Submitted:
16 October 2023
Posted:
17 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Pituitary adenylate cyclase–activating peptide and vasoactive intestinal peptide
2.1. Background
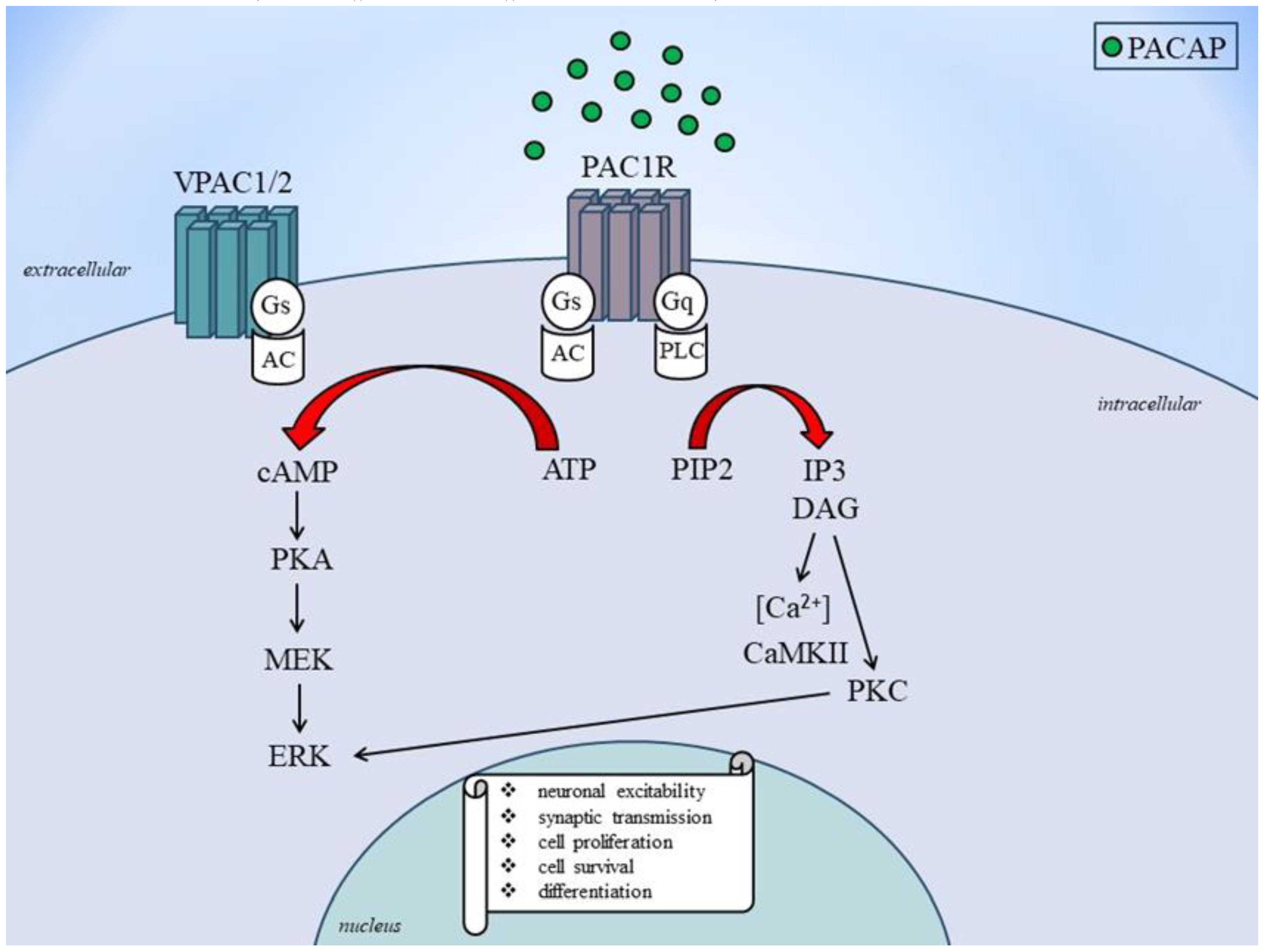
2.2. Receptor and Signaling Mechanisms of PACAP and VIP
2.3. Role of PACAP and VIP in migraine
2.4. Preclinical Studies
2.5. Clinical studies
3. Discussion
4. Conclusion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AC | adenylate cyclase |
| ADM | adrenomedullin |
| cAMP | cyclic adenosine monophosphate |
| CGRP | calcitonin gene-related peptide |
| DAG | diacylglycerol |
| ERK | extracellular signal-regulated kinase |
| GPCR | G protein-coupled receptors |
| IAPP | islet amyloid polypeptide/amylin |
| mAbs | monoclonal antibodies |
| MAPK | mitogen-activated protein kinase |
| MCA | middle cerebral artery |
| MEK: | mitogen-activated protein kinase kinase |
| PACAP | pituitary adenylate cyclase-activating polypeptide |
| PACAP1–38 | 38-amino acid form of PACAP |
| PACAP1–27 | 27-amino acid form of PACAP |
| PACAP6-38 | 6–38-amino acid form of PACAP |
| PIP2 | phosphatidyl inositol 4,5-bisphosphate |
| PKA | protein kinase A |
| PKC | activates protein kinase C |
| PRP | PACAP-related peptide |
| SNV | N-stearyl-[Nle17] neurotensin-(6-11)/VIP-(7-28) |
| TNC | trigeminal nucleus caudalis |
| TS | trigeminovascular system |
| VPAC | vasoactive intestinal peptide receptor |
| VIP | vasoactive intestinal polypeptide |
References
- IHS Classification ICHD-3. Available online: https://ichd-3.org/1-migraine/1-2-migraine-with-aura/#:~:text=Description%3A,headache%20and%20associated%20migraine%20symptoms (accessed on 12 Ocotorber 2023).
- Amiri, P.; Kazeminasab, S.; Nejadghaderi, S.A.; Mohammadinasab, R.; Pourfathi, H.; Araj-Khodaei, M.; Sullman, M.J.M.; Kolahi, A.A.; Safiri, S. Migraine: A Review on Its History, Global Epidemiology, Risk Factors, and Comorbidities. Front. Neurol. 2022, 12, 800605. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.D.; Goadsby, P.J.; Burstein, R.; Kurth, T.; Ayata, C.; Charles, A.; Ashina, M.; van den Maagdenberg, A.M.J.M.; Dodick, D.W. Migraine. Nat Rev Dis Primers. 2022, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Ashina, M. Migraine. N. Engl. J. Med. 2020, 383, 1866–1876. [Google Scholar] [CrossRef]
- Gasparini, C.F.; Sutherland, H.G.; Griffiths, L.R. Studies on the pathophysiology and genetic basis of migraine. Curr. Genomics 2013, 14, 300–315. [Google Scholar] [CrossRef]
- Burstein, R.; Noseda, R.; Borsook, D. Migraine: multiple processes, complex pathophysiology. J. Neurosci. 2015, 35, 6619–6629. [Google Scholar] [CrossRef]
- Dodick, D.W. A Phase-by-Phase Review of Migraine Pathophysiology. Headache 2018, 58 (Suppl. 1), 4–16. [Google Scholar] [CrossRef] [PubMed]
- Schankin, C.J.; Viana, M.; Goadsby, P.J. Persistent and Repetitive Visual Disturbances in Migraine: A Review. Headache 2017, 57, 1–16. [Google Scholar] [CrossRef]
- Khan, J.; Asoom, L.I.A.; Sunni, A.A.; Rafique, N.; Latif, R.; Saif, S.A.; Almandil, N.B.; Almohazey, D.; AbdulAzeez, S.; Borgio, J.F. Genetics, pathophysiology, diagnosis, treatment, management, and prevention of migraine. Biomed. Pharmacother. 2021, 139, 111557. [Google Scholar] [CrossRef]
- Buse, D.C.; Rupnow, M.F.; Lipton, R.B. Assessing and managing all aspects of migraine: migraine attacks, migraine-related functional impairment, common comorbidities, and quality of life. Mayo Clin. Proc. 2009, 84, 422–435. [Google Scholar] [CrossRef]
- Gupta, J.; Gaurkar, S.S. Migraine: An Underestimated Neurological Condition Affecting Billions. Cureus 2022, 14, e28347. [Google Scholar] [CrossRef]
- Villar-Martinez, M.D.; Goadsby, P.J. Pathophysiology and Therapy of Associated Features of Migraine. Cells 2022, 11, 2767. [Google Scholar] [CrossRef]
- Durham, P.L. Calcitonin gene-related peptide (CGRP) and migraine. Headache 2006, 46 (Suppl. 1), S3–S8. [Google Scholar] [CrossRef]
- Waschek, J.A.; Baca, S.M.; Akerman, S. PACAP and migraine headache: immunomodulation of neural circuits in autonomic ganglia and brain parenchyma. J. Headache Pain 2018, 19, 23. [Google Scholar] [CrossRef]
- Pellesi, L.; Al-Karagholi, M.A.; De Icco, R.; Coskun, H.; Elbahi, F.A.; Lopez-Lopez, C.; Snellman, J.; Hannibal, J.; Amin, F.M.; Ashina, M. Effect of Vasoactive Intestinal Polypeptide on Development of Migraine Headaches: A Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e2118543. [Google Scholar] [CrossRef]
- Ghanizada, H.; Al-Karagholi, M.A.; Walker, C.S.; Arngrim, N.; Rees, T.; Petersen, J.; Siow, A.; Mørch-Rasmussen, M.; Tan, S.; O'Carroll, S.J.; et al. Amylin Analog Pramlintide Induces Migraine-like Attacks in Patients. Ann. Neurol. 2021, 89, 1157–1171. [Google Scholar] [CrossRef] [PubMed]
- May, A.; Goadsby, P.J. Substance P receptor antagonists in the therapy of migraine. Expert Opin. Investig. Drugs 2001, 10, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.A.; Birk, S.; Kitamura, K.; Olesen, J. Effect of adrenomedullin on the cerebral circulation: relevance to primary headache disorders. Cephalalgia 2009, 29, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Hay, D.L.; Garelja, M.L.; Poyner, D.R.; Walker, C.S. Update on the pharmacology of calcitonin/CGRP family of peptides: IUPHAR Review 25. Br. J. Pharmacol. 2018, 175, 3–17. [Google Scholar] [CrossRef]
- Poyner, D.R.; Hay, D.L. Secretin family (Class B) G protein-coupled receptors - from molecular to clinical perspectives. Br. J. Pharmacol. 2012, 166, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, L.; Grell, A.S.; Warfvinge, K. Expression of the CGRP Family of Neuropeptides and their Receptors in the Trigeminal Ganglion. J. Mol. Neurosci. 2020, 70, 930–944. [Google Scholar] [CrossRef]
- Dux, M.; Vogler, B.; Kuhn, A.; Mackenzie, K.D.; Stratton, J.; Messlinger, K. The Anti-CGRP Antibody Fremanezumab Lowers CGRP Release from Rat Dura Mater and Meningeal Blood Flow. Cells 2022, 11, 1768. [Google Scholar] [CrossRef]
- Pavelic, A.R.; Wöber, C.; Riederer, F.; Zebenholzer, K. Monoclonal Antibodies against Calcitonin Gene-Related Peptide for Migraine Prophylaxis: A Systematic Review of Real-World Data. Cells 2023, 12, 143. [Google Scholar] [CrossRef]
- Körtési, T.; Spekker, E.; Vécsei, L. Exploring the Tryptophan Metabolic Pathways in Migraine-Related Mechanisms. Cells 2022, 11, 3795. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, S.; Johnson, K.W.; Ossipov, M.H.; Aurora, S.K. CGRP and the Trigeminal System in Migraine. Headache 2019, 59, 659–681. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, L.; Tajti, J.; Szalárdy, L.; Vécsei, L. PACAP and its role in primary headaches. J. Headache Pain 2018, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Körtési, T.; Tuka, B.; Nyári, A.; Vécsei, L.; Tajti, J. The effect of orofacial complete Freund's adjuvant treatment on the expression of migraine-related molecules. J. Headache Pain 2019, 20, 43. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.F. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 533–552. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, E.A.; Russo, A.F. CGRP and migraine: could PACAP play a role too? Neuropeptides 2013, 47, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Kuburas, A.; Mason, B.N.; Hing, B.; Wattiez, A.S.; Reis, A.S.; Sowers, L.P.; Moldovan Loomis, C.; Garcia-Martinez, L.F.; Russo, A.F. PACAP Induces Light Aversion in Mice by an Inheritable Mechanism Independent of CGRP. J. Neurosci. 2021, 26, 41–4697. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.F. CGRP as a neuropeptide in migraine: lessons from mice. Br. J. Clin Pharmacol. 2015, 80, 403–414. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Edvinsson, L.; Ekman, R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann. Neurol. 1988, 23, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Tuka, B.; Helyes, Z.; Markovics, A.; Bagoly, T.; Szolcsányi, J.; Szabó, N.; Tóth, E.; Kincses, Z.T.; Vécsei, L.; Tajti, J. Alterations in PACAP-38-like immunoreactivity in the plasma during ictal and interictal periods of migraine patients. Cephalalgia 2013, 33, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Lassen, L.H; Haderslev, P.A.; Jacobsen, V.B.; Iversen, H.K.; Sperling, B.; Olesen, J. CGRP may play a causative role in migraine. Cephalalgia 2002, 22, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Schytz, H.W.; Birk, S.; Wienecke, T.; Kruuse, C.; Olesen, J.; Ashina, M. PACAP38 induces migraine-like attacks in patients with migraine without aura. Brain 2009, 132 Pt 1, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Vollesen, A.L.H.; Amin, F.M.; Ashina, M. Targeted Pituitary Adenylate Cyclase-Activating Peptide Therapies for Migraine. Neurotherapeutics 2018, 15, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Ghanizada, H.; Al-Karagholi, M.A.; Arngrim, N.; Olesen, J.; Ashina, M. PACAP27 induces migraine-like attacks in migraine patients. Cephalalgia 2020, 40, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Cernuda-Morollón, E.; Martínez-Camblor, P.; Alvarez, R.; Larrosa, D.; Ramón, C.; Pascual, J. Increased VIP levels in peripheral blood outside migraine attacks as a potential biomarker of cranial parasympathetic activation in chronic migraine. Cephalalgia 2015, 35, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Hery, M.; Faudon, M.; Hery, F. Effect of vasoactive intestinal peptide on serotonin release in the suprachiasmatic area of the rat. Modulation by oestradiol. Peptides 1984, 5, 313–317. [Google Scholar] [CrossRef]
- Pellesi, L.; Al-Karagholi, M.A.; De Icco, R.; Chaudhry, B.A.; Lopez, C.L.; Snellman, J.; Hannibal, J.; Amin, F.M.; Ashina, M. Plasma Levels of CGRP During a 2-h Infusion of VIP in Healthy Volunteers and Patients With Migraine: An Exploratory Study. Front. Neurol. 2022, 13, 871176. [Google Scholar] [CrossRef]
- De la Fuente, M.; Delgado, M.; Gomariz, R.P. VIP modulation of immune cell functions. Adv. Neuroimmunol. 1996, 6, 75–91. [Google Scholar] [CrossRef]
- Hoffmann, J.; Baca, S.M.; Akerman, S. Neurovascular mechanisms of migraine and cluster headache. J. Cereb. Blood. Flow Metab. 2019, 39, 573–594. [Google Scholar] [CrossRef]
- Ocheretyaner, E.R.; Kofman, M.; Quattrocchi, E. Calcitonin gene-related peptide (CGRP) receptor antagonists for the acute treatment of migraines in adults. Drugs Context. 2022, 11, 2022–3. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Song, J.; You, C. Efficacy and Safety of Monoclonal Antibody Against Calcitonin Gene-Related Peptide or Its Receptor for Migraine: A Systematic Review and Network Meta-analysis. Front. Pharmacol. 2021, 12, 649143. [Google Scholar] [CrossRef]
- Berger, A.A.; Winnick, A.; Popovsky, D.; Kaneb, A.; Berardino, K.; Kaye, A.M.; Cornett, E.M.; Kaye, A.D.; Viswanath, O. Urits I. Lasmiditan for the Treatment of Migraines With or Without Aura in Adults. Psychopharmacol. Bull. 2020, 50 (Suppl. 1), 163–188. [Google Scholar] [PubMed]
- Rissardo, J.P.; Caprara, A.L.F. Gepants for Acute and Preventive Migraine Treatment: A Narrative Review. Brain Sci. 2022, 12, 1612. [Google Scholar] [CrossRef] [PubMed]
- Ibekwe, A.; Perras, C.; Mierzwinski-Urban, M. Monoclonal Antibodies to Prevent Migraine Headaches. In: CADTH Issues in Emerging Health Technologies. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health, Ottawa, Canada,2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538376/ (accessed on 28 August 2023).
- Nie, L.; Sun, K.; Gong, Z.; Li, H.; Quinn, J.P.; Wang, M. Src Family Kinases Facilitate the Crosstalk between CGRP and Cytokines in Sensitizing Trigeminal Ganglion via Transmitting CGRP Receptor/PKA Pathway. Cells 2022, 11, 3498. [Google Scholar] [CrossRef]
- Greco, R.; Demartini, C.; Francavilla, M.; Zanaboni, A.M.; Tassorelli, C. Antagonism of CGRP Receptor: Central and Peripheral Mechanisms and Mediators in an Animal Model of Chronic Migraine. Cells 2022, 11, 3092. [Google Scholar] [CrossRef] [PubMed]
- Mavridis, T.; Deligianni, C.I.; Karagiorgis, G.; Daponte, A.; Breza, M.; Mitsikostas, D.D. Monoclonal Antibodies Targeting CGRP: From Clinical Studies to Real-World Evidence—What Do We Know So Far? Pharmaceuticals 2021, 14, 700. [Google Scholar] [CrossRef]
- Pellesi, L.; Guerzoni, S.; Pini, L.A. Spotlight on Anti-CGRP Monoclonal Antibodies in Migraine: The Clinical Evidence to Date. Clin. Pharmacol. Drug Dev. 2017, 6, 534–547. [Google Scholar] [CrossRef]
- Nguyen, J.L.; Munshi, K.; Peasah, S.K.; Swart, E.C.S.; Kohli, M.; Henderson, R.; Good, C.B. Trends in utilization and costs of migraine medications, 2017-2020. J. Headache Pain 2022, 23, 111. [Google Scholar] [CrossRef]
- Haanes, K.A. , Edvinsson, L.; Sams, A. Understanding side-effects of anti-CGRP and anti-CGRP receptor antibodies. J. Headache Pain 2020, 21, 26. [Google Scholar] [CrossRef]
- Telegdy, G.; Adamik, A.; Tanaka, M.; Schally, A.V. Effects of the LHRH antagonist Cetrorelix on affective and cognitive functions in rats. Regul. Pept. 2010, 159, 142–147. [Google Scholar] [CrossRef]
- Tanaka, M.; Schally, A.V.; Telegdy, G. Neurotransmission of the antidepressant-like effects of the growth hormone-releasing hormone antagonist MZ-4-71. Behav. Brain Res. 2012, 228, 388–91. [Google Scholar] [CrossRef]
- Tanaka, M.; Telegdy, G. Neurotransmissions of antidepressant-like effects of neuromedin U-23 in mice. Behav. Brain Res. 2014, 259, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Csabafi, K.; Telegdy, G. Neurotransmissions of antidepressant-like effects of kisspeptin-13. Regul. Pept. 2013, 180, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Telegdy, G.; Tanaka, M.; Schally, A.V. Effects of the growth hormone-releasing hormone (GH-RH) antagonist on brain functions in mice. Behav. Brain Res. 2011, 224, 155–158. [Google Scholar] [CrossRef]
- Rákosi, K.; Masaru, T.; Zarándia, M.; Telegdy, G.; Tóth, G.K. Short analogs and mimetics of human urocortin 3 display antidepressant effects in vivo. Peptides 2014, 62, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.N.; Nguyen, N.P.K.; Nguyen, L.T.H.; Shin, H.-M.; Yang, I.-J. Screening for Neuroprotective and Rapid Antidepressant-like Effects of 20 Essential Oils. Biomedicines 2023, 11, 1248. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Kádár, K.; Tóth, G.; Telegdy, G. Antidepressant-like effects of urocortin 3 fragments. Brain Res. Bull. 2011, 84, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Baliellas, D.E.M.; Barros, M.P.; Vardaris, C.V.; Guariroba, M.; Poppe, S.C.; Martins, M.F.; Pereira, Á.A.F.; Bondan, E.F. Propentofylline Improves Thiol-Based Antioxidant Defenses and Limits Lipid Peroxidation following Gliotoxic Injury in the Rat Brainstem. Biomedicines 2023, 11, 1652. [Google Scholar] [CrossRef] [PubMed]
- Montanari, M.; Imbriani, P.; Bonsi, P.; Martella, G.; Peppe, A. Beyond the Microbiota: Understanding the Role of the Enteric Nervous System in Parkinson’s Disease from Mice to Human. Biomedicines 2023, 11, 1560. [Google Scholar] [CrossRef]
- Garifulin, R.; Davleeva, M.; Izmailov, A.; Fadeev, F.; Markosyan, V.; Shevchenko, R.; Minyazeva, I.; Minekayev, T.; Lavrov, I.; Islamov, R. Evaluation of the Autologous Genetically Enriched Leucoconcentrate on the Lumbar Spinal Cord Morpho-Functional Recovery in a Mini Pig with Thoracic Spine Contusion Injury. Biomedicines 2023, 11, 1331. [Google Scholar] [CrossRef]
- Bueno, C.R.d.S.; Tonin, M.C.C.; Buchaim, D.V.; Barraviera, B.; Ferreira Junior, R.S.; Santos, P.S.d.S.; Reis, C.H.B.; Pastori, C.M.; Pereira, E.d.S.B.M.; Nogueira, D.M.B.; et al. Morphofunctional Improvement of the Facial Nerve and Muscles with Repair Using Heterologous Fibrin Biopolymer and Photobiomodulation. Pharmaceuticals 2023, 16, 653. [Google Scholar] [CrossRef]
- Sojka, A,; Żarowski, M. ; Steinborn, B.; Hedzelek, W.; Wiśniewska-Spychała, B.; Dorocka-Bobkowska, B. Temporomandibular disorders in adolescents with headache. Adv. Clin. Exp. Med. 2018, 27, 193–199. [Google Scholar] [CrossRef]
- Polyák, H.; Galla, Z.; Nánási, N.; Cseh, E.K.; Rajda, C.; Veres, G.; Spekker, E.; Szabó, Á.; Klivényi, P.; Tanaka, M.; et al. The Tryptophan-Kynurenine Metabolic System Is Suppressed in Cuprizone-Induced Model of Demyelination Simulating Progressive Multiple Sclerosis. Biomedicines 2023, 11, 945. [Google Scholar] [CrossRef]
- Tanaka, M.; Szabó, Á.; Vécsei, L. Preclinical modeling in depression and anxiety: Current challenges and future research directions. Adv. Clin. Exp. Med. 2023, 32, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.-C.; Huang, C.-S.; Chang, P.-K.; Chen, R.-S.; Chen, K.-T.; Hsieh, T.-H.; Liu, H.-L. Weak Ultrasound Contributes to Neuromodulatory Effects in the Rat Motor Cortex. Int. J. Mol. Sci. 2023, 24, 2578. [Google Scholar] [CrossRef]
- Gecse, K.; Édes, A.E.; Nagy, T.; Demeter, A.K.; Virág, D.; Király, M.; Dalmadi Kiss, B.; Ludányi, K.; Környei, Z.; Denes, A.; et al. Citalopram Neuroendocrine Challenge Shows Altered Tryptophan and Kynurenine Metabolism in Migraine. Cells 2022, 11, 2258. [Google Scholar] [CrossRef]
- Nasini, S.; Tidei, S.; Shkodra, A.; De Gregorio, D.; Cambiaghi, M.; Comai, S. Age-Related Effects of Exogenous Melatonin on Anxiety-like Behavior in C57/B6J Mice. Biomedicines 2023, 11, 1705. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-C.; Wang, T.-S.; Chang, F.-Y.; Chen, P.-A.; Chen, Y.-C. Age, Dose, and Locomotion: Decoding Vulnerability to Ketamine in C57BL/6J and BALB/c Mice. Biomedicines 2023, 11, 1821. [Google Scholar] [CrossRef]
- Statsenko, Y.; Habuza, T.; Smetanina, D.; Simiyu, G.L.; Meribout, S.; King, F.C.; Gelovani, J.G.; Das, K.M.; Gorkom, K.N.-V.; Zaręba, K.; et al. Unraveling Lifelong Brain Morphometric Dynamics: A Protocol for Systematic Review and Meta-Analysis in Healthy Neurodevelopment and Ageing. Biomedicines 2023, 11, 1999. [Google Scholar] [CrossRef]
- Dang, J.; Tao, Q.; Niu, X.; Zhang, M.; Gao, X.; Yang, Z.; Yu, M.; Wang, W.; Han, S. Meta-Analysis of Structural and Functional Brain Abnormalities in Cocaine Addiction. Front. Psychiatry 2022, 13, 927075. [Google Scholar] [CrossRef]
- Balogh, L.; Tanaka, M.; Török, N.; Vécsei, L.; Taguchi, S. Crosstalk between Existential Phenomenological Psychotherapy and Neurological Sciences in Mood and Anxiety Disorders. Biomedicines 2021, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Rassler, B.; Blinowska, K.; Kaminski, M.; Pfurtscheller, G. Analysis of Respiratory Sinus Arrhythmia and Directed Information Flow between Brain and Body Indicate Different Management Strategies of fMRI-Related Anxiety. Biomedicines 2023, 11, 1028. [Google Scholar] [CrossRef] [PubMed]
- Arimura, A. Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn. J. Physiol. 1998, 48, 301–331. [Google Scholar] [CrossRef] [PubMed]
- Holland, P.R.; Barloese, M.; Fahrenkrug, J. PACAP in hypothalamic regulation of sleep and circadian rhythm: importance for headache. J. Headache Pain 2018, 19, 20. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, G.; D’Amico, A.G.; Musumeci, G.; Reglodi, D.; D’Agata, V. Effects of PACAP on Schwann Cells: Focus on Nerve Injury. Int. J. Mol. Sci. 2020, 21, 8233. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.C.; May, V.; Parsons, R.L.; Hammack, S.E. Parallel signaling pathways of pituitary adenylate cyclase activating polypeptide (PACAP) regulate several intrinsic ion channels. Ann. N.Y. Acad. Sci. 2019, 1455, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Clement, A.; Guo, S.; Jansen-Olesen, I.; Christensen, S.L. ATP-Sensitive Potassium Channels in Migraine: Translational Findings and Therapeutic Potential. Cells 2022, 11, 2406. [Google Scholar] [CrossRef] [PubMed]
- Miyata, A.; Arimura, A.; Dahl, R.R.; Minamino, N.; Uehara, A.; Jiang, L.; Culler, M.D.; Coy, D.H. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem. Biophys. Res. Commun. 1989, 164, 567–574. [Google Scholar] [CrossRef]
- Denes, V.; Geck, P.; Mester, A.; Gabriel, R. Pituitary Adenylate Cyclase-Activating Polypeptide: 30 Years in Research Spotlight and 600 Million Years in Service. J. Clin. Med. 2019, 8, 1488. [Google Scholar] [CrossRef] [PubMed]
- Hypophysis Adenylate Cyclase Activating Polypeptide. Handbook of Biologically Active Peptides (Second Edition), 2013. Available online: https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/hypophysis-adenylate-cyclase-activating-polypeptide (accessed on 28 August 2023).
- Tam, J.K.; Lee, L.T.; Chow, B.K. PACAP-related peptide (PRP)--molecular evolution and potential functions. Peptides 2007, 28, 1920–1929. [Google Scholar] [CrossRef] [PubMed]
- Köves, K.; Szabó, E.; Kántor, O.; Heinzlmann, A.; Szabó, F.; Csáki, Á. Current State of Understanding of the Role of PACAP in the Hypothalamo-Hypophyseal Gonadotropin Functions of Mammals. Front. Endocrinol. (Lausanne) 2020, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Seo, S.R. Neuroprotective roles of pituitary adenylate cyclase-activating polypeptide in neurodegenerative diseases. BMB Rep. 2014, 47, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Sadanandan, N.; Cozene, B.; Park, Y.J.; Farooq, J.; Kingsbury, C.; Wang, Z.J.; Moscatello, A.; Saft, M.; Cho, J.; Gonzales-Portillo, B.; Borlongan, C.V. Pituitary Adenylate Cyclase-Activating Polypeptide: A Potent Therapeutic Agent in Oxidative Stress. Antioxidants 2021, 10, 354. [Google Scholar] [CrossRef]
- Waschek, J.A. VIP and PACAP: neuropeptide modulators of CNS inflammation, injury, and repair. Br. J. Pharmacol. 2013, 169, 512–523. [Google Scholar] [CrossRef]
- Xiao, X.; Qiu, P.; Gong, H.Z.; Chen, X.M. , Sun, Y.; Hong, A.; Ma, Y. PACAP ameliorates hepatic metabolism and inflammation through up-regulating FAIM in obesity. J. Cell Mol. Med. 2019, 23, 5970–5980. [Google Scholar] [CrossRef]
- Gonkowski, S. Vasoactive Intestinal Polypeptide in the Carotid Body—A History of Forty Years of Research. A Mini Review. Int. J. Mol. Sci. 2020, 21, 4692. [Google Scholar] [CrossRef]
- Jiang W, Wang H, Li YS, Luo W. Role of vasoactive intestinal peptide in osteoarthritis. J Biomed Sci. 2016, 23, 63. [Google Scholar] [CrossRef]
- Cao SG, Wu WC, Han Z, Wang MY. Effects of psychological stress on small intestinal motility and expression of cholecystokinin and vasoactive intestinal polypeptide in plasma and small intestine in mice. World J Gastroenterol. 2005, 11, 737–40. [Google Scholar] [CrossRef]
- Jacobs B, Dussor G. Neurovascular contributions to migraine: Moving beyond vasodilation. Neuroscience 2016, 338, 130–144. [Google Scholar] [CrossRef]
- Langer, I.; Jeandriens, J.; Couvineau, A.; Sanmukh, S.; Latek, D. Signal Transduction by VIP and PACAP Receptors. Biomedicines 2022, 10, 406. [Google Scholar] [CrossRef]
- Fizanne, L.; Sigaudo-Roussel, D.; Saumet, J.L.; Fromy, B. Evidence for the involvement of VPAC1 and VPAC2 receptors in pressure-induced vasodilatation in rodents. J. Physiol. 2004, 554 Pt 2, 519–528. [Google Scholar] [CrossRef]
- Parsons RL, May V. PACAP-Induced PAC1 Receptor Internalization and Recruitment of Endosomal Signaling Regulate Cardiac Neuron Excitability. J Mol Neurosci. 2019, 68, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Bill, C.A.; Vines, C.M. Phospholipase C. Adv. Exp. Med. Biol. 2020, 1131, 215–242. [Google Scholar] [CrossRef]
- Barloese, M.; Chitgar, M.; Hannibal, J.; Møller, S. Pituitary adenylate cyclase-activating peptide: Potential roles in the pathophysiology and complications of cirrhosis. Liver Int. 2020, 40, 2578–2589. [Google Scholar] [CrossRef]
- Makhinson, M.; Chotiner, J.K.; Watson, J.B.; O'Dell, T.J. Adenylyl cyclase activation modulates activity-dependent changes in synaptic strength and Ca2+/calmodulin-dependent kinase II autophosphorylation. J. Neurosci. 1999, 19, 2500–2510. [Google Scholar] [CrossRef]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef]
- Lu, J.; Piper, S.J.; Zhao, P.; Miller, L.J.; Wootten, D.; Sexton, P.M. Targeting VIP and PACAP Receptor Signaling: New Insights into Designing Drugs for the PACAP Subfamily of Receptors. Int. J. Mol. Sci. 2022, 23, 8069. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, T.; Nakamachi, T.; Shioda, S. Discovery of PACAP and its receptors in the brain. J. Headache Pain, 2018, 19, 28. [Google Scholar] [CrossRef] [PubMed]
- Vasoactive Intestinal Polypeptide Receptor 1. Methods in Enzymology, 2013. Available online: https://www.sciencedirect.com/topics/medicine-and-dentistry/vasoactive-intestinal-polypeptide-receptor-1 (accessed on 28 August 2023).
- Vasoactive Intestinal Polypeptide Receptor. Autonomic Neuroscience, 2007. Available online: https://www.sciencedirect.com/topics/neuroscience/vasoactive-intestinal-polypeptide-receptor (accessed on 28 August 2023).
- May, V.; Buttolph, T.R.; Girard, B.M.; Clason, T.A.; Parsons, R.L. PACAP-induced ERK activation in HEK cells expressing PAC1 receptors involves both receptor internalization and PKC signaling. Am. J. Physiol. Cell Physiol. 2014, 306, C1068–C1079. [Google Scholar] [CrossRef]
- Hou, X.; Yang, D.; Yang, G.; Li., M.; Zhang, J.; Zhang, J.; Zhang, Y.; Liu, Y. Therapeutic potential of vasoactive intestinal peptide and its receptor VPAC2 in type 2 diabetes. Front. Endocrinol. (Lausanne) 2022, 13, 984198. [Google Scholar] [CrossRef]
- Sundrum, T.; Walker, C.S. Pituitary adenylate cyclase-activating polypeptide receptors in the trigeminovascular system: implications for migraine. Br. J. Pharmacol. 2018, 175, 4109–4120. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, G.; Dan, Y.; Liu, X. CGRP and PACAP-38 play an important role in diagnosing pediatric migraine. J. Headache Pain 2022, 23, 68. [Google Scholar] [CrossRef] [PubMed]
- Schytz, H.W.; Olesen, J.; Ashina, M. The PACAP receptor: a novel target for migraine treatment. Neurotherapeutics 2010, 7, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Kuburas, A.; Russo, A.F. Shared and independent roles of CGRP and PACAP in migraine pathophysiology. J. Headache Pain 2023, 24, 34. [Google Scholar] [CrossRef]
- Ernstsen, C.; Christensen, S.L.; Rasmussen, R.H.; Nielsen, B.S.; Jansen-Olesen, I.; Olesen, J.; Kristensen, D.M. The PACAP pathway is independent of CGRP in mouse models of migraine: possible new drug target? Brain 2022, 145, 2450–2460. [Google Scholar] [CrossRef] [PubMed]
- Christensen, C.E.; Ashina, M.; Amin, F.M. Calcitonin Gene-Related Peptide (CGRP) and Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) in Migraine Pathogenesis. Pharmaceuticals 2022, 15, 1189. [Google Scholar] [CrossRef]
- Anapindi, K.D.B.; Yang, N.; Romanova, E.V.; Rubakhin, S.S.; Tipton, A.; Dripps, I.; Sheets, Z.; Sweedler, J.V.; Pradhan, A.A. PACAP and Other Neuropeptide Targets Link Chronic Migraine and Opioid-induced Hyperalgesia in Mouse Models. Mol. Cell Proteomics 2019, 18, 2447–2458. [Google Scholar] [CrossRef] [PubMed]
- Silvestro, M.; Iannone, L.F.; Orologio, I.; Tessitore, A.; Tedeschi, G.; Geppetti, P.; Russo, A. Migraine Treatment: Towards New Pharmacological Targets. Int. J. Mol. Sci. 2023, 24, 12268. [Google Scholar] [CrossRef]
- Pellesi, L.; Chaudhry, B.A.; Vollesen, A.L.H.; Snoer, A.H.; Baumann, K.; Skov, P.S.; Jensen, R.H.; Ashina, M. PACAP38- and VIP-induced cluster headache attacks are not associated with changes of plasma CGRP or markers of mast cell activation. Cephalalgia 2022, 42, 687–695. [Google Scholar] [CrossRef]
- Rasmussen, N.B.; Deligianni, C.; Christensen, C.E.; Karlsson, W.K.; Al-Khazali, H.M.; Van de Casteele, T.; Granhall, C.; Amin, F.M.; Ashina, M. The effect of Lu AG09222 on PACAP38- and VIP-induced vasodilation, heart rate increase, and headache in healthy subjects: an interventional, randomized, double-blind, parallel-group, placebo-controlled study. J. Headache Pain 2023, 24, 60. [Google Scholar] [CrossRef]
- Adenylate Cyclase. Adenylate cyclases are enzymes that catalyze the conversion of ATP to cAMP and pyrophosphate. From: The Senses: A Comprehensive Reference, 2008. Available online: https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/adenylate-cyclase (accessed on 29 August 2023).
- Roberts, R.E. The extracellular signal-regulated kinase (ERK) pathway: a potential therapeutic target in hypertension. J. Exp. Pharmacol. 2012, 4, 77–83. [Google Scholar] [CrossRef]
- Lund, A.M.; Hannibal, J. Localization of the neuropeptides pituitary adenylate cyclase-activating polypeptide, vasoactive intestinal peptide, and their receptors in the basal brain blood vessels and trigeminal ganglion of the mouse CNS; an immunohistochemical study. Front. Neuroanat. 2022, 16, 991403. [Google Scholar] [CrossRef]
- Ivic, I.; Balasko, M.; Fulop, B.D.; Hashimoto, H.; Toth, G.; Tamas, A.; Juhasz, T.; Koller, A.; Reglodi, D.; Solymár, M. VPAC1 receptors play a dominant role in PACAP-induced vasorelaxation in female mice. PLoS One 2019, 14, e0211433. [Google Scholar] [CrossRef]
- VIP and PACAP receptors - IUPHAR/BPS Guide to PHARMACOLOGY. Available online: https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=67 (accessed on 29 August 2023).
- Datki, Z.; Sinka, R. Translational biomedicine-oriented exploratory research on bioactive rotifer-specific biopolymers. Adv. Clin. Exp. Med. 2022, 31, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Palotai, M.; Telegdy, G.; Tanaka, M.; Bagosi, Z.; Jászberényi, M. Neuropeptide AF induces anxiety-like and antidepressant-like behavior in mice. Behav. Brain Res. 2014, 274, 264–269. [Google Scholar] [CrossRef]
- Lieb, A.; Thaler, G.; Fogli, B.; Trovato, O.; Posch, M.A.; Kaserer, T.; Zangrandi, L. Functional Characterization of Spinocerebellar Ataxia Associated Dynorphin A Mutant Peptides. Biomedicines 2021, 9, 1882. [Google Scholar] [CrossRef] [PubMed]
- Skobeleva, K.; Shalygin, A.; Mikhaylova, E.; Guzhova, I.; Ryazantseva, M.; Kaznacheyeva, E. The STIM1/2-Regulated Calcium Homeostasis Is Impaired in Hippocampal Neurons of the 5xFAD Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 14810. [Google Scholar] [CrossRef] [PubMed]
- Martos, D.; Tuka, B.; Tanaka, M.; Vécsei, L.; Telegdy, G. Memory Enhancement with Kynurenic Acid and Its Mechanisms in Neurotransmission. Biomedicines 2022, 10, 849. [Google Scholar] [CrossRef]
- Tanaka, M.; Szabó, Á.; Spekker, E.; Polyák, H.; Tóth, F.; Vécsei, L. Mitochondrial Impairment: A Common Motif in Neuropsychiatric Presentation? The Link to the Tryptophan–Kynurenine Metabolic System. Cells 2022, 11, 2607. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Bohár, Z.; Martos, D.; Telegdy, G.; Vécsei, L. Antidepressant-like effects of kynurenic acid in a modified forced swim test. Pharmacol. Rep. 2020, 72, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Telegdy, G. Involvement of adrenergic and serotonergic receptors in antidepressant-like effect of urocortin 3 in a modified forced swimming test in mice. Brain Res. Bull. 2008, 77, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Spekker, E.; Szabó, Á.; Polyák, H.; Vécsei, L. Modelling the neurodevelopmental pathogenesis in neuropsychiatric disorders. Bioactive kynurenines and their analogues as neuroprotective agents-in celebration of 80th birthday of Professor Peter Riederer. J. Neural. Transm. (Vienna) 2022, 129, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Reducha, P.V.; Edvinsson, L.; Haanes, K.A. Could Experimental Inflammation Provide Better Understanding of Migraines? Cells 2022, 11, 2444. [Google Scholar] [CrossRef] [PubMed]
- Ojala, J.; Tooke, K.; Hsiang, H.; Girard, B.M.; May, V.; Vizzard, M.A. PACAP/PAC1 Expression and Function in Micturition Pathways. J. Mol. Neurosci. 2019, 68, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Tamas, A.; Reglodi, D.; Farkas, O.; Kovesdi, E.; Pal, J.; Povlishock, J.T.; Schwarcz, A.; Czeiter, E.; Szanto, Z.; Doczi, T.; et al. Effect of PACAP in central and peripheral nerve injuries. Int. J. Mol. Sci. 2012, 13, 8430–8448. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Y.; Yang, L.; Wang, Y.; Xiao, Z. PACAP6-38 improves nitroglycerin-induced central sensitization by modulating synaptic plasticity at the trigeminal nucleus caudalis in a male rat model of chronic migraine. J. Headache Pain 2023, 24, 66. [Google Scholar] [CrossRef]
- Takács-Lovász K, Kun J, Aczél T, Urbán P, Gyenesei A, Bölcskei K, Szőke É, Helyes Z. PACAP-38 Induces Transcriptomic Changes in Rat Trigeminal Ganglion Cells Related to Neuroinflammation and Altered Mitochondrial Function Presumably via PAC1/VPAC2 Receptor-Independent Mechanism. Int. J. Mol. Sci. 2022, 23, 2120. [Google Scholar] [CrossRef]
- Frederiksen, S.D.; Haanes, K.A.; Warfvinge, K.; Edvinsson, L. Perivascular neurotransmitters: Regulation of cerebral blood flow and role in primary headaches. J. Cereb. Blood Flow Metab. 2019, 39, 610–632. [Google Scholar] [CrossRef]
- Markovics, A.; Kormos, V.; Gaszner, B.; Lashgarara, A.; Szoke, E.; Sandor, K.; Szabadfi, K.; Tuka, B.; Tajti, J.; Szolcsanyi, J.; et al. Pituitary adenylate cyclase-activating polypeptide plays a key role in nitroglycerol-induced trigeminovascular activation in mice. Neurobiol. Dis. 2012, 45, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, L. PACAP and its receptors in migraine pathophysiology: Commentary on Walker et al., Br. J. Pharmacol. 171: 1521-1533. Br. J. Pharmacol. 2015, 172, 4782–4784. [Google Scholar] [CrossRef] [PubMed]
- Saposnik, G.; Montalban, X.; Selchen, D.; Terzaghi, M.A.; Bakdache, F.; Montoya, A.; Fruns, M.; Caceres, F.; Oh, J. Therapeutic Inertia in Multiple Sclerosis Care: A Study of Canadian Neurologists. Front. Neurol. 2018, 9, 781. [Google Scholar] [CrossRef] [PubMed]
- Harding, S.D.; Armstrong, J.F.; Faccenda, E.; Southan, C.; Alexander, S.P.H.; Davenport, A.P.; Pawson, A.J.; Spedding, M.; Davies, J.A.; NC-IUPHAR. (2021) The IUPHAR/BPS guide to PHARMACOLOGY in 2022: curating pharmacology for COVID-19, malaria and antibacterials. Nucl. Acids Res. 2022, 2022. 50, D1282–D1294. [Google Scholar] [CrossRef]
- Guo S, Jansen-Olesen I, Olesen J, Christensen SL. Role of PACAP in migraine: An alternative to CGRP? Neurobiol Dis. 2023, 176, 105946. [Google Scholar] [CrossRef]
- Granoth R, Fridkin M, Gozes I. VIP and the potent analog, stearyl-Nle(17)-VIP, induce proliferation of keratinocytes. FEBS Lett. 2000, 475, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Gourlet P, De Neef P, Cnudde J, Waelbroeck M, Robberecht P. In vitro properties of a high affinity selective antagonist of the VIP1 receptor. Peptides. 1997, 18, 1555–60. [Google Scholar] [CrossRef] [PubMed]
- Beebe, X.; Darczak, D.; Davis-Taber, R.A.; Uchic, M.E.; Scott, V.E.; Jarvis, M.F.; Stewart, A.O. Discovery and SAR of hydrazide antagonists of the pituitary adenylate cyclase-activating polypeptide (PACAP) receptor type 1 (PAC1-R). Bioorg. Med. Chem. Lett. 2008, 18, 2162–2166. [Google Scholar] [CrossRef]
- Laburthe, M.; Couvineau, A.; Tan, V. Class II G protein-coupled receptors for VIP and PACAP: structure, models of activation and pharmacology. Peptides 2007, 28, 1631–1639. [Google Scholar] [CrossRef]
- Sundrum T, Walker CS. Pituitary adenylate cyclase-activating polypeptide receptors in the trigeminovascular system: implications for migraine. Br J Pharmacol. 2018, 175, 4109–4120. [Google Scholar] [CrossRef]
- Spekker, E.; Tanaka M, Szabó Á, Vécsei L. Neurogenic Inflammation: The Participant in Migraine and Recent Advancements in Translational Research. Biomedicines. 2022, 10, 76. [Google Scholar] [CrossRef]
- Pinho-Ribeiro, F.A.; Verri, W.A. Jr.; Chiu, I.M. Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation. Trends Immunol. 2017, 38, 5–19. [Google Scholar] [CrossRef]
- Guo, S.; Vollesen, A.L.; Hansen, R.D.; Esserlind, A.L.; Amin, F.M.; Christensen, A.F.; Olesen, J.; Ashina, M. Part I: Pituitary adenylate cyclase-activating polypeptide-38 induced migraine-like attacks in patients with and without familial aggregation of migraine. Cephalalgia. 2017, 37, 125–135. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov; PACAP Induced Migraine Attacks in Patients With High and Low Genetic Load. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT02158221 (accessed on 29 August 2023).
- Togha, M.; Ghorbani, Z.; Ramazi, S.; Zavvari, F.; Karimzadeh, F. Evaluation of Serum Levels of Transient Receptor Potential Cation Channel Subfamily V Member 1, Vasoactive Intestinal Polypeptide, and Pituitary Adenylate Cyclase-Activating Polypeptide in Chronic and Episodic Migraine: The Possible Role in Migraine Transformation. Front. Neurol. 2021, 12, 770980. [Google Scholar] [CrossRef] [PubMed]
- Körtési, T.; Tuka, B.; Tajti, J.; Bagoly, T.; Fülöp, F.; Helyes, Z.; Vécsei, L. Kynurenic Acid Inhibits the Electrical Stimulation Induced Elevated Pituitary Adenylate Cyclase-Activating Polypeptide Expression in the TNC. Front. Neurol. 2018, 8, 745. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Vollesen, A.L.; Hansen, Y.B.; Frandsen, E.; Andersen, M.R.; Amin, F.M.; Fahrenkrug, J.; Olesen, J.; Ashina, M. Part II: Biochemical changes after pituitary adenylate cyclase-activating polypeptide-38 infusion in migraine patients. Cephalalgia 2017, 37, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.M.; Asghar, M.S.; Guo, S.; Hougaard, A.; Hansen, A.E.; Schytz, H.W.; van der Geest, R.J.; de Koning, P.J.; Larsson, H.B.; Olesen, J.; Ashina, M. Headache and prolonged dilatation of the middle meningeal artery by PACAP38 in healthy volunteers. Cephalalgia 2012, 32, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Maasz, G.; Zrinyi, Z.; Reglodi, D.; Petrovics, D.; Rivnyak, A.; Kiss, T.; Jungling, A.; Tamas, A.; Pirger, Z. Pituitary adenylate cyclase-activating polypeptide (PACAP) has a neuroprotective function in dopamine-based neurodegeneration in rat and snail parkinsonian models. Dis. Model Mech. 2017, 10, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Beltrán, E.; Correnti, E.; Deen, M.; Kamm, K.; Kelderman, T.; Papetti, L.; Vigneri, S.; MaassenVanDenBrink, A.; Edvinsson, L.; European Headache Federation School of Advanced Studies (EHF-SAS). PACAP38 and PAC1 receptor blockade: a new target for headache? J. Headache Pain 2018, 19, 64. [Google Scholar] [CrossRef] [PubMed]
- Ashina, M.; Doležil, D.; Bonner, J.H.; Zhou, L.; Klatt, J.; Picard, H.; Mikol, D.D. A phase 2, randomized, double-blind, placebo-controlled trial of AMG 301, a pituitary adenylate cyclase-activating polypeptide PAC1 receptor monoclonal antibody for migraine prevention. Cephalalgia 2021, 41, 33–44. [Google Scholar] [CrossRef]
- Study to Evaluate the Efficacy and Safety of AMG 301 in Migraine Prevention 2020. Available online: https://ClinicalTrials.gov/show/NCT03238781 (accessed on 4 September 2023).
- Lundbeck News Room: Lundbeck announced the start of a phase II clinical study to assess Lu AG09222 for migraine prevention. Available online: https://newsroom.lundbeckus.com/news-release/2021/lundbeck-announced-start-of-phase-ii-clinical-study-for-migraine-prevention (accessed on 29 August 2023).
- A Study With Lu AG09222 in Adults With Migraine Who Have Not Been Helped by Prior Preventive Treatments 2023. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05133323 (accessed on 4 September 2023).
- A Study of LY3451838 in Participants with Migraine 2023. Available online: https://ClinicalTrials.gov/show/NCT04498910 (accessed on 4 September 2023).
- ClinicalTrials.gov; The Effects of a Long-lasting Infusion of Vasoactive Intestinal Peptide (VIP) in Episodic Migraine Patients. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04260035 (accessed on 29 August 2023).
- Tanaka, M.; Szabó, Á.; Vécsei, L. Integrating Armchair, Bench, and Bedside Research for Behavioral Neurology and Neuropsychiatry: Editorial. Biomedicines 2022, 10, 2999. [Google Scholar] [CrossRef] [PubMed]
- Gaebler, A.J.; Finner-Prével, M.; Sudar, F.P.; Langer, F.H.; Keskin, F.; Gebel, A.; Zweerings, J.; Mathiak, K. The Interplay between Vitamin D, Exposure of Anticholinergic Antipsychotics and Cognition in Schizophrenia. Biomedicines 2022, 10, 1096. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Mariqueo, L.; Giménez-Llort, L. Impact of Behavioral Assessment and Re-Test as Functional Trainings That Modify Survival, Anxiety and Functional Profile (Physical Endurance and Motor Learning) of Old Male and Female 3xTg-AD Mice and NTg Mice with Normal Aging. Biomedicines 2022, 10, 973. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.C.; Hong, D.-Y.; Lee, D.-H.; Park, S.-W.; Lee, J.Y.; Jeong, J.H.; Kim, E.-Y.; Chung, H.-M.; Hong, K.-S.; Park, S.-P.; et al. Inflammation and Rho-Associated Protein Kinase-Induced Brain Changes in Vascular Dementia. Biomedicines 2022, 10, 446. [Google Scholar] [CrossRef]
- Simonato, M.; Dall’Acqua, S.; Zilli, C.; Sut, S.; Tenconi, R.; Gallo, N.; Sfriso, P.; Sartori, L.; Cavallin, F.; Fiocco, U.; et al. Tryptophan Metabolites, Cytokines, and Fatty Acid Binding Protein 2 in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Biomedicines 2021, 9, 1724. [Google Scholar] [CrossRef]
- Smagin, D.A.; Kovalenko, I.L.; Galyamina, A.G.; Belozertseva, I.V.; Tamkovich, N.V.; Baranov, K.O.; Kudryavtseva, N.N. Chronic Lithium Treatment Affects Anxious Behaviors and theExpression of Serotonergic Genes in Midbrain Raphe Nuclei of Defeated Male Mice. Biomedicines 2021, 9, 1293. [Google Scholar] [CrossRef]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef]
- Tanaka, M.; Török, N.; Tóth, F.; Szabó, Á.; Vécsei, L. Co-Players in Chronic Pain: Neuroinflammation and the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 897. [Google Scholar] [CrossRef]
- Vila-Merkle, H.; González-Martínez, A.; Campos-Jiménez, R.; Martínez-Ricós, J.; Teruel-Martí, V.; Blasco-Serra, A.; Lloret, A.; Celada, P.; Cervera-Ferri, A. The Oscillatory Profile Induced by the Anxiogenic Drug FG-7142 in the Amygdala–Hippocampal Network Is Reversed by Infralimbic Deep Brain Stimulation: Relevance for Mood Disorders. Biomedicines 2021, 9, 783. [Google Scholar] [CrossRef]
- Santana-Santana, M.; Bayascas, J.-R.; Giménez-Llort, L. Fine-Tuning the PI3K/Akt Signaling Pathway Intensity by Sex and Genotype-Load: Sex-Dependent Homozygotic Threshold for Somatic Growth but Feminization of Anxious Phenotype in Middle-Aged PDK1 K465E Knock-In and Heterozygous Mice. Biomedicines 2021, 9, 747. [Google Scholar] [CrossRef]
- Muntsant, A.; Giménez-Llort, L. Genotype Load Modulates Amyloid Burden and Anxiety-Like Patterns in Male 3xTg-AD Survivors despite Similar Neuro-Immunoendocrine, Synaptic and Cognitive Impairments. Biomedicines 2021, 9, 715. [Google Scholar] [CrossRef]
- Giménez-Llort, L.; Marin-Pardo, D.; Marazuela, P.; Hernández-Guillamón, M. Survival Bias and Crosstalk between Chronological and Behavioral Age: Age- and Genotype-Sensitivity Tests Define Behavioral Signatures in Middle-Aged, Old, and Long-Lived Mice with Normal and AD-Associated Aging. Biomedicines 2021, 9, 636. [Google Scholar] [CrossRef]
- Komatsu, H.; Watanabe, E.; Fukuchi, M. Psychiatric Neural Networks and Precision Therapeutics by Machine Learning. Biomedicines 2021, 9, 403. [Google Scholar] [CrossRef]
- Caruso, G.; Godos, J.; Castellano, S.; Micek, A.; Murabito, P.; Galvano, F.; Ferri, R.; Grosso, G.; Caraci, F. The Therapeutic Potential of Carnosine/Anserine Supplementation against Cognitive Decline: A Systematic Review with Meta-Analysis. Biomedicines 2021, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Correia, B.S.B.; Nani, J.V.; Waladares Ricardo, R.; Stanisic, D.; Costa, T.B.B.C.; Hayashi, M.A.F.; Tasic, L. Effects of Psychostimulants and Antipsychotics on Serum Lipids in an Animal Model for Schizophrenia. Biomedicines 2021, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Ikonnikova, A.; Anisimova, A.; Galkin, S.; Gunchenko, A.; Abdukhalikova, Z.; Filippova, M.; Surzhikov, S.; Selyaeva, L.; Shershov, V.; Zasedatelev, A.; et al. Genetic Association Study and Machine Learning to Investigate Differences in Platelet Reactivity in Patients with Acute Ischemic Stroke Treated with Aspirin. Biomedicines 2022, 10, 2564. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Miranda, O.; Qi, X.; Kofler, J.; Sweet, R.A.; Wang, L. Unveiling the Enigma: Exploring Risk Factors and Mechanisms for Psychotic Symptoms in Alzheimer’s Disease through Electronic Medical Records with Deep Learning Models. Pharmaceuticals 2023, 16, 911. [Google Scholar] [CrossRef] [PubMed]
- Parolini, F.; Goethel, M.; Becker, K.; Fernandes, C.; Fernandes, R.J.; Ervilha, U.F.; Santos, R.; Vilas-Boas, J.P. Breaking Barriers: Artificial Intelligence Interpreting the Interplay between Mental Illness and Pain as Defined by the International Association for the Study of Pain. Biomedicines 2023, 11, 2042. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Diano, M.; Battaglia, S. Editorial: Insights into structural and functional organization of the brain: evidence from neuroimaging and non-invasive brain stimulation techniques. Front. Psychiatry. 2023, 14, 1225755. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Soga, T.; Ahemad, N.; Bhuvanendran, S.; Parhar, I. Kisspeptin-10 Rescues Cholinergic Differentiated SHSY-5Y Cells from α-Synuclein-Induced Toxicity In Vitro. Int. J. Mol. Sci. 2022, 23, 5193. [Google Scholar] [CrossRef] [PubMed]
- Okanda Nyatega, C.; Qiang, L.; Jajere Adamu, M.; Bello Kawuwa, H. Altered striatal functional connectivity and structural dysconnectivity in individuals with bipolar disorder: A resting state magnetic resonance imaging study. Front. Psychiatry. 2022, 13, 1054380. [Google Scholar] [CrossRef]
- Liu, N.; Li, Y.; Hong, Y.; Huo, J.; Chang, T.; Wang, H.; Huang, Y.; Li, W.; Zhang, Y. Altered brain activities in mesocorticolimbic pathway in primary dysmenorrhea patients of long-term menstrual pain. Front. Neurosci. 2023, 17, 1098573. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Yang, B.; Wang, H.; Zeng, Y.; Xin, J.; Li, X. The non-linear correlation between the volume of cerebral white matter lesions and incidence of bipolar disorder: A secondary analysis of data from a cross-sectional study. Front. Psychiatry 2023, 14, 1149663. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, R.; DeSouza, J.F.X.; Shen, Y.; Zhang, H.; Zhu, C.; Huang, P.; Wang, C. Differential responses from the left postcentral gyrus, right middle frontal gyrus, and precuneus to meal ingestion in patients with functional dyspepsia. Front. Psychiatry 2023, 14, 1184797. [Google Scholar] [CrossRef]
- Adamu, M.J.; Qiang, L.; Nyatega, C.O.; Younis, A.; Kawuwa, H.B.; Jabire, A.H.; Saminu, S. Unraveling the pathophysiology of schizophrenia: insights from structural magnetic resonance imaging studies. Front. Psychiatry 2023, 14, 1188603. [Google Scholar] [CrossRef]
- Battaglia S, Nazzi C, Thayer JF. Fear-induced bradycardia in mental disorders: Foundations, current advances, future perspectives. Neurosci Biobehav Rev. 2023, 149, 105163. [Google Scholar] [CrossRef]
- Battaglia, S., Nazzi, C., & Thayer, J. F. (2023). Heart's tale of trauma: Fear-conditioned heart rate changes in post-traumatic stress disorder. Acta psychiatrica Scandinavica, 10.1111/acps.13602. Advance online publication. [CrossRef]
- Battaglia S, Di Fazio C, Vicario CM, Avenanti A. Neuropharmacological Modulation of N-methyl-D-aspartate, Noradrenaline and Endocannabinoid Receptors in Fear Extinction Learning: Synaptic Transmission and Plasticity. Int J Mol Sci. 2023, 24, 5926. [Google Scholar] [CrossRef]
- Battaglia, M. R., Di Fazio, C., Battaglia, S. Activated Tryptophan-Kynurenine metabolic system in the human brain is associated with learned fear. Frontiers in Molecular Neuroscience 2023, 16, 1217090. [CrossRef]
- Battaglia S, Garofalo S, di Pellegrino G, Starita F. Revaluing the Role of vmPFC in the Acquisition of Pavlovian Threat Conditioning in Humans. J Neurosci. 2020, 40, 8491–8500. [Google Scholar] [CrossRef]
- Battaglia, S., Harrison, B. J., Fullana, M. A. Does the human ventromedial prefrontal cortex support fear learning, fear extinction or both? A commentary on subregional contributions. Molecular psychiatry 2022, 27, 784–786. [CrossRef]
- Di Gregorio, F., Battaglia, S. Advances in EEG-based functional connectivity approaches to the study of the central nervous system in health and disease. Advances in Clinical and Experimental Medicine 2023, 32, 607–612. [CrossRef]
- Vuralli, D.; Ayata, C.; Bolay, H. Cognitive dysfunction and migraine. J. Headache Pain 2018, 19, 109. [Google Scholar] [CrossRef] [PubMed]
- Minen, M.T.; Begasse De Dhaem, O.; Kroon Van Diest, A.; Powers, S.; Schwedt, T.J.; Lipton, R.; Silbersweig, D. Migraine and its psychiatric comorbidities. J. Neurol. Neurosurg. Psychiatry 2016, 87, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Chen, C. Editorial: Towards a mechanistic understanding of depression, anxiety, and their comorbidity: perspectives from cognitive neuroscience. Front. Behav. Neurosci. 2023, 17, 1268156. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Escamilla, G.; Dörfel, D.; Becke, M.; Trefz, J.; Bonanno, G.A.; Groppa, S. Associating Flexible Regulation of Emotional Expression With Psychopathological Symptoms. Front. Behav. Neurosci. 2022, 16, 924305. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Cardellicchio, P.; Di Fazio, C.; Nazzi, C.; Fracasso, A.; Borgomaneri, S. The Influence of Vicarious Fear-Learning in "Infecting" Reactive Action Inhibition. Front. Behav. Neurosci. 2022, 16, 946263. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Cardellicchio, P.; Di Fazio, C.; Nazzi, C.; Fracasso, A.; Borgomaneri, S. Stopping in (e)motion: Reactive action inhibition when facing valence-independent emotional stimuli. Front Behav. Neurosci. 2022, 16, 998714. [Google Scholar] [CrossRef] [PubMed]
- Ironside, M.; DeVille, D.C.; Kuplicki, R.T.; Burrows, K.P.; Smith, R.; Teed, A.R.; Paulus, M.P.; Khalsa, S.S. The unique face of comorbid anxiety and depression: increased interoceptive fearfulness and reactivity. Front. Behav. Neurosci. 2023, 16, 1083357. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, R.P. Comorbid depression and anxiety: Integration of insights from attachment theory and cognitive neuroscience, and their implications for research and treatment. Front. Behav. Neurosci. 2022, 16, 1104928. [Google Scholar] [CrossRef]
- Vila-Merkle, H.; González-Martínez, A.; Campos-Jiménez, R.; Martínez-Ricós, J.; Teruel-Martí, V.; Lloret, A.; Blasco-Serra, A.; Cervera-Ferri, A. Sex differences in amygdalohippocampal oscillations and neuronal activation in a rodent anxiety model and in response to infralimbic deep brain stimulation. Front. Behav. Neurosci. 2023, 17, 1122163. [Google Scholar] [CrossRef]
- Panov, G.; Panova, P. Obsessive-compulsive symptoms in patient with schizophrenia: The influence of disorganized symptoms, duration of schizophrenia, and drug resistance. Front. Psychiatry. 2023, 14, 1120974. [Google Scholar] [CrossRef]
- Hakamata, Y.; Hori, H.; Mizukami, S.; Izawa, S.; Yoshida, F.; Moriguchi, Y.; Hanakawa, T.; Inoue, Y.; Tagaya, H. Blunted diurnal interleukin-6 rhythm is associated with amygdala emotional hyporeactivity and depression: a modulating role of gene-stressor interactions. Front. Psychiatry 2023, 14, 1196235. [Google Scholar] [CrossRef]
- Fraile-Ramos, J.; Garrit, A.; Reig-Vilallonga, J.; Giménez-Llort, L. Hepatic Oxi-Inflammation and Neophobia as Potential Liver–Brain Axis Targets for Alzheimer’s Disease and Aging, with Strong Sensitivity to Sex, Isolation, and Obesity. Cells 2023, 12, 1517. [Google Scholar] [CrossRef] [PubMed]
- Sobolewska-Nowak, J.; Wachowska, K.; Nowak, A.; Orzechowska, A.; Szulc, A.; Płaza, O.; Gałecki, P. Exploring the Heart–Mind Connection: Unraveling the Shared Pathways between Depression and Cardiovascular Diseases. Biomedicines 2023, 11, 1903. [Google Scholar] [CrossRef] [PubMed]
- Festa, F.; Medori, S.; Macrì, M. Move Your Body, Boost Your Brain: The Positive Impact of Physical Activity on Cognition across All Age Groups. Biomedicines 2023, 11, 1765. [Google Scholar] [CrossRef] [PubMed]
- Spekker, E.; Bohár, Z.; Fejes-Szabó, A.; Szűcs, M.; Vécsei, L.; Párdutz, Á. Estradiol Treatment Enhances Behavioral and Molecular Changes Induced by Repetitive Trigeminal Activation in a Rat Model of Migraine. Biomedicines 2022, 10, 3175. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Lee, S.P.; Kim, D.; Kim, W.J. Gut Dysbiosis: A New Avenue for Stroke Prevention and Therapeutics. Biomedicines 2023, 11, 2352. [Google Scholar] [CrossRef] [PubMed]
- Montanari, M.; Imbriani, P.; Bonsi, P.; Martella, G.; Peppe, A. Beyond the Microbiota: Understanding the Role of the Enteric Nervous System in Parkinson’s Disease from Mice to Human. Biomedicines 2023, 11, 1560. [Google Scholar] [CrossRef] [PubMed]
- Altamura, C.; Corbelli, I.; de Tommaso, M.; Di Lorenzo, C.; Di Lorenzo, G.; Di Renzo A, Filippi, M. ; Jannini, T.B.; Messina, R.; Parisi, P.; et al. Pathophysiological Bases of Comorbidity in Migraine. Front. Hum. Neurosci. 2021, 15, 640574. [Google Scholar] [CrossRef] [PubMed]
- Schwedt, T.J.; Vargas, B. Neurostimulation for Treatment of Migraine and Cluster Headache. Pain Med. 2015, 16, 1827–1834. [Google Scholar] [CrossRef]
- de Albuquerque, L.L.; Pantovic, M.; Clingo, M.; Fischer, K.; Jalene, S.; Landers, M.; Mari, Z.; Poston, B. A Single Application of Cerebellar Transcranial Direct Current Stimulation Fails to Enhance Motor Skill Acquisition in Parkinson’s Disease: A Pilot Study. Biomedicines 2023, 11, 2219. [Google Scholar] [CrossRef]
- Senevirathne, D.K.L.; Mahboob, A.; Zhai, K.; Paul, P.; Kammen, A.; Lee, D.J.; Yousef, M.S.; Chaari, A. Deep Brain Stimulation beyond the Clinic: Navigating the Future of Parkinson’s and Alzheimer’s Disease Therapy. Cells 2023, 12, 1478. [Google Scholar] [CrossRef]
- Chu, P.-C.; Huang, C.-S.; Chang, P.-K.; Chen, R.-S.; Chen, K.-T.; Hsieh, T.-H.; Liu, H.-L. Weak Ultrasound Contributes to Neuromodulatory Effects in the Rat Motor Cortex. Int. J. Mol. Sci. 2023, 24, 2578. [Google Scholar] [CrossRef] [PubMed]
- Adeel, M.; Chen, C.-C.; Lin, B.-S.; Chen, H.-C.; Liou, J.-C.; Li, Y.-T.; Peng, C.-W. Safety of Special Waveform of Transcranial Electrical Stimulation (TES): In Vivo Assessment. Int. J. Mol. Sci. 2022, 23, 6850. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Schmidt, A.; Hassel, S.; Tanaka, M. Editorial: Case reports in neuroimaging and stimulation. Front. Psychiatry. 2023, 14, 1264669. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Wang, W.L.; Shieh, Y.H.; Peng, H.Y.; Ho, C.S.; Tsai, H.C. Case Report: Low-Frequency Repetitive Transcranial Magnetic Stimulation to Dorsolateral Prefrontal Cortex and Auditory Cortex in a Patient With Tinnitus and Depression. Front. Psychiatry 2022, 13, 847618. [Google Scholar] [CrossRef] [PubMed]
- Zakia, H.; Iskandar, S. Case report: Depressive disorder with peripartum onset camouflages suspected intracranial tuberculoma. Front. Psychiatry 2022, 13, 932635. [Google Scholar] [CrossRef] [PubMed]
- Nyatega, C.O.; Qiang, L.; Adamu, M.J.; Kawuwa, H.B. Gray matter, white matter and cerebrospinal fluid abnormalities in Parkinson's disease: A voxel-based morphometry study. Front Psychiatry 2022, 13, 1027907. [Google Scholar] [CrossRef]
- Rymaszewska, J.; Wieczorek, T.; Fila-Witecka, K.; Smarzewska, K.; Weiser, A, Piotrowski P, Tabakow P. Various neuromodulation methods including Deep Brain Stimulation of the medial forebrain bundle combined with psychopharmacotherapy of treatment-resistant depression-Case report. Front. Psychiatry 2023, 13, 1068054. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xie, X.; Xie, J.; Tian, S.; Du, X.; Feng, H.; Zhang, H. Early-onset Alzheimer's disease with depression as the first symptom: a case report with literature review. Front. Psychiatry 2023, 14, 1192562. [Google Scholar] [CrossRef]
- Kim, B.H.; Kim, S.H.; Han, C.; Jeong, H.G.; Lee, M.S.; Kim, J. Antidepressant-induced mania in panic disorder: a single-case study of clinical and functional connectivity characteristics. Front. Psychiatry 2023, 14, 1205126. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Cao, Y.; Deng, G.; Fang, J.; Qiu, C. Transient splenial lesion syndrome in bipolar-II disorder: a case report highlighting reversible brain changes during hypomanic episodes. Front. Psychiatry 2023, 14, 1219592. [Google Scholar] [CrossRef] [PubMed]
- Statsenko, Y.; Habuza, T.; Smetanina, D.; Simiyu, G.L.; Meribout, S.; King, F.C.; Gelovani, J.G.; Das, K.M.; Gorkom, K.N.-V.; Zaręba, K.; et al. Unraveling Lifelong Brain Morphometric Dynamics: A Protocol for Systematic Review and Meta-Analysis in Healthy Neurodevelopment and Ageing. Biomedicines 2023, 11, 1999. [Google Scholar] [CrossRef] [PubMed]
- Gazerani, P. Human Brain Organoids in Migraine Research: Pathogenesis and Drug Development. Int. J. Mol. Sci. 2023, 24, 3113. [Google Scholar] [CrossRef]
- Jalink, P.; Caiazzo, M. Brain Organoids: Filling the Need for a Human Model of Neurological Disorder. Biology 2021, 10, 740. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Song, H.; Ming, G.L. Modeling neurological disorders using brain organoids. Semin. Cell Dev. Biol. 2021, 111, 4–14. [Google Scholar] [CrossRef]
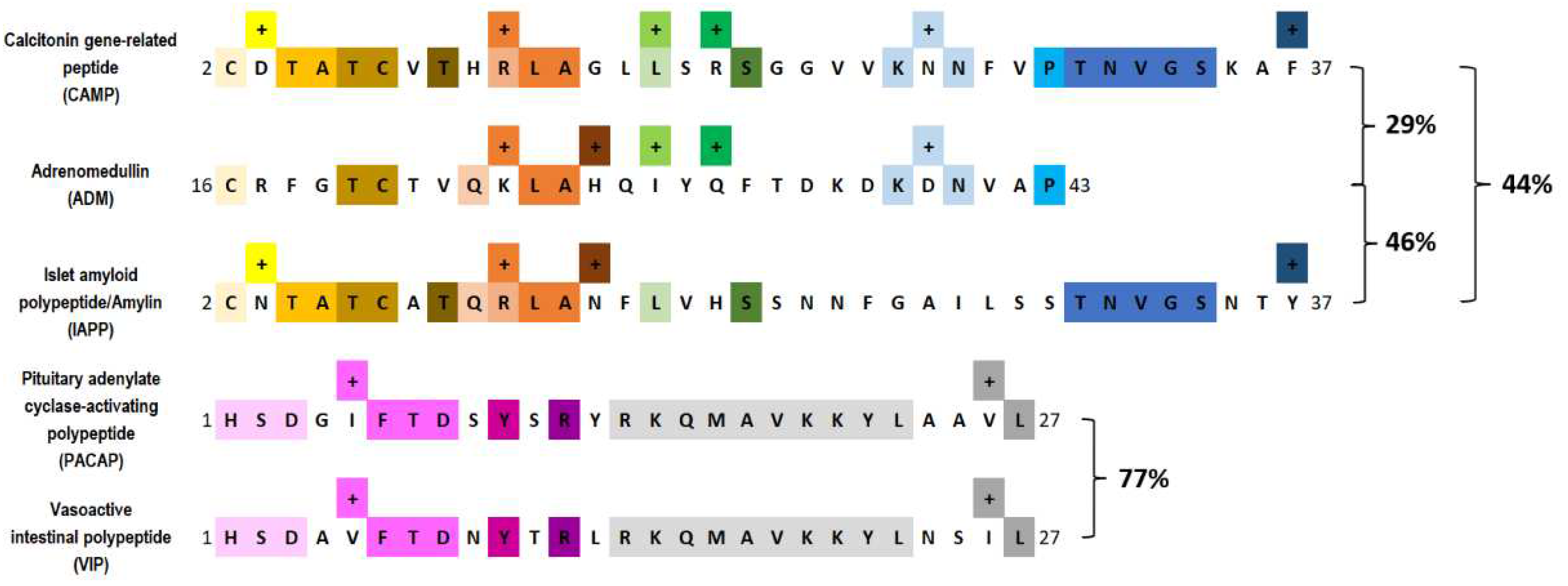
| Peptides | Receptors |
|---|---|
| CGRP | CLR |
| PACAP1–38 | >>PAC1, <VPAC1, <VPAC2 |
| PACAP6-38 | ? |
| PACAP1–27 | >PAC1, <VPAC1, <VPAC2 |
| PRP | ? |
| VIP | >VPAC1, >VPAC2, <PAC1 |
| Antagonists | Characteristics | Ref. |
|---|---|---|
| PACAP6-38 | PAC1 receptor antagonist, affinity for VPAC2 receptors | [142] |
| N-stearyl-[Nle17] neurotensin-(6-11)/VIP-(7-28) | VIP2/PACAP receptor antagonist, mitogen for the keratinocytic cell line and can increase adenylate cyclase activity | [143,144] |
| Maxadilan mutamnts | PAC1 receptor antagonists, increased adenylate cyclase activity | [145,146] |
| PG 97-269 | selective PAC1 receptor antagonists | [144] |
| ClinicalTrials.gov Identifier | Monoclonal antibody | Target | Status | Ref. |
| NCT03238781 | AMG 301 | receptor | No benefit over placebo for migraine prevention | [158,159] |
| NCT05133323 | Lu AG09222 | ligand | No results posted; The press release announced a decrease in the number of migraine days per month | [160,161] |
| NCT04498910 | LY3451838 | receptor | No results posted | [162] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





