Submitted:
08 September 2023
Posted:
11 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Feeding and management
2.2. Sample collection
2.3. Biochemical indexes determination for blood and fermentation parameters for rumen fluid
2.4. DNA extraction, 16S rRNA gene amplification, and high-throughput sequencing for rumen
2.5. Statistical analysis
3. Results
3.1. Growth performance, blood biochemical indices and rumen fermentation parameters
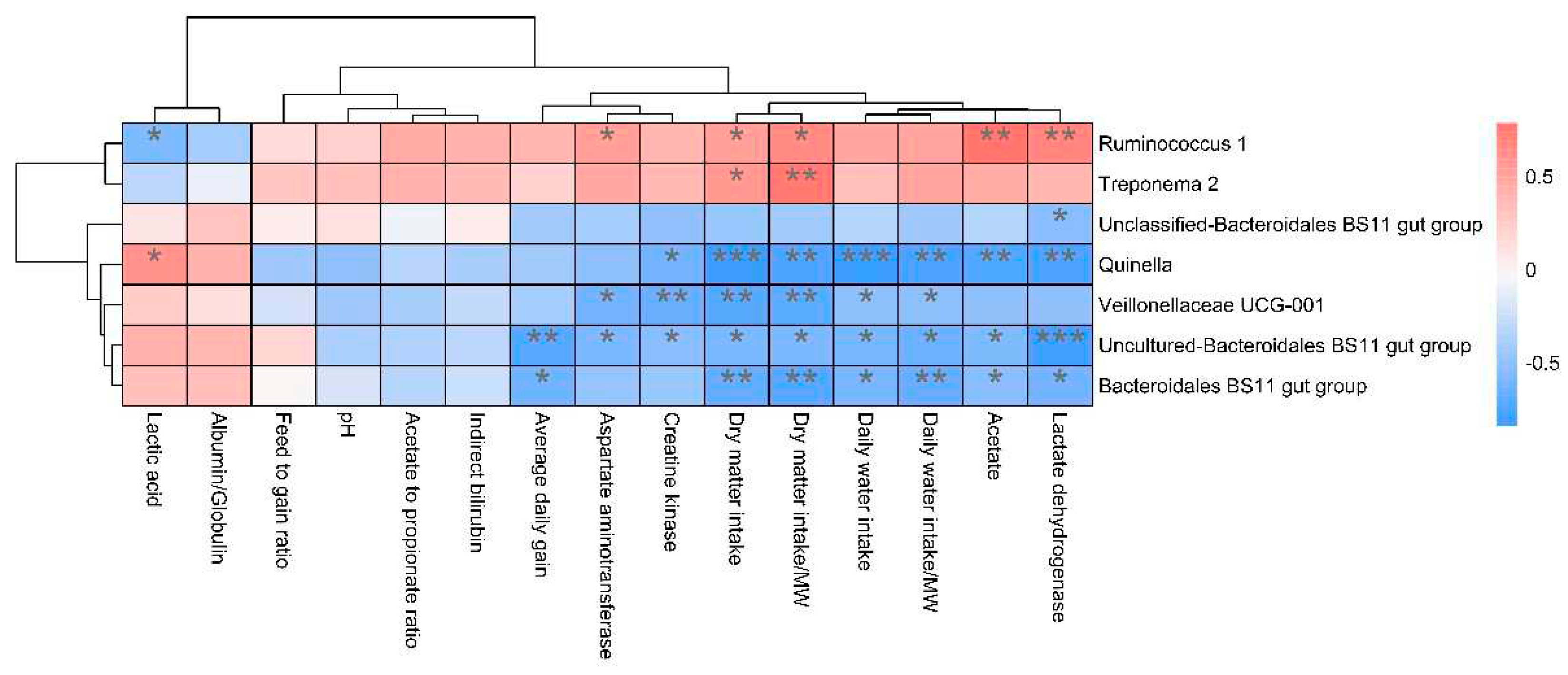
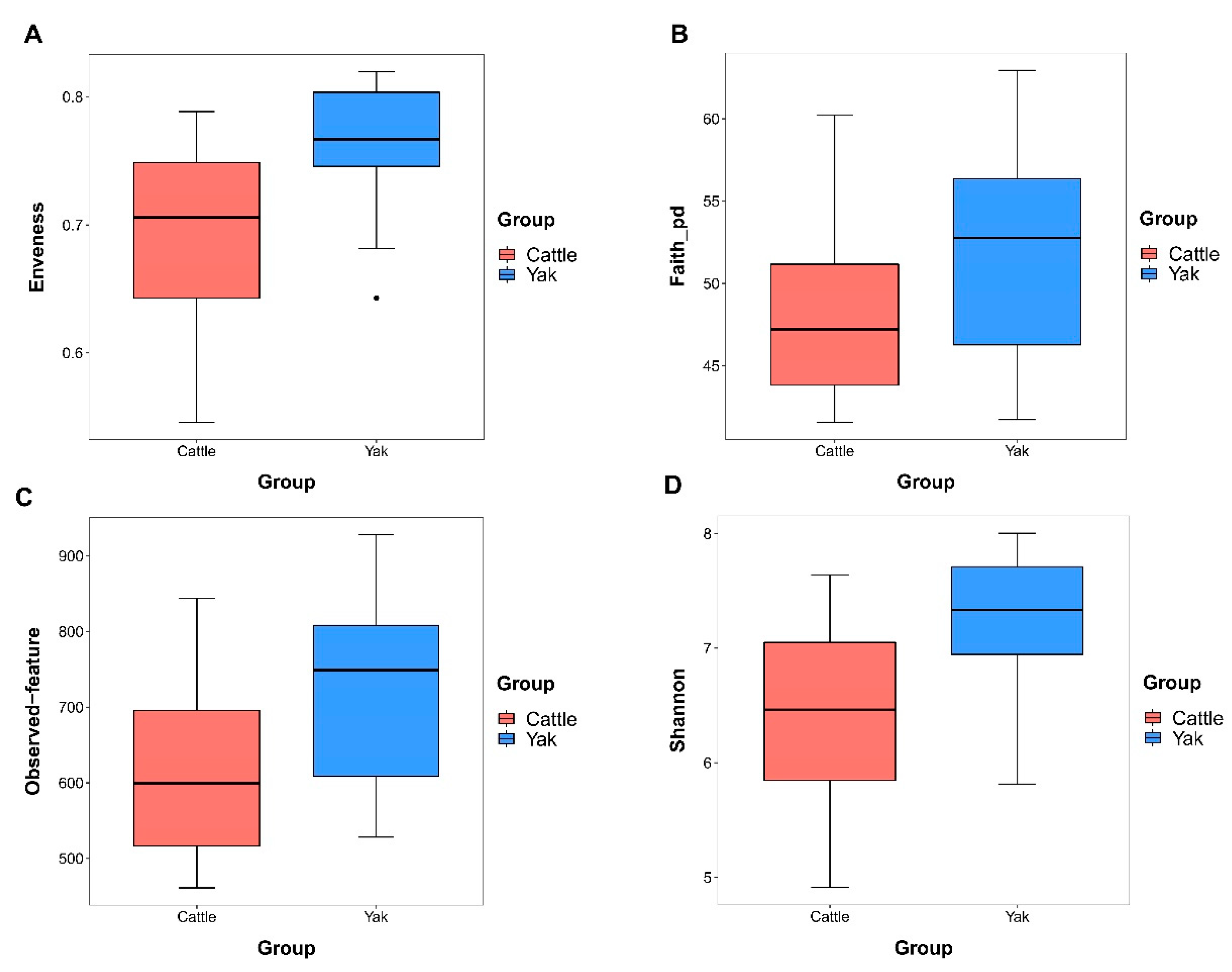
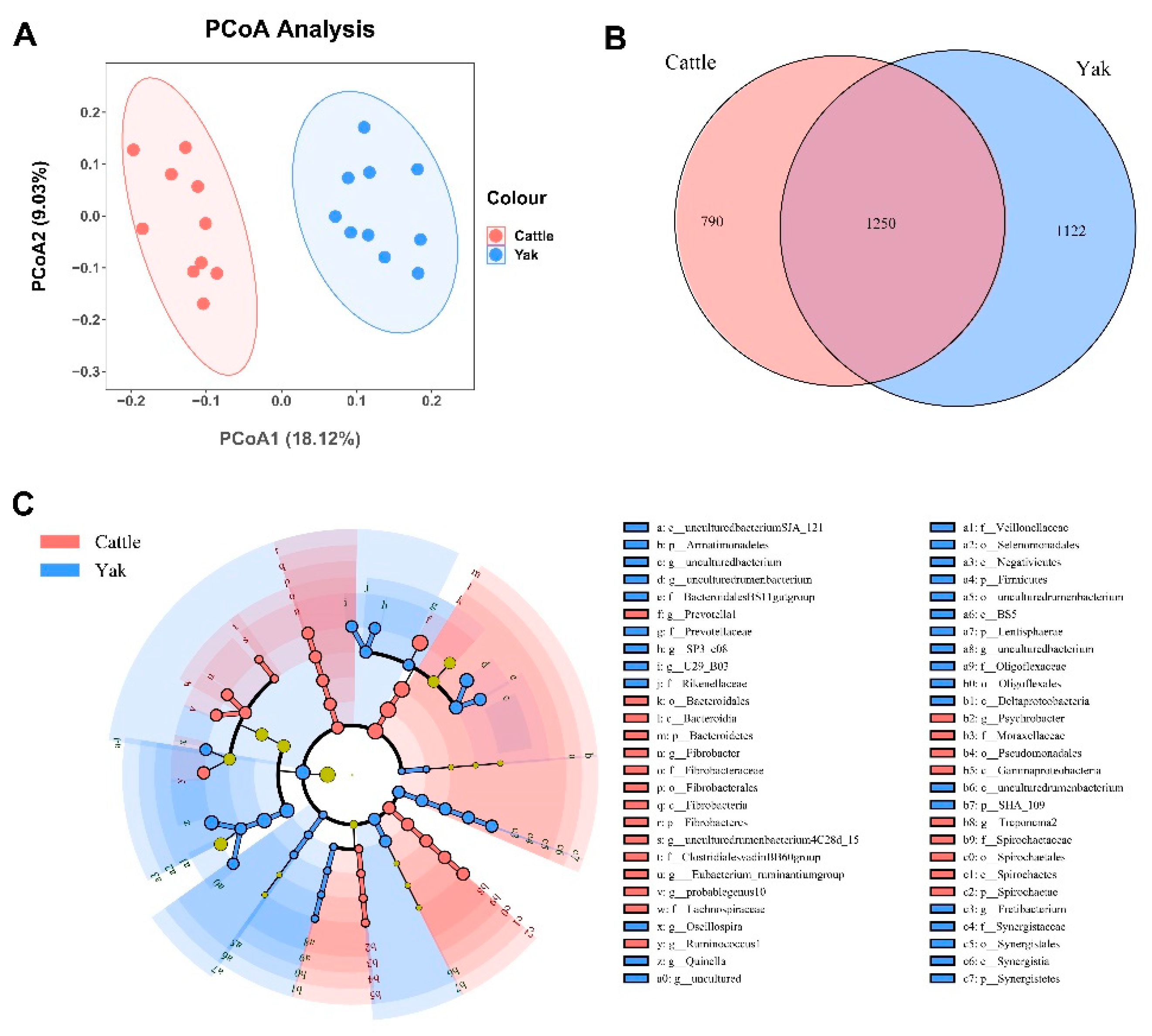
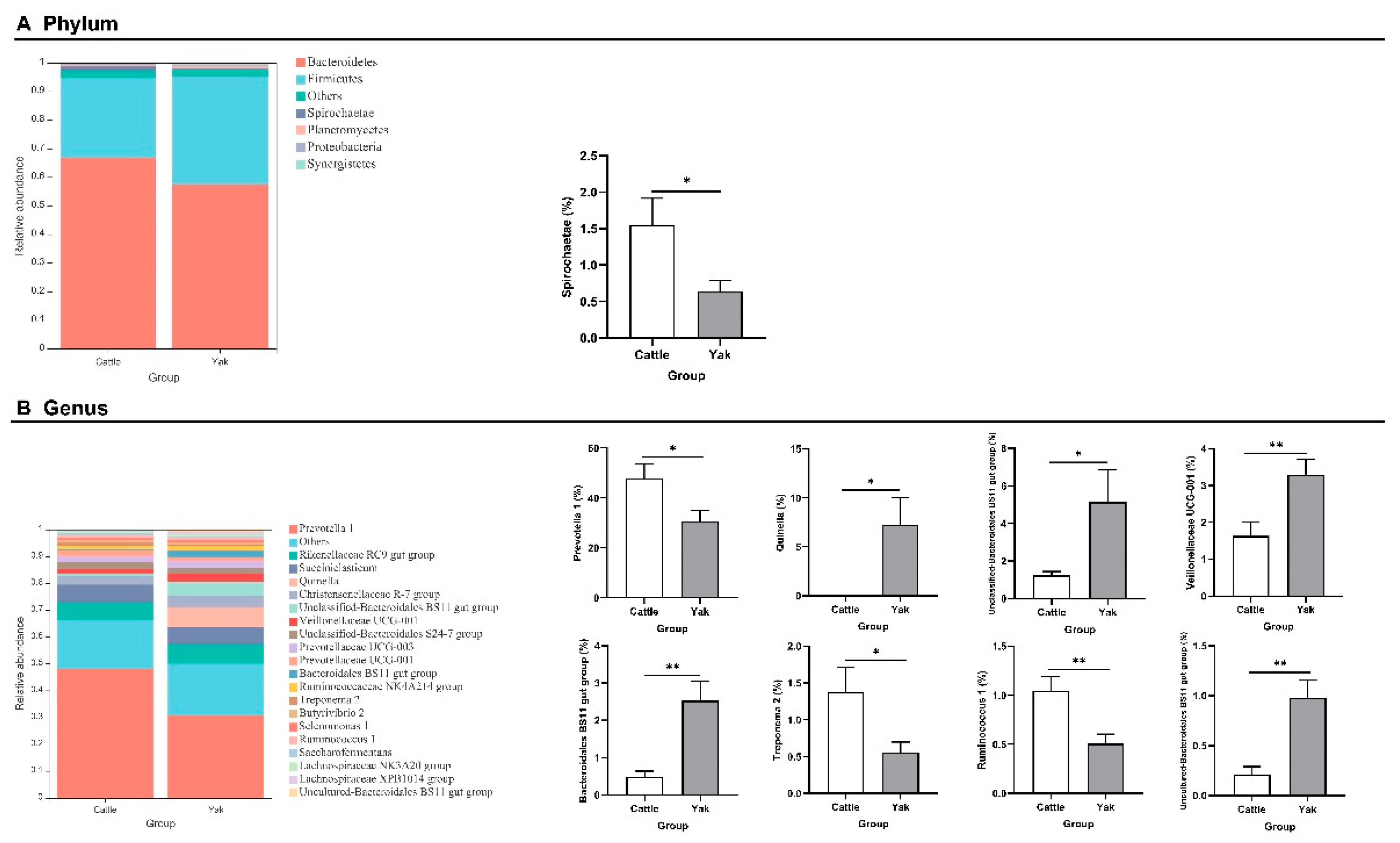
3.2. Ruminal and fecal bacteria structure
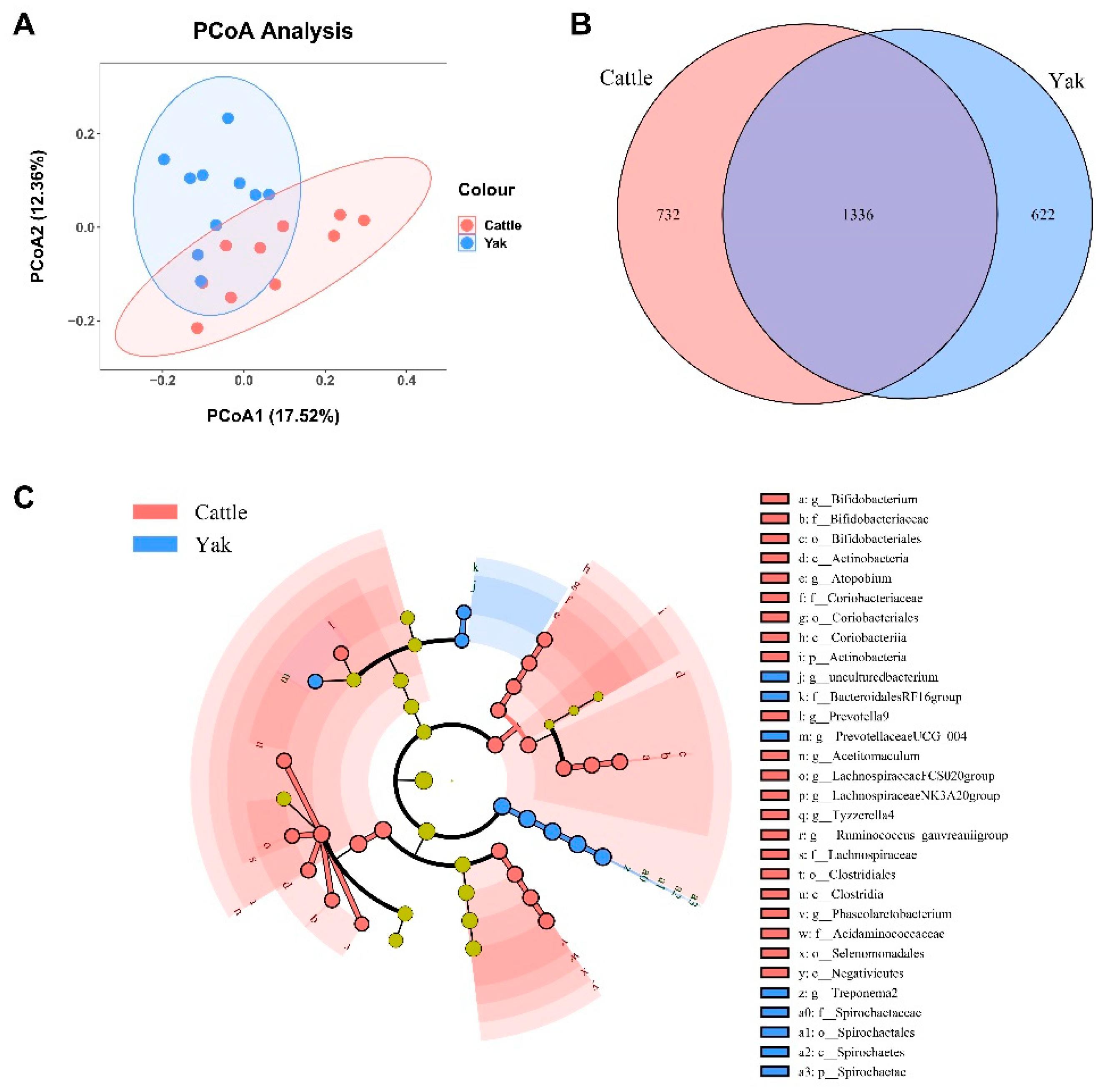
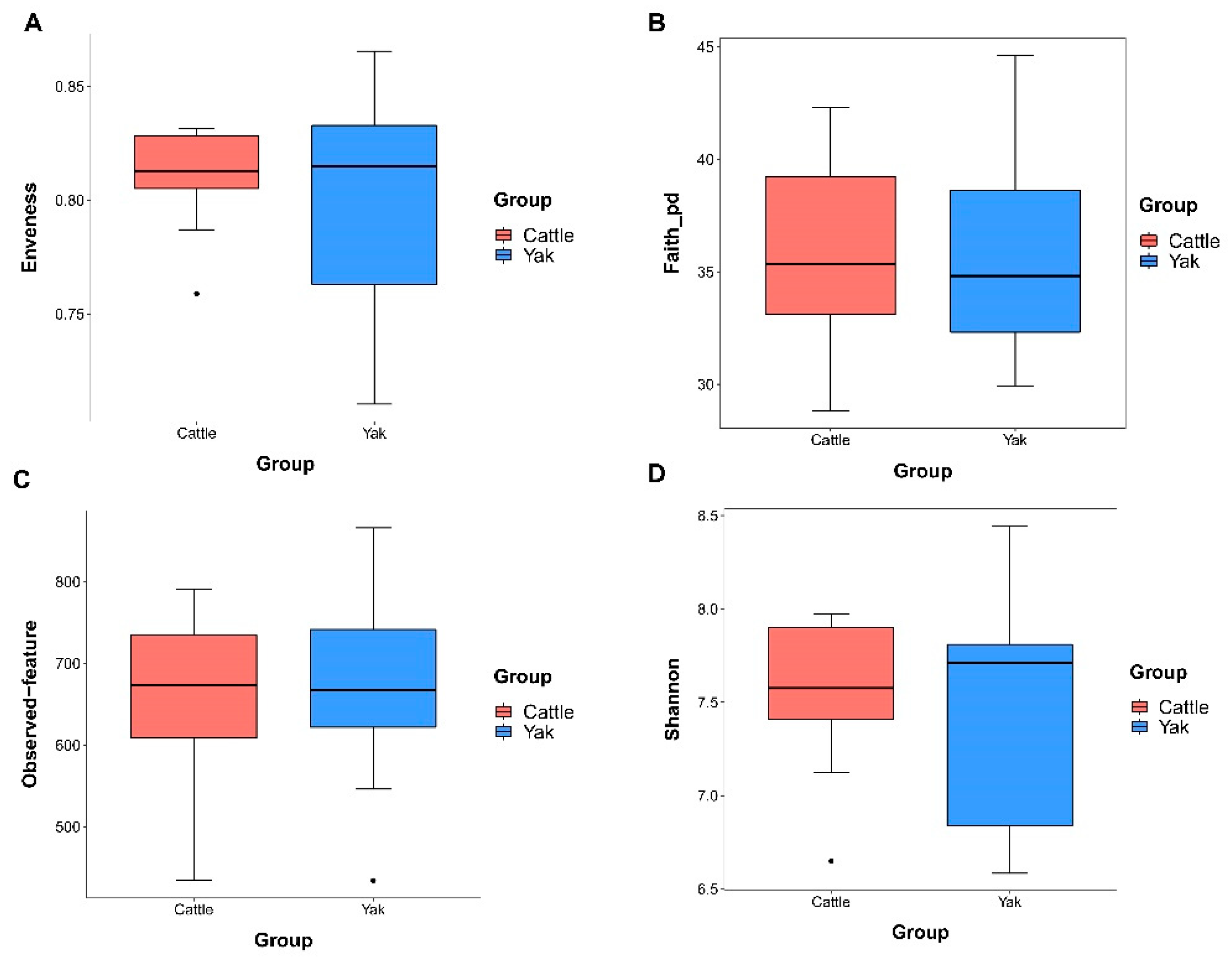
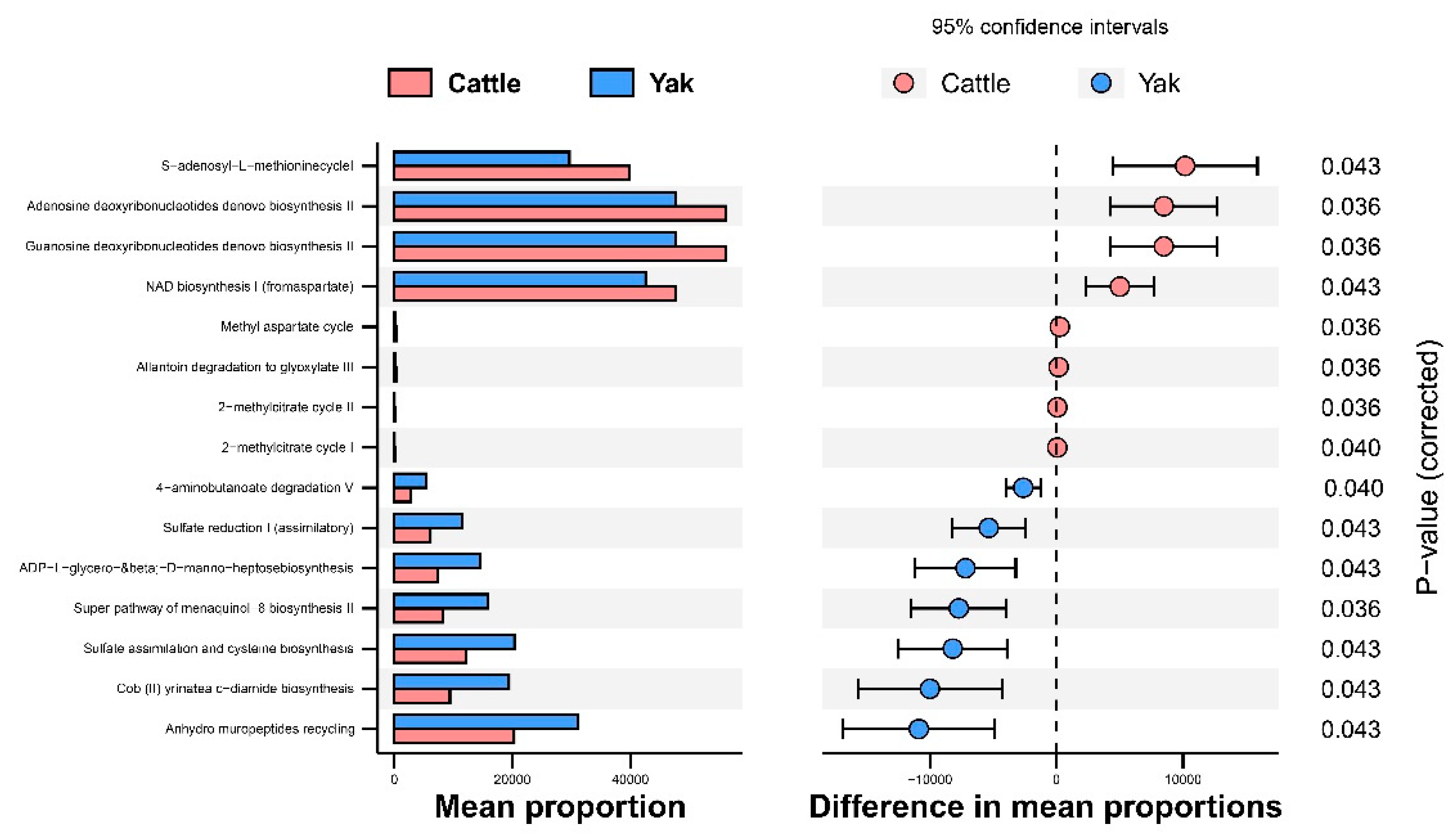
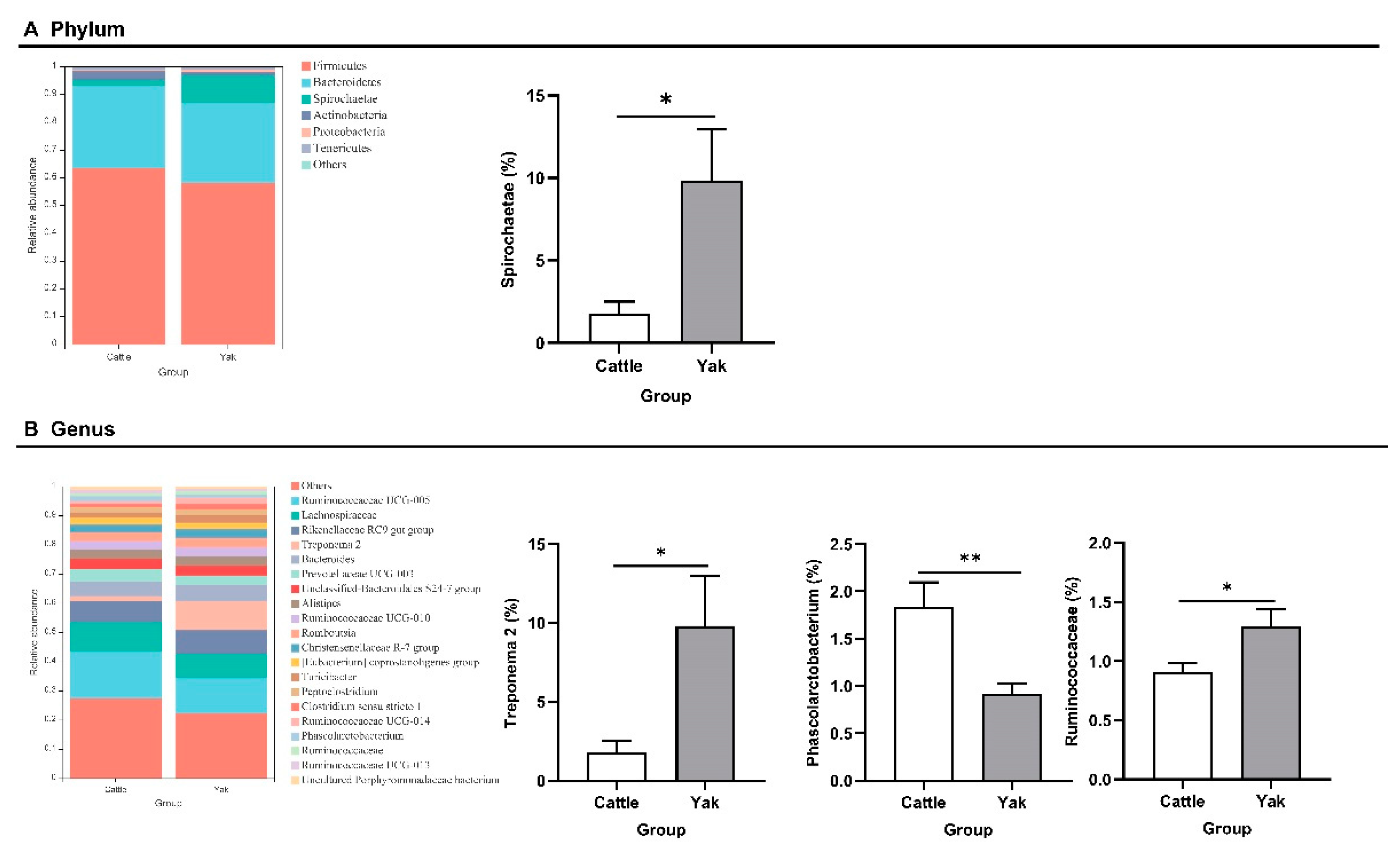
4. Discussion
4.1. Production efficiency, serum biochemistry and rumen fermentation parameters
4.2. Rumen and fecal bacterial structure in cattle and yaks
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiu, Q.; Zhang, G.; Ma, T.; Qian, W.; Wang, J.; Ye, Z.; Cao, C.; Hu, Q.; Kim, J.; Larkin, D.M.; et al. The yak genome and adaptation to life at high altitude. Nat Genet 2012, 44, 946–949. [Google Scholar] [CrossRef] [PubMed]
- Mipam, T.D.; Wen, Y.; Fu, C.; Li, S.; Zhao, H.; Ai, Y.; Li, L.; Zhang, L.; Zou, D. Maternal phylogeny of a newly-found yak population in china. Int J Mol Sci 2012, 13, 11455–11470. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhu, Y.; Luo, Y.; Song, L.; Liu, D.; Liu, L.; Chen, F.; Wang, M.; Li, J.; Zeng, X.; et al. Metagenomic insights into the fibrolytic microbiome in yak rumen. PLoS One 2012, 7, e40430. [Google Scholar] [CrossRef]
- Wei, Y.Q.; Long, R.J.; Yang, H.; Yang, H.J.; Shen, X.H.; Shi, R.F.; Wang, Z.Y.; Du, J.G.; Qi, X.J.; Ye, Q.H. Fiber degradation potential of natural co-cultures of Neocallimastix frontalis and Methanobrevibacter ruminantium isolated from yaks (Bos grunniens) grazing on the Qinghai Tibetan Plateau. Anaerobe 2016, 39, 158–164. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Dong, X.; Dong, Z. Prokaryote diversity in the rumen of yak (Bos grunniens) and Jinnan cattle (Bos taurus) estimated by 16S rDNA homology analyses. Anaerobe 2005, 11, 207–215. [Google Scholar] [CrossRef]
- Long, R.J.; Dong, S.K.; Wei, X.H.; Pu, X.P.J.L.P.S. The effect of supplementary feeds on the bodyweight of yaks in cold season. 2005, 93, 197–204. [Google Scholar] [CrossRef]
- Khafipour, E.; Li, S.; Plaizier, J.C.; Krause, D.O. Rumen microbiome composition determined using two nutritional models of subacute ruminal acidosis. Appl Environ Microbiol 2009, 75, 7115–7124. [Google Scholar] [CrossRef]
- Motoi, Y.; Oohashi, T.; Hirose, H.; Hiramatsu, M.; Miyazaki, S.; Nagasawa, S.; Takahashi, J. Turbidimetric-kinetic assay of endotoxin in rumen fluid or serum of cattle fed rations containing various levels of rolled barley. J Vet Med Sci 1993, 55, 19–25. [Google Scholar] [CrossRef]
- Ohtaki, T.; Ogata, K.; Kajikawa, H.; Sumiyoshi, T.; Asano, S.; Tsumagari, S.; Horikita, T. Effect of high-concentrate corn grain diet-induced elevated ruminal lipopolysaccharide levels on dairy cow liver function. J Vet Med Sci 2020, 82, 971–977. [Google Scholar] [CrossRef]
- Khafipour, E.; Krause, D.O.; Plaizier, J.C. A grain-based subacute ruminal acidosis challenge causes translocation of lipopolysaccharide and triggers inflammation. J Dairy Sci 2009, 92, 1060–1070. [Google Scholar] [CrossRef]
- Wang, D.S.; Zhang, R.Y.; Zhu, W.Y.; Mao, S.Y.J.L.S. Effects of subacute ruminal acidosis challenges on fermentation and biogenic amines in the rumen of dairy cows. 2013, 155, 262–272. [Google Scholar] [CrossRef]
- Helrich, K.; Scott, P.M. Official Methods of Analysis of the Association of Official Analytical Chemist. 2005. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Jin, W.; Cheng, Y.; Zhu, W. The community structure of Methanomassiliicoccales in the rumen of Chinese goats and its response to a high-grain diet. J Anim Sci Biotechnol 2017, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Infascelli, F.; Gigli, S.; Campanile, G. Buffalo meat production: Performance infra vitam and quality of meat. Vet.Res. Commun. 2004, 28, 143–148. [Google Scholar] [CrossRef]
- Mi, J.; Zhou, J.; Huang, X.; Long, R. Lower Methane Emissions from Yak Compared with Cattle in Rusitec Fermenters. PLoS One 2017, 12, e0170044. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, D.; Wang, L.; Hao, J.; Wang, J.; Zhou, X.; Wang, W.; Qiu, Q.; Huang, X.; Zhou, J.; et al. Convergent Evolution of Rumen Microbiomes in High-Altitude Mammals. Curr Biol 2016, 26, 1873–1879. [Google Scholar] [CrossRef]
- A, Y.Z.; A, J.W.Z.; C, X.S.G.B.; A, G.X.C.; C, L.M.D.B.; A, H.C.W.; D, L.W.L.; B, R.J.L.A.J.L.S. Influences of dietary nitrogen and non-fiber carbohydrate levels on apparent digestibility, rumen fermentation and nitrogen utilization in growing yaks fed low quality forage based-diet. 2012, 147, 139–147. [Google Scholar]
- Ma, N.; Abaker, J.A.; Wei, G.; Chen, H.; Shen, X.; Chang, G. A high-concentrate diet induces an inflammatory response and oxidative stress and depresses milk fat synthesis in the mammary gland of dairy cows. J Dairy Sci 2022, 105, 5493–5505. [Google Scholar] [CrossRef]
- Cozzi, G.; Ravarotto, L.; Gottardo, F.; Stefani, A.L.; Contiero, B.; Moro, L.; Brscic, M.; Dalvit, P. Short communication: reference values for blood parameters in Holstein dairy cows: effects of parity, stage of lactation, and season of production. J Dairy Sci 2011, 94, 3895–3901. [Google Scholar] [CrossRef]
- Hatate, K.; Shinya, K.; Matsuo-Sato, A.; Sasaki, S.; Devkota, B.; Takahashi, M.; Hirata, T.; Yamagishi, N. Changes in the plasma levels of several bone markers in newborn calves during the first two days of life. J Vet Med Sci 2016, 78, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Eckersall, P.D.J.C.B.o.D.A. Proteins, Proteomics, and the Dysproteinemias. 2008, 117–155. [Google Scholar]
- Alberghina, D.; Giannetto, C.; Vazzana, I.; Ferrantelli, V.; Piccione, G. Reference intervals for total protein concentration, serum protein fractions, and albumin/globulin ratios in clinically healthy dairy cows. J Vet Diagn Invest 2011, 23, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.J.T.J.o.A.S. Blood-Urea concentration in Relation to Protein Utilization in. 1957, 48, 438–446. [Google Scholar] [CrossRef]
- Lombardi, P.; Musco, N.; Cutrignelli, M.I.; Mollica, M.P.; Infascelli, F. The association of aloe and β-carotene supplementation improves oxidative stress and inflammatory state in pregnant buffalo cows. Buffalo Bulletin. 2017, 36(3), 497–503. [Google Scholar]
- Abd Ellah, M.R.; Hamed, M.I.; Ibrahim, D.R.; Rateb, H.Z. Serum biochemical and haematological reference intervals for water buffalo Bubalus bubalis heifers. J S Afr Vet Assoc 2014, 85, e1–e7. [Google Scholar] [CrossRef]
- Andretta, I.; Kipper, M.; Lehnen, C.R.; Lovatto, P.A. Meta-analysis of the relationship of mycotoxins with biochemical and hematological parameters in broilers. Poult Sci 2012, 91, 376–382. [Google Scholar] [CrossRef]
- Tzouvelekis, A.; Kouliatsis, G.; Anevlavis, S.; Bouros, D. Serum biomarkers in interstitial lung diseases. Respir Res 2005, 6, 78. [Google Scholar] [CrossRef]
- Bilotta, M.T.; Petillo, S.; Santoni, A.; Cippitelli, M. Liver X Receptors: Regulators of Cholesterol Metabolism, Inflammation, Autoimmunity, and Cancer. Front Immunol 2020, 11, 584303. [Google Scholar] [CrossRef]
- Li, T.; Chiang, J.Y. Bile acid signaling in metabolic disease and drug therapy. Pharmacol Rev 2014, 66, 948–983. [Google Scholar] [CrossRef] [PubMed]
- Joyce, S.A.; Gahan, C.G. Bile Acid Modifications at the Microbe-Host Interface: Potential for Nutraceutical and Pharmaceutical Interventions in Host Health. Annu Rev Food Sci Technol 2016, 7, 313–333. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Takeda, K.; Sundrud, M.S. Emerging roles of bile acids in mucosal immunity and inflammation. Mucosal Immunol 2019, 12, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Liu, S.; Xu, F.; Jiang, S.; Yan, J.; He, Q. Corrigendum: powdery mildews are characterized by contracted carbohydrate metabolism and diverse effectors to adapt to obligate biotrophic lifestyle. Front Microbiol 2019, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.B. The importance of pH in the regulation of ruminal acetate to propionate ratio and methane production in vitro. J Dairy Sci 1998, 81(12), 3222–3230. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, P.; Wang, L.; Zhao, Z.; Chen, Y.; Yang, Y. Bacterial community diversity associated with different levels of dietary nutrition in the rumen of sheep. Appl Microbiol Biotechnol 2017, 101(9), 3717–3728. [Google Scholar] [CrossRef]
- Brown, M.S.; Ponce, C.H.; Pulikanti, R. Adaptation of beef cattle to high-concentrate diets: performance and ruminal metabolism. J Anim Sci 2006, 84 Suppl, E25–33. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: a new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef]
- Liu, H.; Xu, T.; Xu, S.; Ma, L.; Han, X.; Wang, X.; Zhang, X.; Hu, L.; Zhao, N.; Chen, Y.; et al. Effect of dietary concentrate to forage ratio on growth performance, rumen fermentation and bacterial diversity of Tibetan sheep under barn feeding on the Qinghai-Tibetan plateau. PeerJ 2019, 7, e7462. [Google Scholar] [CrossRef]
- Stevenson, D.M.; Weimer, P.J. Dominance of Prevotella and low abundance of classical ruminal bacterial species in the bovine rumen revealed by relative quantification real-time PCR. Appl Microbiol Biotechnol 2007, 75, 165–174. [Google Scholar] [CrossRef]
- Henao-Mejia, J.; Elinav, E.; Jin, C.; Hao, L.; Mehal, W.Z.; Strowig, T.; Thaiss, C.A.; Kau, A.L.; Eisenbarth, S.C.; Jurczak, M.J.; et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012, 482, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Si, H.; Yan, X.; Liu, C.; Ding, L.; Long, R.; Li, Z.; Qiu, Q. Bacterial communities in the solid, liquid, dorsal, and ventral epithelium fractions of yak (Bos grunniens) rumen. Microbiologyopen 2020, 9, e963. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Altermann, E.; Leahy, S.C.; Jauregui, R.; Jonker, A.; Henderson, G.; Kittelmann, S.; Attwood, G.T.; Kamke, J.; Waters, S.M.; et al. Genomic insights into the physiology of Quinella, an iconic uncultured rumen bacterium. Nat Commun 2022, 13, 6240. [Google Scholar] [CrossRef]
- Solden, L.M.; Hoyt, D.W.; Collins, W.B.; Plank, J.E.; Daly, R.A.; Hildebrand, E.; Beavers, T.J.; Wolfe, R.; Nicora, C.D.; Purvine, S.O.; et al. New roles in hemicellulosic sugar fermentation for the uncultivated Bacteroidetes family BS11. Isme j 2017, 11, 691–703. [Google Scholar] [CrossRef]
- Wu, Y.; Yao, Y.; Dong, M.; Xia, T.; Li, D.; Xie, M.; Wu, J.; Wen, A.; Wang, Q.; Zhu, G.; et al. Characterisation of the gut microbial community of rhesus macaques in high-altitude environments. BMC Microbiol 2020, 20, 68. [Google Scholar] [CrossRef]
- Nordhoff, M.; Moter, A.; Schrank, K.; Wieler, L.H. High prevalence of treponemes in bovine digital dermatitis-a molecular epidemiology. Vet Microbiol 2008, 131, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Bessman, S.P.; Rossen, J.; Layne, E.C. Gamma-Aminobutyric acid-glutamic acid transamination in brain. J Biol Chem 1953, 201, 385–391. [Google Scholar] [CrossRef]
- van Zijderveld, S.M.; Gerrits, W.J.; Apajalahti, J.A.; Newbold, J.R.; Dijkstra, J.; Leng, R.A.; Perdok, H.B. Nitrate and sulfate: Effective alternative hydrogen sinks for mitigation of ruminal methane production in sheep. J Dairy Sci 2010, 93, 5856–5866. [Google Scholar] [CrossRef]
- Li, Q.S.; Wang, R.; Ma, Z.Y.; Zhang, X.M.; Jiao, J.Z.; Zhang, Z.G.; Ungerfeld, E.M.; Yi, K.L.; Zhang, B.Z.; Long, L.; et al. Dietary selection of metabolically distinct microorganisms drives hydrogen metabolism in ruminants. Isme j 2022, 16, 2535–2546. [Google Scholar] [CrossRef]
- Harada, E.; Yamaguchi, Y.; Koizumi, N.; Hiroshi, S. Cadmium stress induces production of thiol compounds and transcripts for enzymes involved in sulfur assimilation pathways in Arabidopsis. Journal of Plant Physiology 2002, 159, 445–448. [Google Scholar] [CrossRef]
- Dowd, P.; Ham, S.W.; Naganathan, S.; Hershline, R. The mechanism of action of vitamin K. Annu Rev Nutr 1995, 15, 419–440. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, W.H. The cellulosome and cellulose degradation by anaerobic bacteria. Appl Microbiol Biotechnol 2001, 56, 634–649. [Google Scholar] [CrossRef] [PubMed]
- Myer, P.R.; Wells, J.E.; Smith, T.P.; Kuehn, L.A.; Freetly, H.C. Cecum microbial communities from steers differing in feed efficiency. J Anim Sci 2015, 93, 5327–5340. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Yang, C.; Guan, L.L.; Wang, J.; Xue, M.; Liu, J.X. Persistence of Cellulolytic Bacteria Fibrobacter and Treponema After Short-Term Corn Stover-Based Dietary Intervention Reveals the Potential to Improve Rumen Fibrolytic Function. Front Microbiol 2018, 9, 1363. [Google Scholar] [CrossRef] [PubMed]
- Paster, B.J.; Canale-Parola, E. Physiological diversity of rumen spirochetes. Appl Environ Microbiol 1982, 43, 686–693. [Google Scholar] [CrossRef]
- Azad, E.; Derakhshani, H.; Forster, R.J.; Gruninger, R.J.; Acharya, S.; McAllister, T.A.; Khafipour, E. Characterization of the rumen and fecal microbiome in bloated and non-bloated cattle grazing alfalfa pastures and subjected to bloat prevention strategies. Sci Rep 2019, 9, 4272. [Google Scholar] [CrossRef]
- de Oliveira, M.N.; Jewell, K.A.; Freitas, F.S.; Benjamin, L.A.; Tótola, M.R.; Borges, A.C.; Moraes, C.A.; Suen, G. Characterizing the microbiota across the gastrointestinal tract of a Brazilian Nelore steer. Vet Microbiol 2013, 164, 307–314. [Google Scholar] [CrossRef]
| Ingredients, % | Nutrient levels, % | ||
|---|---|---|---|
| Oat hay | 10 | DM | 60.89 |
| Corn silage | 20 | Calcium | 0.49 |
| Corn kernels | 40 | Phosphorus | 0.58 |
| Bran | 17 | Ether extract | 2.77 |
| Soybean meal | 5 | Crude protein | 16.19 |
| Rapeseed cake | 5 | 2ME, MJ/kg | 11.96 |
| Mineral-vitamin premix1 | 2 | NDF | 29.44 |
| Baking soda | 1 | ADF | 16.54 |
| Items | Cattle | Yak | P-value |
|---|---|---|---|
| Initial body weight (kg) | 256.9±8.6 | 194.3±13.7 | 0.001 |
| Dry matter intake (kg/d) | 9.63±0.51 | 5.49±0.32 | <0.001 |
| Daily water intake (kg/d) | 35.59±1.07 | 20.38±0.96 | <0.001 |
| Dry matter intake/MW(g/kgW0.75) | 128.11±5.52 | 92.24±3.76 | <0.001 |
| Daily water intake/MW(g/kgW0.75) | 475.97±19.13 | 343.96±14.50 | <0.001 |
| Average daily gain (kg/d) | 1.09±0.08 | 0.87±0.05 | 0.039 |
| Feed to gain ratio | 9.09±0.60 | 6.41±0.49 | 0.004 |
| Items | Cattle | Yak | P-value |
|---|---|---|---|
| Aspartate aminotransferase (U/L) | 91.80±7.62 | 64.40±3.94 | 0.005 |
| Alanine aminotransferase (U/L) | 27.20±1.97 | 30.50±2.07 | 0.263 |
| Total protein (g/L) | 76.55±2.48 | 73.71±3.17 | 0.491 |
| Albumin (g/L) | 35.50±0.48 | 37.24±0.91 | 0.113 |
| Globulin (g/L) | 41.07±2.70 | 36.48±2.67 | 0.242 |
| Albumin/Globulin | 0.89±0.06 | 1.07±0.06 | 0.048 |
| Alkaline phosphatase (U/L) | 218.50±22.93 | 165.60±13.54 | 0.066 |
| Total bilirubin (μmol/L) | 1.98±0.23 | 1.23±0.33 | 0.082 |
| Direct bilirubin (μmol/L) | 0.53±0.09 | 0.50±0.22 | 0.904 |
| Indirect bilirubin (μmol/L) | 1.45±0.15 | 0.81±0.20 | 0.021 |
| Creatine kinase (U/L) | 477.80±170.74 | 113.00±12.53 | 0.047 |
| Lactate dehydrogenase (IU/L) | 1431.60±55.05 | 940.00±53.21 | <0.001 |
| Glucose (mmol/L) | 4.43±0.12 | 4.58±0.24 | 0.578 |
| Urea (mmol/L) | 2.95±0.33 | 4.29±0.35 | 0.012 |
| Uric acid (μmol/L) | 16.40±1.83 | 34.40±3.06 | <0.001 |
| Calcium (mmol/L) | 2.71±0.06 | 2.53±0.10 | 0.144 |
| Phosphorus (mmol/L) | 2.01±0.13 | 2.24±0.12 | 0.219 |
| Total cholesterol (mmol/L) | 2.81±0.16 | 1.90±0.18 | 0.001 |
| Triglyceride (mmol/L) | 0.26±0.02 | 0.34±0.04 | 0.101 |
| Items | Cattle | Yak | P-value |
|---|---|---|---|
| pH | 6.13±0.03 | 6.00±0.03 | 0.006 |
| Lactate (mmol/L) | 0.25±0.04 | 0.38±0.04 | 0.028 |
| TVFA (mmol/L) | 76.02±7.70 | 85.11±6.88 | 0.390 |
| Acetate (%) | 59.34±0.81 | 56.04±0.50 | 0.003 |
| Propionate (%) | 19.48±0.79 | 21.13±0.65 | 0.125 |
| Butyrate (%) | 15.81±0.87 | 17.14±0.65 | 0.232 |
| Isobutyrate (%) | 1.56±0.20 | 1.51±0.19 | 0.861 |
| Isovalerate (%) | 2.02±0.26 | 2.13±0.26 | 0.773 |
| Valerate (%) | 1.80±0.21 | 2.06±0.24 | 0.428 |
| Acetate:Propionate | 3.10±0.15 | 2.67±0.07 | 0.022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





