Submitted:
04 September 2023
Posted:
06 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and methods
2.1. Species distribution data
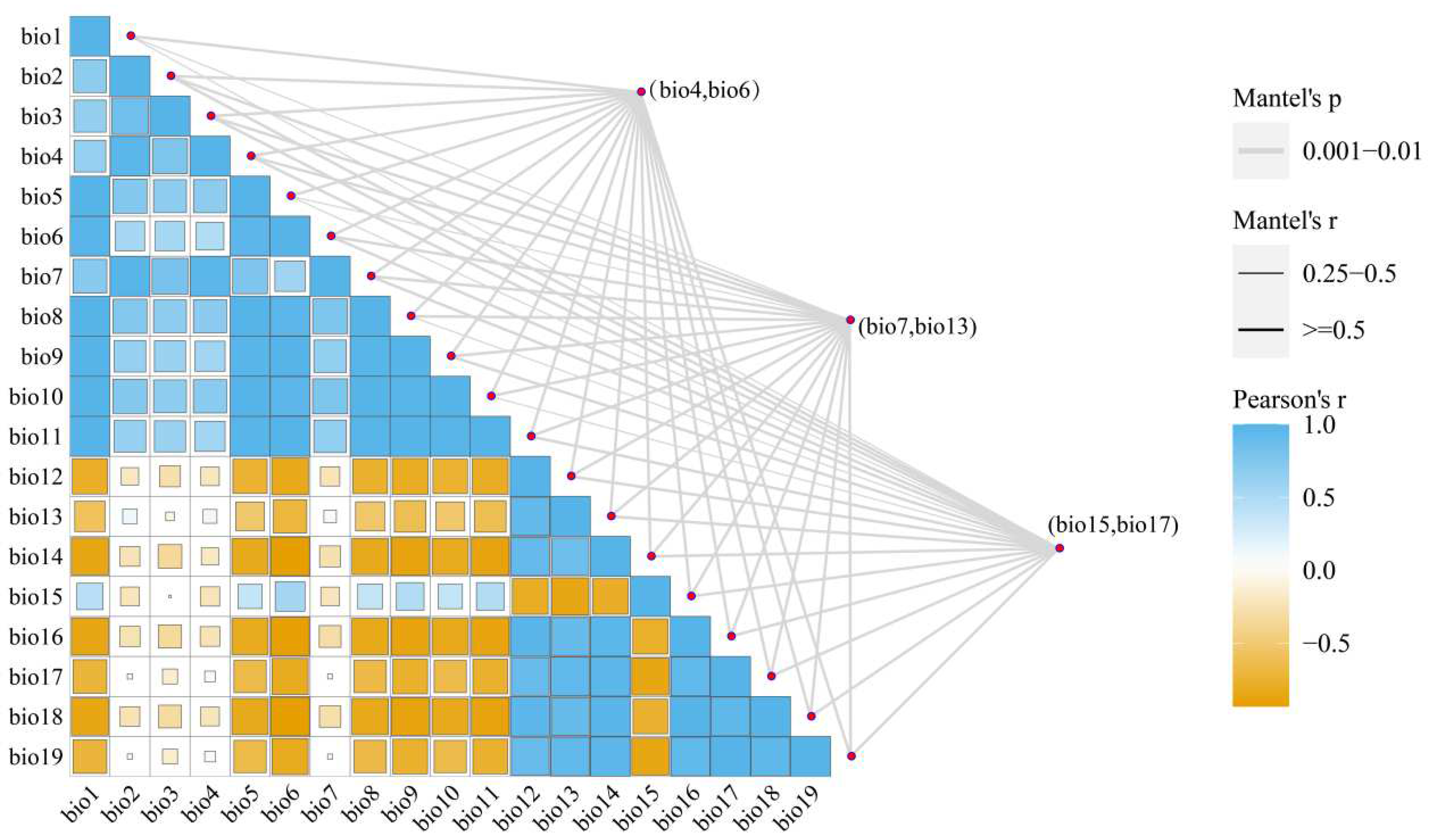
2.2. Bioclimatic data acquisition and screening
2.3. MaxEnt model operation and evaluation
2.4. Classification of habitat suitability
2.5. Analysis of centroid migration in suitable distribution areas
3. Results
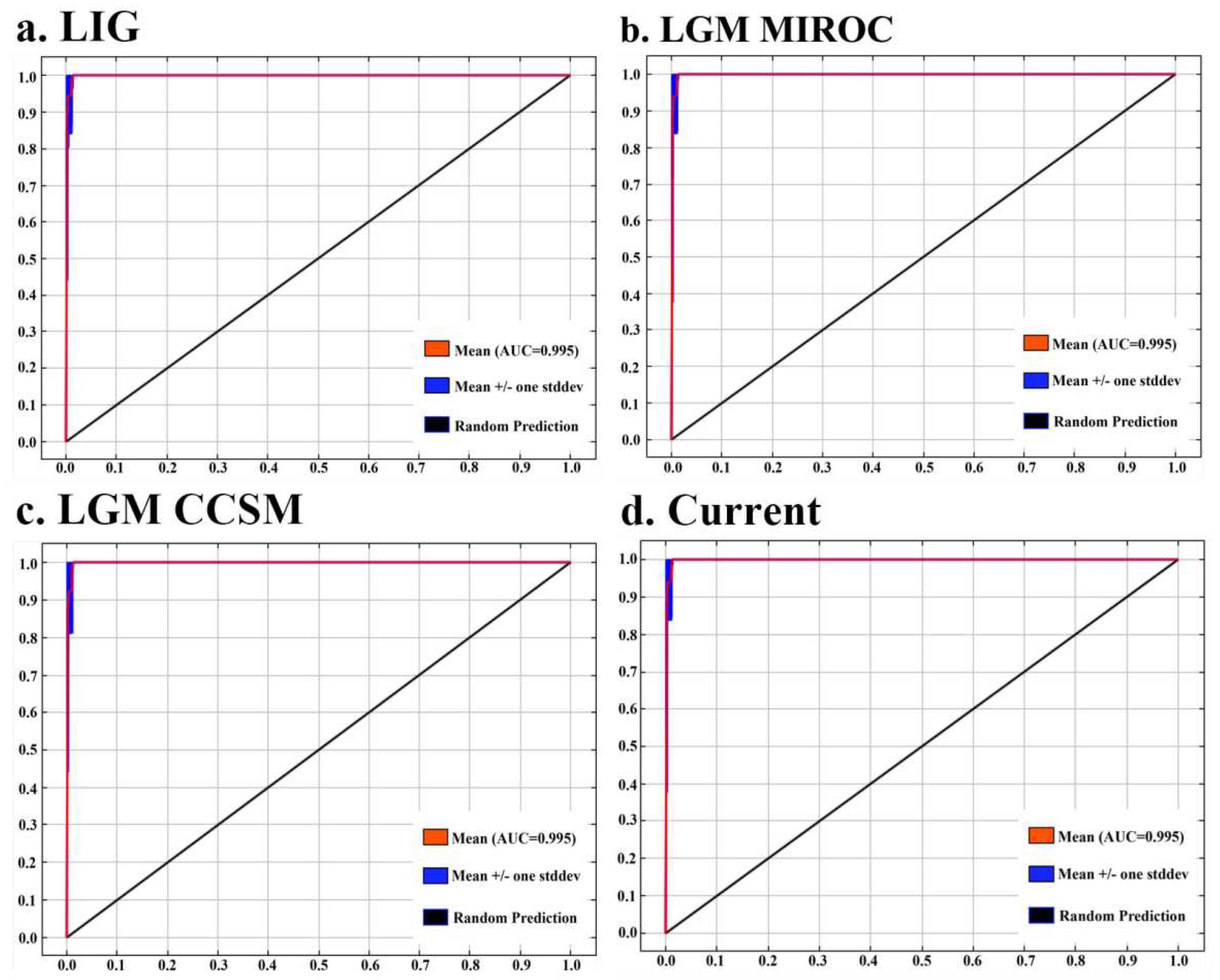
3.1. Accuracy of model analysis
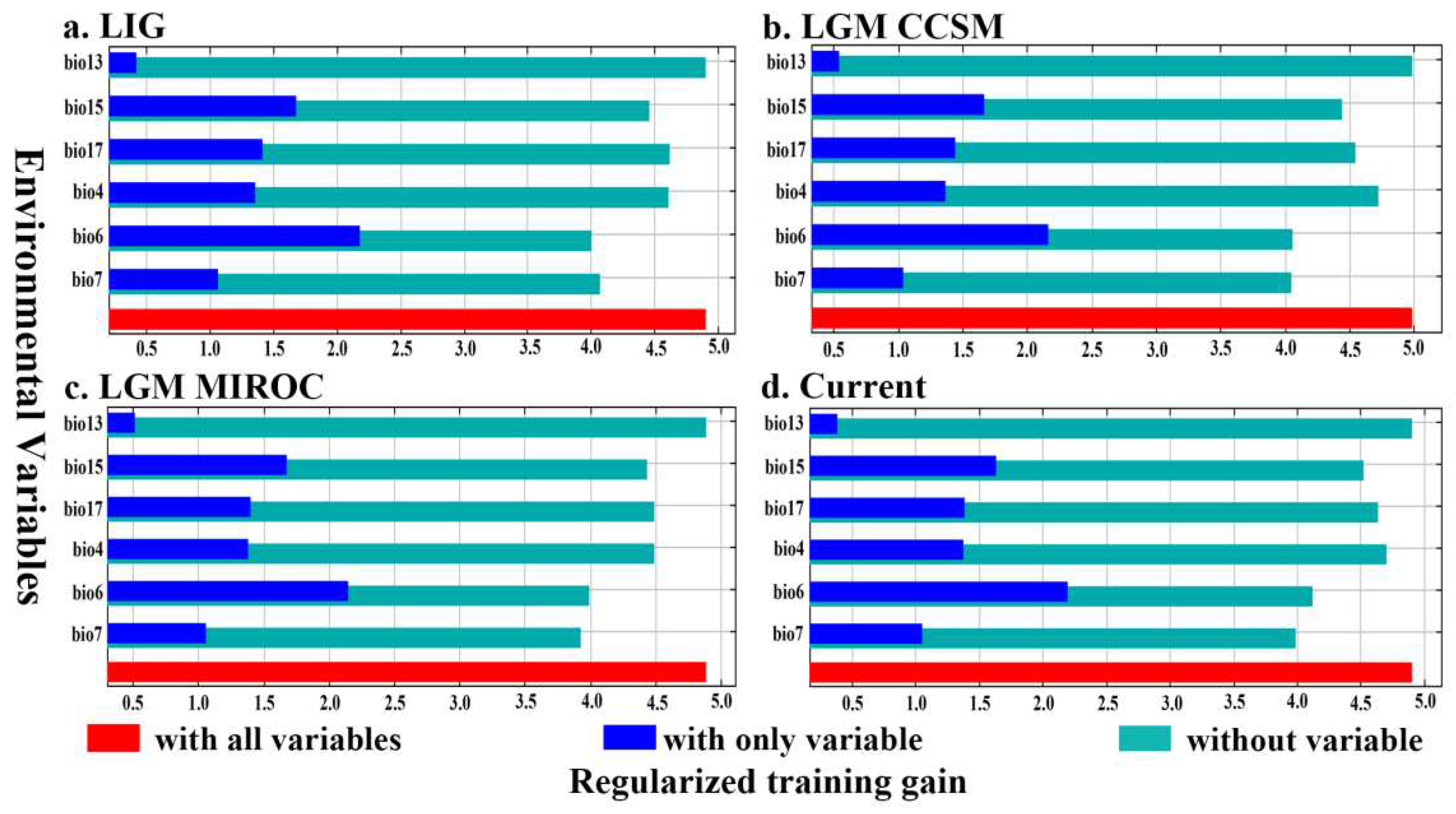
3.2. Dominant environmental factors
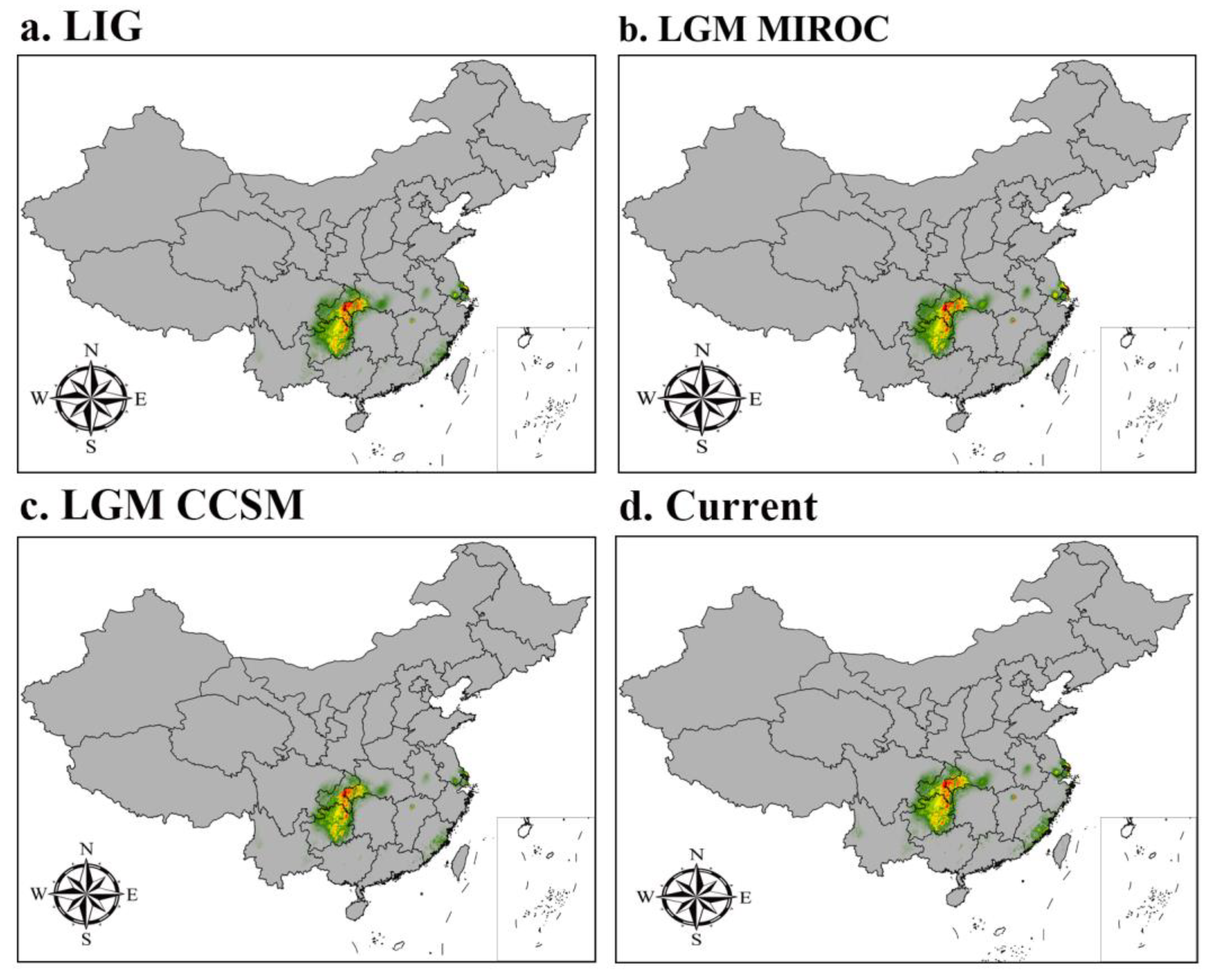
3.3. Suitable species distribution areas
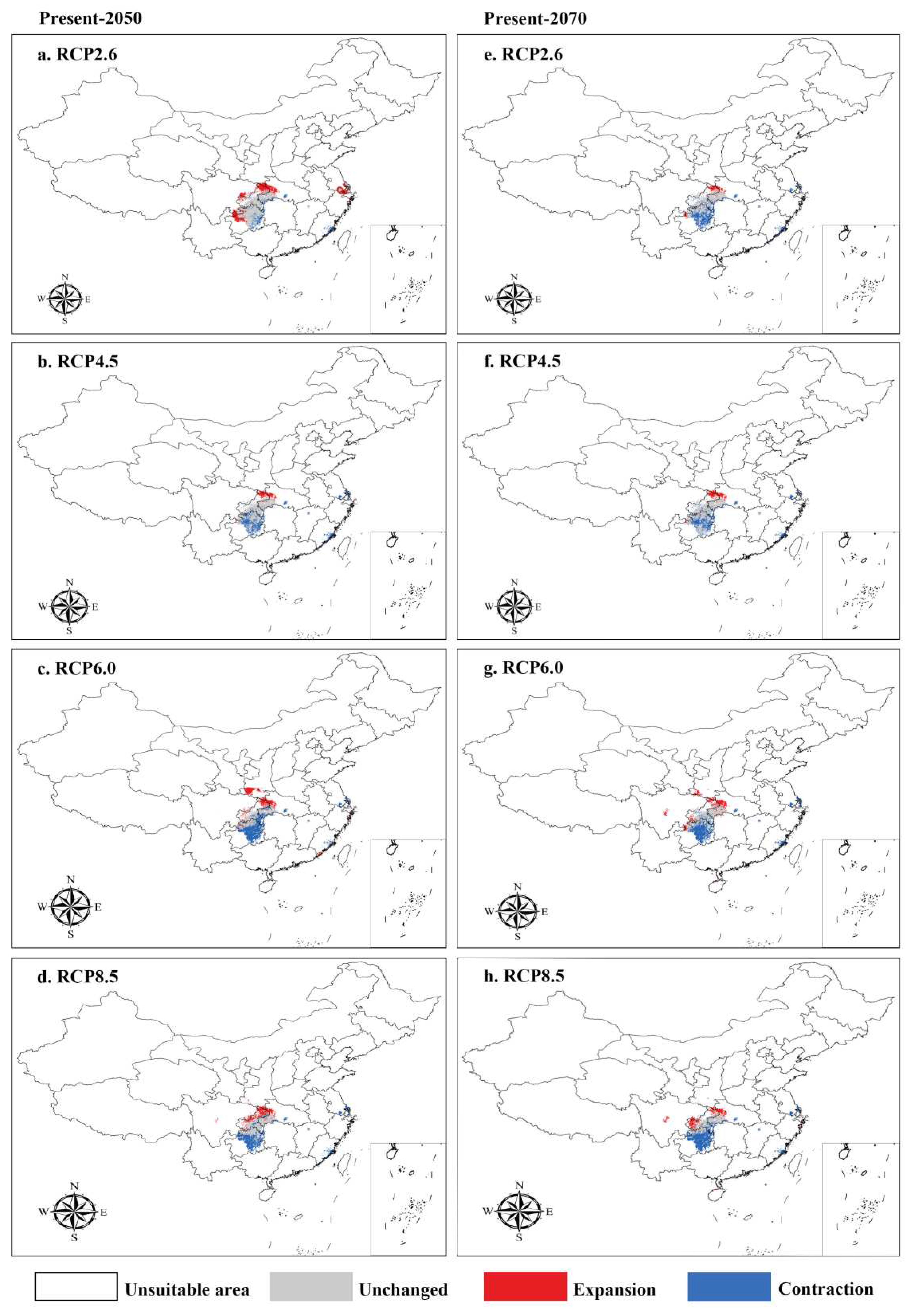
3.4. Suitable distribution under future climate scenarios
3.5. Migration trends of the geometric center of suitable habitat
4. Discussion
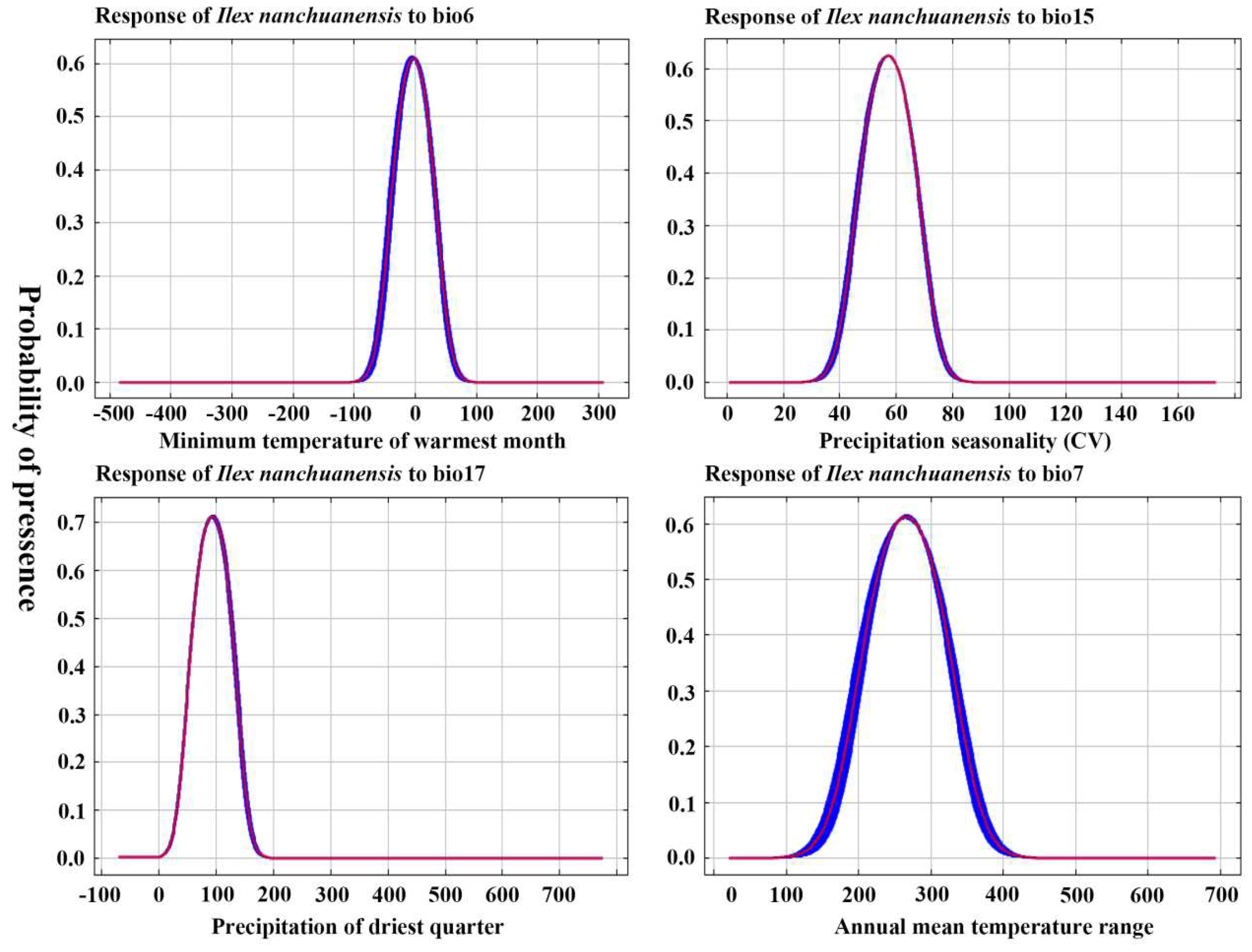
4.1. Response of species distribution to climate change
4.2. Potential distribution area changes and conservation of Ilex nanchuanensis under future climate scenarios
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quantifying the benefit of early climate change mitigation in avoiding biodiversity loss. Nature Climate Change 2013.
- Balfagón, D.; Terán, F.; de Oliveira, T.D.R.; Santa-Catarina, C.; Gómez-Cadenas, A. Citrus rootstocks modify scion antioxidant system under drought and heat stress combination. Plant Cell Rep. 2022, 41, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tian, H.; Wang, Y.; et al. Vulnerability of 208 endemic or endangered species in China to the effects of climate change. Regional Environmental Change 2013, 13, 843–852. [Google Scholar] [CrossRef]
- Garza, G.; Rivera, A.; Venegas Barrera, C.S.; Martinez-Ávalos, J.G.; Dale, J.; Feria Arroyo, T.P. Potential Effects of Climate Change on the Geographic Distribution of the Endangered Plant Species Manihot walkerae. Forests 2020, 11, 689. [Google Scholar] [CrossRef]
- Varela, D.; Romeiras, M.M.; Silva, L. Implications of climate change on the distribution and conservation of Cabo Verde endemic trees. Global Ecology and Conservation 2022, 34. [Google Scholar] [CrossRef]
- Gray, S.B.; Brady, S.M. Plant developmental responses to climate change. Dev Biol 2016. [CrossRef]
- Lambert, A.M.; Abraham, J. Miller-Rushing, Inouye D W. Changes in snowmelt date and summer precipitation affect the flowering phenology of Erythronium grandiflorum (glacier lily; Liliaceae). American Journal of Botany 2010, 97. [Google Scholar] [CrossRef]
- McMahon, S.M.; et al. Improving assessment and modelling of climate change impacts on global terrestrial biodiversity. Trends in Ecology & Evolution 2011, 26, 249–259. [Google Scholar]
- Vitasse Yann and Signarbieux Constant and Fu Yongshuo H. Global warming leads to more uniform spring phenology across elevations. Proceedings of the National Academy of Sciences of the United States of America 2018, 115, 1004–1008. [Google Scholar]
- Alessandro Ferrarini; et al. Redefining the climate niche of plant species: A novel approach for realistic predictions of species distribution under climate change. Science of the Total Environment 2019, 671, 1086–1093. [Google Scholar] [CrossRef]
- Dyderski Marcin, K.; et al. How much does climate change threaten European forest tree species distributions? Global change biology 2018, 24, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- et al. Minimal climate change impacts on the geographic distribution of Nepeta glomerulosa, medicinal species endemic to southwestern and central Asia. Scientific Reports 2022, 12, 19893. [Google Scholar] [CrossRef] [PubMed]
- Wang, Shaokun et al. Increasing precipitation weakened the negative effects of simulated warming on soil microbial community composition in a semi-arid sandy grassland. Frontiers in Microbiology 2023, 13. [Google Scholar]
- Asymmetric sensitivity of first flowering date to warming and cooling in alpine plants. Ecology 2014.
- Dorji, T.; Totland, O.; Moe, S.; et al. Plant functional traits mediate reproductive phenology and success in response to experimental warming and snow addition in Tibet. Glob Chang Biol. 2013, 19, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi Shirin et al. The current and future potential geographical distribution of Nepeta crispa Willd., an endemic, rare and threatened aromatic plant of Iran: Implications for ecological conservation and restoration. Ecological Indicators 2022, 137. [Google Scholar]
- Panetta, A.M.; Stanton, M.L.; Harte, J. Climate warming drives local extinction: Evidence from observation and experimentation. Science Advances 2018, 4, eaaq1819. [Google Scholar] [CrossRef]
- Liu, J.; Rhland, K.; Chen, J.; et al. Aerosol-weakened summer monsoons decrease lake fertilization on the Chinese Loess Plateau. Nature Climate Change 2017, 7. [Google Scholar] [CrossRef]
- Barnosky Anthony, D.; et al. Has the Earth's sixth mass extinction already arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef]
- et al. Changes in daily climate extremes in China and their connection to the large scale atmospheric circulation during 1961–2003. Climate Dynamics 2011, 36, 2399–2417. [Google Scholar] [CrossRef]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; et al. Impacts of climate change on the future of biodiversity. Ecology letters 2012, 15. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Summary for Policymakers. In: Climate Change 2021: The Physical Science Basis. [R]. 2021.
- Hansen, J.; Ruedy, R.; Sato, M.; et al. GLOBAL SURFACE TEMPERATURE CHANGE. Reviews of Geophysics 2010, 48. [Google Scholar] [CrossRef]
- Varela Danilson and Romeiras Maria M. and Silva Luís. Implications of climate change on the distribution and conservation of Cabo Verde endemic trees. Global Ecology and Conservation 2022, 34. [Google Scholar]
- Thomas, C.D.; Cameron, A.; Green, R.E.; et al. Extinction risk from climate change. Nature 2004, 427, 145. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.C. Climate change. Accelerating extinction risk from climate change. Science 2015, 348, 571. [Google Scholar] [CrossRef]
- Gergana N. Daskalova and Isla H. Myers-Smith and John L. Godlee. Rare and common vertebrates span a wide spectrum of population trends. Nature communications 2020, 11, 4394. [Google Scholar] [CrossRef]
- et al. Niche divergence at the intraspecific level in an endemic rare peony (Paeonia rockii): A phylogenetic, climatic and environmental survey. Frontiers in Plant Science 2022, 13, 978011. [Google Scholar] [CrossRef]
- Daskalova, G.N.; Myers-Smith, I.H.; Godlee, J.L. Rare and common vertebrates span a wide spectrum of population trends. Nat. Commun. 2020, 11, 4394. [Google Scholar] [CrossRef]
- Krner, C. Mountain Biodiversity, Its Causes and Function. Ambio 2004, 33, 11–17. [Google Scholar] [CrossRef]
- Perrigo, A.; Hoorn, C.; Antonelli, A. Why mountains matter for biodiversity. Journal of Biogeography 2020, 47. [Google Scholar] [CrossRef]
- D. Nogués-Bravo a b, M.B. Araújo c b, D M P E; et al. Exposure of global mountain systems to climate warming during the 21st Century. Global Environmental Change 2007, 17, 420–428. [Google Scholar] [CrossRef]
- A J P D, A L F, B G R; et al. Shifting mountain snow patterns in a changing climate from remote sensing retrieval. Science of The Total Environment 2014, 493, 1267–1279.
- Mccullough, I.M.; Davis, F.W.; Dingman, J.R.; et al. High and dry: High elevations disproportionately exposed to regional climate change in Mediterranean-climate landscapes. Landscape Ecology 2016, 31, 1063–1075. [Google Scholar] [CrossRef]
- Dyurgerov, M. Mountain and subpolar glaciers show an increase in sensitivity to climate warming and intensification of the water cycle. Journal of Hydrology 2003. [Google Scholar] [CrossRef]
- Stoffel, M.; Tiranti, D.; Huggel, C. Climate change impacts on mass movements - Case studies from the European Alps. Science of the Total Environment 2014, 493, 1255–1266. [Google Scholar] [CrossRef]
- Carroll, C.; Roberts, D.R.; Michalak, J.L.; et al. Scale-dependent complementarity of climatic velocity and environmental diversity for identifying priority areas for conservation under climate change. Journal of Fish Diseases 2017, 40. [Google Scholar] [CrossRef]
- Lenoir, J.; Svenning, J.C. Climate-related range shifts - a global multidimensional synthesis and new research directions. Ecography 2015, 38, 15–28. [Google Scholar] [CrossRef]
- Alexander, J.M.; Chalmandrier, L.; Lenoir, J.; et al. Lags in the response of mountain plant communities to climate change. Global Change Biology 2018, 24. [Google Scholar] [CrossRef]
- Memmott, J.; Craze, P.G.; Waser, N.M.; et al. Global warming and the disruption of plant-pollinator interactions. Ecology Letters 2007, 10. [Google Scholar] [CrossRef]
- Jan, Sauer, Sami; et al. Low mountain ranges: Summit traps for montane freshwater species under climate change. Biodiversity&Conservation 2011. [Google Scholar]
- Liu, H.; Jacquemyn, H.; He, X.; Chen, W.; Huang, Y.; Yu, S.; Lu, Y.; Zhang, Y. The Impact of Human Pressure and Climate Change on the Habitat Availability and Protection of Cypripedium (Orchidaceae) in Northeast China. Plants 2021, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Renwick, K.M.; Curtis, C.; Kleinhesselink, A.R.; et al. Multi-model comparison highlights consistency in predicted effect of warming on a semi-arid shrub. Global Change Biology 2017. [CrossRef]
- Zhao, R.; Chu, X.; He, Q.; Tang, Y.; Song, M.; Zhu, Z. Modeling Current and Future Potential Geographical Distribution of Carpinus tientaiensis, a Critically Endangered Species from China. Forests 2020, 11, 774. [Google Scholar] [CrossRef]
- Qin, A.; Liu, B.; Guo, Q.; et al. Maxent modeling for predicting impacts of climate change on the potential distribution of Thuja sutchuenensis Franch. an extremely endangered conifer from southwestern China. Global Ecology and Conservation 2017. [CrossRef]
- Dong, P.-B.; Wang, L.-Y.; Wang, L.-J.; Jia, Y.; Li, Z.-H.; Bai, G.; Zhao, R.-M.; Liang, W.; Wang, H.-Y.; Guo, F.-X.; Chen, Y. Distributional Response of the Rare and Endangered Tree Species Abies chensiensis to Climate Change in East Asia. Biology 2022, 11, 1659. [Google Scholar] [CrossRef] [PubMed]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annual Review of Ecology Evolution & Systematics 2009, 40, 677–697. [Google Scholar]
- Garza, G.; Rivera, A.; Venegas Barrera, C.S.; Martinez-Ávalos, J.G.; Dale, J.; Feria Arroyo, T.P. Potential Effects of Climate Change on the Geographic Distribution of the Endangered Plant Species Manihot walkerae. Forests 2020, 11, 689. [Google Scholar] [CrossRef]
- Hameed, S.; Din, J.U.; Ali, H.; et al. Identifying priority landscapes for conservation of snow leopards in Pakistan. PLoS ONE 2020, 15, 1–20. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; et al. Novel methods improve prediction of species' distributions from occurrence data. Ecography 2006, 29. [Google Scholar] [CrossRef]
- Jsp, A.; Lep, B.; Cah, A.; et al. Climate change shifts in habitat suitability and phenology of huckleberry (Vaccinium membranaceum) - ScienceDirect. Agricultural and Forest Meteorology 2020, 280, 107803. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecological Modelling 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Marcer A, Lloren Sáez, Molowny-Horas R; et al. Using species distribution modelling to disentangle realised versus potential distributions for rare species conservation. Biological Conservation 2013, 166, 221–230. [Google Scholar] [CrossRef]
- Li, G.; Du, S.; Wen, Z. Mapping the climatic suitable habitat of oriental arborvitae (Platycladus orientalis) for introduction and cultivation at a global scale. Scientific Reports 2016, 6, 30009. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Tsiftsis, S.; Chapagain, D.J.; Khadka, C.; Bhattarai, P.; Kayastha Shrestha, N.; Alicja Kolanowska, M.; Kindlmann, P. Suitability of Habitats in Nepal for Dactylorhiza hatagirea Now and under Predicted Future Changes in Climate. Plants 2021, 10, 467. [Google Scholar] [CrossRef] [PubMed]
- Liu Xu, Chen Yuhan, Yang Yi. New greening product-Nanchuan Holly. Gardening 2008, 69. [Google Scholar]
- Li, Q.M.; Xie, Z.Q.; Sun, Y.L. Study on Seedling Adaptability of Abies chensiensis. Forest Research 2008, 21, 481–485. [Google Scholar]
- Feeley, K.J.; Silman, M.R. Modelling the responses of Andean and Amazonian plant species to climate change: The effects of georeferencing errors and the importance of data filtering. Journal of Biogeography 2010, 37, 733–740. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, L.; Tao, J. Impact of Climate Change on the Distribution of Euscaphis japonica (Staphyleaceae) Trees. Forests 2020, 11, 525. [Google Scholar] [CrossRef]
- Marcer A, Lloren Sáez, Molowny-Horas R; et al. Using species distribution modelling to disentangle realised versus potential distributions for rare species conservation. Biological Conservation 2013, 166, 221–230. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; et al. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 2005, 25. [Google Scholar] [CrossRef]
- Yang, X.Q.; Kushwaha, S.; Saran, S.; et al. Maxent modeling for predicting the potential distribution of medicinal plant, Justicia adhatoda L. in Lesser Himalayan foothills. Ecological Engineering 2013, 51, 83–87. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1 km spatial resolution climate surfaces for global land areas. International Journal of Climatology 2017, 37. [Google Scholar] [CrossRef]
- Taylor, K.E.; Stouffer, R.J.; Meehl, G.A. An Overview of CMIP5 and the Experiment Design. Bulletin of the American Meteorological Society 2012, 93, 485–498. [Google Scholar] [CrossRef]
- Roberto Moreno and Ricardo Zamora and Juan Ramón Molina and Angélica Vasquez and Miguel Ángel Herrera. Predictive modeling of microhabitats for endemic birds in South Chilean temperate forests using Maximum entropy (Maxent). Ecological Informatics 2011. [Google Scholar]
- Moreno, R.; Zamora, R.; Molina, J.R.; Vasquez, A.; Herrera, M.T. Predictive modeling of microhabitats for endemic birds in South Chilean temperate forests using Maximum entropy (Maxent). Ecological informatics: An international journal on ecoinformatics and computational ecology 2011, 6. [Google Scholar] [CrossRef]
- Mcpherson, J.M.; Rogers, D.J. The Effects of Species Range Sizes on the Accuracy of Distribution Models: Ecological Phenomenon or Statistical Artefact? Journal of Applied Ecology 2004, 41, 811–823. [Google Scholar] [CrossRef]
- C A M M A B, B B D B, A P L M G. Ecological relevance of performance criteria for species distribution models. Ecological Modelling 2010, 221, 1995–2002. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; et al. Novel methods improve prediction of species' distributions from occurrence data. Ecography 2006, 29. [Google Scholar] [CrossRef]
- Fielding, A. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ Conser 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Hu, L.-L.; Zhang, H.-Y.; Qin, L.; et al. Current distribution of Schisandra chinensis in China and its predicted responses to climate change. Chinese Journal of Applied Ecology 2012, 23, 2445–2450. [Google Scholar]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2. 0: The next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 2017, 5, e4095. [Google Scholar]
- Laurent, P.; Mouillot, F.; Yue, C.; et al. Data descriptor: FRY, a global database of fire patch functional traits derived from space-borne burned area products [data paper]. 2018.
- Auffret, A.G.; Svenning, J.C. Climate warming has compounded plant responses to habitat conversion in northern Europe. Nature Communications. [CrossRef] [PubMed]
- Ning, H.; Ling, L.; Sun, X.; et al. Predicting the future redistribution of Chinese white pine Pinus armandii Franch. Under climate change scenarios in China using species distribution models. Global Ecology and Conservation 2021, 25, e01420. [Google Scholar]
- Pandey, B.; Kathiwada, J.R.; Zhang, L.; et al. Energy–water and seasonal variations in climate underlie the spatial distribution patterns of gymnosperm species richness in China. Ecology and Evolution 2020. [CrossRef]
- Noce, S.; Caporaso, L.; Santini, M. Climate Change and Geographic Ranges: The Implications for Russian Forests. Frontiers in Ecology and Evolution 2019, 7, 57. [Google Scholar] [CrossRef]
- Hewitt, G. The genetic legacy of the Quaternary ice ages. Nature. [CrossRef]
- IPCC. IPCC Fifth Assessment Report (AR5); Cambridge University Press: Cambridge, 2014. [Google Scholar]
- Lehikoinen, P.; Santangeli, A.; Jaatinen, K.; et al. Protected areas act as a buffer against detrimental effects of climate change-Evidence from large-scale, long-term abundance data. Global Change Biology 2018, 25. [Google Scholar] [CrossRef]
- Bates, O.K.; Ollier, S.; Bertelsmeier, C. Smaller climatic niche shifts in invasive than non-invasive alien ant species. Nature Communications. [CrossRef]
- You, J.; Qin, X.; Ranjitkar, S.; et al. Response to climate change of montane herbaceous plants in the genus Rhodiola predicted by ecological niche modelling. Scientific Reports 2018, 8, 5879. [Google Scholar] [CrossRef]
- Chen, X.; Dimitrov, N.B.; Meyers, L.A. Uncertainty analysis of species distribution models. PLoS ONE 2019, 14, e0214190. [Google Scholar] [CrossRef]
- Li, J.J.; Shu, Q.; Zhou, S.Z.; et al. Review and prospects of Quaternary glaciation research in China. Journal of Glaciol Geocryol 2004, 26, 235–243. [Google Scholar]
- Katherine, J, Willis; et al. The role of Quaternary environmental change in plant macroevolution: The exception or the rule? Philosophical Transactions of the Royal Society B Biological Sciences 2004. [Google Scholar]
- Wang, Q.; Abbott, R.J.; Yu, Q.S.; et al. Pleistocene climate change and the origin of two desert plant species, Pugionium cornutum and Pugioniumdolabratum (Brassicaceae), in northwest China. New Phytologist 2013.
- Qiu, Y.X.; Fu, C.X.; Comes, H.P. Plant molecular phylogeography in China and adjacent regions: Tracing the genetic imprints of Quaternary climate and environmental change in the world's most diverse temperate flora. Molecular phylogenetics and evolution 2011, 59. [Google Scholar] [CrossRef] [PubMed]
- Amelia, C.R.; Fabián, V.M.; Aguilar-Martínez, G.F.; et al. Alternative glacial-interglacial refugia demographic hypotheses tested on Cephalocereus columna-trajani (Cactaceae) in the intertropical Mexican drylands. PLoS ONE 2017, 12, e0175905. [Google Scholar]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31. [Google Scholar] [CrossRef]
- Zeng, M. Study on the distribution and potential suitable area of rare and endangered plant Cercidiphyllum japonicum. Master’s Thesis, China West Normal University, Nanchong, China, 2021. [Google Scholar]
- Hao, H.X. Response of Potential Geographical Distribution of Eight China’s First-Class Rare and Endangered Plants to Climate Change and Analysis of GAP. Master’s Thesis, Northwest Normal University, Lanzhou, China, 2021. [Google Scholar]
- Nzei, J.M.; Mwanzia, V.M.; Ngarega, B.K.; Musili, P.M.; Wang, Q.F.; Chen, J.M.; Li, Z.Z. Ecological Niche Modeling of Water Lily (Nymphaea L.) Species in Australia under Climate Change to Ascertain Habitat Suitability for Conservation Measures. Plants (Basel) 2022, 11, 1874. [Google Scholar] [CrossRef]
- Crowley, T.J. Causes of climate change over the last 1000 years. Science 2000, 289. [Google Scholar]

| Variable | Code | Unit |
|---|---|---|
| Temperature seasonality (standard deviation) | Bio4 | ℃ |
| Minimum temperature of warmest month | Bio6 | ℃ |
| Annual mean temperature range | Bio7 | ℃ |
| Precipitation of wettest month | Bio13 | mm |
| Precipitation seasonality (coefficient of variation) | Bio15 | mm |
| Precipitation of driest quarter | Bio17 | mm |
| Period | Bio4 | Bio6 | Bio7 | Bio13 | Bio15 | Bio17 | |
|---|---|---|---|---|---|---|---|
| Percent contribution | LIG | 4.7 | 41.5 | 15.4 | 2.0 | 23.3 | 13 |
| LGM (CCSM) | 3.7 | 41.3 | 16.1 | 1.5 | 24.1 | 13.3 | |
| LGM (MIROC) | 6.3 | 43.2 | 16.1 | 0.5 | 21.7 | 12.2 | |
| Current | 2.8 | 44.1 | 15.3 | 3.5 | 24.3 | 10.0 | |
| Permutation importance | LIG | 10.2 | 33.3 | 42.0 | 1.5 | 11.5 | 1.5 |
| LGM (CCSM) | 11.5 | 27.8 | 41.0 | 1.9 | 15.6 | 2.2 | |
| LGM (MIROC) | 16.6 | 30.6 | 43.2 | 0 | 8.2 | 1.3 | |
| Current | 13.9 | 29.2 | 40.0 | 2.5 | 12.6 | 1.9 |
| Period | Area of each suitable region (×104 km2) | |||
|---|---|---|---|---|
| Marginally suitable region | Moderately suitable region | Highly suitable region | Total suitable region | |
| LIG | 19.46181 | 10.72569 | 5.185764 | 35.373264 |
| LGM (CCSM) | 17.64583 | 9.892362 | 4.810764 | 32.348956 |
| LGM (MIROC) | 17.97049 | 11.09375 | 5.348958 | 34.413198 |
| Current | 20.02604 | 11.98785 | 5.708333 | 37.722223 |
| 2050RCP2.6 | 20.61979 | 16.17708 | 4.168403 | 40.965273 |
| 2050RCP4.5 | 21.50868 | 12.00868 | 2.602431 | 36.119791 |
| 2050RCP6.0 | 24.05035 | 14.31597 | 8.088542 | 46.454862 |
| 2050RCP8.5 | 18.48958 | 11.60417 | 5.079861 | 35.173611 |
| 2070RCP2.6 | 19.43924 | 11.04688 | 2.881944 | 33.368064 |
| 2070RCP4.5 | 19.10417 | 10.43403 | 2.644097 | 32.182297 |
| 2070RCP6.0 | 21.71701 | 6.925347 | 0.032986 | 28.675343 |
| 2070RCP8.5 | 18.81424 | 10.66667 | 0.03125 | 29.51216 |
| Species | Period | Area of each suitable region (×104 Km2) | |||
|---|---|---|---|---|---|
| Unsuitable region | Unchanged region | Expansion region | Contraction region | ||
| Abies chensiensis | Current vs RCP2.6-2050s | 889.29 | 42.34 | 3.25 | 14.00 |
| Current vs RCP4.5-2050s | 894.40 | 37.14 | 8.45 | 8.90 | |
| Current vs RCP6.0-2050s | 893.47 | 40.52 | 5.07 | 9.82 | |
| Current vs RCP8.5-2050s | 897.51 | 36.61 | 3.81 | 8.86 | |
| Current vs RCP2.6-2070s | 894.11 | 39.40 | 6.18 | 9.19 | |
| Current vs RCP4.5-2070s | 887.94 | 41.85 | 3.74 | 15.36 | |
| Current vs RCP6.0-2070s | 892.25 | 42.52 | 3.66 | 11.04 | |
| Current vs RCP8.5-2070s | 891.08 | 39.23 | 7.24 | 9.24 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




