Submitted:
01 September 2023
Posted:
06 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
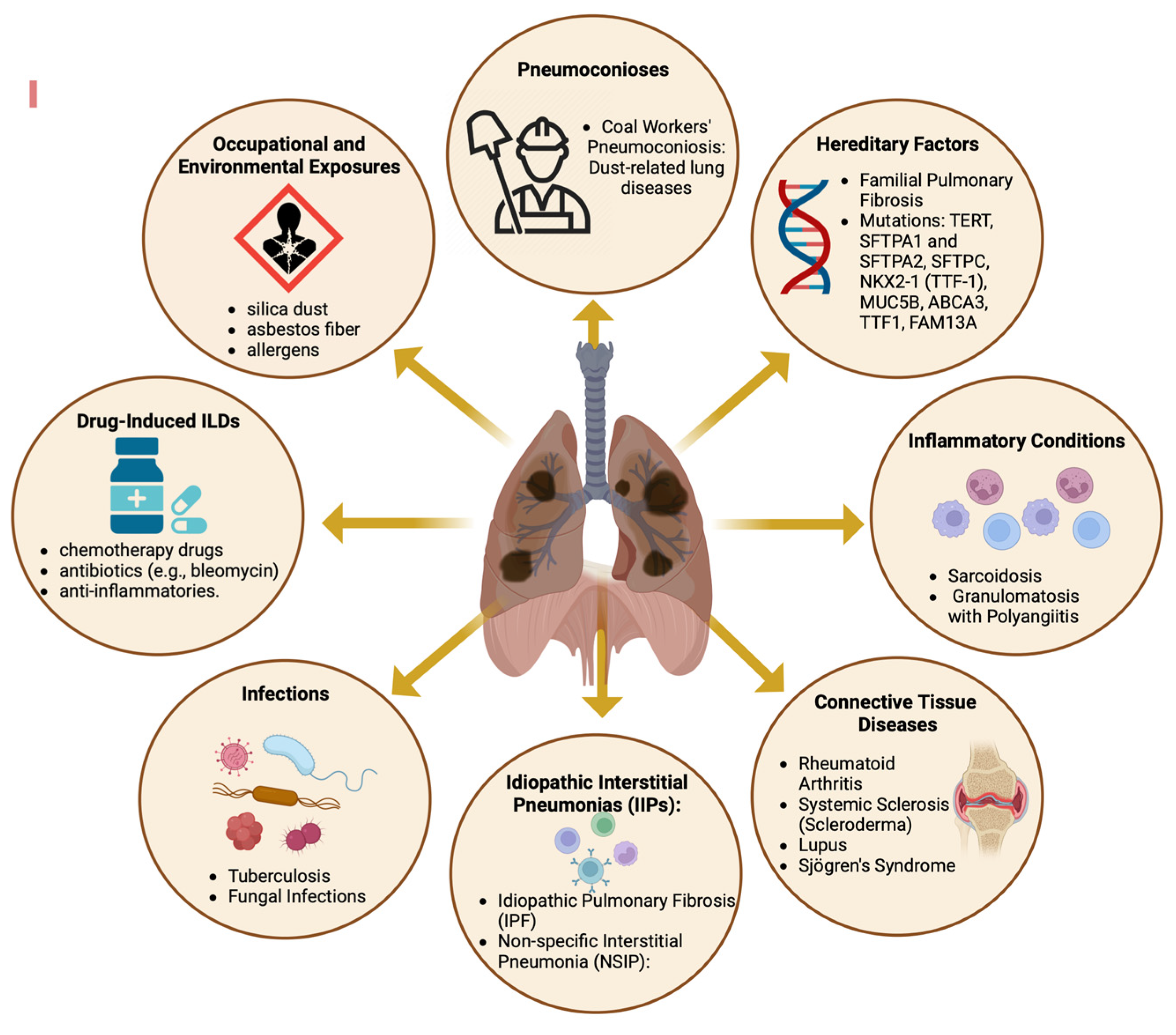
2. Epidemiology
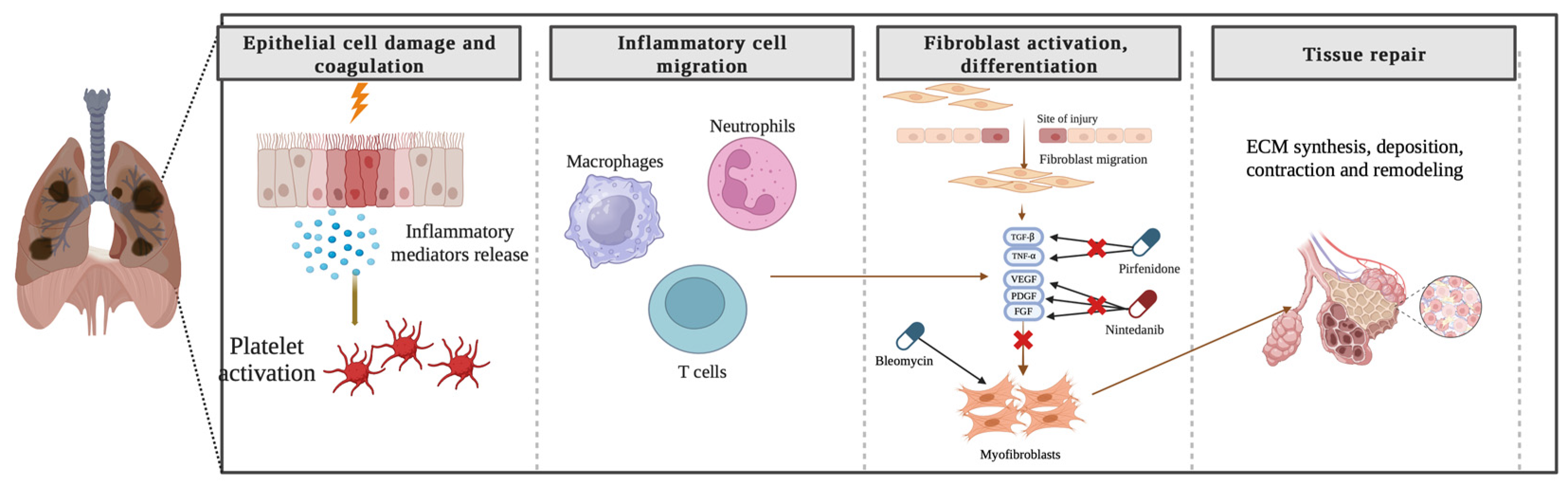
3. Pathogenesis of lung fibrosis
4. CTHRC1—structural characteristics and expression
5. CTHRC1 plays a role in several signaling pathways
6. CTHRC1 as a biomarker for RA
7. Common pathogenic pathways in RA-ILD and pulmonary fibrosis
8. Role of CTHRC1 in pulmonary fibrosis
9. CTHRC1: A Potential Prognostic Marker for Severe Lung Complications in COVID-19 Patients
10. Targeting CTHRC1: Therapeutic options for lung fibrosis
11. Conclusion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tomassetti, S.; Ravaglia, C.; Poletti, V. Diffuse parenchymal lung disease. Eur. Respir. Rev. 2017, 26, 170004. [Google Scholar] [CrossRef] [PubMed]
- Wijsenbeek, M.; Suzuki, A.; Maher, T.M. Interstitial lung diseases. Lancet 2022, 400, 769–786. [Google Scholar] [CrossRef] [PubMed]
- Podolanczuk, A.J.; Wong, A.W.; Saito, S.; Lasky, J.A.; Ryerson, C.J.; Eickelberg, O. Update in Interstitial Lung Disease 2020. Am. J. Respir. Crit. Care Med. 2021, 203, 1343–1352. [Google Scholar] [CrossRef]
- Moss, B.J.; Ryter, S.W.; Rosas, I.O. Pathogenic Mechanisms Underlying Idiopathic Pulmonary Fibrosis. Annu. Rev. Pathol. Mech. Dis. 2022, 17, 515–546. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef] [PubMed]
- Rockey, D.C.; Bell, P.D.; Hill, J.A. Fibrosis — A Common Pathway to Organ Injury and Failure. New Engl. J. Med. 2015, 372, 1138–1149. [Google Scholar] [CrossRef]
- Wolters, P.J.; Blackwell, T.S.; Eickelberg, O.; E Loyd, J.; Kaminski, N.; Jenkins, G.; Maher, T.M.; Molina-Molina, M.; Noble, P.W.; Raghu, G.; et al. Time for a change: is idiopathic pulmonary fibrosis still idiopathic and only fibrotic? Lancet Respir. Med. 2018, 6, 154–160. [Google Scholar] [CrossRef]
- Wells, A.U.; Brown, K.K.; Flaherty, K.R.; Kolb, M.; Thannickal, V.J. What’s in a name? That which we call IPF, by any other name would act the same. Eur. Respir. J. 2018, 51, 1800692. [Google Scholar] [CrossRef]
- Gochuico, B.R.; Avila, N.A.; Chow, C.K.; Novero, L.J.; Wu, H.-P.; Ren, P.; MacDonald, S.D.; Travis, W.D.; Stylianou, M.P.; Rosas, I.O. Progressive Preclinical Interstitial Lung Disease in Rheumatoid Arthritis. Arch. Intern. Med. 2008, 168, 159–166. [Google Scholar] [CrossRef]
- Brown, K.K. Roger S. Mitchell Lecture. Rheumatoid Lung Disease. Proc. Am. Thorac. Soc. 2007, 4, 443–448. [Google Scholar] [CrossRef]
- Lurje, I.; Gaisa, N.T.; Weiskirchen, R.; Tacke, F. Mechanisms of organ fibrosis: Emerging concepts and implications for novel treatment strategies. Mol. Asp. Med. 2023, 92, 101191. [Google Scholar] [CrossRef]
- Bauer, Y.; Tedrow, J.; de Bernard, S.; Birker-Robaczewska, M.; Gibson, K.F.; Guardela, B.J.; Hess, P.; Klenk, A.; Lindell, K.O.; Poirey, S.; et al. A Novel Genomic Signature with Translational Significance for Human Idiopathic Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2015, 52, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Togo, S.; Kadoya, K.; Tulafu, M.; Namba, Y.; Iwai, M.; Watanabe, J.; Nagahama, K.; Okabe, T.; Hidayat, M.; et al. Pirfenidone attenuates lung fibrotic fibroblast responses to transforming growth factor-β1. Respir. Res. 2019, 20, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Melms, J.C.; Biermann, J.; Huang, H.; Wang, Y.; Nair, A.; Tagore, S.; Katsyv, I.; Rendeiro, A.F.; Amin, A.D.; Schapiro, D.; et al. A molecular single-cell lung atlas of lethal COVID-19. Nature 2021, 595, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Tsukui, T.; Sun, K.-H.; Wetter, J.B.; Wilson-Kanamori, J.R.; Hazelwood, L.A.; Henderson, N.C.; Adams, T.S.; Schupp, J.C.; Poli, S.D.; Rosas, I.O.; et al. Collagen-producing lung cell atlas identifies multiple subsets with distinct localization and relevance to fibrosis. Nat. Commun. 2020, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Duchemann, B.; Annesi-Maesano, I.; de Naurois, C.J.; Sanyal, S.; Brillet, P.-Y.; Brauner, M.; Kambouchner, M.; Huynh, S.; Naccache, J.M.; Borie, R.; et al. Prevalence and incidence of interstitial lung diseases in a multi-ethnic county of Greater Paris. Eur. Respir. J. 2017, 50, 1602419. [Google Scholar] [CrossRef]
- Nannini, C.; Ryu, J.H.; Matteson, E.L. Lung disease in rheumatoid arthritis. Curr. Opin. Rheumatol. 2008, 20, 340–346. [Google Scholar] [CrossRef]
- Ascherman, D.P. Interstitial Lung Disease in Rheumatoid Arthritis. Curr. Rheumatol. Rep. 2010, 12, 363–369. [Google Scholar] [CrossRef]
- Kadura, S.; Raghu, G. Rheumatoid arthritis-interstitial lung disease: manifestations and current concepts in pathogenesis and management. Eur. Respir. Rev. 2021, 30, 210011. [Google Scholar] [CrossRef]
- Distler, J.H.W.; Györfi, A.-H.; Ramanujam, M.; Whitfield, M.L.; Königshoff, M.; Lafyatis, R. Shared and distinct mechanisms of fibrosis. Nat. Rev. Rheumatol. 2019, 15, 705–730. [Google Scholar] [CrossRef]
- Meyer, K.C. Pulmonary fibrosis, part I: Epidemiology, pathogenesis, and diagnosis. Expert Rev. Respir. Med. 2017, 11, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Adegunsoye, A.; Vij, R.; Noth, I. Integrating Genomics Into Management of Fibrotic Interstitial Lung Disease. Chest 2019, 155, 1026–1040. [Google Scholar] [CrossRef] [PubMed]
- Juge, P.-A.; Lee, J.S.; Ebstein, E.; Furukawa, H.; Dobrinskikh, E.; Gazal, S.; Kannengiesser, C.; Ottaviani, S.; Oka, S.; Tohma, S.; et al. MUC5B Promoter Variant and Rheumatoid Arthritis with Interstitial Lung Disease. N. Engl. J. Med. 2018, 379, 2209–2219. [Google Scholar] [CrossRef] [PubMed]
- Ley, B.; Torgerson, D.G.; Oldham, J.M.; Adegunsoye, A.; Liu, S.; Li, J.; Elicker, B.M.; Henry, T.S.; Golden, J.A.; Jones, K.D.; et al. Rare Protein-Altering Telomere-related Gene Variants in Patients with Chronic Hypersensitivity Pneumonitis. Am. J. Respir. Crit. Care Med. 2019, 200, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, J.; Grutters, J.C.; Arkema, E.V.; Saketkoo, L.A.; Moller, D.R.; Müller-Quernheim, J. Sarcoidosis. Nat. Rev. Dis. Prim. 2019, 5, 45. [Google Scholar] [CrossRef]
- Pyagay, P.; Heroult, M.; Wang, Q.; Lehnert, W.; Belden, J.; Liaw, L.; Friesel, R.E.; Lindner, V. Collagen Triple Helix Repeat Containing 1, a Novel Secreted Protein in Injured and Diseased Arteries, Inhibits Collagen Expression and Promotes Cell Migration. Circ. Res. 2005, 96, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Stohn, J.P.; Perreault, N.G.; Wang, Q.; Liaw, L.; Lindner, V. Cthrc1, a Novel Circulating Hormone Regulating Metabolism. PLOS ONE 2012, 7, e47142. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Nishimura, O.; Misaki, K.; Nishita, M.; Minami, Y.; Yonemura, S.; Tarui, H.; Sasaki, H. Cthrc1 Selectively Activates the Planar Cell Polarity Pathway of Wnt Signaling by Stabilizing the Wnt-Receptor Complex. Dev. Cell 2008, 15, 23–36. [Google Scholar] [CrossRef]
- Smith, J.D.; Bryant, S.R.; Couper, L.L.; Vary, C.P.H.; Gotwals, P.J.; Koteliansky, V.E.; Lindner, V. Soluble Transforming Growth Factor-β Type II Receptor Inhibits Negative Remodeling, Fibroblast Transdifferentiation, and Intimal Lesion Formation But Not Endothelial Growth. Circ. Res. 1999, 84, 1212–1222. [Google Scholar] [CrossRef]
- Bryant, S.R.; Bjercke, R.J.; Erichsen, D.A.; Rege, A.; Lindner, V. Vascular Remodeling in Response to Altered Blood Flow Is Mediated by Fibroblast Growth Factor-2. Circ. Res. 1999, 84, 323–328. [Google Scholar] [CrossRef]
- Durmus, T.; LeClair, R.J.; Park, K.-S.; Terzic, A.; Yoon, J.K.; Lindner, V. Expression analysis of the novel gene collagen triple helix repeat containing-1 (Cthrc1). Gene Expr. Patterns 2006, 6, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lee, M.; Yu, G.; Lee, H.; Han, X.; Kim, D. CTHRC1 activates pro-tumorigenic signaling pathways in hepatocellular carcinoma. Oncotarget 2017, 8, 105238–105250. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Ren, F.; Xu, M.; Tan, C.; Weng, W.; Huang, Z.; Sheng, W.; Huang, D. CTHRC1 overexpression predicts poor survival and enhances epithelial-mesenchymal transition in colorectal cancer. Cancer Med. 2018, 7, 5643–5654. [Google Scholar] [CrossRef]
- Liu, J.; Li, W.; Liu, S.; Zheng, X.; Shi, L.; Zhang, W.; Yang, H. Knockdown of Collagen Triple Helix Repeat Containing 1 (CTHRC1) Inhibits Epithelial-Mesenchymal Transition and Cellular Migration in Glioblastoma Cells. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2017, 25, 225–232. [Google Scholar] [CrossRef]
- Jiang, N.; Cui, Y.; Liu, J.; Zhu, X.; Wu, H.; Yang, Z.; Ke, Z. Multidimensional Roles of Collagen Triple Helix Repeat Containing 1 (CTHRC1) in Malignant Cancers. J. Cancer 2016, 7, 2213–2220. [Google Scholar] [CrossRef]
- Myngbay, A.; Bexeitov, Y.; Adilbayeva, A.; Assylbekov, Z.; Yevstratenko, B.P.; Aitzhanova, R.M.; Matkarimov, B.; Adarichev, V.A.; Kunz, J. CTHRC1: A New Candidate Biomarker for Improved Rheumatoid Arthritis Diagnosis. Front. Immunol. 2019, 10, 1353. [Google Scholar] [CrossRef]
- Myngbay, A.; Manarbek, L.; Ludbrook, S.; Kunz, J. The Role of Collagen Triple Helix Repeat-Containing 1 Protein (CTHRC1) in Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22, 2426. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yang, Q.; Sun, H. Collagen triple helix repeat containing-1: a novel biomarker associated with disease activity in Systemic lupus erythematosus. Lupus 2018, 27, 2076–2085. [Google Scholar] [CrossRef]
- Spector, I.; Zilberstein, Y.; Lavy, A.; Genin, O.; Barzilai-Tutsch, H.; Bodanovsky, A.; Halevy, O.; Pines, M. The Involvement of Collagen Triple Helix Repeat Containing 1 in Muscular Dystrophies. Am. J. Pathol. 2013, 182, 905–916. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Ma, M.; Jiang, S.; Zhang, X.; Zhang, Y.; Yang, X.; Xu, C.; Tian, G.; Li, Q.; et al. Autocrine CTHRC1 activates hepatic stellate cells and promotes liver fibrosis by activating TGF-β signaling. EBioMedicine 2019, 40, 43–55. [Google Scholar] [CrossRef]
- Binks, A.P.; Beyer, M.; Miller, R.; LeClair, R.J. Cthrc1 lowers pulmonary collagen associated with bleomycin-induced fibrosis and protects lung function. Physiol. Rep. 2017, 5, e13115. [Google Scholar] [CrossRef]
- El-Mallah, R.; Farrag, D.A.; Safwat, N.A. Potential value of collagen triple helix repeat containing-1 (CTHRC1) in systemic lupus erythematosus (SLE) patients with arthritis detected clinically or by musculoskeletal ultrasound. Egypt. Rheumatol. 2023, 45, 197–202. [Google Scholar] [CrossRef]
- Feng, Y.; Hu, J.; Liu, F.; Shang, Y. Collagen Triple Helix Repeat Containing 1 Deficiency Protects Against Airway Remodeling and Inflammation in Asthma Models In Vivo and In Vitro. Inflammation 2023, 46, 925–940. [Google Scholar] [CrossRef] [PubMed]
- LeClair, R.; Lindner, V. The Role of Collagen Triple Helix Repeat Containing 1 in Injured Arteries, Collagen Expression, and Transforming Growth Factor β Signaling. Trends Cardiovasc. Med. 2007, 17, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Zheng, J.-H.; Xia, Z.-H.; Qian, J.; Deng, C.-L.; Yang, S.-L. CTHRC1 promotes wound repair by increasing M2 macrophages via regulating the TGF-β and notch pathways. BioMedicine 2019, 113, 108594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yin, Z.; Zhang, F.; Cao, K.; Sun, H. CTHRC1 mediates IL-1β-induced apoptosis in chondrocytes via JNK1/2 signaling. Int. J. Mol. Med. 2018, 41, 2270–2278. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-R.; Stohn, J.P.; Wang, Q.; Nagano, K.; Baron, R.; Bouxsein, M.L.; Rosen, C.J.; Adarichev, V.A.; Lindner, V. Inhibition of osteoclast differentiation and collagen antibody-induced arthritis by CTHRC1. Bone 2017, 97, 153–167. [Google Scholar] [CrossRef]
- Stohn, J.P.; Wang, Q.; Siviski, M.E.; Kennedy, K.; Jin, Y.-R.; Kacer, D.; DeMambro, V.; Liaw, L.; Vary, C.P.; Rosen, C.J.; et al. Cthrc1 controls adipose tissue formation, body composition, and physical activity. Obesity 2015, 23, 1633–1642. [Google Scholar] [CrossRef]
- Adarichev, V.A.; Nesterovitch, A.B.; Bárdos, T.; Biesczat, D.; Chandrasekaran, R.; Vermes, C.; Mikecz, K.; Finnegan, A.; Glant, T.T. Sex effect on clinical and immunologic quantitative trait loci in a murine model of rheumatoid arthritis. Arthritis Rheum. 2003, 48, 1708–1720. [Google Scholar] [CrossRef]
- Kudryavtseva, E.; Forde, T.S.; Pucker, A.D.; Adarichev, V.A. Wnt signaling genes of murine chromosome 15 are involved in sex-affected pathways of inflammatory arthritis. Arthritis Rheum. 2011, 64, 1057–1068. [Google Scholar] [CrossRef]
- Hu, T.; Liu, Y.; Tan, L.; Huang, J.; Yu, J.; Wu, Y.; Pei, Z.; Zhang, X.; Li, J.; Song, L.; et al. Value of serum collagen triple helix repeat containing-1(CTHRC1) and 14-3-3η protein compared to anti-CCP antibodies and anti-MCV antibodies in the diagnosis of rheumatoid arthritis. Br. J. Biomed. Sci. 2020, 78, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Shekhani, M.T.; Forde, T.S.; Adilbayeva, A.; Ramez, M.; Myngbay, A.; Bexeitov, Y.; Lindner, V.; Adarichev, V.A. Collagen triple helix repeat containing 1 is a new promigratory marker of arthritic pannus. Arthritis Res. Ther. 2016, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Li, X.; Zhu, H.; Luo, H. Single-Cell Sequencing in Rheumatic Diseases: New Insights from the Perspective of the Cell Type. Aging Dis. 2022, 13, 1633–1651. [Google Scholar] [CrossRef]
- Mizoguchi, F.; Slowikowski, K.; Wei, K.; Marshall, J.L.; Rao, D.A.; Chang, S.K.; Nguyen, H.N.; Noss, E.H.; Turner, J.D.; Earp, B.E.; et al. Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Abuwarwar, M.H.; Knoblich, K.; Fletcher, A.L. A pathogenic hierarchy for synovial fibroblasts in rheumatoid arthritis. Ann. Transl. Med. 2018, 6, S75–S75. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Nawata, M.; Wakitani, S. Expression Profiles and Functional Analyses of Wnt-Related Genes in Human Joint Disorders. Am. J. Pathol. 2005, 167, 97–105. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Kim, D.W.; Ha, Y.; Ihm, M.H.; Kim, H.; Song, K.; Lee, I. Wnt5a Induces Endothelial Inflammation via β-Catenin–Independent Signaling. J. Immunol. 2010, 185, 1274–1282. [Google Scholar] [CrossRef]
- Rauner, M.; Stein, N.; Winzer, M.; Goettsch, C.; Zwerina, J.; Schett, G.; Distler, J.H.; Albers, J.; Schulze, J.; Schinke, T.; et al. WNT5A is induced by inflammatory mediators in bone marrow stromal cells and regulates cytokine and chemokine production. J. Bone Miner. Res. 2011, 27, 575–585. [Google Scholar] [CrossRef]
- Yu, M.; Guo, Y.; Zhang, P.; Xue, J.; Yang, J.; Cai, Q.; You, X.; Ma, J.; Yang, D.; Jia, Y.; et al. Increased circulating Wnt5a protein in patients with rheumatoid arthritis-associated interstitial pneumonia (RA-ILD). Immunobiology 2019, 224, 551–559. [Google Scholar] [CrossRef]
- Deane, K.D.; Demoruelle, M.K.; Kelmenson, L.B.; Kuhn, K.A.; Norris, J.M.; Holers, V.M. Genetic and environmental risk factors for rheumatoid arthritis. Best Pr. Res. Clin. Rheumatol. 2017, 31, 3–18. [Google Scholar] [CrossRef]
- Reynisdottir, G.; Karimi, R.; Joshua, V.; Olsen, H.; Hensvold, A.H.; Harju, A.; Engström, M.; Grunewald, J.; Nyren, S.; Eklund, A.; et al. Structural Changes and Antibody Enrichment in the Lungs Are Early Features of Anti-Citrullinated Protein Antibody-Positive Rheumatoid Arthritis. Arthritis Rheumatol. 2013, 66, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, H.; Kusaba, T.; Yamaguchi, M. [Cause of death in autopsied RA patients]. Ryumachi. [Rheumatism] 1993, 33. [Google Scholar]
- Kelly, C.A.; Saravanan, V.; Nisar, M.; Arthanari, S.; Woodhead, F.A.; Price-Forbes, A.N.; Dawson, J.; Sathi, N.; Ahmad, Y.; Koduri, G.; et al. Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics--a large multicentre UK study. Rheumatol. 2014, 53, 1676–1682. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Koga, Y.; Sugimoto, M. Different risk factors between interstitial lung disease and airway disease in rheumatoid arthritis. Respir. Med. 2012, 106, 1591–1599. [Google Scholar] [CrossRef]
- Catrina, A.I.; Ytterberg, A.J.; Reynisdottir, G.; Malmström, V.; Klareskog, L. Lungs, joints and immunity against citrullinated proteins in rheumatoid arthritis. Nat. Rev. Rheumatol. 2014, 10, 645–653. [Google Scholar] [CrossRef]
- Sparks, J.A.; Karlson, E.W. The Roles of Cigarette Smoking and the Lung in the Transitions Between Phases of Preclinical Rheumatoid Arthritis. Curr. Rheumatol. Rep. 2016, 18, 1–17. [Google Scholar] [CrossRef]
- Thannickal, V.J.; Toews, G.B.; White, E.S.; Iii, J.P.L.; Martinez, F.J. Mechanisms of Pulmonary Fibrosis. Annu. Rev. Med. 2004, 55, 395–417. [Google Scholar] [CrossRef]
- Larsen, J.M.; Steen-Jensen, D.B.; Laursen, J.M.; Søndergaard, J.N.; Musavian, H.S.; Butt, T.M.; Brix, S. Divergent pro-inflammatory profile of human dendritic cells in response to commensal and pathogenic bacteria associated with the airway microbiota. PLoS ONE 2012, 7, e31976. [Google Scholar] [CrossRef]
- Van Steendam, K.; Tilleman, K.; De Ceuleneer, M.; De Keyser, F.; Elewaut, D.; Deforce, D. Citrullinated vimentin as an important antigen in immune complexes from synovial fluid of rheumatoid arthritis patients with antibodies against citrullinated proteins. Arthritis Res. Ther. 2010, 12, R132–R132. [Google Scholar] [CrossRef]
- Van Steendam, K.; Tilleman, K.; Deforce, D. The relevance of citrullinated vimentin in the production of antibodies against citrullinated proteins and the pathogenesis of rheumatoid arthritis. Rheumatol. 2011, 50, 830–837. [Google Scholar] [CrossRef]
- Liao, M.; Liu, Y.; Yuan, J.; Wen, Y.; Xu, G.; Zhao, J.; Cheng, L.; Li, J.; Wang, X.; Wang, F.; et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020, 26, 842–844. [Google Scholar] [CrossRef] [PubMed]
- Pairo-Castineira, E.; Clohisey, S.; Klaric, L.; Bretherick, A.D.; Rawlik, K.; Pasko, D.; Walker, S.; Parkinson, N.; Fourman, M.H.; Russell, C.D.; et al. Genetic mechanisms of critical illness in COVID-19. Nature 2021, 591, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Dorward, D.A.; Russell, C.D.; Um, I.H.; Elshani, M.; Armstrong, S.D.; Penrice-Randal, R.; Millar, T.; Lerpiniere, C.E.B.; Tagliavini, G.; Hartley, C.S.; et al. Tissue-Specific Immunopathology in Fatal COVID-19. Am. J. Respir. Crit. Care Med. 2021, 203, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Barra, L.; Scinocca, M.; Saunders, S.; Bhayana, R.; Rohekar, S.; Racapé, M.; Coles, R.; Cairns, E.; Bell, D.A. Anti-Citrullinated Protein Antibodies in Unaffected First-Degree Relatives of Rheumatoid Arthritis Patients. Arthritis Rheum. 2013, 65, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Mikuls, T.R.; Payne, J.B.; Deane, K.D.; Thiele, G.M. Autoimmunity of the lung and oral mucosa in a multisystem inflammatory disease: The spark that lights the fire in rheumatoid arthritis? J. Allergy Clin. Immunol. 2016, 137, 28–34. [Google Scholar] [CrossRef]
- Rangel-Moreno, J.; Hartson, L.; Navarro, C.; Gaxiola, M.; Selman, M.; Randall, T.D. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J. Clin. Investig. 2006, 116, 3183–3194. [Google Scholar] [CrossRef]
- Ossipova, E.; Cerqueira, C.F.; Reed, E.; Kharlamova, N.; Israelsson, L.; Holmdahl, R.; Nandakumar, K.S.; Engström, M.; Harre, U.; Schett, G.; et al. Affinity purified anti-citrullinated protein/peptide antibodies target antigens expressed in the rheumatoid joint. Thromb. Haemost. 2014, 16, R167–11. [Google Scholar] [CrossRef]
- Janssen, K.M.J.; de Smit, M.J.; Brouwer, E.; Kok, F.A.C.d.; Kraan, J.; Altenburg, J.; Verheul, M.K.; A Trouw, L.; van Winkelhoff, A.J.; Vissink, A.; et al. Rheumatoid arthritis–associated autoantibodies in non–rheumatoid arthritis patients with mucosal inflammation: a case–control study. Arthritis Res. Ther. 2015, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Demoruelle, M.K.; Deane, K.D.; Holers, V.M. When and where does inflammation begin in rheumatoid arthritis? Curr. Opin. Rheumatol. 2014, 26, 64–71. [Google Scholar] [CrossRef]
- Juge, P.-A.; Borie, R.; Kannengiesser, C.; Gazal, S.; Revy, P.; Wemeau-Stervinou, L.; Debray, M.-P.; Ottaviani, S.; Marchand-Adam, S.; Nathan, N.; et al. Shared genetic predisposition in rheumatoid arthritis-interstitial lung disease and familial pulmonary fibrosis. Eur. Respir. J. 2017, 49, 1602314. [Google Scholar] [CrossRef]
- Seibold, M.A.; Wise, A.L.; Speer, M.C.; Steele, M.P.; Brown, K.K.; Loyd, J.E.; Fingerlin, T.E.; Zhang, W.; Gudmundsson, G.; Groshong, S.D.; et al. A CommonMUC5BPromoter Polymorphism and Pulmonary Fibrosis. New Engl. J. Med. 2011, 364, 1503–1512. [Google Scholar] [CrossRef]
- Lehmann, M.; Hu, Q.; Hu, Y.; Hafner, K.; Costa, R.; Berg, A.v.D.; Königshoff, M. Chronic WNT/β-catenin signaling induces cellular senescence in lung epithelial cells. Cell. Signal. 2020, 70, 109588–109588. [Google Scholar] [CrossRef] [PubMed]
- Ley, B.; Collard, H.R.; King, T.E., Jr. Clinical Course and Prediction of Survival in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2011, 183, 431–440. [Google Scholar] [CrossRef]
- Tager, A.M.; LaCamera, P.; Shea, B.S.; Campanella, G.S.; Selman, M.; Zhao, Z.; Polosukhin, V.; Wain, J.; A Karimi-Shah, B.; Kim, N.D.; et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat. Med. 2007, 14, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Tsukui, T.; Ueha, S.; Abe, J.; Hashimoto, S.-I.; Shichino, S.; Shimaoka, T.; Shand, F.H.; Arakawa, Y.; Oshima, K.; Hattori, M.; et al. Qualitative Rather than Quantitative Changes Are Hallmarks of Fibroblasts in Bleomycin-Induced Pulmonary Fibrosis. Am. J. Pathol. 2013, 183, 758–773. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Y.; Yan, H.; Peng, X.; Zou, J. NEDD4L-induced β-catenin ubiquitination suppresses the formation and progression of interstitial pulmonary fibrosis via inhibiting the CTHRC1/HIF-1α axis. Int. J. Biol. Sci. 2021, 17, 3320–3330. [Google Scholar] [CrossRef]
- Hesselstrand, R.; Andréasson, K.; Wuttge, D.M.; Bozovic, G.; Scheja, A.; Saxne, T. Increased serum COMP predicts mortality in SSc: results from a longitudinal study of interstitial lung disease. Rheumatology 2012, 51, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Kanaoka, M.; Yamaguchi, Y.; Komitsu, N.; Feghali-Bostwick, C.A.; Ogawa, M.; Arima, K.; Izuhara, K.; Aihara, M. Pro-fibrotic phenotype of human skin fibroblasts induced by periostin via modulating TGF-β signaling. J. Dermatol. Sci. 2018, 90, 199–208. [Google Scholar] [CrossRef]
- Gur, C.; Wang, S.-Y.; Sheban, F.; Zada, M.; Li, B.; Kharouf, F.; Peleg, H.; Aamar, S.; Yalin, A.; Kirschenbaum, D.; et al. LGR5 expressing skin fibroblasts define a major cellular hub perturbed in scleroderma. Cell 2022, 185, 1373–1388. [Google Scholar] [CrossRef]
- Valenzi, E.; Bulik, M.; Tabib, T.; Morse, C.; Sembrat, J.; Bittar, H.T.; Rojas, M.; Lafyatis, R. Single-cell analysis reveals fibroblast heterogeneity and myofibroblasts in systemic sclerosis-associated interstitial lung disease. Rheumatology 2019, 78, 1379–1387. [Google Scholar] [CrossRef]
- Shen, Z.; Su, T.; Chen, J.; Xie, Z.; Li, J. Collagen triple helix repeat containing-1 exerts antifibrotic effects on human skin fibroblast and bleomycin-induced dermal fibrosis models. Ann. Transl. Med. 2021, 9, 801–801. [Google Scholar] [CrossRef]
- Mehandru, S.; Merad, M. Pathological sequelae of long-haul COVID. Nat. Immunol. 2022, 23, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Rendeiro, A.F.; Ravichandran, H.; Bram, Y.; Chandar, V.; Kim, J.; Meydan, C.; Park, J.; Foox, J.; Hether, T.; Warren, S.; et al. The spatial landscape of lung pathology during COVID-19 progression. Nature 2021, 593, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Dolby, H.W.; Potey, P.; Wilder-Smith, A.B.; Clohisey, S.; E Millar, J.; Baillie, J.K.; A Dorward, D.; Lucas, C.D.; Russell, C.D. Histological Evidence of Pulmonary Microthrombosis and Vasculitis in Life-Threatening Respiratory Virus Diseases. Open Forum Infect. Dis. 2020, 8, ofaa640. [Google Scholar] [CrossRef] [PubMed]
- Phetsouphanh, C.; Darley, D.R.; Wilson, D.B.; Howe, A.; Munier, C.M.L.; Patel, S.K.; Juno, J.A.; Burrell, L.M.; Kent, S.J.; Dore, G.J.; et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat. Immunol. 2022, 23, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.J.; Coutavas, E.; Pine, A.B.; Lee, A.I.; Yu, V.L.; Shallow, M.K.; Giovacchini, C.X.; Mathews, A.M.; Stephenson, B.; Que, L.G.; et al. Immunofibrotic drivers of impaired lung function in postacute sequelae of SARS-CoV-2 infection. J. Clin. Investig. 2021, 6. [Google Scholar] [CrossRef]
- Schneider, C.; Nobs, S.P.; Kurrer, M.; Rehrauer, H.; Thiele, C.; Kopf, M. Induction of the nuclear receptor PPAR-γ by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages. Nat. Immunol. 2014, 15, 1026–1037. [Google Scholar] [CrossRef]
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Cao, Y.; Yousif, A.S.; Bals, J.; Hauser, B.M.; et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell 2020, 181, 1016–1035. [Google Scholar] [CrossRef]
- Hou, Y.; Okuda, K.; Edwards, C.E.; Martinez, D.R.; Asakura, T.; Dinnon, K.H., 3rd; Kato, T.; Lee, R.E.; Yount, B.L.; Mascenik, T.M.; et al. SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell 2020, 182, 429–446. [Google Scholar] [CrossRef]
- Pérez-Mies, B.; Caniego-Casas, T.; Bardi, T.; Carretero-Barrio, I.; Benito, A.; García-Cosío, M.; González-García, I.; Pizarro, D.; Rosas, M.; Cristóbal, E.; et al. Progression to lung fibrosis in severe COVID-19 patients: A morphological and transcriptomic study in postmortem samples. Front. Med. 2022, 9, 976759. [Google Scholar] [CrossRef]
- Coker, R.K.; Laurent, G.J.; Jeffery, P.K.; du Bois, R.M.; Black, C.M.; McAnulty, R.J. Localisation of transforming growth factor beta1 and beta3 mRNA transcripts in normal and fibrotic human lung. Thorax 2001, 56, 549–556. [Google Scholar] [CrossRef]
- Chua, R.L.; Lukassen, S.; Trump, S.; Hennig, B.P.; Wendisch, D.; Pott, F.; Debnath, O.; Thürmann, L.; Kurth, F.; Völker, M.T.; et al. COVID-19 severity correlates with airway epithelium–immune cell interactions identified by single-cell analysis. Nat. Biotechnol. 2020, 38, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Sikkema, L.; Ramírez-Suástegui, C.; Strobl, D.C.; Gillett, T.E.; Zappia, L.; Madissoon, E.; Markov, N.S.; Zaragosi, L.-E.; Ji, Y.; Ansari, M.; et al. An integrated cell atlas of the lung in health and disease. Nat. Med. 2023, 29, 1563–1577. [Google Scholar] [CrossRef] [PubMed]
- Jyothula, S.S.; Peters, A.; Liang, Y.; Bi, W.; Shivshankar, P.; Yau, S.; Garcha, P.S.; Yuan, X.; Akkanti, B.; Collum, S.; et al. Fulminant lung fibrosis in non-resolvable COVID-19 requiring transplantation. EBioMedicine 2022, 86. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, M.; Ramachandran, P. Immunology of human fibrosis. Nat. Immunol. 2023, 24, 1423–1433. [Google Scholar] [CrossRef]
- Li, A.; Chen, J.-Y.; Hsu, C.-L.; Oyang, Y.-J.; Huang, H.-C.; Juan, H.-F. A Single-Cell Network-Based Drug Repositioning Strategy for Post-COVID-19 Pulmonary Fibrosis. Pharmaceutics 2022, 14, 971. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, L.; Wang, M.; Zhou, S.; Lu, Y.; Cui, H.; Racanelli, A.C.; Zhang, L.; Ye, T.; Ding, B.; et al. Targeting fibrosis: mechanisms and clinical trials. Signal Transduct. Target. Ther. 2022, 7, 1–21. [Google Scholar] [CrossRef]
- Amati, F.; Stainer, A.; Polelli, V.; Mantero, M.; Gramegna, A.; Blasi, F.; Aliberti, S. Efficacy of Pirfenidone and Nintedanib in Interstitial Lung Diseases Other than Idiopathic Pulmonary Fibrosis: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 7849. [Google Scholar] [CrossRef]
- Behr, J.; Prasse, A.; Kreuter, M.; Johow, J.; Rabe, K.F.; Bonella, F.; Bonnet, R.; Grohe, C.; Held, M.; Wilkens, H.; et al. Pirfenidone in patients with progressive fibrotic interstitial lung diseases other than idiopathic pulmonary fibrosis (RELIEF): a double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir. Med. 2021, 9, 476–486. [Google Scholar] [CrossRef]
- Redente, E.F.; Aguilar, M.A.; Black, B.P.; Edelman, B.L.; Bahadur, A.N.; Humphries, S.M.; Lynch, D.A.; Wollin, L.; Riches, D.W.H. Nintedanib reduces pulmonary fibrosis in a model of rheumatoid arthritis-associated interstitial lung disease. Am. J. Physiol. Cell. Mol. Physiol. 2018, 314, L998–L1009. [Google Scholar] [CrossRef] [PubMed]
- Deterding, R.; Young, L.R.; DeBoer, E.M.; Warburton, D.; Cunningham, S.; Schwerk, N.; Flaherty, K.R.; Brown, K.K.; Dumistracel, M.; Erhardt, E.; et al. Nintedanib in children and adolescents with fibrosing interstitial lung diseases. Eur. Respir. J. 2022, 61, 2201512. [Google Scholar] [CrossRef] [PubMed]
- Distler, O.; Highland, K.B.; Gahlemann, M.; Azuma, A.; Fischer, A.; Mayes, M.D.; Raghu, G.; Sauter, W.; Girard, M.; Alves, M.; et al. Nintedanib for Systemic Sclerosis–Associated Interstitial Lung Disease. New Engl. J. Med. 2019, 380, 2518–2528. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.R.; Wells, A.U.; Cottin, V.; Devaraj, A.; Walsh, S.L.; Inoue, Y.; Richeldi, L.; Kolb, M.; Tetzlaff, K.; Stowasser, S.; et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. New Engl. J. Med. 2019, 381, 1718–1727. [Google Scholar] [CrossRef]
- Liu, Z.-W.; Zhao, M.-Y.; Su, X.-L.; Ye, T.-H.; Zhuang, Y.-J.; Zhang, Y.; Zhang, Z.-Z.; Yang, J.-L.; Chen, L.-J.; Long, C.-F.; et al. The antifibrotic effect and mechanism of a novel tyrosine kinase inhibitor, ZSP1603, in preclinical models of pulmonary fibrosis. . 2020, 24, 1481–1491. [Google Scholar]
- Sgalla, G.; Franciosa, C.; Simonetti, J.; Richeldi, L. Pamrevlumab for the treatment of idiopathic pulmonary fibrosis. Expert Opin. Investig. Drugs 2020, 29, 771–777. [Google Scholar] [CrossRef]
- Wells, A.U. Pamrevlumab in idiopathic pulmonary fibrosis. Lancet Respir. Med. 2019, 8, 2–3. [Google Scholar] [CrossRef]
- Behr, J.; Prasse, A.; Kreuter, M.; Johow, J.; Rabe, K.F.; Bonella, F.; Bonnet, R.; Grohe, C.; Held, M.; Wilkens, H.; et al. Pirfenidone in patients with progressive fibrotic interstitial lung diseases other than idiopathic pulmonary fibrosis (RELIEF): a double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir. Med. 2021, 9, 476–486. [Google Scholar] [CrossRef]
- Malik, B.; Abdelazeem, B.; Ghatol, A. Pulmonary Fibrosis After COVID-19 Pneumonia. Cureus 2021, 13. [Google Scholar] [CrossRef]
- Boshra, M.S.; Warda, A.E.A.; Sayed, M.A.; Elkomy, M.H.; Alotaibi, N.H.; Mohsen, M.; Sarhan, R.M. Effect of Pirfenidone on Risk of Pulmonary Fibrosis in COVID-19 Patients Experiencing Cytokine Storm. Healthcare 2022, 10, 2387. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, D.; Kong, X.; Wei, C.; LvQiu, S.; Wang, L.; Lin, Y.; Yin, Z.; Zhou, Z.; Luo, H. Case Report: Pirfenidone in the Treatment of Post-COVID-19 Pulmonary Fibrosis. Front. Med. 2022, 9, 925703. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




