Submitted:
07 September 2023
Posted:
11 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
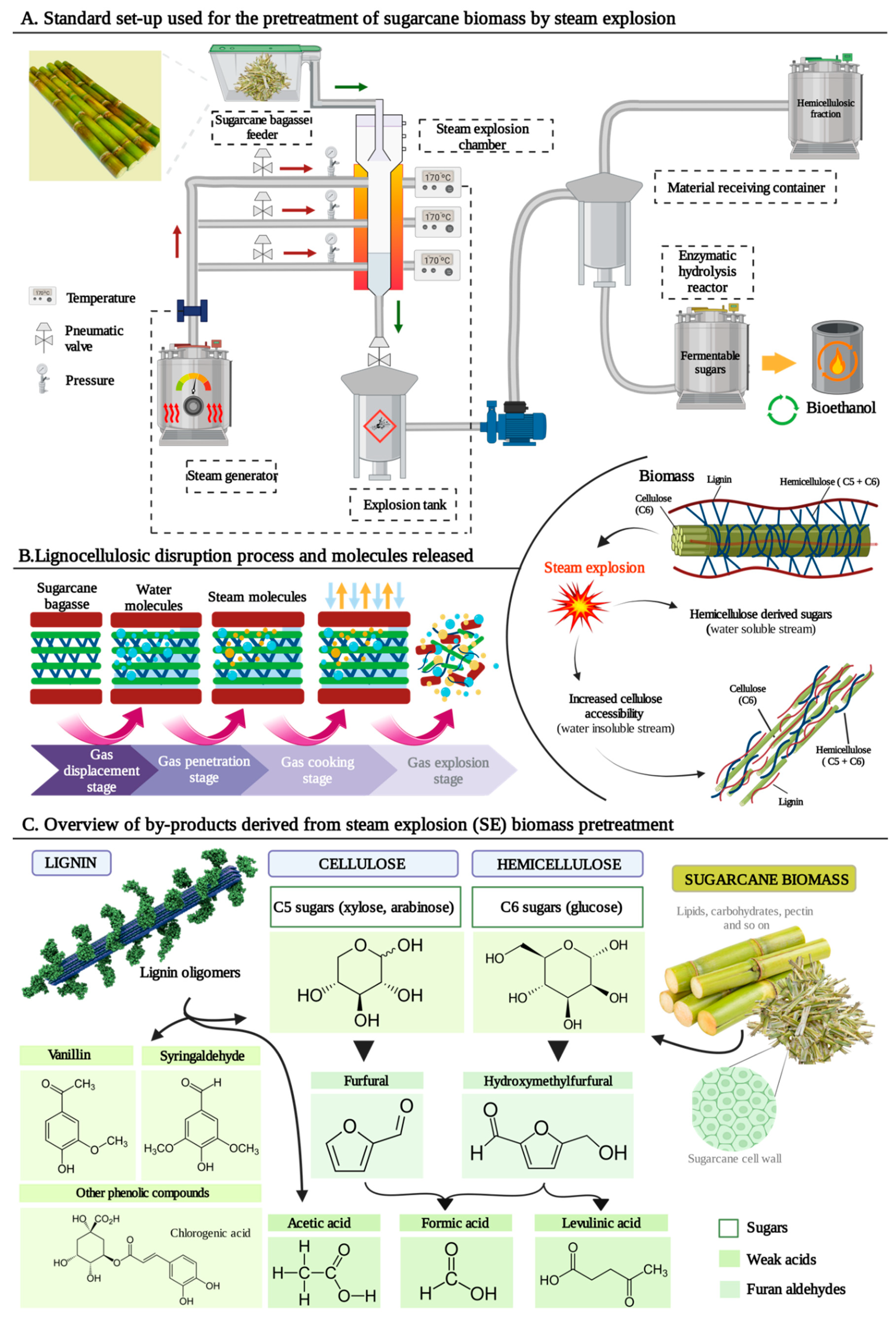
2. Steam explosion (SE) as lignocellulosic biomass pretreatment
3. Sugarcane bagasse (SCB) as a potential matrix for bioethanol production
| Pretreatment | Operational conditions | Recovery (%) | Ref. | ||
|---|---|---|---|---|---|
| Temperature (ºC) | Time (min) | Pressure (atm) | |||
| SE+AHS | 195 | 7.5 | 18 | 73.8 bEtOH, 0.58g/L/h EtOH | [6] |
| AHS | 200 | 10 | - | 51.88-66.67 bEtOH (11.96 g/L) | [68] |
| K3PO4 6.4% | 144 | 60 | - | 53.04 bEtOH | [69] |
| SE | 160 | 30 | 6.805 | >150mg/g TC, 87.16 mg/mL EtOH | [65] |
| H2SO4 10% | 100 | 60 | - | 251.1 mg/g TC, 58.7 mg/mL EtOH | |
| SE+H2O2 | 210 | 15 | - | 86.9 C; 92.4 HM; 29.7 Lig | [41] |
| SF-CO2+H2O2 | 186.85 | 40 | 153.96 | 97.8 Glu | [70] |
| NaOH 0.7% | 70 | 360 | - | 53.3-68.8 Glu; 67.8-74.7 xylose → 10.69 g/L | [71] |
| NH4-OH-H2O2+IL | 100 | 360 | - | 87.4 Glu; 55.5 glucan; 19.8 xylan0.42 g EtOH/g G, 14.1 g/L EtOH | [61] |
| Imidazole | 160 | 60 | - | 55.7 solid | |
| HOAc | 107 | 30-90 | - | 80 bEtOH | [72] |
| Na2CO3 | 195 | 15 | - | 69.1 C; 4.1 HM; 9.5 lignin → 16.1 g EtOH/100 g biomass | [62] |
| SE | 200 | 10.5 | 14.2 | 52 C; 3.9 HM; 33.1 Lig | [64] |
| SE+H2SO4 | 180 | 4 | 10 | 50.5 C; 6.9 HM; 30.8 Lig | |
| SE+H3PO4 | 195 | 7.5 | 14.2 | 50.2 C; 2.7 HM; 35.2 Lig | |
4. Steam explosion (SE) bioethanol production applications in diverse matrices
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soares de Carvalho Freitas, E.; Xavier, L.H.; Oliveira, L.B.; Guarieiro, L.L.N. System Dynamics Applied to Second Generation Biofuel in Brazil: A Circular Economy Approach. Sustain. Energy Technol. Assessments 2022, 52, 102288. [Google Scholar] [CrossRef]
- D’Amato, D.; Korhonen, J.; Toppinen, A. Circular, Green, and Bio Economy: How Do Companies in Land-Use Intensive Sectors Align with Sustainability Concepts? Ecol. Econ. 2019, 158, 116–133. [Google Scholar] [CrossRef]
- Khaire, K.C.; Moholkar, V.S.; Goyal, A. Bioconversion of Sugarcane Tops to Bioethanol and Other Value Added Products: An Overview. Mater. Sci. Energy Technol. 2021, 4, 54–68. [Google Scholar] [CrossRef]
- Kesharwani, R.; Sun, Z.; Dagli, C.; Xiong, H. Moving Second Generation Biofuel Manufacturing Forward: Investigating Economic Viability and Environmental Sustainability Considering Two Strategies for Supply Chain Restructuring. Appl. Energy 2019, 242, 1467–1496. [Google Scholar] [CrossRef]
- Kirshner, J.; Brown, E.; Dunlop, L.; Franco Cairo, J.P.; Redeker, K.; Veneu, F.; Brooks, S.; Kirshner, S.; Walton, P.H. “A Future beyond Sugar”: Examining Second-Generation Biofuel Pathways in Alagoas, Northeast Brazil. Environ. Dev. 2022, 44, 100739. [Google Scholar] [CrossRef]
- Neves, P. V.; Pitarelo, A.P.; Ramos, L.P. Production of Cellulosic Ethanol from Sugarcane Bagasse by Steam Explosion: Effect of Extractives Content, Acid Catalysis and Different Fermentation Technologies. Bioresour. Technol. 2016, 208, 184–194. [Google Scholar] [CrossRef]
- Vaish, S.; Kaur, G.; Sharma, N.K.; Gakkhar, N. Estimation for Potential of Agricultural Biomass Sources as Projections of Bio-Briquettes in Indian Context. Sustain. 2022, 14. [Google Scholar] [CrossRef]
- Niju, S.; Swathika, M. Delignification of Sugarcane Bagasse Using Pretreatment Strategies for Bioethanol Production. Biocatal. Agric. Biotechnol. 2019, 20, 101263. [Google Scholar] [CrossRef]
- Espírito Santo, M.C. do; Cardoso, E.B.; Guimaraes, F.E.G.; deAzevedo, E.R.; Cunha, G.P. da; Novotny, E.H.; Pellegrini, V. de O.A.; Chandel, A.K.; Silveira, M.H.L.; Polikarpov, I. Multifaceted Characterization of Sugarcane Bagasse under Different Steam Explosion Severity Conditions Leading to Distinct Enzymatic Hydrolysis Yields. Ind. Crops Prod. 2019, 139, 111542. [Google Scholar] [CrossRef]
- Silva, T.A.L.; Zamora, H.D.Z.; Varão, L.H.R.; Prado, N.S.; Baffi, M.A.; Pasquini, D. Effect of Steam Explosion Pretreatment Catalysed by Organic Acid and Alkali on Chemical and Structural Properties and Enzymatic Hydrolysis of Sugarcane Bagasse. Waste and Biomass Valorization 2018, 9, 2191–2201. [Google Scholar] [CrossRef]
- Auxenfans, T.; Crônier, D.; Chabbert, B.; Paës, G. Understanding the Structural and Chemical Changes of Plant Biomass Following Steam Explosion Pretreatment. Biotechnol. Biofuels 2017, 10, 1–16. [Google Scholar] [CrossRef]
- Algayyim, S.J.M.; Yusaf, T.; Hamza, N.H.; Wandel, A.P.; Fattah, I.M.R.; Laimon, M.; Rahman, S.M.A. Sugarcane Biomass as a Source of Biofuel for Internal Combustion Engines (Ethanol and Acetone-Butanol-Ethanol): A Review of Economic Challenges. Energies 2022, 15. [Google Scholar] [CrossRef]
- Kaur, P.; Bohidar, H.B.; Pfeffer, F.M.; Williams, R.; Agrawal, R. A Comparative Assessment of Biomass Pretreatment Methods for the Sustainable Industrial Upscaling of Rice Straw into Cellulose. Cellulose 2023, 30, 4247–4261. [Google Scholar] [CrossRef]
- Vallejo, M.; Cordeiro, R.; Dias, P.A.N.; Moura, C.; Henriques, M.; Seabra, I.J.; Malça, C.M.; Morouço, P. Recovery and Evaluation of Cellulose from Agroindustrial Residues of Corn, Grape, Pomegranate, Strawberry-Tree Fruit and Fava. Bioresour. Bioprocess. 2021, 8. [Google Scholar] [CrossRef]
- Qasim, U.; Ali, Z.; Nazir, M.S.; Ul Hassan, S.; Rafiq, S.; Jamil, F.; Al-Muhtaseb, A.H.; Ali, M.; Khan Niazi, M.B.; Ahmad, N.M.; et al. Isolation of Cellulose from Wheat Straw Using Alkaline Hydrogen Peroxide and Acidified Sodium Chlorite Treatments: Comparison of Yield and Properties. Adv. Polym. Technol. 2020, 2020. [Google Scholar] [CrossRef]
- Sankhla, S.; Sardar, H.H.; Neogi, S. Greener Extraction of Highly Crystalline and Thermally Stable Cellulose Micro-Fibers from Sugarcane Bagasse for Cellulose Nano-Fibrils Preparation. Carbohydr. Polym. 2021, 251, 117030. [Google Scholar] [CrossRef]
- Akizuki, S.; Suzuki, H.; Fujiwara, M.; Toda, T. Impacts of Steam Explosion Pretreatment on Semi-Continuous Anaerobic Digestion of Lignin-Rich Submerged Macrophyte. J. Clean. Prod. 2023, 385, 135377. [Google Scholar] [CrossRef]
- Bandyopadhyay-Ghosh, S.; Ghosh, S.B.; Sain, M. The Use of Biobased Nanofibres in Composites. In Biofiber Reinforcements in Composite Materials; Faruk, O.M.S., Ed.; Elsevier Ltd 571: Pilani, India, 2015; p. 647; ISBN 9781782421276. [Google Scholar]
- Yu, Y.; Wu, J.; Ren, X.; Lau, A.; Rezaei, H.; Takada, M.; Bi, X.; Sokhansanj, S. Steam Explosion of Lignocellulosic Biomass for Multiple Advanced Bioenergy Processes: A Review. Renew. Sustain. Energy Rev. 2022, 154, 111871. [Google Scholar] [CrossRef]
- Galbe, M.; Wallberg, O. Pretreatment for Biorefineries: A Review of Common Methods for Efficient Utilisation of Lignocellulosic Materials. Biotechnol. Biofuels 2019, 12, 1–26. [Google Scholar] [CrossRef]
- Michalak, L.; Knutsen, S.H.; Aarum, I.; Westereng, B. Effects of PH on Steam Explosion Extraction of Acetylated Galactoglucomannan from Norway Spruce. Biotechnol. Biofuels 2018, 11, 1–12. [Google Scholar] [CrossRef]
- Zhao, Z.M.; Yu, W.; Huang, C.; Xue, H.; Li, J.; Zhang, D.; Li, G. Steam Explosion Pretreatment Enhancing Enzymatic Digestibility of Overground Tubers of Tiger Nut (Cyperus Esculentus L.). Front. Nutr. 2023, 9. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Ni, L.; Guo, Z.; Zeng, H.; Wu, M.; Zhang, M.; Zheng, B. Principle and Application of Steam Explosion Technology in Modification of Food Fiber. Foods 2022, 11, 1–19. [Google Scholar] [CrossRef]
- Ziegler-Devin, I.; Chrusciel, L.; Brosse, N. Steam Explosion Pretreatment of Lignocellulosic Biomass: A Mini-Review of Theorical and Experimental Approaches. Front. Chem. 2021, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhang, B.; Yu, F.; Xu, G.; Song, A. A Real Explosion: The Requirement of Steam Explosion Pretreatment. Bioresour. Technol. 2012, 121, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Capolupo, L.; Faraco, V. Green Methods of Lignocellulose Pretreatment for Biorefinery Development. Appl. Microbiol. Biotechnol. 2016, 100, 9451–9467. [Google Scholar] [CrossRef] [PubMed]
- Bhukya, B.; Keshav, P.K. An Evaluation of Steam Explosion Pretreatment to Enhance the Digestibility of Lignocellulosic Biomass. In Lignocellulose Bioconversion Through White Biotechnology; Chandel, A.K., Ed.; John Wiley & Sons Ltd: Telangana, India, 2022; pp. 83–98; ISBN 9781119735984. [Google Scholar]
- Akizuki, S.; Suzuki, H.; Fujiwara, M.; Toda, T. Impacts of Steam Explosion Pretreatment on Semi-Continuous Anaerobic Digestion of Lignin-Rich Submerged Macrophyte. J. Clean. Prod. 2023, 385, 135377. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Galbe, M.; Garrote, G.; Ramirez-Gutierrez, D.M.; Ximenes, E.; Sun, S.N.; Lachos-Perez, D.; Rodríguez-Jasso, R.M.; Sun, R.C.; Yang, B.; et al. Severity Factor Kinetic Model as a Strategic Parameter of Hydrothermal Processing (Steam Explosion and Liquid Hot Water) for Biomass Fractionation under Biorefinery Concept. Bioresour. Technol. 2021, 342. [Google Scholar] [CrossRef]
- Steinbach, D.; Kruse, A.; Sauer, J. Pretreatment Technologies of Lignocellulosic Biomass in Water in View of Furfural and 5-Hydroxymethylfurfural Production- A Review. Biomass Convers. Biorefinery 2017, 7, 247–274. [Google Scholar] [CrossRef]
- Sulzenbacher, D.; Atzmüller, D.; Hawe, F.; Richter, M.; Cristobal-Sarramian, A.; Zwirzitz, A. Optimization of Steam Explosion Parameters for Improved Biotechnological Use of Wheat Straw. Biomass Convers. Biorefinery 2023, 13, 1035–1046. [Google Scholar] [CrossRef]
- Shrotri, A.; Kobayashi, H.; Fukuoka, A. Catalytic Conversion of Structural Carbohydrates and Lignin to Chemicals; 1st ed.; Elsevier Inc., 2017; Vol. 60.
- Leskinen, T.; Kelley, S.S.; Argyropoulos, D.S. E-Beam Irradiation & Steam Explosion as Biomass Pretreatment, and the Complex Role of Lignin in Substrate Recalcitrance. Biomass and Bioenergy 2017, 103, 21–28. [Google Scholar] [CrossRef]
- Steinbach, D.; Kruse, A.; Sauer, J.; Storz, J. Is Steam Explosion a Promising Pretreatment for Acid Hydrolysis of Lignocellulosic Biomass? Processes 2020, 8, 1–12. [Google Scholar] [CrossRef]
- He, Q.; Ziegler-Devin, I.; Chrusciel, L.; Obame, S.N.; Hong, L.; Lu, X.; Brosse, N. Lignin-First Integrated Steam Explosion Process for Green Wood Adhesive Application. ACS Sustain. Chem. Eng. 2020, 8, 5380–5392. [Google Scholar] [CrossRef]
- Onyenwoke, C.; Tabil, L.G.; Dumonceaux, T.; Cree, D.; Mupondwa, E.; Adapa, P.; Karunakaran, C. Investigation of Steam Explosion Pretreatment of Sawdust and Oat Straw to Improve Their Quality as Biofuel Pellets. Energies 2022, 15, 1–19. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Barakat, A.; Quéméneur, M.; Trably, E.; Steyer, J.P.; Carrère, H. Do Furanic and Phenolic Compounds of Lignocellulosic and Algae Biomass Hydrolyzate Inhibit Anaerobic Mixed Cultures? A Comprehensive Review. Biotechnol. Adv. 2014, 32, 934–951. [Google Scholar] [CrossRef]
- Basak, B.; Jeon, B.H.; Kim, T.H.; Lee, J.C.; Chatterjee, P.K.; Lim, H. Dark Fermentative Hydrogen Production from Pretreated Lignocellulosic Biomass: Effects of Inhibitory Byproducts and Recent Trends in Mitigation Strategies. Renew. Sustain. Energy Rev. 2020, 133, 110338. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Rajendran, K.; Pugazhendhi, A.; Rao, C. V.; Atabani, A.E.; Kumar, G.; Yang, Y.H. Renewable Biohydrogen Production from Lignocellulosic Biomass Using Fermentation and Integration of Systems with Other Energy Generation Technologies. Sci. Total Environ. 2021, 765, 144429. [Google Scholar] [CrossRef]
- Li, X.; Xu, R.; Yang, J.; Nie, S.; Liu, D.; Liu, Y.; Si, C. Production of 5-Hydroxymethylfurfural and Levulinic Acid from Lignocellulosic Biomass and Catalytic Upgradation. Ind. Crops Prod. 2019, 130, 184–197. [Google Scholar] [CrossRef]
- Rabelo, S.C.; Vaz Rossell, C.E.; de Moraes Rocha, G.J.; Zacchi, G. Enhancement of the Enzymatic Digestibility of Sugarcane Bagasse by Steam Pretreatment Impregnated with Hydrogen Peroxide. Biotechnol. Prog. 2012, 28, 1207–1217. [Google Scholar] [CrossRef]
- Baksi, S.; Saha, D.; Saha, S.; Sarkar, U.; Basu, D.; Kuniyal, J.C. Pre-Treatment of Lignocellulosic Biomass: Review of Various Physico-Chemical and Biological Methods Influencing the Extent of Biomass Depolymerization. Int. J. Environ. Sci. Technol. 2023. [Google Scholar] [CrossRef]
- Gao, Z.; Alshehri, K.; Li, Y.; Qian, H.; Sapsford, D.; Cleall, P.; Harbottle, M. Advances in Biological Techniques for Sustainable Lignocellulosic Waste Utilization in Biogas Production. Renew. Sustain. Energy Rev. 2022, 170, 112995. [Google Scholar] [CrossRef]
- Zhao, G.; Kuang, G.; Wang, Y.; Yao, Y.; Zhang, J.; Pan, Z.H. Effect of Steam Explosion on Physicochemical Properties and Fermentation Characteristics of Sorghum (Sorghum Bicolor (L.) Moench). Lwt 2020, 129, 109579. [Google Scholar] [CrossRef]
- Seidel, C.M.; Brethauer, S.; Gyenge, L.; Rudolf Von Rohr, P.; Studer, M.H. Two-Stage Steam Explosion Pretreatment of Softwood with 2-Naphthol as Carbocation Scavenger. Biotechnol. Biofuels 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Lizasoain, J.; Rincón, M.; Theuretzbacher, F.; Enguídanos, R.; Nielsen, P.J.; Potthast, A.; Zweckmair, T.; Gronauer, A.; Bauer, A. Biogas Production from Reed Biomass: Effect of Pretreatment Using Different Steam Explosion Conditions. Biomass and Bioenergy 2016, 95, 84–91. [Google Scholar] [CrossRef]
- Ahmad, E.; Pant, K.K. Lignin Conversion: A Key to the Concept of Lignocellulosic Biomass-Based Integrated Biorefinery. In Waste Biorefinery: Potential and Perspectives; Elsevier B.V., 2018; pp. 409–444 ISBN 9780444639929.
- Kim, D. Physico-Chemical Conversion of Lignocellulose: Inhibitor Effects and Detoxification Strategies: A Mini Review. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yuan, Q.; Cheng, G. Deconstruction of Corncob by Steam Explosion Pretreatment: Correlations between Sugar Conversion and Recalcitrant Structures. Carbohydr. Polym. 2017, 156, 351–356. [Google Scholar] [CrossRef]
- Antunes, F.; Mota, I.F.; da Silva Burgal, J.; Pintado, M.; Costa, P.S. A Review on the Valorization of Lignin from Sugarcane By-Products: From Extraction to Application. In Biomass and Bioenergy; Pergamon, 2022; Vol. 166, p. 106603.
- Moore, P.H. Sci-Hub | Sugarcane and Sugarbeet. Encyclopedia of Applied Plant Sciences, 273–280 | ; Elselvier, 2017. [CrossRef]
- Bhardwaj, N.K.; Kaur, D.; Chaudhry, S.; Sharma, M.; Arya, S. Approaches for Converting Sugarcane Trash, a Promising Agro Residue, into Pulp and Paper Using Soda Pulping and Elemental Chlorine-Free Bleaching. In Journal of Cleaner Production; Elsevier, 2019; Vol. 217, pp. 225–233.
- del Río, J.C.; Lino, A.G.; Colodette, J.L.; Lima, C.F.; Gutiérrez, A.; Martínez, Á.T.; Lu, F.; Ralph, J.; Rencoret, J. Differences in the Chemical Structure of the Lignins from Sugarcane Bagasse and Straw. In Biomass and Bioenergy; Elsevier Ltd, 2015; Vol. 81, pp. 322–338.
- Farias, J.P.; Okeke, B.C.; Ávila, F.D. De; Demarco, C.F.; Silva, M.S.; Camargo, F.A. de O.; Menezes Bento, F.; Pieniz, S.; Andreazza, R. Biotechnology Process for Microbial Lipid Synthesis from Enzymatic Hydrolysate of Pre-Treated Sugarcane Bagasse for Potential Bio-Oil Production. Renew. Energy 2023, 205, 174–184. [Google Scholar] [CrossRef]
- Qiu, Z.; Han, X.; Fu, A.; Jiang, Y.; Zhang, W.; Jin, C.; Li, D.; Xia, J.; He, J.; Deng, Y.; et al. Enhanced Cellulosic D-Lactic Acid Production from Sugarcane Bagasse by Pre-Fermentation of Water-Soluble Carbohydrates before Acid Pretreatment. Bioresour. Technol. 2023, 368, 128324. [Google Scholar] [CrossRef]
- Pereira Marques, F.; Lima Soares, A.K.; Lomonaco, D.; Alexandre e Silva, L.M.; Tédde Santaella, S.; de Freitas Rosa, M.; Carrhá Leitão, R. Steam Explosion Pretreatment Improves Acetic Acid Organosolv Delignification of Oil Palm Mesocarp Fibers and Sugarcane Bagasse. Int. J. Biol. Macromol. 2021, 175, 304–312. [Google Scholar] [CrossRef]
- Hongrattanavichit, I.; Aht-Ong, D. Nanofibrillation and Characterization of Sugarcane Bagasse Agro-Waste Using Water-Based Steam Explosion and High-Pressure Homogenization. J. Clean. Prod. 2020, 277, 123471. [Google Scholar] [CrossRef]
- Da Silva, A.S.A.; Inoue, H.; Endo, T.; Yano, S.; Bon, E.P.S. Milling Pretreatment of Sugarcane Bagasse and Straw for Enzymatic Hydrolysis and Ethanol Fermentation. Bioresour. Technol. 2010, 101, 7402–7409. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.M.; Mazzola, P.G.; Silva, J.C.A.R.; Pahl, R.; Pessoa, A.; Costa, S.A. Use of Sugar Cane Straw as a Source of Cellulose for Textile Fiber Production. Ind. Crops Prod. 2013, 42, 189–194. [Google Scholar] [CrossRef]
- Gómez, E.O.; Souza, R.T.G. de; Rocha, G.J. de M.; Almeida, E. de; Cortez, L.A.B. SUGARCANE TRASH AS FEEDSTOCK FOR SECOND GENERATION PROCESSES. In Sugarcane bioethanol — R&D for Productivity and Sustainability; Editora Edgard Blücher, 2014; pp. 637–660.
- Zhu, Z.; Zhu, M.; Wu, Z. Pretreatment of Sugarcane Bagasse with NH4OH–H2O2 and Ionic Liquid for Efficient Hydrolysis and Bioethanol Production. Bioresour. Technol. 2012, 119, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Martín, C.; Klinke, H.B.; Thomsen, A.B. Wet Oxidation as a Pretreatment Method for Enhancing the Enzymatic Convertibility of Sugarcane Bagasse. Enzyme Microb. Technol. 2007, 40, 426–432. [Google Scholar] [CrossRef]
- Haghighi Mood, S.; Hossein Golfeshan, A.; Tabatabaei, M.; Salehi Jouzani, G.; Najafi, G.H.; Gholami, M.; Ardjmand, M. Lignocellulosic Biomass to Bioethanol, a Comprehensive Review with a Focus on Pretreatment. Renew. Sustain. Energy Rev. 2013, 27, 77–93. [Google Scholar] [CrossRef]
- Espirito Santo, M.C.; Fockink, D.H.; Pellegrini, V.O.A.; Guimaraes, F.E.G.; DeAzevedo, E.R.; Ramos, L.P.; Polikarpov, I. Physical Techniques Shed Light on the Differences in Sugarcane Bagasse Structure Subjected to Steam Explosion Pretreatments at Equivalent Combined Severity Factors. Ind. Crops Prod. 2020, 158, 113003. [Google Scholar] [CrossRef]
- Bernier-Oviedo, D.J.; Rincón-Moreno, J.A.; Solanilla-Duqué, J.F.; Muñoz-Hernández, J.A.; Váquiro-Herrera, H.A. Comparison of Two Pretreatments Methods to Produce Second-Generation Bioethanol Resulting from Sugarcane Bagasse. Ind. Crops Prod. 2018, 122, 414–421. [Google Scholar] [CrossRef]
- Joseph, A.M.; Tulasi, Y.; Shrivastava, D.; Kiran, B. Techno-Economic Feasibility and Exergy Analysis of Bioethanol Production from Waste. Energy Convers. Manag. X 2023, 18, 100358. [Google Scholar] [CrossRef]
- Junqueira, T.L.; Dias, M.O.S.; Cavalett, O.; Jesus, C.D.F.; Cunha, M.P.; Rossell, C.E.V.; Maciel Filho, R.; Bonomi, A. Economic and Environmental Assessment of Integrated 1 St and 2 Nd Generation Sugarcane Bioethanol Production Evaluating Different 2 Nd Generation Process Alternatives. Comput. Aided Chem. Eng. 2012, 30, 177–181. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Lei, F.; Jiang, J. Co-Production Bioethanol and Xylooligosaccharides from Sugarcane Bagasse via Autohydrolysis Pretreatment. Renew. Energy 2020, 162, 2297–2305. [Google Scholar] [CrossRef]
- Fu, Y.; Gao, H.; Yu, H.; Yang, Q.; Peng, H.; Liu, P.; Li, Y.; Hu, Z.; Zhang, R.; Li, J.; et al. Specific Lignin and Cellulose Depolymerization of Sugarcane Bagasse for Maximum Bioethanol Production under Optimal Chemical Fertilizer Pretreatment with Hemicellulose Retention and Liquid Recycling. Renew. Energy 2022, 200, 1371–1381. [Google Scholar] [CrossRef]
- Phan, D.T.; Tan, C.S. Innovative Pretreatment of Sugarcane Bagasse Using Supercritical CO2 Followed by Alkaline Hydrogen Peroxide. Bioresour. Technol. 2014, 167, 192–197. [Google Scholar] [CrossRef]
- Jin, Y.; Shi, Z.; Xu, G.; Yang, H.; Yang, J. A Stepwise Pretreatment of Sugarcane Bagasse by Alkaline and Hydroxymethyl Reagent for Bioethanol Production. Ind. Crops Prod. 2020, 145, 112136. [Google Scholar] [CrossRef]
- Zhao, X.; Wen, J.; Chen, H.; Liu, D. The Fate of Lignin during Atmospheric Acetic Acid Pretreatment of Sugarcane Bagasse and the Impacts on Cellulose Enzymatic Hydrolyzability for Bioethanol Production. Renew. Energy 2018, 128, 200–209. [Google Scholar] [CrossRef]
- da Fonseca, Y.A.; Silva, N.C.S.; Fernandes, A.R.A.C.; Faria, M. V.; Adarme, O.F.H.; Passos, F.; Baêta, B.E.L. Steam Explosion Pretreatment of Coffee Husks: A Strategy towards Decarbonization in a Biorefinery Approach. J. Chem. Technol. Biotechnol. 2022, 97, 1567–1574. [Google Scholar] [CrossRef]
- Baêta, B.E.L.; Cordeiro, P.H. de M.; Passos, F.; Gurgel, L.V.A.; de Aquino, S.F.; Fdz-Polanco, F. Steam Explosion Pretreatment Improved the Biomethanization of Coffee Husks. Bioresour. Technol. 2017, 245, 66–72. [Google Scholar] [CrossRef]
- Barbanera, M.; Buratti, C.; Cotana, F.; Foschini, D.; Lascaro, E. Effect of Steam Explosion Pretreatment on Sugar Production by Enzymatic Hydrolysis of Olive Tree Pruning. Energy Procedia 2015, 81, 146–154. [Google Scholar] [CrossRef]
- Barbanera, M.; Lascaro, E.; Foschini, D.; Cotana, F.; Buratti, C. Optimization of Bioethanol Production from Steam Exploded Hornbeam Wood (Ostrya Carpinifolia) by Enzymatic Hydrolysis. Renew. Energy 2018, 124, 136–143. [Google Scholar] [CrossRef]
- Varga, E.; Réczey, K.; Zacchi, G. Optimization of Steam Pretreatment of Corn Stover to Enhance Enzymatic Digestibility. In Proceedings of the Applied Biochemistry and Biotechnology - Part A Enzyme Engineering and Biotechnology; Vol. 114, pp. 509–523.
| Advantages | Ref. |
|---|---|
| Alternative method for the reutilization of agro-industrial by products to create value-added products | [23] |
| Solubilization of hemicellulose into monomers and oligomers enhances microbial enzymatic hydrolysis | [46] |
| Strong ability to compromise carbohydrate recovery and cellulose hydrolysis performances | [19] |
| Further processing of phenolic monomers presents in lignin | [47] |
| Elevated solid loadings are operable by large-scale autocatalyzed steam pretreatment | [42] |
| Absence of organic solvents and corrosive chemicals | [32] |
| Friendly-environmentally technique: 1.5 kg of water to treat 1 kg of biomass | [30] |
| Low-energy treatment: <70% energy requirements to reach same particle size than mechanical processes | [23,48] |
| Physical pretreatments generate no toxic by-products, they are sulfur-free processes | [3,47] |
| Low environmental impact, fast pretreatment, and high potential for energy efficiency | [9,10,44] |
| Relatively economic because of no external catalyst requirement | [34,40] |
| Broad applicability: high short-term efficacy, industrial scale-up and affordable technique | [23] |
| Numerous scientific reports strongly support outcomes and continuously disclose new application fields | [29] |
| Alternative method for the reutilization of agro-industrial by products to create value-added products | [23] |
| Disadvantages | |
| Difficult control of strength and consistency of treatment that may degrade other effective components | [23] |
| Hemicellulose fraction may be partially degraded due to severe pretreatment conditions | [45] |
| Potential capacity of destroying cellulose connection | [23] |
| Over-degradation of hemicellulose and cellulose may create inhibitory by-products limiting the effectiveness | [38] |
| Poor pulp yields necessitate further research into the application of pretreatment technique | [47] |
| Some studies have found no correlation between crystallinity and sugar conversion after SE pretreatment | [49] |
| High energy requirements may need the use of silencing devices and a waste heat recovery device | [3,23] |
| High temperature and pressure can trigger the Maillard reaction and denaturation of nutrients | [23] |
| Commercial application still under development and has not been proved yet | [47] |
| Matrix | Pretreatment before the measurement |
Cellulose | Hemicellulose | Lignin | Xylan | Sucrose | Ash | Ref. |
|---|---|---|---|---|---|---|---|---|
| SCB | Not specified | 57.68 | 12.41 | 7.89 | - | - | 2.20 | [54] |
| SCB | Dried in an oven at 65ºC | 29.19 | - | - | 16.51 | 25.75 | - | [55] |
| SCB | Air-dried at 50 ºC | 36.4 | 20.1 | 29.9 | - | - | 5.4 | [56] |
| SCF | Dehydration in hot air oven at 60ºC | 39.70 | 36.39 | 7.37 | - | - | 5.63 | [57] |
| SCB | Dried at 105ºC | 38.8 | 26 | 32.4 | - | - | 2.8 | [58] |
| SCS | Air-dried until a 10% final humidity | 33.5 | 27.1 | 25.8 | - | - | 2.5 | [59] |
| SCS | Not specified | 44.5 | 30.4 | 12.3 | - | - | 7.5 | [60] |
| SCB | No treated | 40.1 | 23.8 | 23.6 | - | - | 3.5 | [9] |
| SCB | Non-treated | 38.7 | 23 | 16.9 | - | - | - | [61] |
| SCB | Air-dried at NST | 43.1 | 31.1 | 11.4 | - | - | 5.5 | [62] |
| SCB | Not specified | 57.68 | 12.41 | 7.89 | - | - | 2.20 | [54] |
| Biomass matrix | Temp (ºC) | Time (min) | P (bar) |
SF (S0) |
Results | Highlights | Ref. |
|---|---|---|---|---|---|---|---|
| Coffee husks | 210 | 15 | - | 4.41 | %RM: 62.2 C; 54.1 HM; 43.3 Lig; 3.49 (C+HM)/Lig (g/g) |
48.6% EH | [73] |
| Coffee husks | 120 | 60 | 2 | 2.37 | %RM: 28.9 C; 16 HM; 38.9 Lig; electricity production 0.59 kWh kg/CH |
methane: 144.96 NmL CH4 g/COD (yield); | [74] |
| OTP* | 210 | 15 | 20 | 4.41 | 4.23 Glu; 3.72 xmg; 0.55 AR; 0.67 HCOOH; 1.87 HOAc (g/100 g RM) | 144.1 g bEtOH/kg dry raw material |
[75] |
| Reed | 200 | 15 | - | 4.12 | %DM: 21.5 (non-pretreated); 93.8 DM (pretreated) 43.4 C; 0.1 HM; 15.2 Lig | 89% methane (yield) | [46] |
| Hornbeam wood* | 190 | - | 28 | 4.08 | %DM: 32.1 glucan; 16 xmg; 25.4 Lig; 7 TS; 13 EL; 98.4 Glu and 64.6 FS | 251 L bEtOH/ton of DM | [76] |
| Sorghum | - | 5 | 15 | - | bEtOH yield: 20.5 g/100g; reducing sugar yield: 49.6 mg/g | 43 g residues | [44] |
| Corn | 200 | 2 | - | - | 90.3% bEtOH; %RM: 60 HM | 0.5% of H2SO4 was used | [77] |
| Corncob | - | 5 | 10 | - | %Conversion: 83.4 sugars; 90 glucan; 41 xylan |
Interactions of recalcitrant factors | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




