Submitted:
05 September 2023
Posted:
07 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Gene Search Strategy
2.2. Data Extraction and methodology assessment
2.3. Genetic study
2.4. Rechecking data in genetic resources
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wells, S.A. Progress in Endocrine Neoplasia. Clin Cancer Res 2016, 22, 4981–4988. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, H.; Wang, M.; Li, N.; Tian, T.; Wu, Y.; Xu, P.; Yang, S.; Zhai, Z.; Zhou, L.; et al. Global Burden of Thyroid Cancer From 1990 to 2017. JAMA Netw Open 2020, 3, e208759. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.; Morris, L.G.T.; Haymart, M.; Chen, A.Y.; Goldenberg, D.; Morris, J.; Ogilvie, J.B.; Terris, D.J.; Netterville, J.; Wong, R.J.; et al. American Association of Clinical Endocrinologists and American College of Endocrinology Disease State Clinical Review: The Increasing Incidence of Thyroid Cancer. Endocrine Practice 2015, 21, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Megwalu, U.C.; Moon, P.K. Thyroid Cancer Incidence and Mortality Trends in the United States: 2000-2018. Thyroid 2022, 32, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Tofe, S.; Argüelles, I.; Forteza, A.; Alvarez, C.; Repetto, A.; Masmiquel, L.; Rodríguez, I.; Losada, E.; Sukunza, N.; Cabrer, M.; et al. Age-Standardized Incidence, Mortality Rate and Trend Changes of Thyroid Cancer in the Balearic Islands during the 2000-2020 Period: A Population-Based Study. Eur Thyroid J 2023, ETJ-22-0183. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Vriens, M.R.; Suh, I.; Moses, W.; Kebebew, E. Clinical Features and Genetic Predisposition to Hereditary Nonmedullary Thyroid Cancer. Thyroid 2009, 19, 1343–1349. [Google Scholar] [CrossRef]
- Hińcza, K.; Kowalik, A.; Kowalska, A. Current Knowledge of Germline Genetic Risk Factors for the Development of Non-Medullary Thyroid Cancer. Genes 2019, 10, 482. [Google Scholar] [CrossRef]
- Borowczyk, M.; Szczepanek-Parulska, E.; Dębicki, S.; Budny, B.; Janicka-Jedyńska, M.; Gil, L.; Verburg, F.A.; Filipowicz, D.; Wrotkowska, E.; Majchrzycka, B.; et al. High Incidence of FLT3 Mutations in Follicular Thyroid Cancer: Potential Therapeutic Target in Patients with Advanced Disease Stage. Ther Adv Med Oncol 2020, 12, 1758835920907534. [Google Scholar] [CrossRef]
- Capezzone, M.; Robenshtok, E.; Cantara, S.; Castagna, M.G. Familial Non-Medullary Thyroid Cancer: A Critical Review. J Endocrinol Invest 2021, 44, 943–950. [Google Scholar] [CrossRef]
- Guilmette, J.; Nosé, V. Hereditary and Familial Thyroid Tumours. Histopathology 2018, 72, 70–81. [Google Scholar] [CrossRef]
- Kamani, T.; Charkhchi, P.; Zahedi, A.; Akbari, M.R. Genetic Susceptibility to Hereditary Non-Medullary Thyroid Cancer. Hered Cancer Clin Pract 2022, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network Integrated Genomic Characterization of Papillary Thyroid Carcinoma. Cell 2014, 159, 676–690. [CrossRef]
- Prete, A.; Borges de Souza, P.; Censi, S.; Muzza, M.; Nucci, N.; Sponziello, M. Update on Fundamental Mechanisms of Thyroid Cancer. Front Endocrinol (Lausanne) 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- 1000 Genomes Project Consortium; Auton, A.; Brooks, L.D.; Durbin, R.M.; Garrison, E.P.; Kang, H.M.; Korbel, J.O.; Marchini, J.L.; McCarthy, S.; McVean, G.A.; et al. A Global Reference for Human Genetic Variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef]
- Popejoy, A.B.; Fullerton, S.M. Genomics Is Failing on Diversity. Nature 2016, 538, 161–164. [Google Scholar] [CrossRef]
- Kaja, E.; Lejman, A.; Sielski, D.; Sypniewski, M.; Gambin, T.; Dawidziuk, M.; Suchocki, T.; Golik, P.; Wojtaszewska, M.; Mroczek, M.; et al. The Thousand Polish Genomes-A Database of Polish Variant Allele Frequencies. Int J Mol Sci 2022, 23, 4532. [Google Scholar] [CrossRef]
- Bonora, E.; Tallini, G.; Romeo, G. Genetic Predisposition to Familial Nonmedullary Thyroid Cancer: An Update of Molecular Findings and State-of-the-Art Studies. J Oncol 2010, 2010, 385206. [Google Scholar] [CrossRef]
- Goldgar, D.E.; Easton, D.F.; Cannon-Albright, L.A.; Skolnick, M.H. Systematic Population-Based Assessment of Cancer Risk in First-Degree Relatives of Cancer Probands. J Natl Cancer Inst 1994, 86, 1600–1608. [Google Scholar] [CrossRef]
- Moses, W.; Weng, J.; Kebebew, E. Prevalence, Clinicopathologic Features, and Somatic Genetic Mutation Profile in Familial versus Sporadic Nonmedullary Thyroid Cancer. Thyroid 2011, 21, 367–371. [Google Scholar] [CrossRef]
- Mazeh, H.; Benavidez, J.; Poehls, J.L.; Youngwirth, L.; Chen, H.; Sippel, R.S. In Patients with Thyroid Cancer of Follicular Cell Origin, a Family History of Nonmedullary Thyroid Cancer in One First-Degree Relative Is Associated with More Aggressive Disease. Thyroid 2012, 22, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Peiling Yang, S.; Ngeow, J. Familial Non-Medullary Thyroid Cancer: Unraveling the Genetic Maze. Endocrine-Related Cancer 2016, 23, R577–R595. [Google Scholar] [CrossRef]
- Park, Y.J.; Ahn, H.Y.; Choi, H.S.; Kim, K.W.; Park, D.J.; Cho, B.Y. The Long-Term Outcomes of the Second Generation of Familial Nonmedullary Thyroid Carcinoma Are More Aggressive than Sporadic Cases. Thyroid 2012, 22, 356–362. [Google Scholar] [CrossRef]
- Klubo-Gwiezdzinska, J.; Yang, L.; Merkel, R.; Patel, D.; Nilubol, N.; Merino, M.J.; Skarulis, M.; Sadowski, S.M.; Kebebew, E. Results of Screening in Familial Non-Medullary Thyroid Cancer. Thyroid 2017, 27, 1017–1024. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated Guidance for Trusted Systematic Reviews: A New Edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 2019, 10, ED000142. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.K.-L.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing Reviewers’ to Authors’ Assessments. BMC Med Res Methodol 2014, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- McLaren, W.; Gil, L.; Hunt, S.E.; Riat, H.S.; Ritchie, G.R.S.; Thormann, A.; Flicek, P.; Cunningham, F. The Ensembl Variant Effect Predictor. Genome Biol 2016, 17, 122. [Google Scholar] [CrossRef]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Hoover, J.; et al. ClinVar: Public Archive of Interpretations of Clinically Relevant Variants. Nucleic Acids Res 2016, 44, D862–868. [Google Scholar] [CrossRef]
- Sherry, S.T.; Ward, M.H.; Kholodov, M.; Baker, J.; Phan, L.; Smigielski, E.M.; Sirotkin, K. DbSNP: The NCBI Database of Genetic Variation. Nucleic Acids Res 2001, 29, 308–311. [Google Scholar] [CrossRef]
- Rentzsch, P.; Witten, D.; Cooper, G.M.; Shendure, J.; Kircher, M. CADD: Predicting the Deleteriousness of Variants throughout the Human Genome. Nucleic Acids Res 2019, 47, D886–D894. [Google Scholar] [CrossRef]
- Middleton, D.; Menchaca, L.; Rood, H.; Komerofsky, R. New Allele Frequency Database: Http://Www.Allelefrequencies.Net. Tissue Antigens 2003, 61, 403–407. [Google Scholar] [CrossRef]
- Cetta, F. FAP Associated Papillary Thyroid Carcinoma: A Peculiar Subtype of Familial Nonmedullary Thyroid Cancer. Patholog Res Int 2015, 2015, 309348. [Google Scholar] [CrossRef]
- Figlioli, G.; Köhler, A.; Chen, B.; Elisei, R.; Romei, C.; Cipollini, M.; Cristaudo, A.; Bambi, F.; Paolicchi, E.; Hoffmann, P.; et al. Novel Genome-Wide Association Study–Based Candidate Loci for Differentiated Thyroid Cancer Risk. The Journal of Clinical Endocrinology & Metabolism 2014, 99, E2084–E2092. [Google Scholar] [CrossRef]
- Dombernowsky, S.L.; Weischer, M.; Allin, K.H.; Bojesen, S.E.; Tybjaerg-Hansen, A.; Nordestgaard, B.G. Risk of Cancer by ATM Missense Mutations in the General Population. J Clin Oncol 2008, 26, 3057–3062. [Google Scholar] [CrossRef]
- Akulevich, N.M.; Saenko, V.A.; Rogounovitch, T.I.; Drozd, V.M.; Lushnikov, E.F.; Ivanov, V.K.; Mitsutake, N.; Kominami, R.; Yamashita, S. Polymorphisms of DNA Damage Response Genes in Radiation-Related and Sporadic Papillary Thyroid Carcinoma. Endocr Relat Cancer 2009, 16, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Yu, Y.; Ai, L.; Shi, J.; Liu, X.; Sun, H.; Liu, Y. Association of the ATM Gene Polymorphisms with Papillary Thyroid Cancer. Endocrine 2014, 45, 454–461. [Google Scholar] [CrossRef]
- Wójcicka, A.; Czetwertyńska, M.; Świerniak, M.; Długosińska, J.; Maciąg, M.; Czajka, A.; Dymecka, K.; Kubiak, A.; Kot, A.; Płoski, R.; et al. Variants in the ATM-CHEK2-BRCA1 Axis Determine Genetic Predisposition and Clinical Presentation of Papillary Thyroid Carcinoma. Genes Chromosomes Cancer 2014, 53, 516–523. [Google Scholar] [CrossRef]
- Wang, G.; Li, Z.; Li, X.; Zhang, C.; Peng, L. RASAL1 Induces to Downregulate the SCD1, Leading to Suppression of Cell Proliferation in Colon Cancer via LXRα/SREBP1c Pathway. Biol Res 2019, 52, 60. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Singh, P.; Yin, K.; Wang, J.; Bao, Y.; Wu, M.; Pathak, K.; McKinley, S.K.; Braun, D.; Lubitz, C.C.; et al. Non-Medullary Thyroid Cancer Susceptibility Genes: Evidence and Disease Spectrum. Ann Surg Oncol 2021, 28, 6590–6600. [Google Scholar] [CrossRef]
- Siołek, M.; Cybulski, C.; Gąsior-Perczak, D.; Kowalik, A.; Kozak-Klonowska, B.; Kowalska, A.; Chłopek, M.; Kluźniak, W.; Wokołorczyk, D.; Pałyga, I.; et al. CHEK2 Mutations and the Risk of Papillary Thyroid Cancer. International Journal of Cancer 2015, 137, 548–552. [Google Scholar] [CrossRef]
- Rutter, M.M.; Jha, P.; Schultz, K.A.P.; Sheil, A.; Harris, A.K.; Bauer, A.J.; Field, A.L.; Geller, J.; Hill, D.A. DICER1 Mutations and Differentiated Thyroid Carcinoma: Evidence of a Direct Association. J Clin Endocrinol Metab 2016, 101, 1–5. [Google Scholar] [CrossRef]
- Son, H.-Y.; Hwangbo, Y.; Yoo, S.-K.; Im, S.-W.; Yang, S.D.; Kwak, S.-J.; Park, M.S.; Kwak, S.H.; Cho, S.W.; Ryu, J.S.; et al. Genome-Wide Association and Expression Quantitative Trait Loci Studies Identify Multiple Susceptibility Loci for Thyroid Cancer. Nat Commun 2017, 8, 15966. [Google Scholar] [CrossRef]
- Lai, X.; Umbricht, C.B.; Fisher, K.; Bishop, J.; Shi, Q.; Chen, S. Identification of Novel Biomarker and Therapeutic Target Candidates for Diagnosis and Treatment of Follicular Carcinoma. J Proteomics 2017, 166, 59–67. [Google Scholar] [CrossRef]
- Yang, X.; Wu, Y.; Xu, S.; Li, H.; Peng, C.; Cui, X.; Dhoomun, D.K.; Wang, G.; Xu, T.; Dong, M.; et al. Targeting the Inward Rectifier Potassium Channel 5.1 in Thyroid Cancer: Artificial Intelligence-Facilitated Molecular Docking for Drug Discovery. BMC Endocrine Disorders 2023, 23, 113. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, H.; Li, W.; Phay, J.; Shen, R.; Yu, L.; Hancioglu, B.; de la Chapelle, A. MYH9 Binds to LncRNA Gene PTCSC2 and Regulates FOXE1 in the 9q22 Thyroid Cancer Risk Locus. Proc Natl Acad Sci U S A 2017, 114, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Kamihara, J.; Zhou, J.; LaDuca, H.; Wassner, A.J.; Dalton, E.; Garber, J.E.; Black, M.H. Germline Pathogenic Variants in Cancer Risk Genes among Patients with Thyroid Cancer and Suspected Predisposition. Cancer Medicine 2022, 11, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Bakhsh, A.D.; Ladas, I.; Hamshere, M.L.; Bullock, M.; Kirov, G.; Zhang, L.; Taylor, P.N.; Gregory, J.W.; Scott-Coombes, D.; Völzke, H.; et al. An InDel in Phospholipase-C-B-1 Is Linked with Euthyroid Multinodular Goiter. Thyroid 2018, 28, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Sarquis, M.; Moraes, D.C.; Bastos-Rodrigues, L.; Azevedo, P.G.; Ramos, A.V.; Reis, F.V.; Dande, P.V.; Paim, I.; Friedman, E.; De Marco, L. Germline Mutations in Familial Papillary Thyroid Cancer. Endocr Pathol 2020, 31, 14–20. [Google Scholar] [CrossRef]
- Ngeow, J.; Eng, C. PTEN in Hereditary and Sporadic Cancer. Cold Spring Harb Perspect Med 2020, 10, a036087. [Google Scholar] [CrossRef]
- Pilarski, R.; Burt, R.; Kohlman, W.; Pho, L.; Shannon, K.M.; Swisher, E. Cowden Syndrome and the PTEN Hamartoma Tumor Syndrome: Systematic Review and Revised Diagnostic Criteria. J Natl Cancer Inst 2013, 105, 1607–1616. [Google Scholar] [CrossRef]
- Hendricks, L.A.J.; Hoogerbrugge, N.; Schuurs-Hoeijmakers, J.H.M.; Vos, J.R. A Review on Age-Related Cancer Risks in PTEN Hamartoma Tumor Syndrome. Clin Genet 2021, 99, 219–225. [Google Scholar] [CrossRef]
- Bubien, V.; Bonnet, F.; Brouste, V.; Hoppe, S.; Barouk-Simonet, E.; David, A.; Edery, P.; Bottani, A.; Layet, V.; Caron, O.; et al. High Cumulative Risks of Cancer in Patients with PTEN Hamartoma Tumour Syndrome. J Med Genet 2013, 50, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Jonker, L.A.; Lebbink, C.A.; Jongmans, M.C.J.; Nievelstein, R. a. J.; Merks, J.H.M.; Nieveen van Dijkum, E.J.M.; Links, T.P.; Hoogerbrugge, N.; van Trotsenburg, A.S.P.; van Santen, H.M. Recommendations on Surveillance for Differentiated Thyroid Carcinoma in Children with PTEN Hamartoma Tumor Syndrome. Eur Thyroid J 2020, 9, 234–242. [Google Scholar] [CrossRef]
- Yehia, L.; Niazi, F.; Ni, Y.; Ngeow, J.; Sankunny, M.; Liu, Z.; Wei, W.; Mester, J.L.; Keri, R.A.; Zhang, B.; et al. Germline Heterozygous Variants in SEC23B Are Associated with Cowden Syndrome and Enriched in Apparently Sporadic Thyroid Cancer. Am J Hum Genet 2015, 97, 661–676. [Google Scholar] [CrossRef]
- Vierlinger, K.; Mansfeld, M.H.; Koperek, O.; Nöhammer, C.; Kaserer, K.; Leisch, F. Identification of SERPINA1 as Single Marker for Papillary Thyroid Carcinoma through Microarray Meta Analysis and Quantification of Its Discriminatory Power in Independent Validation. BMC Med Genomics 2011, 4, 30. [Google Scholar] [CrossRef] [PubMed]
- Makhlouf, A.-M.; Chitikova, Z.; Pusztaszeri, M.; Berczy, M.; Delucinge-Vivier, C.; Triponez, F.; Meyer, P.; Philippe, J.; Dibner, C. Identification of CHEK1, SLC26A4, c-KIT, TPO and TG as New Biomarkers for Human Follicular Thyroid Carcinoma. Oncotarget 2016, 7, 45776–45788. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, J.; Thorleifsson, G.; Sigurdsson, J.K.; Stefansdottir, L.; Jonasson, J.G.; Gudjonsson, S.A.; Gudbjartsson, D.F.; Masson, G.; Johannsdottir, H.; Halldorsson, G.H.; et al. A Genome-Wide Association Study Yields Five Novel Thyroid Cancer Risk Loci. Nat Commun 2017, 8, 14517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.; Li, D.; Jing, S. Notch and TGF-β/Smad3 Pathways Are Involved in the Interaction between Cancer Cells and Cancer-Associated Fibroblasts in Papillary Thyroid Carcinoma. Tumour Biol 2014, 35, 379–385. [Google Scholar] [CrossRef]
- Buryk, M.A.; Picarsic, J.L.; Creary, S.E.; Shaw, P.H.; Simons, J.P.; Deutsch, M.; Monaco, S.E.; Nikiforov, Y.E.; Witchel, S.F. Identification of Unique, Heterozygous Germline Mutation, STK11 (p.F354L), in a Child with an Encapsulated Follicular Variant of Papillary Thyroid Carcinoma within Six Months of Completing Treatment for Neuroblastoma. Pediatr Dev Pathol 2015, 18, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; LiVolsi, V.A.; Brose, M.S.; Montone, K.T.; Morrissette, J.J.D.; Baloch, Z.W. STK11 Mutation Identified in Thyroid Carcinoma. Endocr Pathol 2016, 27, 65–69. [Google Scholar] [CrossRef]
- Alzahrani, A.S.; Alswailem, M.; Murugan, A.K.; Alghamdi, B.; Al-Hindi, H. Papillary Thyroid Cancer and a TERT Promotor Mutation-Positive Paraganglioma in a Patient With a Germline SDHB Mutation. J Endocr Soc 2022, 6, bvac076. [Google Scholar] [CrossRef] [PubMed]
- Abe, I.; Lam, A.K.-Y. Anaplastic Thyroid Carcinoma: Current Issues in Genomics and Therapeutics. Curr Oncol Rep 2021, 23, 31. [Google Scholar] [CrossRef]
- Lauper, J.M.; Krause, A.; Vaughan, T.L.; Monnat, R.J. Spectrum and Risk of Neoplasia in Werner Syndrome: A Systematic Review. PLoS One 2013, 8, e59709. [Google Scholar] [CrossRef] [PubMed]
- Risch, N. The Genetic Epidemiology of Cancer: Interpreting Family and Twin Studies and Their Implications for Molecular Genetic Approaches. Cancer Epidemiol Biomarkers Prev 2001, 10, 733–741. [Google Scholar]
- Liyanarachchi, S.; Gudmundsson, J.; Ferkingstad, E.; He, H.; Jonasson, J.G.; Tragante, V.; Asselbergs, F.W.; Xu, L.; Kiemeney, L.A.; Netea-Maier, R.T.; et al. Assessing Thyroid Cancer Risk Using Polygenic Risk Scores. Proc. Natl. Acad. Sci. U.S.A. 2020, 117, 5997–6002. [Google Scholar] [CrossRef]
- Abdullah Suhaimi, S.N.; Nazri, N.; Nani Harlina, M.L.; Md Isa, N.; Muhammad, R. Familial Adenomatous Polyposis-Associated Papillary Thyroid Cancer. Malays J Med Sci 2015, 22, 69–72. [Google Scholar]
- Xu, M.; Zheng, Y.; Zuo, Z.; Zhou, Q.; Deng, Q.; Wang, J.; Wang, D. De Novo Familial Adenomatous Polyposis Associated Thyroid Cancer with a c.2929delG Frameshift Deletion Mutation in APC: A Case Report and Literature Review. World Journal of Surgical Oncology 2023, 21, 73. [Google Scholar] [CrossRef]
- Chenbhanich, J.; Atsawarungruangkit, A.; Korpaisarn, S.; Phupitakphol, T.; Osataphan, S.; Phowthongkum, P. Prevalence of Thyroid Diseases in Familial Adenomatous Polyposis: A Systematic Review and Meta-Analysis. Familial Cancer 2019, 18, 53–62. [Google Scholar] [CrossRef]
- Expression of ARSB in Cancer - Summary - The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000113273-ARSB/pathology (accessed on 15 August 2023).
- Pstrąg, N.; Ziemnicka, K.; Bluyssen, H.; Wesoły, J. Thyroid Cancers of Follicular Origin in a Genomic Light: In-Depth Overview of Common and Unique Molecular Marker Candidates. Molecular Cancer 2018, 17, 116. [Google Scholar] [CrossRef] [PubMed]
- Nagy, R.; Ringel, M.D. Genetic Predisposition for Nonmedullary Thyroid Cancer. HORM CANC 2015, 6, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Tobacman, J.K. Arylsulfatase B Regulates Colonic Epithelial Cell Migration by Effects on MMP9 Expression and RhoA Activation. Clin Exp Metastasis 2009, 26, 535–545. [Google Scholar] [CrossRef]
- Xu, L.; Morari, E.C.; Wei, Q.; Sturgis, E.M.; Ward, L.S. Functional Variations in the ATM Gene and Susceptibility to Differentiated Thyroid Carcinoma. The Journal of Clinical Endocrinology & Metabolism 2012, 97, 1913–1921. [Google Scholar] [CrossRef]
- CBioPortal for Cancer Genomics. Available online: http://www.cbioportal.org/ (accessed on 15 August 2023).
- Tissue Expression of ATM - Staining in Thyroid Gland - The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000149311-ATM/tissue/thyroid+gland (accessed on 15 August 2023).
- Miasaki, F.Y.; Saito, K.C.; Yamamoto, G.L.; Boguszewski, C.L.; de Carvalho, G.A.; Kimura, E.T.; Kopp, P.A. Thyroid and Breast Cancer in 2 Sisters With Monoallelic Mutations in the Ataxia Telangiectasia Mutated (ATM) Gene. Journal of the Endocrine Society 2022, 6, bvac026. [Google Scholar] [CrossRef]
- PubChem BRCA1 - BRCA1 DNA Repair Associated (Human). Available online: https://pubchem.ncbi.nlm.nih.gov/gene/BRCA1/human (accessed on 15 August 2023).
- Cieszyńska, M.; Kluźniak, W.; Wokołorczyk, D.; Cybulski, C.; Huzarski, T.; Gronwald, J.; Falco, M.; Dębniak, T.; Jakubowska, A.; Derkacz, R.; et al. Risk of Second Primary Thyroid Cancer in Women with Breast Cancer. Cancers 2022, 14, 957. [Google Scholar] [CrossRef]
- Swierniak, M.; Pfeifer, A.; Stokowy, T.; Rusinek, D.; Chekan, M.; Lange, D.; Krajewska, J.; Oczko-Wojciechowska, M.; Czarniecka, A.; Jarzab, M.; et al. Somatic Mutation Profiling of Follicular Thyroid Cancer by next Generation Sequencing. Mol Cell Endocrinol 2016, 433, 130–137. [Google Scholar] [CrossRef]
- De Toledo, M.; Coulon, V.; Schmidt, S.; Fort, P.; Blangy, A. The Gene for a New Brain Specific RhoA Exchange Factor Maps to the Highly Unstable Chromosomal Region 1p36.2-1p36.3. Oncogene 2001, 20, 7307–7317. [Google Scholar] [CrossRef]
- Azzedine, H.; Zavadakova, P.; Planté-Bordeneuve, V.; Vaz Pato, M.; Pinto, N.; Bartesaghi, L.; Zenker, J.; Poirot, O.; Bernard-Marissal, N.; Arnaud Gouttenoire, E.; et al. PLEKHG5 Deficiency Leads to an Intermediate Form of Autosomal-Recessive Charcot-Marie-Tooth Disease. Hum Mol Genet 2013, 22, 4224–4232. [Google Scholar] [CrossRef]
- Kai, J.-D.; Cheng, L.-H.; Li, B.-F.; Kang, K.; Xiong, F.; Fu, J.-C.; Wang, S. MYH9 Is a Novel Cancer Stem Cell Marker and Prognostic Indicator in Esophageal Cancer That Promotes Oncogenesis through the PI3K/AKT/MTOR Axis. Cell Biology International 2022, 46, 2085–2094. [Google Scholar] [CrossRef]
- Cameselle-Teijeiro, J.M.; Mete, O.; Asa, S.L.; LiVolsi, V. Inherited Follicular Epithelial-Derived Thyroid Carcinomas: From Molecular Biology to Histological Correlates. Endocr Pathol 2021, 32, 77–101. [Google Scholar] [CrossRef] [PubMed]
- Bakhsh, A.; Kirov, G.; Gregory, J.W.; Williams, E.D.; Ludgate, M. A New Form of Familial Multi-Nodular Goitre with Progression to Differentiated Thyroid Cancer. Endocr Relat Cancer 2006, 13, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Yehia, L.; Plitt, G.; Tushar, A.M.; Joo, J.; Burke, C.A.; Campbell, S.C.; Heiden, K.; Jin, J.; Macaron, C.; Michener, C.M.; et al. Longitudinal Analysis of Cancer Risk in Children and Adults With Germline PTEN Variants. JAMA Netw Open 2023, 6, e239705. [Google Scholar] [CrossRef] [PubMed]
- Saenko, V.A.; Rogounovitch, T.I. Genetic Polymorphism Predisposing to Differentiated Thyroid Cancer: A Review of Major Findings of the Genome-Wide Association Studies. Endocrinol Metab (Seoul) 2018, 33, 164–174. [Google Scholar] [CrossRef]
- Wang, Q.; Shen, Y.; Ye, B.; Hu, H.; Fan, C.; Wang, T.; Zheng, Y.; Lv, J.; Ma, Y.; Xiang, M. Gene Expression Differences between Thyroid Carcinoma, Thyroid Adenoma and Normal Thyroid Tissue. Oncol Rep 2018. [Google Scholar] [CrossRef]
- Jiang, Y.-L.; Zhao, Z.-Y.; Li, B.-R.; Yang, F.; Li, J.; Jin, X.-W.; Wang, H.; Yu, E.-D.; Sun, S.-H.; Ning, S.-B. The Altered Activity of P53 Signaling Pathway by STK11 Gene Mutations and Its Cancer Phenotype in Peutz-Jeghers Syndrome. BMC Medical Genetics 2018, 19, 141. [Google Scholar] [CrossRef]
- Lardelli, R.M.; Schaffer, A.E.; Eggens, V.R.C.; Zaki, M.S.; Grainger, S.; Sathe, S.; Van Nostrand, E.L.; Schlachetzki, Z.; Rosti, B.; Akizu, N.; et al. Biallelic Mutations in the 3′ Exonuclease TOE1 Cause Pontocerebellar Hypoplasia and Uncover a Role in SnRNA Processing. Nat Genet 2017, 49, 457–464. [Google Scholar] [CrossRef]
- Expression of TOE1 in Thyroid Cancer - The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000132773-TOE1/pathology/thyroid+cancer (accessed on 15 August 2023).
- Liu, X.; Bishop, J.; Shan, Y.; Pai, S.; Liu, D.; Murugan, A.K.; Sun, H.; El-Naggar, A.K.; Xing, M. Highly Prevalent TERT Promoter Mutations in Aggressive Thyroid Cancers. Endocr Relat Cancer 2013, 20, 603–610. [Google Scholar] [CrossRef]
- Matsuse, M.; Yabuta, T.; Saenko, V.; Hirokawa, M.; Nishihara, E.; Suzuki, K.; Yamashita, S.; Miyauchi, A.; Mitsutake, N. TERT Promoter Mutations and Ki-67 Labeling Index as a Prognostic Marker of Papillary Thyroid Carcinomas: Combination of Two Independent Factors. Sci Rep 2017, 7, 41752. [Google Scholar] [CrossRef]
- Muftuoglu, M.; Oshima, J.; von Kobbe, C.; Cheng, W.-H.; Leistritz, D.F.; Bohr, V.A. The Clinical Characteristics of Werner Syndrome: Molecular and Biochemical Diagnosis. Hum Genet 2008, 124, 369–377. [Google Scholar] [CrossRef]
- Jazdzewski, K.; Murray, E.L.; Franssila, K.; Jarzab, B.; Schoenberg, D.R.; de la Chapelle, A. Common SNP in Pre-MiR-146a Decreases Mature MiR Expression and Predisposes to Papillary Thyroid Carcinoma. Proceedings of the National Academy of Sciences 2008, 105, 7269–7274. [Google Scholar] [CrossRef] [PubMed]
- Zuk, O.; Hechter, E.; Sunyaev, S.R.; Lander, E.S. The Mystery of Missing Heritability: Genetic Interactions Create Phantom Heritability. Proceedings of the National Academy of Sciences 2012, 109, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
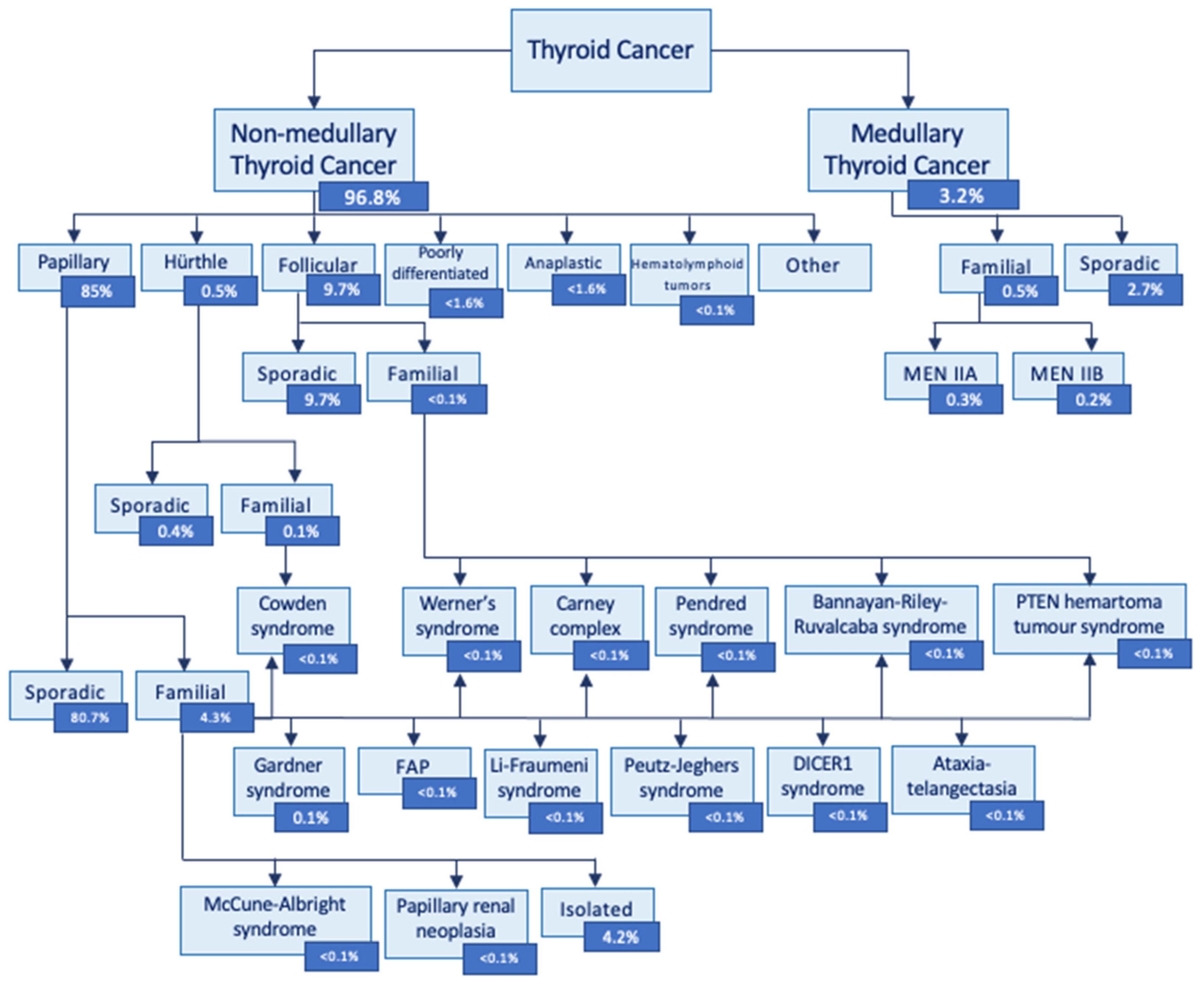
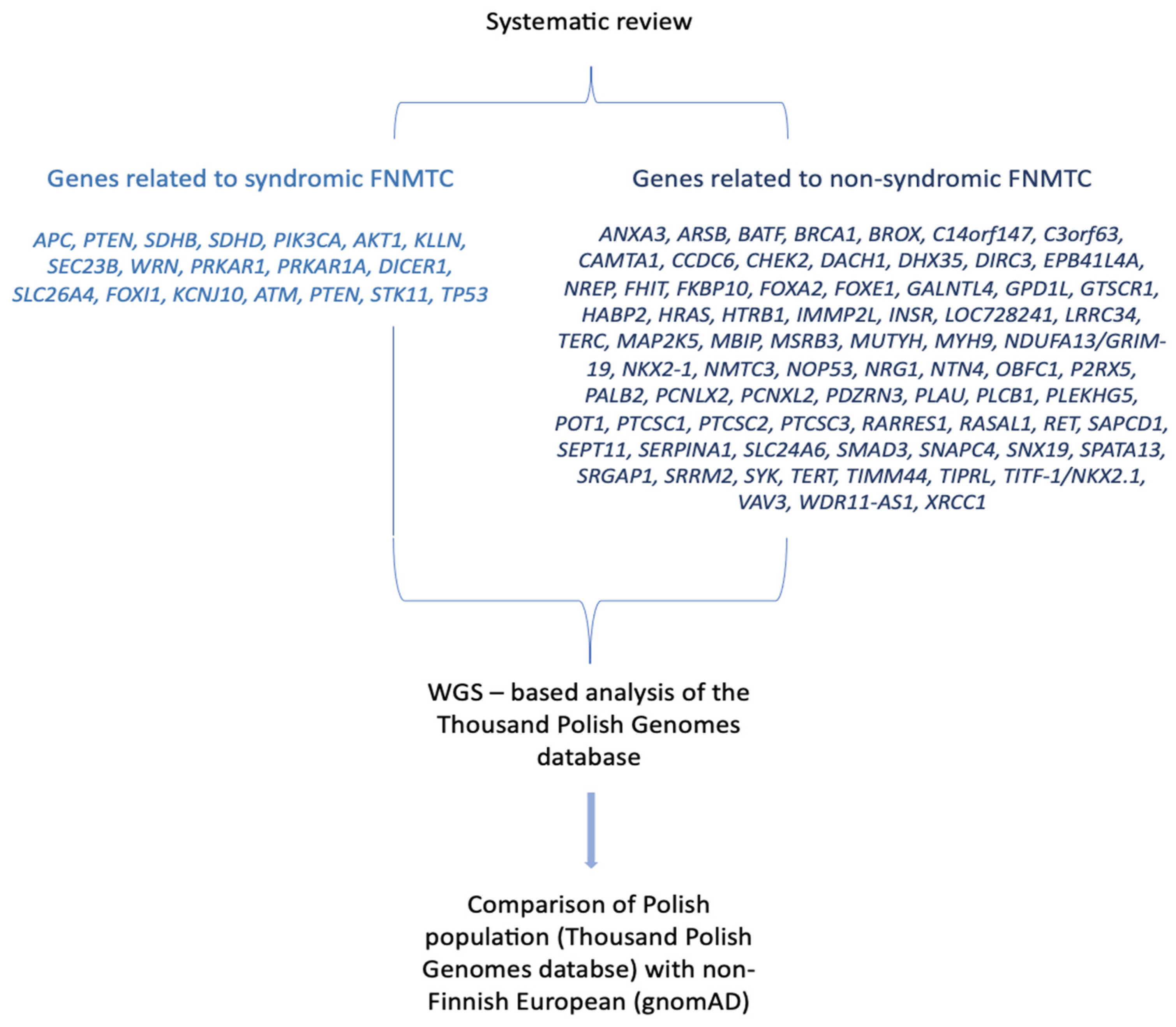
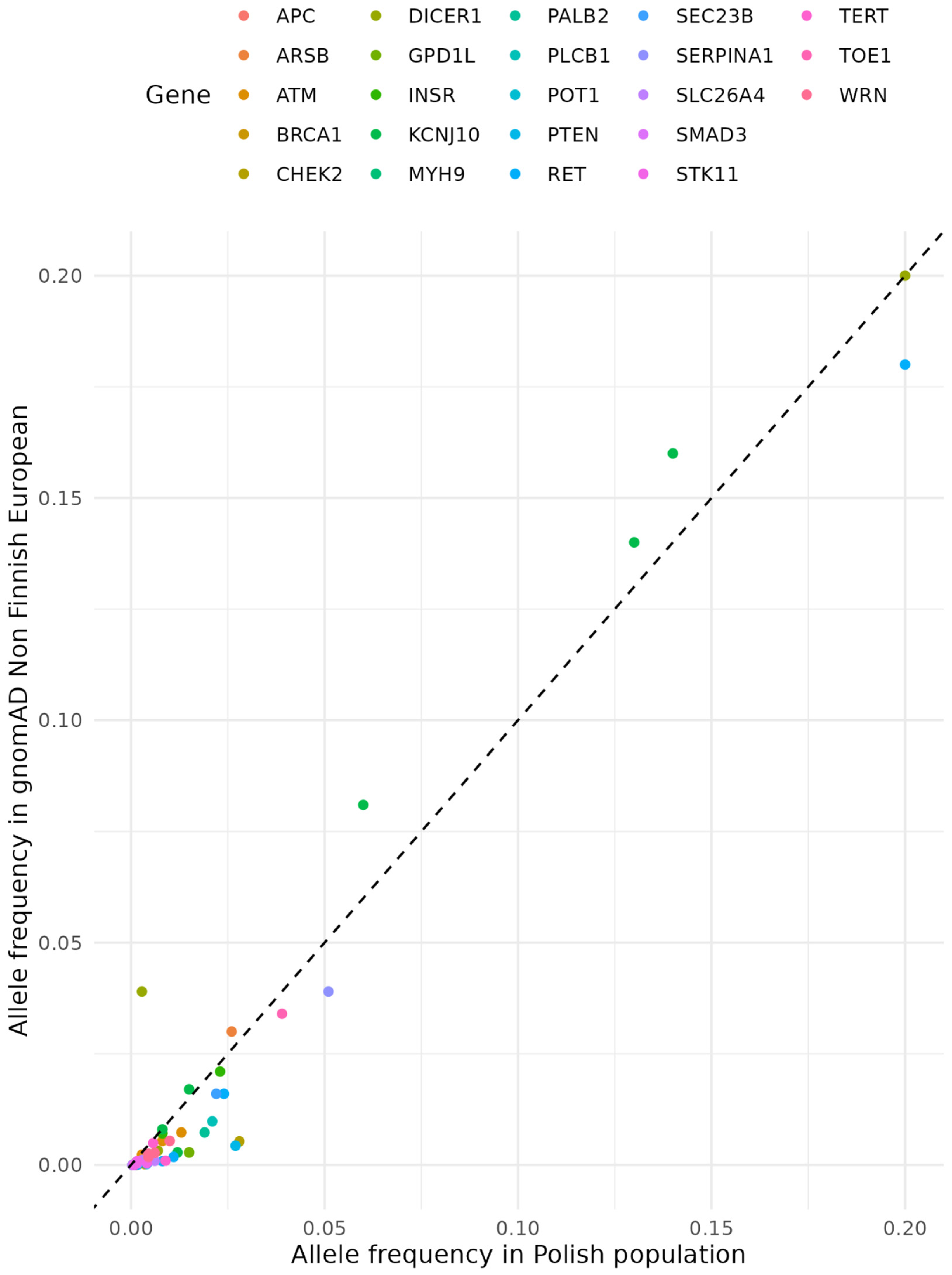
| Name | Gene | Mode of Inheritance | Thyroid cancer histological subtype* | Phenotypes other than thyroid cancer |
|---|---|---|---|---|
| FAP and Gardner’s syndrome | APC | Autosomal dominant | PTC | Colorectal carcinoma, ampullary carcinoma, hepatoblastoma, medulloblastoma |
| Cowden syndrome |
PTEN, SDHB-D, PIK3CA, AKT1, KLLN, SEC23B |
Autosomal dominant | cPTC, fvPTC, FTC |
Multiple hamartomas, follicular thyroid carcinoma, benign thyroid nodules, breast cancer, endometrial cancer |
| Werner syndrome | WRN | Autosomal recessive | PTC, FTC, ATC |
Premature ageing, scleroderma-like skin changes, cataracts, subcutaneous calcifications, muscular atrophy, diabetes |
| Carney complex | PRKAR1 | Autosomal dominant | PTC, FTC | Spotty skin pigmentation, cardiac myxomas, endocrine tumors |
| DICER1 syndrome | DICER1 | Autosomal dominant | PTC, DTC | Endocrine tumors (thyroid, parathyroid, pituitary, pineal gland, endocrine pancreas, paragangliomas, medullary, adrenocortical, ovarian, and testicular tumors |
| Pendred syndrome | SLC26A4, FOXI1, KCNJ10 | Autosomal recessive | PTC, FTC, ATC | Sensorineural deafness/hearing impairment, goiter, and an abnormal organification of iodide with or without hypothyroidism |
| Ataxia-telangiectasia | ATM | Autosomal recessive | PTC | Cerebellar degeneration, telangiectasia, immunodeficiency, recurrent sinopulmonary infections, radiation sensitivity, premature aging, lymphoid cancer, poor growth, gonadal atrophy, insulin resistant diabetes |
| Bannayan- Riley- Ruvalcaba syndrome | PTEN | Autosomal dominant | PTC, FTC | Macrocephaly, hamartomatous tissue overgrowth, lipomas, pigmented macules on the penis, developmental delay, large birth weight, joint hyperextensibility, endometrial cancer, renal cell carcinoma, Lhermitte–Duclos disease |
| Peutz-Jeghers syndrome | STK11 | Autosomal dominant | PTC, DTC | Gastrointestinal polyposis, mucocutaneous pigmented macules, breast cancer, uterine cancer, cervical cancer, lung cancer, ovarian cancer, testicular cancers |
| PTEN hamartoma tumor syndrome | PTEN | Autosomal dominant | FTC, PTC, fvPTC |
Breast cancer, endometrial cancer, gastrointestinal hamartomas, Lhermitte-Duclos disease, macrocephaly, macular pigmentation of the glans penis, multiple mucocutaneous lesions, autism spectrum disorder, colon cancer, esophageal glycogenic acanthosis, lipomas, mental retardation, renal cell carcinoma, testicular lipomatosis, thyroid adenoma, multinodular goiter |
| Li-Fraumeni syndrome | TP53 | Autosomal dominant | cPTC, fvPTC | Adrenocortical carcinomas, breast cancer, central nervous system tumors, osteosarcomas, soft-tissue sarcomas, leukemia, lymphoma, gastrointestinal cancers, cancers of head and neck, kidney, larynx, lung, skin, ovary, pancreas, prostate, and testis |
| Gene | Type of thyroid cancer* | Hereditary syndromes | Other cancers | Reference |
|---|---|---|---|---|
| APC | PTC with cribriform pattern | FAP and Gardner’s syndrome | Colorectal cancer, ampullary carcinoma, hepatoblastoma, medulloblastoma | Kamani et al. 2022 [34]; Cetta et al. 2015 [34]2023-10-07 12:25:00 PM |
| ARSB | DTC | N/A | N/A | Figlioli et al. 2014 [35] |
| ATM | PTC | Ataxia-telangiectasia | Cerebellar degeneration, telangiectasia, immunodeficiency, recurrent sinopulmonary infections, radiation sensitivity, premature ageing, lymphoid cancer, poor growth, gonadal atrophy, insulin resistant diabetes | Kamani et al. 2022 [12] |
| PTC, DTC | N/A | N/A | Dombernowsky et al. 2008 [36]; Akulevich et al. 2009 [37]; Gu et al. 2014 [38]; Wójcicka 2014 [39] |
|
| BRCA1 | PTC | N/A | N/A | Wójcicka et al. 2014 [39] |
| CHEK2 | Non-syndromic DTC | N/A | Breast cancer, prostate cancer | Wang et al. 2019 [40] |
| PTC | N/A | N/A | Wójcicka et al. 2014 [41]; Siołek et al. 2015 [42]; Zhou et al. 2021[41] |
|
| DICER1 | PTC, DTC | DICER1 syndrome | Endocrine tumors (parathyroid, pituitary, pineal gland, endocrine pancreas, paragangliomas, medullary, adrenocortical, ovarian, and testicular tumors | Rutter et al. 2016 [43] |
| Nephroblastoma, NMTC pleuropulmonary blastoma, cystic nephroma, multinodular goiter, thyroid adenoma, sex cord tumor | Zhou et al.2021 [41] | |||
| GPD1L | DTC | N/A | N/A | Figloli et al. 2014 [35] |
| INSR | PTC | N/A | N/A | Son et al. 2017 [44] |
| FTC | N/A | N/A | Lai et al. 2017 [45] | |
| KCNJ10 | ATC | Pendred | Sensorineural deafness/hearing impairment, goiter, and an abnormal organification of iodide with or without hypothyroidism | Liu et al. 2016 [46]; Yang et al. 2023 0/0/00 0:00:00 AM |
| MYH9 | PTC, FTC | N/A | N/A | Wang et al. 2017 [47] |
| PALB2 | PTC | N/A | N/A | Kamihara et al. 2022 [48] |
| PLCB1 | PTC | N/A | N/A | Bakhsh et al. 2018 [49] |
| PLEKHG5 | PTC | N/A | N/A | Sarquis et al. 2020 [50] |
| PTEN | cPTC, fvPTC, FTC | Cowden syndrome | Multiple hamartomas, follicular thyroid carcinoma, benign thyroid nodules, breast cancer, endometrial cancer | Bevan et al. 2001 [51]; Pilarski et al. 2013 [52]; Hendricks et al. 2021 [53]; Bubien et al. 2013 [54]; Jonker et al. 2020 [55]; Ngeow et al. 2020 0/0/00 0:00:00 AM |
| PTC, FTC | Bannayan-Riley-Ruvalcaba syndrome | Macrocephaly, hamartomatous tissue overgrowth, lipomas, pigmented macules on the penis, developmental delay, large birth weight, joint hyperextensibility, endometrial cancer, renal cell carcinoma, Lhermitte–Duclos disease | ||
| FTC, PTC, fvPTC |
PTEN hamartoma tumor syndrome | Breast cancer, Endometrial cancer, FTC, Gastrointestinal hamartomas, Lhermitte-Duclos disease, Macrocephaly, Macu- lar pigmentation of the glans penis, Multiple mucocutaneous lesions, Autism spectrum disorder, Colon cancer, Esophageal glycogenic acanthosis, Lipomas, Mental retardation, Renal cell carcinoma, Testicular lipomatosis, PTC, fvPTC, thyroid adenoma, MNG | ||
| RET | PTC | FAP | Colorectal carcinoma, ampullary carcinoma, hepatoblastoma, medulloblastoma | Cetta et al. 2015 [34] |
| SEC23B | cPTC, fvPTC, FTC | Cowden syndrome | Multiple hamartomas, follicular thyroid carcinoma, benign thyroid nodules, breast cancer, endometrial cancer | Yehia et al. 2015 [56] |
| SERPINA1 | PTC | N/A | N/A | Vierlinger et al. 2011 [57] |
| SLC26A4 | FTC, DTC | Pendred syndrome | Sensorineural deafness/hearing impairment, goiter, and an abnormal organification of iodide with or without hypothyroidism | Makhlouf et al. 2016 [58] |
| SMAD3 | DTC | N/A | N/A | Gudmundsson et al. 2017 [59] |
| PTC | N/A | N/A | Zhang et al. 2014 [60]; | |
| STK11 | PTC, DTC | Peutz-Jeghers syndrome | Gastrointestinal (GI) polyposis, mucocutaneous pigmented macules, breast cancer, uterine cancer, cervical cancer, lung cancer, ovarian cancer, testicular cancers | Buryk et al. 2015 [61]; Wei et al. 2016 [62] |
| TERT | DTC | N/A | N/A | Gudmundsson et al. 2017 [59] |
| PTC | N/A | N/A | Kim et al. 2022 [63]; Alzahrani et al. 2022 0/0/00 0:00:00 AM |
|
| ATC | N/A | N/A | Abe et al. 2021 [64] | |
| TOE1 | PTC | FAP | Colorectal carcinoma, ampullary carcinoma, hepatoblastoma, medulloblastoma | ClinVar [30] |
| WRN | PTC, FTC, ATC |
Werner syndrome | Premature aging, scleroderma-like skin changes, cataracts, subcutaneous calcifications, muscular atrophy, diabetes | Lauper et al. 2013 [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





