Submitted:
06 September 2023
Posted:
08 September 2023
You are already at the latest version
Abstract
Keywords:
INTRODUCTION
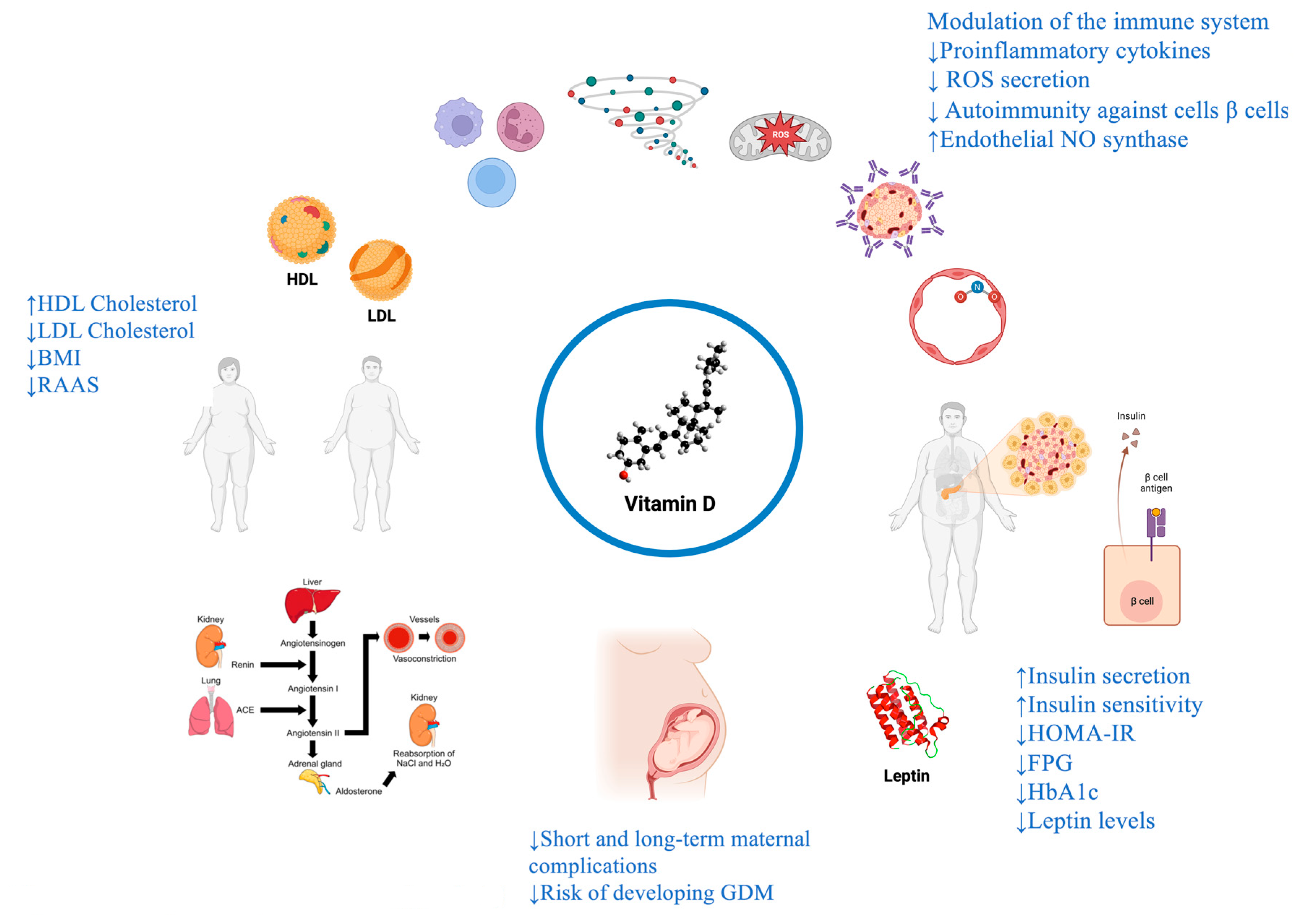
1. VITAMIN D METABOLISM
2. VITAMIN D AND INSULIN RESISTANCE
2.1. Vitamin D, Insulin-resistance and molecular mechanisms
2.2. Studies and research
3. VITAMIN D AND TYPE 2 DIABETES MELLITUS (T2DM)
3.1. Vitamin D, T2DM and molecular mechanisms
3.2. Studies and research
4. VITAMIN D AND TYPE 1 DIABETES MELLITUS (T1DM)
4.1. Vitamin D, T1DM and molecular mechanisms
4.2. Studies and research
5. VITAMIN D AND GESTATIONAL DIABETES MELLITUS (GDM)
5.1. Pathophysiology of vitamin D levels in pregnancy
5.2. Vitamin D, GDM and molecular mechanisms
5.3. Studies and research
6. VITAMIN D, METABOLIC SYNDROME (MetS) AND CARDIOVASCULAR DISEASE (CVD)
6.1. MetS and CVD: burden of the problem
6.2. Vitamin D, MetS and CVD and molecular mechanisms
6.3. Studies and research
DISCUSSION AND CONCLUSIONS
References
- Dattola, A.; Silvestri, M.; Bennardo, L.; Passante, M.; Scali, E.; Patruno, C.; Nisticò, S.P. Role of Vitamins in Skin Health: A Systematic Review. Curr Nutr Rep 2020, 9, 226–235. [CrossRef]
- Umar, M.; Sastry, K.S.; Chouchane, A.I. Role of Vitamin D Beyond the Skeletal Function: A Review of the Molecular and Clinical Studies. Int J Mol Sci 2018, 19, 1618. [CrossRef]
- Maddaloni, E.; Cavallari, I.; Napoli, N.; Conte, C. Vitamin D and Diabetes Mellitus. Front Horm Res 2018, 50, 161–176. [CrossRef]
- Mitri, J.; Muraru, M.D.; Pittas, A.G. Vitamin D and Type 2 Diabetes: A Systematic Review. Eur J Clin Nutr 2011, 65, 1005–1015. [CrossRef]
- Targher, G.; Bertolini, L.; Scala, L.; Cigolini, M.; Zenari, L.; Falezza, G.; Arcaro, G. Associations between Serum 25-Hydroxyvitamin D3 Concentrations and Liver Histology in Patients with Non-Alcoholic Fatty Liver Disease. Nutr Metab Cardiovasc Dis 2007, 17, 517–524. [CrossRef]
- Chen, X.; Zhou, M.; Yan, H.; Chen, J.; Wang, Y.; Mo, X. Association of Serum Total 25-Hydroxy-Vitamin D Concentration and Risk of All-Cause, Cardiovascular and Malignancies-Specific Mortality in Patients with Hyperlipidemia in the United States. Front Nutr 2022, 9, 971720. [CrossRef]
- Wimalawansa, S.J. Non-Musculoskeletal Benefits of Vitamin D. J Steroid Biochem Mol Biol 2018, 175, 60–81. [CrossRef]
- Teleni, L.; Baker, J.; Koczwara, B.; Kimlin, M.G.; Walpole, E.; Tsai, K.; Isenring, E.A. Clinical Outcomes of Vitamin D Deficiency and Supplementation in Cancer Patients. Nutr Rev 2013, 71, 611–621. [CrossRef]
- Khademi, Z.; Hamedi-Shahraki, S.; Amirkhizi, F. Vitamin D Insufficiency Is Associated with Inflammation and Deregulation of Adipokines in Patients with Metabolic Syndrome. BMC Endocrine Disorders 2022, 22, 223. [CrossRef]
- Argano, C.; Mallaci Bocchio, R.; Natoli, G.; Scibetta, S.; Lo Monaco, M.; Corrao, S. Protective Effect of Vitamin D Supplementation on COVID-19-Related Intensive Care Hospitalization and Mortality: Definitive Evidence from Meta-Analysis and Trial Sequential Analysis. Pharmaceuticals (Basel) 2023, 16, 130. [CrossRef]
- Corrao, S.; Mallaci Bocchio, R.; Lo Monaco, M.; Natoli, G.; Cavezzi, A.; Troiani, E.; Argano, C. Does Evidence Exist to Blunt Inflammatory Response by Nutraceutical Supplementation during COVID-19 Pandemic? An Overview of Systematic Reviews of Vitamin D, Vitamin C, Melatonin, and Zinc. Nutrients 2021, 13, 1261. [CrossRef]
- Argano, C.; Mallaci Bocchio, R.; Lo Monaco, M.; Scibetta, S.; Natoli, G.; Cavezzi, A.; Troiani, E.; Corrao, S. An Overview of Systematic Reviews of the Role of Vitamin D on Inflammation in Patients with Diabetes and the Potentiality of Its Application on Diabetic Patients with COVID-19. Int J Mol Sci 2022, 23, 2873. [CrossRef]
- Barragan, M.; Good, M.; Kolls, J.K. Regulation of Dendritic Cell Function by Vitamin D. Nutrients 2015, 7, 8127–8151. [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr Hypertens Rep 2018, 20, 12. [CrossRef]
- Argano, C.; Natoli, G.; Mularo, S.; Nobili, A.; Monaco, M.L.; Mannucci, P.M.; Perticone, F.; Pietrangelo, A.; Corrao, S. Impact of Diabetes Mellitus and Its Comorbidities on Elderly Patients Hospitalized in Internal Medicine Wards: Data from the RePoSi Registry. Healthcare (Basel) 2022, 10, 86. [CrossRef]
- Diabetes Is “a Pandemic of Unprecedented Magnitude” Now Affecting One in 10 Adults Worldwide. Diabetes Research and Clinical Practice 2021, 181. [CrossRef]
- Dong, J.-Y.; Zhang, W.; Chen, J.J.; Zhang, Z.-L.; Han, S.-F.; Qin, L.-Q. Vitamin D Intake and Risk of Type 1 Diabetes: A Meta-Analysis of Observational Studies. Nutrients 2013, 5, 3551–3562. [CrossRef]
- Littorin, B.; Blom, P.; Schölin, A.; Arnqvist, H.J.; Blohmé, G.; Bolinder, J.; Ekbom-Schnell, A.; Eriksson, J.W.; Gudbjörnsdottir, S.; Nyström, L.; et al. Lower Levels of Plasma 25-Hydroxyvitamin D among Young Adults at Diagnosis of Autoimmune Type 1 Diabetes Compared with Control Subjects: Results from the Nationwide Diabetes Incidence Study in Sweden (DISS). Diabetologia 2006, 49, 2847–2852. [CrossRef]
- Song, Y.; Wang, L.; Pittas, A.G.; Del Gobbo, L.C.; Zhang, C.; Manson, J.E.; Hu, F.B. Blood 25-Hydroxy Vitamin D Levels and Incident Type 2 Diabetes: A Meta-Analysis of Prospective Studies. Diabetes Care 2013, 36, 1422–1428. [CrossRef]
- Afzal, S.; Bojesen, S.E.; Nordestgaard, B.G. Low 25-Hydroxyvitamin D and Risk of Type 2 Diabetes: A Prospective Cohort Study and Meta-analysis. Clin Chem 2013, 59, 381–391. [CrossRef]
- Lee, C.J.; Iyer, G.; Liu, Y.; Kalyani, R.R.; Bamba, N.; Ligon, C.B.; Varma, S.; Mathioudakis, N. The Effect of Vitamin D Supplementation on Glucose Metabolism in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Intervention Studies. J Diabetes Complications 2017, 31, 1115–1126. [CrossRef]
- Sharma, S.; Biswal, N.; Bethou, A.; Rajappa, M.; Kumar, S.; Vinayagam, V. Does Vitamin D Supplementation Improve Glycaemic Control In Children With Type 1 Diabetes Mellitus? - A Randomized Controlled Trial. J Clin Diagn Res 2017, 11, SC15–SC17. [CrossRef]
- Ataie-Jafari, A.; Loke, S.-C.; Rahmat, A.B.; Larijani, B.; Abbasi, F.; Leow, M.K.S.; Yassin, Z. A Randomized Placebo-Controlled Trial of Alphacalcidol on the Preservation of Beta Cell Function in Children with Recent Onset Type 1 Diabetes. Clin Nutr 2013, 32, 911–917. [CrossRef]
- Contreras-Bolívar, V.; García-Fontana, B.; García-Fontana, C.; Muñoz-Torres, M. Mechanisms Involved in the Relationship between Vitamin D and Insulin Resistance: Impact on Clinical Practice. Nutrients 2021, 13, 3491. [CrossRef]
- Gysemans, C.A.; Cardozo, A.K.; Callewaert, H.; Giulietti, A.; Hulshagen, L.; Bouillon, R.; Eizirik, D.L.; Mathieu, C. 1,25-Dihydroxyvitamin D3 Modulates Expression of Chemokines and Cytokines in Pancreatic Islets: Implications for Prevention of Diabetes in Nonobese Diabetic Mice. Endocrinology 2005, 146, 1956–1964. [CrossRef]
- Park, S.; Kim, D.S.; Kang, S. Vitamin D Deficiency Impairs Glucose-Stimulated Insulin Secretion and Increases Insulin Resistance by Reducing PPAR-γ Expression in Nonobese Type 2 Diabetic Rats. The Journal of Nutritional Biochemistry 2016, 27, 257–265. [CrossRef]
- Melguizo-Rodríguez, L.; Costela-Ruiz, V.J.; García-Recio, E.; De Luna-Bertos, E.; Ruiz, C.; Illescas-Montes, R. Role of Vitamin D in the Metabolic Syndrome. Nutrients 2021, 13, 830. [CrossRef]
- Mancuso, P.; Rahman, A.; Hershey, S.D.; Dandu, L.; Nibbelink, K.A.; Simpson, R.U. 1,25-Dihydroxyvitamin-D3 Treatment Reduces Cardiac Hypertrophy and Left Ventricular Diameter in Spontaneously Hypertensive Heart Failure-Prone (Cp/+) Rats Independent of Changes in Serum Leptin. J Cardiovasc Pharmacol 2008, 51, 559–564. [CrossRef]
- Pieńkowska, A.; Janicka, J.; Duda, M.; Dzwonnik, K.; Lip, K.; Mędza, A.; Szlagatys-Sidorkiewicz, A.; Brzeziński, M. Controversial Impact of Vitamin D Supplementation on Reducing Insulin Resistance and Prevention of Type 2 Diabetes in Patients with Prediabetes: A Systematic Review. Nutrients 2023, 15, 983. [CrossRef]
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium Dietary Reference Intakes for Calcium and Vitamin D; Ross, A.C., Taylor, C.L., Yaktine, A.L., Del Valle, H.B., Eds.; The National Academies Collection: Reports funded by National Institutes of Health; National Academies Press (US): Washington (DC), 2011;
- Haussler, M.R.; Haussler, C.A.; Jurutka, P.W.; Thompson, P.D.; Hsieh, J.C.; Remus, L.S.; Selznick, S.H.; Whitfield, G.K. The Vitamin D Hormone and Its Nuclear Receptor: Molecular Actions and Disease States. J Endocrinol 1997, 154 Suppl, S57-73.
- Adams, J.S.; Rafison, B.; Witzel, S.; Reyes, R.E.; Shieh, A.; Chun, R.; Zavala, K.; Hewison, M.; Liu, P.T. Regulation of the Extrarenal CYP27B1-Hydroxylase. J Steroid Biochem Mol Biol 2014, 144PA, 22–27. [CrossRef]
- Klopot, A.; Hance, K.W.; Peleg, S.; Barsony, J.; Fleet, J.C. Nucleo-Cytoplasmic Cycling of the Vitamin D Receptor in the Enterocyte-Like Cell Line, Caco-2. J Cell Biochem 2007, 100, 617–628. [CrossRef]
- Charoenngam, N.; Shirvani, A.; Holick, M.F. Vitamin D for Skeletal and Non-Skeletal Health: What We Should Know. J Clin Orthop Trauma 2019, 10, 1082–1093. [CrossRef]
- Battault, S.; Whiting, S.J.; Peltier, S.L.; Sadrin, S.; Gerber, G.; Maixent, J.M. Vitamin D Metabolism, Functions and Needs: From Science to Health Claims. Eur J Nutr 2013, 52, 429–441. [CrossRef]
- Chagas, C.E.A.; Borges, M.C.; Martini, L.A.; Rogero, M.M. Focus on Vitamin D, Inflammation and Type 2 Diabetes. Nutrients 2012, 4, 52–67. [CrossRef]
- Hewison, M. An Update on Vitamin D and Human Immunity. Clin Endocrinol (Oxf) 2012, 76, 315–325. [CrossRef]
- Selvaraj, P.; Harishankar, M.; Afsal, K. Vitamin D: Immuno-Modulation and Tuberculosis Treatment. Can J Physiol Pharmacol 2015, 93, 377–384. [CrossRef]
- Wang, S.; Cai, B.; Han, X.; Gao, Y.; Zhang, X.; Wang, R.; Zhang, Y.; Chen, Q. Vitamin D Supplementation for Non-alcoholic Fatty Liver Disease in Type 2 Diabetes Mellitus: A Protocol for a Systematic Review and Meta-Analysis. Medicine (Baltimore) 2020, 99, e20148. [CrossRef]
- Han, F.; Lv, Y.; Gong, L.; Liu, H.; Wan, Z.; Liu, L. VDR Gene Variation and Insulin Resistance Related Diseases. Lipids Health Dis 2017, 16, 157. [CrossRef]
- Sindhughosa, D.A.; Wibawa, I.D.N.; Mariadi, I.K.; Somayana, G. Additional Treatment of Vitamin D for Improvement of Insulin Resistance in Non-Alcoholic Fatty Liver Disease Patients: A Systematic Review and Meta-Analysis. Sci Rep 2022, 12, 7716. [CrossRef]
- Rafiq, S.; Jeppesen, P.B. Vitamin D Deficiency Is Inversely Associated with Homeostatic Model Assessment of Insulin Resistance. Nutrients 2021, 13, 4358. [CrossRef]
- Mo, M.; Shao, B.; Xin, X.; Luo, W.; Si, S.; Jiang, W.; Wang, S.; Shen, Y.; Wu, J.; Yu, Y. The Association of Gene Variants in the Vitamin D Metabolic Pathway and Its Interaction with Vitamin D on Gestational Diabetes Mellitus: A Prospective Cohort Study. Nutrients 2021, 13, 4220. [CrossRef]
- Wang, M.; Chen, Z.; Hu, Y.; Wang, Y.; Wu, Y.; Lian, F.; Li, H.; Yang, J.; Xu, X. The Effects of Vitamin D Supplementation on Glycemic Control and Maternal-Neonatal Outcomes in Women with Established Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Clin Nutr 2021, 40, 3148–3157. [CrossRef]
- Huang, S.; Fu, J.; Zhao, R.; Wang, B.; Zhang, M.; Li, L.; Shi, C. The Effect of Combined Supplementation with Vitamin D and Omega-3 Fatty Acids on Blood Glucose and Blood Lipid Levels in Patients with Gestational Diabetes. Ann Palliat Med 2021, 10, 5652–5658. [CrossRef]
- Asbaghi, O.; Khosroshahi, M.Z.; Kashkooli, S.; Abbasnezhad, A. Effect of Calcium-Vitamin D Co-Supplementation on Insulin, Insulin Sensitivity, and Glycemia: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Horm Metab Res 2019, 51, 288–295. [CrossRef]
- Muoio, D.M.; Newgard, C.B. Mechanisms of Disease:Molecular and Metabolic Mechanisms of Insulin Resistance and Beta-Cell Failure in Type 2 Diabetes. Nat Rev Mol Cell Biol 2008, 9, 193–205. [CrossRef]
- Lotfy, M.; Adeghate, J.; Kalasz, H.; Singh, J.; Adeghate, E. Chronic Complications of Diabetes Mellitus: A Mini Review. Curr Diabetes Rev 2017, 13, 3–10. [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global Aetiology and Epidemiology of Type 2 Diabetes Mellitus and Its Complications. Nat Rev Endocrinol 2018, 14, 88–98. [CrossRef]
- Rooney, M.R.; Fang, M.; Ogurtsova, K.; Ozkan, B.; Echouffo-Tcheugui, J.B.; Boyko, E.J.; Magliano, D.J.; Selvin, E. Global Prevalence of Prediabetes. Diabetes Care 2023, 46, 1388–1394. [CrossRef]
- Blaak, E.E.; Antoine, J.-M.; Benton, D.; Björck, I.; Bozzetto, L.; Brouns, F.; Diamant, M.; Dye, L.; Hulshof, T.; Holst, J.J.; et al. Impact of Postprandial Glycaemia on Health and Prevention of Disease. Obes Rev 2012, 13, 923–984. [CrossRef]
- Zakharova, I.; Klimov, L.; Kuryaninova, V.; Nikitina, I.; Malyavskaya, S.; Dolbnya, S.; Kasyanova, A.; Atanesyan, R.; Stoyan, M.; Todieva, A.; et al. Vitamin D Insufficiency in Overweight and Obese Children and Adolescents. Front Endocrinol (Lausanne) 2019, 10, 103. [CrossRef]
- Kabadi, S.M.; Lee, B.K.; Liu, L. Joint Effects of Obesity and Vitamin D Insufficiency on Insulin Resistance and Type 2 Diabetes. Diabetes Care 2012, 35, 2048–2054. [CrossRef]
- Gagnon, C.; Lu, Z.X.; Magliano, D.J.; Dunstan, D.W.; Shaw, J.E.; Zimmet, P.Z.; Sikaris, K.; Grantham, N.; Ebeling, P.R.; Daly, R.M. Serum 25-Hydroxyvitamin D, Calcium Intake, and Risk of Type 2 Diabetes after 5 Years: Results from a National, Population-Based Prospective Study (the Australian Diabetes, Obesity and Lifestyle Study). Diabetes Care 2011, 34, 1133–1138. [CrossRef]
- Mirhosseini, N.; Vatanparast, H.; Mazidi, M.; Kimball, S.M. Vitamin D Supplementation, Glycemic Control, and Insulin Resistance in Prediabetics: A Meta-Analysis. J Endocr Soc 2018, 2, 687–709. [CrossRef]
- Borissova, A.M.; Tankova, T.; Kirilov, G.; Dakovska, L.; Kovacheva, R. The Effect of Vitamin D3 on Insulin Secretion and Peripheral Insulin Sensitivity in Type 2 Diabetic Patients. Int J Clin Pract 2003, 57, 258–261.
- Fadda, G.Z.; Akmal, M.; Lipson, L.G.; Massry, S.G. Direct Effect of Parathyroid Hormone on Insulin Secretion from Pancreatic Islets. Am J Physiol 1990, 258, E975-984. [CrossRef]
- Sherman, M.H.; Yu, R.T.; Engle, D.D.; Ding, N.; Atkins, A.R.; Tiriac, H.; Collisson, E.A.; Connor, F.; Van Dyke, T.; Kozlov, S.; et al. Vitamin D Receptor-Mediated Stromal Reprogramming Suppresses Pancreatitis and Enhances Pancreatic Cancer Therapy. Cell 2014, 159, 80–93. [CrossRef]
- Pittas, A.G.; Lau, J.; Hu, F.B.; Dawson-Hughes, B. The Role of Vitamin D and Calcium in Type 2 Diabetes. A Systematic Review and Meta-Analysis. J Clin Endocrinol Metab 2007, 92, 2017–2029. [CrossRef]
- Cigolini, M.; Iagulli, M.P.; Miconi, V.; Galiotto, M.; Lombardi, S.; Targher, G. Serum 25-Hydroxyvitamin D3 Concentrations and Prevalence of Cardiovascular Disease among Type 2 Diabetic Patients. Diabetes Care 2006, 29, 722–724. [CrossRef]
- Scragg, R.; Sowers, M.; Bell, C.; Third National Health and Nutrition Examination Survey Serum 25-Hydroxyvitamin D, Diabetes, and Ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care 2004, 27, 2813–2818. [CrossRef]
- Di Cesar, D.J.; Ploutz-Snyder, R.; Weinstock, R.S.; Moses, A.M. Vitamin D Deficiency Is More Common in Type 2 than in Type 1 Diabetes. Diabetes Care 2006, 29, 174. [CrossRef]
- Intensive Blood-Glucose Control with Sulphonylureas or Insulin Compared with Conventional Treatment and Risk of Complications in Patients with Type 2 Diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998, 352, 837–853.
- Hu, Z.; Chen, J.; Sun, X.; Wang, L.; Wang, A. Efficacy of Vitamin D Supplementation on Glycemic Control in Type 2 Diabetes Patients: A Meta-Analysis of Interventional Studies. Medicine (Baltimore) 2019, 98, e14970. [CrossRef]
- Mitri, J.; Dawson-Hughes, B.; Hu, F.B.; Pittas, A.G. Effects of Vitamin D and Calcium Supplementation on Pancreatic β Cell Function, Insulin Sensitivity, and Glycemia in Adults at High Risk of Diabetes: The Calcium and Vitamin D for Diabetes Mellitus (CaDDM) Randomized Controlled Trial. Am J Clin Nutr 2011, 94, 486–494. [CrossRef]
- Nazarian, S.; St Peter, J.V.; Boston, R.C.; Jones, S.A.; Mariash, C.N. Vitamin D3 Supplementation Improves Insulin Sensitivity in Subjects with Impaired Fasting Glucose. Transl Res 2011, 158, 276–281. [CrossRef]
- Pramono, A.; Jocken, J.W.E.; Blaak, E.E. Vitamin D Deficiency in the Aetiology of Obesity-Related Insulin Resistance. Diabetes Metab Res Rev 2019, 35, e3146. [CrossRef]
- Alvarez, J.A.; Ashraf, A. Role of Vitamin D in Insulin Secretion and Insulin Sensitivity for Glucose Homeostasis. Int J Endocrinol 2010, 2010, 351385. [CrossRef]
- Larrick, B.M.; Kim, K.-H.; Donkin, S.S.; Teegarden, D. 1,25-Dihydroxyvitamin D Regulates Lipid Metabolism and Glucose Utilization in Differentiated 3T3-L1 Adipocytes. Nutr Res 2018, 58, 72–83. [CrossRef]
- Karkeni, E.; Bonnet, L.; Marcotorchino, J.; Tourniaire, F.; Astier, J.; Ye, J.; Landrier, J.-F. Vitamin D Limits Inflammation-Linked MicroRNA Expression in Adipocytes in Vitro and in Vivo: A New Mechanism for the Regulation of Inflammation by Vitamin D. Epigenetics 2018, 13, 156–162. [CrossRef]
- Altieri, B.; Grant, W.B.; Della Casa, S.; Orio, F.; Pontecorvi, A.; Colao, A.; Sarno, G.; Muscogiuri, G. Vitamin D and Pancreas: The Role of Sunshine Vitamin in the Pathogenesis of Diabetes Mellitus and Pancreatic Cancer. Crit Rev Food Sci Nutr 2017, 57, 3472–3488. [CrossRef]
- Cade, C.; Norman, A.W. Vitamin D3 Improves Impaired Glucose Tolerance and Insulin Secretion in the Vitamin D-Deficient Rat in Vivo. Endocrinology 1986, 119, 84–90. [CrossRef]
- Zeitz, U.; Weber, K.; Soegiarto, D.W.; Wolf, E.; Balling, R.; Erben, R.G. Impaired Insulin Secretory Capacity in Mice Lacking a Functional Vitamin D Receptor. FASEB J 2003, 17, 509–511. [CrossRef]
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and Human Health: Lessons from Vitamin D Receptor Null Mice. Endocr Rev 2008, 29, 726–776. [CrossRef]
- Maestro, B.; Campión, J.; Dávila, N.; Calle, C. Stimulation by 1,25-Dihydroxyvitamin D3 of Insulin Receptor Expression and Insulin Responsiveness for Glucose Transport in U-937 Human Promonocytic Cells. Endocr J 2000, 47, 383–391. [CrossRef]
- Maestro, B.; Molero, S.; Bajo, S.; Dávila, N.; Calle, C. Transcriptional Activation of the Human Insulin Receptor Gene by 1,25-Dihydroxyvitamin D(3). Cell Biochem Funct 2002, 20, 227–232. [CrossRef]
- Pittas, A.G.; Harris, S.S.; Stark, P.C.; Dawson-Hughes, B. The Effects of Calcium and Vitamin D Supplementation on Blood Glucose and Markers of Inflammation in Nondiabetic Adults. Diabetes Care 2007, 30, 980–986. [CrossRef]
- Bland, R.; Markovic, D.; Hills, C.E.; Hughes, S.V.; Chan, S.L.F.; Squires, P.E.; Hewison, M. Expression of 25-Hydroxyvitamin D3-1alpha-Hydroxylase in Pancreatic Islets. J Steroid Biochem Mol Biol 2004, 89–90, 121–125. [CrossRef]
- Reusch, J.E.; Begum, N.; Sussman, K.E.; Draznin, B. Regulation of GLUT-4 Phosphorylation by Intracellular Calcium in Adipocytes. Endocrinology 1991, 129, 3269–3273. [CrossRef]
- Ryan, Z.C.; Craig, T.A.; Folmes, C.D.; Wang, X.; Lanza, I.R.; Schaible, N.S.; Salisbury, J.L.; Nair, K.S.; Terzic, A.; Sieck, G.C.; et al. 1α,25-Dihydroxyvitamin D3 Regulates Mitochondrial Oxygen Consumption and Dynamics in Human Skeletal Muscle Cells. J Biol Chem 2016, 291, 1514–1528. [CrossRef]
- Baron, A.D.; Brechtel, G.; Wallace, P.; Edelman, S.V. Rates and Tissue Sites of Non-Insulin- and Insulin-Mediated Glucose Uptake in Humans. Am J Physiol 1988, 255, E769-774. [CrossRef]
- DeFronzo, R.A.; Jacot, E.; Jequier, E.; Maeder, E.; Wahren, J.; Felber, J.P. The Effect of Insulin on the Disposal of Intravenous Glucose. Results from Indirect Calorimetry and Hepatic and Femoral Venous Catheterization. Diabetes 1981, 30, 1000–1007. [CrossRef]
- Jefferson, G.E.; Schnell, D.M.; Thomas, D.T.; Bollinger, L.M. Calcitriol Concomitantly Enhances Insulin Sensitivity and Alters Myocellular Lipid Partitioning in High Fat-Treated Skeletal Muscle Cells. J Physiol Biochem 2017, 73, 613–621. [CrossRef]
- Krul-Poel, Y.H.M.; Ter Wee, M.M.; Lips, P.; Simsek, S. MANAGEMENT OF ENDOCRINE DISEASE: The Effect of Vitamin D Supplementation on Glycaemic Control in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Eur J Endocrinol 2017, 176, R1–R14. [CrossRef]
- Earthman, C.P.; Beckman, L.M.; Masodkar, K.; Sibley, S.D. The Link between Obesity and Low Circulating 25-Hydroxyvitamin D Concentrations: Considerations and Implications. Int J Obes (Lond) 2012, 36, 387–396. [CrossRef]
- Bajaj, S.; Singh, R.P.; Dwivedi, N.C.; Singh, K.; Gupta, A.; Mathur, M. Vitamin D Levels and Microvascular Complications in Type 2 Diabetes. Indian J Endocrinol Metab 2014, 18, 537–541. [CrossRef]
- Zhao, J.; Dong, J.; Wang, H.; Shang, H.; Zhang, D.; Liao, L. Efficacy and Safety of Vitamin D3 in Patients with Diabetic Nephropathy: A Meta-Analysis of Randomized Controlled Trials. Chin Med J (Engl) 2014, 127, 2837–2843.
- Shehab, D.; Al-Jarallah, K.; Mojiminiyi, O.A.; Al Mohamedy, H.; Abdella, N.A. Does Vitamin D Deficiency Play a Role in Peripheral Neuropathy in Type 2 Diabetes? Diabet Med 2012, 29, 43–49. [CrossRef]
- Assy, M.H.; Draz, N.A.; Fathy, S.E.; Hamed, M.G. Impact of Vitamin D Level in Diabetic People with Peripheral Neuropathy. The Egyptian Journal of Neurology, Psychiatry and Neurosurgery 2021, 57, 117. [CrossRef]
- Al-Shoumer, K.A.; Al-Essa, T.M. Is There a Relationship between Vitamin D with Insulin Resistance and Diabetes Mellitus? World J Diabetes 2015, 6, 1057–1064. [CrossRef]
- Mohd Saffian, S.; Jamil, N.A.; Mohd Tahir, N.A.; Hatah, E. Vitamin D Insufficiency Is High in Malaysia: A Systematic Review and Meta-Analysis of Studies on Vitamin D Status in Malaysia. Front Nutr 2022, 9, 1050745. [CrossRef]
- Ding, Y.-H.; Wei, T.-M.; Qian, L.-Y.; Ma, Y.; Lao, D.-B.; Yao, B.; Pang, J. Association between Serum 25-Hydroxyvitamin D and Carotid Atherosclerotic Plaque in Chinese Type 2 Diabetic Patients. Medicine (Baltimore) 2017, 96, e6445. [CrossRef]
- Paschou, S.A.; Papadopoulou-Marketou, N.; Chrousos, G.P.; Kanaka-Gantenbein, C. On Type 1 Diabetes Mellitus Pathogenesis. Endocr Connect 2018, 7, R38–R46. [CrossRef]
- Soltesz, G.; Patterson, C.C.; Dahlquist, G.; EURODIAB Study Group Worldwide Childhood Type 1 Diabetes Incidence--What Can We Learn from Epidemiology? Pediatr Diabetes 2007, 8 Suppl 6, 6–14. [CrossRef]
- Cernea, S.; Dobreanu, M.; Raz, I. Prevention of Type 1 Diabetes: Today and Tomorrow. Diabetes Metab Res Rev 2010, 26, 602–605. [CrossRef]
- Huber, A.; Menconi, F.; Corathers, S.; Jacobson, E.M.; Tomer, Y. Joint Genetic Susceptibility to Type 1 Diabetes and Autoimmune Thyroiditis: From Epidemiology to Mechanisms. Endocr Rev 2008, 29, 697–725. [CrossRef]
- Mathieu, C.; Badenhoop, K. Vitamin D and Type 1 Diabetes Mellitus: State of the Art. Trends Endocrinol Metab 2005, 16, 261–266. [CrossRef]
- Takiishi, T.; Gysemans, C.; Bouillon, R.; Mathieu, C. Vitamin D and Diabetes. Endocrinol Metab Clin North Am 2010, 39, 419–446, table of contents. [CrossRef]
- Storm, T.L.; Sørensen, O.H.; Lund, B.; Lund, B.; Christiansen, J.S.; Andersen, A.R.; Lumholtz, I.B.; Parving, H.H. Vitamin D Metabolism in Insulin-Dependent Diabetes Mellitus. Metab Bone Dis Relat Res 1983, 5, 107–110. [CrossRef]
- Luong, K. vinh quoc; Nguyen, L.T.H.; Nguyen, D.N.P. The Role of Vitamin D in Protecting Type 1 Diabetes Mellitus. Diabetes Metab Res Rev 2005, 21, 338–346. [CrossRef]
- Danescu, L.G.; Levy, S.; Levy, J. Vitamin D and Diabetes Mellitus. Endocrine 2009, 35, 11–17. [CrossRef]
- Yang, C.-Y.; Leung, P.S.C.; Adamopoulos, I.E.; Gershwin, M.E. The Implication of Vitamin D and Autoimmunity: A Comprehensive Review. Clin Rev Allergy Immunol 2013, 45, 217–226. [CrossRef]
- Norris, J.M.; Johnson, R.K.; Stene, L.C. Type 1 Diabetes-Early Life Origins and Changing Epidemiology. Lancet Diabetes Endocrinol 2020, 8, 226–238. [CrossRef]
- Jiang, X.; Kiel, D.P.; Kraft, P. The Genetics of Vitamin D. Bone 2019, 126, 59–77. [CrossRef]
- Holick, M.F. Vitamin D Deficiency. N Engl J Med 2007, 357, 266–281. [CrossRef]
- Bikle, D.D. Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chem Biol 2014, 21, 319–329. [CrossRef]
- Dankers, W.; Colin, E.M.; van Hamburg, J.P.; Lubberts, E. Vitamin D in Autoimmunity: Molecular Mechanisms and Therapeutic Potential. Front Immunol 2016, 7, 697. [CrossRef]
- Metabolic Bone Disease Available online: https://accessmedicine.mhmedical.com/content.aspx?sectionid=166248750&bookid=2178 (accessed on 23 August 2023).
- Penna, G.; Adorini, L. 1 Alpha,25-Dihydroxyvitamin D3 Inhibits Differentiation, Maturation, Activation, and Survival of Dendritic Cells Leading to Impaired Alloreactive T Cell Activation. J Immunol 2000, 164, 2405–2411. [CrossRef]
- Zhang, X.; Zhou, M.; Guo, Y.; Song, Z.; Liu, B. 1,25-Dihydroxyvitamin D₃ Promotes High Glucose-Induced M1 Macrophage Switching to M2 via the VDR-PPARγ Signaling Pathway. Biomed Res Int 2015, 2015, 157834. [CrossRef]
- Unger, W.W.J.; Laban, S.; Kleijwegt, F.S.; van der Slik, A.R.; Roep, B.O. Induction of Treg by Monocyte-Derived DC Modulated by Vitamin D3 or Dexamethasone: Differential Role for PD-L1. Eur J Immunol 2009, 39, 3147–3159. [CrossRef]
- Bock, G.; Prietl, B.; Mader, J.K.; Höller, E.; Wolf, M.; Pilz, S.; Graninger, W.B.; Obermayer-Pietsch, B.M.; Pieber, T.R. The Effect of Vitamin D Supplementation on Peripheral Regulatory T Cells and β Cell Function in Healthy Humans: A Randomized Controlled Trial. Diabetes Metab Res Rev 2011, 27, 942–945. [CrossRef]
- Lemire, J.M.; Adams, J.S.; Sakai, R.; Jordan, S.C. 1 Alpha,25-Dihydroxyvitamin D3 Suppresses Proliferation and Immunoglobulin Production by Normal Human Peripheral Blood Mononuclear Cells. J Clin Invest 1984, 74, 657–661. [CrossRef]
- Casteels, K.M.; Mathieu, C.; Waer, M.; Valckx, D.; Overbergh, L.; Laureys, J.M.; Bouillon, R. Prevention of Type I Diabetes in Nonobese Diabetic Mice by Late Intervention with Nonhypercalcemic Analogs of 1,25-Dihydroxyvitamin D3 in Combination with a Short Induction Course of Cyclosporin A. Endocrinology 1998, 139, 95–102. [CrossRef]
- Mathieu, C.; Waer, M.; Laureys, J.; Rutgeerts, O.; Bouillon, R. Prevention of Autoimmune Diabetes in NOD Mice by 1,25 Dihydroxyvitamin D3. Diabetologia 1994, 37, 552–558. [CrossRef]
- Zella, J.B.; McCary, L.C.; DeLuca, H.F. Oral Administration of 1,25-Dihydroxyvitamin D3 Completely Protects NOD Mice from Insulin-Dependent Diabetes Mellitus. Arch Biochem Biophys 2003, 417, 77–80. [CrossRef]
- Fronczak, C.M.; Barón, A.E.; Chase, H.P.; Ross, C.; Brady, H.L.; Hoffman, M.; Eisenbarth, G.S.; Rewers, M.; Norris, J.M. In Utero Dietary Exposures and Risk of Islet Autoimmunity in Children. Diabetes Care 2003, 26, 3237–3242. [CrossRef]
- Corrao, S.; Colomba, D.; Arnone, S.; Argano, C.; Di Chiara, T.; Scaglione, R.; Licata, G. Improving Efficacy of PubMed Clinical Queries for Retrieving Scientifically Strong Studies on Treatment. J Am Med Inform Assoc 2006, 13, 485–487. [CrossRef]
- Corrao, S.; Colomba, D.; Argano, C.; Calvo, L.; Scaglione, R.; Licata, G. Optimized Search Strategy for Detecting Scientifically Strong Studies on Treatment through PubMed. Intern Emerg Med 2012, 7, 283–287. [CrossRef]
- Hou, Y.; Song, A.; Jin, Y.; Xia, Q.; Song, G.; Xing, X. A Dose–Response Meta-Analysis between Serum Concentration of 25-Hydroxy Vitamin D and Risk of Type 1 Diabetes Mellitus. Eur J Clin Nutr 2021, 75, 1010–1023. [CrossRef]
- Bener, A.; Alsaied, A.; Al-Ali, M.; Al-Kubaisi, A.; Basha, B.; Abraham, A.; Guiter, G.; Mian, M. High Prevalence of Vitamin D Deficiency in Type 1 Diabetes Mellitus and Healthy Children. Acta Diabetol 2009, 46, 183–189. [CrossRef]
- Omar, D.F.; Kamal, M.M.; El-Hefnawy, M.H.; El-Mesallamy, H.O. Serum Vitamin D and Its Upregulated Protein, Thioredoxin Interacting Protein, Are Associated With Beta-Cell Dysfunction in Adult Patients With Type 1 and Type 2 Diabetes. Can J Diabetes 2018, 42, 588–594. [CrossRef]
- ALkharashi, N.A. Estimation of Vitamin D Deficiency Prevalence among Saudi Children in Armed Forces Hospital and Riyadh Care Hospital in Riyadh, Kingdom of Saudi Arabia and Its Relation to Type 1 Diabetes Mellitus. Saudi Med J 2019, 40, 1290–1293. [CrossRef]
- Hyppönen, E.; Läärä, E.; Reunanen, A.; Järvelin, M.R.; Virtanen, S.M. Intake of Vitamin D and Risk of Type 1 Diabetes: A Birth-Cohort Study. Lancet 2001, 358, 1500–1503. [CrossRef]
- Norris, J.M.; Lee, H.-S.; Frederiksen, B.; Erlund, I.; Uusitalo, U.; Yang, J.; Lernmark, Å.; Simell, O.; Toppari, J.; Rewers, M.; et al. Plasma 25-Hydroxyvitamin D Concentration and Risk of Islet Autoimmunity. Diabetes 2018, 67, 146–154. [CrossRef]
- Vitamin D Supplement in Early Childhood and Risk for Type I (Insulin-Dependent) Diabetes Mellitus. The EURODIAB Substudy 2 Study Group. Diabetologia 1999, 42, 51–54. [CrossRef]
- Wei, Z.; Yoshihara, E.; He, N.; Hah, N.; Fan, W.; Pinto, A.F.M.; Huddy, T.; Wang, Y.; Ross, B.; Estepa, G.; et al. Vitamin D Switches BAF Complexes to Protect β Cells. Cell 2018, 173, 1135-1149.e15. [CrossRef]
- Yu, J.; Sharma, P.; Girgis, C.M.; Gunton, J.E. Vitamin D and Beta Cells in Type 1 Diabetes: A Systematic Review. Int J Mol Sci 2022, 23, 14434. [CrossRef]
- Najjar, L.; Sutherland, J.; Zhou, A.; Hyppönen, E. Vitamin D and Type 1 Diabetes Risk: A Systematic Review and Meta-Analysis of Genetic Evidence. Nutrients 2021, 13, 4260. [CrossRef]
- von Websky, K.; Hasan, A.A.; Reichetzeder, C.; Tsuprykov, O.; Hocher, B. Impact of Vitamin D on Pregnancy-Related Disorders and on Offspring Outcome. J Steroid Biochem Mol Biol 2018, 180, 51–64. [CrossRef]
- American Diabetes Association 2. Classification and Diagnosis of Diabetes. Diabetes Care 2017, 40, S11–S24. [CrossRef]
- Jenum, A.K.; Mørkrid, K.; Sletner, L.; Vangen, S.; Torper, J.L.; Nakstad, B.; Voldner, N.; Rognerud-Jensen, O.H.; Berntsen, S.; Mosdøl, A.; et al. Impact of Ethnicity on Gestational Diabetes Identified with the WHO and the Modified International Association of Diabetes and Pregnancy Study Groups Criteria: A Population-Based Cohort Study. Eur J Endocrinol 2012, 166, 317–324. [CrossRef]
- Noctor, E.; Crowe, C.; Carmody, L.A.; Kirwan, B.; O’Dea, A.; Glynn, L.G.; McGuire, B.E.; O’Shea, P.M.; Dunne, F.P. ATLANTIC-DIP: Prevalence of Metabolic Syndrome and Insulin Resistance in Women with Previous Gestational Diabetes Mellitus by International Association of Diabetes in Pregnancy Study Groups Criteria. Acta Diabetol 2015, 52, 153–160. [CrossRef]
- Milajerdi, A.; Tehrani, H.; Haghighatdoost, F.; Larijani, B.; Surkan, P.J.; Azadbakht, L. Associations between Higher Egg Consumption during Pregnancy with Lowered Risks of High Blood Pressure and Gestational Diabetes Mellitus. Int J Vitam Nutr Res 2018, 88, 166–175. [CrossRef]
- Groof, Z.; Garashi, G.; Husain, H.; Owayed, S.; AlBader, S.; Mouhsen, H.; Mohammad, A.; Ziyab, A.H. Prevalence, Risk Factors, and Fetomaternal Outcomes of Gestational Diabetes Mellitus in Kuwait: A Cross-Sectional Study. Journal of Diabetes Research 2019, 2019, e9136250. [CrossRef]
- Verma, A.; Boney, C.M.; Tucker, R.; Vohr, B.R. Insulin Resistance Syndrome in Women with Prior History of Gestational Diabetes Mellitus. J Clin Endocrinol Metab 2002, 87, 3227–3235. [CrossRef]
- Andersson-Hall, U.; Gustavsson, C.; Pedersen, A.; Malmodin, D.; Joelsson, L.; Holmäng, A. Higher Concentrations of BCAAs and 3-HIB Are Associated with Insulin Resistance in the Transition from Gestational Diabetes to Type 2 Diabetes. J Diabetes Res 2018, 2018, 4207067. [CrossRef]
- Nutrition Therapy in Gestational Diabetes Mellitus: Time to Move Forward | Diabetes Care | American Diabetes Association Available online: https://diabetesjournals.org/care/article/41/7/1343/36441/Nutrition-Therapy-in-Gestational-Diabetes-Mellitus (accessed on 23 August 2023).
- Ismail, N.A.; Mohamed Ismail, N.A.; Bador, K.M. Vitamin D in Gestational Diabetes Mellitus and Its Association with Hyperglycaemia, Insulin Sensitivity and Other Factors. J Obstet Gynaecol 2021, 41, 899–903. [CrossRef]
- Magnusdottir, K.S.; Tryggvadottir, E.A.; Magnusdottir, O.K.; Hrolfsdottir, L.; Halldorsson, T.I.; Birgisdottir, B.E.; Hreidarsdottir, I.T.; Hardardottir, H.; Gunnarsdottir, I. Vitamin D Status and Association with Gestational Diabetes Mellitus in a Pregnant Cohort in Iceland. Food & nutrition Research 2021. [CrossRef]
- Agüero-Domenech, N.; Jover, S.; Sarrión, A.; Baranda, J.; Quesada-Rico, J.A.; Pereira-Expósito, A.; Gil-Guillén, V.; Cortés-Castell, E.; García-Teruel, M.J. Vitamin D Deficiency and Gestational Diabetes Mellitus in Relation to Body Mass Index. Nutrients 2021, 14, 102. [CrossRef]
- Khambule, L.; George, J.A. The Role of Inflammation in the Development of GDM and the Use of Markers of Inflammation in GDM Screening. Adv Exp Med Biol 2019, 1134, 217–242. [CrossRef]
- Wolf, M.; Sauk, J.; Shah, A.; Vossen Smirnakis, K.; Jimenez-Kimble, R.; Ecker, J.L.; Thadhani, R. Inflammation and Glucose Intolerance: A Prospective Study of Gestational Diabetes Mellitus. Diabetes Care 2004, 27, 21–27. [CrossRef]
- López-Tinoco, C.; Roca, M.; García-Valero, A.; Murri, M.; Tinahones, F.J.; Segundo, C.; Bartha, J.L.; Aguilar-Diosdado, M. Oxidative Stress and Antioxidant Status in Patients with Late-Onset Gestational Diabetes Mellitus. Acta Diabetol 2013, 50, 201–208. [CrossRef]
- Shang, M.; Zhao, J.; Yang, L.; Lin, L. Oxidative Stress and Antioxidant Status in Women with Gestational Diabetes Mellitus Diagnosed by IADPSG Criteria. Diabetes Res Clin Pract 2015, 109, 404–410. [CrossRef]
- Peuchant, E.; Brun, J.-L.; Rigalleau, V.; Dubourg, L.; Thomas, M.-J.; Daniel, J.-Y.; Leng, J.-J.; Gin, H. Oxidative and Antioxidative Status in Pregnant Women with Either Gestational or Type 1 Diabetes. Clin Biochem 2004, 37, 293–298. [CrossRef]
- Grissa, O.; Atègbo, J.-M.; Yessoufou, A.; Tabka, Z.; Miled, A.; Jerbi, M.; Dramane, K.L.; Moutairou, K.; Prost, J.; Hichami, A.; et al. Antioxidant Status and Circulating Lipids Are Altered in Human Gestational Diabetes and Macrosomia. Transl Res 2007, 150, 164–171. [CrossRef]
- Maged, A.M.; Torky, H.; Fouad, M.A.; GadAllah, S.H.; Waked, N.M.; Gayed, A.S.; Salem, A.K. Role of Antioxidants in Gestational Diabetes Mellitus and Relation to Fetal Outcome: A Randomized Controlled Trial. J Matern Fetal Neonatal Med 2016, 29, 4049–4054. [CrossRef]
- Haidari, F.; Zakerkish, M.; Karandish, M.; Saki, A.; Pooraziz, S. Association between Serum Vitamin D Level and Glycemic and Inflammatory Markers in Non-Obese Patients with Type 2 Diabetes. Iran J Med Sci 2016, 41, 367–373.
- Wang, W.; Zhang, J.; Wang, H.; Wang, X.; Liu, S. Vitamin D Deficiency Enhances Insulin Resistance by Promoting Inflammation in Type 2 Diabetes. Int J Clin Exp Pathol 2019, 12, 1859–1867.
- Berridge, M.J. Vitamin D Deficiency and Diabetes. Biochem J 2017, 474, 1321–1332. [CrossRef]
- Johannesson, B.; Sui, L.; Freytes, D.O.; Creusot, R.J.; Egli, D. Toward Beta Cell Replacement for Diabetes. EMBO J 2015, 34, 841–855. [CrossRef]
- Taheri, E.; Saedisomeolia, A.; Djalali, M.; Qorbani, M.; Madani Civi, M. The Relationship between Serum 25-Hydroxy Vitamin D Concentration and Obesity in Type 2 Diabetic Patients and Healthy Subjects. J Diabetes Metab Disord 2012, 11, 16. [CrossRef]
- Milajerdi, A.; Abbasi, F.; Mousavi, S.M.; Esmaillzadeh, A. Maternal Vitamin D Status and Risk of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Clin Nutr 2021, 40, 2576–2586. [CrossRef]
- Wu, C.; Song, Y.; Wang, X. Vitamin D Supplementation for the Outcomes of Patients with Gestational Diabetes Mellitus and Neonates: A Meta-Analysis and Systematic Review. Int J Clin Pract 2023, 2023, 1907222. [CrossRef]
- Akbari, M.; Moosazaheh, M.; Lankarani, K.B.; Tabrizi, R.; Samimi, M.; Karamali, M.; Jamilian, M.; Kolahdooz, F.; Asemi, Z. The Effects of Vitamin D Supplementation on Glucose Metabolism and Lipid Profiles in Patients with Gestational Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Horm Metab Res 2017, 49, 647–653. [CrossRef]
- Wang, H.; Xia, N.; Yang, Y.; Peng, D.-Q. Influence of Vitamin D Supplementation on Plasma Lipid Profiles: A Meta-Analysis of Randomized Controlled Trials. Lipids Health Dis 2012, 11, 42. [CrossRef]
- Kron-Rodrigues, M.R.; Rudge, M.V.C.; Lima, S.A.M. Supplementation of Vitamin D in the Postdelivery Period of Women with Previous Gestational Diabetes Mellitus: Systematic Review and Meta-Analysis of Randomized Trials. Rev Bras Ginecol Obstet 2021, 43, 699–709. [CrossRef]
- Saha, S.; Saha, S. Changes in Anthropometric and Blood 25-Hydroxyvitamin D Measurements in Antenatal Vitamin Supplemented Gestational Diabetes Mellitus Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Turk Ger Gynecol Assoc 2021, 22, 217–234. [CrossRef]
- Wang, M.M.; Chen, Z.J.; Wang, Y.; Xu, X.R.; Li, H.J.; Yang, J. [Effects of Vitamin D Supplementation on Serum Lipid Profiles and Neonatal Outcomes in Gestational Diabetes Mellitus:a Meta-analysis]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2021, 43, 82–91. [CrossRef]
- Yang, C.; Jing, W.; Ge, S.; Sun, W. Vitamin D Status and Vitamin D Deficiency Risk Factors among Pregnancy of Shanghai in China. BMC Pregnancy and Childbirth 2021, 21, 431. [CrossRef]
- Chatzakis, C.; Sotiriadis, A.; Tsakmaki, E.; Papagianni, M.; Paltoglou, G.; Dinas, K.; Mastorakos, G. The Effect of Dietary Supplements on Oxidative Stress in Pregnant Women with Gestational Diabetes Mellitus: A Network Meta-Analysis. Nutrients 2021, 13, 2284. [CrossRef]
- Wang, L.; Zhang, C.; Song, Y.; Zhang, Z. Serum Vitamin D Deficiency and Risk of Gestational Diabetes Mellitus: A Meta-Analysis. Arch Med Sci 2020, 16, 742–751. [CrossRef]
- Jin, S.; Sha, L.; Dong, J.; Yi, J.; Liu, Y.; Guo, Z.; Hu, B. Effects of Nutritional Strategies on Glucose Homeostasis in Gestational Diabetes Mellitus: A Systematic Review and Network Meta-Analysis. J Diabetes Res 2020, 2020, 6062478. [CrossRef]
- Soheilykhah, S.; Mojibian, M.; Rashidi, M.; Rahimi-Saghand, S.; Jafari, F. Maternal Vitamin D Status in Gestational Diabetes Mellitus. Nutr Clin Pract 2010, 25, 524–527. [CrossRef]
- Vitamin D and Gestational Diabetes Mellitus: A Systematic Review Based on Data Free of Hawthorne Effect - Zhang - 2018 - BJOG: An International Journal of Obstetrics & Gynaecology - Wiley Online Library Available online: https://obgyn.onlinelibrary.wiley.com/doi/abs/10.1111/1471-0528.15060 (accessed on 23 August 2023).
- Vaidya, A.; Williams, J.S. Vitamin D and Insulin Sensitivity: Can Gene Association and Pharmacogenetic Studies of the Vitamin D Receptor Provide Clarity? Metabolism 2012, 61, 759–761. [CrossRef]
- Lu, M.; Xu, Y.; Lv, L.; Zhang, M. Association between Vitamin D Status and the Risk of Gestational Diabetes Mellitus: A Meta-Analysis. Arch Gynecol Obstet 2016, 293, 959–966. [CrossRef]
- Chen, Y.; Zhu, B.; Wu, X.; Li, S.; Tao, F. Association between Maternal Vitamin D Deficiency and Small for Gestational Age: Evidence from a Meta-Analysis of Prospective Cohort Studies. BMJ Open 2017, 7, e016404. [CrossRef]
- Licata, G.; Argano, C.; Di Chiara, T.; Parrinello, G.; Scaglione, R. Obesity: a main factor of metabolic syndrome? Panminerva Med. 2006 Jun;48(2):77-85. PMID: 16953145.
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int J Mol Sci 2022, 23, 786. [CrossRef]
- 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2022 | Diabetes Care | American Diabetes Association Available online: https://diabetesjournals.org/care/article/45/Supplement_1/S17/138925/2-Classification-and-Diagnosis-of-Diabetes (accessed on 23 August 2023).
- Wang, H.; Naghavi, M.; Allen, C.; Barber, R.M.; Bhutta, Z.A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; Coates, M.M.; et al. Global, Regional, and National Life Expectancy, All-Cause Mortality, and Cause-Specific Mortality for 249 Causes of Death, 1980–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. The Lancet 2016, 388, 1459–1544. [CrossRef]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018, 8, 80. [CrossRef]
- Hojs, R.; Ekart, R.; Bevc, S.; Hojs, N. Markers of Inflammation and Oxidative Stress in the Development and Progression of Renal Disease in Diabetic Patients. Nephron 2016, 133, 159–162. [CrossRef]
- Flaim, C.; Kob, M.; Di Pierro, A.M.; Herrmann, M.; Lucchin, L. Effects of a Whey Protein Supplementation on Oxidative Stress, Body Composition and Glucose Metabolism among Overweight People Affected by Diabetes Mellitus or Impaired Fasting Glucose: A Pilot Study. J Nutr Biochem 2017, 50, 95–102. [CrossRef]
- Mannarino, E.; Pirro, M. Molecular Biology of Atherosclerosis. Clin Cases Miner Bone Metab 2008, 5, 57–62.
- Hussin, A.M.; Ashor, A.W.; Schoenmakers, I.; Hill, T.; Mathers, J.C.; Siervo, M. Effects of Vitamin D Supplementation on Endothelial Function: A Systematic Review and Meta-Analysis of Randomised Clinical Trials. Eur J Nutr 2017, 56, 1095–1104. [CrossRef]
- Jacobson, T.A.; Maki, K.C.; Orringer, C.E.; Jones, P.H.; Kris-Etherton, P.; Sikand, G.; La Forge, R.; Daniels, S.R.; Wilson, D.P.; Morris, P.B.; et al. National Lipid Association Recommendations for Patient-Centered Management of Dyslipidemia: Part 2. J Clin Lipidol 2015, 9, S1-122.e1. [CrossRef]
- Cholesterol Treatment Trialists’ (CTT) Collaboration; Fulcher, J.; O’Connell, R.; Voysey, M.; Emberson, J.; Blackwell, L.; Mihaylova, B.; Simes, J.; Collins, R.; Kirby, A.; et al. Efficacy and Safety of LDL-Lowering Therapy among Men and Women: Meta-Analysis of Individual Data from 174,000 Participants in 27 Randomised Trials. Lancet 2015, 385, 1397–1405. [CrossRef]
- Bovolini, A.; Garcia, J.; Andrade, M.A.; Duarte, J.A. Metabolic Syndrome Pathophysiology and Predisposing Factors. Int J Sports Med 2021, 42, 199–214. [CrossRef]
- Castro-Barquero, S.; Ruiz-León, A.M.; Sierra-Pérez, M.; Estruch, R.; Casas, R. Dietary Strategies for Metabolic Syndrome: A Comprehensive Review. Nutrients 2020, 12, 2983. [CrossRef]
- Pan, G.-T.; Guo, J.-F.; Mei, S.-L.; Zhang, M.-X.; Hu, Z.-Y.; Zhong, C.-K.; Zeng, C.-Y.; Liu, X.-H.; Ma, Q.-H.; Li, B.-Y.; et al. Vitamin D Deficiency in Relation to the Risk of Metabolic Syndrome in Middle-Aged and Elderly Patients with Type 2 Diabetes Mellitus. J Nutr Sci Vitaminol (Tokyo) 2016, 62, 213–219. [CrossRef]
- Faraji, S.; Alizadeh, M. Mechanistic Effects of Vitamin D Supplementation on Metabolic Syndrome Components in Patients with or without Vitamin D Deficiency. J Obes Metab Syndr 2020, 29, 270–280. [CrossRef]
- Sepidarkish, M.; Farsi, F.; Akbari-Fakhrabadi, M.; Namazi, N.; Almasi-Hashiani, A.; Maleki Hagiagha, A.; Heshmati, J. The Effect of Vitamin D Supplementation on Oxidative Stress Parameters: A Systematic Review and Meta-Analysis of Clinical Trials. Pharmacol Res 2019, 139, 141–152. [CrossRef]
- Jain, S.K.; Micinski, D. Vitamin D Upregulates Glutamate Cysteine Ligase and Glutathione Reductase, and GSH Formation, and Decreases ROS and MCP-1 and IL-8 Secretion in High-Glucose Exposed U937 Monocytes. Biochem Biophys Res Commun 2013, 437, 7–11. [CrossRef]
- Brandenburg, V.M.; Vervloet, M.G.; Marx, N. The Role of Vitamin D in Cardiovascular Disease: From Present Evidence to Future Perspectives. Atherosclerosis 2012, 225, 253–263. [CrossRef]
- Siti, H.N.; Kamisah, Y.; Kamsiah, J. The Role of Oxidative Stress, Antioxidants and Vascular Inflammation in Cardiovascular Disease (a Review). Vascul Pharmacol 2015, 71, 40–56. [CrossRef]
- Sies, H. Total Antioxidant Capacity: Appraisal of a Concept. J Nutr 2007, 137, 1493–1495. [CrossRef]
- Namazi, N.; Larijani, B.; Azadbakht, L. Alpha-Lipoic Acid Supplement in Obesity Treatment: A Systematic Review and Meta-Analysis of Clinical Trials. Clin Nutr 2018, 37, 419–428. [CrossRef]
- Heshmati, J.; Farsi, F.; Shokri, F.; Rezaeinejad, M.; Almasi-Hashiani, A.; Vesali, S.; Sepidarkish, M. A Systematic Review and Meta-Analysis of the Probiotics and Synbiotics Effects on Oxidative Stress. Journal of Functional Foods 2018, 46, 66–84. [CrossRef]
- Slominski, A.T.; Kim, T.-K.; Hobrath, J.V.; Janjetovic, Z.; Oak, A.S.W.; Postlethwaite, A.; Lin, Z.; Li, W.; Takeda, Y.; Jetten, A.M.; et al. Characterization of a New Pathway That Activates Lumisterol in Vivo to Biologically Active Hydroxylumisterols. Sci Rep 2017, 7, 11434. [CrossRef]
- Mahmood, S.F.; Idiculla, J.; Joshi, R.; Joshi, S.; Kulkarni, S. Vitamin D Supplementation in Adults with Vitamin D Deficiency and Its Effect on Metabolic Syndrome - A Randomized Controlled Study. Int J Vitam Nutr Res 2016, 86, 121–126. [CrossRef]
- Teixeira, J.S.; Bull Ferreira Campos, A.; Cordeiro, A.; Pereira, S.E.; Saboya, C.J.; Ramalho, A. Vitamin D Nutritional Status and Its Relationship with Metabolic Changes in Adolescents and Adults with Severe Obesity. Nutr Hosp 2018, 35, 847–853. [CrossRef]
- Ferreira, P.P.; Cangussu, L.; Bueloni-Dias, F.N.; Orsatti, C.L.; Schmitt, E.B.; Nahas-Neto, J.; Nahas, E. a. P. Vitamin D Supplementation Improves the Metabolic Syndrome Risk Profile in Postmenopausal Women. Climacteric 2020, 23, 24–31. [CrossRef]
- Xu, Y.; Zhou, Y.; Liu, J.; Wang, C.; Qu, Z.; Wei, Z.; Zhou, D. Genetically Increased Circulating 25(OH)D Level Reduces the Risk of Type 2 Diabetes in Subjects with Deficiency of Vitamin D. Medicine (Baltimore) 2020, 99, e23672. [CrossRef]
- Zhu, W.; Heil, D.P. Associations of Vitamin D Status with Markers of Metabolic Health: A Community-Based Study in Shanghai, China. Diabetes Metab Syndr 2018, 12, 727–732. [CrossRef]
- Jafari, T.; Fallah, A.A.; Barani, A. Effects of Vitamin D on Serum Lipid Profile in Patients with Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. Clin Nutr 2016, 35, 1259–1268. [CrossRef]
- Li, X.; Liu, Y.; Zheng, Y.; Wang, P.; Zhang, Y. The Effect of Vitamin D Supplementation on Glycemic Control in Type 2 Diabetes Patients: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 375. [CrossRef]
- Wu, Z.; Wang, T.; Zhu, S.; Li, L. Effects of Vitamin D Supplementation as an Adjuvant Therapy in Coronary Artery Disease Patients. Scand Cardiovasc J 2016, 50, 9–16. [CrossRef]
- Ganji, V.; Sukik, A.; Alaayesh, H.; Rasoulinejad, H.; Shraim, M. Serum Vitamin D Concentrations Are Inversely Related to Prevalence of Metabolic Syndrome in Qatari Women. Biofactors 2020, 46, 180–186. [CrossRef]
- Wang, N.; Wang, C.; Chen, X.; Wan, H.; Chen, Y.; Chen, C.; Han, B.; Lu, Y. Vitamin D, Prediabetes and Type 2 Diabetes: Bidirectional Mendelian Randomization Analysis. Eur J Nutr 2020, 59, 1379–1388. [CrossRef]
- Zheng, J.-S.; Luan, J.; Sofianopoulou, E.; Sharp, S.J.; Day, F.R.; Imamura, F.; Gundersen, T.E.; Lotta, L.A.; Sluijs, I.; Stewart, I.D.; et al. The Association between Circulating 25-Hydroxyvitamin D Metabolites and Type 2 Diabetes in European Populations: A Meta-Analysis and Mendelian Randomisation Analysis. PLoS Med 2020, 17, e1003394. [CrossRef]
- Ostadmohammadi, V.; Milajerdi, A.; Ghayour-Mobarhan, M.; Ferns, G.; Taghizadeh, M.; Badehnoosh, B.; Mirzaei, H.; Asemi, Z. The Effects of Vitamin D Supplementation on Glycemic Control, Lipid Profiles and C-Reactive Protein Among Patients with Cardiovascular Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Curr Pharm Des 2019, 25, 201–210. [CrossRef]
- Qi, K.-J.; Zhao, Z.-T.; Zhang, W.; Yang, F. The Impacts of Vitamin D Supplementation in Adults with Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Frontiers in Pharmacology 2022, 13.
- de Paula, T.P.; Kramer, C.K.; Viana, L.V.; Azevedo, M.J. Effects of Individual Micronutrients on Blood Pressure in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Sci Rep 2017, 7, 40751. [CrossRef]
- McGreevy, C.; Williams, D. New Insights about Vitamin D and Cardiovascular Disease: A Narrative Review. Ann Intern Med 2011, 155, 820–826. [CrossRef]
- Grossman, E. Ambulatory Blood Pressure Monitoring in the Diagnosis and Management of Hypertension. Diabetes Care 2013, 36 Suppl 2, S307-311. [CrossRef]
- Hajhashemy, Z.; Shahdadian, F.; Moslemi, E.; Mirenayat, F.S.; Saneei, P. Serum Vitamin D Levels in Relation to Metabolic Syndrome: A Systematic Review and Dose-Response Meta-Analysis of Epidemiologic Studies. Obes Rev 2021, 22, e13223. [CrossRef]
| AUTHOR/YEAR | DESIGN | DURATION | PARTICIPANTS (I/C) |
DOSE OF VITAMIN D | RESULTS |
|---|---|---|---|---|---|
| VITAMIN D AND INSULIN-RESISTANCE (IR) | |||||
|
Asbaghi et al. 2019 46 |
MT (12 RCTs) | From 6 to 312 weeks | 8946 healthy subjects or patients with overweight/obesity, IFG, prediabetes, GDM, T2DM, PCOS, HIV infection (4395/4551) |
From 200 IU/day Vitamin D3 to 50.000 IU/week Vitamin D3 (with supplementation dose of calcium, that ranged from 500 mg/day to 1000 mg/day) | Reducing effects on FBG, circulating levels of insulin and HOMA-IR |
| Sindhughosa et al. 2022 41 | MT (7 RCTs) | From 10 to 52 weeks | 735 patients with NAFLD (423/312) | From 1.000 IU/day Vitamin D3 to 50.000 IU/week Vitamin D3 | Improvement on IR (marked by decrease of HOMA-IR), decrement in ALT levels |
| Pienkowska et al. 202329 | SR (8 RCTs) | From 12 to 260 weeks | From 66 to 2423 patients with prediabetes | From 1.000 IU/day Vitamin D3 to 88.000 IU/week Vitamin D3 | Only one trial showed improvements in FBG and HOMA-IR |
| VITAMIN D AND TYPE 2 DIABETES MELLITUS (T2DM) | |||||
|
Pittas et al. 2007 59 |
MT (13 Case Control Studies; 15 Cross-sectional studies; 12 RCTs) | N/A | Patients with T2DM or prediabetes | 2.000 IU/day Vitamin D3 or Vitamin D3 700 IU/day with supplementation dose of 500 mg/day calcium citrate |
Vitamin D and calcium insufficiency may negatively influence glycemia, whereas combined supplementation with both nutrients may be beneficial in optimizing glucose metabolism |
| Krul-Poel et al. 2017 84 | MT (23 RCTs) | From 4 to 52 weeks | 1797 patients with T2DM: for the effect on HbA1c 1475 patients (755/720), for the effect on FBG 1180 patients (608/572) | From 1.000 IU/day Vitamin D3 to 45.000 IU/week Vitamina D3 or 11.200 IU/day Vitamin D3 for 2 weeks followed by 5.600 IU/day for 10 weeks or from 100.000 to 300.000 IU Vitamin D3 single dose | Significant effect on FBG in a subgroup of studies (n = 4); no significant effect in change of HbA1c |
| Mirhosseini et al. 2018 55 | MT (28 RCTs) | From 8 to 260 weeks | 3848 healthy subjects or patients with prediabetes and/or overweight or obesity, NAFLD, arterial hypertension, cervical intraepithelial neoplasia, premenopausal and postmenopausal women | From 420 IU/day to 88.880 IU/week Vitamin D3 | Significant reduction in HbA1c, FBG and HOMA-IR |
|
Hu et al. 2019 64 |
MT (19 RCTs) | From 4 to 24 weeks | 1374 patients with T2DM (747/627) |
Up to 50.000 UI/weekly Vitamin D3 or 300.000 UI single injection Vitamin D3 | Significant reduction in HbA1c, IR (marked by decrease of HOMA-IR) and insulin levels in the short-term vitamin D supplementation group |
| VITAMIN D AND TYPE 1 DIABETES MELLITUS (T1DM) | |||||
| Najjar et al. 2021129 | MT (10 studies: 3 Cohort; 5 Case-control; 2 Matched case-control) |
N/A | 39884 patients with T1DM (16370/23514) |
N/A | No large effect of a genetically determined reduction in 25(OH)D concentrations by selected polymorphisms on T1D risk |
|
Hou et al. 2021120 |
MT (16 studies: 12 case-control studies; 1 cross-sectional case-control study; 2 nested case- control study; 1 case-cohort study) |
N/A | 10605 patients with T1DM (3913/6692) | N/A | Results demonstrated a significant inverse association between the 25(OH)D concentration in circulation and the risk of T1DM |
|
Yu et al. 2022 128 |
SR (13 studies: 9 RCTs; 2 Open label case-control; 1 Open label; 1 Cohort ) |
From 4 to 12 weeks | 527 patients with T1DM | The following therapeutic regimens were used: 1,25D 0.25 μg 2nd daily; 25D 2.000 IU daily; 25D to achieve serum 25D > 125 nmol/L; Alfacalcidol 0,25 μg bd 25D; 60.000 IU monthly; Ergocalciferol (D2) 2 m of 50.000 IU/w; 25D 2.000 IU/d; 25D. 3.000 IU/d; Calciferol 2.000 IU/d + etanercept + GAD-alum |
The maintenance of optimal circulating 25D levels may reduce the risk of T1D and that it may have potential for benefits in delaying the development of absolute or near-absolute C-peptide deficiency |
| VITAMIN D AND GESTATIONAL DIABETES MELLITUS (GDM) | |||||
|
Rodrigues et al. 2019 158 |
MT (6 studies RCTs) | From 6 to 24 weeks and a study until delivery | 456 pregnant women with GDM diagnosed in the second or third trimester of pregnancy |
50.000 IU of vitamin D3 every 2 weeks or 1.000 UI daily |
Improves adverse maternal and neonatal outcomes related to GDM |
|
Milajerdi et al. 2021 134 |
MT (29 studies: 18 Cohort; 9 Nested case-control;1 Prospective cross-sectional; 1 Retrospective cohort) |
N/A | 42668 patients with GDM or not | Blood vitamin D levels | The lowest risk of GDM was found among those with a serum vitamin D levels of 40 and 90 nmol/L |
|
Wang et al. 2021 44 |
MT (19 RCTs of these 13 concerned GDM) | From 6 to 12 weeks | 1198 patients with GDM | From 50.000 IU of vitamin D3 2 times/day to 1.200 IU daily |
The results showed that vitamin D supplementation during pregnancy could significantly reduce maternal cesarean section rate, maternal hospitalization rate, and postpartum hemorrhage in women with GDM |
|
Chatzakis et al 2021 162 |
MT (15 studies: 9 Cohort; 6 Nested case- control) |
N/A | 42636 pregnant women (1848/40788) |
Blood vitamin D levels | The result showed that lower levels of serum 25(OH)D were associated with a higher chance of GDM |
|
Wu et al. 2023 155 |
MT (20 studies RCTs) | From 2 to 16 weeks | 1682 pregnant women with GDM diagnosed (837/845) |
From 50.000 IU of vitamin D3 2 times/day to 1.200 IU daily |
Reduce serum LDL-C, TG, and TC levels and increase the serum HDL-C level. Reduce maternal and neonatal hyperbilirubinemia and hospitalization risk. |
| VITAMIN D, METABOLIC SYNDROME (MetS) AND CARDIOVASCULAR DISEASE (CVD) | |||||
|
De Paula TP et al. 2017 205 |
MT (7 RCTs) | From 3 to 52 weeks | 542 patients with T2DM (472/70) | A single dose of vitamin D2 (100.000 IU) or vitamin D3 (100.000 IU or 200.000 IU) | Reduction in BP, especially in systolic BP |
|
Ostadmohammadi et al. 2019 203 |
MT (8 RCTs) | From 8 to 24 weeks | 630 adults with CVD (305/325) | 50.000 IU/week Vitamin D3 or 50.000 IU every two weeks or 300.000 IU single dose |
Improving glycemic control, HDL-C and CRP levels; it did not affect TG, TC and LDL-C levels |
|
Hajhashemy Z et al. 2021208 |
Dose–response MT (43 epidemiological studies: 38 cross-sectional 1 nested case control, and 4 cohorts studies) |
N/A | 309.206 adults with o without MetS | Blood Vitamin D levels in adults | Inverse association between serum vitamin D concentrations and risk of MetS |
|
Qi KJ et al. 2022 204 |
MT (13 RCTs) | From 8 to 24 weeks | 1.076 adults with MetS (530/546) | From 1.000 IU/day Vitamin D3 to 50.000 IU/week | Decreased BP, FPG, HOMA-IR and CRP levels; it did not affect HDL-C, LDL-C, TC, and TG levels |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




