Submitted:
04 September 2023
Posted:
06 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Wheat dwarf virus (WDV)
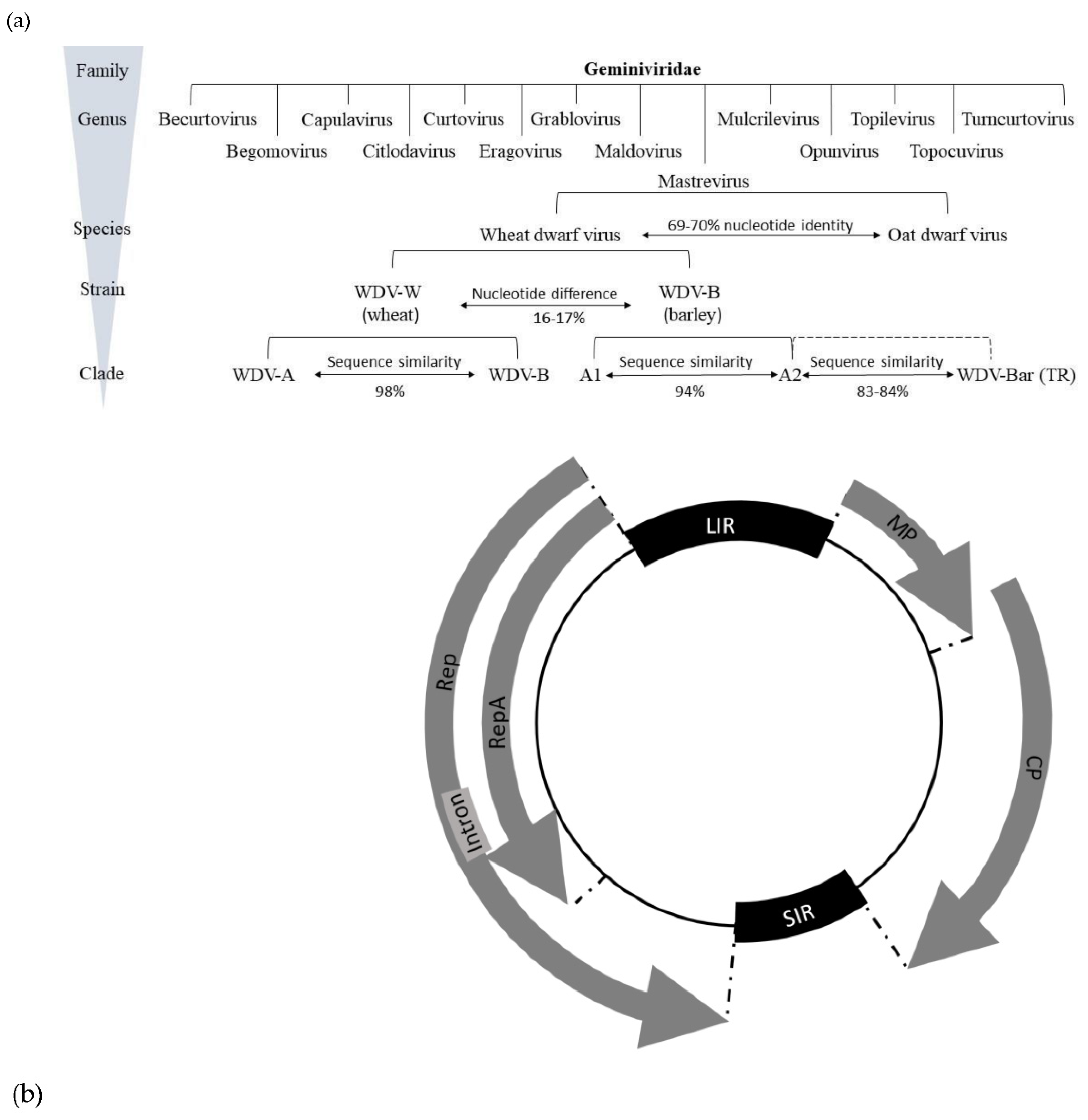
2.1. Classification and Genomic Organization of WDV
2.2. Life cycle of the virus
2.3. Phylogenetics
3. Wheat dwarf disease (WDD)
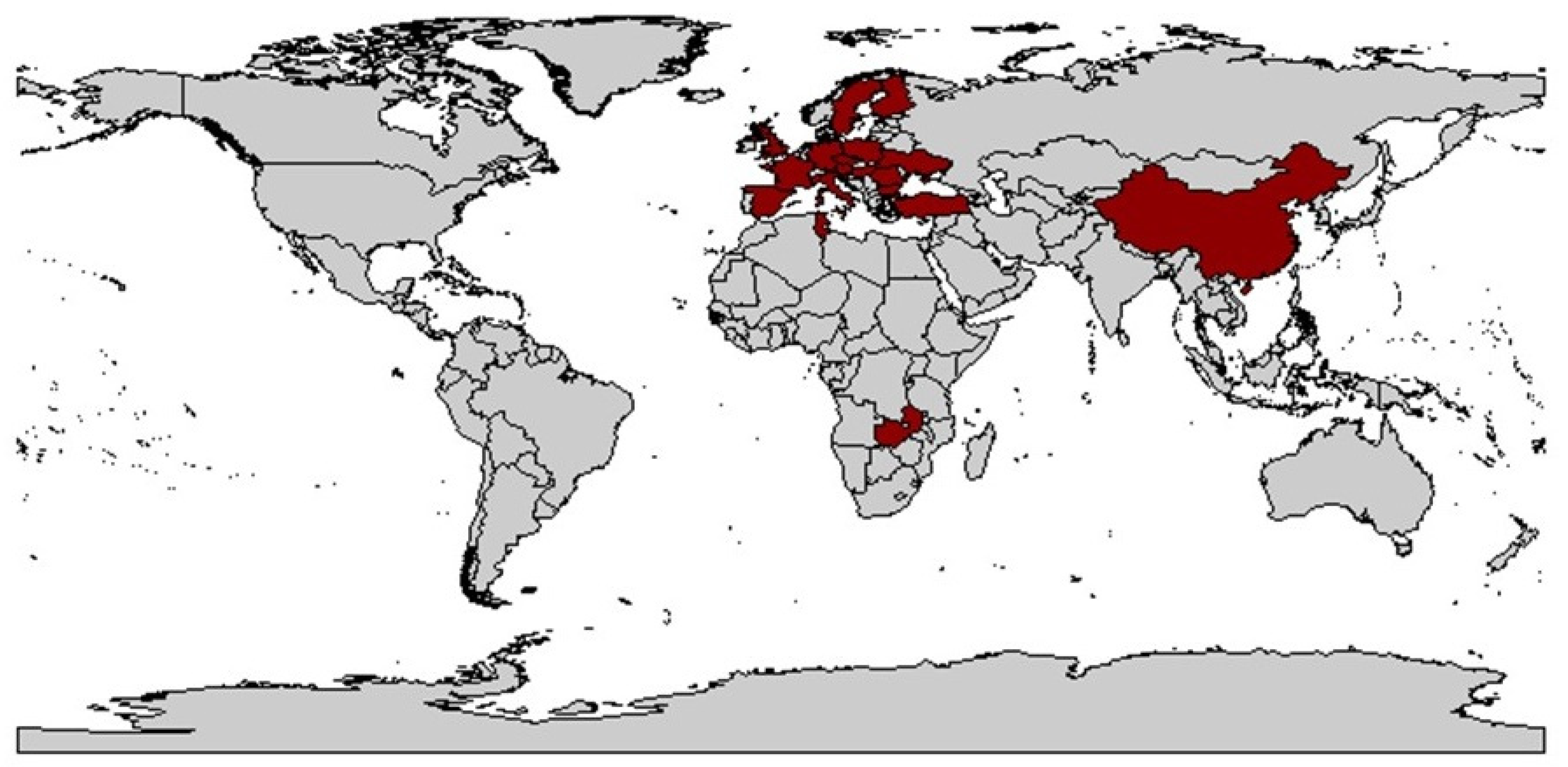
3.1. History
| Time | Event | Reference |
| Early 20th century | first observed dwarfing of wheat, called slidsjuka | 143, 112 |
| Early 20th century | relatively field prevalence of WDV, only few symptoms of dwarfing have been described in scientific literature | 113, 114, 115, 116, 117 |
| 1918 | leafhopper P. alienus was made responsible for WDV | 143 |
| Early 1950s | less undersowing in wheat, increased use of combine harvesters | 121 |
| Around 1950 | decline of slidsjuka due to changes in agricultural practices | 121, 118, 119, 120 |
| 1950-1980/1990 | Slidsjuka occured sporadically | 118, 119, 120 |
| 1961 | first report of direct relationship between virus, vector, symptoms, no virus particle detected | 10, 122 |
| 1980 | increased incidence of disease in european countries | 121 |
| 1980 | identification and taxonomic classification of WDV | 121 |
| Late 1980s | new disease (pieds chétifs) occured in France in association with P. alienus, disease was identified as WDV | 123, 124 |
3.2. Host range
3.3. Symptoms of WDD
4. WDV and its vector
4.1. Taxonomy and virus transmission of P. alienus
4.2. Morphology of P. alienus
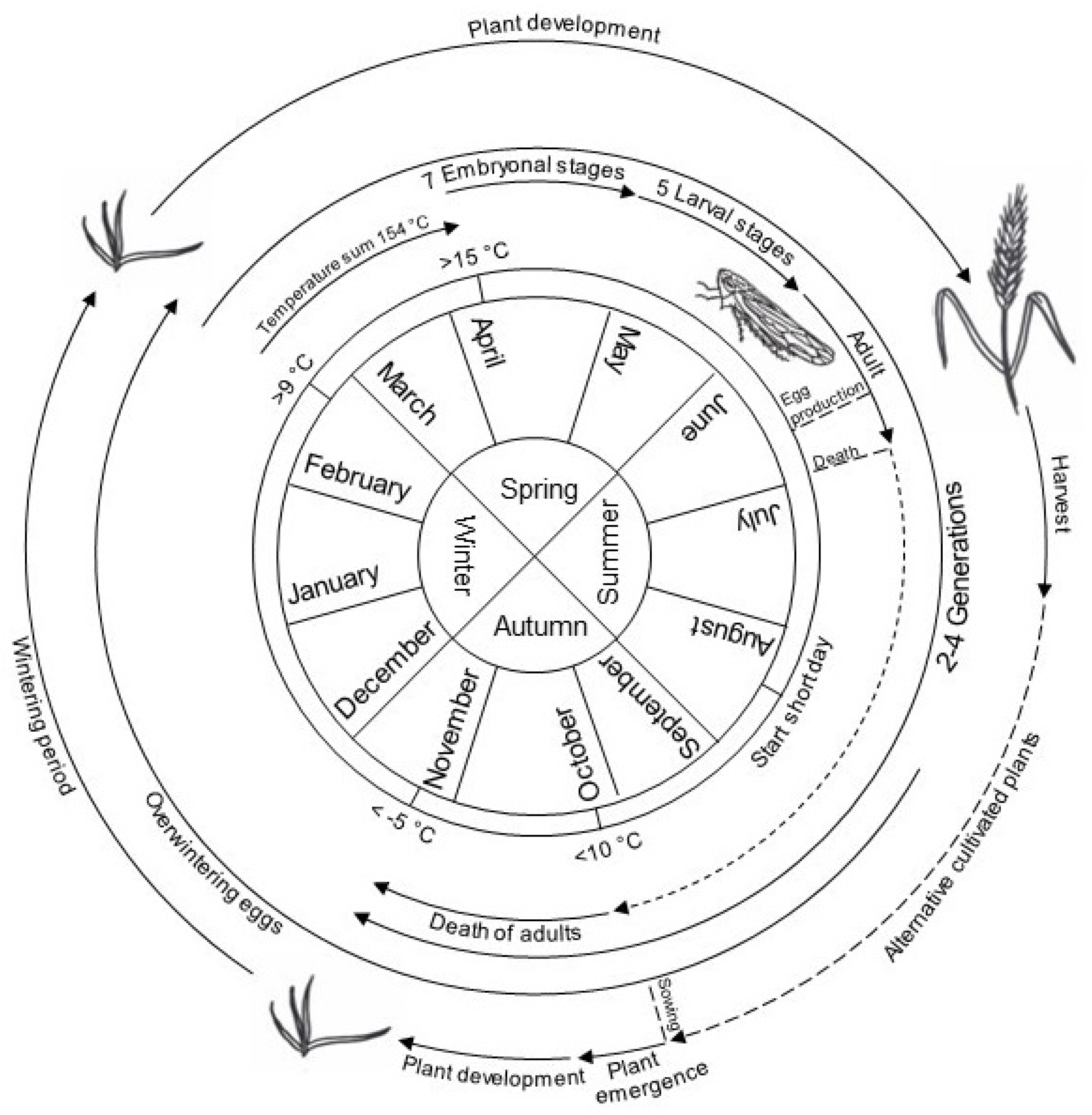
4.3. Life cycle of P. alienus
4.4. Process of Virus Transmission
4.5. Host Range and Wild Reservoirs
4.6. Studies of Insect-Plant Interactions
5. Possibilities of WDV Control
6. Resistance Research
6.1. Status quo of the resistance in wheat
7. Perspective
Author Contributions
Conflicts of Interest
References
- Buck, K. W. Geminiviruses (Geminiviridae). Encyclopedia of Virology 1999, 597–606. [CrossRef]
- Canto, T.; Aranda; M. A.; Fereres, A. Climate change effects on physiology and population processes of hosts and vectors that influence the spread of hemipteran-borne plant viruses. Global Change Biology 2009, 15. Jg., Nr. 8, 1884-1894. [CrossRef]
- Habekuß, A.; Riedel, C.; Schliephake, E.; Ordon, F. Breeding for resistance to insect-transmitted viruses in barley- an emerging challenge due to global warming. Journal für Kulturpflanzen 2009, 61. Jg., Nr. 2, 53-61. [CrossRef]
- Roos, J.; Hopkins, R.; Kvarnheden, A.; Dixelius, C. The impact of global warming on plant diseases and insect vectors in Sweden. European Journal of Plant Pathology 2011, 129. Jg., 9-19. [CrossRef]
- Ziesche, T. M.; Bell, J.; Ordon, F.; Schliephake, E.; Will, T. Long-term monitoring of insects in agricultural landscapes. Mitteilungen der DGaaE 2020, 22. Jg., 101-106.
- Barnett, O. W. & Main, C. E. Plant Virus Disease - Economic Aspects. Encyclopedia of Virology 1999, 1318–1326. [CrossRef]
- Waterworth, H. E.; Hadidi, A. Economic losses due to plant viruses. Plant virus disease control. APS, St. Paul 1998.
- Fraser, R. S. S. Plant Resistance to Viruses | Natural Resistance. In Encyclopedia of Virology, 2nd ed.; Granoff, A., Webster, R.G.; Publisher: Elsevier, 1999, 1300–1307. [CrossRef]
- van Regenmortel, M. H.; Fauquet, C. M.; Bishop, D. H.; Carstens, E. B.; Estes, M. K.; Lemon, S. M.; ... ; Wickner, R. B. Virus taxonomy: classification and nomenclature of viruses. Seventh report of the International Committee on Taxonomy of Viruses. Academic Press 2000.
- Vacke, J. Wheat dwarf virus disease. Biologia Plantarum 1961, 3. Jg., Nr. 3, 228-233. [CrossRef]
- Mehner, S.; Manurung, B.; Gruntzig, M.; Habekuss, A.; Witsack, W.; Fuchs, E. Investigations into the ecology of the Wheat dwarf virus (WDV) in Saxony-Anhalt, Germany. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz/Journal of Plant Diseases and Protection 2003, 313-323.
- Manurung, B.; Witsack, W.; Mehner, S.; Gruntzig, M.; Fuchs, E. Studies on biology and population dynamics of the leafhopper Psammotettix alienus Dahlb. (Homoptera: Auchenorrhyncha) as vector of Wheat dwarf virus (WDV) in Saxony-Anhalt, Germany. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz/Journal of Plant Diseases and Protection 2005, 497-507.
- Vacke, J. Host plants range and symptoms of wheat dwarf virus. Věd Pr Výz Ústavú Rostl Výroby Praha-Ruzyně 1972, 17. Jg., 151-162.
- MacDowell, S. W.; Macdonald, H.; Hamilton, W. D. O.; Coutts, R. H. A.; Buck, K. W. The nucleotide sequence of cloned wheat dwarf virus DNA. The EMBO journal 1985, 4. Jg., Nr. 9, 2173-2180. [CrossRef]
- Macdonald, H.; Coutts, R. H. A.; Buck, K. W. Characterization of a Subgenomic DNA Isolated from Triticum Aestivum Plants Infected with Wheat Dwarf. Journal of general virology 1988, 69. Jg., Nr. 6, 1339-1344. [CrossRef]
- Schalk H.J.; Matzeit V.; Schiller B.; Schell J.; Gronenborn B. Wheat dwarf virus, a geminivirus of graminaceous plants needs splicing for replication. The EMBO journal 1989, 8. Jg., Nr. 2, 359-364. [CrossRef]
- Lindblad, M.; Waern, P. Correlation of wheat dwarf incidence to winter wheat cultivation practices. Agriculture, ecosystems & environment 2002, 92. Jg., Nr. 2-3, 115-122. [CrossRef]
- Lemmetty, A.; Huusela-Veistola, E. First Report of Wheat dwarf virus in Winter Wheat in Finland. Plant disease 2005, 89. Jg., Nr. 8, 912-912. [CrossRef]
- Wang, J.; Guan, Y.; Wu, L.; Guan, X.; Cai, W.; Huang, J.; Dong, W.; Zhang, B. Changing Lengths of the Four Seasons by Global Warming. Geophysical Research Letters 2021, 48. Jg., Nr. 6. [CrossRef]
- Lindsten, K.; Lindsten, B.; Abdelmoeti, M.; Junti, N. Purification and some properties of wheat dwarf virus. Proceedings of the 3rd conference on virus diseases of Gramineae in Europe, Rothamsted. 1980. 27-31.
- Fauquet, C. M.; Briddon, R. W.; Brown, J. K.; Moriones, E.; Stanley, J.; Zerbini, M.; Zhou, X. Geminivirus strain demarcation and nomenclature. Archives of virology 2008, 153. Jg., 783-821. [CrossRef]
- Bernardo, P.; Golden, M.; Akram, M.; Naimuddin, Nadarajan, N.; Fernandez, E.; Granier, M.; Rebelo, A. G.; Peterschmitt, M.; Martin, D. P.; Roumagnac, P. Identification and characterisation of a highly divergent geminivirus: Evolutionary and taxonomic implications. Virus Research 2013, 177. Jg., Nr. 1, 35-45. [CrossRef]
- Varsani, A.; Navas-Castillo, J.; Moriones, E.; Hernández-Zepeda, C.; Idris, A.; Brown, J. K.; Murilo Zerbini, F.; Martin, D. P. Establishment of three new genera in the family Geminiviridae: Becurtovirus, Eragrovirus and Turncurtovirus. Archives of virology 2014, 159. Jg., 2193-2203. [CrossRef]
- Agrios, G. N. (1988). in Plant Pathology, 3rdEdition Edn, ed. G. N. Agrios (New York, NY: Academic Press), 3–39. [CrossRef]
- Matzeit, V. Wheat dwarf virus – Ein Geminivirus monokotyledoner Pflanzen-DNA-Sequenz, Replikation und Einsatz seines Genoms zur Amplifikation und Expression fremder Gene. Ph.D. Dissertation, Universität zu Köln 1988.
- Zhang, W.; Olson, N. H.; Baker, T. S.; Faulkner, L.; Agbandje-McKenna, M.; Boulton, M. I.; Davies, J. W.; McKenna, R. Structure of the Maize Streak Virus Geminate Particle. Virology 2001, 279. Jg., Nr. 2, 471-477. [CrossRef]
- Boulton, M. I. Functions and interactions of mastrevirus gene products. Physiological and molecular plant pathology 2002, 60. Jg., Nr. 5, 243-255. [CrossRef]
- Drews, G.; Adam, G.; Heinze, C. Molekulare Pflanzenvirologie. Springer-Verlag: Berlin Heidelberg, Germany, 2004. [CrossRef]
- Adejare, G. O.; Coutts, R. H. A. The Isolation and Characterisation of a Virus from Nigerian Cassava Plants Affected by the Cassava Mosaic Disease, and Attempted Transmission of the Disease. Journal of Phytopathology 1982, 103. Jg., Nr. 3, 198-210. [CrossRef]
- Harrison, B. D. Advances in Geminivirus Research. Annual Review of Phytopathology 1985, 23. Jg., Nr. 1, 55-82. [CrossRef]
- Damsteegt, V. D.; Igwegbe, E. C. K. Epidemiology and Control of Maize streak disease. Plant Virus Disease Control 1998, 484-494.
- Moffat, A. S. Geminiviruses Emerge as Serious Crop Threat. Science 1999, 286. Jg., Nr. 5446, 1835-1835. [CrossRef]
- Lefkowitz E.J.; Dempsey D.M.; Hendrickson R.C.; Orton R.J.; Siddell S.G.; Smith D.B. Virus taxonomy: the database of the International Committee on Taxonomy of Viruses (ICTV). Nucleic acids research 2018, 46. Jg., Nr. D1, D708-D717. [CrossRef]
- Fiallo-Olivé, E.; Lett, J.-M.; Martin, D. P.; Roumagnac, P.; Varsani, A.; Zerbini, F. M.; Navas-Castillo, J. ICTV Virus Taxonomy Profile: Geminiviridae 2021. Journal of General Virology 2021, 102. Jg., Nr. 12, 001696. [CrossRef]
- Family: Geminiviridae. Available online: https://ictv.global/report/chapter/geminiviridae/geminiviridae (accessed on 12.11.2022).
- Fauquet, C. M.; Bisaro, D. M.; Briddon, R. W.; Brown, J. K.; Harrison, B. D.; Rybicki, E. P.; Stenger, D. C.; Stanley, J. Virology division news: Revision of taxonomic criteria for species demarcation in the family Geminiviridae, and an updated list of begomovirus species. Archives of virology 2003, 148. Jg., Nr. 2, 405-420. [CrossRef]
- Morris, B. A. M.; Richardson, K. A.; Haley, A.; Zhan, X.; Thomas, J. E. The nucleotide sequence of the infectious cloned dna component of tobacco yellow dwarf virus reveals features of geminiviruses infecting monocotyledonous plants. Virology 1992, 187. Jg., Nr. 2, 633-642. [CrossRef]
- Gutierrez, C. Geminivirus DNA replication. Molecular Life Sciences CMLS 1999, 56. Jg., 313-329. [CrossRef]
- Thomas, J. E.; Parry, J. N.; Schwinghamer, M. W.; Dann, E. K. Two novel mastreviruses from chickpea (Cicer arietinum) in Australia. Archives of virology 2010, 155. Jg., 1777-1788. [CrossRef]
- Zerbini, F. M.; Briddon, R. W.; Idris, A.; Martin, D. P.; Moriones, E.; Navas-Castillo, J.; Rivera-Bustamante, R.; Roumagnac, P.; Varsani, A. ICTV Virus Taxonomy Profile: Geminiviridae. Journal of general virology 2017, 98. Jg., Nr. 2, 131-133. [CrossRef]
- Gafni, Y.; Epel, B. L. The role of host and viral proteins in intra- and inter-cellular trafficking of geminiviruses. Physiological and Molecular Plant Pathology 2002, 60. Jg., Nr. 5, 231-241. [CrossRef]
- Ramsell, J.N.E. Genetic Variability of Wheat dwarf virus. Ph.D. dissertation, Swedish University of Agricultural Sciences, 2007.
- Woolston, C. J.; Barker, R.; Gunn, H.; Boulton, M. I.; Mullineaux, P. M. Agroinfection and nucleotide sequence of cloned wheat dwarf virus DNA. Plant Molecular Biology 1988, 11. Jg., 35-43. [CrossRef]
- Bendahmane, M.; Schalk, H. J.; Gronenborn, B. Identification and characterization of wheat dwarf virus from France using a rapid method for geminivirus DNA preparation. Phytopathology 1995, 85. Jg., Nr. 11, 1449-1455.
- Dickinson, V. J.; Halder, J.; Woolston, C. J. The Product of Maize Streak Virus ORF V1 Is Associated with Secondary Plasmodesmata and Is First Detected with the Onset of Viral Lesions. Virology 1996, 220. Jg., Nr. 1, 51-59. [CrossRef]
- Gutierrez, C. Geminiviruses and the plant cell cycle. Plant molecular biology 2000, 43. Jg., 763-772. [CrossRef]
- Gutierrez, C. DNA replication and cell cycle in plants: learning from geminiviruses. The EMBO journal 2000, 19. Jg., Nr. 5, 792-799. [CrossRef]
- Gutierrez, C.; Ramirez-Parra, E.; Mar Castellano, M.; Sanz-Burgos, A. P.; Luque, A.; Missich, R. Geminivirus DNA replication and cell cycle interactions. Veterinary microbiology 2004, 98. Jg., Nr. 2, 111-119. [CrossRef]
- Rojas, M.R.; Hagen, C.; Lucas, W.J.; Gilbertson, R.L. Exploiting chinks in the plant’s armor: Evolution and emergence of geminiviruses. Annu. Rev. Phytopathol. 2005, 43. Jg., 361-394. [CrossRef]
- Briddon, R. W.; Martin, D. P.; Owor, B. E.; Donaldson, L.; Markham, P. G.; Greber, R. S.; Varsani, A. A novel species of mastrevirus (family Geminiviridae) isolated from Digitaria didactyla grass from Australia. Archives of virology 2010, 155. Jg., 1529-1534. [CrossRef]
- Hofer, J. M. I.; Dekker, E. L.; Reynolds, H. V.; Woolston, C. J.; Cox, B. S.; Mullineaux, P. M. Coordinate Regulation of Replication and Virion Sense Gene Expression in Wheat Dwarf Virus. The plant cell 1992, 4. Jg., Nr. 2, 213-223. [CrossRef]
- Morris-Krsinich, B. A. M.; Mullineaux, P. M.; Donson, J.; Boulton, M. I.; Markham, P. G.; Short, M. N.; Davies, J. W. Bidirectional transcription of maize streak virus DNA and identification of the coat protein gene. Nucleic acids research 1985, 13. Jg., Nr. 20, 7237-7256. [CrossRef]
- Dekker, E. L.; Woolston, C. J.; Xue, Y.; Cox, B.; Mullineaux, P. M. Transcript mapping reveals different expression strategies for the bicistronic RNAs of the geminivirus wheat dwarf virus. Nucleic acids research 1991, 19. Jg., Nr. 15, 4075-4081. [CrossRef]
- Fenoll, C.; Black, D. M.; Howell, S. H. The intergenic region of maize streak virus contains promoter elements involved in rightward transcription of the viral genome. The EMBO Journal 1988, 7. Jg., Nr. 6, 1589-1596. [CrossRef]
- Accotto, G. P.; Donson, J.; Mullineaux, P. M. Mapping of Digitaria streak virus transcripts reveals different RNA species from the same transcription unit. The EMBO Journal 1989, 8. Jg., Nr. 4, 1033-1039. [CrossRef]
- Mullineaux, P. M.; Guerineau, F.; Accotto, G.-P. Processing of complementary sense RNAs of Digitariastreak virus in its host and in transgenic tobacco. Nucleic acids research 1990, 18. Jg., Nr. 24, 7259-7265. [CrossRef]
- Wright, E. A.; Heckel, T.; Groenendijk, J.; Davies, J. W.; Boulton, M. I. Splicing features in maize streak virus virion- and complementary-sense gene expression. The plant journal 1997, 12. Jg., Nr. 6, 1285-1297. [CrossRef]
- Palmer, K. E.; Rybicki, E. P. The Molecular Biology of Mastreviruses. Advances in virus research 1998, 50. Jg., 183-234. [CrossRef]
- Wang, Y.; Mao, Q.; Liu, W.; Mar, T.; Wei, T.; Liu, Y.; Wang, X. Localization and Distribution of Wheat dwarf virus in Its Vector Leafhopper, Psammotettix alienus. Phytopathology 2014, 104. Jg., Nr. 8, 897-904. [CrossRef]
- Noueiry, A. O.; Lucas, W. J.; Gilbertson, R. L. Two proteins of a plant DNA virus coordinate nuclear and plasmodesmal transport. Cell 1994, 76. Jg., Nr. 5, 925-932. [CrossRef]
- Liu, H.; Boulton, M. I.; Oparka, K. J.; Davies, J. W. Interaction of the movement and coat proteins of Maize streak virus: implications for the transport of viral DNA Journal of general virology 2001, 82. Jg., Nr. 1, 35-44. [CrossRef]
- Liu, H.; Andrew, Lucy, P.; Davies, J. W.; Boulton, M. I. A single amino acid change in the coat protein of Maize streak virus abolishes systemic infection, but not interaction with viral DNA or movement protein. Molecular plant pathology 2001, 2. Jg., Nr. 4, 223-228. [CrossRef]
- Noris, E.; Vaira, A. M.; Caciagli, P.; Masenga, V.; Gronenborn, B. & Accotto, G. P. Amino Acids in the Capsid Protein of Tomato Yellow Leaf Curl Virus That Are Crucial for Systemic Infection, Particle Formation, and Insect Transmission. Journal of virology 1998, 72. Jg., Nr. 12, 10050-10057. [CrossRef]
- Liu, H.; Boulton, M. I.; Thomas, C. L.; Prior, D. A. M.; Oparka, K. J.; Davies, J. W. Maize Streak Virus Coat Protein Is Karyophyllic and Facilitates Nuclear Transport of Viral DNA. Molecular plant-microbe interactions 1999, 12. Jg., Nr. 10, 894-900. [CrossRef]
- Kotlizky, G.; Boulton, M. I.; Pitaksutheepong, C.; Davies, J. W.; Epel, B. L. Intracellular and Intercellular Movement of Maize Streak Geminivirus V1 and V2 Proteins Transiently Expressed as Green Fluorescent Protein Fusions. Virology 2000, 274. Jg., Nr. 1, 32-38. [CrossRef]
- Sunter, G.; Bisaro, D. M. Transactivation of Geminivirus AR1 and BR1 Gene Expression by the Viral AL2 Gene Product Occurs at the Level of Transcription. The Plant Cell 1992, 4. Jg., Nr. 10, 1321-1331. [CrossRef]
- Hong, Y.; Saunders, K.; Hartley, M. R.; Stanley, J. Resistance to Geminivirus Infection by Virus-Induced Expression of Dianthin in Transgenic Plants. Virology 1996, 220. Jg., Nr. 1, 119-127. [CrossRef]
- Voinnet, O.; Pinto, Y. M.; Baulcombe, D. C. Suppression of gene silencing: A general strategy used by diverse DNA and RNA viruses of plants. Proceedings of the National Academy of Sciences 1999, 96. Jg., Nr. 24, 14147-14152. [CrossRef]
- Shivaprasad, P. V.; Akbergenov, R.; Trinks, D.; Rajeswaran, R.; Veluthambi, K.; Hohn, T.; Pooggin, M. M. Promoters, Transcripts, and Regulatory Proteins of Mungbean Yellow Mosaic Geminivirus. Journal of virology 2005, 79. Jg., Nr. 13, 8149-8163. [CrossRef]
- Trinks, D.; Rajeswaran, R.; Shivaprasad, P. V.; Akbergenov, R.; Oakeley, E. J.; Veluthambi, K.; Hohn, T.; Pooggin, M. M. Suppression of RNA Silencing by a Geminivirus Nuclear Protein, AC2, Correlates with Transactivation of Host Genes. Journal of virology 2005, 79. Jg., Nr. 4, 2517-2527. [CrossRef]
- Wang, H.; Buckley, K. J.; Yang, X.; Buchmann, R. C.; Bisaro, D. M. Adenosine Kinase Inhibition and Suppression of RNA Silencing by Geminivirus AL2 and L2 Proteins. Journal of virology 2005, 79. Jg., Nr. 12, 7410-7418. [CrossRef]
- Chowda-Reddy, R. V.; Dong, W.; Felton, C.; Ryman, D.; Ballard, K.; Fondong, V. N. Characterization of the cassava geminivirus transcription activation protein putative nuclear localization signal. Virus research 2009, 145. Jg., Nr. 2, 270-278. [CrossRef]
- Castillo-González, C.; Liu, X.; Huang, C.; Zhao, C.; Ma, Z.; Hu, T.; Sun, F.; Zhou, X.; Wang, X.J.; Zhang, X. Geminivirus-Encoded TrAP Suppressor Inhibits the Histone Methyltransferase SUVH4/KYP to Counter Host Defense. Elife 2015, 4. Jg., S. e06671. [CrossRef]
- Kumar, V.; Mishra, S. K.; Rahman, J.; Taneja, J.; Sundaresan, G.; Mishra, N. S.; Mukherjee, S. K. Mungbean yellow mosaic Indian virus encoded AC2 protein suppresses RNA silencing by inhibiting Arabidopsis RDR6 and AGO1 activities. Virology 2015, 486. Jg., 158-172. [CrossRef]
- Kvarnheden, A.; Lindblad, M.; Lindsten, K.; Valkonen, J. P. T. Genetic diversity of Wheat dwarf virus. Archives of virology 2002, 147. Jg., 205-216. [CrossRef]
- Koch, C. Die Bestimmung der DNA-Sequenz des Geminivirus WDV-ER Genoms und Versuche zur Übertragung des Virus auf Gerste mit Agrobacterium tumefaciens. Diploma thesis, Universität Köln, 1990.
- Schubert, J.; Habekuß, A.; Rabenstein, F. Investigation of differences between wheat and barley forms of Wheat dwarf virus and their distribution in host plants. Plant Protection Science-Prague 2003, 38. Jg., 43-48. [CrossRef]
- Jeske, H. Geminiviruses. TT Viruses: The Still Elusive Human Pathogens 2009, 185-226. [CrossRef]
- Hanley-Bowdoin, L.; Bejarano, E. R.; Robertson, D.; Mansoor, S. Geminiviruses: masters at redirecting and reprogramming plant processes. Nature Reviews Microbiology 2013, 11. Jg., Nr. 11, 777-788. [CrossRef]
- Wu, B.; Shang, X.; Schubert, J.; Habekuß, A.; Elena, S. F.; Wang, X. Global-scale computational analysis of genomic sequences reveals the recombination pattern and coevolution dynamics of cereal-infecting geminiviruses. Scientific reports 2015, 5. Jg., Nr. 1, 8153. [CrossRef]
- Van Bel, A. J. E. The phloem, a miracle of ingenuity. Plant, Cell & Environment 2003, 26. Jg., Nr. 1, 125-149. [CrossRef]
- Kammann, M.; Schalk, H.-J.; Matzeit, V.; Schaefer, S.; Schell, J.; Gronenborn, B. DNA replication of wheat dwarf virus, a geminivirus, requires two cis-acting signals. Virology 1991, 184. Jg., Nr. 2, 786-790. [CrossRef]
- Heyraud, F.; Matzeit, V.; Schaefer, S.; Schell, J.; Gronenborn, B. The conserved nonanucleotide motif of the geminivirus stem-loop sequence promotes replicational release of virus molecules from redundant copies. Biochimie 1993, 75. Jg., Nr. 7, 605-615. [CrossRef]
- Laufs, J.; Jupin, I.; David, C.; Schumacher, S.; Heyraud-Nitschke, F.; Gronenborn, B. Geminivirus replication: Genetic and biochemical characterization of Rep protein function, a review. Biochimie 1995, 77. Jg., Nr. 10, 765-773. [CrossRef]
- Hanley-Bowdoin, L.; Settlage, S. B.; Orozco, B. M.; Nagar, S.; Robertson, D. Geminiviruses: Models for Plant DNA Replication, Transcription, and Cell Cycle Regulation. Critical Reviews in Plant Sciences 1999, 18. Jg., Nr. 1, 71-106. [CrossRef]
- Bosque-Pérez, N. A. Eight decades of maize streak virus research. Virus research 2000, 71. Jg., Nr. 1-2, 107-121. [CrossRef]
- Astier, S.; Albouy J.; Maury Y.; Robaglia C.; Lecoq H. Principles of plant virology: genome, pathogenicity, virus ecology. Ecology. Paris: Science Publisher 2007.
- Tomenius, K.; Oxelfelt, P. Preliminary Observations of Viruslike Particles in Nuclei in Cells of Wheat Infected with the Wheat Dwarf Disease. Journal of Phytopathology 1981, 101. Jg., Nr. 2, 163-167. [CrossRef]
- Huth, W.; Lesemann, D.-E. Nachweis des wheat dwarf virus in Deutschland. Nachrichtenblatt des Deutschen Pflanzenschutzdienstes 1994, 46. Jg., Nr. 5, 105-106.
- Hehnle, S.; Wege, C.; Jeske, H. Interaction of DNA with the Movement Proteins of Geminiviruses Revisited. Journal of virology 2004, 78. Jg., Nr. 14, 7698-7706. [CrossRef]
- Evert, R. F.; Russin, W. A.; Botha, C. E. J. Distribution and frequency of plasmodesmata in relation to photoassimilate pathways and phloem loading in the barley leaf. Planta 1996, 198. Jg., 572-579. [CrossRef]
- Aoki, N.; Scofield, G. N.; Wang, X.-D.; Patrick, J. W.; Offler, C. E.; Furbank, R. T. Expression and localisation analysis of the wheat sucrose transporter TaSUT1 in vegetative tissues. Planta 2004, 219. Jg., 176-184. [CrossRef]
- Crawford, K. M.; Zambryski, P. C. Non-Targeted and Targeted Protein Movement through Plasmodesmata in Leaves in Different Developmental and Physiological States. Plant Physiology 2001, 125. Jg., Nr. 4, 1802-1812. [CrossRef]
- Peterschmitt, M.; Quiot, J. B.; Reynaud, B.; Baudin, P. Detection of maize streak virus antigens over time in different parts of maize plants of a sensitive and a so-called tolerant cultivar by ELISA. Annals of applied biology 1992, 121. Jg., Nr. 3, 641-653. [CrossRef]
- Waigmann et al. 2000 cited in Mariano, A. C.; Andrade, M. O.; Santos, A. A.; Carolino, S. M. B.; Oliveira, M. L.; Baracat-Pereira, M. C.; Brommonshenkel, S. H.; Fontes, E. P. B. Identification of a novel receptor-like protein kinase that interacts with a geminivirus nuclear shuttle protein. Virology 2004, 318. Jg., Nr. 1, 24-31. [CrossRef]
- Maule, A.; Leh, V.; Lederer, C. The dialogue between viruses and hosts in compatible interactions. Current opinion in plant biology 2002, 5. Jg., Nr. 4, 279-284. [CrossRef]
- Mariano, A. C.; Andrade, M. O.; Santos, A. A.; Carolino, S. M. B.; Oliveira, M. L.; Baracat-Pereira, M. C.; Brommonshenkel, S. H. & Fontes, E. P. B. Identification of a novel receptor-like protein kinase that interacts with a geminivirus nuclear shuttle protein. Virology 2004, 318. Jg., Nr. 1, 24-31. [CrossRef]
- Mehner, S. Zur Ökologie des Wheat dwarf virus (WDV) in Sachsen-Anhalt. Ph.D. Dissertation, Martin-Luther-Universität Halle-Wittenberg, 2005.
- Lindsten, K.; Lindsten, B. Wheat dwarf - an old disease with new outbreaks in Sweden. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz/Journal of Plant Diseases and Protection 1999, 325-332.
- Commandeur U.; Huth W. Differentiation of strains of Wheat dwarf virus in infected wheat and barley plants by means of polymerase chain reaction. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz/Journal of Plant Diseases and Protection 1999, 550-552.
- Schubert, J.; Habekuß, A.; Kazmaier, K.; Jeske, H. Surveying cereal-infecting geminiviruses in Germany—Diagnostics and direct sequencing using rolling circle amplification. Virus research 2007, 127. Jg., Nr. 1, 61-70. [CrossRef]
- Muhire, B.; Martin, D. P.; Brown, J. K.; Navas-Castillo, J.; Moriones, E.; Zerbini, F. M.; Rivera-Bustamante, R.; Malathi, V. G.; Briddon, R. W.; Varsani, A. A genome-wide pairwise-identity-based proposal for the classification of viruses in the genus Mastrevirus (family Geminiviridae). Archives of virology 2013, 158. Jg., 1411-1424. [CrossRef]
- Wu, B.; Melcher, U.; Guo, X.; Wang, X.; Fan, L.; Zhou, G. Assessment of codivergence of Mastreviruses with their plant hosts. BMC evolutionary biology 2008, 8. Jg., Nr. 1, 1-13. [CrossRef]
- Mishchenko L.T.; Dunich A.A.; Mishchenko I.A.; Dashchenko A.V.; Kozub N.O.; Kyslykh T.M.; Molodchenkova O.O. Wheat dwarf virus in Ukraine: occurrence, molecular characterization and impact on the yield. Journal of Plant Diseases and Protection 2022, 129. Jg., Nr. 1, 107-116. [CrossRef]
- Shepherd, D. N.; Martin, D. P.; McGivern, D. R.; Boulton, M. I.; Thomson, J. A.; Rybicki, E. P. A three-nucleotide mutation altering the Maize streak virus Rep pRBR-interaction motif reduces symptom severity in maize and partially reverts at high frequency without restoring pRBR–Rep binding. Journal of general virology 2005, 86. Jg., Nr. 3, 803-813. [CrossRef]
- Schubert J.; Habekuß A.; Wu B.; Thieme T.; Wang X. Analysis of complete genomes of isolates of the Wheat dwarf virus from new geographical locations and descriptions of their defective forms. Virus Genes 2014, 48. Jg., 133-139. [CrossRef]
- Köklü, G.; Ramsell, J. N. E.; Kvarnheden, A. The complete genome sequence for a Turkish isolate of Wheat dwarf virus (WDV) from barley confirms the presence of two distinct WDV strains. Virus Genes 2007, 34. Jg., 359-366. [CrossRef]
- Ramsell, J. N. E.; Boulton, M. I.; Martin, D. P.; Valkonen, J. P. T.; Kvarnheden, A. Studies on the host range of the barley strain of Wheat dwarf virus using an agroinfectious viral clone. Plant pathology 2009, 58. Jg., Nr. 6, 1161-1169. [CrossRef]
- Wu, X.; Weigel, D.; Wigge, P. A. Signaling in plants by intercellular RNA and protein movement. Genes & development 2002, 16. Jg., Nr. 2, 151-158. [CrossRef]
- Owor, B. E.; Shepherd, D. N.; Taylor, N. J.; Edema, R.; Monjane, A. L.; Thomson, J. A.; Martin, D. P.; Varsani, A. Successful application of FTA® Classic Card technology and use of bacteriophage ϕ29 DNA polymerase for large-scale field sampling and cloning of complete maize streak virus genomes. Journal of virological methods 2007, 140. Jg., Nr. 1-2, 100-105. [CrossRef]
- Jungner J. Die Zwergzikade (Cicadula sexnotata Fall.) und ihre Bekämpfung. Berlin DLG 1906.
- Lindsten, K.; Vacke, J.; Gerhardson, B. A preliminary report on three cereal virus diseases new to Sweden spread by Macrosteles and Psammotettix leafhoppers. Meddelanden fran Statens Vaxtskyddsanstalt 1970, 14. Jg., Nr. 123, 285-297.
- Gaborjanyi, R.; Vacke, J. and Bisztray, G. Wheat dwarf virus: a new cereal pathogen in Hungary. Novenytermeles (Hungary) 1988.
- Lapierre, H.; Cousin, M.T.; Della Giustina, W.; Moreau, J.P.; Khogali, M.; et al. Nanisme blé: agent pathogéne et vecteur. Description, biologie, interaction. Phytoma 432 1991, 26–28.
- Conti, M. Leafhopper-borne plant viruses in Italy. Memorie della Societá Entomologica Italiana 72, 1993, 541–547.
- Jilaveanu, A.; Vacke, J. Isolation and identification of wheat dwarf virus (WDV) in Romania. Probleme de protectia plantelor 1995, 23. Jg., 51-62.
- Najar, A.; Makkouk, K.M.; Boudhir, H.; Kumari, S.G.; Zarouk, R.; Bessai, R.; Othman, F.B. Viral diseases of cultivated legume and cereal crops in Tunisia. Viral Diseases of Cultivated Legume and Cereal Crops in Tunisia 2000, 1000-1010.
- Sandgren, M.; Lindblad, M. Field studies of Wheat dwarf virus. In 7th International Congress of Plant Pathology, Edinburgh, UK, 9-16 August 1998.
- Lindsten, K.; Lindsten, B. Occurrence and transmission of Wheat dwarf virus (WDV) in France. In Proceedings of Third International Conference on Pest in Agriculture. Montpellier, France. 1993. 7-9.
- Lindblad, M. What happened to the wheat dwarf disease. Växtskyddsnotiser 2000, 64. Jg., Nr. 1, 11-13.
- Lindsten, K. Wheat dwarf – an old disease caused by a unique and earlier unknown virus. Vaextskyddsnotiser, 1980.
- Dlabola, J. Zur Schädlichkeit der Zikaden in Getreidefeldern. Nachrichtenblatt Deutscher Pflanzenschutzdienst (Berlin), 1961, 14. Jg., 120-122.
- Moreau, J.-P.; Lapierre, H.; Navarro, D.; Debray, P.; Fohrer, F.; Lebrun, I. Distinction des effets du nanisme et de la jaunisse sur le blé. Phytoma, la défense des végétaux 1992, Nr. 443, 21-25.
- Lindsten, K. and Vacke, J. A possible barley adapted strain of wheat dwarf virus (WDV). Acta phytopathologica et entomologica Hungarica 1991, 26. Jg., Nr. 1-2, 175-180.
- Giustina, W. D.; Lebrun, I.; Lapierre, H.; Lochon, S. et le Groupe de travail „Biologie et écologie de P. alienus “. Distribution géographique du vecteur et du virus. Phytoma – La défense des végétaux 1991, 432: 30-34.
- Anonym. New Knowledges about wheat dwarf virus. Phytoma – La défense des végétaux 1992, 443: 17-20.
- Vacher, C.; Felix, I.; Bonnand, E. Lutte contre Psammotettix alienus, Cicadelle vectrice de la maladie des „pieds chétifs “. Perspectives Agricoles 1991, 162: 86-89.
- Pridanceva 1965 cited in Bisztray, G.; Gaborjanyi, R.; Vacke, J. Isolation and characterization of wheat dwarf virus found for the first time in Hungary. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz/Journal of Plant Diseases and Protection 1989, 449-454.
- Radulescu & Munteanu, 1970 cited in Vacke, J. Host plants range and symptoms of wheat dwarf virus. Věd Pr Výz Ústavú Rostl Výroby Praha-Ruzyně 1972, 17. Jg., 151-162.
- Stephanov & Dimov 1981 cited in Bisztray, G.; Gaborjanyi, R.; Vacke, J. Isolation and characterization of wheat dwarf virus found for the first time in Hungary. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz/Journal of Plant Diseases and Protection 1989, 449-454.
- Bisztray, G.; Gaborjanyi, R.; Vacke, J. Isolation and characterization of wheat dwarf virus found for the first time in Hungary. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz/Journal of Plant Diseases and Protection 1989, 449-454.
- Jezewska, J. First report of Wheat dwarf virus occurring in Poland. Phytopathologia Polonica 2001, Nr. 21, S. 93-100.
- Achon, M. A.; Serrano, L.; Ratti, C.; Rubies-Autonell, C. First Detection of Wheat dwarf virus in Barley in Spain Associated with an Outbreak of Barley Yellow Dwarf. Plant Disease 2006, 90. Jg., Nr. 7, 970-970. [CrossRef]
- Viršček Marn, M.; Mavrič Pleško, I. First Report of the Occurrence of Wheat dwarf virus Infecting Wheat in Slovenia. Plant Disease 2017, 101. Jg., Nr. 7, 1336. [CrossRef]
- Behjatnia, S. A. A.; Afsharifar, A. R.; Tahan, V.; Motlagh, M. H. A.; Gandomani, O. E.; Niazi, A.; Izadpanah, K. Widespread occurrence and molecular characterization of Wheat dwarf virus in Iran. Australasian Plant Pathology 2011, 40. Jg., 12-19. [CrossRef]
- Kapooria, R. G.; Ndunguru, J. Occurrence of viruses in irrigated wheat in Zambia. EPPO Bulletin 2004, 34. Jg., Nr. 3, 413-419. [CrossRef]
- Ekzayez, A. M.; Kumari, S. G.; Ismail, I. First Report of Wheat dwarf virus and Its Vector (Psammotettix provincialis) Affecting Wheat and Barley Crops in Syria. Plant Disease 2011, 95. Jg., Nr. 1, 76-76. [CrossRef]
- Xie, J.; Wang, X.; Liu, Y.; Peng, Y.; Zhou, G. First Report of the Occurrence of Wheat dwarf virus in Wheat in China. Plant disease 2007, 91. Jg., Nr. 1, 111-111. [CrossRef]
- Wang X.; Wu B.; Wang J.F.; First report of Wheat dwarf virus infecting barley in Yunnan, China. Journal of Plant Pathology 2008, 90. Jg., Nr. 2, 400-400. [CrossRef]
- Bivand, R.; Lewin-Koh, N.; Pebesma, E.; Archer, E.; Baddeley, A.; Bearman, N.; ... & Golicher, D. Package ‘maptools’.
- pers. Mitteilung Peder Waren in Lindsten, K.; Lindsten, B. (1999) Wheat dwarf - an old disease with new outbreaks in Sweden. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz/Journal of Plant Diseases and Protection 1999, 325-332.
- Felix, I.; Larcher, J.M.; Maraby, J.; Philippeau, G.; Vinatier, K. Risques d’attaques de cicadelles et conditions d’efficacité des insecticides. Perspectives agricoles (Paris) 1992, Nr. 173, 98-106.
- Tullgren, A. Zur Morphologie und Systematik der Hemipteren I. Entomologisk Tidskrift / Entomologiska Föreningen i Stockholm 1918, 113–133. [CrossRef]
- ICTV Report Virus taxonomy: classification and nomenclature of viruses: Ninth Report of the International Committee on Taxonomy of Viruses. King, A.M.Q.; Adams, M.J.; Carstens, E.B. and Lefkowitz, E.J (eds). Elsevier Academic Press 2012.
- Ramsell, J. N. E.; Lemmetty, A.; Jonasson, J.; Andersson, A.; Sigvald, R.; Kvarnheden, A. Sequence analyses of Wheat dwarf virus isolates from different hosts reveal low genetic diversity within the wheat strain. Plant pathology 2008, 57. Jg., Nr. 5, 834-841. [CrossRef]
- Vacke J.; Cibulka, R. Silky bent grass (Apera spica-venti [L.] Beauv.) – a new host and reservoir of wheat dwarf virus. Plant protection science 1999, 35. Jg., Nr. 2, 47-50. [CrossRef]
- Lindsten 1991 cited in Brunt, A.; Crabtree, K.; Dallwitz, M.; Gibbs, A.; Watson, L. Viruses of Plants 1996. [CrossRef]
- Fohrer, F.; Lebrun, I.; Lapierre E, H. Acquisitions recéntes sur le virus du nanisme du blé. Phytoma, la défense des végétaux 1992, Nr. 443, S. 18-20.
- Vacke, J.; Cibulka, R. Response of selected winter wheat varieties to wheat dwarf virus infection at an early growth stage. Czech Journal of Genetics and Plant Breeding 2000, 36. Jg., Nr. 1, 1-4.
- Manurung, B.; Witsack, W.; Mehner, S.; Gruntzig, M.; Fuchs, E. The epidemiology of Wheat dwarf virus in relation to occurrence of the leafhopper Psammotettix alienus in Middle-Germany. Virus Research 2004, 100. Jg., Nr. 1, 109-113. [CrossRef]
- Širlová, L.; Vacke, J.; Chaloupková, M. Reaction of selected winter wheat varieties to autumnal infection with Wheat dwarf virus. Plant Prot. Sci 2005, 41. Jg., 1-7. [CrossRef]
- Jones, R. A. C. Global Plant Virus Disease Pandemics and Epidemics. Plants 2021, 10. Jg., Nr. 2, 233. [CrossRef]
- Huth W. Weizenverzwergung - bisher übersehen? Pflanzenschutz-Praxis 1994, 4, 37-39.
- Áy, Z.; Kerényi, Z.; Takács, A.; Papp, M.; Petróczi, I.; Gáborjányi, R.; Silhavy, D.; Pauk, J.; Kertész, Z. Detection of cereal viruses in wheat (Triticum aestivumL.) by serological and molecular methods. Cereal Research Communications 2008, 36. Jg., Nr. 2, 215-224. [CrossRef]
- Lindblad, M.; Arenö, P. Temporal and spatial population dynamics of Psammotettix alienus, a vector of wheat dwarf virus. International journal of pest management 2002, 48. Jg., Nr. 3, 233-238. [CrossRef]
- Nault, L. R. & Ammar, E. D. Leafhopper and Planthopper Transmission of Plant Viruses. Annual review of entomology 1989, 34. Jg., Nr. 1, 503-529. [CrossRef]
- Whitfield, A. E.; Falk, B. W.; Rotenberg, D. Insect vector-mediated transmission of plant viruses. Virology 2015, 479. Jg., 278-289. [CrossRef]
- Dietzgen, R. G.; Kondo, H.; Goodin, M. M.; Kurath, G.; Vasilakis, N. The family Rhabdoviridae: mono- and bipartite negative-sense RNA viruses with diverse genome organization and common evolutionary origins. Virus research 2017, 227. Jg., 158-170. [CrossRef]
- Liu, B.; Yuan, R.; Liang, Z.; Zhang, T.; Zhu, M.; Zhang, X.; Geng, W.; Fang, P.; Jiang, M.; Wang, Z.; Feng, Y.; Liu, X.; Zhou, Y.; Xue, R.; Cao, G.; Chen, H.; Hu, X.; Gong, C. Comprehensive analysis of circRNA expression pattern and circRNA–mRNA–miRNA network in Ctenopharyngodon idellus kidney (CIK) cells after grass carp reovirus (GCRV) infection. Aquaculture 2019, 512. Jg., 734349. [CrossRef]
- Du, Z.; Fu, Y.; Liu, Y.; Wang, X. Transmission Characteristics of Wheat Yellow Striate Virus by its Leafhopper Vector Psammotettix alienus. Plant disease 2020, 104. Jg., Nr. 1, 222-226. [CrossRef]
- Liu, Y.; Du, Z.; Wang, H.; Zhang, S.; Cao, M.; Wang, X. Identification and Characterization of Wheat Yellow Striate Virus, a Novel Leafhopper-Transmitted Nucleorhabdovirus Infecting Wheat. Frontiers in microbiology 2018, 9. Jg., 468. [CrossRef]
- Lundsgaard, T. Filovirus-like particles detected in the leafhopper Psammotettix alienus. Virus research 1997, 48. Jg., Nr. 1, 5-40. [CrossRef]
- Bock, J. O.; Lundsgaard, T.; Pedersen, P. A.; Christensen, L. S. Identification and partial characterization of Taastrup virus: a newly identified member species of the Mononegavirales. Virology 2004, 319. Jg., Nr. 1, 49-59. [CrossRef]
- Wang H.; Liu Y.; Liu W.; Cao M.; Wang X. Full genome sequence of a novel iflavirus from the leafhopper Psammotettix alienus. Archives of virology 2019, 164. Jg., 309-311. [CrossRef]
- Wang H.; Liu Y.; Liu W.; Cao M.; Wang X. Sequence analysis and genomic organization of a novel chuvirus, Tàiyuán leafhopper virus. Archives of virology 2019, 164. Jg., 617-620. [CrossRef]
- Han, X.; Wang, H.; Wu, N.; Liu, W.; Cao, M.; Wang, X. Leafhopper Psammotettix alienus hosts chuviruses with different genomic structures. Virus research 2020, 285. Jg., 197-992. [CrossRef]
- Fu, Y.; Cao, M.; Wang, H.; Du, Z.; Liu, Y.; Wang, X. Discovery and characterization of a novel insect-specific reovirus isolated from Psammotettix alienus. Journal of General Virology 2020, 101. Jg., Nr. 8, 884-892. [CrossRef]
- Harding, R. M.; Burns, P.; Geijskes, R. J.; McQualter, R. M.; Dale, J. L.; Smith, G. R. Molecular Analysis of Fiji Disease Virus Segments 2, 4 and 7 Completes the Genome Sequence. Virus Genes 2006, 32. Jg., 43-47. [CrossRef]
- Lot, H.; Delecolle, B.; Boccardo, G.; Marzachi, C.; Milne, R. G. Partial characterization of reovirus-like particles associated with garlic dwarf disease. Plant pathology 1994, 43. Jg., Nr. 3, 537-546. [CrossRef]
- Xie, L.; LV, M.-F.; Yang, J.; Chen, J.-P.; Zhang, H.-M. Genomic and phylogenetic evidence that Maize rough dwarf and Rice black-streaked dwarf fijiviruses should be classified as different geographic strains of a single species. Acta virologica 2017, 61. Jg., Nr. 4, 453-462. [CrossRef]
- Distéfano, A. J.; Conci, L. R.; Muñoz Hidalgo, M.; Guzmán, F. A.; Hopp, H. E. & del Vas, M. Sequence and phylogenetic analysis of genome segments S1, S2, S3 and S6 of Mal de Rı́o Cuarto virus, a newly accepted Fijivirus species. Virus research 2003, 92. Jg., Nr. 1, 113-121. [CrossRef]
- Isogai, M.; Lindsten, K.; Uyeda, I. Taxonomic characteristics of fijiviruses based on nucleotide sequences of the oat sterile dwarf virus genome. Journal of general virology 1998, 79. Jg., Nr. 6, 1479-1485. [CrossRef]
- Teakle, D.; Hicks, S.; Karan, M.; Hacker, J.; Greber, R.; Donaldson, J. Host range and geographic distribution of pangola stunt virus and its planthopper vectors in Australia. Australian Journal of Agricultural Research 1991, 42. Jg., Nr. 5, 819-826. [CrossRef]
- Zhou, G.; Wen, J.; Cai, D.; Li, P.; Xu, D.; Zhang, S. Southern rice black-streaked dwarf virus: A new proposed Fijivirus species in the family Reoviridae. Chinese science bulletin 2008, 53. Jg., Nr. 23, 3677-3685. [CrossRef]
- Nakashima, N.; Koizumi, M.; Watanabe, H.; Noda, H. Complete nucleotide sequence of the Nilaparvata lugens reovirus: a putative member of the genus Fijivirus. Journal of General Virology 1996, 77. Jg., Nr. 1, 139-146. [CrossRef]
- Zhang, X.; Zhou, G.; Wang, X. Detection of wheat dwarf virus (WDV) in wheat and vector leafhopper (Psammotettix alienus Dahlb.) by real-time PCR. Journal of virological methods 2010, 169. Jg., Nr. 2, 416-419. [CrossRef]
- Le Roux, J. J.; Rubinoff, D. Molecular data reveals California as the potential source of an invasive leafhopper species, Macrostelessp. nr. severini, transmitting the aster yellows phytoplasma in Hawaii. Annals of Applied Biology, 154(3), 419–427. [CrossRef]
- Derlink, M.; Pavlovčič, P.; Stewart, A. J. A.; Virant-Doberlet, M. Mate recognition in duetting species: the role of male and female vibrational signals. Animal behaviour 2014, 90. Jg., 181-193. [CrossRef]
- Abt, I.; Jacquot, E. (2015). Wheat dwarf. In Virus Diseases of Tropical and Subtropical Crops, Tennant, P., Fermin, R., Eds.; Plant Protection Series; CAB International: Boston, MA, USA, 2015; pp. 27–41. [CrossRef]
- Vilbaste, J. Preliminary key for the identification of the nymphs of North European Homoptera, Cicadinea. II. Cicadelloidea. Annales Zoologici Fennici 19 1982, 1–20.
- Della Giustina, W. Homoptères Cicadellidae 3: compléments Faune de France 73. Fédération Française des Sociétés de Sciences Naturelles, INRA éditions 1989.
- Biedermann R.; Niedrighaus, R. Die Zikaden Deutschlands. In Bestimmungstafeln für alle Arten. Wissenschaftlich Akademischer Buchvertrieb-Fründ 2004.
- Kunz G., Nickel H.; Niedrighaus, R. In Photographic atlas of the planthoppers and leafhoppers of Germany. Wissenschaftlich Akademischer Buchvertrieb-Fründ 2011.
- Tishechkin, D. Yu. The variability of acoustic signals and some morphological characters in Psammotettix striatus (Homoptera, Cicadellidae) from Russia and adjacent countries. Зooлoгический журнал 1999, 78. Jg., Nr. 11.
- Biedermann, R.; Niedringhaus, R. The Plant- and Leafhoppers of Germany: Identification Key to all Species. Wabv Fründ, 2009.
- Heady, S. E.; Nault, L. R.; Shambaugh, G. F.; Fairchild, L. Acoustic and Mating Behavior of Dalbulus Leaf hoppers (Homoptera: Cicadellidae). Annals of the Entomological Society of America 1986, 79. Jg., Nr. 4, 727-736. [CrossRef]
- Gillham, M. C. Variation in acoustic signals within and among leafhopper species of the genus Alebra (Homoptera, Cicadellidae). Biological Journal of the Linnean Society 1992, 45. Jg., Nr. 1, 1-15. [CrossRef]
- Tishechkin, D. Yu. Vibrational communication in Aphrodinae leafhoppers (Deltocephalinae auct.; Homoptera: Cicadellidae) and related groups with notes on classification of higher taxa. Russian Entomological Journal 2000, 9. Jg., Nr. 1, 1-66.
- Percy, D. M.; Boyd, E. A.; Hoddle, M. S. Observations of acoustic signaling in three sharpshooters: Homalodisca vitripennis, Homalodisca liturata, and Graphocephala atropunctata (Hemiptera: Cicadellidae). Annals of the Entomological Society of America 2008, 101. Jg., Nr. 1, 253-259. [CrossRef]
- Tishechkin, D. Yu. The use of bioacoustic characters for distinguishing between cryptic species in insects: Potentials, restrictions, and prospects. Entomological Review 2014, 94. Jg., 289-309. [CrossRef]
- Derlink, M.; Abt, I.; Mabon, M.; Julian, C.; Virant-Doberlet, M.; Jacquot, E. Mating behaviour of Psammotettix alienus Dahlbom (Hemiptera: Cicadellidae). Insect Science 2018, 25. Jg., Nr. 1, 148-160. [CrossRef]
- Schlick-Steiner, B. C.; Steiner, F. M.; Seifert, B.; Stauffer, C.; Christian, E.; Crozier, R. H. Integrative Taxonomy: A Multisource Approach to Exploring Biodiversity. Annual review of entomology 2010, 55. Jg., 421-438. [CrossRef]
- Bluemel, J. K.; Derlink, M.; Pavlovcic, P.; Russio, I.-R. M.; Andrew King, R.; Corbett, E.; Sherrard-Smith, E.; Blejec, A.; Wilson, M. R.; Stewart, A. J. A.; Symondson, W. O. C.; Virant-Doberlet, M. Integrating vibrational signals, mitochondrial DNA and morphology for species determination in the genus Aphrodes (Hemiptera: Cicadellidae). Systematic Entomology 2014, 39. Jg., Nr. 2, 304-324. [CrossRef]
- Kamitani, S. (2011). DNA Barcodes of Japanese Leafhoppers. ESAKIA 2011, 50, 81–88. [CrossRef]
- Gwiazdowski, R. A.; Foottit, R. G.; Maw, H. E. L.; Hebert, P. D. N. The Hemiptera (Insecta) of Canada: Constructing a Reference Library of DNA Barcodes. PLoS One 2015, 10. Jg., Nr. 4, S. e0125635. [CrossRef]
- Abt, I.; Derlink, M.; Mabon, R.; Virant-Doberlet, M.; Jacquot, E. Integrating multiple criteria for the characterization of Psammotettix populations in European cereal fields. Bulletin of entomological research 2018, 108. Jg., Nr. 2, 185-202. [CrossRef]
- Guglielmino, A.; Virla, E.G. Postembryonic development and biology of Psammotettix alienus (Dahlbom) (Homoptera, Cicadellidae) under laboratory conditions. Bollettino di Zoologia, Agraria e di Bachicoltura 1997, 29. Jg., 65-80.
- Manurung, B. Untersuchungen zur Biologie und Ökologie der Zwergzikade Psammotettix alienus Dahlb. (Auchenorrhyncha) und zu ihrer Bedeutung als Vektor des Wheat dwarf virus (Weizenverzwergungs-Virus, WDV). Ph.D. Dissertation, Martin–Luther-Universität Halle-Wittenberg, 2002.
- Schiemenz, H.; Emmrich, R.; Witsack, W. Beiträge zur Insektenfauna Ost- deutschlands: Homoptera – Auchenorrhyncha (Cicadina) (Insecta) Teil IV: Unterfamilie Deltocephalinae. Faunistische Abhandlungen 1996, 20. Jg., 153-258.
- Alla, S.; Moreau, J. P.; Frerot, B. Effects of the aphid Rhopalosiphum padi on the leafhopper Psammotettix alienus under laboratory conditions. Entomologia Experimentalis et Applicata 2001, 98. Jg., Nr. 2, 203-209. [CrossRef]
- Van Nieuwenhove, G. A.; Frías, E. A.; Virla, E. G. Effects of temperature on the development, performance and fitness of the corn leafhopperDalbulus maidis (DeLong) (Hemiptera: Cicadellidae): implications on its distribution under climate change. Agricultural and Forest Entomology 2016, 18. Jg., Nr. 1, 1-10. [CrossRef]
- Schiemenz, H. Die Zikadenfauna mitteleuropäischer Trockenrasen (Homoptera, Auchenorrhyncha). Entomologische Abhandlungen Staatliches Museum für Tierkunde in Dresden 1969, 36. Jg., 201-280.
- Ghodoum Parizipour M.H.; Behjatnia S.A.A.; Afsharifar A.; Izadpanah K. Natural hosts and efficiency of leafhopper vector in transmission of Wheat dwarf virus. Journal of Plant Pathology 2016, 483-492. [CrossRef]
- Lindblad, M.; Sigvald, R. Temporal spread of wheat dwarf virus and mature plant resistance in winter wheat. Crop protection 2004, 23. Jg., Nr. 3, 229-234. [CrossRef]
- Backus, E. A. Sensory systems and behaviours which mediate hemipteran plant-feeding: A taxonomic overview. Journal of Insect Physiology 1988, 34. Jg., Nr. 3, 151-165. [CrossRef]
- Zhao, L.; Dai, W.; Zhang, C.; Zhang, Y. Morphological characterization of the mouthparts of the vector leafhopper Psammotettix striatus (L.) (Hemiptera: Cicadellidae). Micron 2010, 41. Jg., Nr. 7, 754-759. [CrossRef]
- Hewer A.; Becker A.; van Bel A.J.E. An aphid’s Odyssey –the cortical quest for the vascular bundle. Journal of Experimental Biology 2011, 214. Jg., Nr. 22, 3868-3879. [CrossRef]
- Storey, H. H. Transmission studies of maize streak disease. Annals of applied biology 1928, 15. Jg., Nr. 1, 1-25. [CrossRef]
- Wang, Y.; Dang, M.; Hou, H.; Mei, Y.; Qian, Y.; Zhou, X. Identification of an RNA silencing suppressor encoded by a mastrevirus. Journal of General Virology 2014, 95. Jg., Nr. 9, 2082-2088. [CrossRef]
- Reynaud, B.; Peterschmitt, M. A study of the mode of transmission of maize streak virus by Cicadulina mbila using an enzyme-linked immunosorbent assay. Annals of applied biology 1992, 121. Jg., Nr. 1, 85-94. [CrossRef]
- Tholt, G.; Samu, F.; Kiss, B. Feeding behaviour of a virus-vector leafhopper on host and non-host plants characterised by electrical penetration graphs. Entomologia Experimentalis et Applicata 2015, 155. Jg., Nr. 2, 123-136. [CrossRef]
- Hogenhout, S. A.; Ammar, E.-D.; Whitfield, A. E.; Redinbaugh, M. G. Insect Vector Interactions with Persistently Transmitted Viruses. Annu. Rev. Phytopathol. 2008, 46. Jg., 327-359. [CrossRef]
- Brault, V.; Uzest, M.; Monsion, B.; Jacquot, E.; Blanc, S. Aphids as transport devices for plant viruses. Comptes rendus biologies 2010, 333. Jg., Nr. 6-7, 524-538. [CrossRef]
- Yazdkhasti, E.; Hopkins, R. J.; Kvarnheden, A. Reservoirs of plant virus disease: Occurrence of wheat dwarf virus and barley/cereal yellow dwarf viruses in Sweden. Plant Pathology 2021, 70. Jg., Nr. 7, 1552-1561. [CrossRef]
- Nickel H.; Remane R. Artenliste der Zikaden Deutschands, (Checklist of the planthoppers and leafhoppers of Germany, with notes on food plants, diet width, life cycles, geographic range and conservation status). Beiträge zur Zikadenkunde 2002, 5. Jg., 27-64.
- Ossiannilsson, F. The Auchenorrhyncha (Homoptera) of Fennoscandia and Denmark. Part 2: The Families Cicadidae, Cercopidae, Membracidae, and Cicadellidae (excl. Deltocephalinae). Scandinavian Science Press 1981, 7. Jg., Nr. 2, 223-593. [CrossRef]
- Koppányi, T. A lucernásban kalakuló Cicadinea együttes évszakos és állományok korával járó változásának vizsgálata. A study into the seasonal and crop age related changes of Cicadinea assemblages in alfalfa.). Debreceni Agrártudományi Egyetem Tudományos Közleményei 1976, 18. Jg., 27-60.
- Drobnjakovic, T.; Peric, P.; Marcic, D.; Picciau, L.; Alma, A.; Mitrovic, J.; Duduk, B.; Bertaccini, A. Leafhoppers and cixiids in phytoplasma-infected carrot fields: Species composition and potential phytoplasma vectors. Pesticidi i Fitomedicina 2010, 25(4), 311–318. [CrossRef]
- Kiss B.; Redei D.; Koczor S. Occurrence and feeding of hemipterans on common ragweed (Ambrosia artemisiifolia) in Hungary. Bulletin of Insectology 2008, 61. Jg., Nr. 1, 195-196.
- Riedle-Bauer M.; Tiefenbrunner W.; Otreba J.; Hanak K.; Schildberger B.; Regner F. Epidemiological observations on Bois Noir in Austrian vineyards. Mitteilungen Klosterneuburg 2006, 56:177–181.
- Landi, L.; Isidoro, N.; Riolo, P. Natural Phytoplasma Infection of Four Phloem-Feeding Auchenorrhyncha Across Vineyard Agroecosystems in Central-Eastern Italy. Journal of Economic Entomology 2013, 106. Jg., Nr. 2, 604-613. [CrossRef]
- Tholt, G.; Kiss, B. Host range of Psammotettix alienus (Dahlbom). Növényvédelem 2011, 47. Jg., Nr. 6, 229-235.
- Stafford, C. A.; Walker, G. P. Characterization and correlation of DC electrical penetration graph waveforms with feeding behavior of beet leafhopper, Circulifer tenellus. Entomologia Experimentalis et Applicata 2009, 130. Jg., Nr. 2, 113-129. [CrossRef]
- Alvarez, A. E.; Tjallingii, W. F.; Garzo, E.; Vleeshouwers, V.; Dicke, M.; Vosman, B. Location of resistance factors in the leaves of potato and wild tuber-bearing Solanum species to the aphid Myzus persicae. Entomologia Experimentalis et Applicata 2006, 121. Jg., Nr. 2, 145-157. [CrossRef]
- Lei, H.; Lenteren, J. C.; Tjallingii, W. F. Analysis of resistance in tomato and sweet pepper against the greenhouse whitefly using electrically monitored and visually observed probing and feeding behaviour. Entomologia Experimentalis et Applicata 1999, 92. Jg., Nr. 3, 299-309. [CrossRef]
- Lei, H.; Van Lenteren, J. C.; Xu, R. M. Effects of plant tissue factors on the acceptance of four greenhouse vegetable host plants by the greenhouse whitefly: an Electrical Penetration Graph (EPG) study. European Journal of Entomology 2001, 98. Jg., Nr. 1, 31-36. [CrossRef]
- McLean, D. L.; Kinsey, M. G. A Technique for Electronically Recording Aphid Feeding and Salivation. Nature 1964, 202. Jg., Nr. 4939, 1358-1359. [CrossRef]
- Tjallingii, W. F. Electronic recording of penetration behaviour by aphids. Entomologia experimentalis et applicata 1978, 24. Jg., Nr. 3, 721-730. [CrossRef]
- Tjallingii, W. F. Electrical nature of recorded signals during stylet penetration by aphids. Entomologia experimentalis et applicata 1985, 38. Jg., Nr. 2, 177-186. [CrossRef]
- Tjallingii, W. F. Electrical recording of stylet penetration activities. In Aphids, their biology, natural enemies and control. Elsevier Science Publishers 1988. 95-108.
- Prado, E.; Tjallingii, W. F. Aphid activities during sieve element punctures. Entomologia experimentalis et applicata 1994, 72. Jg., Nr. 2, S. 157-165. [CrossRef]
- Harrewijn, P.; Kayser, H. Pymetrozine, a Fast-Acting and Selective Inhibitor of Aphid Feeding. In-situStudies with Electronic Monitoring of Feeding Behaviour. Pesticide Science 1997, 49. Jg., Nr. 2, S. 130-140. [CrossRef]
- Mesfin T.; Bosque-Pérez N.A. Feeding behavior of Cicadulina storeyi China (Homoptera: Cicadellidae) on maize varieties susceptible or resistant to maize streak virus. African entomology 1998, 6. Jg., Nr. 2, S. 185-191.
- Helden, M. van; Tjallingii, W. F. Experimental Design and Analysis in EPG Experiments with Emphasis on Plant Resistance Research. In Principles and applications of electronic monitoring and other techniques in the study of homopteran feeding behavior. Entomological Society of America 2000. 144. [CrossRef]
- Liu, X. D.; Zhai, B. P.; Zhang, X. X.; Zong, J. M. Impact of transgenic cotton plants on a non-target pest, Aphis gossypii Glover. Ecological Entomology 2005, 30. Jg., Nr. 3, 307-315. [CrossRef]
- Prado, E.; Fred Tjallingii, W. Behavioral Evidence for Local Reduction of Aphid-Induced Resistance. Journal of Insect Science 2007, 7. Jg., Nr. 1, 48. [CrossRef]
- Martin, B.; Fereres, A.; Tjallingii, W. F.; Collar, J. L. Intracellular ingestion and salivation by aphids may cause the acquisition and inoculation of non-persistently transmitted plant viruses. Journal of General Virology 1997, 78. Jg., Nr. 10, 2701-2705. [CrossRef]
- Sauge M.H.; Lambert P.; Pascal T. Co-localisation of host plant resistance QTLs affecting the performance and feeding behaviour of the aphid Myzus persicae in the peach tree. Heredity 2012, 108. Jg., Nr. 3, 292-301. [CrossRef]
- Giordanengo, P. EPG-Calc: a PHP-based script to calculate electrical penetration graph (EPG) parameters. Arthropod-Plant Interactions 2014, 8. Jg., 163-169. [CrossRef]
- Tjallingii, W. F.; Esch, TH. H. Fine structure of aphid stylet routes in plant tissues in correlation with EPG signals. Physiological entomology 1993, 18. Jg., Nr. 3, 317-328. [CrossRef]
- Khan, Z. R.; Saxena, R. C. Probing Behavior of Three Biotypes of Nilaparvata lugens (Homoptera: Delphacidae) on Different Resistant and Susceptible Rice Varieties. Journal of Economic Entomology 1988, 81. Jg., Nr. 5, 1338-1345. [CrossRef]
- Kimmins, F. M. Electrical penetration graphs from Nilaparvata lugens on resistant and susceptible rice varieties. Entomologia experimentalis et applicata 1989, 50. Jg., Nr. 1, 69-79. [CrossRef]
- Powell, K. S.; Gatehouse, J. A. Mechanism of mannose-binding snowdrop lectin for use against brown planthopper in rice. Rice Genetics III: (In 2 Parts) 1996. 753-758. [CrossRef]
- Seo, B. Y.; Kwon, Y.-H.; Jung, J. K.; Kim, G.-H. Electrical penetration graphic waveforms in relation to the actual positions of the stylet tips of Nilaparvata lugens in rice tissue. Journal of Asia-Pacific Entomology 2009, 12. Jg., Nr. 2, 89-95. [CrossRef]
- Calatayud, P.-A.; Seligmann, C. D.; Polania, M. A.; Bellotti, A. C. Influence of parasitism by encyrtid parasitoids on the feeding behaviour of the cassava mealybug Phenacoccus herreni. Entomologia Experimentalis et Applicata 2001, 98. Jg., Nr. 3, 271-278. [CrossRef]
- Harrewijn P.; Piron P.G.M.; Ponsen M.B. Evolution of vascular feeding in aphids: an electrophysiological study. Entomologia Experimentalis et Applicata 1998, 9. Jg., 29-34.
- Kingston K.B. Digestive and feeding physiology of grape phylloxera (Daktulosphaira vitifoliae Fitch), Ph.D. thesis, Australian National University, Canberra, 2007.
- Joost P.H.; Riley D.G. Imidacloprid effects on probing and settling behavior of Frankliniella fusca and Frankliniella occidentalis (Thysanoptera: Thripidae) in tomato. Journal of economic entomology 2005, 98. Jg., Nr. 5, 1622-1629. [CrossRef]
- Kindt, F.; Joosten, N. N.; Tjallingii, W. F. Electrical penetration graphs of thrips revised: Combining DC- and AC-EPG signals. Journal of Insect Physiology 2006, 52. Jg., Nr. 1, 1-10. [CrossRef]
- Walker, G. P.; Janssen, J. A. M. Electronic Recording of Whitefly (Homoptera: Aleyrodidae) Feeding and Oviposition Behavior. Principles and Applications of Electronic Monitoring and Other Techniques in the Study of Homopteran Feeding Behavior. In Principles and applications of electronic monitoring and other techniques in the study of Homopteran feeding behavior. Entomological Society of America 2000. 172. [CrossRef]
- Kimmins, F. M.; Bosque-Perez, N. A. Electrical penetration graphs from Cicadulina spp. and the inoculation of a persistent virus into maize. Proceedings of the 9th International Symposium on Insect-Plant Relationships 1996. 46–49. [CrossRef]
- Lett, J.-M.; Granier, M.; Grondin, M.; Turpin, P.; Molinaro, F.; Chiroleu, F.; Peterschmitt, M.; Reynaud, B. Electrical penetration graphs from Cicadulina mbila on maize, the fine structure of its stylet pathways and consequences for virus transmission efficiency. Entomologia Experimentalis et Applicata 2001, 101. Jg., Nr. 2, 93-109. [CrossRef]
- Miranda, M. P.; Fereres, A.; Appezzato-da-Gloria, B.; Lopes, J. R. S. Characterization of electrical penetration graphs of Bucephalogonia xanthophis, a vector of Xylella fastidiosain citrus. Entomologia Experimentalis et Applicata 2010, 134. Jg., Nr. 1, 35-49. [CrossRef]
- Tjallingii, W. F.; Esch, TH. H. Fine structure of aphid stylet routes in plant tissues in correlation with EPG signals. Physiological entomology 1993, 18. Jg., Nr. 3, 317-328. [CrossRef]
- Tjallingii, W. F. Salivary secretions by aphids interacting with proteins of phloem wound responses. Journal of experimental botany 2006, 57. Jg., Nr. 4, 739-745. [CrossRef]
- Wayadande, A. C. Leafhopper Probing Behavior Associated with Maize Chlorotic Dwarf Virus Transmission to Maize. Phytopathology 1993, 83. Jg., Nr. 5, 522-526. [CrossRef]
- Backus, E. A.; Holmes, W. J.; Schreiber, F.; Reardon, B. J.; Walker, G. P. Sharpshooter X Wave: Correlation of an Electrical Penetration Graph Waveform with Xylem Penetration Supports a Hypothesized Mechanism for Xylella fastidiosa Inoculation. Annals of the Entomological Society of America 2009, 102. Jg., Nr. 5, 847-867. [CrossRef]
- Pillon, O. Importance en Champagne de deux parasitoides de la cicadelle vectrice du nanisme du ble. In ANPP – 3rd International Conference on Pests in Agriculture, France, 7-9 December 1993.
- Samu, F.; Beleznai, O.; Tholt, G. A potential spider natural enemy against virus vector leafhoppers in agricultural mosaic landscapes – Corroborating ecological and behavioral evidence. Biological Control 2013, 67. Jg., Nr. 3, 390-396. [CrossRef]
- Kogan, M. Integrated Pest Management: Historical Perspectives and Contemporary Developments. Annual review of entomology 1998, 43. Jg., Nr. 1, S. 243-270. [CrossRef]
- Shepherd, D. N.; Martin, D. P.; van der Walt, E.; Dent, K.; Varsani, A.; Rybicki, E. P. Maize streak virus: an old and complex ‘emerging’ 62 pathogen. Molecular plant pathology 2010, 11. Jg., Nr. 1, 1-12. [CrossRef]
- Yazdkhasti, E. (2022). Epidemiology of wheat dwarf virus. Ph.D. Dissertation, Acta Universitatis Agriculturae Sueciae, 2022.
- Eweida, M.; Oxelfelt, P.; Tomenius, K. Concentration of virus and ultrastructural changes in oats at various stages of barley yellow dwarf virus infection. Annals of applied biology 1988, 112. Jg., Nr. 2, 313-321. [CrossRef]
- Aranda, M. A.; Freitas-Astúa, J. Ecology and diversity of plant viruses, and epidemiology of plant virus-induced diseases. Annals of Applied Biology 2017, 171. Jg., Nr. 1, 1-4. [CrossRef]
- Weibull, A.-C.; Östman, Ö. Species composition in agroecosystems: The effect of landscape, habitat, and farm management. Basic and Applied Ecology 2003, 4. Jg., Nr. 4, 349-361. [CrossRef]
- Ansari, M. S.; Moraiet, M. A.; Ahmad, S. Insecticides: Impact on the Environment and Human Health. Environmental deterioration and human health: natural and anthropogenic determinants 2014, 99-123. [CrossRef]
- Biondi, A.; Desneux, N.; Siscaro, G.; Zappalà, L. Using organic-certified rather than synthetic pesticides may not be safer for biological control agents: Selectivity and side effects of 14 pesticides on the predator Orius laevigatus. Chemosphere 2012, 87. Jg., Nr. 7, 803-812. [CrossRef]
- Dudley, N.; Attwood, S. J.; Goulson, D.; Jarvis, D.; Bharucha, Z. P. Pretty, J. How should conservationists respond to pesticides as a driver of biodiversity loss in agroecosystems? Biological Conservation 2017, 209. Jg., 449-453. [CrossRef]
- Guimarães-Cestaro, L.; Martins, M. F.; Martínez, L. C.; Alves, M. L. T. M. F.; Guidugli-Lazzarini, K. R.; Nocelli, R. C. F.; Malaspina, O.; Serrão, J. E.; Teixeira, É. W. Occurrence of virus, microsporidia, and pesticide residues in three species of stingless bees (Apidae: Meliponini) in the field. The Science of Nature 2020, 107. Jg., 1-14. [CrossRef]
- Staskawicz, B. J. Genetics of Plant-Pathogen Interactions Specifying Plant Disease Resistance. Plant Physiology 2001, 125. Jg., Nr. 1, 73-76. [CrossRef]
- Wang, H.; Hao, L.; Shung, C.-Y.; Sunter, G.; Bisaro, D. M. Adenosine Kinase Is Inactivated by Geminivirus AL2 and L2 Proteins. The Plant Cell 2003, 15. Jg., Nr. 12, 3020-3032. [CrossRef]
- Leke, W.N. Molecular Epidemiology of Begomoviruses that Infect Vegetable Crops in Southwestern Cameroon. Ph.D. Thesis, Acta Universitatis Agriculturae Sueciae, Uppsala, Sweden, 2010.
- Wulff, B. B. H.; Moscou, M. J. Strategies for transferring resistance into wheat: from wide crosses to GM cassettes. Frontiers in Plant Science 2014, 5. Jg., 692. [CrossRef]
- Kanyuka, K.; Lovell, D. J.; Mitrofanova, O. P.; Hammond-Kosack, K.; Adams, M. J. A controlled environment test for resistance to Soil-borne cereal mosaic virus (SBCMV) and its use to determine the mode of inheritance of resistance in wheat cv. Cadenza and for screening Triticum monococcum genotypes for sources of SBCMV resistance. Plant Pathology 2004, 53. Jg., Nr. 2, 154-160. [CrossRef]
- Ward E.; Kanyuka K.; Motteram J.; Kornyukhin D.; Adams M.J. The use of conventional and quantitative real-time PCR assays for Polymyxa graminis to examine host plant resistance, inoculum levels and intraspecific variation. New Phytologist 2005, 875-885. [CrossRef]
- Hall, M. D.; Brown-Guedira, G.; Klatt, A.; Fritz, A. K. Genetic analysis of resistance to soil-borne wheat mosaic virus derived from Aegilops tauschii. Euphytica 2009, 169. Jg., 169-176. [CrossRef]
- Zaharieva, M.; Monneveux, P.; Henry, M.; Rivoal, R.; Valkoun, J.; Nachit, M. M. Evaluation of a Collection of Wild Wheat Relative Aegilops Geniculata Roth and Identification of Potential Sources for Useful Traits. Developments in Plant Breeding 2001. 739–746. [CrossRef]
- Pfrieme, A.-K.; Ruckwied, B.; Habekuß, A.; Will, T.; Stahl, A.; Pillen, K.; Ordon, F. Identification and Validation of Quantitative Trait Loci for Wheat Dwarf Virus Resistance in Wheat (Triticum spp.). Frontiers in Plant Science 2022, 13. Jg., 828639. [CrossRef]
- Pfrieme, A. K.; Stahl, A.; Pillen, K.; Will, T. Comparison of two different experimental environments for resistance screenings for leafhopper-transmitted wheat dwarf virus in wheat. Journal of Plant Diseases and Protection 2023, submitted.
- Nygren, J.; Shad, N.; Kvarnheden, A.; Westerbergh, A. Variation in Susceptibility to Wheat dwarf virus among Wild and Domesticated Wheat. PloS one 2015, 10. Jg., Nr. 4, e0121580. ttps://doi.org/10.1371/journal.pone.0121580.
- Riedel, C.; Habekuß, A.; Schliephake, E.; Niks, R.; Broer, I.; Ordon, F. Pyramiding of Ryd2 and Ryd3 conferring tolerance to a German isolate of Barley yellow dwarf virus-PAV (BYDV-PAV-ASL-1) leads to quantitative resistance against this isolate. Theoretical and applied genetics 2011, 123. Jg., Nr. 1, 69-76. [CrossRef]
- Scheurer, K. S.; Friedt, W.; Huth, W.; Waugh, R.; Ordon, F. QTL analysis of tolerance to a German strain of BYDV-PAV in barley (Hordeum vulgare L.). Theoretical and Applied Genetics 2001, 103. Jg., 1074-1083. [CrossRef]
- Clark, M. F.; Adams, A. N. Characteristics of the Microplate Method of Enzyme-Linked Immunosorbent Assay for the Detection of Plant Viruses. Journal of general virology 1977, 34. Jg., Nr. 3, 475-483. [CrossRef]
- Hayes, R. J.; Macdonald, H.; Coutts, R. H. A.; Buck, K. W. Agroinfection of Triticum aestivum with Cloned DNA of Wheat Dwarf Virus. Journal of general virology 1988, 69. Jg., Nr. 4, 891-896. [CrossRef]
- Boulton, M. I. Agrobacterium-Mediated Transfer of Geminiviruses to Plant Tissues. Plant Gene Transfer and Expression Protocols 1995, 77-93. [CrossRef]
- Boulton, M. I. Construction of Infectious Clones for DNA Viruses: Mastreviruses. Plant Virology Protocols: From Viral Sequence to Protein Function 2008, 503-523. [CrossRef]
- Bukvayová, N.; Henselová, M.; Vajcíková, V.; Kormanová, T. Occurrence of dwarf virus of winter wheat and barley in several regions of Slovakiaduring the growing seasons 2001-2004. Plant Soil and Environment 2006, 52. Jg., Nr. 9, 392. [CrossRef]
- Rabenstein, F.; Sukhacheva, E.; Habekuß, A.; Schubert, J. Differentiation of Wheat dwarf virus isolates from wheat, triticale, and barley by means of a monoclonal antibody. In Proceedings of the X Conference on Viral Diseases of Gramineae in Europe, Louvain-la-Neuve, Belgium, 2005. 60.
- Kundu, J. K.; Gadiou, S.; Červená, G. Discrimination and genetic diversity of Wheat dwarf virus in the Czech Republic. Virus genes 2009, 38. Jg., 468-474. [CrossRef]
- Glais, L.; Jacquot, E. Detection and Characterization of Viral Species/Subspecies Using Isothermal Recombinase Polymerase Amplification (RPA) Assays. Plant Pathology: Techniques and Protocols 2015, 207-225. [CrossRef]
- Thresh, J. M. Cropping Practices and Virus Spread. Annual Review of Phytopathology 1982, 20. Jg., Nr. 1, 193-216. [CrossRef]
- Benkovics, A. H.; Vida, G.; Nelson, D.; Veisz, O.; Bedford, I.; Silhavy, D.; Boulton, M. I. Partial resistance to Wheat dwarf virus in winter wheat cultivars. Plant pathology 2010, 59. Jg., Nr. 6, 1144-1151. [CrossRef]
- Schneider, A.; Molnár-Láng, M. Detection of the 1RS chromosome arm in Martonvásár wheat genotypes containing 1Bl.1Rs or 1Al.1Rs translocations using SSR and STS markers. Acta Agronomica Hungarica 2009, 57. Jg., Nr. 4, 409-416. [CrossRef]
- Schlegel R. Current list of wheats with rye and alien introgression. From http://www.ryegene-map.de/rye-introgression/html/entry_m.html. Retrieved 22/05/2020.
- Ruckwied, B. Sources of resistance and identification of QTLs for Wheat dwarf virus (WDV) resistance in wheat (Triticum spp.) and wild relatives. Julius Kühn-Institut, Bundesforschungsinstitut für Kulturpflanzen, 2022.
- Kumar, R. V. Plant Antiviral Immunity Against Geminiviruses and Viral Counter-Defense for Survival. Frontiers in Microbiology 2019, 10. Jg., 1460. [CrossRef]
- Sharaf, A.; Nuc, P.; Ripl, J.; Alquicer, G.; Ibrahim, E.; Wang, X.; Maruthi, M. N.; Kundu, J. K. Transcriptome Dynamics in Triticum aestivum Genotypes Associated with Resistance against the Wheat Dwarf Virus. Viruses 2023, 15. Jg., Nr. 3, 689. [CrossRef]
- Schoelz, J. E.; Harries, P. A.; Nelson, R. S. Intracellular Transport of Plant Viruses: Finding the Door out of the Cell. Molecular plant 2011, 4. Jg., Nr. 5, 813-831. [CrossRef]
- Frederickson Matika, D. E.; Loake, G. J. Redox Regulation in Plant Immune Function. Antioxidants & redox signaling 2014, 21. Jg., Nr. 9, 1373-1388. [CrossRef]
- Mayer, M. P. Recruitment of Hsp70 chaperones: a crucial part of viral survival strategies. Reviews of physiology, biochemistry and pharmacology 2005, 1-46. [CrossRef]
- Nagy, P. D.; Pogany, J. The dependence of viral RNA replication on co-opted host factors. Nature Reviews Microbiology 2012, 10. Jg., Nr. 2, 137-149. [CrossRef]
- Yuan, W.; Jiang, T.; Du, K.; Chen, H.; Cao, Y.; Xie, J.; Li, M.; Carr, J. P.; Wu, B.; Fan, Z.; Zhou, T. Maize phenylalanine ammonia-lyases contribute to resistance toSugarcane mosaic virusinfection, most likely through positive regulation of salicylic acid accumulation. Molecular plant pathology 2019, 20. Jg., Nr. 10, 1365-1378. [CrossRef]
- Zhu, F.; Yuan, S.; Wang, S.-D.; Xi, D.-H.; Lin, H.-H. The higher expression levels of dehydroascorbate reductase and glutathione reductase in salicylic acid-deficient plants may contribute to their alleviated symptom infected with RNA viruses. Plant signaling & behavior 2011, 6. Jg., Nr. 9, 1402-1404. [CrossRef]
- Selway, J.W. Antiviral activity of flavones and flavans. Progress in clinical and biological research 1986, 213. Jg., 521-536.
- Hrmova, M.; Hussain, S. S. Plant Transcription Factors Involved in Drought and Associated Stresses. International Journal of molecular sciences 2021, 22. Jg., Nr. 11, 5662. [CrossRef]
- Pandey, A.; Khan, Mohd. K.; Hamurcu, M.; Brestic, M.; Topal, A.; Gezgin, S. Insight into the Root Transcriptome of a Boron-Tolerant Triticum zhukovskyi Genotype Grown under Boron Toxicity. Agronomy 2022, 12. Jg., Nr. 10, S. 2421. [CrossRef]
- Li Y, Liu K, Tong G, Xi C, Liu J, Zhao H, Wang Y, Ren D, Han S. MPK3/MPK6-mediated phosphorylation of ERF72 positively regulates resistance to Botrytis cinerea through directly and indirectly activating the transcription of camalexin biosynthesis enzymes. Journal of Experimental Botany 2022, 73. Jg., Nr. 1, 413-428. [CrossRef]
- Liu, Y.; Jin, W.; Wang, L.; Wang, X. Replication-associated proteins encoded by Wheat dwarf virus act as RNA silencing suppressors. Virus research 2014, 190. Jg., 34-39. [CrossRef]
- Chellappan P.; Vanitharani R.; Pita J.; Fauquet C.M. Short interfering RNA accumulation correlates with host recovery in DNA virus-infected hosts, and gene silencing targets specific viral sequences. Journal of virology 2004, 78. Jg., Nr. 14, 7465-7477. [CrossRef]
- Li, F.; Wang, Y. & Zhou, X. SGS3 Cooperates with RDR6 in Triggering Geminivirus-Induced Gene Silencing and in Suppressing Geminivirus Infection in Nicotiana Benthamiana. Viruses 2017, 9. Jg., Nr. 9, 247. [CrossRef]
- Ding, S.-W.; Li, H.; Lu, R.; Li, F.; Li, W.-X. RNA silencing: a conserved antiviral immunity of plants and animals. Virus research 2004, 102. Jg., Nr. 1, 109-115. [CrossRef]
- Shen, Q.; Bao, M.; Zhou, X. A plant kinase plays roles in defense response against geminivirus by phosphorylation of a viral pathogenesis protein. Plant signaling & behavior 2012, 7. Jg., Nr. 7, S. 888-892. [CrossRef]
- Zhang, D.; Bai, G.; Hunger, R. M.; Bockus, W. W.; Yu, J.; Carver, B. F.; Brown-Guedira, G. Association Study of Resistance to Soilborne wheat mosaic virus in U.S. Winter Wheat. Phytopathology 2011, 101. Jg., Nr. 11, 1322-1329. [CrossRef]
- Liu, S.; Yang, X.; Zhang, D.; Bai, G.; Chao, S.; Bockus, W. Genome-wide association analysis identified SNPs closely linked to a gene resistant to Soil-borne wheat mosaic virus. Theoretical and Applied Genetics 2014, 127. Jg., 1039-1047. [CrossRef]
- Hourcade, D.; Bogard, M.; Bonnefoy, M.; Savignard, F.; Mohamadi, F.; Lafarge, S.; Du Cheyron, P.; Mangel, N.; Cohan, J. P. Genome-wide association analysis of resistance to wheat spindle streak mosaic virus in bread wheat. Plant Pathology 2019, 68. Jg., Nr. 3, 609-616. [CrossRef]
- Pfrieme, A. K.; Ruckwied, B; Habekuß, A.; Will, T.; Stahl, A.; Pillen, K.; Ordon, F. Corrigendum: Identification and validation of Quantitative Trait Loci for Wheat dwarf virus resistance in wheat (Triticum spp.). Frontiers in Plant Science, 2022, 13. Jg., 1088938. [CrossRef]
- Buerstmayr, M.; Buerstmayr, H. Two major quantitative trait loci control wheat dwarf virus resistance in four related winter wheat populations. Theoretical and Applied Genetics 2023, 136. Jg., Nr. 5, 103. [CrossRef]
- Li, H.; Conner, R. L.; Liu, Z.; Li, Y.; Chen, Y.; Zhou, Y.; Duan, X.; Shen, T.; Chen, Q.; Graf, R. J.; Jia, X. Characterization of Wheat-Triticale Lines Resistant to Powdery Mildew, Stem Rust, Stripe Rust, Wheat Curl Mite, and Limitation on Spread of WSMV. Plant disease 2007, 91. Jg., Nr. 4, 368-374. [CrossRef]
- Parlevliet, J.E. Durability of resistance against fungal, bacterial and viral pathogens; present situation. Euphytica 2002, 124. Jg., 147-156. [CrossRef]
- Palloix, A.; Ayme, V.; Moury, B. Durability of plant major resistance genes to pathogens depends on the genetic background, experimental evidence and consequences for breeding strategies. New Phytologist 2009, 183. Jg., Nr. 1, 190-199. [CrossRef]
- Brown J.K.; Idris A.M.; Ostrow K.M.; Goldberg N.; French R.; Stenger D.C. Genetic and phenotypic variation of the Pepper golden mosaic virus Complex. Phytopathology 2005, 95. Jg., Nr. 10, 1217-1224. [CrossRef]
- Riedel, C. Pyramidisierung von QTL im Hinblick auf eine Verbesserung der Barley yellow dwarf virus Toleranz der Gerste und genetische Analyse der Toleranz gegenüber Wheat dwarf virus. Ph.D. dissertation, university of Rostock, 2013.
- Ayalew, H.; Tsang, P.W.; Chu, C.; Wang, J.; Liu, S.; Chen, C.; et al. Comparison of TaqMan, KASP and rhAmp SNP genotyping platforms in hexaploid wheat. PloS one 2019, 14. Jg., Nr. 5, e0217222. [CrossRef]
- Karlstedt, F. (2020). Identification and mapping of QTL for resistance against Zymoseptoria tritici in the winter wheat accession HTRI1410 (Triticum aestivum L. subsp. Spelta) Julius Kühn-Institut, Bundesforschungsinstitut für Kulturpflanzen, 2020.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





