Submitted:
06 September 2023
Posted:
07 September 2023
You are already at the latest version
Abstract
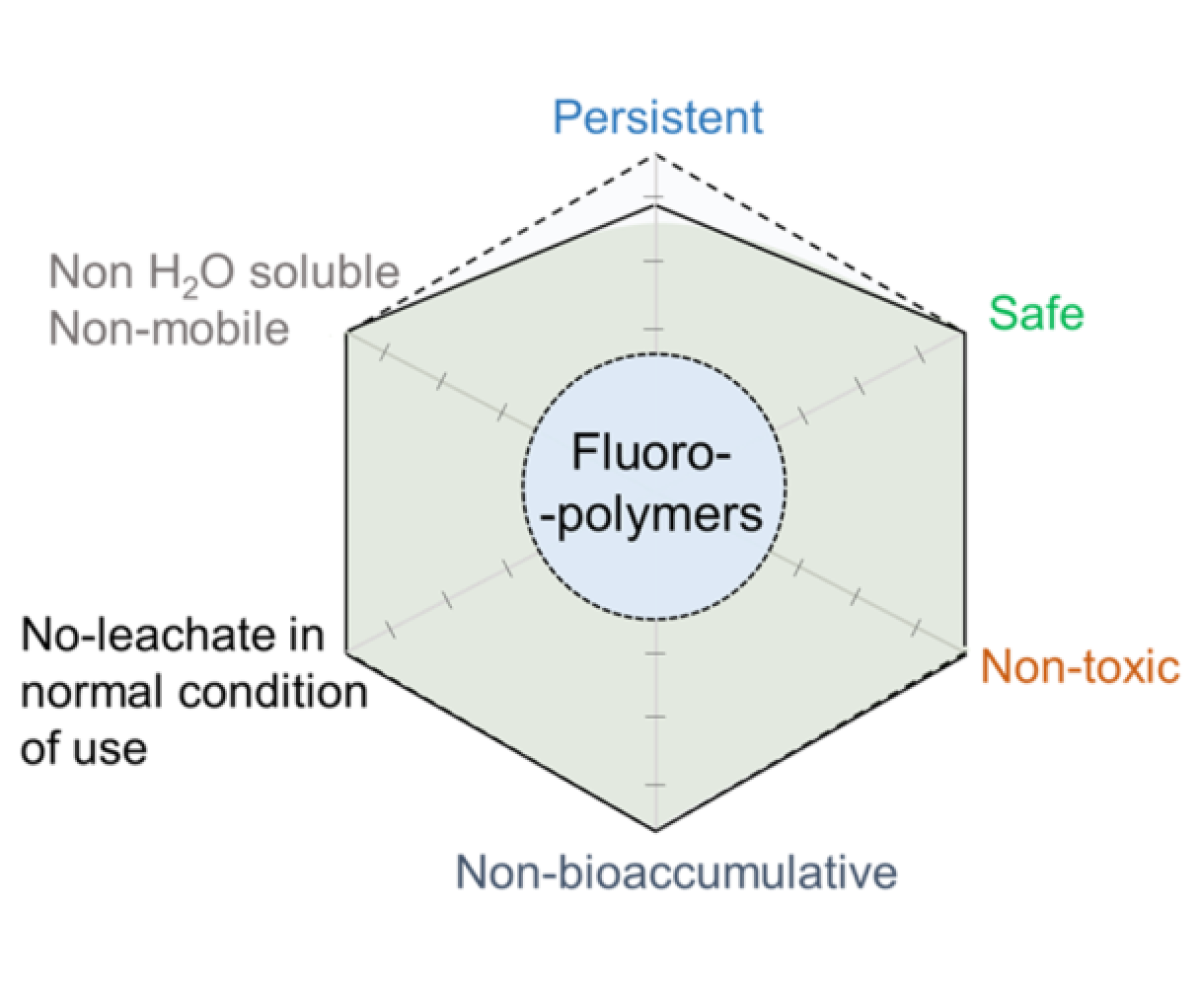
Keywords:
1. Introduction
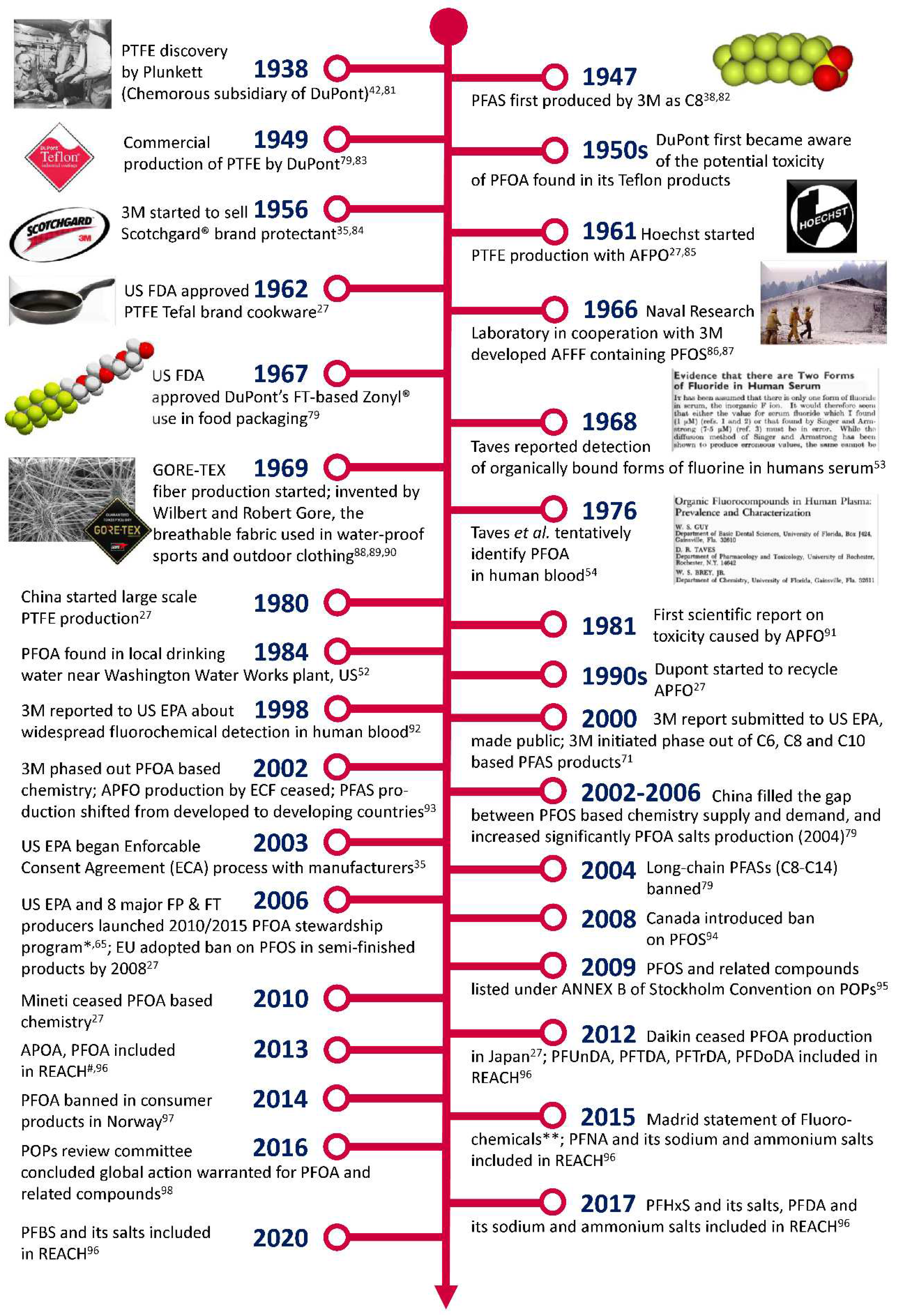
2. History of PFASs
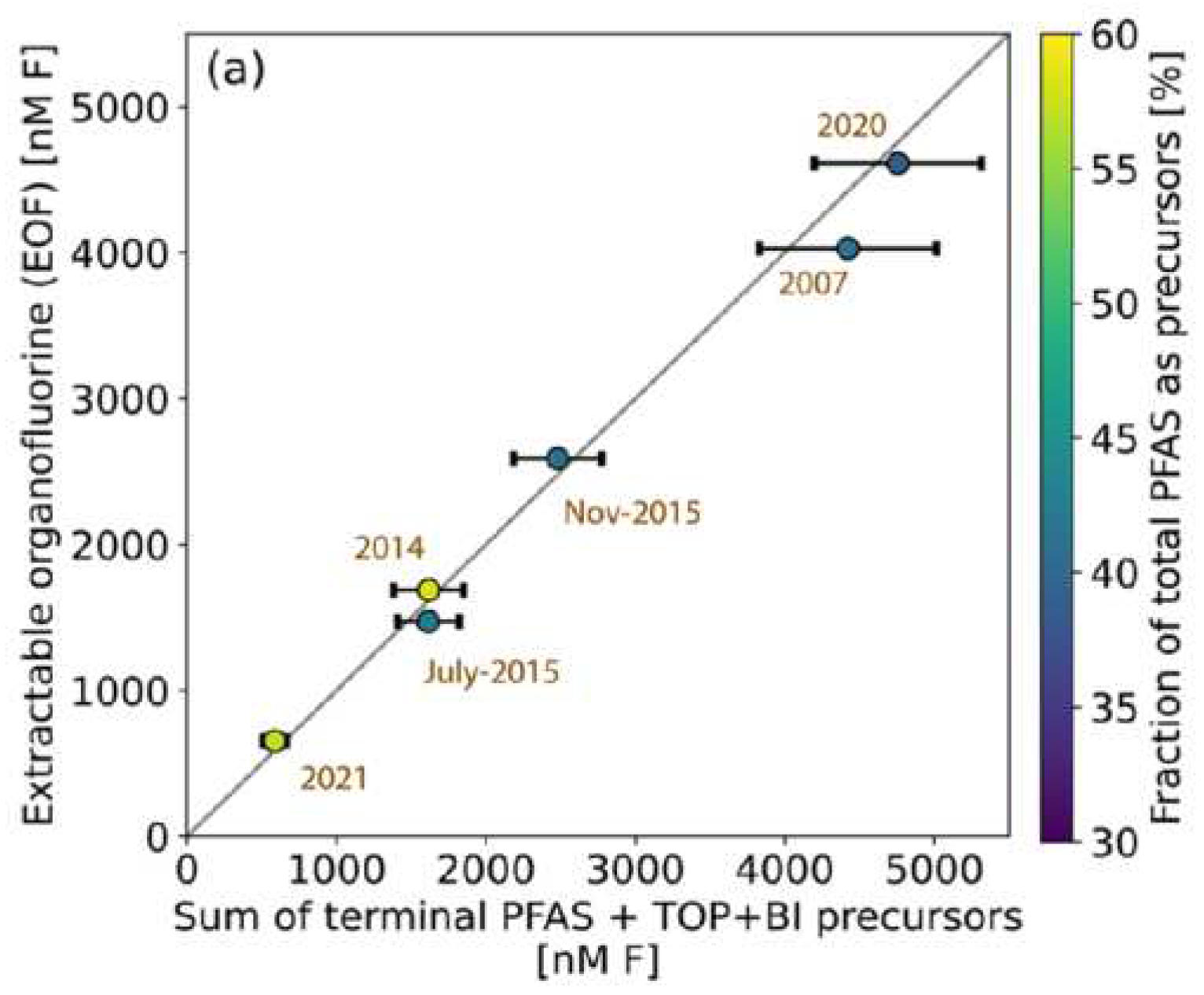
3. Issues on Mobility, Persistency, Toxicity and Bioaccumulation of PFASs
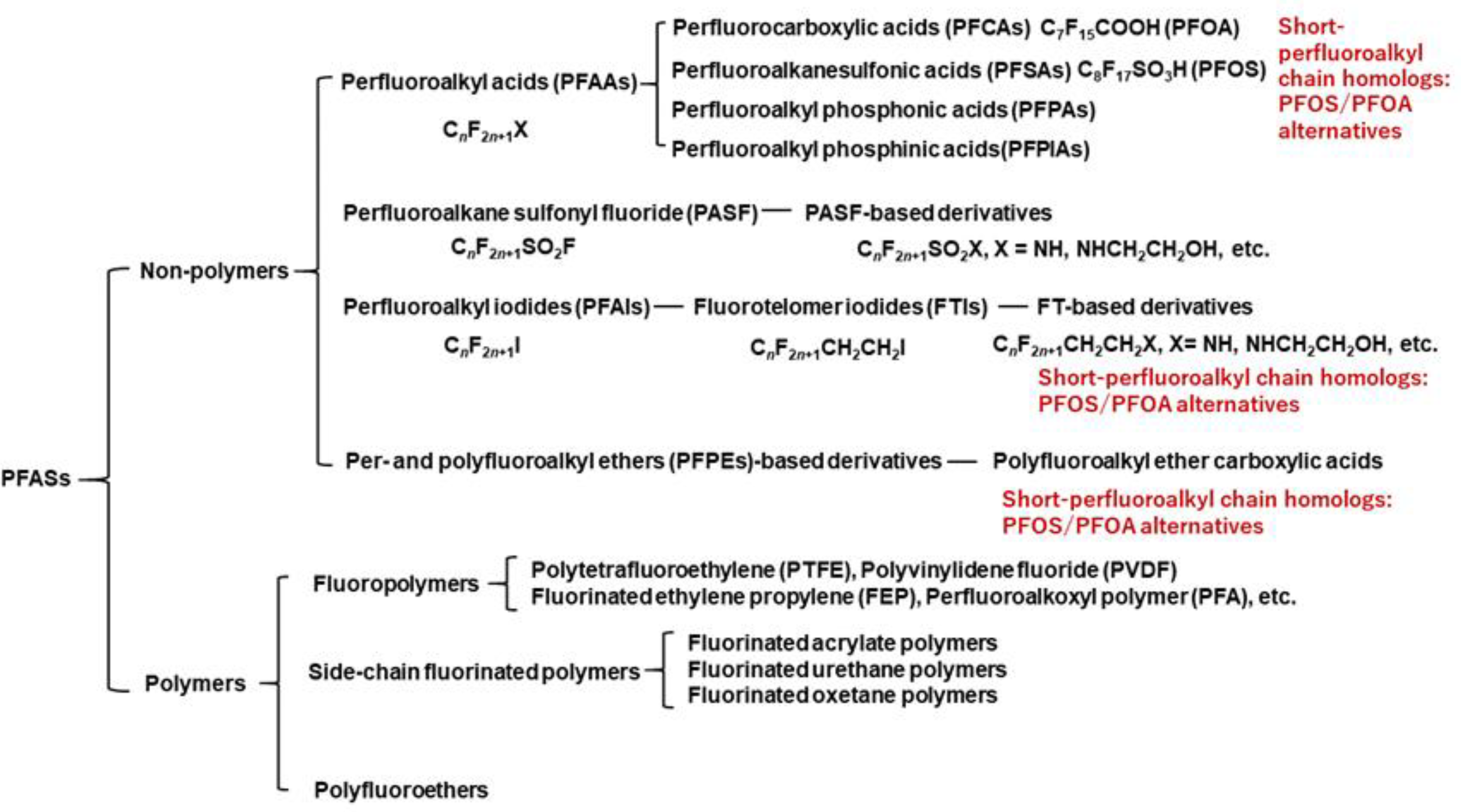
4. Different Categories of PFASs
4.1. Non-Polymeric PFASs
4.1.1. Introduction
4.1.2. Strategies to eliminate low molar- mass PFASs.
4.2. Fluorinated Polymers
4.2.1. FPs containing fluorinated side chain or Oxygen atoms
4.2.2. Fluoropolymers (i.e. backbone containing C-F bonds)
4.2.2.1. Valuable Fluoropolymers with exceptional properties
4.2.2.2. Improvement of FP production in more environmentally friendly processes
4.2.2.3. Polymer of Low Concern criteria
4.2.2.4. Recycling, End of Life and Reuse of FPs
4.2.2.5. Reuse of degraded FP
4.2.2.6. Incineration
4.2.2.7. End-of-life
4.2.2.8. Mineralization
Conclusions and Discussion
Acknowledgments
References
- Organisation for Economic Co-operation and Development (OECD). Toward a new comprehensive global database of per- and polyfluoroalkyl substances (PFASs). Series on Risk Management No. 39, 2018.
- Wang, Z.; DeWitt, J.C.; Higgins, C. P.; Cousins, I.T. A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)? Environ. Sci. Technol. 2017, 51, 2508−18. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. PFAS Strategic Roadmap: EPA’s Commitments to Action 2021−2024. Available online: https://www.epa.gov/pfas/pfas-strategic-roadmap-epascommitments-action-2021-2024 (accessed May 2023).
- Kwiatkowski, C. F.; Andrews, D.Q.; Birnbaum, L. S.; Bruton, T. A.; DeWitt, J. C.; Knappe, D.R.U.; Maffini, M. V.; Miller, M. F.; Pelch, K.E.; Reade, A.; Soehl, A.; Trier, X.; Venier, M.; Wagner, C.C.; Wang, Z.; Blum, A. Scientific Basis for Managing PFAS as a Chemical Class; Environ. Sci. Technol. Lett. 2022, 7, 532−543. [Google Scholar]
- Ng, C.; Cousins, I.T.; DeWitt, J.C.; Glüge, J.; Goldenman, G.; Herzke, D.; Lohmann, R.; Miller, M.; Patton, S.; Scheringer, M.; et al. Addressing Urgent Questions for PFAS in the 21st Century. Environ. Sci. Technol. 2021, 55, 12755–12765. [Google Scholar] [CrossRef] [PubMed]
- Guelfo, G.L.; Korzeniowski, H.S.; Mills, M.A.; Anderson, J.; Anderson, R.H.; Arblaster, J.A.; Conder, J.M.; Cousins, I.T.; Dasu, K.; Henry, B.J.; Lee, L.S.; Liu, J.; McKenzie, E.R.; Willeyn, J. Environmental Sources, Chemistry, Fate, and Transport of Per- and Polyfluoroalkyl Substances: State of the Science, Key Knowledge Gaps, and Recommendations Presented at the August 2019 SETAC Focus Topic Meeting, Environ Toxic Chem. 2021, 40, 3234-3260.
- Buck, R.C.; Korzeniowski, S.; Laganis, E.; Adamsky, F. Perfluoroalkyl and Poly-fluoroalkyl Substances in the Environment:Terminology, Classification, and Origins, Integr. Environ. Assess. Manage., 2021, 17, 1045–1055. [Google Scholar]
- Abunada, Z.; Alazaiza, M. Y. D.; Bashir, M. J. K. An Overview of Per- and Polyfluoroalkyl Substances (PFAS) in the Environment: Source, Fate, Risk and Regulations, Water 2020, 12, 3590-3601.
- Ameduri, B.; ed. Perfluoroalkyl Substances: Synthesis, Properties, Applications and Regulations, Royal Society of Chemistry, Oxford, 2022.
- Glüge, J.; London, R.; Cousins, I.T.; DeWitt, J.; Goldenman, G.; Herzke, D.; Lohmann, R.; Miller, M.; Ng, C.A.; Patton, S.; et al. Information Requirements under the Essential-Use Concept: PFAS Case Studies. Environ. Sci. Technol. 2021, 56, 6232–6242. [Google Scholar] [CrossRef]
- Available online: https://www.epa.gov/system/files/documents/2021-09/cq2_br2_thayer.pdf (accessed January 2023).
- Salvatore, D.; Mok, K. ; Garrett, K K. ; Poudrier, G.; Brown, L.; Birnbaum, L.S.; Goldenman, G.; Miller, M.F.; Patton, S.; Poehlein, M.; Varshavsky, J.; Cordner, A. Presumptive Contamination: A New Approach to PFAS Contamination Based on Likely Sources; Environ. Sci. Technol. Lett. 2022, 9, 983−90. [Google Scholar]
- Kempisty, D.M.; Xing, Y.; Racz, L.A. ; Perfluoroalkyl substances in the Environment, Theory, Practice and Innovation; 2018, CRC Press, Boca Raton (ISBN 9781498764186).
- Richter, L.; Cordner, A.; Brown, P. Non-stick science: Sixty years of research and (in)action on fluorinated compounds. Soc. Stud. Sci. 2018, 48, 691–714. [Google Scholar] [CrossRef]
- Bock, R.; Laird, B.E. ; PFAS Regulations: past and present and their impact on fluoropolymers. In Ameduri, B. ed., Issues, Challenges, Regulations and Applications of Perfluoroalkyl Substances, Chapter 1, RSC, Oxford, 2022, pp 1-21.
- European Chemical Agency. Available online: https://echa.europa.eu/fr/-/echa-receives-pfass-restriction-proposal-from-five-national-authorities (accessed May 2023).
- Thomas, T.; Malek, A.; Arokianathar, J.; Haddad, E.; Matthew, J. Global Regulations Around PFAS: The Past, the Present and the Future; Intern. Chem. Regul. Law Rev., 2023, 6, 3–17. [Google Scholar]
- List of New Pollutants under Key Control (2023 Edition) (Order No. 2022 of the Ministry of Ecology and Environment, the Ministry of Industry and Information Technology, the Ministry of Agriculture and Rural Affairs, the Ministry of Commerce, the General Administration of Customs, and the State Administration for Market Regulation promulgated on December 12, 29, effective from March 28, 2023).
- Glüge, J.; Scheringer, M.; Cousins, I.T.; DeWitt, J.C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Carla, A.; Ng, C.; Trieri, X.; Wangj, Z. ; An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ. Sci.: Proc. & Impacts, 2020, 22, 2345. [Google Scholar]
- Peshoria, S. Nandini, D. ; Tanwar, K. R.; Narang, R.; Short-chain and long-chain fluorosurfactants in firefighting foam: a review, Environ. Chem. Lett. 2020, 18, 1277–1300. [Google Scholar]
- Peshoria, S.; Nandini, D.; Understanding Aqueous Film-forming Foam Components, in ref. 9, chapter 7, pp 357-387. [CrossRef]
- Hu, X. C.; Andrews, D. Q.; Lindstrom, A. B.; Bruton, T. A.; Schaider, L. A.; Grandjean, P.; Lohmann, R.; Carignan, C. C.; Blum, A.; Balan, S. A.; Higgins, C. P.; Sunderland, E. M. ; Detection of Poly- and Perfluoroalkyl Substances (PFASs) in U. S. Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants. Environ. Sci. Technol. Lett. 2016, 3, 344–350. [Google Scholar] [PubMed]
- Ruyle, B. J.; Thackray, C. P.; Butt, C. M.; LeBlanc, D. R.; Tokranov, A. K.; Vecitis, C. D.; Sunderland, E.M. Centurial Persistence of Forever Chemicals at Military Fire Training Sites; Enviro. Sci. Techn. 2023, 57, 8096–106. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.aviationpros.com/aoa/aircraft-rescue-firefighting-arff/article/21092898/the-evolving-concern-of-pfas-at-airports (accessed May 2023).
- Adamson, D.T.; Nickerson, A.; Kulkarni, P.R.; Higgins, C.P.; Popovic, J.; Field, J.; Rodowa, A.; Newell, C.; DeBlanc, P.; Kornuc, J.J. Mass-Based, Field-Scale Demonstration of PFAS Retention within AFFF-Associated Source Areas. Environ. Sci. Technol. 2020, 54, 15768–15777. [Google Scholar] [CrossRef]
- Fiedler, H.; Kennedy, T.; Henry, B.J. A Critical Review of a Recommended Analytical and Classification Approach for Organic Fluorinated Compounds with an Emphasis on Per- and Polyfluoroalkyl Substances. Integr. Environ. Assess. Manag. 2020, 17, 331–351. [Google Scholar] [CrossRef]
- Cousins, I. T.; Ng, C.A.; Wang, Z.; Scheringer, M. ; Why is high persistence alone a major cause of concern? Environ. Sci.: Processes, Impacts 2019, 21, 781–792. [Google Scholar] [CrossRef]
- Cousins, I.T.; DeWitt, J.C.; Gluege, J.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.; Scheringer, M.; Wang, Z. The High Persistence of PFAS is Sufficient for their Management as a Chemical Class. [CrossRef]
- Scheringer, M.; Johansson, J.H.; Salter, M. E.; Sha, B.; Cousins, I.T. ; Stories of Global Chemical Pollution: Will We Ever Understand Environmental Persistence? Environ. Sci. Technol. 2022, 56, 17498−501. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.; Brecher, R.; Cousins, I.; DeWitt, J.; Fiedler, H.; Kannan, K.; Kirman, C.; Lipscomb, J.; Priestly, B.; Schoeny, R.; et al. Grouping of PFAS for human health risk assessment: Findings from an independent panel of experts. Regul. Toxicol. Pharmacol. 2022, 134, 105226. [Google Scholar] [CrossRef]
- Buck, R.C.; Franklin, J.; Berger, J.; Conder, J. M.; Cousins, I.T.; de Voogt, J.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P.J. ; Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–41. [Google Scholar] [CrossRef]
- For PFAS restriction Dossier. Available online: https://echa.europa.eu/restrictions-under-consideration/-/substance-rev/72301/term (accessed on March 2023).
- Qian, L.; Huang, M.; Guo, Y.; Chen, Q.-Y. Surface Properties and Biological Effects of Fluoroether Surfactants. 2022, 146–165. [CrossRef]
- Carlson, L.M.; Angrish, M.; Shirke, A.V.; Radke, E.G.; Schulz, B.; Kraft, A.; Judson, R.; Patlewicz, G.; Blain, R.; Lin, C.; et al. Systematic Evidence Map for Over One Hundred and Fifty Per- and Polyfluoroalkyl Substances (PFAS). Environ. Heal. Perspect. 2020, 130, 56001. [Google Scholar] [CrossRef]
- Henry, B. J.; Carlin, J. P.; Hammerschmidt, J. A.; Buck, R.C.; Buxton, L.W.; Fiedler, L.; Seed, J.; Hernandez, O. ; A critical review of the application of polymer of low concern and regulatory criteria to fluoropolymers; Integr. Environ. Assess. Manag., 2018, 14, 316–334. [Google Scholar]
- Ameduri, B.; Boutevin, B. ; Well-Architectured Fluoropolymers, Elsevier, Amsterdam, 2004. Chapt. 1, pp 1-99.
- Freeling, F.; Björnsdotter, M. K. ; Assessing the environmental occurrence of the anthropogenic contaminant trifluoroacetic acid (TFA); Curr. Opinion Green Sust. Chem.; 2023, 41, 100807. [Google Scholar] [CrossRef]
- Available online: https://echa.europa.eu/documents/10162/17086/report_pfcas_additional_derogation_en.pdf/527979b6-87ea-c9b7-a504-4ae2a4da73bf (accessed January 2023).
- Trang, B.; Li, Y.; Xue, X.-S.; Ateia, M.; Houk, K.N.; Dichtel, W.R. Low-temperature mineralization of perfluorocarboxylic acids. 2022, 377, 839–+. [CrossRef]
- Wang, J.; Lin, Z.; He, X.; Song, M.; Westerhoff, P.; Doudrick, K.; Hanigan, D. Critical Review of Thermal Decomposition of Per- and Polyfluoroalkyl Substances: Mechanisms and Implications for Thermal Treatment Processes. Environ. Sci. Technol. 2022, 56, 5355–5370. [Google Scholar] [CrossRef] [PubMed]
- DiStefano, R.; Feliciano, T.; Mimna, R.A.; Redding, A.M.; Matthis, J. Thermal destruction of PFAS during full-scale reactivation of PFAS-laden granular activated carbon. Remediat. J. 2022, 32, 231–238. [Google Scholar] [CrossRef]
- Grgas, D.; Petrina, A.; Štefanac, T.; Bešlo, D.; Dragicevi, T.L. ; A Review: Per- and Polyfluoroalkyl Substances— Biological Degradation; Toxics, 2023, 11, 446-63.
- Liu, J.; Avendaño, S.M. Microbial degradation of polyfluoroalkyl chemicals in the environment: A review. Environ. Int. 2013, 61, 98–114. [Google Scholar] [CrossRef]
- Hori, H.; Nagaoka, Y.; Yamamoto, A.; Sano, T.; Yamashita, N.; Taniyasu, S.; Kutsuna, S.; Osaka, I.; Arakawa, R. Efficient Decomposition of Environmentally Persistent Perfluorooctanesulfonate and Related Fluorochemicals Using Zerovalent Iron in Subcritical Water. Environ. Sci. Technol. 2006, 40, 1049–1054. [Google Scholar] [CrossRef]
- Mueller, R.; Yingling, V. Naming Conventions and Physical and Chemical Properties of Per- and Polyfluoroalkyl Substances (PFAS), Interstate Technology Regulatory Council, April 2020. PFAS Technical and Regulatory Guidance Document and Fact Sheets PFAS-1. 2022, Washington, D.C. Available online: https://pfas-1.itrcweb.org/12-treatment-technologies/#12_1 (accessed November 2022).
- Kumarasamy, E.; Manning, I.M.; Collins, L.B.; Coronell, O.; Leibfarth, F.A. Ionic Fluorogels for Remediation of Per- and Polyfluorinated Alkyl Substances from Water. ACS Central Sci. 2020, 6, 487–492. [Google Scholar] [CrossRef]
- Tan, X.; Sawczyk, M.; Chang, Y.; Wang, Y.; Usman, A.; Fu, C.; Král, P.; Peng, H.; Zhang, C.; Whittaker, A.K. Revealing the Molecular-Level Interactions between Cationic Fluorinated Polymer Sorbents and the Major PFAS Pollutant PFOA. Macromolecules 2022, 55, 1077–1087. [Google Scholar] [CrossRef]
- Choudhary, A.; Bedrov, D. , Interaction of Short-Chain PFAS with Polycationic Gels: How Much Fluorination is Necessary for Efficient Adsorption? ACS Macro Lett. 2022, 11, 1123–28. [Google Scholar] [CrossRef]
- Manning, I. M.; Guan Pin Chew, N.; Macdonald, H. P.; Miller, K. E.; Strynar, M. J.; Coronell, O.; Leibfarth, F. A. Hydrolytically Stable Ionic Fluorogels for High-Performance Remediation of Per- and Polyfluoroalkyl Substances (PFAS) from Natural Water; Angew. Chem. Int. Ed. 2022, 61, e202208150. [Google Scholar] [CrossRef]
- Verduzco, R.; Wong, M.S. Fighting PFAS with PFAS. ACS Central Sci. 2020, 6, 453–455. [Google Scholar] [CrossRef]
- Koda, Y.; Terashima, T.; Sawamoto, M. Fluorous Microgel Star Polymers: Selective Recognition and Separation of Polyfluorinated Surfactants and Compounds in Water. J. Am. Chem. Soc. 2014, 136, 15742–15748. [Google Scholar] [CrossRef] [PubMed]
- Koda, Y.; Terashima, T.; Nomura, A.; Ouchi, M.; Sawamoto, M. Fluorinated Microgel-Core Star Polymers as Fluorous Compartments for Molecular Recognition. Macromolecules 2011, 44, 4574–4578. [Google Scholar] [CrossRef]
- Song, D.; Qiao, B.; Wang, X.; Zhao, L.; Li, X.; Zhang, P.; Yao, Y.; Chen, H.; Zhu, L.; Sun, H. Degradation of Perfluorooctanoic Acid by Chlorine Radical Triggered Electrochemical Oxidation System. Environ. Sci. Technol. 2023, 57, 9416–9425. [Google Scholar] [CrossRef] [PubMed]
- Quan, Q.; Wen, H.; Han, S.; Wang, Z.; Shao, Z.; Chen, M. Fluorous-Core Nanoparticle-Embedded Hydrogel Synthesized via Tandem Photo-Controlled Radical Polymerization: Facilitating the Separation of Perfluorinated Alkyl Substances from Water. ACS Appl. Mater. Interfaces 2020, 12, 24319–24327. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Ling, Y.; Alsbaiee, A.; Li, C.; Helbling, D.E.; Dichtel, W.R. β-Cyclodextrin Polymer Network Sequesters Perfluorooctanoic Acid at Environmentally Relevant Concentrations. J. Am. Chem. Soc. 2017, 139, 7689–7692. [Google Scholar] [CrossRef] [PubMed]
- Ebnesajjad, S. (Ed.) Introduction to Fluoropolymers: Materials, Technology, and Applications, Elsevier, Amsterdam, 2013, chapter 6, pp. 63−89.
- Drobny, J. G. (Ed.) Technology of Fluoropolymers, 2nd ed; CRC Press: Boca Raton, FL, 2009. [Google Scholar]
- Smith, D.W.; Iacono, S.T.; Iyer, S.S. (Eds.) ; Encyclopedia of Fluoropolymers Science and Technologies; Wiley: New-York, 2014. [Google Scholar]
- Ameduri, B.; Fomin, S. (Eds.) ; Fascinating Fluoropolymers and their Applications; Elsevier, Amsterdam, 2020.
- Gardiner, J. Fluoropolymers: Origin, Production, and Industrial and Commercial Applications. Aust. J. Chem. 2015, 68, 13. [Google Scholar] [CrossRef]
- Puts, G.J.; Crouse, P.; Ameduri, B.M. Polytetrafluoroethylene: Synthesis and Characterization of the Original Extreme Polymer. Chem. Rev. 2019, 119, 1763–1805. [Google Scholar] [CrossRef]
- Ameduri, B. The Promising Future of Fluoropolymers Macromol. Chem. Phys. 2020, 221, 1900573. [Google Scholar]
- Sales, J.; Hernandez, D.; Kapoor, D.; Van den Noort, M. Fluoropolymers: the safe science that society needs, Intern. Chem. Regul. Law Rev. 2023, 6, 29–36. [Google Scholar]
- Lohman, R.; Letcher, R.J. ; The universe of fluorinated polymers and polymeric substances and potential environmental impacts and concerns. Curr. Opinion Green Sustain. Chem. 2023, 41, 100795. [Google Scholar] [CrossRef]
- Yao, W.; Li, Y.; Huang, X. Fluorinated poly(meth)acrylate: Synthesis and properties. Polymer 2014, 55, 6197–6211. [Google Scholar] [CrossRef]
- van der Veen, I.; Hanning, A.-C.; Stare, A.; Leonards, P.E.; de Boer, J.; Weiss, J.M. The effect of weathering on per- and polyfluoroalkyl substances (PFASs) from durable water repellent (DWR) clothing. Chemosphere 2020, 249, 126100. [Google Scholar] [CrossRef] [PubMed]
- Friesen, C.M.; Améduri, B. Outstanding telechelic perfluoropolyalkylethers and applications therefrom. Prog. Polym. Sci. 2018, 81, 238–280. [Google Scholar] [CrossRef]
- Wang, S.; Legare, J.M. ; Perfluoroelastomer and fluoroelastomer seals for semiconductor wafer processing equipment; J. Fluorine Chem., 2003, 122, 113–9. [Google Scholar] [CrossRef]
- Available online: https://www.space.com/31732-space-shuttle-challenger-disaster-explained-infographic.html (accessed Sept. 2023).
- Fluoropolymer Market Overview; Future Market Insights: Newark, 2022. https://www.futuremarketinsights.com/reports/fluoropolymers-market (accessed November 2022).
- Available online: file:///C:/Users/b.ameduri/Downloads/2019_06_Aerospace_catalog.pdf (assessed 3 September 2023).
- Ameduri, B. Fluorinated Elastomers; Encyclop. Polym. Sci. Tech. 2023, 1-22, (10.1002/0471440264.pst137.pub2).
- Qian, X.; Chen, X.; Zhu, L.; Zhang, Q.M. ; Fluoropolymer ferroelectrics: Multifunctional platform for polar-structured energy conversion; Science 2023, 380, 1-12.
- Bouharras, F.; Lopez, G.; Raihane, M.; Ameduri, B.; in Gnanou, Y.; Matyjaszewski, K.; Hadjichristidis, N.; Muthukumar, M. (Eds.) ; Macromolecular Engineering: From Precise Synthesis to Macroscopic Materials and Applications, Wiley-VCH GmbH, 2022, chapt. 26, pp. 1371-1402.
- Morel, C. ; Les cables sous-marins: Enjeux et Perspectives du XXIe siècle; 2023, CNRS Editions, Paris.
- Ober, C.K.; Käfer, F.; Deng, J. Review of essential use of fluorochemicals in lithographic patterning and semiconductor processing. J. Micro/Nanopatterning, Mater. Metrol. 2022, 21, 010901. [Google Scholar] [CrossRef]
- Cardoso, V.F.; Correia, D.M.; Ribeiro, C.; Fernandes, M.M.; Lanceros-Méndez, S. Fluorinated Polymers as Smart Materials for Advanced Biomedical Applications. Polymers 2018, 10, 161. [Google Scholar] [CrossRef]
- Lv, J.; Cheng, Y. ; Fluoropolymers in biomedical applications: state-of-the-art and future perspectives; Chem. Soc. Rev., 2021, 50, 5435–67. [Google Scholar] [CrossRef]
- Koguchi, R.; Jankova, K.; Tanaka, M. Fluorine-containing bio-inert polymers: Roles of intermediate water. Acta Biomater. 2021, 138, 34–56. [Google Scholar] [CrossRef]
- Roina, Y.; Auber, F.; Hocquet, D.; Herlem, G. ePTFE functionalization for medical applications. Mater. Today Chem. 2021, 20, 100412. [Google Scholar] [CrossRef]
- Roina, Y.; Auber, F.; Hocquet, D.; Herlem, G. ; ePTFE-based biomedical devices: An overview of surgical efficiency. J. Biomed. Mater. Res. Part B: Appl. Biomater., 2022, 110, 302–20. [Google Scholar] [CrossRef]
- Lim, X.Z. ; Can the world leave forever chemicals behind? Nature, 2023, 620, 24–2779. [Google Scholar] [CrossRef] [PubMed]
- Ameduri, B. Fluoropolymers: A special class of per- and polyfluoroalkyl substances (PFASs) essential for our daily life J. Fluorine Chem., 2023, 267, 110117. [Google Scholar] [CrossRef]
- Spyrakis, F.; Dragani, T.A.; The EU’s Per- and Polyfluoroalkyl Substances (PFAS) Ban: A Case of Policy over Science; Toxics, 2023, 11, 721-733.
- Goldbach, J. T.; Amin-Sanayei, R.; He, W.; Henry, J.; Kosar, W.; Lefebvre, A.; O’Brien, G. ; Vaessen; D. Wood, D.K. Zerafati, S.; in Ameduri, B.; Sawada, H., eds; Fluorinated Polymer. Applications. Royal Society of Chemistry, Oxford; 2016, Vol. 2; Chapt. 6; pp. 127-57.
- Boschet, F.; Ameduri, B. ; Copolymers of chlorotrifluoroethylene: recent developments and future trends, Chem. Rev. 2014, 114, 927–80. [Google Scholar]
- Hercules, D.A., Parrish, C.A.; Thrasher, J.S.; Research and Non-major Commercial Co- and Terpolymers of Tetrafluoroethylene. Ameduri, B.; Sawada, H. eds. In Fluorinated Polymers: Volume 2: Applications. Royal Society of Chemistry; Oxford, 2016. [CrossRef]
- Ameduri, B. ; Radical copolymerization of Vinylidene fluoride: recent tendencies and future trends; Progr Polym Sci. , 2022, 133, 101591. [Google Scholar]
- Lohmann, R. ; Cousins. I.T.; DeWitt, C.; Glüge, J.; Goldenman, G.; Herzke, D.; Lindstrom, A.B.; Miller, M.F.; Ng, C.A.; Patton, S.; Scheringer, M.; Trier, X.; Wang, Z. Are Fluoropolymers Really of Low Concern for Human and Environmental Health and Separate from Other PFAS? Environ. Sci. Technol., 2020, 54, 12820–28. [Google Scholar]
- Korzeniowski, S.H.; Buck, R.C.; Newkold, R.M.; El Kassmi, A.; Laganis, E.; Matsuoka, Y.; Dinelli, B.; Beauchet, S.; Adamsky, F.; Weilandt, K.; et al. A critical review of the application of polymer of low concern regulatory criteria to fluoropolymers II: Fluoroplastics and fluoroelastomers. Integr. Environ. Assess. Manag. 2022, 19, 326–354. [Google Scholar] [CrossRef]
- Zipplies, T.C.; Hintzer, K.; Dadalas, M.C.M; Frey, O.; Lochlaas, K.H.; Fluoropolymer compositions containing a polyol compound and methods of making them. U.S. Patent 2015/9,139,668; 22 Sep. 2015(assigned to 3M).
- Toyoda, M.; Nagai, H. Method for producing aqueous fluorinated polymer dispersion, aqueous fluorinated polymer dispersion and fluorinated polymer. U.S. Patent 2015/984,355. 30 Dec 2015 (assigned to AGC).
- Amin-Sanayei, R., Hedhli, L., Durali, M., and Wille, R.A. Method of producing fluoropolymers using alkyl sulfate surfactants. U.S. Patent 2016/9,447,256; 20 Sep. 2016 (assigned to Arkema).
- Lyons, D. F.; Chemours, Production of fluoroelastomers by radical emulsion polymerization in the presence of anionic phosphate ester surfactants, US2008/0262177, 2008. (assigned to Chemours).
- Brothers, P.D., Subhash V.G., Dipti, D. K.; Aqueous polymerization of perfluoromonomer using hydrocarbon surfactant. U.S. Patent 2016/9,255,164; 9 Feb. 2016 and EP2019/11787983.3A; 3 Jul 2019 (assigned to Chemours).
- Otsuka, M.; Tokuno, S.; Nakai, K.; Process for preparing fluoropolymer. U.S. Patent 2009/7,566,762; 28 Jul. 2009 (assigned to Daikin).
- Chauhan, R.; Kumar, G.; Rao, P.S.; Soni, V. K.; Bhattacharya, B.S. Dutta, D.; Shukla, A.; Patel, A.M.;Process for preparing fluoropolymers and fluoroelastomers in presence of a non-fluorinated sulfonate type hydrocarbon containing surfactant thereof; EP2022/20873509.2A 17 Aug 2022 (assigned to Gujarat Fluorochemicals Limited).
- Gupta, B.; Bhattacharya, B. S.; Chauhan, R.; Rathour, J. K.; Kumar, G.; Verma, C. ; Process for producing fluoropolymers using 2-alkoxyacetate surfactant; WO2020/136679 (assigned to Gujarat Fluorochemicals Limited).
- Kumar, G.; Soni, V. K.; Chauhan, R.; Mehta, M.; Bhattacharya, B.S.; Dutta, A.; Peddinti, S. ; Process for polymerizing fluoromonomers using a combination of fluorinated and non-fluorinated surfactant; WO2021/149022A1 (assigned to Gujarat Fluorochemicals Limited).
- Carella, S., Ieva, E., Mazzola, M., Brinati, G. Method for stabilizing aqueous dispersions of fluorinated polymers. U.S. Patent Application 16/638,388. 12 Feb 2020 (assigned to Solvay).
- Ameduri, B.; Sales, J.; Schlipf, M. ; Developments in fluoropolymer manufacturing technology to remove intentional use of PFAS as polymerization aids, ITRC Intern. Chem. Regul. Law Rev., 2023, 6, 18–28. [Google Scholar]
- Dams, R.; Hintzer, K. Industrial Aspect of Fluorinated Oligomers and Polymers. In Fluorinated Polymers, Vol. 2: Applications; in Ameduri, B.; Sawada, H., eds.; Royal Society of Chemistry, Cambridge, 2017; Chapt. 1; pp 3–31.
- Thermal oxidizer destroying PFAS at greater than 99.99% efficiency. Available online: https://www.chemours.com/en/-/media/files/corporate/fayetteville-works/2020-0320-thermal-oxidizer-efficiency-results-announced.pdf?rev=87dbfd0ebb9c45aeaa475fddd2a899b4&hash=05916EC2AC2B5A3C41135773A3FCDED0.
- Dauchy, X. ; Evidence of large-scale deposition of airborne emissions of per- and polyfluoroalkyl substances (PFASs) near a fluoropolymer production plant in an urban area; Chemosphere 2023, 337, 139407.
- Arkema. Available online: https://www.arkema.com/global/en/media/newslist/news/global/corporate/2023/20230221-arkema-position-on-european-proposal-to-restrict-pfas/ (accessed on May2023).
- Chemours. Available online: https://www.chemours.com/en/news-media-center/all-news/press-releases/2022/chemours-announces-process-innovation-with-new-viton-fluoroelastomers-advanced-polymer-architecture (accessed on May 2023).
- Available online: https://www.daikinchemicals.com/company/sustainability/pfas.html (accessed on September 2022).
- Daikin, A Responsible Steward for PFAS Emission Reductions and Safe Alternatives. Available online: https://www.daikinchem.de/daikin-responsible-steward-pfas-emission-reductions-and-safe-alternatives (accessed November 2022).
- Available online:. Available online: https://www.gfl.co.in/assets/pdf/Announcement-on-NFPA-technology-FKM-27-Aug-2022.pdf (accessed on March 2023).
- Gujarat Fluorochemicals Limited. (2022). Announcement on the development of a non-fluorinated polymerization aid. Available online: https://gfl.co.in/upload/pages/ebce5fed9030753d0ee651bf1f48d0a0.pdf (consulted on May 2023).
- Solvay’s announcement producing new fluoropolymers without fluorosurfactants. Available online: https://www.solvay.com/en/article/eliminating-pfas (consulted on May 2023).
- Solvay (2022). Innovating with non-fluorosurfactant technologies. Available online: https://www.solvay.com/en/innovation/science-solutions/pfas (consulted May 2023).
- Evich, M.G.; Davis, M.J.B.; McCord, J.P.; Acrey, B.; Awkerman, J.A.; Knappe, D.R.U.; Lindstrom, A.B.; Speth, T.F.; Tebes-Stevens, C.; Strynar, M.J.; et al. Per- and polyfluoroalkyl substances in the environment. Science 2022, 375, 512. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development, Data analysis of the identification of correlations between polymer characteristics and potential for health or ecotoxicological concern, Environment Directorate, OECD, 2009. Available online: https://www.oecd.org/env/ehs/risk-assessment/42081261.pdf, (accessed 2022 September).
- BIO by Deloitte, Technical assistance related to the review of REACH with regard to the registration requirements on polymer Final report, 2015. Available online: https://ec.europa.eu/environment/chemicals/reach/pdf/FINAL%20REPORT%20POLYMER%20SI671025.pdf, (accessed September 2022).
- Australian Industrial Chemicals, Polymer of low concern (PLC) criteria. Available online: https://www.industrialchemicals.gov.au/help-and-guides/polymer-low-concern-plc-criteria, (accessed November, 2022).
- Alberts, B.; Bray, D.; Lewis, J.; Raff, M.; Roberts, K.; Watson, J.D. Molecular Biology of the Cell, 3rd edition, Garland, New York, 1994, pp. 958-63.
- Beyer, E. C. ; in Molecular Biology of Receptors and Transporters: Pumps, Transporters and Channels, Friedlander, M.; Mueckler, M., eds; Academic Press, San Diego, 1993, pp. 1–38.
- De Mello, W. C., ed.; Cell-to-Cell Communication, Plenum, New York, 1987, p. 34.
- Available online: https://www.plasticstoday.com/materials-research/new-study-gives-most-fluoropolymers-clean-bill-health (accessed March 2023).
- US Pharmacopeial Convention, US Pharmacopeia National Formulary 2011: USP 34 NF 29. Rockville, General Notices, Section, 5.3.0, 2011, pp. 6.
- European Commission, Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), 2006.
- Ebnesajjad, S. ; Safety, disposal, and recycling of fluoropolymers, Introduction to Fluoropolymers: Materials, Technology, and Applications, Elsevier, Amsterdam, 2013, Chapt. 13, pp. 293-309.
- The Society of the Plastics Industry, The guide to the safe handling of fluoropolymer resins–fourth edition, Bp-101. Washington, 2005. Available online: https://intechservices.com/content/SPI_Guide_for_Safe_Handling_of_Fluoropolymer_Resins.pdf, (accessed November 2022).
- Guidoin, R.; King, M.W.; Wang, L.; Zhang, Z. Guzman, R.; Marinov, G.; Douville, Y.; Biotextiles as medical implants, in Vascular Prostheses for Open Surgery, Woodhood, Cambridge, 2013, pp. 434–484. [CrossRef]
- USEPA, Premanufacture notification exemption for polymers; Amendment of polymer exemption rule to exclude certain perfluorinated polymers, US Federal Resister 40 CFR Part 723. EPA-HQ-OPP-2002-0051; FRL-8805-5. R 2010, 75, 4295–305.
- USEPA, Interim guidance on the destruction and disposal of perfluoroalkyl and polyfluoroalkyl substances and materials containing perfluoroalkyl and polyfluoroalkyl substances, 2020. Available online: https://www.epa.gov/system/files/documents/2021-11/epa-hq-olem-2020-0527-0002_content.pdf, (accessed November 2022).
- Schyns, Z. O. G.; Shaver, M.P. Mechanical Recycling of Packaging Plastics: A Review; Macromol. Rapid Commun. 2021, 42, 2000415. [Google Scholar] [CrossRef]
- Geyer, R. ; Jambeck; J. R.; Law, K.L.; Production, use, and fate of all plastics ever made Adv. Sci. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Science to enable sustainable plastics; a white paper from the 8th Chemical Sciences and Society Summit (CS3), June 2020. Available online: rsc.li/progressive-plastics-report (accessed on March 2023).
- Li, H.; Aguirre-Villegas, H.A.; Allen, R.D.; Bai, X.; Benson, C. H.; Beckham, G. T.; Bradshaw, L.S.; Brown, J. L.; Brown, R. C.; Cecon, V.S.; Curley, J.B.; Curtzwiler, G.W.; Dong, S.; Gaddameedi, S.; García, J.E.; Hermans, I.; Kim, M.S.; Ma, J.; Mark, L.O.; Mavrikakis, M. ; Expanding plastics recycling technologies: chemical aspects, technology status and challenges; Green Chem. 2022, 24, 8899-9002.
- Available online: https://www.oecd.org/environment/plastics/plastics-lifecycle-is-far-from-circular.html (accessed March 2023).
- MacLeod, M.; Arp, H.P.H.; Tekman, M.B.; Jahnke, A. The global threat from plastic pollution. Science 2021, 373, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Conversio Market & Strategy GmbH, Fluoropolymer waste in Europe 2020–End of life (EOL) analysis of fluoropolymer applications, products and associated waste streams, 2020.
- Pro-K Fluoropolymer Group (2018). Recycling of fluoropolymers. Available online: https://www.pro-kunststoff.de/assets/Merkbl%C3%A4tter%20und%20Co/FP%20TM-10-Recycling-of-fluoropolymers.
- Ameduri, B.; Hori, H. ; Recycling and End of life assessment of Fluoropolymers: Recent Developments, Challenges and Future Trends; Chem. Soc. Rev. 2023, 52, 4208–4247 (. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.E.; Naylor, M.A. Pyrolysis of Polytetrafluoroethylene. J. Am. Chem. Soc. 1947, 69, 1968–1970. [Google Scholar] [CrossRef]
- Wall, L.; Michaelsen, J. Thermal decomposition of polytetrafluoroethylene in various gaseous atmospheres. J. Res. Natl. Bur. Stand. 1956, 56, 27. [Google Scholar] [CrossRef]
- Simon, C.; Kaminsky, W. Chemical recycling of polytetrafluoroethylene by pyrolysis. Polym. Degrad. Stab. 1998, 62, 1–7. [Google Scholar] [CrossRef]
- Ellis, D.A.; Mabury, S.A.; Martin, J.W.; Muir, D.C.G. Thermolysis of fluoropolymers as a potential source of halogenated organic acids in the environment. Nature 2001, 412, 321–324. [Google Scholar] [CrossRef]
- Schlipf, M.; Schwalm, T. ; Closing the recycling loop. Kunststoffe Int. 2014, 6, 58−60. [Google Scholar]
- Puts, G. J.; Crouse, P.L. ; The influence of inorganic materials on the pyrolysis of polytetrafluoroethylene. Part 1: The sulphates and fluorides of Al, Zn, Cu, Ni, Co, Fe and Mn, J. Fluorine Chem., 2014, 168, 260–267. [Google Scholar]
- Puts, G. J.; Crouse, P.L. ; The influence of inorganic materials on the pyrolysis of polytetrafluoroethylene. Part 2: The common oxides of Al, Ga, In, Zn, Cu, Ni, Co, Fe, Mn, Cr, V, Zr and La, J. Fluorine Chem., 2014, 168, 9–15. [Google Scholar]
- Lakshman A., Chakraborty S.K.; Recycling of Polytetrafluoroethylene (PTFE) Scrap Materials’, in: Lakshmanan, A. (ed.), Sintering Techniques of Materials, 2015, InTech. pp. 200. [CrossRef]
- 3M Dyneon, Fluoropolymers Up-Cycling. Closing the loop. Available online: https://multimedia.3m.com/mws/media/907323O/up-cycling-fluoropolymers-brochure.pdf?fn=Up-Cycling_Brochure_EN.pdf#:~:text=In%20March%202015%2C%20Dyneon%20opened%20the%20world%E2%80%99s%20first,thereby%20having%20a%20positive%20impact%20on%20the%20environment, (accessed November 2022).
- Invertec, Pilot project: Recycling of fluoropolymers (PTFE). Available online: https://www.invertec-ev.de/en/projects/environmental-care/ptfe-recycling/, (accessed November 2022).
- Shin-Ren, D. L.; O’Brien, G.S.; Seilers, D.A.; 3D-printed fluoropolymer structures; US Patent 2020/10633468 (April 2020) assigned to Arkema.
- Seiler, D. A.; Lowrie, R.; Henry, J.; Cavalier, C.; DeAngelo, M. ; Study of the Positive Effects Seen When Using Fluoropolymer Polymer Processing and Recycling Aids (PPRA) in Reprocessed LLDPE; presented at SPE International Polyolefins Conference, Galveston, TX, Feb. 2022. [Google Scholar]
- An, Y.; Kajiwara, T.; Padermshoke, A.; Van Nguyen, T.; Feng, S.; Mokudai, H.; Masaki, T.; Takigawa, M.; Van Nguyen, T.; Masunaga, H.; Sasaki, S.; Takahara, A. ;Environmental Degradation of Nylon, Poly(ethylene terephthalate) (PET), and Poly(vinylidene fluoride) (PVDF) Fishing Line Fibers; ACS Appl. Polym. Mater. 2023, 5, 4427–4436. [Google Scholar]
- Schuster, J.; Lutz, J.; Shaik, Y.P.; Yadavalli, V.R. ; Recycling of fluorocarbon-elastomers – A review; Adv. Indust. Engin. Polym. Res., 2022, 5, 248–254. [Google Scholar]
- Curtin, D.E.; Lousenberg, R.D.; Henry, T.J.; Tangeman, P.C.; Tisack, M.E. Advanced materials for improved PEMFC performance and life. J. Power Sources 2004, 131, 41–48. [Google Scholar] [CrossRef]
- Feng, M.; Qu, R.; Wei, Z.; Wang, L.; Sun, P.; Wang, Z. Characterization of the thermolysis products of Nafion membrane: A potential source of perfluorinated compounds in the environment. Sci. Rep. 2015, 5, 9859–9859. [Google Scholar] [CrossRef] [PubMed]
- Zatoń, M.; Rozière, J.; Jones, D.J. Current understanding of chemical degradation mechanisms of perfluorosulfonic acid membranes and their mitigation strategies: a review. Sustain. Energy Fuels 2017, 1, 409–438. [Google Scholar] [CrossRef]
- Grot, S.; Grot, W.; Recycling of used perfluorosulfonic acid membranes; US 2005/0211630 A1; 29 September 2005 (assigned to Ion Power, Inc.).
- Park, A.; Sustainable NafionTM: Opportunities in Recycling and Re-use of Membranes and Ionomers; Manufacturing Automation and Recycling for Clean Hydrogen Technologies Experts Meeting May 26th, 2022.
- Frankenstack project, UK Research and Innovation. Available online: https://gtr.ukri.org/projects?ref=133704 (accessed June 2023).
- Puts, G. ; PhD Dissertation, Synthesis of novel low-molecular weight polytetrafluoroethylene and tetrafluoroethylene copolymers for pyrotechnic applications. The University of Pretoria, 2017.
- Kili, T.; The synthesis and characterisation of fluorinated ethylene propylene (FEP) copolymers; MSc dissertation in Chemical Technologies, The University of Pretoria, South Africa, 2015.
- Puts, G.; Venner, V.; Améduri, B.; Crouse, P. Conventional and RAFT Copolymerization of Tetrafluoroethylene with Isobutyl Vinyl Ether. Macromolecules 2018, 51, 6724–6739. [Google Scholar] [CrossRef]
- Hoshino, T.; Morizawa, Y.; Fluorinated Specialty Chemicals – Fluorinated Copolymers for Paints and Perfluoropolyethers for Coatings, in Ameduri, B.; Sawada, H., eds; Fluorinated Polymers: Volume 2: Applications, 2016, RSC, Oxford.
- Wang, H.S.; Truong, N.P.; Pei, Z.; Coote, M.L.; Anastasaki, A. Reversing RAFT Polymerization: Near-Quantitative Monomer Generation Via a Catalyst-Free Depolymerization Approach; J. Amer. Chem. Soc. 2022, 144, 4678–4684. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, Y.; Zheng, X. ; Towards new environmentally friendly fluoroelastomers: from facile chemical degradation to efficient photo-cross-linkable reaction, Polym. Intern., 2019, 68, 1952−1960. [Google Scholar]
- Patko, D.; Yang, Q.; Liu, Y.; Falireas, P.; Briou, B.; Tawade, B.V.; George, T.S.; Daniell, T.J.; MacDonald, M.P.; Ladmiral, V.; Ameduri, B.; Dupuy, L.X.; Smart soils track the formation of pH gradients across the rhizosphere; Plant Soil, 2023, (in press.
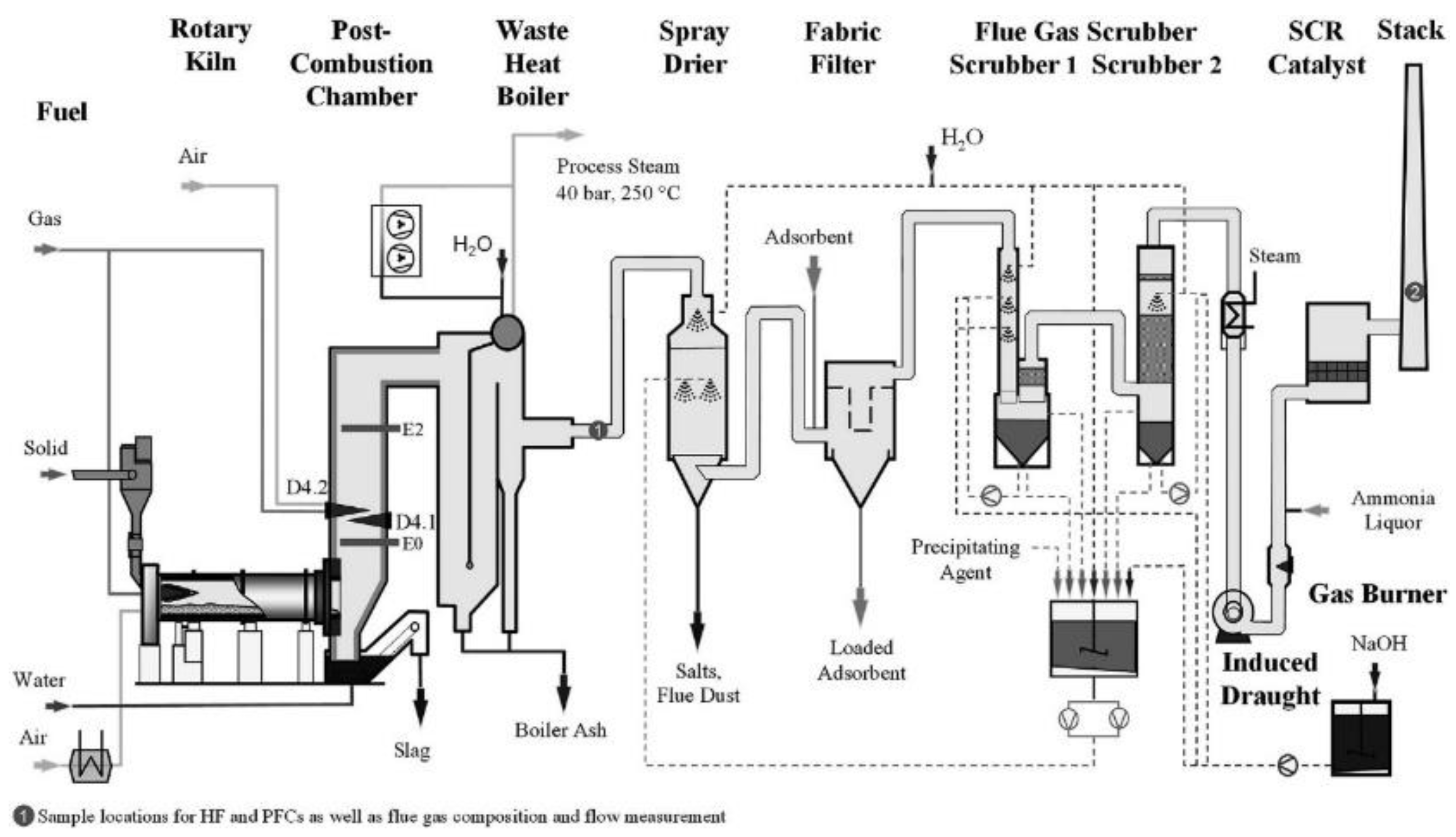
- Dutch Institute for Public Health and Environment (RIVM). Per- and polyfluorinated substances in waste incinerator flue gases. Rijksinstituut voor Volksgezondheid en Milieu. Report 2021-0143. (DOI10.21945/RIVM-2021-0143).
- Yli-Rantala, E.; Behringer, E.; Herzke, D.; Mudge, S.M.; Beekman, M.; de Blaeij, A.; Devilee, J.; Gabbert, S.; van Kuppevelt, M.; Zare Jeddi, M.; Gabrielsen, P.; Trier, X.; Fluorinated polymers in a low carbon, circular and toxic-free economy, Wahlström, M.; Pohjalainen, E.; eds.; Eionet Report – ETC/WMGE 2021/9. Available online: https://www.eionet.europa.eu/etcs/etc-wmge/products/etc-wmge-reports/fluorinated-polymers-in-a-low-carbon-circular-and-toxic-free-economy, (accessed November, 2022).
- Waritz, R. S. ; An industrial approach to evaluation of pyrolysis and combustion hazards. Environ. Health Perspect., 1975, 11, 197–202. [Google Scholar] [CrossRef]
- Chen, D.M.; Hsieh, W.H.; Snyder, T.S.; Yang, V.; Litzinger, T.A.; Kuo, K.K. Combustion behavior and thermophysical properties of metal-based solid fuels. J. Propuls. Power 1991, 7, 250–257. [Google Scholar] [CrossRef]
- Kitahara, Y.; Takahashi, S.; Kuramoto, N.; Šala, M.; Tsugoshi, T.; Sablier, M.; Fujii, T. ; Ion attachment mass spectrometry combined with infrared image furnace for thermal analysis: Evolved gas analysis studies. Anal. Chem., 2009, 81, 3155–3158. [Google Scholar] [CrossRef] [PubMed]
- Bhadury, P.S.; Singh, S.; Sharma, M.; Palit, M. Flash pyrolysis of polytetrafluoroethylene (teflon) in a quartz assembly. J. Anal. Appl. Pyrolysis 2007, 78, 288–290. [Google Scholar] [CrossRef]
- García, A.N.; Viciano, N.; Font, R. Products obtained in the fuel-rich combustion of PTFE at high temperature. J. Anal. Appl. Pyrolysis 2007, 80, 85–91. [Google Scholar] [CrossRef]
- Clarke, F. B.; Vankuijk, M.; Valentine, R.; Makovec, G. T.; Seidel, W. C.; Baker, B. B.; Kasprzak, D. J.; Bonesteel, J.K.; Janssens, M.; Herpol, C. ; The toxicity of smoke from fires involving perFPs: full-scale fire studies. J. Fire Sci. 1992, 10, 488–527. [Google Scholar] [CrossRef]
- Wang, F.; Lu, X.; Li, X.-Y.; Shih, K. Effectiveness and Mechanisms of Defluorination of Perfluorinated Alkyl Substances by Calcium Compounds during Waste Thermal Treatment. Environ. Sci. Technol. 2015, 49, 5672–5680. [Google Scholar] [CrossRef]
- Taylor, P.; Yamada, T.; Striebich, R.; Graham, J.; Giraud, R. Investigation of waste incineration of fluorotelomer-based polymers as a potential source of PFOA in the environment. Chemosphere 2014, 110, 17–22. [Google Scholar] [CrossRef]
- Aleksandrov, K.; Gehrmann, H.-J.; Hauser, M.; Mätzing, H.; Pigeon, D.; Stapf, D.; Wexler, M. Waste incineration of Polytetrafluoroethylene (PTFE) to evaluate potential formation of per- and Poly-Fluorinated Alkyl Substances (PFAS) in flue gas. Chemosphere 2019, 226, 898–906. [Google Scholar] [CrossRef]
- Kucharzyk, K.H.; Darlington, R.; Benotti, M.; Deeb, R.; Hawley, E. Novel treatment technologies for PFAS compounds: A critical review. J. Environ. Manag. 2017, 204, 757–764. [Google Scholar] [CrossRef]
- Hintzer, K. , Schwertfeger, W.; Fluoropolymers—Environmental aspects. In Smith, D. W.; Iacono, S. T.; Iyer, S.S. (eds.), Handbook of fluoropolymer science and technology. Chapt; 21, 2014, pp. 495-520; Wiley, New-York.
- Olsavsky, N. J.; Kearns, V. M.; Beckman, C.P.; Sheehan, P.L.; Burpo, F. J.; Bahaghighat H. D.; Nagelli, E.A.; Research and regulatory advancements on remediation and degradation of fluorinated polymer compounds, Appl. Sci., 2020, 10, 6921-6933. [CrossRef]
- Hori, H.; Honma, R.; in Opportunities for Fluoropolymers, Ameduri, B.; Fomin, S.; eds. Elsevier, Amsterdam, 2020, Chapter 11, pp. 303−331. [CrossRef]
- Hori, H.; Tanaka, H.; Watanabe, K.; Tsuge, T.; Sakamoto, T.; Manseri, A.; Ameduri, B. ; Hydrogen peroxide induced efficient mineralisation of poly(vinylidene fluoride) and related copolymers in subcritical water, Ind. Eng. Chem. Res., 2015, 54, 8650−8658. [Google Scholar] [CrossRef]
- Hamaura, J.; Honma, R.; Hori, H.; Manseri, A.; Ameduri, B. Efficient fluoride recovery from poly(vinylidene fluoride), poly(vinylidene fluoride-co-hexafluoropropylene) copolymer and poly(ethylene-co-tetrafluoroethylene) copolymer using superheated water with alkaline reagent. Eur. Polym. J. 2023, 182, 111724. [Google Scholar] [CrossRef]
- Honma, R.; Hori, H. ; Reis da Cunha, F; Horiike, N. ; Steinbach, L.; Ameduri, B.; Permanganate-Induced Efficient Mineralization of PVDF and VDF Based Copolymers in Low Temperature Subcritical Water Ind. Eng. Chem. Res. 2019, 58, 13030−13040. [Google Scholar]
- Hori, H.; Honma, R.; Igarashi, K.; Manseri, A.; Ameduri, B. Oxidative Mineralization of Poly[vinylidene fluoride-co-2-(trifluoromethyl)acrylic acid] Copolymers in Superheated Water. Ind. Eng. Chem. Res. 2022, 61, 1386–1397. [Google Scholar] [CrossRef]
- Hori, H.; Tanaka, H.; Tsuge, T.; Honma, R.; Banerjee, S.; Ameduri, B. ; Decomposition of fluoroelastomer: Poly(vinylidene fluoride-ter-hexafluoropropylene-ter-tetrafluoroethylene) terpolymer in subcritical water; Eur. Polym. J., 2017, 94, 322−31. [Google Scholar] [CrossRef]
- Hori, H.; Murayama, M.; Sano, T.; Kutsuna, S. Decomposition of Perfluorinated Ion-Exchange Membrane to Fluoride Ions Using Zerovalent Metals in Subcritical Water. Ind. Eng. Chem. Res. 2009, 49, 464–471. [Google Scholar] [CrossRef]
- G. Escobedo, Enabling commercial PEM fuel cells with breakthrough lifetime improvements. 2005 DOE hydrogen Program Review. Project ID #FC9. U. S. Department of Energy, Washington, DC. Available online: http://www.hydrogen.energy.gov/pdfs/review05/fc9_escobedo.pdf, (accessed November 2022).
- Yanagihara, N.; Katoh, T. ; Mineralisation of poly(tetrafluoroethylene) and other fluoropolymers using molten sodium hydroxide, Green Chem. , 2022, 24, 6255–63. [Google Scholar]
- Sheldon, D.J.; Parr, J.M.; Crimmin, M.R. Room Temperature Defluorination of Poly(tetrafluoroethylene) by a Magnesium Reagent; J. Am. Chem. Soc. 2023, 145, 10486−10490. [Google Scholar] [CrossRef]
- Hintzer, K.; Hirschberg, M.E. ; Fluoropolymers: Environmental aspects; Proceeding Fluorine Days in Bremen, 2006.
- De Smit, K.; Wieme, T.; Marien, Y. W.; Van Steenberge, P. H. M.; D’Hooge, D.R.; Edeleva, M. ; Multi-scale reactive extrusion modelling approaches to design polymer synthesis, modification and mechanical recycling, React. Chem. Eng., 2022, 7, 245–263. [Google Scholar]
- Global Fluoropolymers Markets Report 2022-2026 - 5G Communication Technology Boosts Demand for PTFE/Demands of Membrane Market Being Addressed by PVDF. Available online: https://www.prnewswire.com/news-releases/global-fluoropolymers-markets-report-2022-2026---5g-communication-technology-boosts-demand-for-ptfe--demands-of-membrane-market-being-addressed-by-pvdf-301600681.html (accessed October 2022).
- FPP4EU Available online: https://www.fpp4eu.eu/ (consulted on June2023).
- Plastics Europe – Fluoropolymer Group, Socio-economic Analysis of the European Fluoropolymer Industry – Executive Summary, May 2017. Available online: https://fluoropolymers.plasticseurope.org/application/files/7816/1167/4026/Final_SEA_Fluoropolymers_summary2017_3.pdf (accessed September 2022).
- Tyrrell, N.D. A Proposal That Would Ban Manufacture, Supply, and Use of All Fluoropolymers and Most Fluorinated Reagents within the Entire EU; Org. Process. Res. Dev. 2023, 27, 1422–1426. [Google Scholar] [CrossRef]
- Available online: https://fluoropolymers.plasticseurope.org/application/files/1316/7957/3228/21_March_FPG_Statement_on_the_PFAS_REACH_restriction_report.pdf (accessed on March 2023).
- Hydrogen Europe Position Paper on PFAS; The importance of fluoropolymers across the hydrogen value chain, and impacts of the proposed PFAS restriction for the hydrogen sector. Available online: https://hydrogeneurope.eu/wp-content/uploads/2023/02/Hydrogen-Europe-position-paper-on-PFAS-ban_v12_FINAL.pdf.
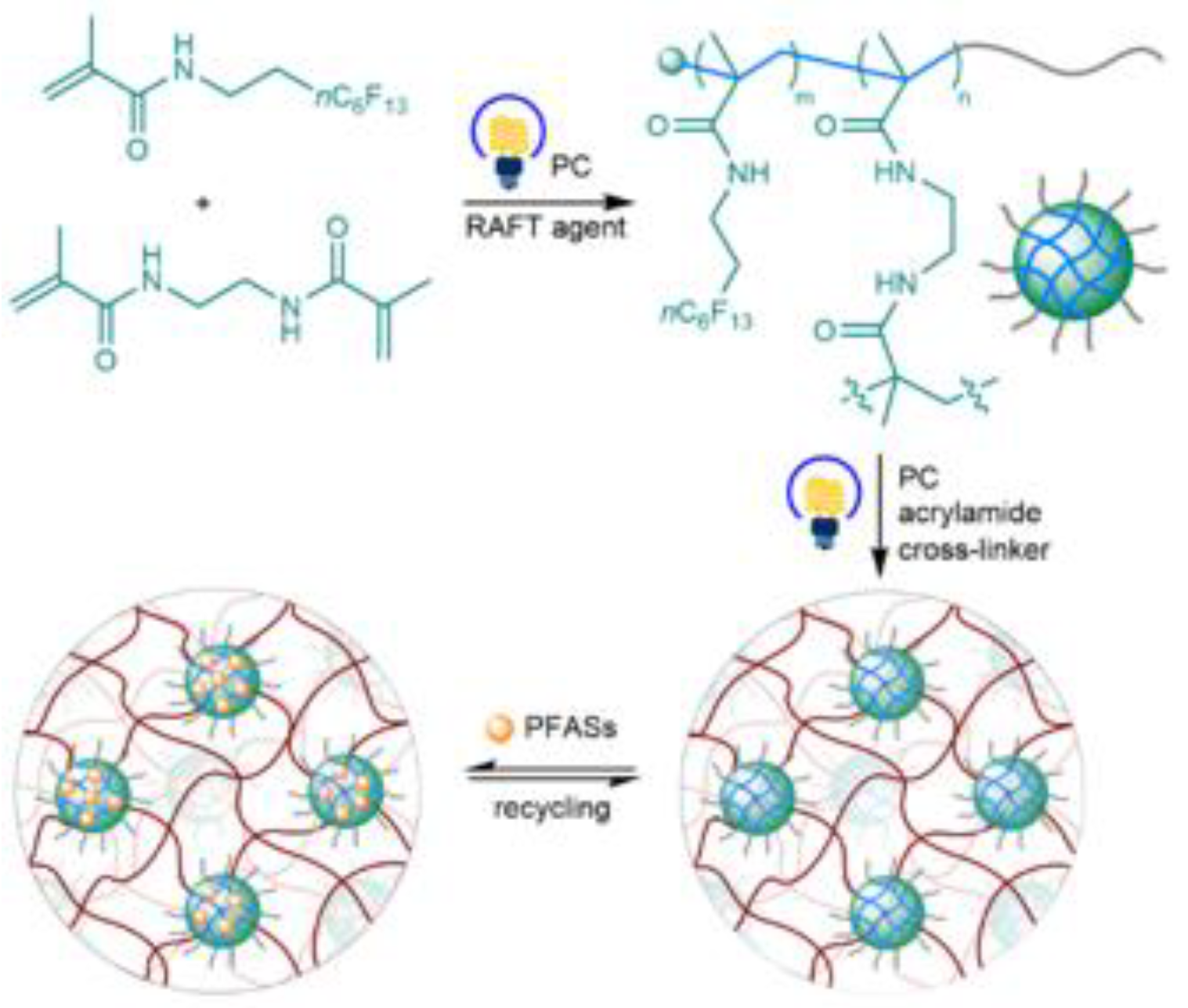
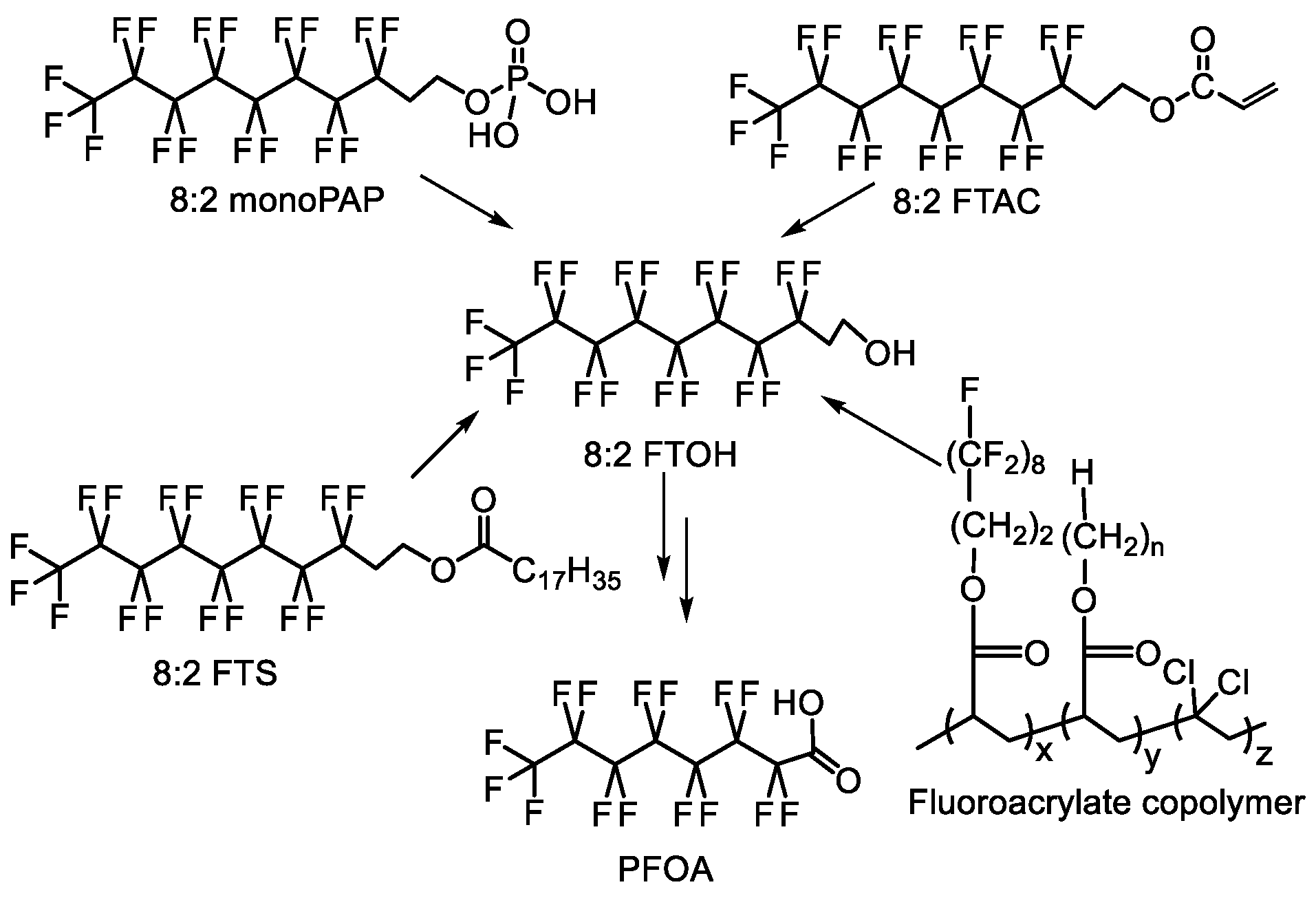
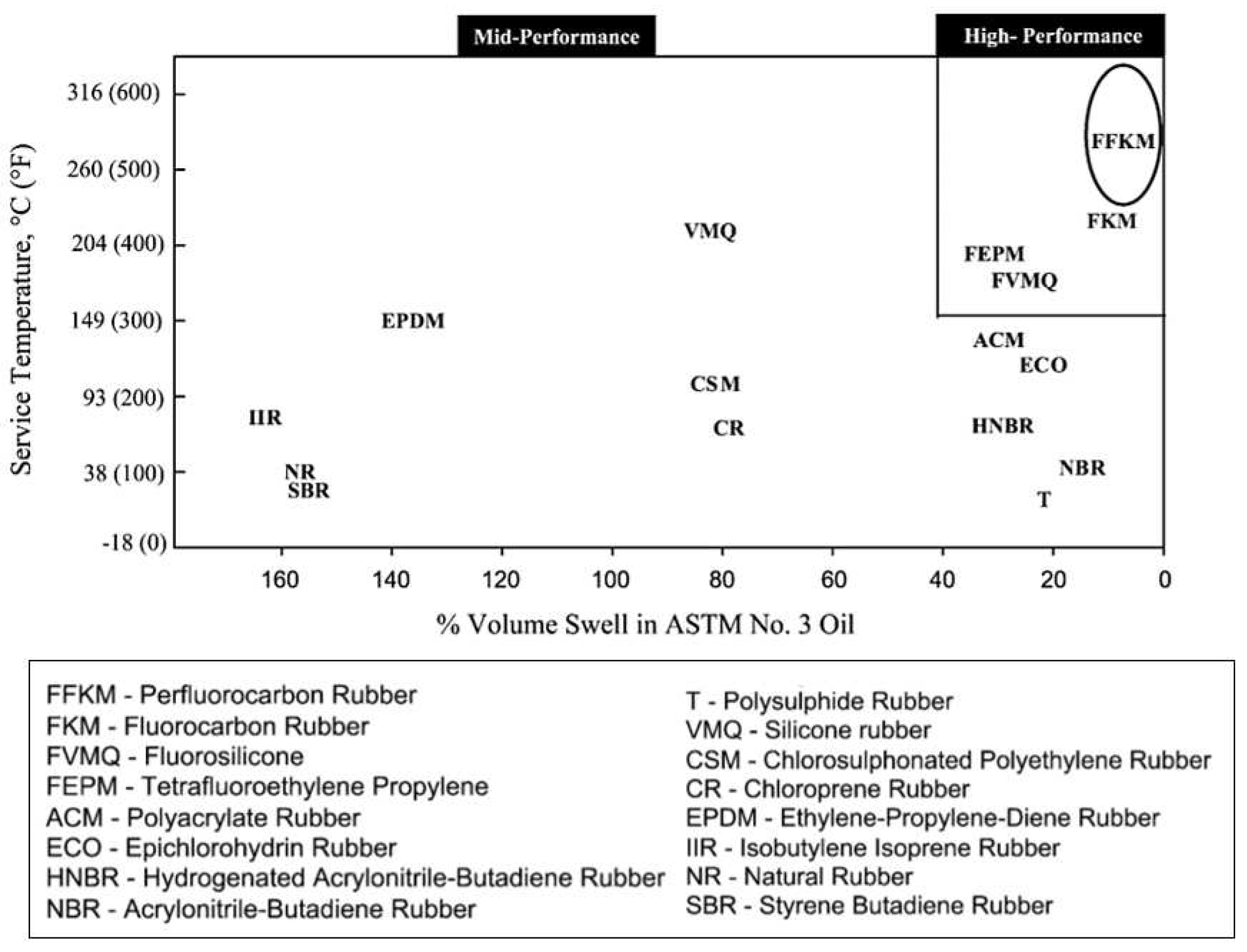

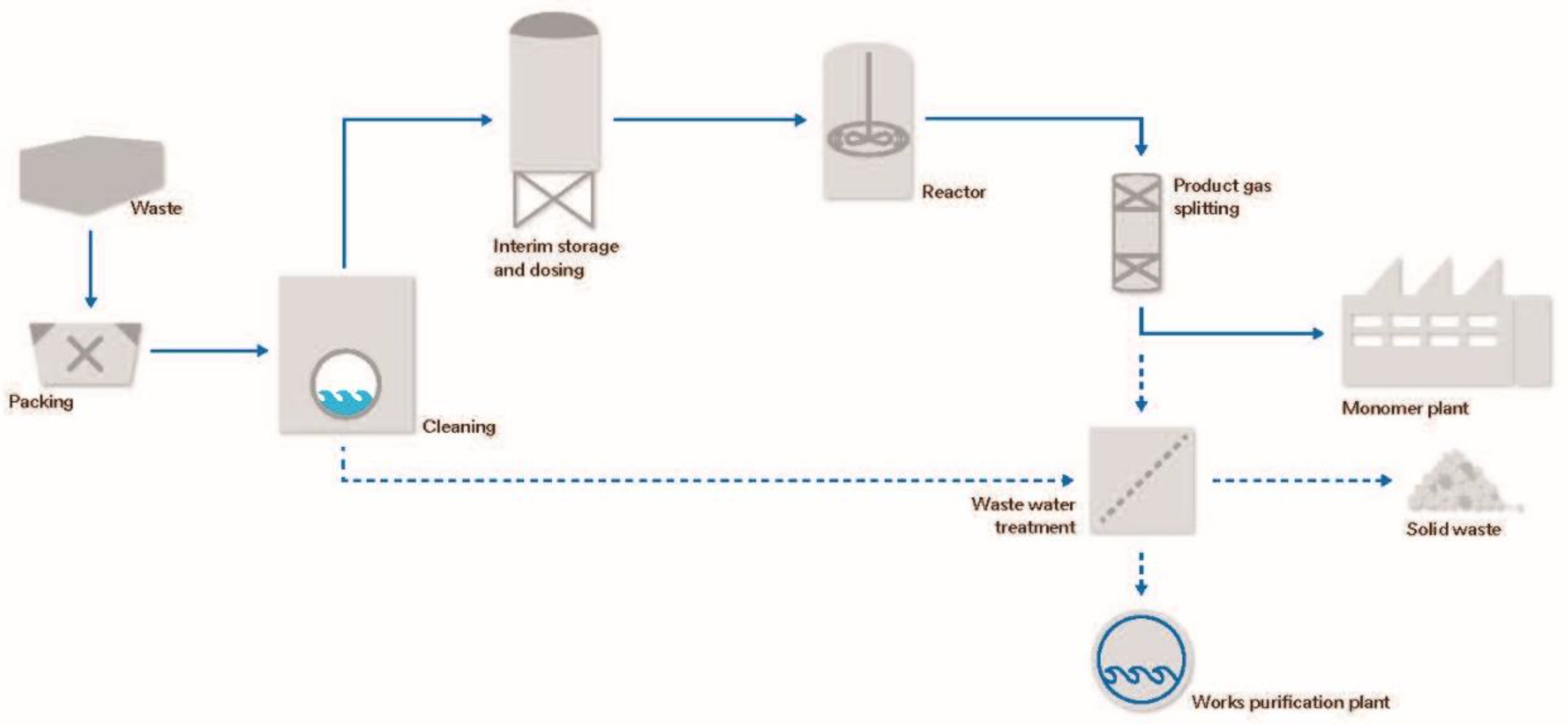
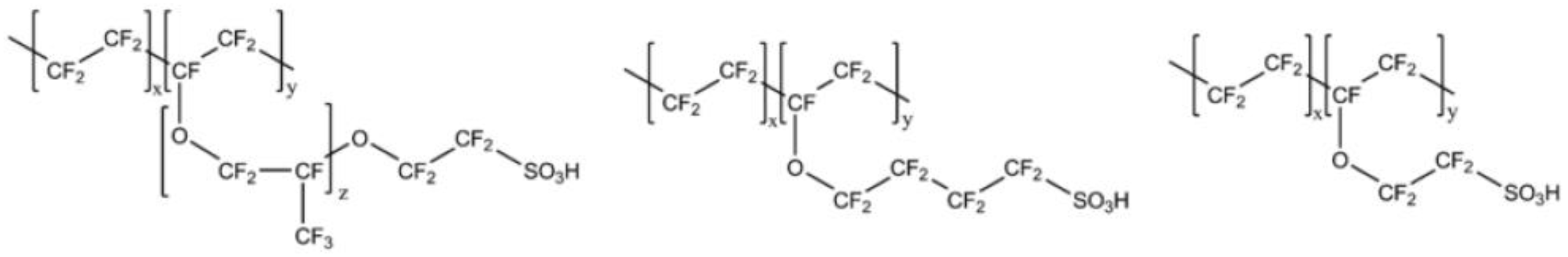
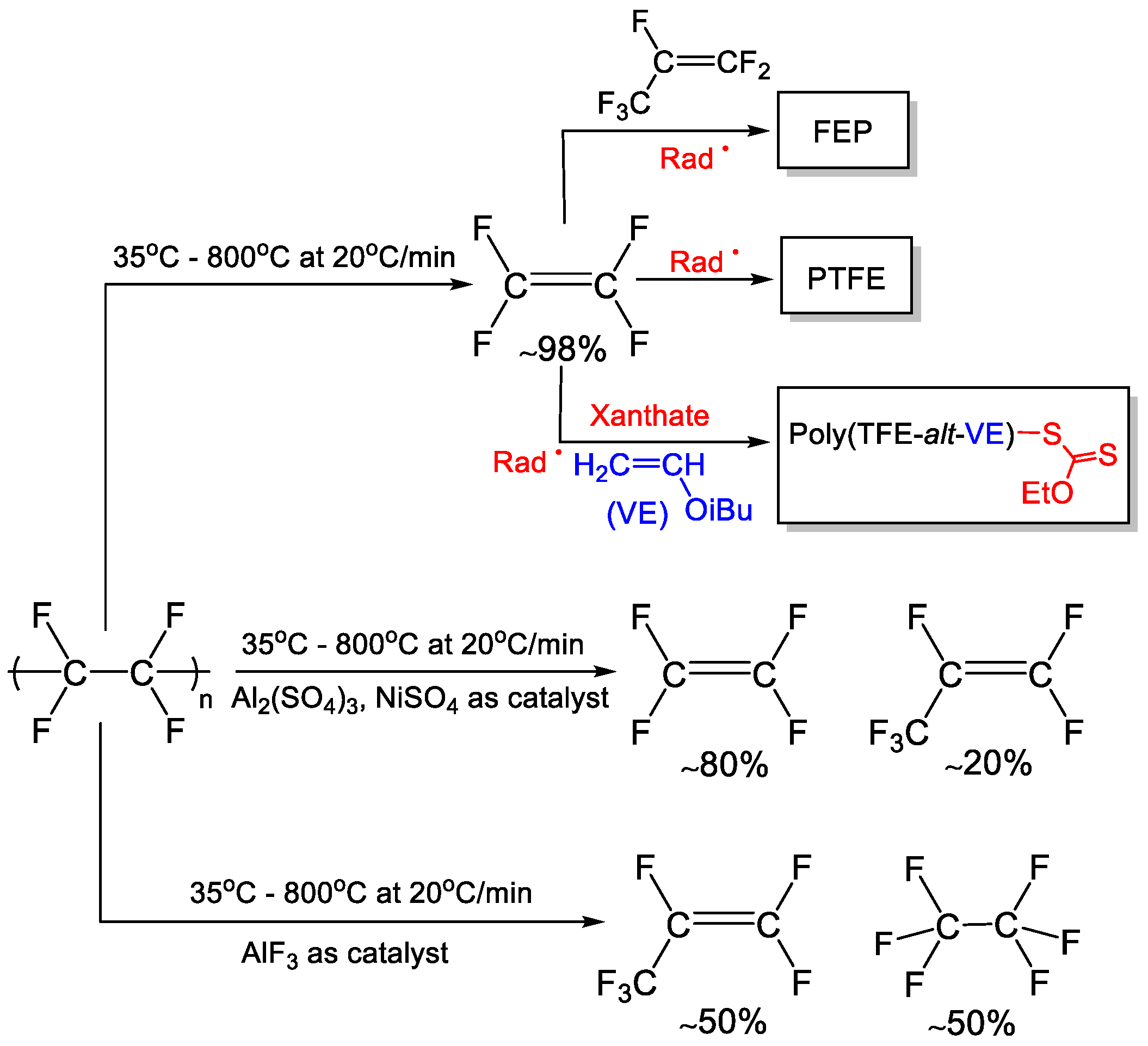
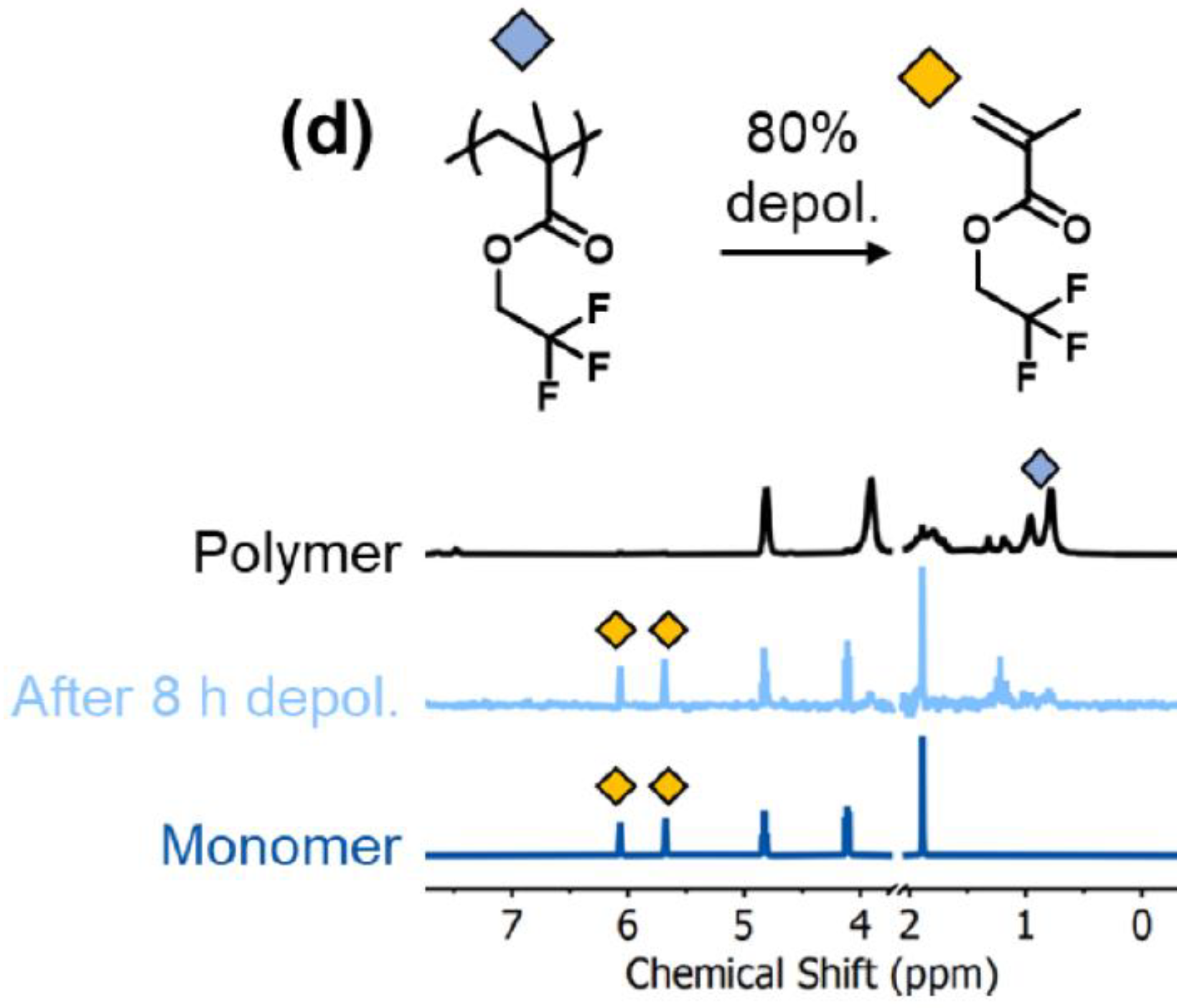
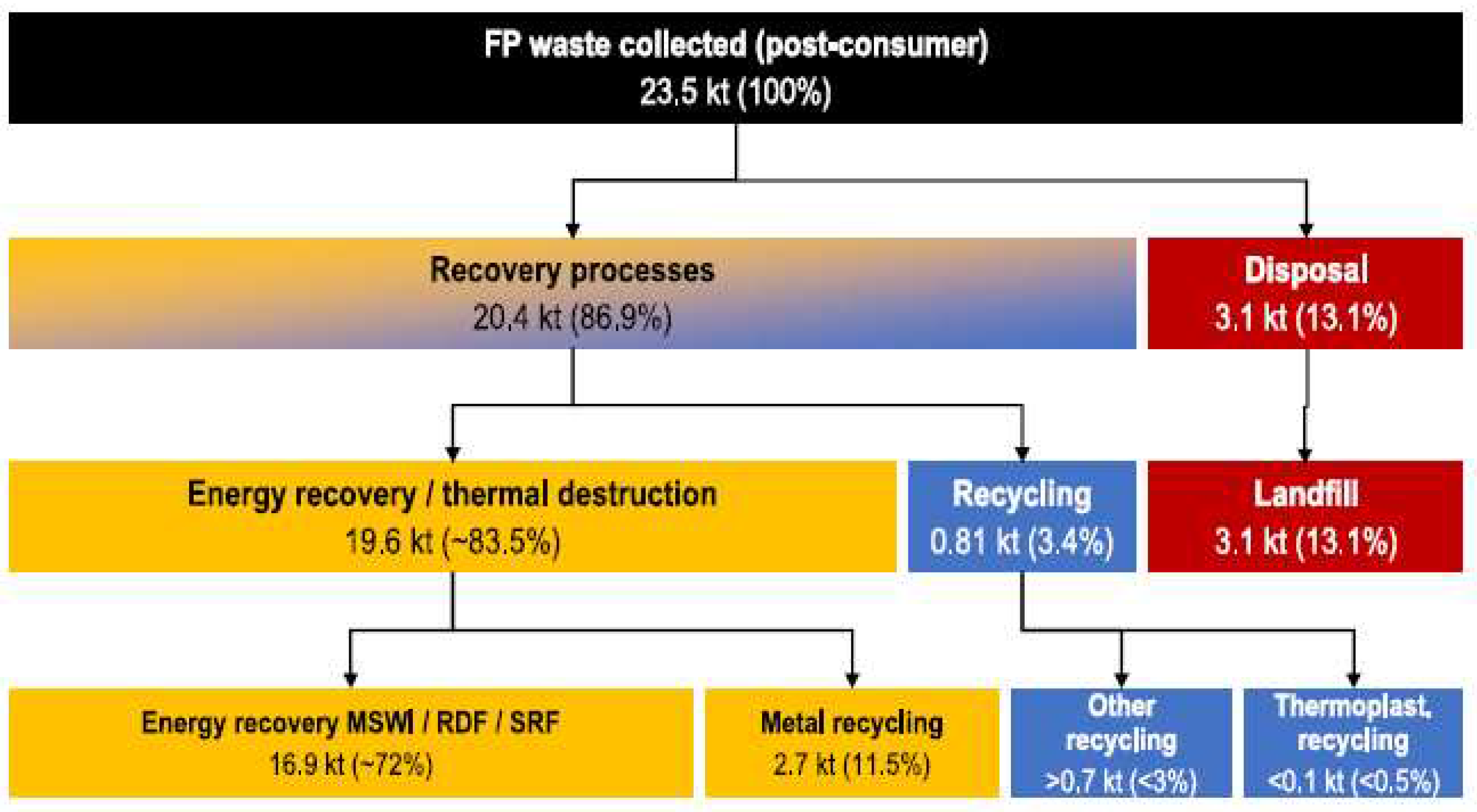
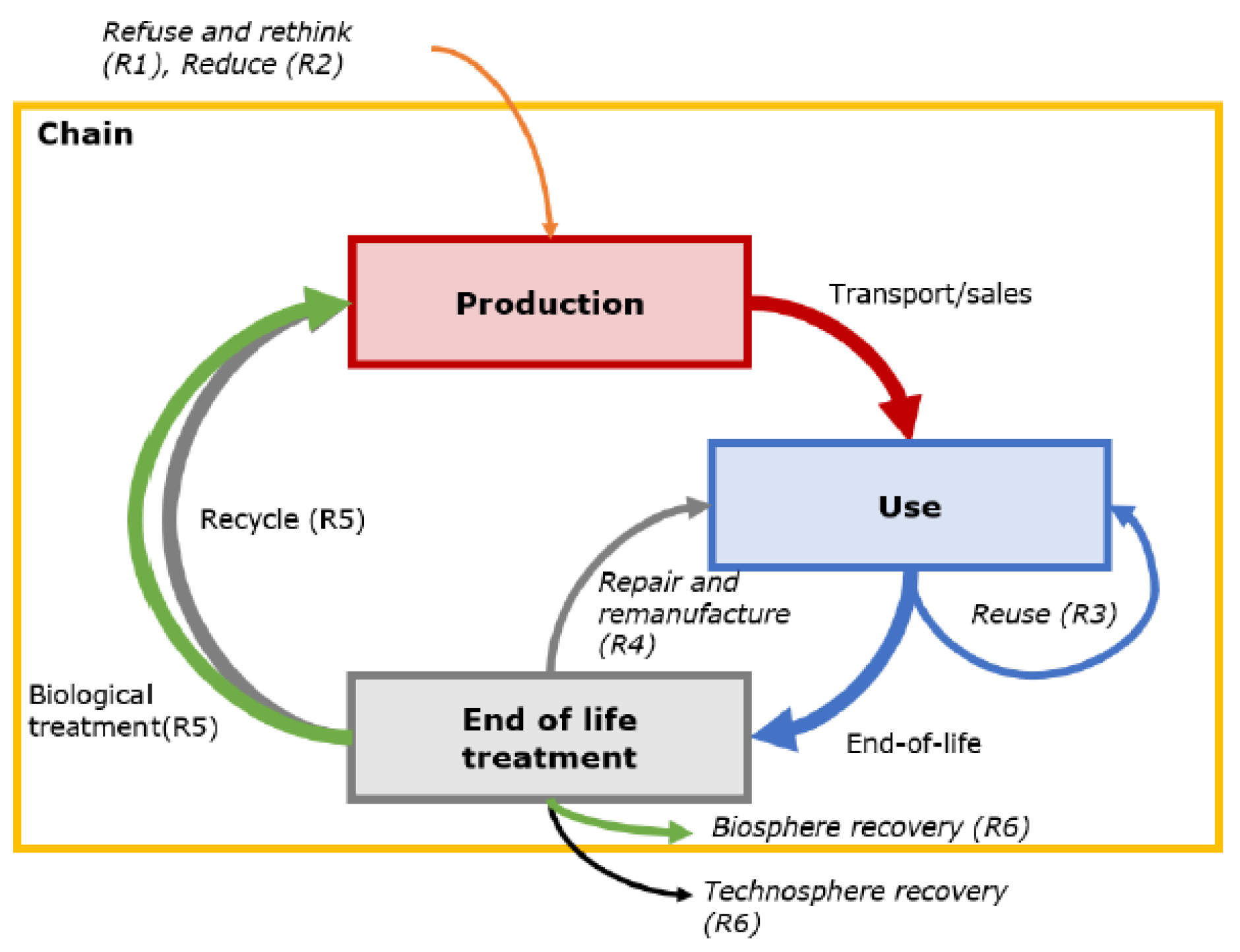
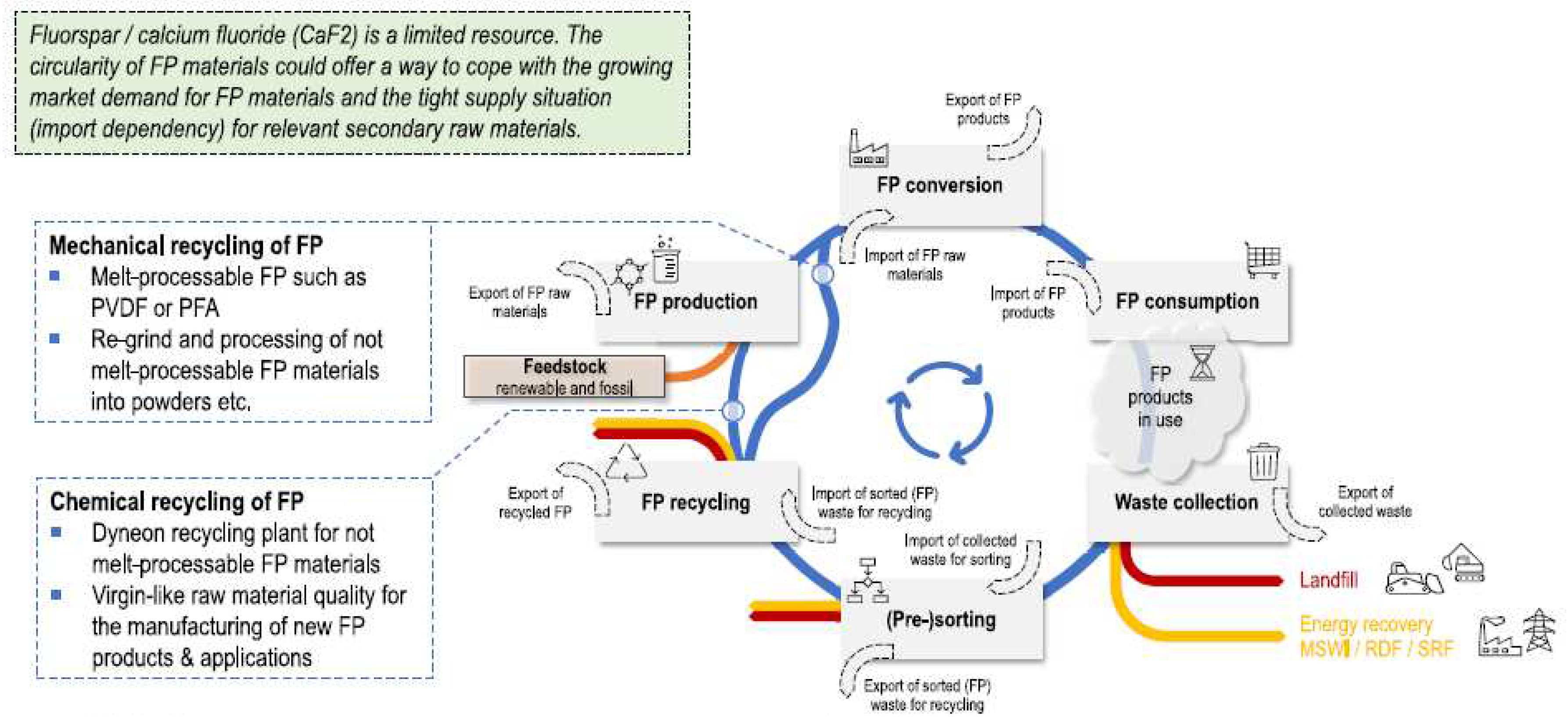
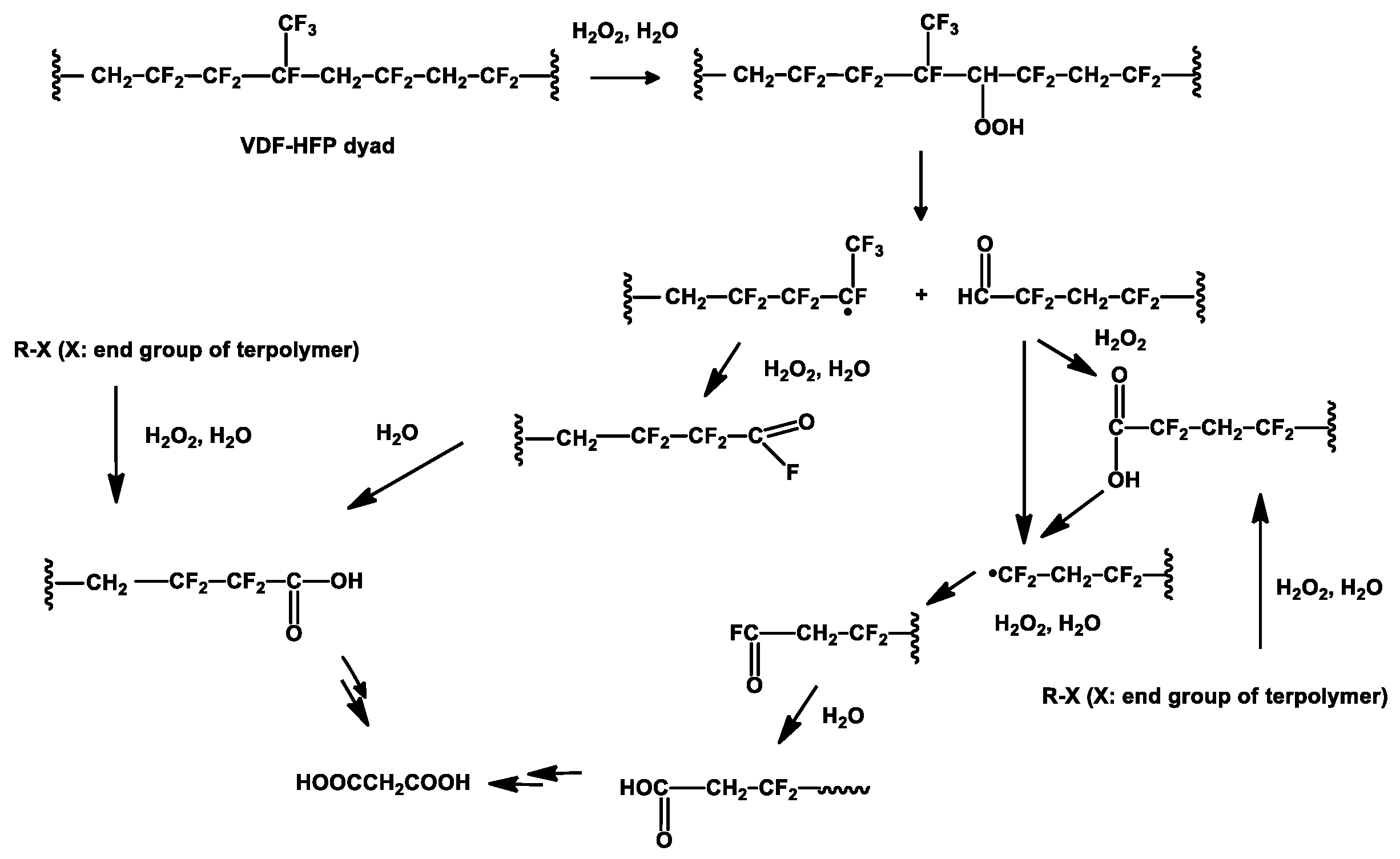
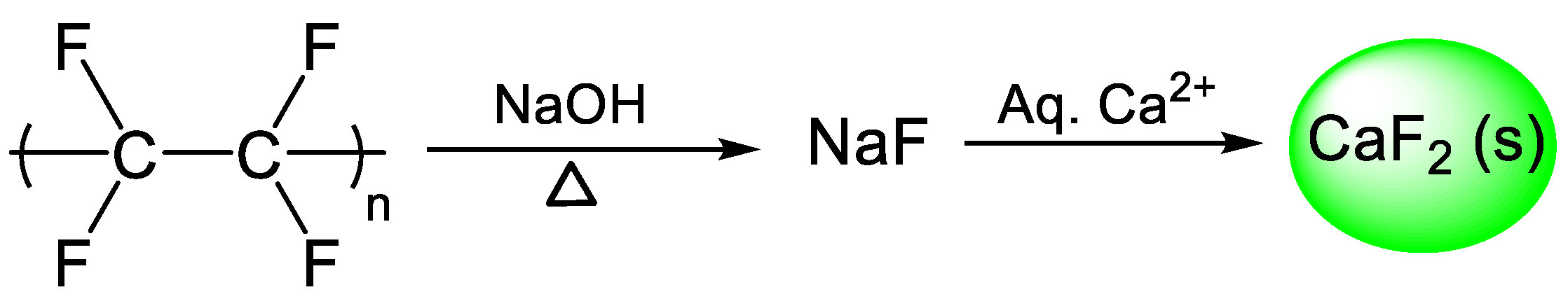
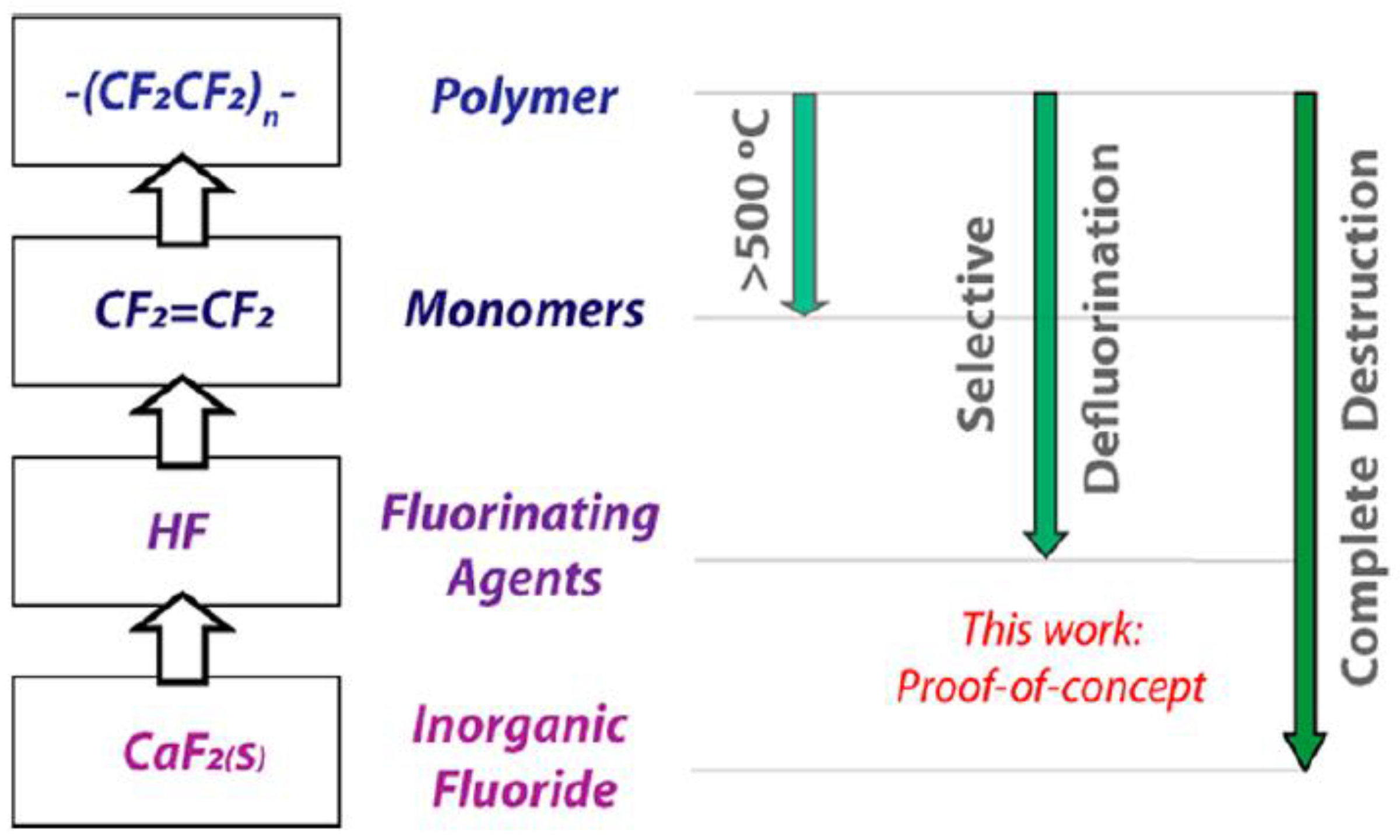
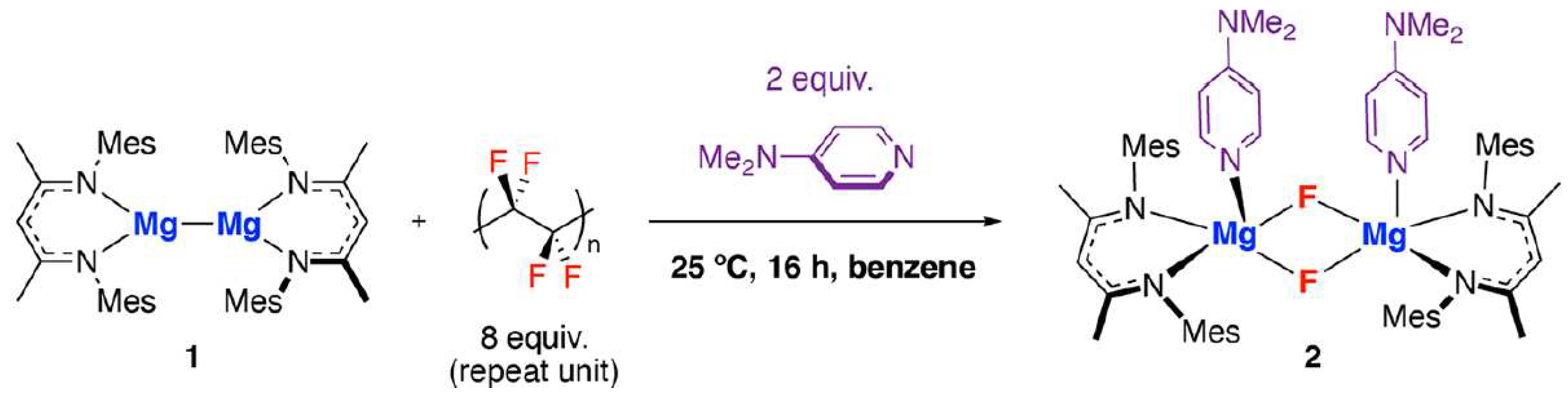
| Type of Fluoropolymer | Reference |
|---|---|
| PTFE Aqueous dispersions | 89,93 |
| PTFE Fine powders | 90,94 |
| PVDF | 88,93,95 |
| FKM | 89,92,95 |
| PFA | 94 |
| Final PTFE Results | ||
|---|---|---|
| Properties of Interest | Concentration | PLC Criteria |
| *% oligomer | Not detected | < 2% wt/wt (20,000 ppm) |
| **residual monomers | Not detected | No Limit Established by OECD, 2009 |
| low molar mass leachables & extractables | #2 ppm | No Limit Established by OECD, 2009 |
| *Polymers with potential health concern have an increased incidence of higher oligomer content that began at 5% for <1000 Da and 2% for <500 Da oligomeric content (OECD, 2009). The table lists the lower limit, 2%. | ||
| **The data set used by OECD114 to establish the PLC criteria was insufficient to establish a universal limit for all residual monomers, though residual monomer content was established as a PLC criteria.108 According to U.S. EPA’s Safer Choice criteria (SCP, 2015), TFE is a residual of concern, which is not allowed to be present in Safer Choice recognized products at 0.01% or higher. There is no specific limit on residual monomer in the PLC criteria.114 | ||
| #Isopar K, an unavoidable ambient air contaminant adsorbed to the PTFE fine powder, was detected at < 2 ppm. | ||
| Polymer | T (°C) | Main products | References |
| PTFE | 450 | COF2. HF | 124 |
| 400~500 | TFE, HFP, PFIB | 166 | |
| 500 | HFP, TFA | 140 | |
| 530 | CF4, C2F6, TFE, HFP, c-C4F8 (c-OFCB) | 167 | |
| 550 | CF2O, C2F6, CF3CFO, C5F4, CF3CF2CFO, (CF2)3O2 |
168 | |
| 600-700 | TFE, OFCB | 169 | |
| 750-800 | HFP | 169 | |
| 850-900 | PFIB | 169 | |
| 800 °C | CF4 | 124 | |
| >900 °C | C2F6 | 124 | |
| 850 | HFP, TFE | 170 | |
| 750-1050 | C2F6, CF4 | 170 | |
| ETFE | 350 | COF2, PFBE, TFE, CO | 124 124 124 124 |
| ECTFE | 500 | TFA, CDFA | |
| FEP | 400 | COF2, HFP, TFE, PFIB | |
| PFA | 400 | COF2 | |
| PEEPE | 500 | TFA | 140 124 |
| CPTFE/PCTFE | 500 | CPFP, CDFA | |
| PTFE/PFA + PTFE/FEP | 800 | CH4, CHF3 (HFC-23), C2F6 (PFC-116) | 171 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





