Submitted:
05 September 2023
Posted:
07 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistical analysis
3. Results
3.1. Demographic characteristics
3.2. Surgical characteristics
3.3. Early results
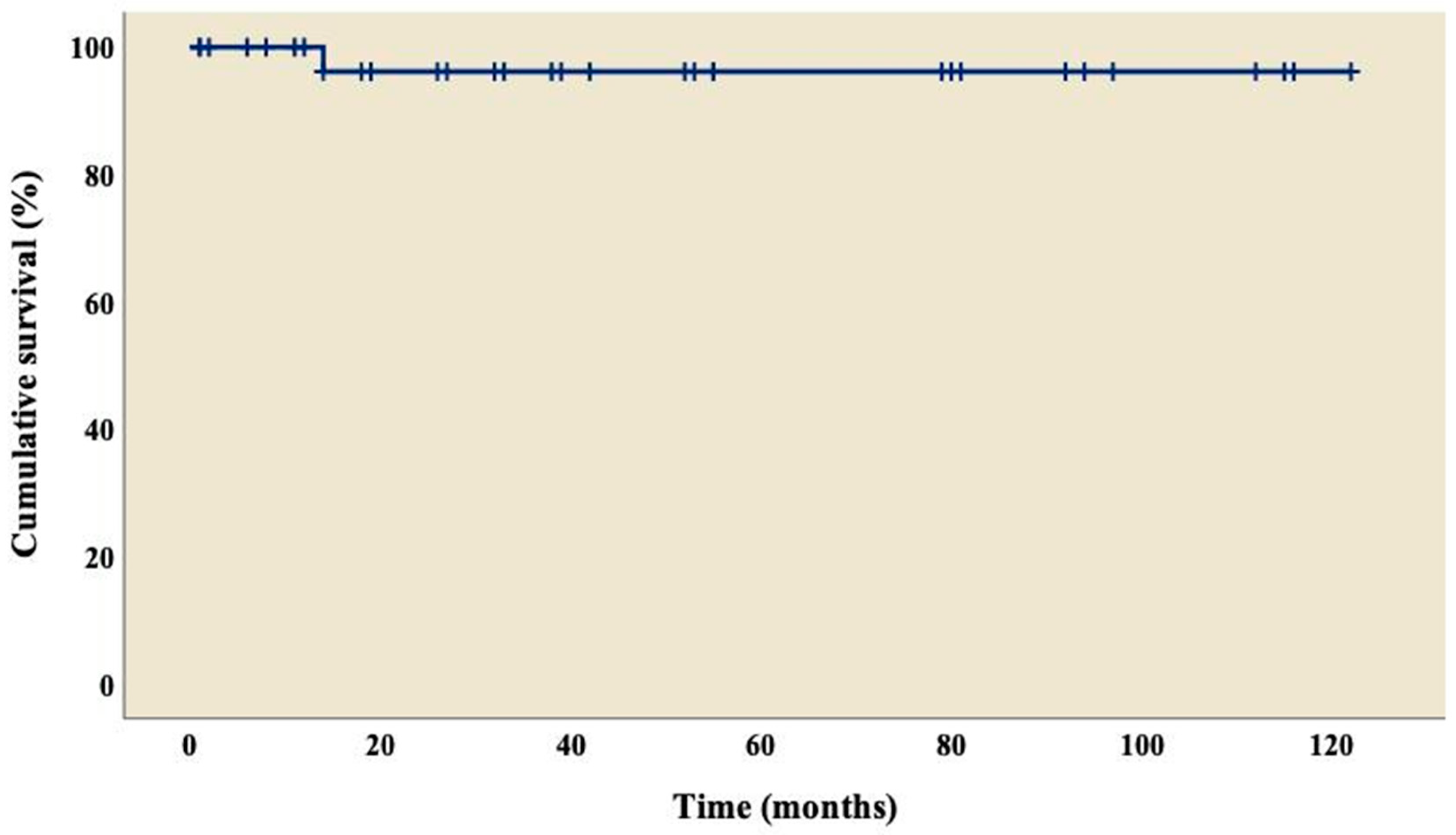
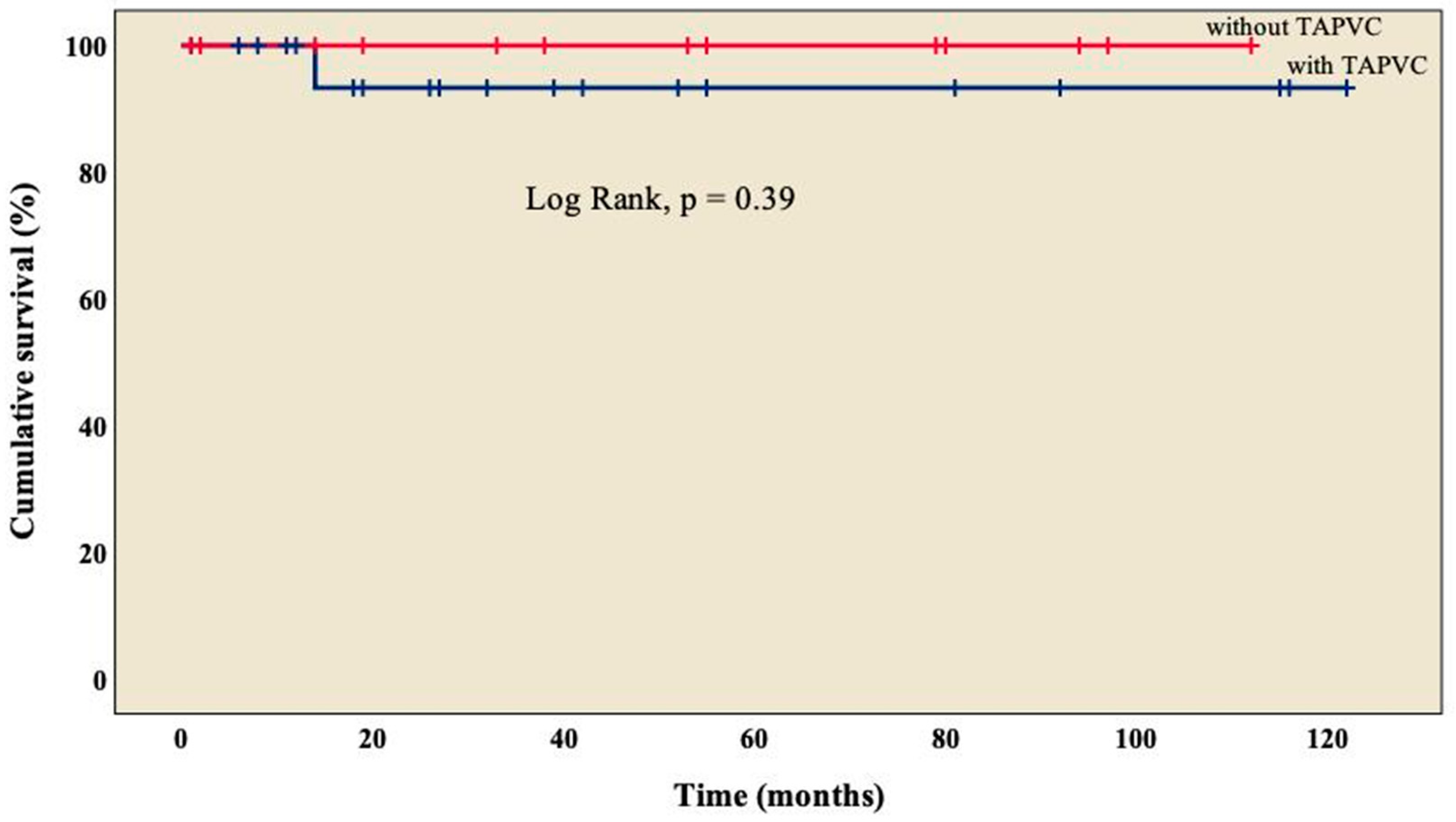
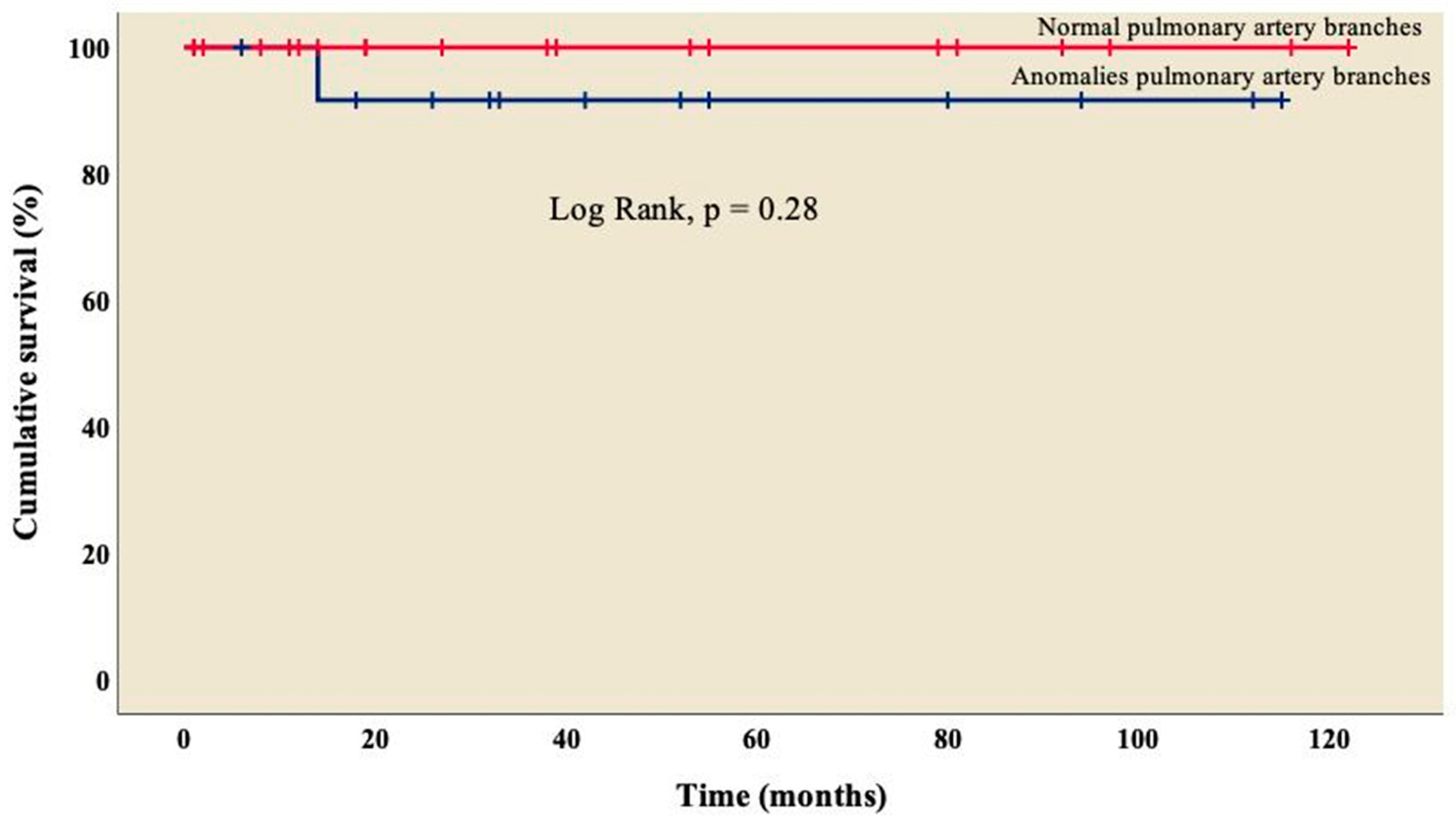
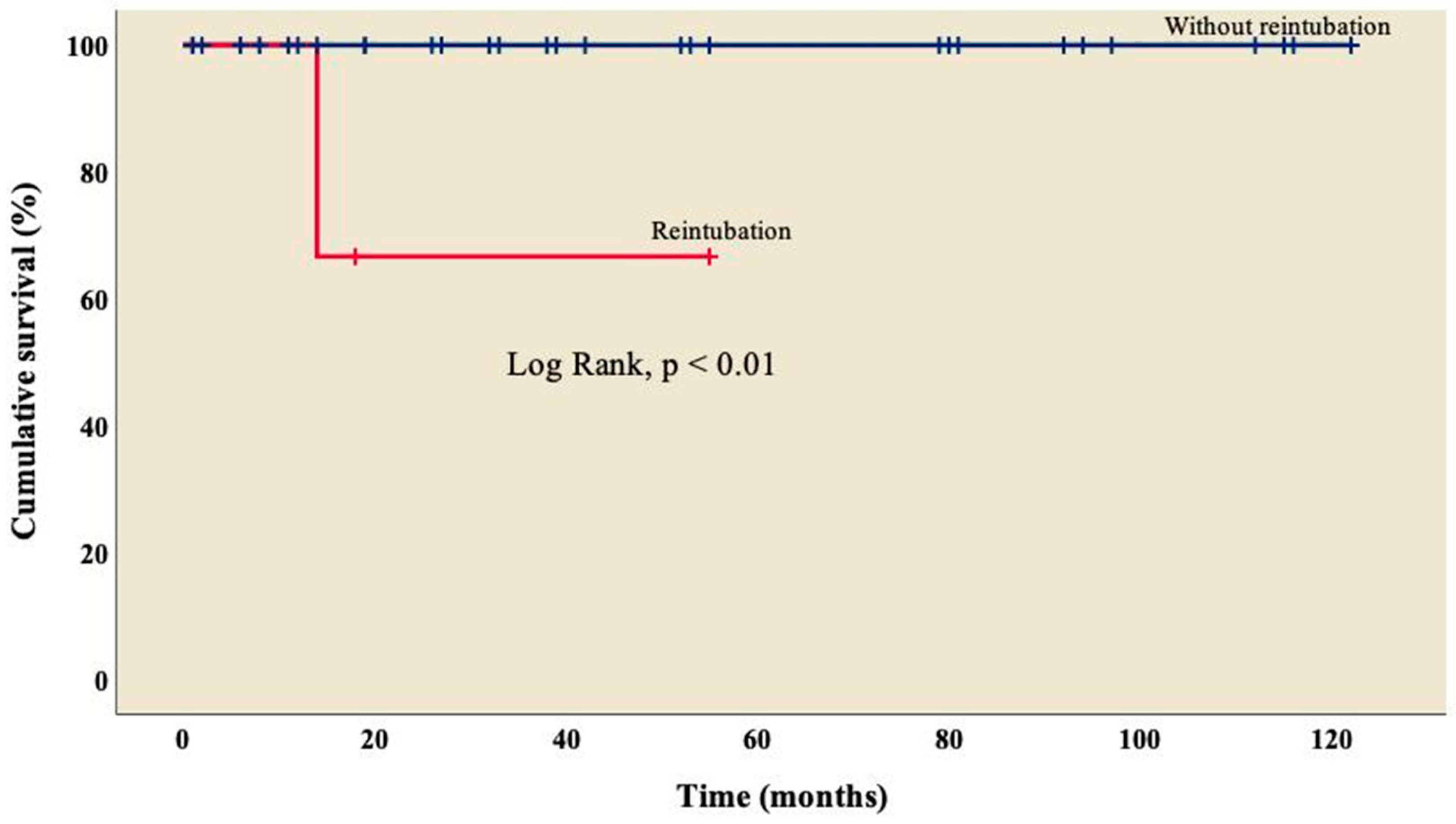
3.4. Follow-up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García-Flores, E.; Calderón-Colmenero, J.; Borgonio-Cuadra, VM, Sandoval, J. P.; García-Montes, J.A.; Cazarín-Santos, B.G.; Miranda-Duarte, A.; Gamboa-Domínguez, A.; Rodríguez-Pérez, J.M, Pérez-Hernández, N. Epigenetic Evaluation of the TBX20 Gene and Environmental Risk Factors in Mexican Paediatric Patients with Congenital Septal Defects. Cells 2023, 12, 586. [Google Scholar] [PubMed]
- Monroy-Muñoz, I.E.; Pérez-Hernández, N.; Vargas-Alarcón, G.; Ortiz-San Juan, G.; Buendía-Hernández, A.; Calderón-Colmenero, J.; Ramírez-Marroquín, S.; Cervantes-Salazar, J.L.; Curi-Curi, P.; Martínez-Rodríguez, N.; et al. Cambiando el paradigma en las cardiopatías congénitas: de la anatomía a la etiología molecular [Changing the paradigm of congenital heart disease: from the anatomy to the molecular etiology]. Gac Med Mex 2013, 149, 212–219. [Google Scholar]
- Icardo, J.M.; Rincón, J.M.G.; Ros, M.Á. Malformaciones cardíacas, heterotaxia y lateralidad. Rev Esp Cardiol 2002, 55, 962–974. [Google Scholar] [CrossRef]
- Calderón-Colmenero, J.; Cervantes-Salazar, J.L.; Curi-Curi, P.J.; Ramírez-Marroquín, S. Problemática de las cardiopatías congénitas en México: Propuesta de regionalización [Congenital heart disease in Mexico. Regionalization proposal]. Arch Cardiol Mex 2010, 80, 133–140. [Google Scholar] [PubMed]
- Cervantes-Salazar, J.; Calderón-Colmenero, J.; Ramírez-Marroquín, S.; Palacios-Macedo, A.; Cerdán, A.B.; Alarcón, A.V.; Curi-Curi, P.; de la Llata Romero, M.; Orellana, J.E.; Palacios, J.G. El Registro Mexicano de Cirugía Cardíaca Pediátrica. Primer informe. Evid Med Invest Salud 2014, 7, 56–62. [Google Scholar]
- Madrigal, S.; Bonilla, C.; Sánchez, E. Heterotaxia: Situs ambiguo, síndrome de Ivermark o síndrome de asplenia-poliesplenia. Rev Clín Esc Med UCR-HSJD 2019, 9, 70–76. [Google Scholar] [CrossRef]
- Yan, S.; Jianpeng, W.; Xin, Q.; Minghui, Z.; Li, Z.; Hao, W. Right atrial isomerism in children older than 3 years. Springer Plus 2016, 5, 1–6. [Google Scholar] [CrossRef]
- Kim, S.-J. Heterotaxy syndrome. Korean Circ J 2011, 41, 227–232. [Google Scholar] [CrossRef]
- Loomba, R.S.; Hlavacek, A.M.; Spicer, D.E.; Anderson, R.H. Isomerism or heterotaxy: which term leads to better understanding? Cardiol Young 2015, 25, 1037–1043. [Google Scholar] [CrossRef]
- Ramsdell, A.F. Left-right asymmetry and congenital cardiac defects: getting to the heart of the matter in vertebrate left-right axis determination. Dev Biol 2005, 288, 1–20. [Google Scholar] [CrossRef]
- Blum, M.; Feistel, K, Thumberger, T. ; Schweickert, A. The evolution and conservation of left-right patterning mechanisms. Development 2014, 141, 1603–1613. [Google Scholar] [CrossRef]
- Zhu, L.; Belmont, J.W.; Ware, S.M. Genetics of human heterotaxias. Eur J Hum Genet 2006, 14, 17–25. [Google Scholar] [CrossRef]
- Carro Hevia, A. , Santamarta Liébana, E., & Martín Fernández, M. Síndrome de heterotaxia. Cardiocore, 2011, 46, 23–26. [Google Scholar]
- Friedberg, M.K.; Silverman, N.H.; Moon-Grady, A.J.; Tong, E.; Nourse, J.; Sorenson, B.; Lee, J.; Hornberger, L.K. Prenatal detection of congenital heart disease. J Pediatr 2009, 155, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Pepes, S.; Zidere, V.; Allan, L. Prenatal diagnosis of left atrial isomerism. Heart 2009, 95, 1974–1977. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.W.; Geva,T. ; Shirali, G.S.; Frommelt, P.C.; Humes, R.A.; Brook, M.M..; Pignatelli, R.H.; Rychik, J.Task Force of the Pediatric Council of the American Society of Echocardiography; Paediatric Council of the American Society of Echocardiography. Guidelines and standards for performance of a pediatric echocardiogram: A report from the Task Force of the Paediatric Council of the American Society of Echocardiography. J Am Soc Echocardiogr. 2006, 19, 1413–1430. [Google Scholar]
- Ortega-Zhindón, D.B.; Flores-Sarria, I.P.; Minakata-Quiróga, M.A.; Angulo-Cruzado, S.T.; Romero-Montalvo, L.A.; Cervantes-Salazar, J.L. Atrial isomerism: A multidisciplinary perspective [Isomorfismo cardiaco: Una perspectiva multidisciplinaria]. Arch Cardiol Mex 2021, 91, 470–479. [Google Scholar]
- Carrillo-Álvarez, A.; Martínez-Gutiérrez, A.; Salvat-Germán, F. Reconocimiento del niño con riesgo de parada cardiorrespiratoria. An Pediatr (Barc) 2006, 65, 147–153. [Google Scholar] [CrossRef]
- Faraoni, D. Definition of postoperative bleeding in children undergoing cardiac surgery with cardiopulmonary bypass: One size doesn't fit all. J Thorac Cardiovasc Surg 2018, 155, 2125–2126. [Google Scholar] [CrossRef]
- Chen, W.; Ma, L.; Cui, H.; Yang, S.; Xia, Y.; Zou, M.; Chen, X. Early-and middle-term surgical outcomes in patients with heterotaxy syndrome. Cardiology 2016, 133, 141–146. [Google Scholar] [CrossRef]
- Jacobs, J.P.; Pasquali, S.K.; Morales, D.L.; Jacobs, M.L.; Mavroudis, C.; Chai, P.J.; Tchervenkov, C.I.; Lacour-Gayet, F.G.; Walters III, H.; Quintessenza, J.A. Heterotaxy: lessons learned about patterns of practice and outcomes from the congenital heart surgery database of the society of thoracic surgeons. World J Pediatr Congenit Heart Surg 2011, 2, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.; Abu-Sulaiman, R.; McCrindle, B.W.; Smallhorn, J.F.; Williams, W.G.; Freedom, R.M. Management and outcomes of right atrial isomerism: a 26-year experience. J Am Coll Cardiol 1998, 31, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Baban, A.; Cantarutti, N.; Adorisio, R.; Lombardi, R.; Calcagni, G.; Mortari, E.P.; Dallapiccola, B.; Marino, B.; Iorio, F.S.; Carsetti, R. Long-term survival and phenotypic spectrum in heterotaxy syndrome: A 25-year follow-up experience. Int J Cardiol 2018, 268, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Alongi, A.M.; Kirklin, J.K.; Deng, L.; Padilla, L.; Pavnica, J.; Romp, R.L.; Mauchley, D.C.; Cleveland, D.C.; Dabal, R. Surgical management of heterotaxy syndrome: current challenges and opportunities. World J Pediatr Congenit Heart Surg 2020, 11, 166–176. [Google Scholar] [CrossRef]
- Lafuente, M.; Villalba, C.; Mouratian, M.; Villa, A.; Sciegatamtsac A; García, P. Presentación clínica y evolución del isomerismo derecho. Rev Argent Cardiol 2015, 83, 400–405. [Google Scholar] [CrossRef]
- Loomba, R.S.; Nijhawan, K.; Anderson, R. Impact of era, type of isomerism, and ventricular morphology on survival in heterotaxy: implications for therapeutic management. World J Pediatr Congenit Heart Surg 2016, 7, 54–62. [Google Scholar] [CrossRef]
- Bhaskar, J.; Galati, J.C.; Brooks, P.; Oppido, G.; Konstantinov, I.E.; Brizard, C.P.; d'Udekem, Y. Survival into adulthood of patients with atrial isomerism undergoing cardiac surgery. J Thorac Cardiovasc Surg 2015, 149, 1509–1514. [Google Scholar] [CrossRef]
- Vigneswaran, T.V.; Jones, C.B.; Zidere, V.; Charakida, M.; Miller, O.I.; Simpson, J.M.; Sharland, G. Effect of prenatal laterality disturbance and its accompanying anomalies on survival. Am J Cardiol 2018, 122, 663–671. [Google Scholar] [CrossRef]
- Alsoufi, B.; McCracken, C.; Schlosser, B.; Sachdeva, R.; Well, A.; Kogon, B.; Border, W.; Kanter, K. Outcomes of multistage palliation of infants with functional single ventricle and heterotaxy syndrome. J Thorac Cardiovasc Surg 2016, 151, 1369–1377e2. [Google Scholar] [CrossRef]
- Gilljam, T.; McCrindle, B.W.; Smallhorn, J.F.; Williams, W.G.; Freedom, R.M. Outcomes of left atrial isomerism over a 28-year period at a single institution. J Am Coll Cardiol 2000, 36, 908–916. [Google Scholar] [CrossRef]
- Banka, P.; Adar, A.; Schaetzle, B.; Sleeper, L.A.; Emani, S.; Geva, T. Changes in prognosis of heterotaxy syndrome over time. Pediatrics. 2020, 146, e20193345. [Google Scholar] [CrossRef]
- Acherman, R.J.; Evans, W.N. Persistent right umbilical vein in isomerism. Prenat Diagn 2019, 39, 1220–1224. [Google Scholar] [CrossRef]
- Knowles, M.R.; Zariwala, M.; Leigh, M. Primary Ciliary Dyskinesia. Clin Chest Med 2016, 37, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.P.; Omran, H.; Leigh, M.W.; Dell, S.; Morgan, L.; Molina, P.L.; Robinson, B.V.; Minnix, S.L.; Olbrich, H, Severin, T. ; et al. Congenital heart disease and other heterotaxic defects in a large cohort of patients with primary ciliary dyskinesia. Circulation 2007, 115, 2814–2821. [Google Scholar] [CrossRef] [PubMed]
- Nakhleh, N.; Francis, R.; Giese, R.A.; Tian, X.; Li, Y.; Zariwala, M.A.; Yagi, H.; Khalifa, O.; Kureshi, S.; Chatterjee, B.; et al. High prevalence of respiratory ciliary dysfunction in congenital heart disease patients with heterotaxy. Circulation 2012, 125, 2232–2242. [Google Scholar] [CrossRef] [PubMed]
- Swisher, M.; Jonas, R.; Tian, X.; Lee, E.S.; Lo, C.W.; Leatherbury, L. Increased postoperative and respiratory complications in patients with congenital heart disease associated with heterotaxy. J Thorac Cardiovasc Surg 2011, 141, 637–644, 644.e1-3. [Google Scholar] [CrossRef]
- McGovern, E.; Kelleher, E.; Potts, J.E.; O'Brien, J.; Walsh, K.; Nolke, L.; McMahon, C.J. Predictors of poor outcome among children with heterotaxy syndrome: a retrospective review. Open Heart 2016, 3, e000328. [Google Scholar] [CrossRef]
- Hosseinpour, A.R.; González-Calle, A.; Adsuar-Gómez, A. ¿Qué queremos decir con el término univentricular? Cir Cardiov 2015, 22, 193–194. [Google Scholar] [CrossRef]
- Serrano, F.; Caffarena, J.M. Cirugía del corazón univentricular en segundo estadio: técnicas y resultados. Cir Cardiov 2008, 15, 351–360. [Google Scholar] [CrossRef]
| Characteristics | Total n = 38 |
|---|---|
| Sex, n (%) | |
| Male | 22 (57.9) |
| Female | 16 (42.1) |
| Age (years), median (IQR) | 4 (2 - 9.2) |
| Weight (kg), median (IQR) | 14.3 (9.8 - 22.1) |
| Height (cm), mean (±SD) | 102 (29.1) |
| Previous surgery, n (%) | |
| 0 | 25 (65.8) |
| 1 | 12 (31.6) |
| 2 | 1 (2.6) |
| RACHS-1, n (%) | |
| 2 | 5 (13.2) |
| 3 | 33 (86.8) |
| Cardiac intrathoracic position, n (%) | |
| Levocardia | 21 (55.3) |
| Dextrocardia | 17 (44.7) |
| Atrioventricular valve regurgitation, n (%) | |
| None | 8 (21.1) |
| Mild | 27 (71) |
| Moderate | 1 (2.6) |
| Severe | 2 (5.3) |
| Anomalous pulmonary venous connection, n (%) | |
| Partial | 2 (5.3) |
| Total | 13 (34.3) |
| Pulmonary venous connection, n (%) | |
| Right atrium | 15 (39.5) |
| Left atrium | 20 (52.6) |
| Both atriums | 3 (7.9) |
| Pulmonary artery branches, n (%) | |
| Normal | 15 (39.5) |
| Stenosis | 23 (60.5) |
| Characteristic, n (%) | Total n = 38 |
|---|---|
| Diagnosis | |
| AV canal | 24 (63.2) |
| Pulmonary stenosis | 21 (55.3) |
| PDA | 17 (44.7) |
| Pulmonary atresia | 15 (39.5) |
| TAPVC | 13 (34.3) |
| DORV | 9 (23.7) |
| Hypoplastic left ventricle | 6 (15.9) |
| PAPVC | 2 (5.3) |
| Surgery | |
| MBTS | 13 (34.3) |
| MBTS + TAPVC repair | 10 (26.3) |
| TCPC with an extracardiac conduit fenestrated without CPB | 6 (15.9) |
| BCPC without CPB | 3 (7.9) |
| MBTS + thrombectomy of pulmonary artery + TAPVC repair | 1 (2.6) |
| BCPC with CPB + TAPVC repair | 1 (2.6) |
| BCPC with CPB + RPA angioplasty + PAPVC repair | 1 (2.6) |
| TCPC with an extracardiac conduit fenestrated + PAPVC repair | 1 (2.6) |
| Mechanical AV valve replacement + MBTS + TAPVC repair | 1 (2.6) |
| BCPC takedown + MBTS | 1 (2.6) |
| Characteristic | Total n = 38 |
|---|---|
| Surgery with CPB, n (%) | 15 (39.5) |
| CPB (min), median (IQR) | 89 (67-107) |
| Aortic cross-clamp (min), median (IQR) | 55 (22-83) |
| Mechanical ventilation in PICU, n (%) | 34 (89.5) |
| Mechanical ventilation time (h); median (IQR) | 22 (10-126) |
| Reintubation, n (%) | 3 (7.9) |
| Days in PICU, n (%) | |
| < 1 day | 2 (5.3) |
| 1 - 7 days | 27 (71) |
| 8 - 15 days | 8 (21.1) |
| > 15 days | 1 (2.6) |
| Variable | OR | CI 95% | p | |
|---|---|---|---|---|
| Lower | Upper | |||
| Previous surgery | 1.33 | 0.19 | 9.18 | 0.77 |
| Anomalous pulmonary venous connection | 0.81 | 0.65 | 0.97 | 0.08 |
| Anomalies pulmonary artery branches | 0.38 | 0.05 | 2.61 | 0.31 |
| Atrioventricular valve regurgitation | 0.33 | 0.04 | 2.45 | 0.26 |
| Atrioventricular valve replacement | 0.11 | 0.04 | 0.27 | < 0.01 |
| Preoperative intubation | 4.83 | 0.61 | 38.38 | 0.11 |
| Preoperative inotrope use | 6.66 | 0.77 | 57.06 | 0.05 |
| Transoperative inotrope use | 1.19 | 1.02 | 1.39 | 0.25 |
| Postoperative inotrope use | 1.17 | 1.02 | 1.36 | 0.87 |
| Inhaled nitric oxide use | 10.33 | 1.04 | 102.08 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





