Submitted:
05 September 2023
Posted:
07 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
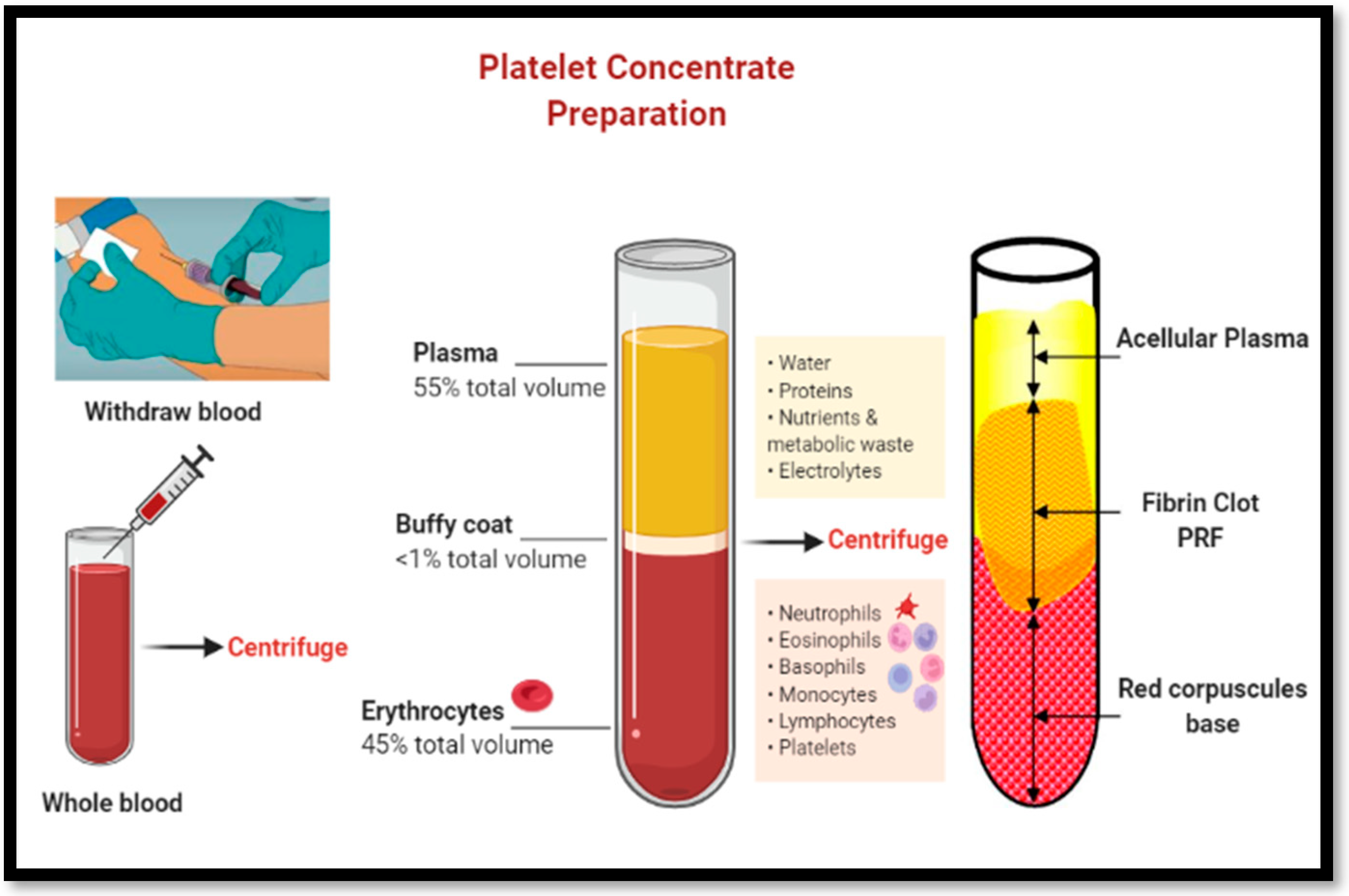
1.1. Use of Platelet-Rich Fibrin (PRF) in Dentistry:
1.2. Improved PRF protocols- A-PRF, A-PRF+ and i-PRF
| PRF Preparation | Tube | RCF (g) | Time (mins) | Speed (rpm) | Evidence |
| Solid Matrix | |||||
| L-PRF | Glass or Silica coated | 408 | 12 | 2700 | Choukroun, 2001 [7] |
| A-PRF | Glass or Silica coated | 194 | 14 | 1500 | Ghanaati et al, 2014 [14] |
| A-PRF+ | Glass or Silica coated | 145 | 8 | 1300 | Fujioka-Kobayashi et al 2017 [15] |
| Liquid/Flowable matrix | |||||
| i-PRF | Plastic (PET) | 60 | 3 | 700 | Miron et al 2017 [9] |
| C-PRF | Plastic (PET) | 408 | 12 | 2700 | Miron et al 2020 [10] |
| Radius (cm) | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| Speed (rpm) | ||||||||||
| 1000 | 45 | 56 | 67 | 78 | 89 | 101 | 112 | 123 | 134 | 145 |
| 1500 | 101 | 126 | 151 | 176 | 201 | 226 | 252 | 277 | 302 | 327 |
| 2000 | 179 | 224 | 268 | 313 | 358 | 402 | 447 | 492 | 537 | 581 |
| 2500 | 280 | 349 | 419 | 489 | 559 | 629 | 699 | 769 | 839 | 908 |
| 3000 | 402 | 503 | 604 | 704 | 805 | 906 | 1006 | 1107 | 1207 | 1308 |
| Growth factors | Functions |
| Transforming Growth factor (TGF) | Growth of endothelial vascular cells, cell recruitment and proliferation in wound healing. Inhibits osteoclast formation and bone resorption. Stimulates fibronectin and collagen production. |
| Epidermal Growth Factor (EGF) | Promotion of mesenchymal cell proliferation and differentiation, epithelial cell growth and angiogenesis |
| Vascular Endothelial Growth Fcator (VEGF) | Restores oxygen supply to the injured tissue. Promotes repair and growth of vascular endothelial cells, angiogenesis |
| Platelet Derived Growth Factors (PDGF) | Cell growth, proliferation of smooth muscle cells within vascular tissue, angiogenesis, collagen production Provokes proliferation of mesenchymal cell lineage, enablers macrophage chemotaxis |
| Insulin-like Growth Factor (IGF) | Cell proliferation, cell to cell communications, stimulates chemotaxis and activation of osteoblasts and bone formation, induces mitogenesis of mesenchymal cells |
| Fibroblast Growth Factor (FGF) | Tissue Repair, Cell Growth, Hyaluronic acid and Collagen production |
1.3. Mode of action and biological effects of PRF:
2. Materials and Methods
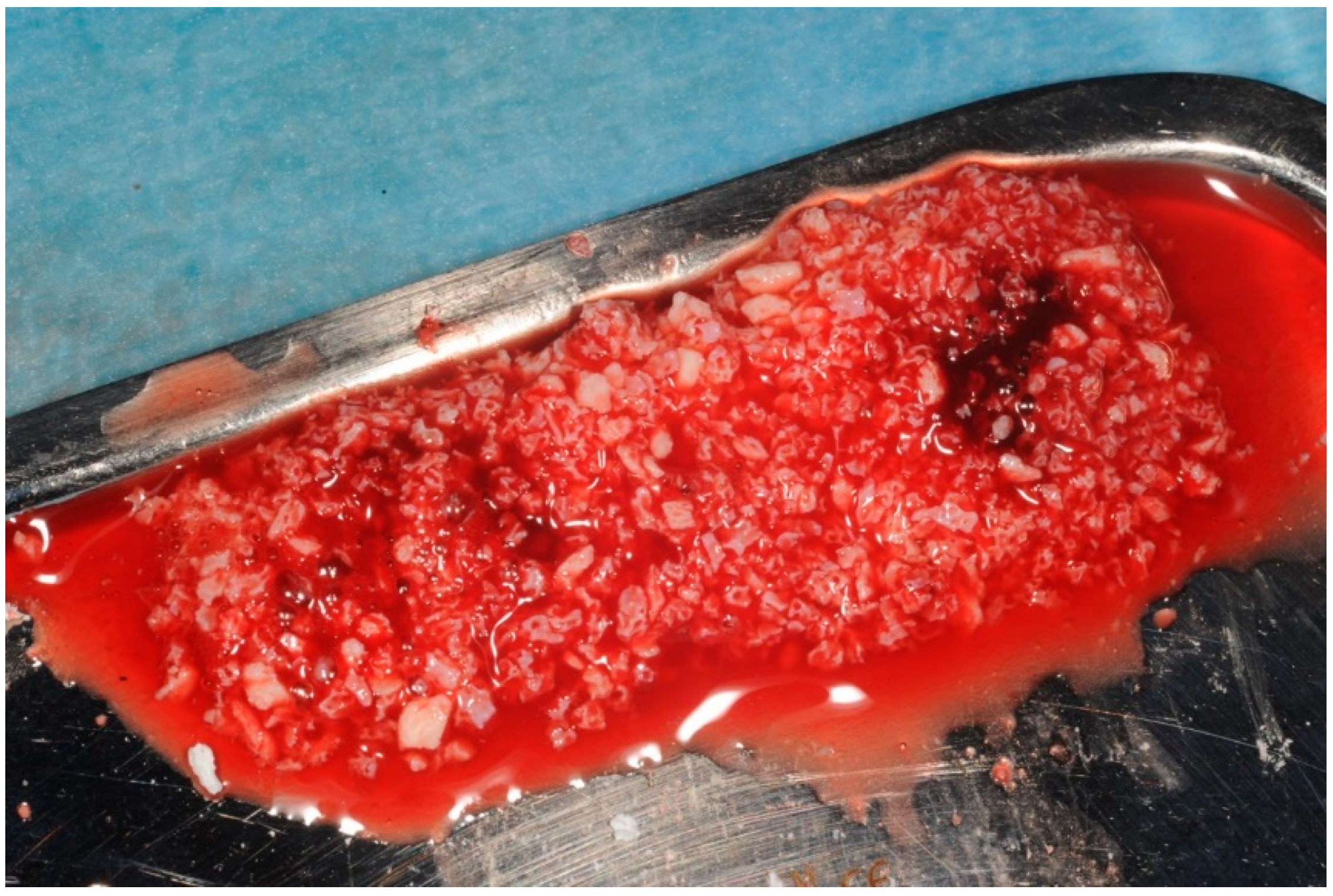
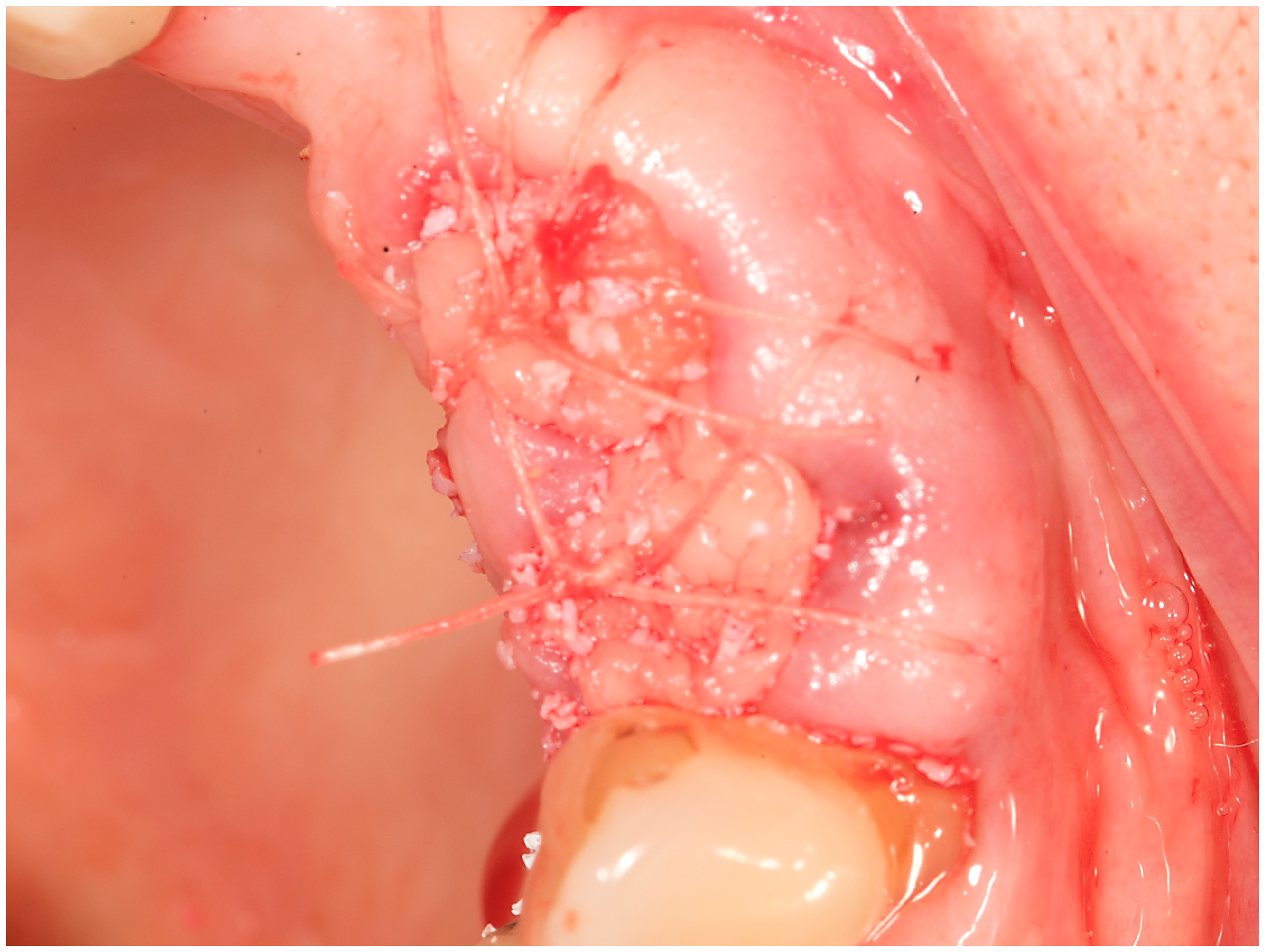
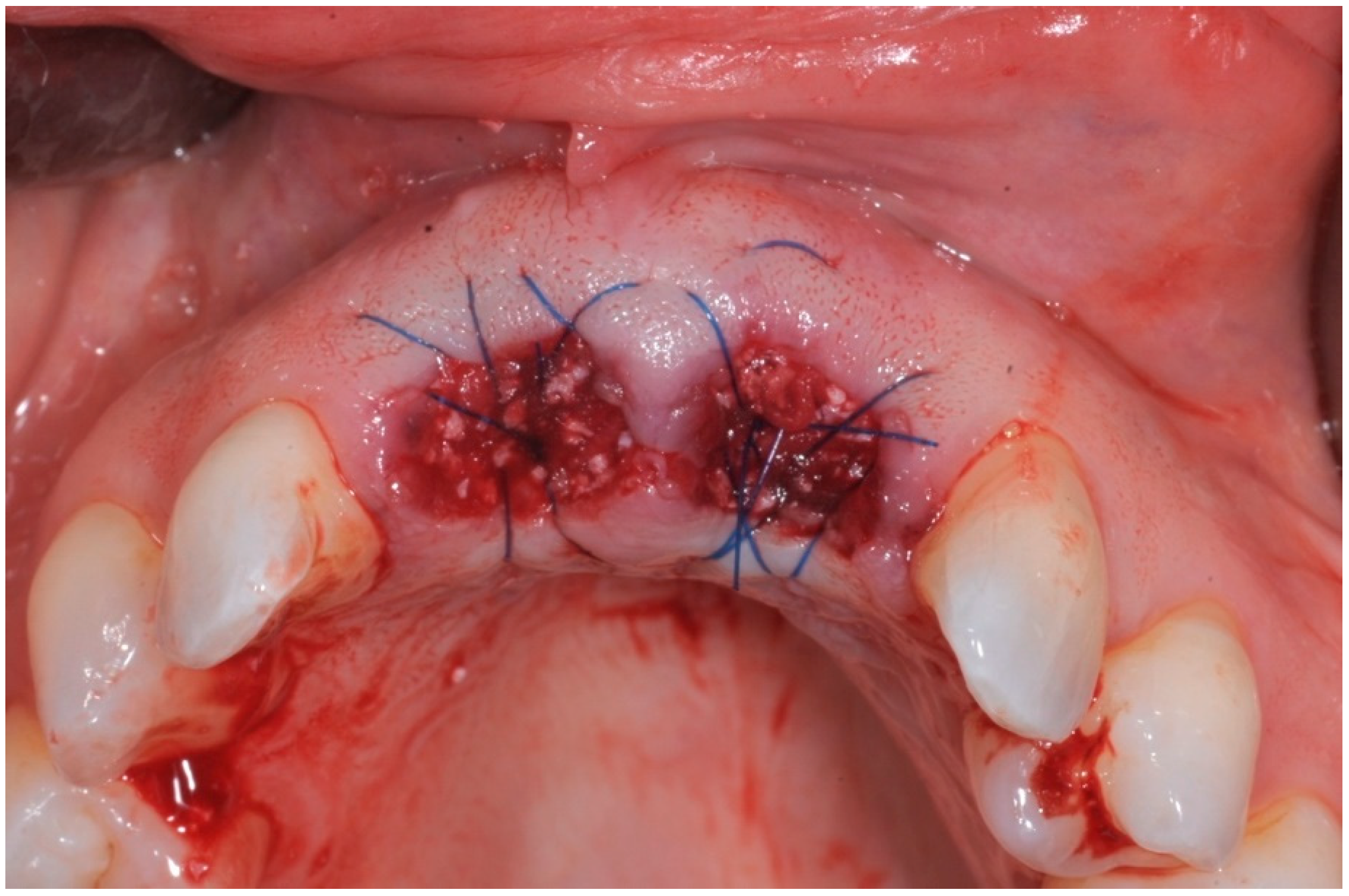
- I)
-
Primary outcome measures:
- a)
- radiological assessment of bone quality and quantity after SA using cone beam computerized tomography (CBCT),
- b)
- bone quality and primary implant stability during implant placement surgery,
- c)
- early placement and loading of implants and
- II)
-
Secondary outcome measures:
- a)
- implant mobility at the time of loading
- b)
- need for additional grafting at the time of implantation
- c)
- radiological assessment of implant integration and marginal bone integrity after loading.
Results
Case study 1
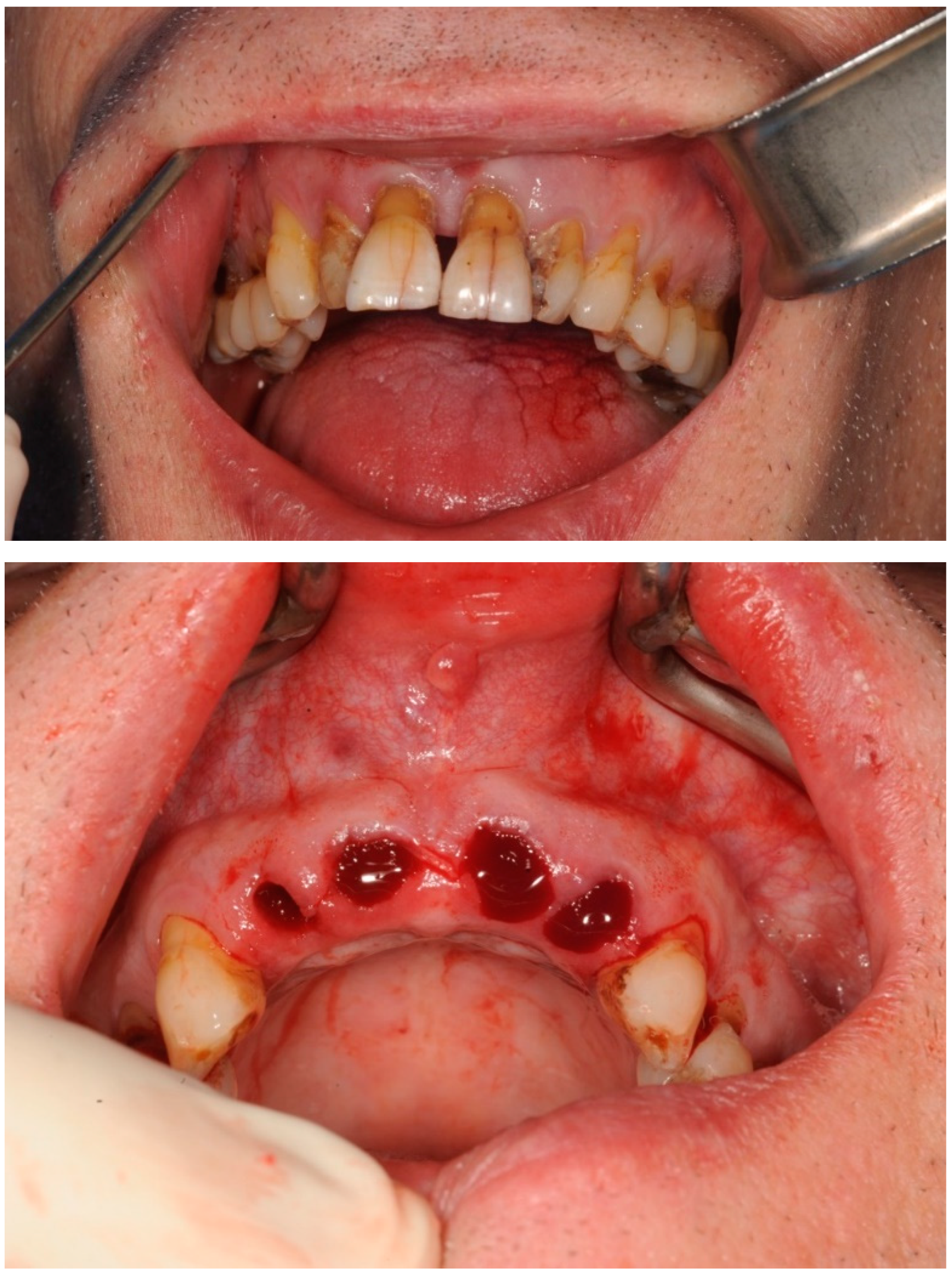
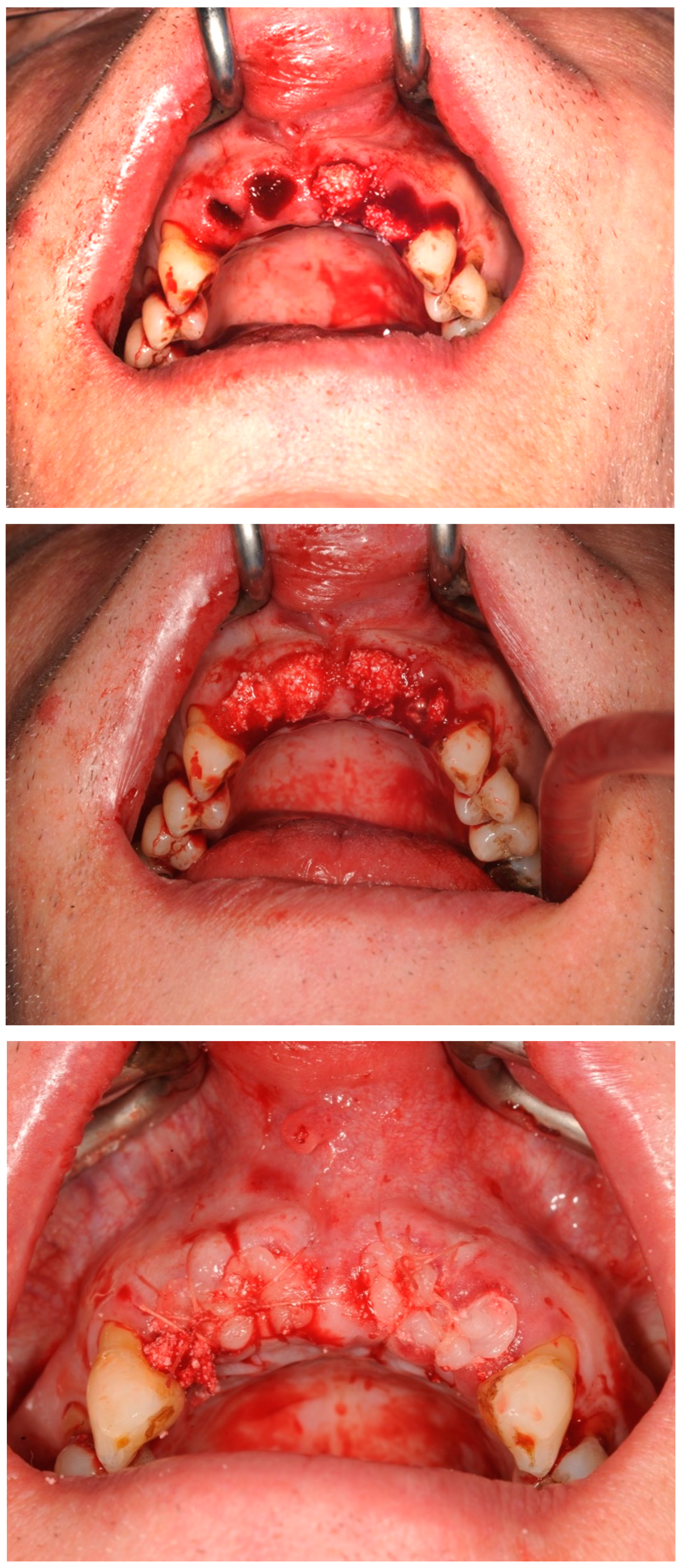
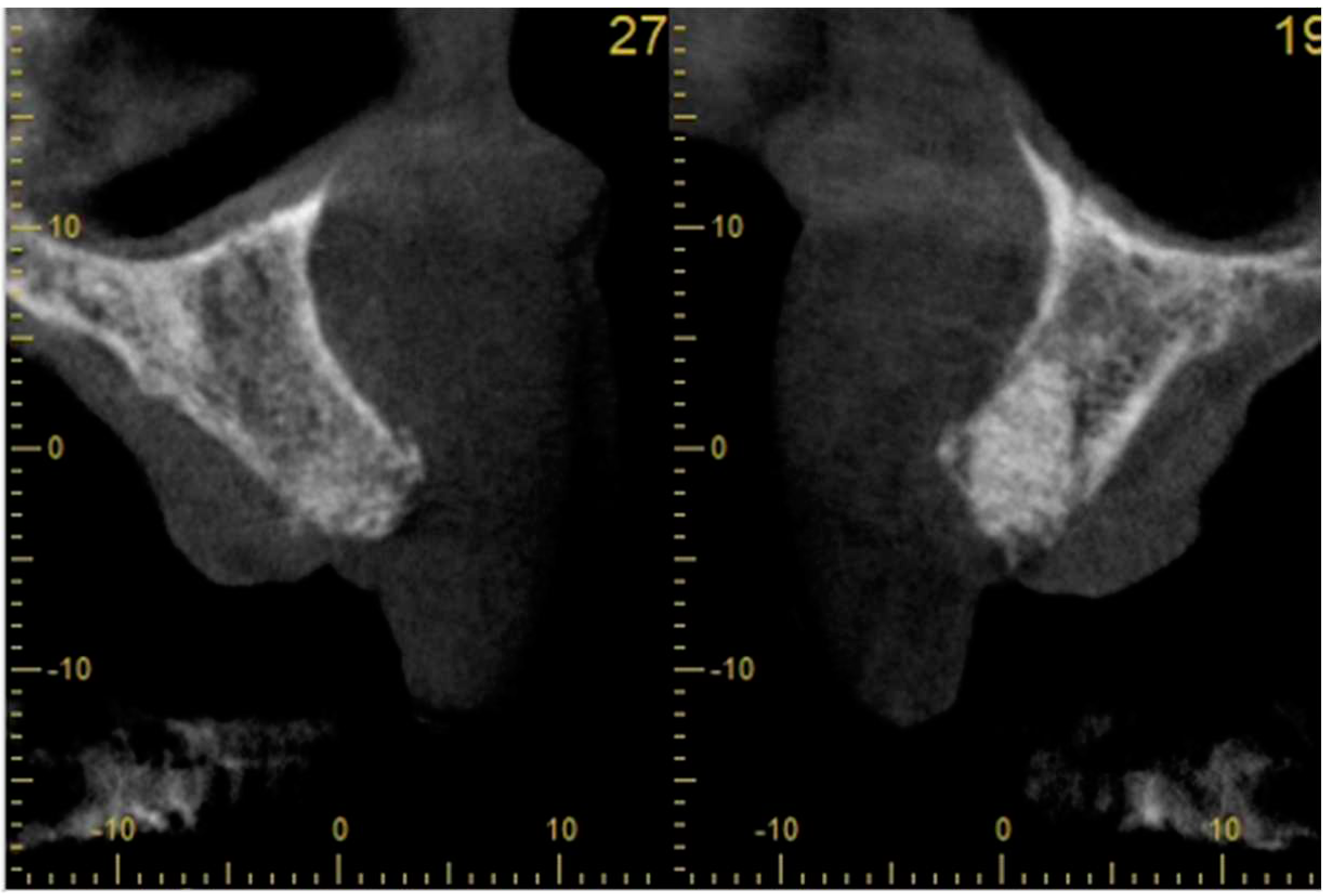
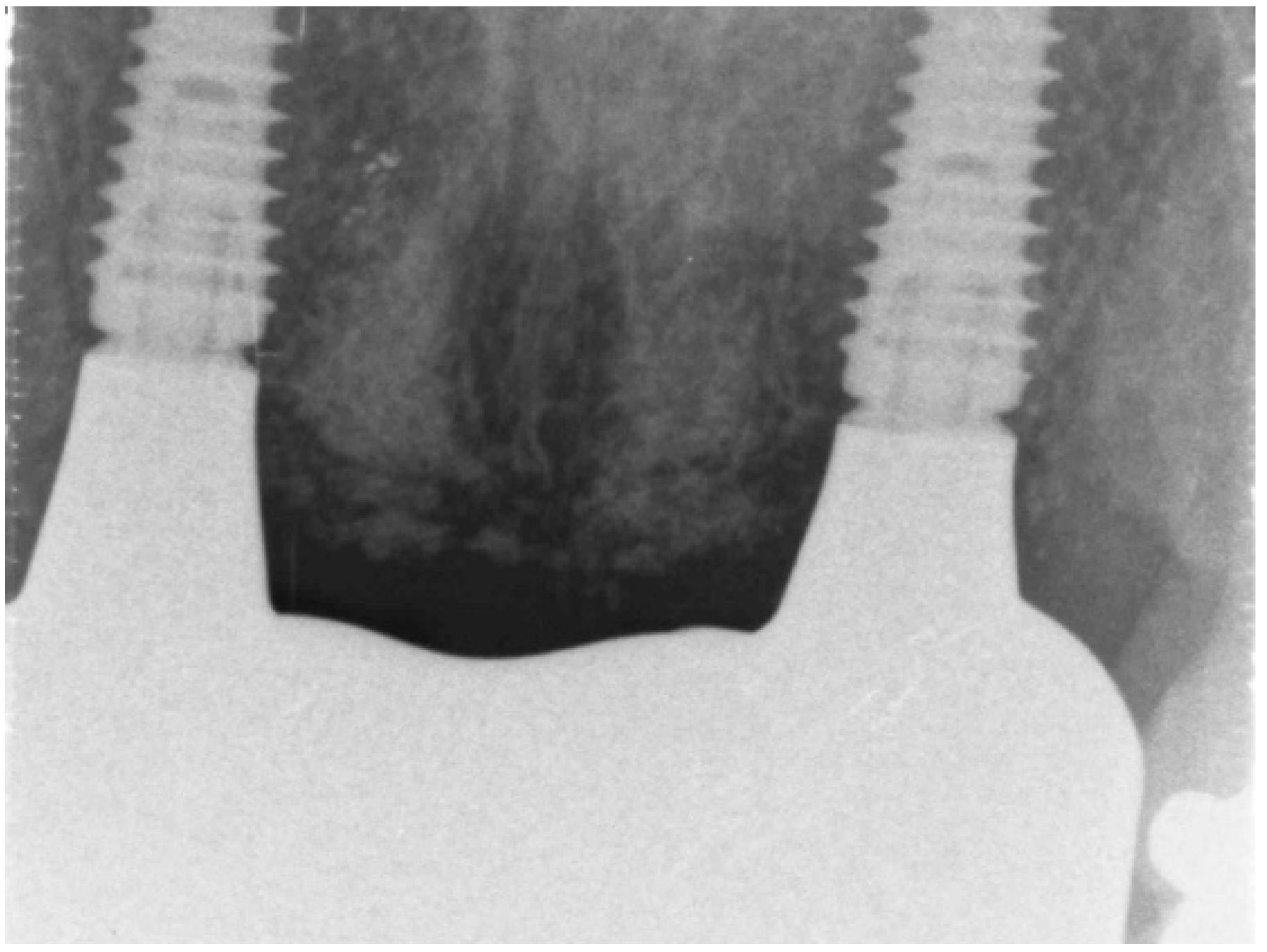
3.2. Case Study 2
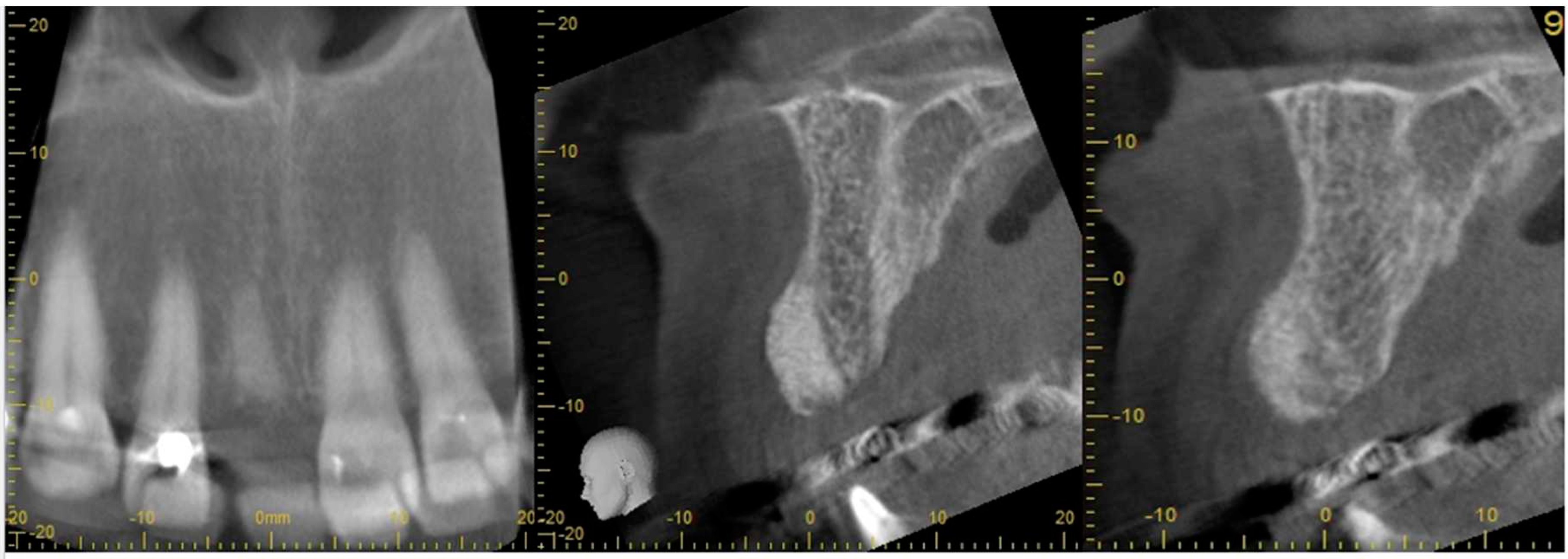
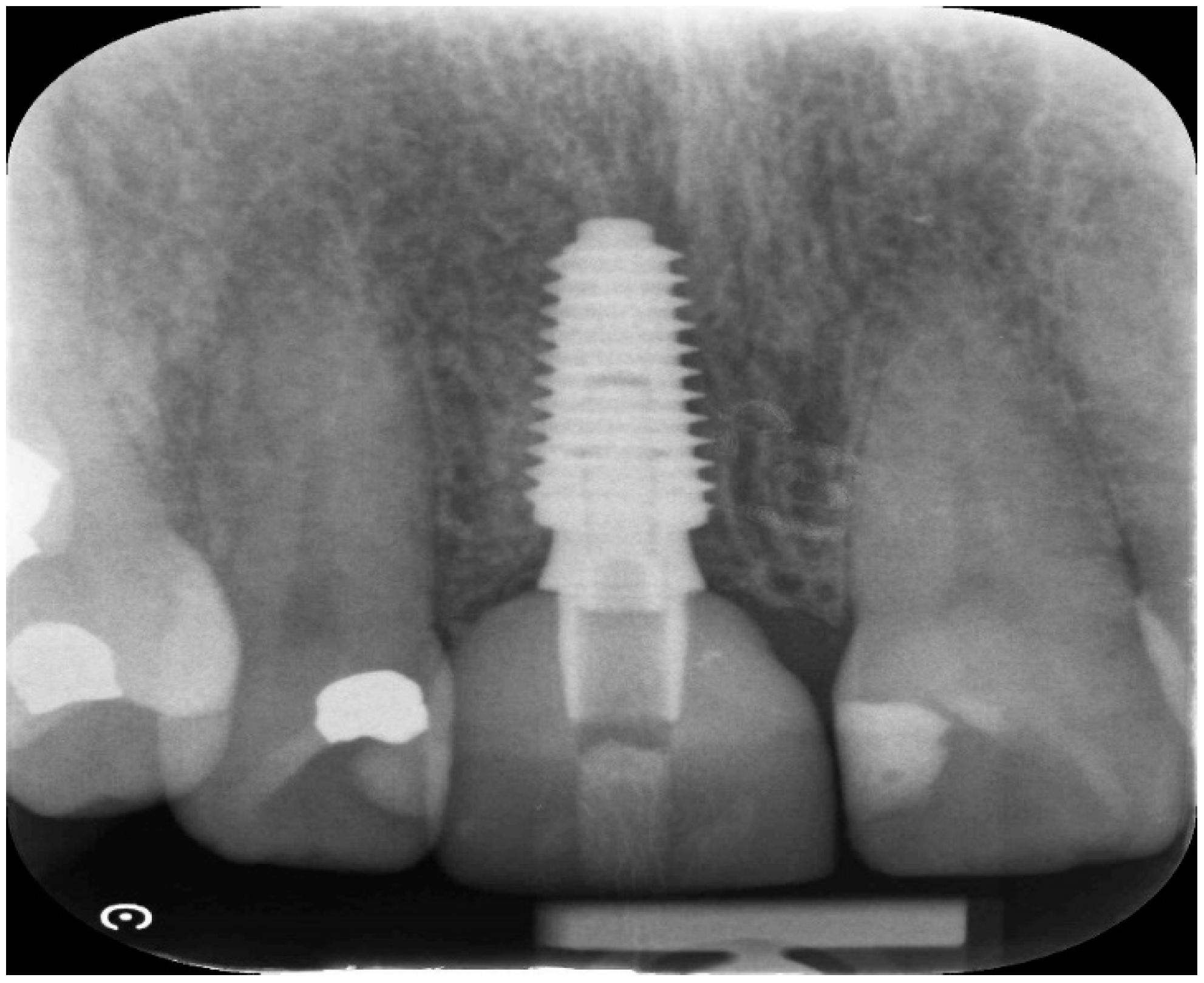
| Case No | Outcomes: | ||||||||
| Case No: | SA | BQn | BQ | PS | Surgery BQ | Secondary Grafting | Time since SA | Early Loading | CBS |
| Case 1 | open SA | Full contour | excellent | high | D2-3 | None | 8 weeks | 6 weeks | No crestal bone loss |
| Case 2 implant 1 | open SA | Full contour | excellent | high | D2-4 | None | 9 weeks | 7 weeks | No crestal bone loss |
| Case 2 implant 2 | open SA | Full contour | excellent | high | D2-5 | None | 10 weeks | 8 weeks | No crestal bone loss |
4. Discussion
Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, H. Y., & Chang, Y. C. (2022). A Bibliometric Analysis of Platelet-Rich Fibrin in Dentistry. International journal of environmental research and public health, 19(19), 12545. https://doi.org/10.3390/ijerph191912545. [CrossRef]
- Wang, X., Zhang, Y., Choukroun, J., Ghanaati, S., & Miron, R. J. (2018). Effects of an injectable platelet-rich fibrin on osteoblast behavior and bone tissue formation in comparison to platelet-rich plasma. Platelets, 29(1), 48–55. https://doi.org/10.1080/09537104.2017.1293807. [CrossRef]
- Pavlovic V, Ciric M, Jovanovic V, Trandafilovic M, Stojanovic P. Platelet-rich fibrin: Basics of biological actions and protocol modifications. Open Med (Wars). 2021 Mar 22;16(1):446-454. doi: 10.1515/med-2021-0259. PMID: 33778163; PMCID: PMC7985567. [CrossRef] [PubMed]
- Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998;85(6):638-646.
- ANITUA, E., TEJERO, R., ALKHRAISAT, M. H. & ORIVE, G. 2013. Platelet-rich plasma to improve the bio-functionality of biomaterials. BioDrugs, 27, 97-111.
- Miron, R. J., Zucchelli, G., Pikos, M. A., Salama, M., Lee, S., Guillemette, V., Fujioka-Kobayashi, M., Bishara, M., Zhang, Y., Wang, H. L., Chandad, F., Nacopoulos, C., Simonpieri, A., Aalam, A. A., Felice, P., Sammartino, G., Ghanaati, S., Hernandez, M. A., & Choukroun, J. (2017). Use of platelet-rich fibrin in regenerative dentistry: a systematic review. Clinical oral investigations, 21(6), 1913–1927. https://doi.org/10.1007/s00784-017-2133-z. [CrossRef]
- Choukroun, J., & Ghanaati, S. (2018). Reduction of relative centrifugation force within injectable platelet-rich-fibrin (PRF) concentrates advances patients’ own inflammatory cells, platelets and growth factors: the first introduction to the low speed centrifugation concept. European journal of trauma and emergency surgery : official publication of the European Trauma Society, 44(1), 87–95. https://doi.org/10.1007/s00068-017-0767-9. [CrossRef]
- Fujioka-Kobayashi, M., Miron, R. J., Hernandez, M., Kandalam, U., Zhang, Y., & Choukroun, J. (2017). Optimized Platelet-Rich Fibrin With the Low-Speed Concept: Growth Factor Release, Biocompatibility, and Cellular Response. Journal of periodontology, 88(1), 112–121. https://doi.org/10.1902/jop.2016.160443. [CrossRef]
- Miron, R. J., Fujioka-Kobayashi, M., Hernandez, M., Kandalam, U., Zhang, Y., Ghanaati, S., & Choukroun, J. (2017). Injectable platelet rich fibrin (i-PRF): opportunities in regenerative dentistry?. Clinical oral investigations, 21(8), 2619–2627. https://doi.org/10.1007/s00784-017-2063-9. [CrossRef]
- Miron, R. J., Chai, J., Zhang, P., Li, Y., Wang, Y., Mourão, C. F. A. B., Sculean, A., Fujioka Kobayashi, M., & Zhang, Y. (2020). A novel method for harvesting concentrated platelet-rich fibrin (C-PRF) with a 10-fold increase in platelet and leukocyte yields. Clinical oral investigations, 24(8), 2819–2828. https://doi.org/10.1007/s00784-019-03147-w. [CrossRef]
- Quirynen M, Pinto NR. Leukocyte-and Platelet-Rich Fibrin in Oral Regenerative Procedures: Evidence-Based Clinical Guidelines. Quintessenz Verlag; 2022 Jun 14.
- (adapted from https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/232/100/rpm-converted-to-g-force-mk.pdf last accessed 06 04 2023 ).
- Xuzhu Wang, Yufeng Zhang, Joseph Choukroun, Shahram Ghanaati & Richard J. Miron (2017): Effects of an injectable platelet-rich fibrin on osteoblast behavior and bone tissue formation in comparison to platelet-rich plasma, Platelets, DOI: 10.1080/09537104.2017.1293807. [CrossRef]
- Choukroun, J., & Ghanaati, S. (2018). Reduction of relative centrifugation force within injectable platelet-rich-fibrin (PRF) concentrates advances patients’ own inflammatory cells, platelets and growth factors: the first introduction to the low speed centrifugation concept. European journal of trauma and emergency surgery : official publication of the European Trauma Society, 44(1), 87–95. https://doi.org/10.1007/s00068-017-0767-9. [CrossRef]
- Fujioka-Kobayashi, M., Miron, R. J., Hernandez, M., Kandalam, U., Zhang, Y., & Choukroun, J. (2017). Optimized Platelet-Rich Fibrin With the Low-Speed Concept: Growth Factor Release, Biocompatibility, and Cellular Response. Journal of periodontology, 88(1), 112–121. https://doi.org/10.1902/jop.2016.160443. [CrossRef]
- Kobayashi E, Flückiger L, Fujioka-Kobayashi M, Sawada K, Sculean A, Schaller B, Miron RJ. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig. 2016 Dec;20(9):2353-2360. doi: 10.1007/s00784-016-1719-1. Epub 2016 Jan 25. PMID: 26809431. [CrossRef] [PubMed]
- Fujioka-Kobayashi M, Miron RJ, Hernandez M, Kandalam U, Zhang Y, Choukroun J. Optimized Platelet-Rich Fibrin With the Low-Speed Concept: Growth Factor Release, Biocompatibility, and Cellular Response. J Periodontol. 2017 Jan;88(1):112-121. doi: 10.1902/jop.2016.160443. Epub 2016 Sep 2. PMID: 27587367. [CrossRef] [PubMed]
- Miron RJ, Dham A, Dham U, Zhang Y, Pikos MA, Sculean A. The effect of age, gender, and time between blood draw and start of centrifugation on the size outcomes of platelet-rich fibrin (PRF) membranes. Clin Oral Investig. 2019 May;23(5):2179-2185. doi: 10.1007/s00784-018-2673-x. Epub 2018 Oct 2. PMID: 30280327. [CrossRef] [PubMed]
- Miron, R. J., Chai, J., Zheng, S., Feng, M., Sculean, A., & Zhang, Y. (2019). A novel method for evaluating and quantifying cell types in platelet rich fibrin and an introduction to horizontal centrifugation. Journal of biomedical materials research. Part A, 107(10), 2257–2271. https://doi.org/10.1002/jbm.a.36734. [CrossRef]
- Miron, R. J., Fujioka-Kobayashi, M., Hernandez, M., Kandalam, U., Zhang, Y., Ghanaati, S., & Choukroun, J. (2017). Injectable platelet rich fibrin (i-PRF): opportunities in regenerative dentistry?. Clinical oral investigations, 21(8), 2619–2627. https://doi.org/10.1007/s00784-017-2063-9. [CrossRef]
- Kobayashi, E., Flückiger, L., Fujioka-Kobayashi, M., Sawada, K., Sculean, A., Schaller, B., & Miron, R. J. (2016). Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clinical oral investigations, 20(9), 2353–2360. https://doi.org/10.1007/s00784-016-1719-1. [CrossRef]
- Miron, R. J., Chai, J., Zhang, P., Li, Y., Wang, Y., Mourão, C. F. A. B., Sculean, A., Fujioka Kobayashi, M., & Zhang, Y. (2020). A novel method for harvesting concentrated platelet-rich fibrin (C-PRF) with a 10-fold increase in platelet and leukocyte yields. Clinical oral investigations, 24(8), 2819–2828. https://doi.org/10.1007/s00784-019-03147-w. [CrossRef]
- Tunalı M, Özdemir H, Küçükodacı Z, Akman S, Yaprak E, Toker H, Fıratlı E. A novel platelet concentrate: titanium-prepared platelet-rich fibrin. Biomed Res Int. 2014;2014:209548. doi: 10.1155/2014/209548. Epub 2014 Jan 21. PMID: 24563860; PMCID: PMC3915853. [CrossRef] [PubMed]
- You JS, Kim SG, Oh JS, Kim JS. Effects of Platelet-Derived Material (Platelet-Rich Fibrin) on Bone Regeneration. Implant Dent. 2019 Jun;28(3):244-255. doi: 10.1097/ID.0000000000000877. PMID: 31124821. [CrossRef] [PubMed]
- Şimşek S, Özeç İ, Kürkçü M, Benlidayı E. Histomorphometric Evaluation of Bone Formation in Peri-Implant Defects Treated With Different Regeneration Techniques: An Experimental Study in a Rabbit Model. J Oral Maxillofac Surg. 2016 Sep;74(9):1757-64. doi: 10.1016/j.joms.2016.05.026. Epub 2016 Jun 7. PMID: 27351696. [CrossRef] [PubMed]
- Oliveira MR, deC Silva A, Ferreira S, Avelino CC, Garcia IR Jr, Mariano RC. Influence of the association between platelet-rich fibrin and bovine bone on bone regeneration. A histomorphometric study in the calvaria of rats. Int J Oral Maxillofac Surg. 2015 May;44(5):649-55. doi: 10.1016/j.ijom.2014.12.005. Epub 2014 Dec 30. PMID: 25553712. [CrossRef] [PubMed]
- Miron, R. J., Zucchelli, G., Pikos, M. A., Salama, M., Lee, S., Guillemette, V., Fujioka-Kobayashi, M., Bishara, M., Zhang, Y., Wang, H. L., Chandad, F., Nacopoulos, C., Simonpieri, A., Aalam, A. A., Felice, P., Sammartino, G., Ghanaati, S., Hernandez, M. A., & Choukroun, J. (2017). Use of platelet-rich fibrin in regenerative dentistry: a systematic review. Clinical oral investigations, 21(6), 1913–1927. https://doi.org/10.1007/s00784-017-2133-z. [CrossRef]
- Phuong Tran TT, Vu Pham TA. Effect of advanced and injectable platelet-rich fibrins against Aggregatibacter actinomycetemcomitans in subjects with or without periodontal diseases. J Dent Sci. 2023 Apr;18(2):491-496. doi: 10.1016/j.jds.2022.09.014. Epub 2022 Oct 6. PMID: 37021261; PMCID: PMC10068356. [CrossRef] [PubMed]
- Zwittnig, K., Kirnbauer, B., Jakse, N., Schlenke, P., Mischak, I., Ghanaati, S., Al-Maawi, S., Végh, D., Payer, M., & Zrnc, T. A. (2022). Growth Factor Release within Liquid and Solid PRF. Journal of clinical medicine, 11(17), 5070. https://doi.org/10.3390/jcm11175070. [CrossRef]
- ANITUA, E., TEJERO, R., ALKHRAISAT, M. H. & ORIVE, G. 2013. Platelet-rich plasma to improve the bio-functionality of biomaterials. BioDrugs, 27, 97-111.
- Sammartino, G., Dohan Ehrenfest, D. M., Carile, F., Tia, M., & Bucci, P. (2011). Prevention of hemorrhagic complications after dental extractions into open heart surgery patients under anticoagulant therapy: the use of leukocyte- and platelet-rich fibrin. The Journal of oral implantology, 37(6), 681–690. https://doi.org/10.1563/AAID-JOI-D-11-00001. [CrossRef]
- Yuan, S., Li, Q., Chen, K. et al. Ridge preservation applying a novel hydrogel for early angiogenesis and osteogenesis evaluation: an experimental study in canine. J Biol Eng 15, 19 (2021). https://doi.org/10.1186/s13036-021-00271-8. [CrossRef]
- Temmerman, A., Vandessel, J., Castro, A., Jacobs, R., Teughels, W., Pinto, N., & Quirynen, M. (2016). The use of leucocyte and platelet-rich fibrin in socket management and ridge preservation: a split-mouth, randomized, controlled clinical trial. Journal of clinical periodontology, 43(11), 990–999. https://doi.org/10.1111/jcpe.12612. [CrossRef]
- Chenchev IL, Ivanova VV, Neychev DZ, Cholakova RB. Application of Platelet-Rich Fibrin and Injectable Platelet-Rich Fibrin in Combination of Bone Substitute Material for Alveolar Ridge Augmentation - a Case Report. Folia Med (Plovdiv). 2017 Sep 1;59(3):362-366. doi: 10.1515/folmed-2017-0044. PMID: 28976904. [CrossRef] [PubMed]
- Anitua E, Murias-Freijo A, Alkhraisat MH, Orive G. Clinical, radiographical, and histological outcomes of plasma rich in growth factors in extraction socket: a randomized controlled clinical trial. Clin Oral Investig. 2015 Apr;19(3):589-600. doi: 10.1007/s00784-014-1278-2. Epub 2014 Jul 8. PMID: 24998770. [CrossRef] [PubMed]
- Chappuis, V., Engel, O., Reyes, M., Shahim, K., Nolte, L. P., & Buser, D. (2013). Ridge alterations post-extraction in the esthetic zone: a 3D analysis with CBCT. Journal of dental research, 92(12 Suppl), 195S–201S. https://doi.org/10.1177/0022034513506713. [CrossRef]
- Castro, A. B., Van Dessel, J., Temmerman, A., Jacobs, R., & Quirynen, M. (2021). Effect of different platelet-rich fibrin matrices for ridge preservation in multiple tooth extractions: A split-mouth randomized controlled clinical trial. Journal of clinical periodontology, 48(7), 984–995. https://doi.org/10.1111/jcpe.13463. [CrossRef]
- Wu CL, Lee SS, Tsai CH, Lu KH, Zhao JH, Chang YC. Platelet-rich fibrin increases cell attachment, proliferation and collagen-related protein expression of human osteoblasts. Aust Dent J. 2012 Jun;57(2):207-12. doi: 10.1111/j.1834-7819.2012.01686.x. PMID: 22624763. [CrossRef] [PubMed]
- Al-Maawi, S., Becker, K., Schwarz, F., Sader, R., & Ghanaati, S. (2021). Efficacy of platelet-rich fibrin in promoting the healing of extraction sockets: a systematic review. International journal of implant dentistry, 7(1), 117. https://doi.org/10.1186/s40729-021-00393-0. [CrossRef]
- Clark D, Rajendran Y, Paydar S, Ho S, Cox D, Ryder M, Dollard J, Kao RT. Advanced platelet-rich fibrin and freeze-dried bone allograft for ridge preservation: A randomized controlled clinical trial. J Periodontol. 2018 Apr;89(4):379-387. doi: 10.1002/JPER.17-0466. PMID: 29683498; PMCID: PMC6483085. [CrossRef] [PubMed]
- Yu, H. Y., & Chang, Y. C. (2022). A Bibliometric Analysis of Platelet-Rich Fibrin in Dentistry. International journal of environmental research and public health, 19(19), 12545. https://doi.org/10.3390/ijerph191912545. [CrossRef]
- Bennardo, F., Gallelli, L., Palleria, C. et al. Can platelet-rich fibrin act as a natural carrier for antibiotics delivery? A proof-of-concept study for oral surgical procedures. BMC Oral Health 23, 134 (2023). https://doi.org/10.1186/s12903-023-02814-5. [CrossRef]
- Hauser F, Gaydarov N, Badoud I, Vazquez L, Bernard JP, Ammann P. Clinical and histological evaluation of postextraction platelet-rich fibrin socket filling: a prospective randomized controlled study. Implant Dent. 2013 Jun;22(3):295-303. doi: 10.1097/ID.0b013e3182906eb3. PMID: 23644909. [CrossRef] [PubMed]
- Sisti A, Canullo L, Mottola MP, Covani U, Barone A, Botticelli D. Clinical evaluation of a ridge augmentation procedure for the severely resorbed alveolar socket: multicenter randomized controlled trial, preliminary results. Clin Oral Implants Res. 2012 May;23(5):526-35. doi: 10.1111/j.1600-0501.2011.02386.x. Epub 2011 Dec 8. PMID: 22150876. [CrossRef] [PubMed]
- Mardas N, Trullenque-Eriksson A, MacBeth N, Petrie A, Donos N. Does ridge preservation following tooth extraction improve implant treatment outcomes: a systematic review: Group 4: Therapeutic concepts & methods. Clin Oral Implants Res. 2015 Sep;26 Suppl 11:180-201. doi: 10.1111/clr.12639. Epub 2015 Jun 16. PMID: 26078004. [CrossRef] [PubMed]
- Ucer, C.; Khan, R.S. Extraction Socket Augmentation with Autologous Platelet-Rich Fibrin (PRF): The Rationale for Socket Augmentation. Dent. J. 2023, 11, 196. https://doi.org/10.3390/dj11080196. [CrossRef]
- Chenchev IL, Ivanova VV, Neychev DZ, Cholakova RB. Application of Platelet-Rich Fibrin and Injectable Platelet-Rich Fibrin in Combination of Bone Substitute Material for Alveolar Ridge Augmentation - a Case Report. Folia Med (Plovdiv). 2017 Sep 1;59(3):362-366. doi: 10.1515/folmed-2017-0044. PMID: 28976904. [CrossRef] [PubMed]
- Temmerman A, Vandessel J, Castro A, Jacobs R, Teughels W, Pinto N, Quirynen M. The use of leucocyte and platelet-rich fibrin in socket management and ridge preservation: a split-mouth, randomized, controlled clinical trial. J Clin Periodontol. 2016 Nov;43(11):990-999. doi: 10.1111/jcpe.12612. Epub 2016 Sep 21. PMID: 27509214. [CrossRef] [PubMed]
- Öncü E, Alaaddinoğlu EE. The effect of platelet-rich fibrin on implant stability. Int J Oral Maxillofac Implants. 2015 May-Jun;30(3):578-82. doi: 10.11607/jomi.3897. PMID: 26009908. [CrossRef] [PubMed]
- Öncü E, Erbeyoğlu AA. Enhancement of Immediate Implant Stability and Recovery Using Platelet-Rich Fibrin. Int J Periodontics Restorative Dent. 2019 March/April;39(2):e58–e63. doi: 10.11607/prd.2505. Epub 2017 Feb 14. PMID: 28196154. [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





