Submitted:
07 September 2023
Posted:
11 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
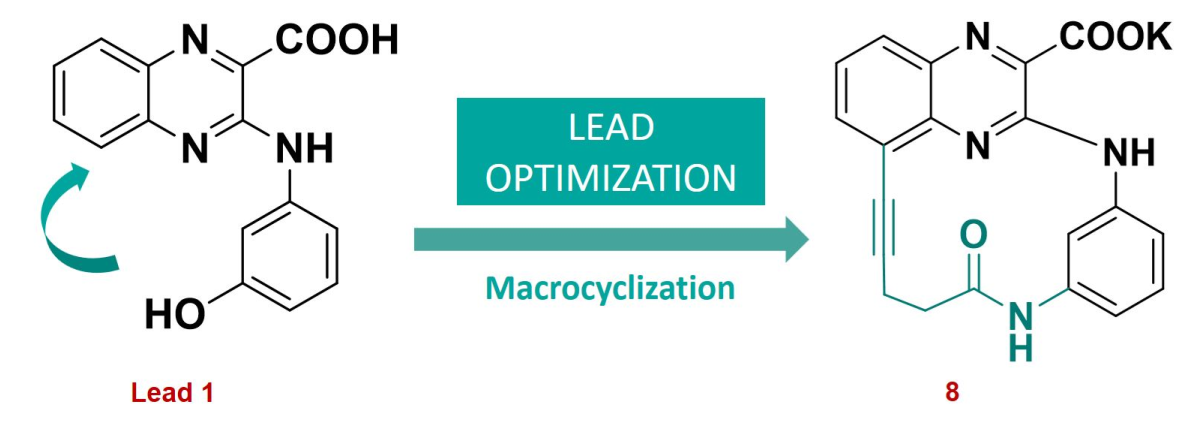
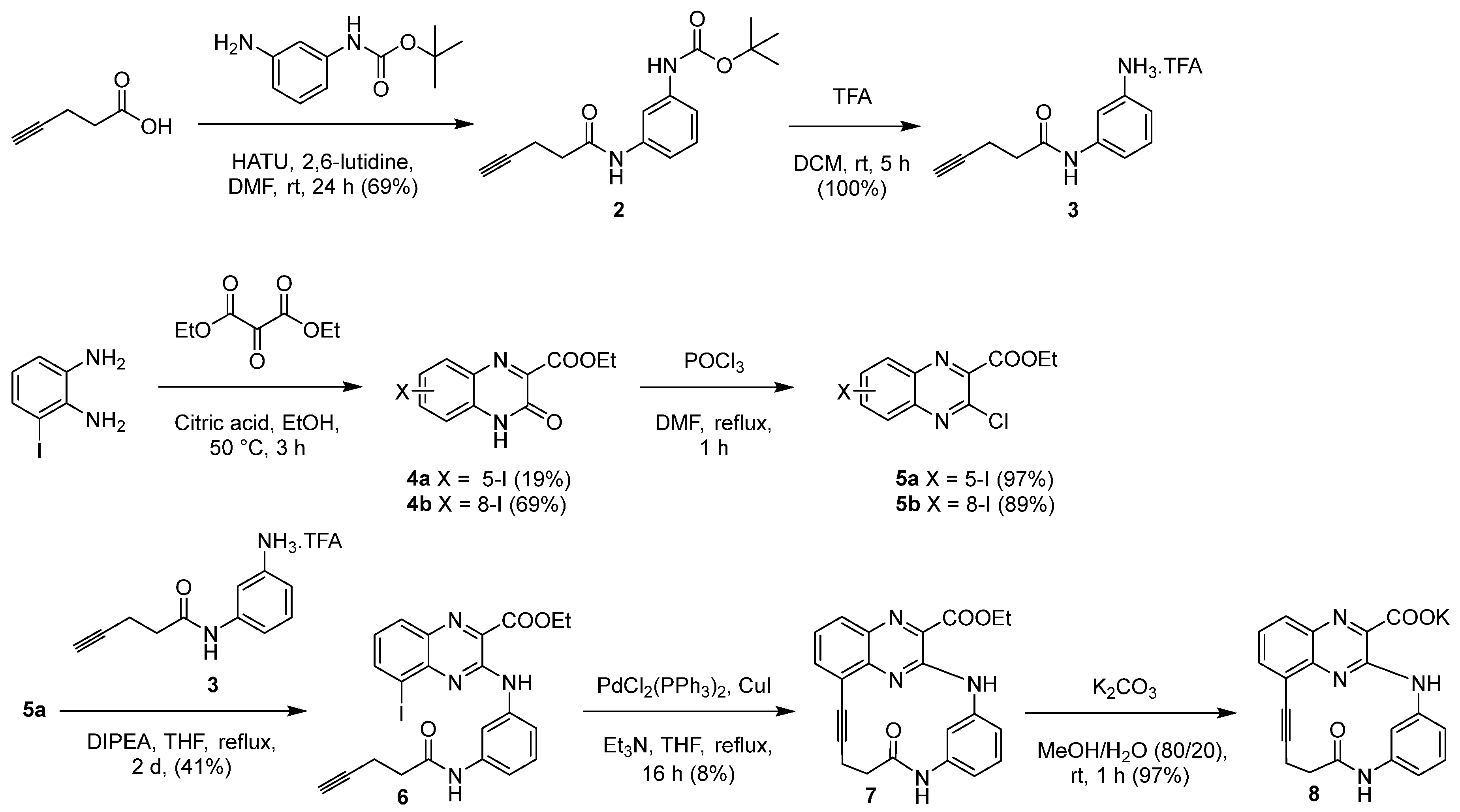
2.1. Potassium 6-oxo-7,13,16,22-tetraazatetracyclo[12.6.2.18,12.017,21]tricosa-1(20),8(23),9,11,14,16,18,21-octaen-2-yne-15-carboxylate
2.2. Protein Kinase Assays
3. Materials and Methods
3.1. Tert-butyl (3-(pent-4-ynamido)phenyl)carbamate (2)
3.2. 3-(Pent-4-ynamido)benzenaminium trifluoroacetic salt (3)
3.3. Ethyl 5-iodo-3-oxo-3,4-dihydroquinoxaline-2-carboxylate (4a) and ethyl 8-iodo-3-oxo-3,4-dihydroquinoxaline-2-carboxylate (4b)
3.4. Ethyl 3-chloro-5-iodoquinoxaline-2-carboxylate (5a)
3.5. Ethyl 3-chloro-8-iodoquinoxaline-2-carboxylate (5b)
3.6. Ethyl 5-iodo-3-((3-(pent-4-ynamido)phenyl)amino)quinoxaline-2-carboxylate (6)
3.7. Ethyl 6-oxo-7,13,16,22-tetraazatetracyclo[12.6.2.18,12.017,21]tricosa-1(20),8(23),9,11,14,16,18,21-octaen-2-yne-15-carboxylate (7)
3.8. Potassium 6-oxo-7,13,16,22-tetraazatetracyclo[12.6.2.18,12.017,21]tricosa-1(20),8(23),9,11,14,16,18,21-octaen-2-yne-15-carboxylate (8)
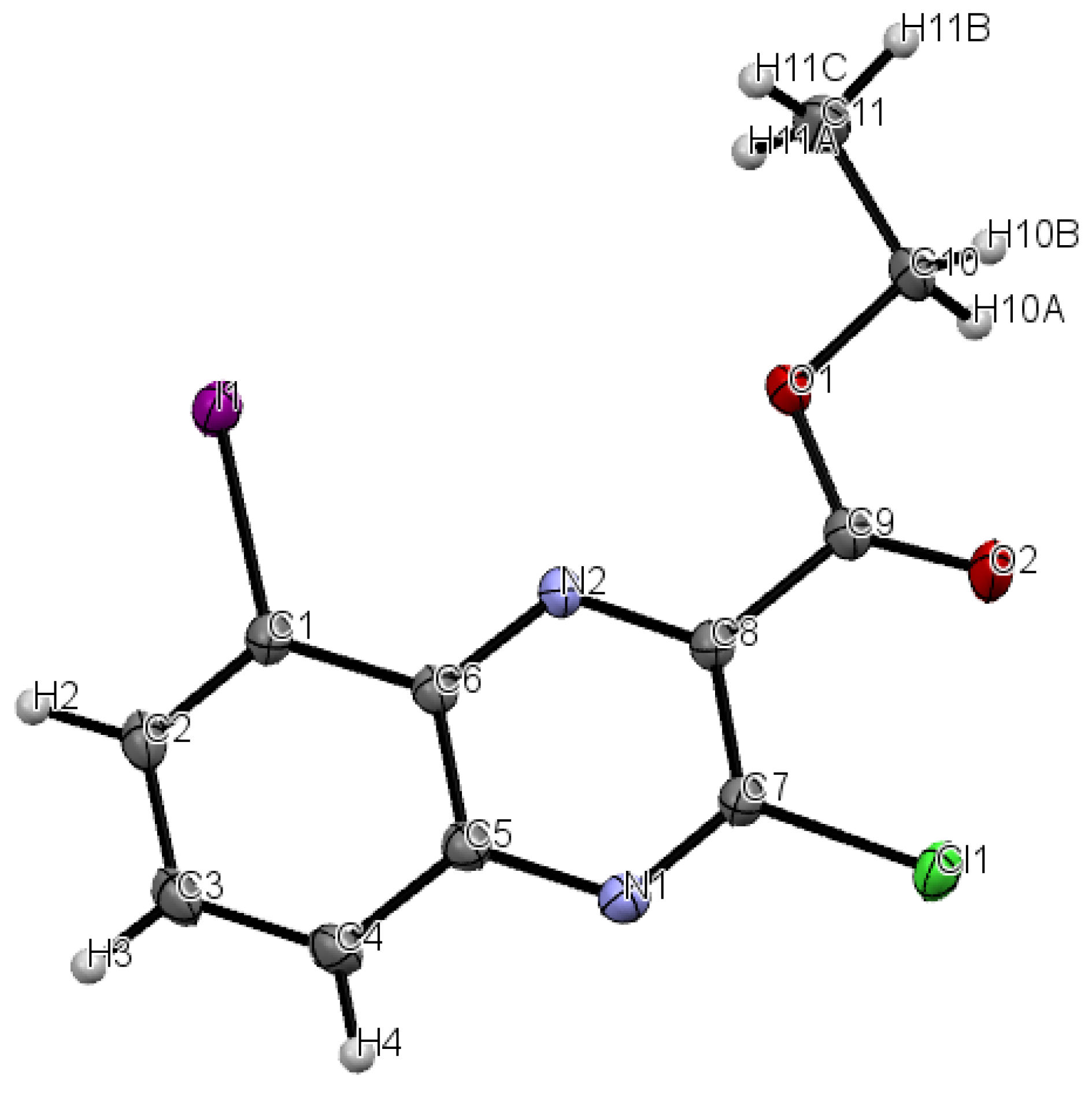
3.9. X-Ray Data
3.10. Protein Kinase Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mochizuki, T.; Kitanaka, C.; Noguchi, K.; Muramatsu, T.; Asai, A.; Kuchino, Y. Physical and Functional Interactions between Pim-1 Kinase and Cdc25A Phosphatase. Implications for the Pim-1-Mediated Activation of the c-Myc Signaling Pathway. J. Biol. Chem. 1999, 274, 18659–18666. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.; Kosan, C.; Xing, P.X.; Montenarh, M.; Hoffmann, I.; Möröy, T. The Oncogenic Serine/Threonine Kinase Pim-1 Directly Phosphorylates and Activates the G2/M Specific Phosphatase Cdc25C. Int. J. Biochem. Cell Biol. 2006, 38, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Theo Cuypers, H.; Selten, G.; Quint, W.; Zijlstra, M.; Maandag, E.R.; Boelens, W.; van Wezenbeek, P.; Melief, C.; Berns, A. Murine Leukemia Virus-Induced T-Cell Lymphomagenesis: Integration of Proviruses in a Distinct Chromosomal Region. Cell 1984, 37, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Maja Narlik-Grassow, Carmen Blanco-Aparicio, and A.C. The PIM Family of Serine/Threonine Kinases in Cancer. Med. Res. Rev. 2014, 34, 136–159. [Google Scholar] [CrossRef] [PubMed]
- Toth, R.K.; Warfel, N.A. Targeting PIM Kinases to Overcome Therapeutic Resistance in Cancer. Mol. Cancer Ther. 2021, 20, 3–10. [Google Scholar] [CrossRef]
- Keane, N.A.; Reidy, M.; Natoni, A.; Raab, M.S.; O’Dwyer, M. Targeting the Pim Kinases in Multiple Myeloma. Blood Cancer J. 2015, 5(7), e325. [Google Scholar] [CrossRef]
- Amson, R.; Sigaux, F.; Przedborski, S.; Flandrin, G.; Givol, D.; Telerman, A. The Human Protooncogene Product P33pim Is Expressed during Fetal Hematopoiesis and in Diverse Leukemias. Proc. Natl. Acad. Sci. U. S. A. 1989, 86, 8857–8861. [Google Scholar] [CrossRef]
- Koblish, H.; Li, Y. long; Shin, N.; Hall, L.; Wang, Q.; Wang, K.; Covington, M.; Marando, C.; Bowman, K.; Boer, J.; et al. Preclinical Characterization of INCB053914, a Novel Pan-PIM Kinase Inhibitor, Alone and in Combination with Anticancer Agents, in Models of Hematologic Malignancies. PLoS One 2018, 13, 1–22. [Google Scholar] [CrossRef]
- Czardybon, W.; Windak, R.; Gołas, A.; Gałezowski, M.; Sabiniarz, A.; Dolata, I.; Salwińska, M.; Guzik, P.; Zawadzka, M.; Gabor-Worwa, E.; et al. A Novel, Dual Pan-PIM/FLT3 Inhibitor SEL24 Exhibits Broad Therapeutic Potential in Acute Myeloid Leukemia. Oncotarget 2018, 9, 16917–16931. [Google Scholar] [CrossRef]
- Bellon, M.; Nicot, C. Targeting Pim Kinases in Hematological Cancers: Molecular and Clinical Review. Mol. Cancer 2023, 22, 1–25. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, C.; Du, E.; Wang, A.; Yang, Y.; Guo, J.; Wang, A.; Zhang, Z.; Xu, Y. Pim-3 Is a Critical Risk Factor in Development and Prognosis of Prostate Cancer. Med. Sci. Monit. 2016, 22, 4254–4260. [Google Scholar] [CrossRef] [PubMed]
- Mikkers, H.; Nawijn, M.; Allen, J.; Brouwers, C.; Verhoeven, E.; Jonkers, J.; Berns, A. Mice Deficient for All PIM Kinases Display Reduced Body Size and Impaired Responses to Hematopoietic Growth Factors. Mol. Cell. Biol. 2004, 24, 6104–6115. [Google Scholar] [CrossRef] [PubMed]
- An, N.; Kraft, A.S.; Kang, Y. Abnormal Hematopoietic Phenotypes in Pim Kinase Triple Knockout Mice. J. Hematol. Oncol. 2013, 6, 1. [Google Scholar] [CrossRef]
- Kumar, A.; Mandiyan, V.; Suzuki, Y.; Zhang, C.; Rice, J.; Tsai, J.; Artis, D.R.; Ibrahim, P.; Bremer, R. Crystal Structures of Proto-Oncogene Kinase Pim1: A Target of Aberrant Somatic Hypermutations in Diffuse Large Cell Lymphoma. J. Mol. Biol. 2005, 348, 183–193. [Google Scholar] [CrossRef]
- Bullock, A.N.; Russo, S.; Amos, A.; Pagano, N.; Bregman, H.; Debreczeni, J.É.; Lee, W.H.; von Delft, F.; Meggers, E.; Knapp, S. Crystal Structure of the PIM2 Kinase in Complex with an Organoruthenium Inhibitor. PLoS One 2009, 4(10), e7112. [Google Scholar] [CrossRef] [PubMed]
- Oyallon, B.; Brachet-Botineau, M.; Logé, C.; Robert, T.; Bach, S.; Ibrahim, S.; Raoul, W.; Croix, C.; Berthelot, P.; Guillon, J.; et al. New Quinoxaline Derivatives as Dual Pim-1/2 Kinase Inhibitors: Design, Synthesis and Biological Evaluation. Molecules 2021, 26, 867. [Google Scholar] [CrossRef] [PubMed]
- Oyallon, B.; Brachet-Botineau, M.; Logé, C.; Bonnet, P.; Souab, M.; Robert, T.; Ruchaud, S.; Bach, S.; Berthelot, P.; Gouilleux, F.; et al. Structure-Based Design of Novel Quinoxaline-2-Carboxylic Acids and Analogues as Pim-1 Inhibitors. Eur. J. Med. Chem. 2018, 154, 101–109. [Google Scholar] [CrossRef]
- Liang, Y.; Fang, R.; Rao, Q. An Insight into the Medicinal Chemistry Perspective of Macrocyclic Derivatives with Antitumor Activity: A Systematic Review. Molecules 2022, 27(9), 2837. [Google Scholar] [CrossRef]
- Basit, S.; Ashraf, Z.; Lee, K.; Latif, M. First Macrocyclic 3rd-Generation ALK Inhibitor for Treatment of ALK/ROS1 Cancer: Clinical and Designing Strategy Update of Lorlatinib. Eur. J. Med. Chem. 2017, 134, 348–356. [Google Scholar] [CrossRef]
- Verstovsek, S.; Komrokji, R.S. A Comprehensive Review of Pacritinib in Myelofibrosis. Futur. Oncol. 2015, 11, 2819–2830. [Google Scholar] [CrossRef]
- Mahesh, R.; Dhar, A.K.; Sasank T.v.n.v., T.; Thirunavukkarasu, S.; Devadoss, T. Citric Acid: An Efficient and Green Catalyst for Rapid One Pot Synthesis of Quinoxaline Derivatives at Room Temperature. Chinese Chem. Lett. 2011, 22, 389–392. [Google Scholar] [CrossRef]
- Mahesh, R.; Devadoss, T.; Dhar, A.K.; Venkatesh, S.M.; Mundra, S.; Pandey, D.K.; Bhatt, S.; Jindal, A.K. Ligand-Based Design, Synthesis, and Pharmacological Evaluation of 3-Methoxyquinoxalin-2-Carboxamides as Structurally Novel Serotonin Type-3 Receptor Antagonists. Arch. Pharm. (Weinheim). 2012, 345, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Zegzouti, H.; Zdanovskaia, M.; Hsiao, K.; Goueli, S.A. ADP-Glo: A Bioluminescent and Homogeneous Adp Monitoring Assay for Kinases. Assay Drug Dev. Technol. 2009, 7, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Aparicio, C.; Carnero, A. Pim Kinases in Cancer: Diagnostic, Prognostic and Treatment Opportunities. Biochem. Pharmacol. 2013, 85, 629–643. [Google Scholar] [CrossRef]
- Supplementary X-ray crystallographic data: Cambridge Crystallographic Data Centre, University Chemical Lab, Lensfield Road, Cambridge, CB2 1EW, UK. Available online: https://www.ccdc.cam.ac.uk/ (accessed on 15 May 2023).
- Sheldrick, G.M. (1996) SADABS, University of Göttingen, Germany.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
| Compound | Kinase enzymatic IC50 (µM) (a) | |||||||
| Pim-1 | Pim-2 | DYRK1A | CDK5/p25 |
CDK9/ CyclinT |
Haspin | CK1ε | GSK3β | |
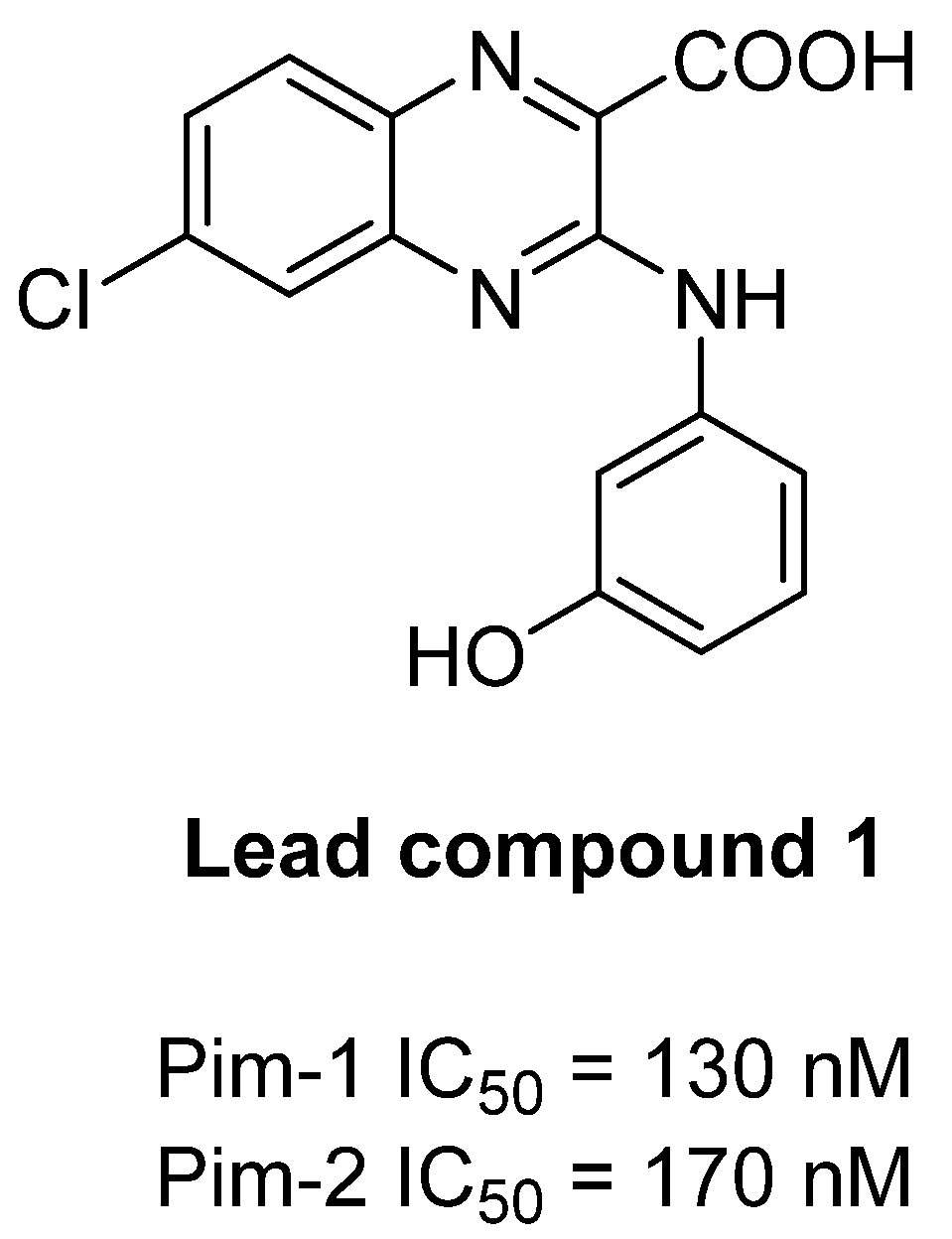
| 1 | 0.13 | 0.17 | 2.58 | > 10 | > 10 | > 10 | > 10 | 2.80 |
| 8 | 0.40 | 0.10 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 |
| SGI-1776 | 0.05 | 0.10 | 3.80 | 9.53 | 1.08 | 0.05 | 6.54 | > 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





