levels correlate with myocardial inflammation, improved left ventricular function, and clinical outcome in patients with inflammatory cardiomyopathy. Eur J Heart Fail. 2016, 18, 1442–1451. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Domínguez, R.; Sánchez-Díaz, R.; de la Fuente, H.; Jiménez-Borreguero, L.J.; Matesanz-Marín, A.; Relaño, M.; Jiménez-Alejandre, R.; Linillos-Pradillo, B.; Tsilingiri, K.; Martín-Mariscal, M.L.; et al. A Novel Circulating MicroRNA for the Detection of Acute Myocarditis. N Engl J Med. 2021, 384, 2014–2027. [Google Scholar] [CrossRef]
- Aleshcheva, G.; Pietsch, H.; Escher, F.; Schultheiss, H.P. MicroRNA profiling as a novel diagnostic tool for identification of patients with inflammatory and/or virally induced cardiomyopathies. ESC Heart Fail. 2021, 8, 408–422. [Google Scholar] [CrossRef] [PubMed]
- Grodzka, O.; Procyk, G.; Gąsecka, A. The Role of MicroRNAs in Myocarditis-What Can We Learn from Clinical Trials? Int J Mol Sci. 2022, 23, 16022. [Google Scholar] [CrossRef] [PubMed]
- Adeboye, A.; Alkhatib, D.; Butt, A.; Yedlapati, N.; Garg, N. A Review of the Role of Imaging Modalities in the Evaluation of Viral Myocarditis with a Special Focus on COVID-19-Related Myocarditis. Diagnostics 2022, 12, 549. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.M.; Boehmer, J.P.; Hruban, R.H.; Hutchins, G.M.; Kasper, E.K.; Baughman, K.L.; Hare, J.M. ; Echocardiographic findings in fulminant and acute myocarditis. J Am Coll Cardiol. 2000, 36, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Condliffe, R.; Swift, A.J.; Alabed, S.; Kiely, D.G.; Charalampopoulos, A. Assessment of Right Ventricular Function-a State of the Art. Curr Heart Fail Rep. 2023, 20, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Grossman, S.M.; Pravda, N.S.; Orvin, K.; Hamdan, A.; Vaturi, M.; Bengal, T.; Kornowski, R.; Weissler-Snir, A. Characterization and long-term outcomes of patients with myocarditis: a retrospective observational study. Postepy Kardiol Interwencyjnej. 2021, 17, 60–67. [Google Scholar] [CrossRef]
- Meindl, C.; Paulus, M.; Poschenrieder, F.; Zeman, F.; Maier, L.S.; Debl, K. Patients with acute myocarditis and preserved systolic left ventricular function: comparison of global and regional longitudinal strain imaging by echocardiography with quantification of late gadolinium enhancement by CMR. Clin Res Cardiol. 2021, 110, 1792–1800. [Google Scholar] [CrossRef]
- Tünnemann-Tarr, A.; Stöbe, S.; Laufs, U.; Hagendorff, A.; Tayal, B. Speckle tracking echocardiography in a patient with viral myocarditis and acute myocardial infarction. J Cardiol Cases. 2020, 22, 184–191. [Google Scholar] [CrossRef]
- Løgstrup, B.B.; Nielsen, J.M.; Kim, W.Y.; Poulsen, S.H. Myocardial oedema in acute myocarditis detected by echocardiographic 2D myocardial deformation analysis. Eur Heart J Cardiovasc Imaging. 2016, 17, 1018–1026. [Google Scholar] [CrossRef]
- Sturmberger, T.; Niel, J.; Aichinger, J.; Ebner, C. Acute myocarditis with normal wall motion detected with 2D speckle tracking echocardiography. Echo Res Pract. 2016, 3, K15–K19. [Google Scholar] [CrossRef]
- Chinali, M.; Franceschini, A.; Ciancarella, P.; Lisignoli, V.; Curione, D.; Ciliberti, P.; Esposito, C.; Del Pasqua, A.; Rinelli, G.; Secinaro, A. Echocardiographic two-dimensional speckle tracking identifies acute regional myocardial edema and sub-acute fibrosis in pediatric focal myocarditis with normal ejection fraction: comparison with cardiac magnetic resonance. Sci Rep. 2020, 10, 11321. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.G.; Marcotte, F. Cardiac magnetic resonance assessment of myocarditis. Circ Cardiovasc Imaging. 2013, 6, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J Am Coll Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef] [PubMed]
- Luetkens, J.A.; Faron, A.; Isaak, A.; Dabir, D.; Kuetting, D.; Feisst, A.; Schmeel, F.C.; Sprinkart, A.M.; Thomas, D. Comparison of Original and 2018 Lake Louise Criteria for Diagnosis of Acute Myocarditis: Results of a Validation Cohort. Radiol Cardiothorac Imaging. 2019, 1, e190010. [Google Scholar] [CrossRef] [PubMed]
- Francone, M.; Chimenti, C.; Galea, N.; Scopelliti, F.; Verardo, R.; Galea, R.; Carbone, I.; Catalano, C.; Fedele, F.; Frustaci, A. CMR sensitivity varies with clinical presentation and extent of cell necrosis in biopsy-proven acute myocarditis. JACC Cardiovasc Imaging. 2014, 7, 254–263. [Google Scholar] [CrossRef]
- Polte, C.L.; Bobbio, E.; Bollano, E.; Bergh, N.; Polte, C.; Himmelman, J.; Lagerstrand, K.M.; Gao, S.A. Cardiovascular Magnetic Resonance in Myocarditis. Diagnostics 2022, 12, 399. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Piechnik, S.K.; Robson, M.D.; Neubauer, S.; Karamitsos, T.D. Myocardial tissue characterization by magnetic resonance imaging: novel applications of T1 and T2 mapping. J Thorac Imaging. 2014, 29, 147–154. [Google Scholar] [CrossRef]
- Mewton, N.; Liu, C.Y.; Croisille, P.; Bluemke, D.; Lima, J.A. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011, 57, 891–903. [Google Scholar] [CrossRef]
- Jiang, L.; Zuo, H.; Liu, J.; Wang, J.; Zhang, K.; Zhang, C.; Peng, X.; Liu, Y.; Wang, D.; Li, H.; et al. The pattern of late gadolinium enhancement by cardiac MRI in fulminant myocarditis and its prognostic implication: a two-year follow-up study. Front Cardiovasc Med. 2023, 10, 1144469. [Google Scholar] [CrossRef]
- Friedrich, M.G.; Strohm, O.; Schulz-Menger, J.; Marciniak, H.; Luft, F.C.; Dietz, R. Noninvasive diagnosis of acute myocarditis by contrast-enhanced magnetic resonance imaging - response to the author. Circulation. 1999, 99, 459–460. [Google Scholar]
- Menacho, K.D.; Ramirez, S.; Perez, A.; Dragonetti, L.; Perez de Arenaza, D.; Katekaru, D.; Illatopa, V.; Munive, S.; Rodriguez, B.; Shimabukuro, A. Improving cardiovascular magnetic resonance access in low- and middle-income countries for cardiomyopathy assessment: rapid cardiovascular magnetic resonance. Eur Heart J. 2022, 7, 2496–2507. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Batrice, A.; Rischpler, C.; Eiber, M.; Ibrahim, T.; Nekolla, S.G. Utility of multimodal cardiac imaging with PET/MRI in cardiac sarcoidosis: Implications for diagnosis, monitoring and treatment. Eur. Heart J. 2014, 35, 312. [Google Scholar] [CrossRef] [PubMed]
- von Olshausen, G.; Hyafil, F.; Langwieser, N.; Laugwitz, K.L.; Schwaiger, M.; Ibrahim, T. Detection of acute inflammatory myocarditis in Epstein Barr virus infection using hybrid 18F-fluoro-deoxyglucose-positron emission tomography/magnetic resonance imaging. Circulation 2014, 130, 925–926. [Google Scholar] [CrossRef] [PubMed]
- Lamacie, M.M.; Almufleh, A.; Nair, V.; Stadnick, E.; Birnie, D.; Beanlands, R.; Chih, S. Serial 18F-Fluorodeoxyglucose Positron Emission Tomography Imaging in a Patient with Giant Cell Myocarditis. Circ. Cardiovasc. Imaging 2020, 13, e009940. [Google Scholar] [CrossRef] [PubMed]
- Mathijssen, H.; Tjoeng, T.W.H.; Keijsers, R.G.M.; Bakker, A.L.M.; Akdim, F.; van Es, H.W.; van Beek, F.T.; Veltkamp, M.V.; Grutters, J.C.; Post, M.C. The usefulness of repeated CMR and FDG PET/CT in the diagnosis of patients with initial possible cardiac sarcoidosis. EJNMMI Res. 2021, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Nensa, F.; Kloth, J.; Tezgah, E.; Poeppel, T.D.; Heusch, P.; Goebel, J.; Nassenstein, K.; Schlosser, T. Feasibility of FDG-PET in myocarditis: Comparison to CMR using integrated PET/MRI. J Nucl Cardiol. 2018, 25, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, V.; Aimo, A.; Todiere, G.; Barison, A.; Fabiani, I.; Panichella, G.; Genovesi, D.; Bonino, L.; Clemente, A.; Cademartiri, F. Role of Imaging in Cardiomyopathies. Card Fail Rev. 2023, 9, e08. [Google Scholar] [CrossRef]
- Chen, W.; Jeudy, J. Assessment of Myocarditis: Cardiac MR, PET/CT, or PET/MR? Curr Cardiol Rep. 2019, 21, 76. [Google Scholar] [CrossRef]
- Vita, T.; Okada, D.R.; Veillet-Chowdhury, M.; Bravo, P.E.; Mullins, E.; Hulten, E.; Agrawal, M.; Madan, R.; Taqueti, V.R.; Steigner, M.; et al. Complementary Value of Cardiac Magnetic Resonance Imaging and Positron Emission Tomography/Computed Tomography in the Assessment of Cardiac Sarcoidosis. Circ Cardiovasc Imaging. 2018, 11, e007030. [Google Scholar] [CrossRef]
- Ammirati, E.; Veronese, G.; Bottiroli, M.; Wang, D.W.; Cipriani, M.; Garascia, A.; Pedrotti, P.; Adler, E.D.; Frigerio, M. Update on acute myocarditis. Trends Cardiovasc Med. 2021, 31, 370–379. [Google Scholar] [CrossRef]
- Pasupathy, S.; Air, T.; Dreyer, R.P.; Tavella, R.; Beltrame, J.F. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 2015, 131, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, S.; Chenaghlou, M.; Mirtajaddini, M.; Naderi, N.; Amin, A. Takotsubo syndrome without major stress mimicking myocarditis. Anatol J Cardiol. 2020, 23, 349–350. [Google Scholar] [CrossRef] [PubMed]
- Lasica, R.; Spasic, J.; Djukanovic, L.; Trifunovic-Zamaklar, D.; Orlic, D.; Nedeljkovic-Arsenovic, O.; Asanin, M. Case report: Acute toxic myocardial damage caused by 5-fluorouracil-from enigma to success. Front Cardiovasc Med. 2022, 9, 991886. [Google Scholar] [CrossRef] [PubMed]
- Rapezzi, C.; Arbustini, E.; Caforio, A.L.; Charron, P.; Gimeno-Blanes, J.; Heliö, T.; Linhart, A.; Mogensen, J.; Pinto, Y.; Ristic, A.; et al. Diagnostic work-up in cardiomyopathies: bridging the gap between clinical phenotypes and final diagnosis. A position statement from the ESC Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013, 34, 1448–1458. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Peretto, G.; Sala, S.; Rizzo, S.; Palmisano, A.; Esposito, A.; De Cobelli, F.; Campochiaro, C.; De Luca, G.; Foppoli, L.; Dagna, L. Ventricular Arrhythmias in Myocarditis: Characterization and Relationships with Myocardial Inflammation. J Am Coll Cardiol. 2020, 75, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Mirna, M.; Schmutzler, L.; Topf, A.; Boxhammer, E.; Sipos, B.; Hoppe, U.C.; Lichtenauer, M. Treatment with Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Does Not Affect Outcome in Patients with Acute Myocarditis or Myopericarditis. J Cardiovasc Dev Dis. 2022, 19, 32. [Google Scholar] [CrossRef]
- Berg, J.; Lovrinovic, M.; Baltensperger, N.; Kissel, C.K.; Kottwitz, J.; Manka, R.; Patriki, D.; Scherff, F.; Schmied, C.; Landmesser, U.; et al. Non-steroidal anti-inflammatory drug use in acute myopericarditis: 12-month clinical follow-up. Open Heart. 2019, 6, e000990. [Google Scholar] [CrossRef]
- Montero, S.; Abrams, D.; Ammirati, E.; Huang, F.; Donker, D.W.; Hekimian, G.; García-García, C.; Bayes-Genis, A.; Combes, A.; Schmidt, M. Fulminant myocarditis in adults: a narrative review. J Geriatr Cardiol. 2022, 19, 137–151. [Google Scholar]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. ESC Scientific Document Group. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef]
- Bang, V.; Ganatra, S.; Shah, S.P.; Dani, S.S.; Neilan, T.G.; Thavendiranathan, P.; Resnic, F.S.; Piemonte, T.C.; Barac, A.; Patel, R.; et al. Management of Patients with Giant Cell Myocarditis: JACC Review Topic of the Week. J Am Coll Cardiol. 2021, 77, 1122–1134. [Google Scholar] [CrossRef] [PubMed]
- Maury, P.; Chilon, T.; Dumonteil, N.; Fontan, A. Complete atrioventricular block persisting after regression of infectious myocarditis. J Electrocardiol. 2008, 41, 665–667. [Google Scholar] [CrossRef] [PubMed]
- Pelargonio, G.; Pinnacchio, G.; Narducci, M.L.; Pieroni, M.; Perna, F.; Bencardino, G.; Comerci, G.; Dello Russo, A.; Casella, M.; Bartoletti, S.; et al. Long-Term Arrhythmic Risk Assessment in Biopsy-Proven Myocarditis. JACC Clin Electrophysiol. 2020, 6, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Bardy, G.H.; Lee, K.L.; Mark, D.B.; Poole, J.E.; Packer, D.L.; Boineau, R.; Domanski, M.; Troutman, C.; Anderson, J.; Johnson, G.; et al. Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005, 352, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Solberg, E.E.; Papadakis, M.; Adami, P.E.; Biffi, A.; Caselli, S.; La Gerche, A.; Niebauer, J.; Pressler, A.; Schmied, C.M.; et al. Recommendations for participation in competitive and leisure time sport in athletes with cardiomyopathies, myocarditis, and pericarditis: position statement of the Sport Cardiology Section of the European Association of Preventive Cardiology (EAPC). Eur Heart J. 2019, 40, 19–33. [Google Scholar] [CrossRef]
- Kühl, U.; Pauschinger, M.; Schwimmbeck, P.L.; Seeberg, B.; Lober, C.; Noutsias, M.; Poller, W.; Schultheiss, H.P. Interferon-beta treatment eliminates cardiotropic viruses and improves left ventricular function in patients with myocardial persistence of viral genomes and left ventricular dysfunction. Circulation. 2003, 107, 2793–2798. [Google Scholar] [CrossRef]
- Abdenasser, D.; Amine, E. Rare case of subacute herpetic myocarditis. IDCases. 2023, 33, e01828. [Google Scholar] [CrossRef]
- Deonarain, R.; Cerullo, D.; Fuse, K.; Liu, P.P.; Fish, E.N. Protective role for interferon-beta in coxsackievirus B3 infection. Circulation. 2004, 110, 3540–3543. [Google Scholar] [CrossRef]
- Goland, S.; Czer, L.S.; Siegel, R.J.; Tabak, S.; Jordan, S.; Luthringer, D.; Mirocha, J.; Coleman, B.; Kass, R.M.; Trento, A. Intravenous immunoglobulin treatment for acute fulminant inflammatory cardiomyopathy: series of six patients and review of literature. Can J Cardiol. 2008, 24, 571–574. [Google Scholar] [CrossRef]
- Kishimoto, C.; Shioji, K.; Hashimoto, T.; Nonogi, H.; Lee, J.D.; Kato, S.; Hiramitsu, S.; Morimoto, S.I. Therapy with immunoglobulin in patients with acute myocarditis and cardiomyopathy: analysis of leukocyte balance. Heart Vessels. 2014, 29, 336–342. [Google Scholar] [CrossRef]
- Fuchs, K.; Rummler, S.; Ries, W.; Helmschrott, M.; Selbach, J.; Ernst, F.; Morath, C.; Gauly, A.; Atiye, S.; Stauss-Grabo, M.; et al. Performance, clinical effectiveness, and safety of immunoadsorption in a wide range of indications. Ther Apher Dial. 2022, 26, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Yang, Z.; Peng, Y.; Wang, L.; Yuan, X. Diagnosis and treatment of eosinophilic myocarditis. J Transl Autoimmun. 2021, 4, 100118. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ortiz, M.; Anguita-Sánchez, M.; Bonilla-Palomas, J.L.; Fernández-Pérez, C.; Bernal-Sobrino, J.L.; Cequier-Fillat, A.; Bueno-Zamora, H.; Marín, F.; Elola-Somoza, F.J. Incidence and outcomes of hospital treated acute myocarditis from 2003 to 2015 in Spain. Eur J Clin Invest. 2021, 51, e13444. [Google Scholar] [CrossRef] [PubMed]
- Alamri, A.S.; Khayat, L.T.; Alzahrani, A.J.; Kurdi, L.K.; Alkhameesi, N.F.; Bahaidarah, S.A. Clinical Presentation of Myocarditis in the Pediatric Age Group and Predictors of Poor Early and Late Outcomes: Academic Hospital Experience. Cureus. 2022, 14, e31643. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




