Submitted:
05 September 2023
Posted:
07 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Synthesis of MgO from cassava starch
2.2. Synthesis of NixMg1-x(OH)2 pigments
2.3. Characterization of compounds
2.4. Microbiological testing
2.4.1. Minimal Inhibitory Concentration (MIC)
2.4.2. Minimal Bactericidal or Fungicidal Concentration (MBC/MFC)
3. Results
3.1. Characterization
3.1.1. Chemical analysis of samples by EDXRF
| Nickel precursor salt | Mg (%) | Ni (%) | Estimated composition |
|---|---|---|---|
| MgOst | 99.841 | - | [Mg0.99O] |
| Acetate | 90.867 | 8.789 | [Ni0.087Mg0.91(OH)2]ace |
| Chloride | 90.784 | 9.050 | [Ni0.090Mg0.91(OH)2]chl |
| Nitrate | 90.342 | 9.437 | [Ni0.094Mg0.90(OH)2]nit |
3.1.2. Structural analysis by X-ray diffraction (XDR)
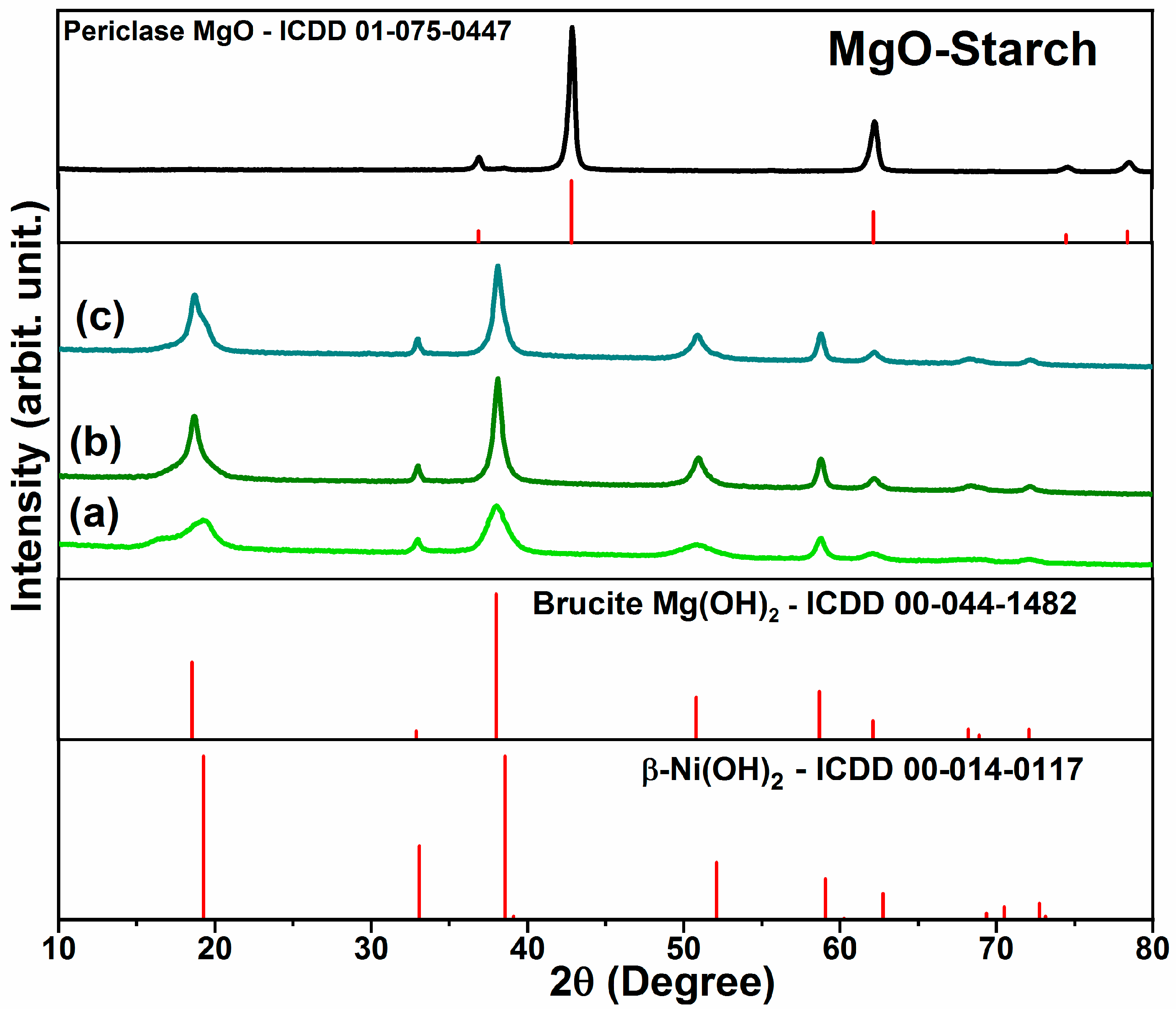
3.1.3. Morphological characteristics by scanning electronic microscopy (SEM)
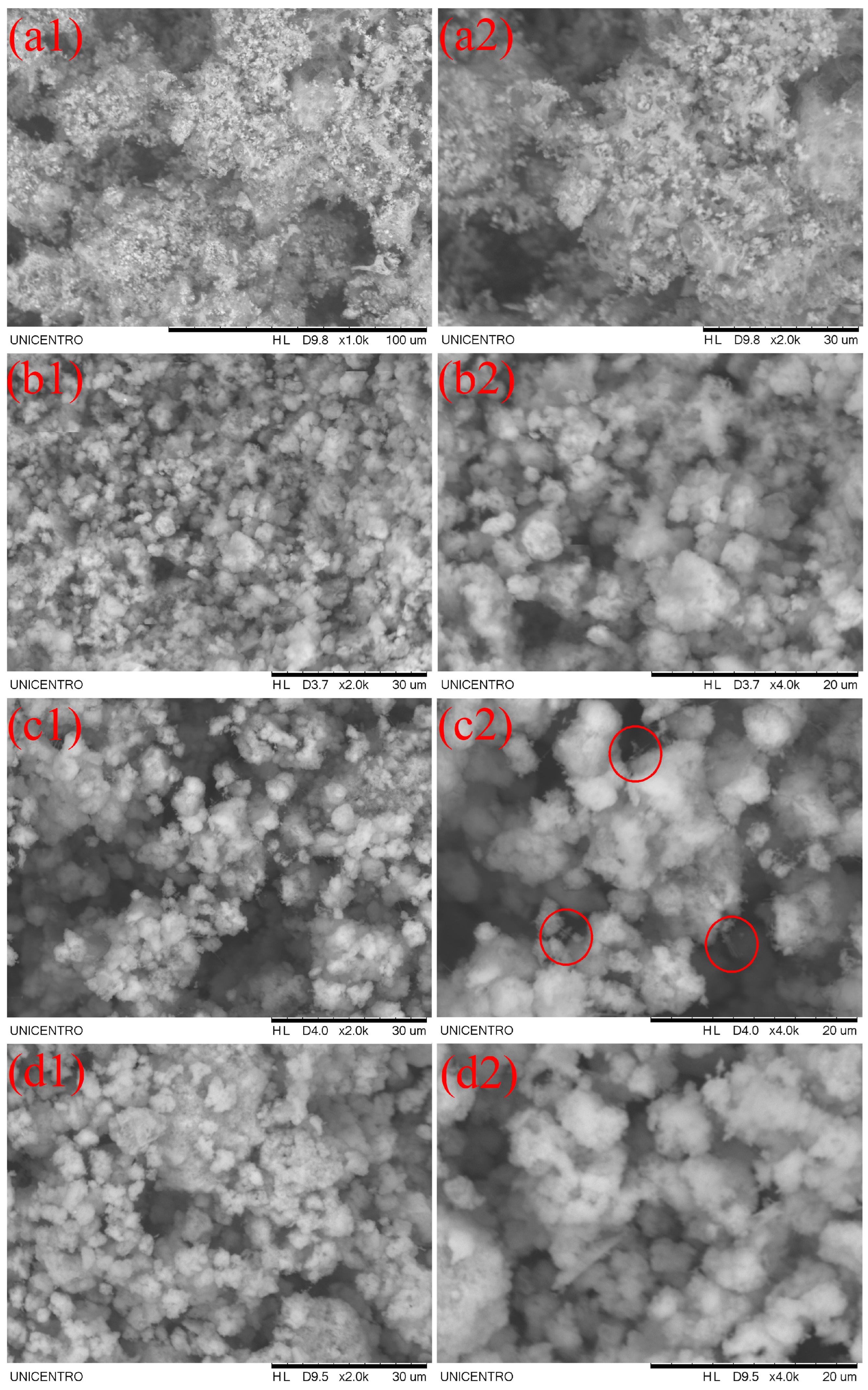
3.1.4. Fourier transform infrared spectroscopy (FTIR)
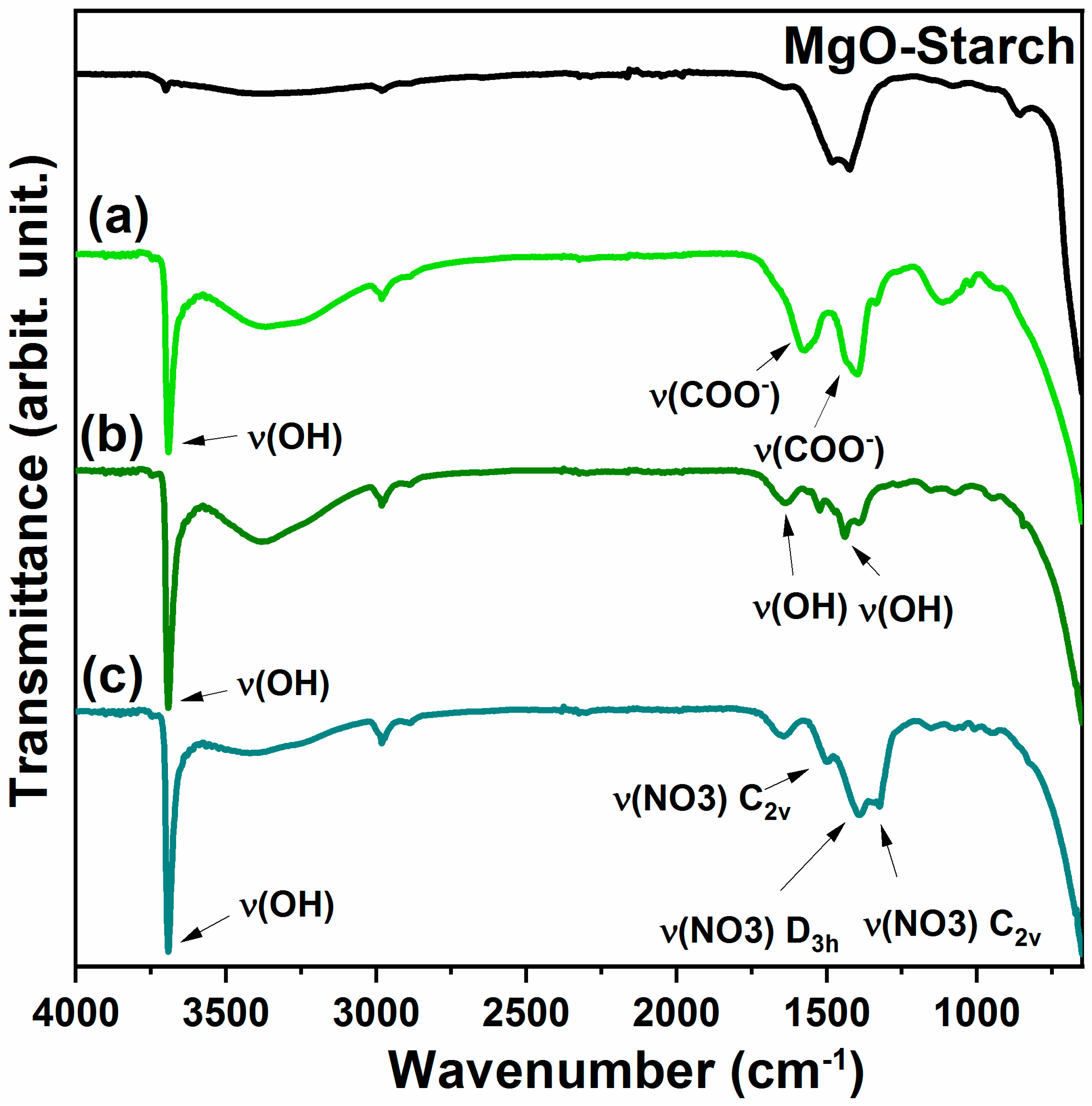
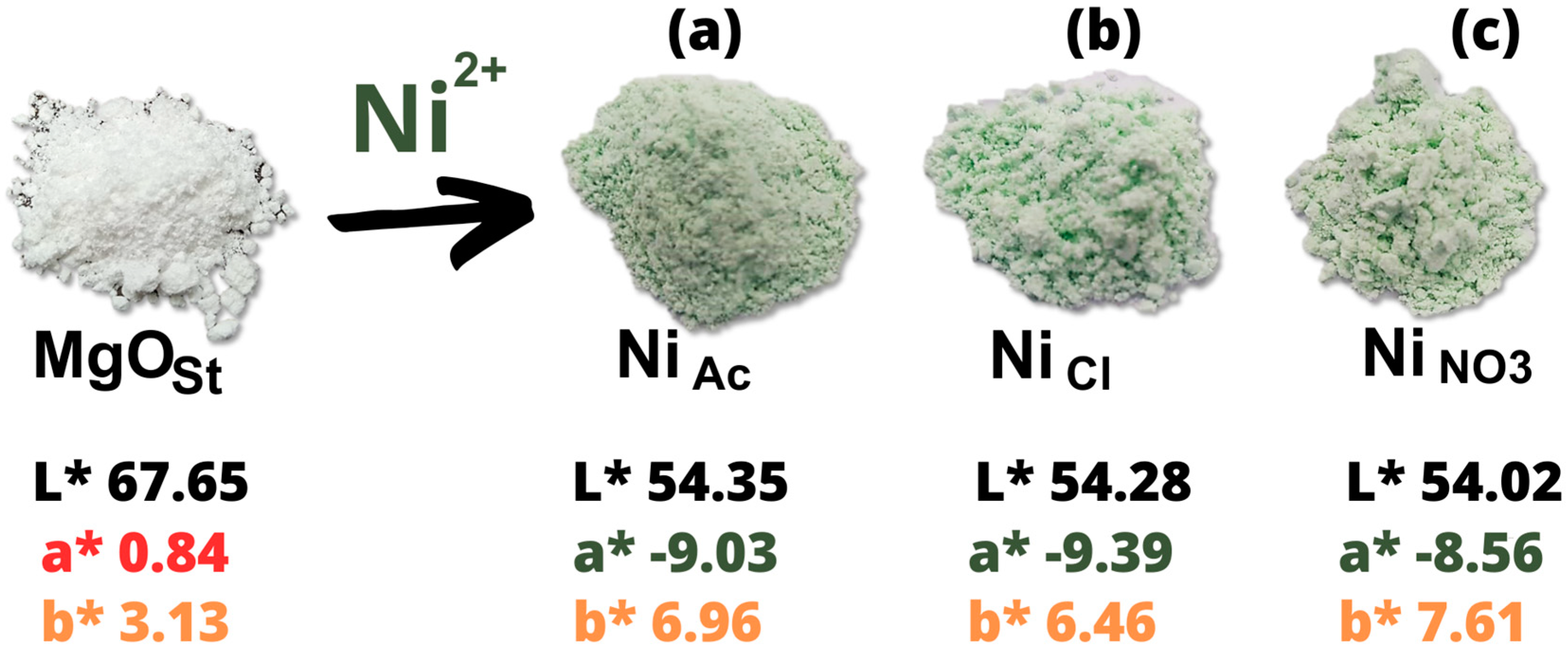
3.1.5. Colorimetric analysis and spectroscopy VIS-NIR
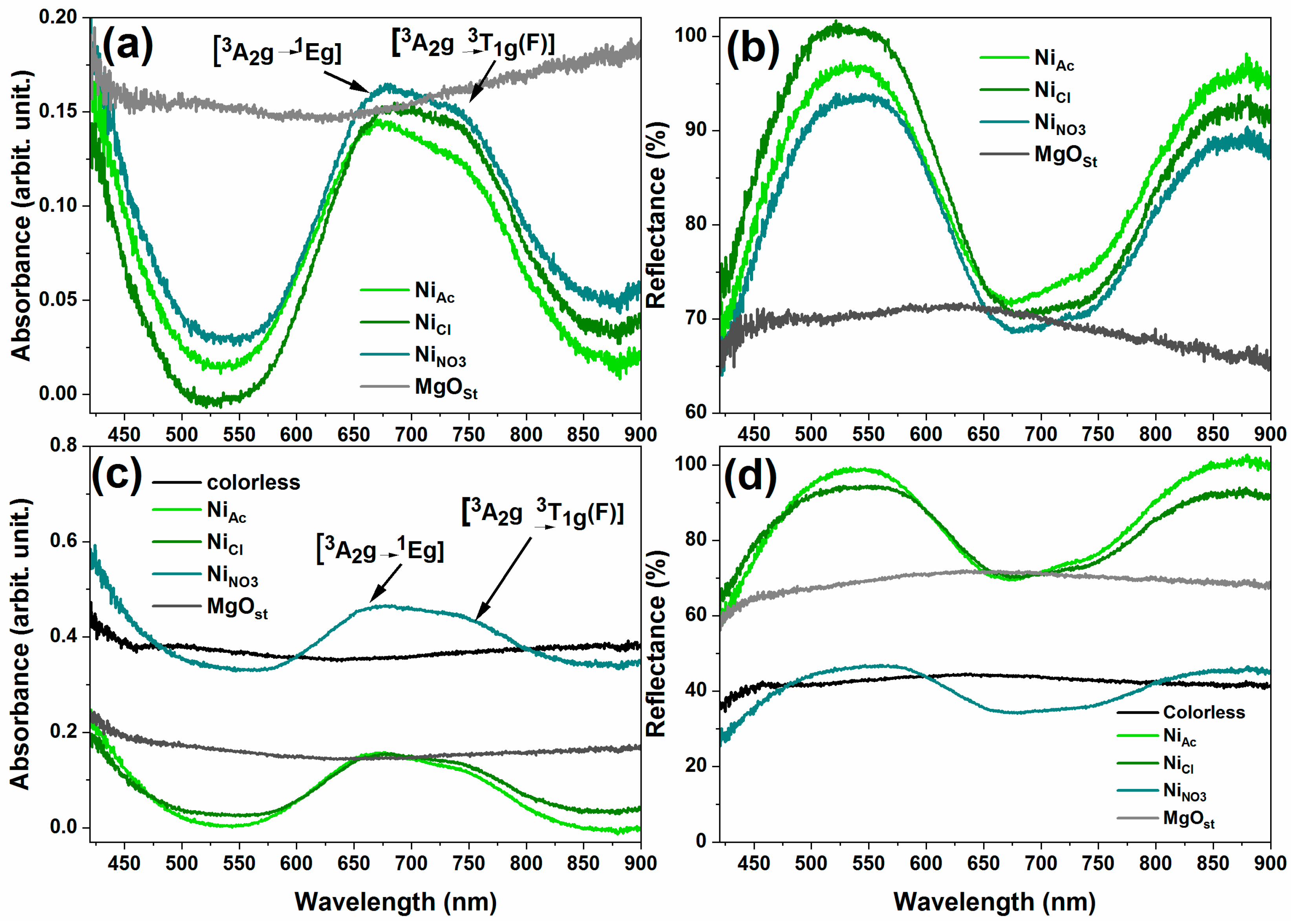
| Sample | L* | a* | b* | C* | h* | ∆E | Software color |
|---|---|---|---|---|---|---|---|
| MgO | 67.65 | 0.84 | 3.13 | 3.24 | 104.98 | - |  |
| Acetate | 54.02 | -8.56 | 7.61 | 11.45 | 138.34 | 17.15 |  |
| Chloride | 54.28 | -9.39 | 6.46 | 11.40 | 145.48 | 17.16 |  |
| Nitrate | 54.35 | -9.03 | 6.96 | 11.40 | 142.37 | 17.00 |  |
| Sample | L* | a* | b* | C* | h* | ∆E | Software color |
|---|---|---|---|---|---|---|---|
| Colorless paint | 65.04 | -0.95 | 2.91 | 3.06 | 108.14 | - |  |
| MgO | 78.49 | 0.54 | 4.42 | 4.45 | 96.90 | 13.62 |  |
| Acetate | 70.60 | -13.07 | 10.67 | 16.87 | 140.77 | 15.43 |  |
| Chloride | 64.59 | -9.20 | 7.64 | 11.96 | 140.31 | 9.52 |  |
| Nitrate | 72.36 | -12.22 | 11.13 | 16.53 | 137.68 | 15.75 |  |
| Sample | L* | a* | b* | C* | h* | ∆E | Software color |
|---|---|---|---|---|---|---|---|
| Colorless paint | 88.00 | -0.06 | 4.37 | 4.37 | 90.34 | - |  |
| MgO | 86.99 | 0.53 | 4.42 | 4.45 | 96.90 | 1.17 |  |
| Acetate | 82.87 | -7.48 | 10.65 | 13.01 | 125.07 | 11.00 |  |
| Chloride | 83.46 | -6.77 | 9.07 | 11.31 | 126.75 | 9.37 |  |
| Nitrate | 85.49 | -5.83 | 10.03 | 11.60 | 120.16 | 8.46 |  |
3.2. Microbiological tests (MIC, MBC and MFC)
| Sample | S. aureus | E. coli | S. gallinarum | C. albicans |
|---|---|---|---|---|
| MgO | 0.625 (bc) | 0.625 (bs) | 0.625 (bs) | * |
| NixMg1-x(OH)2(Ac.) | 0.625 (bc) | 0.625 (bs) | 0.625 (bs) | 0.625 (fs) |
| NixMg1-x(OH)2(Cl) | 0.625 (bc) | 0.312 (bs) | 0.625 (bs) | * |
| NixMg1-x(OH)2(NO3) | 0.625 (bc) | 0.625 (bs) | 0.625 (bc) | 0.625 (fs) |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Monrós, G.; Llusar, M.; García, A.; Gargori, C.; Galindo, R. Development of New Ceramic Dyes.; 2010; pp. 182–193. 27 October.
- Llusar, M.; Gargori, C.; Cerro, S.; Badenes, J.A.; Monrós, G. New Ceramic Pigments for the Coloration of Ceramic Glazes.; October 31 2014; pp. 148–158.
- Pilarska, A.A.; Klapiszewski, Ł.; Jesionowski, T. Recent Development in the Synthesis, Modification and Application of Mg(OH)2 and MgO: A Review. Powder Technol. 2017, 319, 373–407. [Google Scholar] [CrossRef]
- Ansari, A.; Ali, A.; Asif, M.; Shamsuzzaman, S. Microwave-Assisted MgO NP Catalyzed One-Pot Multicomponent Synthesis of Polysubstituted Steroidal Pyridines. New J. Chem. 2018, 42, 184–197. [Google Scholar] [CrossRef]
- Yin, J.; Zhou, G.; Gao, X.; Chen, J.; Zhang, L.; Xu, J.; Zhao, P.; Gao, F. α- and β-Phase Ni-Mg Hydroxide for High Performance Hybrid Supercapacitors. Nanomaterials 2019, 9, 1686. [Google Scholar] [CrossRef]
- Balaba, N.; Jaerger, S.; Horsth, D.F.L.; Primo, J. de O.; Correa, J. de S.; Bittencourt, C.; Zanette, C.M.; Anaissi, F.J. Polysaccharides as Green Fuels for the Synthesis of MgO: Characterization and Evaluation of Antimicrobial Activities. Molecules 2023, 28. [Google Scholar] [CrossRef]
- Tang, Z.-X.; Lv, B.-F. MgO Nanoparticles as Antibacterial Agent: Preparation and Activity. Brazilian J. Chem. Eng. 2014, 31, 591–601. [Google Scholar] [CrossRef]
- Ohira, T.; Yamamoto, O. Correlation between Antibacterial Activity and Crystallite Size on Ceramics. Chem. Eng. Sci. 2012, 68, 355–361. [Google Scholar] [CrossRef]
- Zhang, S.; Cheng, F.; Tao, Z.; Gao, F.; Chen, J. Removal of Nickel Ions from Wastewater by Mg(OH)2/MgO Nanostructures Embedded in Al2O3 Membranes. J. Alloys Compd. 2006, 426, 281–285. [Google Scholar] [CrossRef]
- Nobre, J.; Ahmed, H.; Bravo, M.; Evangelista, L.; de Brito, J. Magnesia (MgO) Production and Characterization, and Its Influence on the Performance of Cementitious Materials: A Review. Materials (Basel). 2020, 13, 4752. [Google Scholar] [CrossRef] [PubMed]
- Kumari, L.; Li, W.Z.; Vannoy, C.H.; Leblanc, R.M.; Wang, D.Z. Synthesis, Characterization and Optical Properties of Mg(OH)2 Micro-/Nanostructure and Its Conversion to MgO. Ceram. Int. 2009, 35, 3355–3364. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Lv, L.; Cui, Y.; Wei, C.; Pang, G. Preparation of Mg(OH)2 Hybrid Pigment by Direct Precipitation and Graft onto Cellulose Fiber via Surface-Initiated Atom Transfer Radical Polymerization. Appl. Surf. Sci. 2016, 363, 189–196. [Google Scholar] [CrossRef]
- Primo, J. de O.; Bittencourt, C.; Acosta, S.; Sierra-Castillo, A.; Colomer, J.F.; Jaerger, S.; Teixeira, V.C.; Anaissi, F.J. Synthesis of Zinc Oxide Nanoparticles by Ecofriendly Routes: Adsorbent for Copper Removal From Wastewater. Front. Chem. 2020, 8, 571790. [Google Scholar] [CrossRef] [PubMed]
- Brito, G.F.; Agrawal, P.; Araújo, E.M.; Mélo, T.J.A. Biopolímeros, Polímeros Biodegradáveis e Polímeros Verdes. Rev. Eletrônoca Mater. e Process. 2011, 6, 127–139. [Google Scholar]
- Hajjaji, W.; Costa, G.; Zanelli, C.; Ribeiro, M.J.; Seabra, M.P.; Dondi, M.; Labrincha, J.A. An Overview of Using Solid Wastes for Pigment Industry. J. Eur. Ceram. Soc. 2012, 32, 753–764. [Google Scholar] [CrossRef]
- Zou, J.; Chen, Y.; Zhang, P. Influence of Crystallite Size on Color Properties and NIR Reflectance of TiO2@NiTiO3 Inorganic Pigments. Ceram. Int. 2021, 47, 12661–12666. [Google Scholar] [CrossRef]
- Wei, X.; Zou, X.; Deng, Z.; Bao, W.; Ai, T.; Zhang, Q. Synthesis and Characterisation of Mg2+ and Al3+ Co-Doped CoCr2O4 Inorganic Pigments With High Near-Infrared Reflectance. Front. Mater. 2022, 9. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, P. Ni-Doped BaTi5O11: New Brilliant Yellow Pigment with High NIR Reflectance as Solar Reflective Fillers. Ceram. Int. 2020, 46, 3490–3497. [Google Scholar] [CrossRef]
- Balaba, N.; Jaerger, S.; Horsth, D.F.L.; Primo, J. de O.; Correa, J. de S.; Bittencourt, C.; Zanette, C.M.; Anaissi, F.J. Polysaccharides as Green Fuels for the Synthesis of MgO: Characterization and Evaluation of Antimicrobial Activities. Molecules 2022, 28, 142. [Google Scholar] [CrossRef]
- Bible, B.B.; Singha, S. Canopy Position Influences CIELAB Coordinates of Peach Color. Hortscience 1993, 28, 992–993. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standarts Institute (CLSI) Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Third edit.; Wayne, Pa, USA, 2008; ISBN 610.688.0700.
- Clinical and Laboratory Standarts Institute (CLSI) Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Sixty Edit.; . NCCLS document M7-A6, Suite 1400, Wayne, Pennsylvania, USA, 2003; ISBN 610.688.0700.
- Haynes, W.M.; Lide, D.R.; Bruno, T.L. CRC Handbook of Chemistry and Physics; 95th ed.; Taylor & Francis Group, 2014; ISBN 978-1-4822-0868-9.
- Vinokurov, S.E.; Kulikova, S.A.; Krupskaya, V. V.; Tyupina, E.A. Effect of Characteristics of Magnesium Oxide Powder on Composition and Strength of Magnesium Potassium Phosphate Compound for Solidifying Radioactive Waste. Russ. J. Appl. Chem. 2019, 92, 490–497. [Google Scholar] [CrossRef]
- Yousefi, S.; Ghasemi, B.; Nikolova, M.P. Opto-Structural Characterization of Mg(OH)2 and MgO Nanostructures Synthesized through a Template-Free Sonochemical Method. Appl. Phys. A 2021, 127, 549. [Google Scholar] [CrossRef]
- Kovalenko, V.; Kotok, V. Synthesis of Nickel Hydroxide in the Presence of Acetate Ion as a «soft» Ligand for Application in Chemical Power Sources. Eastern-European J. Enterp. Technol. 2019, 6, 6–12. [Google Scholar] [CrossRef]
- de Oliveira, E.F.; Hase, Y. Infrared Study of Magnesium–Nickel Hydroxide Solid Solutions. Vib. Spectrosc. 2003, 31, 19–24. [Google Scholar] [CrossRef]
- Prorok, R.; Ramult, J.; Nocun-Wczelik, W.; Madej, D. The Effect of Chelate Compounds on the Hydration Process of MgO–Al2O3 Phase System under Hydrothermal Conditions. Appl. Sci. 2021, 11, 2834. [Google Scholar] [CrossRef]
- Yousefi, S.; Ghasemi, B.; Tajally, M.; Asghari, A. Optical Properties of MgO and Mg(OH) 2 Nanostructures Synthesized by a Chemical Precipitation Method Using Impure Brine. J. Alloys Compd. 2017, 711, 521–529. [Google Scholar] [CrossRef]
- Sajilal, K.; Moses Ezhil Raj, A. Effect of Thickness on Physico-Chemical Properties of p-NiO (Bunsenite) Thin Films Prepared by the Chemical Spray Pyrolysis (CSP) Technique. Optik (Stuttg). 2016, 127, 1442–1449. [Google Scholar] [CrossRef]
- Taibi, M.; Ammar, S.; Jouini, N.; Fiévet, F.; Molinié, P.; Drillon, M. Layered Nickel Hydroxide Salts: Synthesis, Characterization and Magnetic Behaviour in Relation to the Basal Spacing. J. Mater. Chem. 2002, 12, 3238–3244. [Google Scholar] [CrossRef]
- Andrade, T.M. de; Mariani, F.Q.; Nunes Júnior, C.V.; Dalpasquale, M.; Danczuk, M.; Anaissi, F.J. Compreendendo as Propriedades (Estrutural, Espectroscópica, Colorimétrica e Térmica) de Sais de Níquel. Matéria (Rio Janeiro) 2018, 23. [Google Scholar] [CrossRef]
- Qi, Y.; Qi, H.; Li, J.; Lu, C. Synthesis, Microstructures and UV–Vis Absorption Properties of β-Ni(OH)2 Nanoplates and NiO Nanostructures. J. Cryst. Growth 2008, 310, 4221–4225. [Google Scholar] [CrossRef]
- Gottardi, W.; Nagl, M. Chlorine Covers on Living Bacteria: The Initial Step in Antimicrobial Action of Active Chlorine Compounds. J. Antimicrob. Chemother. 2005, 55, 475–482. [Google Scholar] [CrossRef]
- Brackett, R.E. Antimicrobial Effect of Chlorine on Listeria Monocytogenes. J. Food Prot. 1987, 50, 999–1004. [Google Scholar] [CrossRef]
- Bassioni, G.; Farid, R.; Mohamed, M.; Hammouda, R.M.; Kühn, F.E. Effect of Different Parameters on Caustic Magnesia Hydration and Magnesium Hydroxide Rheology: A Review. Mater. Adv. 2021, 2, 6519–6531. [Google Scholar] [CrossRef]
- Hao, L.; Zhu, C.; Mo, X.; Jiang, W.; Hu, Y.; Zhu, Y.; Chen, Z. Preparation and Characterization of Mg(OH) 2 Nanorods by Liquid–Solid Arc Discharge Technique. Inorg. Chem. Commun. 2003, 6, 229–232. [Google Scholar] [CrossRef]
- Kurt, H.I.; Ergul, E.; Yilmaz, N.F.; Oduncuoglu, M. Surface Functionalization of Nano MgO Particles with Nickel and Cobalt. Mater. Res. Express 2019, 6, 0850f1. [Google Scholar] [CrossRef]
- Murtaza, M.; Aqib, A.I.; Khan, S.R.; Muneer, A.; Ali, M.M.; Waseem, A.; Zaheer, T.; Al-Keridis, L.A.; Alshammari, N.; Saeed, M. Sodium Alginate-Based MgO Nanoparticles Coupled Antibiotics as Safe and Effective Antimicrobial Candidates against Staphylococcus Aureus of Houbara Bustard Birds. Biomedicines 2023, 11, 1959. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.-Y.; Pereira, J.; Huang, Z.; Fan, Q.; Santra, S.; White, J.C.; De La Torre-Roche, R.; Da Silva, S.; Vallad, G.E.; Freeman, J.H.; et al. Potential of Novel Magnesium Nanomaterials to Manage Bacterial Spot Disease of Tomato in Greenhouse and Field Conditions. Plants 2023, 12, 1832. [Google Scholar] [CrossRef]
- Nguyen, N.-Y.T.; Grelling, N.; Wetteland, C.L.; Rosario, R.; Liu, H. Antimicrobial Activities and Mechanisms of Magnesium Oxide Nanoparticles (NMgO) against Pathogenic Bacteria, Yeasts, and Biofilms. Sci. Rep. 2018, 8, 16260. [Google Scholar] [CrossRef]
- Chaudhari, V.P.; Rajput, K.; Mondal Roy, S.; Chaudhuri, T.K.; Roy, D.R. Experimental and First-Principles Investigation on the Structural, Electronic and Antimicrobial Properties of Nickel Hydroxide Nanoparticles. J. Phys. Chem. Solids 2022, 160, 110367. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





