Submitted:
07 September 2023
Posted:
11 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
- bio-cementation can be produced at room temperature, thus the amount of energy needed for its manufacturing is significantly less than that required for conventional cement, resulting in a 43–95% decrease in embodied energy [33];
- the carbon footprint from the bio-cementation process is about 18–49.6% lower than that from traditional cement [33];
- due to the relatively low viscosity of the cementation solution and bacterial suspension, the bacterium can flow like water during the bio-cementation process and move through the pores of the concrete [34];
- bacterial sizes are less than 10 µm, which is considerably smaller than the sizes of cement (<40 µm), therefore the pore openings can be as small as 6 mm [35];
1.1. Bio-cementing Agents
- (i)
- (ii)
- the cell-free approach, which uses bacterial fraction components in the absence of viable cells [40];
- (iii)
1.2. Application Methods of Bio-cementation
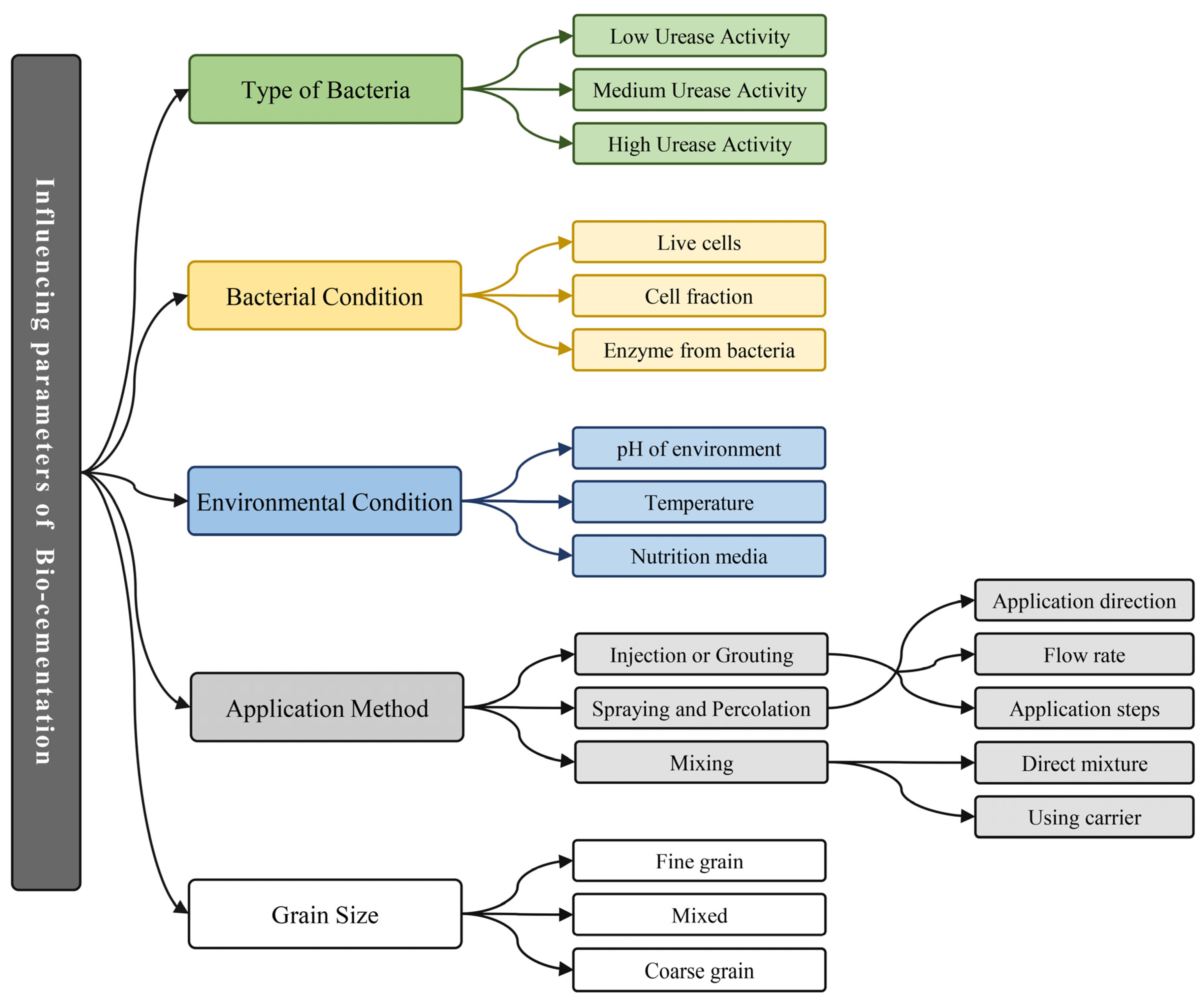
1.3. Parameters That Influence Bio-cementation
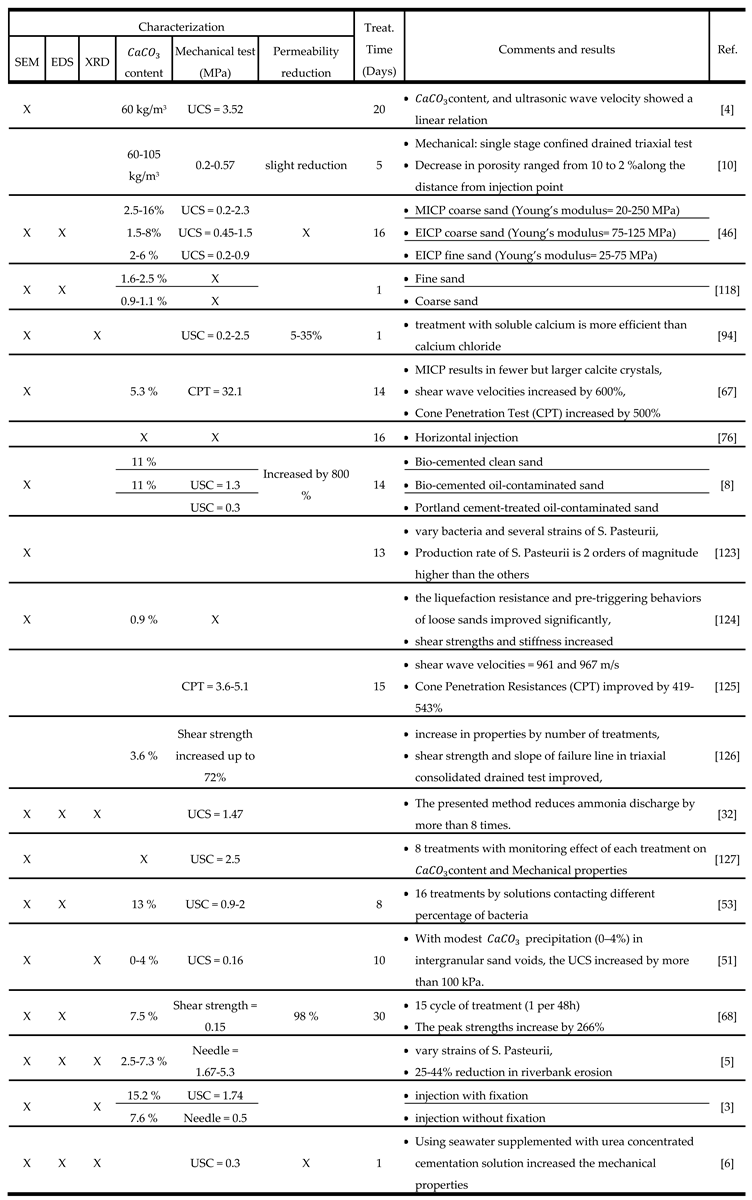
1.4. Characterization Techniques
1.5. Focus of This Review and Bibliometric Analysis
- The four keywords, namely "bio-cementation," "Sporosarcina Pasteurii," "Microbially Induced Calcium Carbonate Precipitation (MICP)," and "Enzymatic Induced Calcium Carbonate Precipitation (EICP)," were searched on Google Scholar and Scopus.
- The pre-screening process was conducted to determine the relevance of the search results. A total of 194 articles were identified as primarily relevant within the scope of this review article.
- The articles that were chosen have been classified into two categories: "review articles" and "original research".
- The original research articles were classified according to two criteria: "the type of bacteria or enzyme studied", and the specific "application field" in which the research was conducted.
- For the selected original articles, the "application method," the primary "experimental methodology" used for characterization, and the "key outcomes" were highlighted. Following this secondary screening, 143 publications were chosen to be included in this manuscript.
- The review articles and original research articles that were not specifically related to Sporosarcina Pasteurii but presented results that highlighted specific outcomes for bio-cementation, such as the effect of influencing parameters or application methods or specific outcomes for experimental methods used in a creative or critical manner, were used in the introduction section to depict a clear background of bio-cementation and governing parameters for the reader.
- Articles that focus on the specific characteristics of S. Pasteurii and its behavior in various environments, as well as the results obtained from its application in soil stabilization and building construction, are used in Sections 3 and 4.
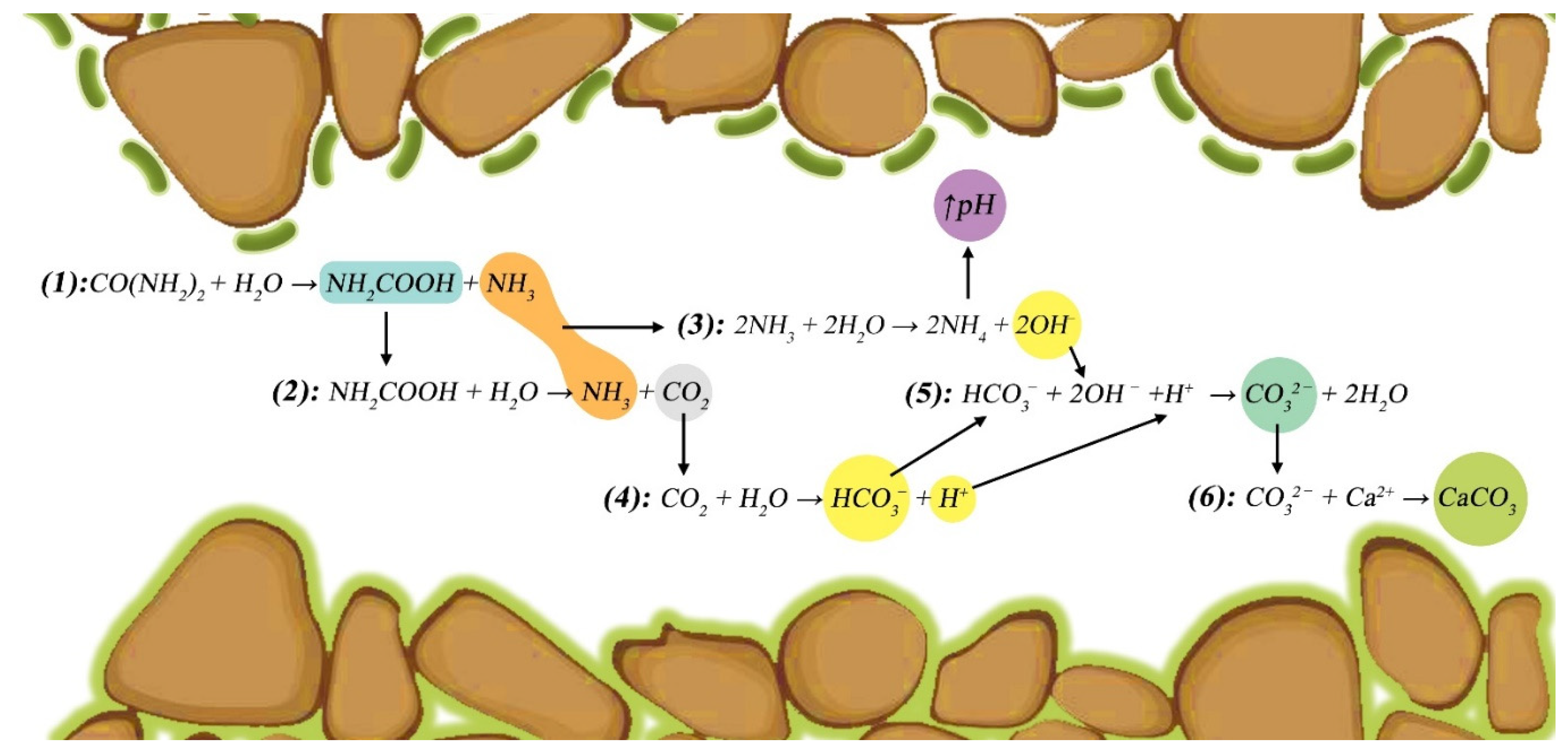
2. Bio-cementation Mechanism
- the hydrolysis of urea () under the catalysis of the bacteria’s urease produces ammonia () and carbamic acid ();
- carbamic acid hydrolysis leads to ammonia and carbon dioxide;
- ammonia interacts with water and generates hydroxide (), and ammonium () leading to a pH increase of about 1-2 pH;
- meanwhile carbon dioxide in the system reacts with water resulting bicarbonate () and hydrogen ion ();
- the system reaches equilibrium and generates carbonate ions () and water;
- eventually, the reaction of carbonate with calcium ions () in the environment results in the precipitation of crystals.
3. Sporosarcina Pasteurii (S. Pasteurii)
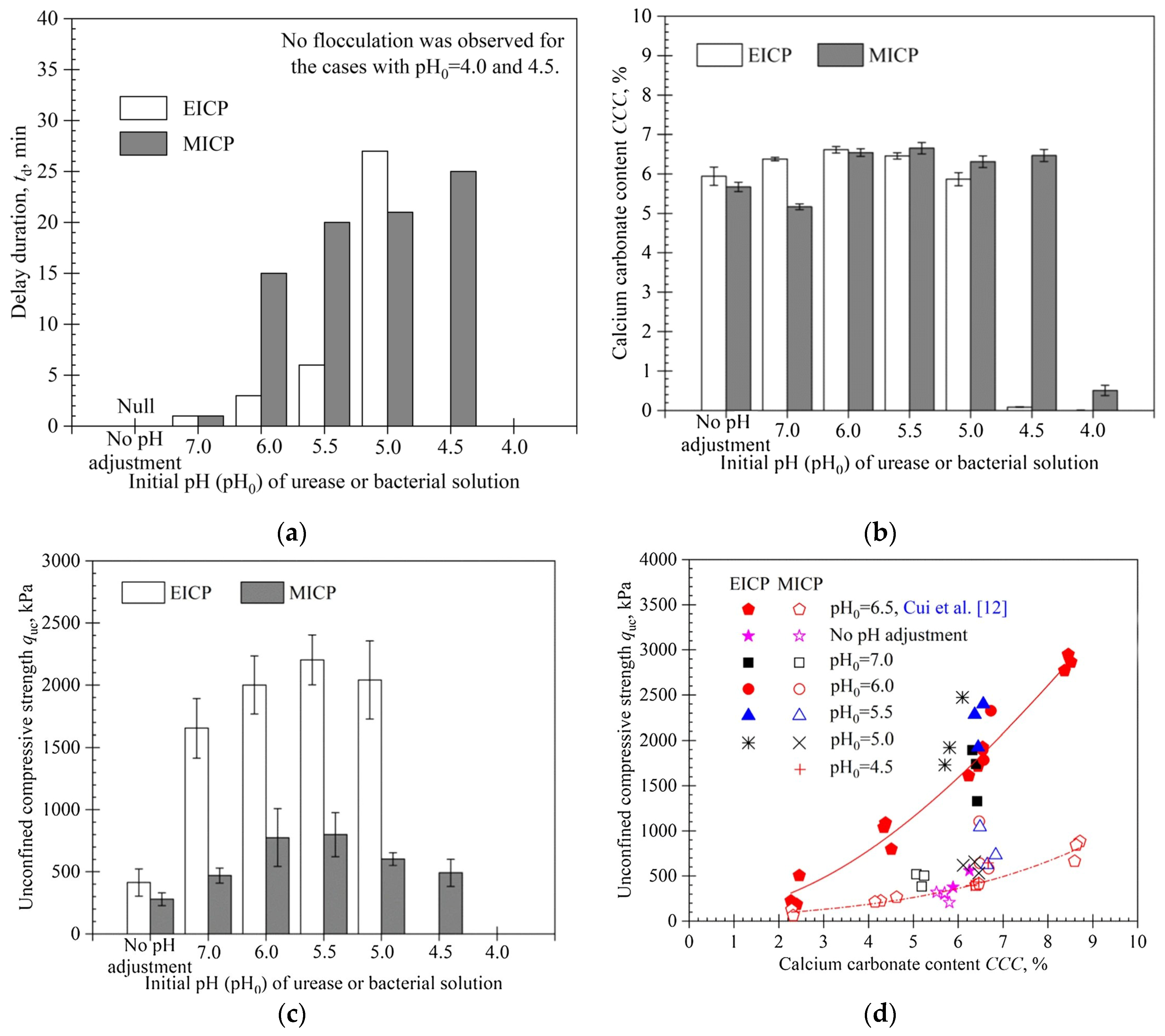
- MICP strategy creates large crystal clusters with a thickness ranging from 50 to 100 µm, whereas the size of crystals via EICP are significantly smaller, ranging from 5 to 20 µm,
- the same number of treatments resulted in a higher content for MICP, and at a constant percentage of , the mechanical properties of EICP-treated samples were superior to those treated by MICP
- while precipitation levels differed (2.5–16%) and (1.5–8%), the peak stress for UCS tests of treated samples in both cases ranged from 200–2400 kPa,
- permeability levels for EICP-treated samples were slightly lower in the range of 1.5%-4% precipitation compared to MICP-treated, while the MICP-treated permeability decreased by 3 to 4 orders of magnitude between 13-16% content.
4. Recent advancements in exploiting S. Pasteurii
4.1. Soil Stabilization
4.2. Building Construction
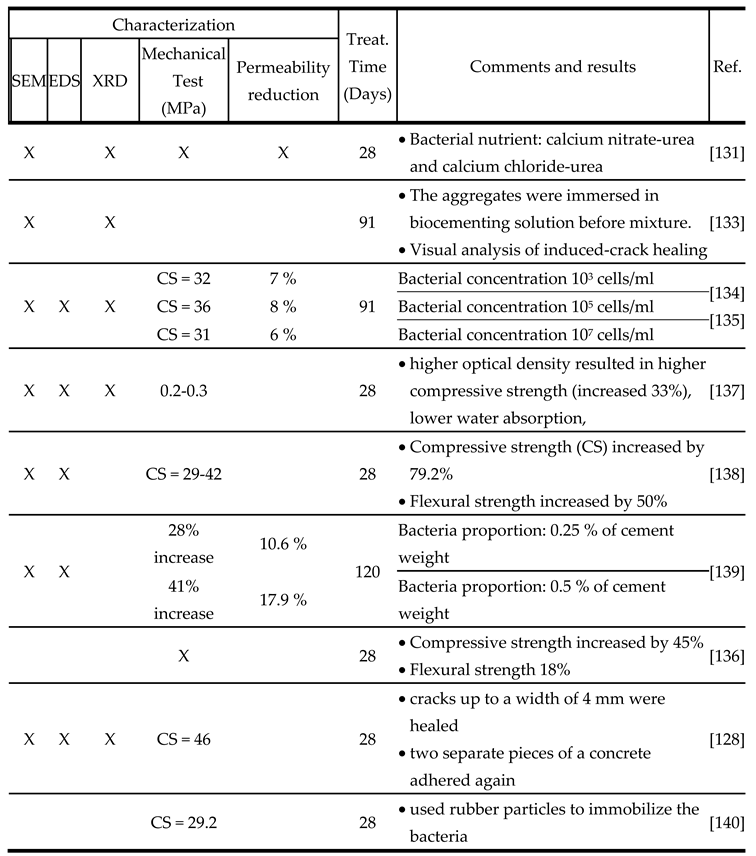
- Introducing bacteria as an admixture component in concrete.
- Curing the cast concrete for a period of 28 days.
- Inducing cracks in the concrete by applying either uniaxial or flexural force.
- Treating the damaged concrete with a bacterial nutrient media or treatment solution.
- Monitoring the healing progress of the crack over time.
- Conducting mechanical and permeability tests to characterize the healed concrete.
 |
5. Conclusions
5.1. Concluding Remarks
- The application of bio-cementation has demonstrated notable efficiency in different engineering sectors, exhibiting enhancements in mechanical properties and reduction in the permeability of construction materials at a laboratory scale. Moreover, the reduction in embodied energy (43-95%), carbon footprint (18-49.6%), and lifecycle cost-effectiveness render bio-cementation a significant step toward sustainable construction.
- Bio-cementation, whether produced by microbial or enzymatic agents (MICP or EICP), is a complex phenomenon whose kinetics can be influenced by various parameters related to the characteristics of bio-cementing agents (rate of urease activity and bio-cementing agent condition), environmental conditions (temperature, pH, and availability of nutrition and nucleation sites), and even the method that has been used to employ bio-cementation. The increase in urease activity rate of the bio-cementing agent, which is influenced by factors such as the type of bio-cementing agent, nutrition media, pH, and temperature, leads to an increase in the number of precipitated crystals and a simultaneous decrease in the crystals' dimensions. The influence of pH and temperature on bio-cementation depends upon the specific bio-cementing agent employed, as each bacteria possesses its own distinct ideal pH and temperature conditions for enzymatic activity.
- Among the different methods for introducing the bio-cementation agent into the system, the injection method is the primary technique used in soil stabilization. Despite the presence of several drawbacks and obstacles, which appear to be resolvable through the fine-tuning of the injection process, this method appears to be better suited for large-scale applications in this sector. Building construction, on the other hand, mainly uses bacteria or enzyme solutions and nutrition media as admixture components of construction material.
- Sporosarcina Pasteurii is widely recognized as the predominant microorganism employed for bio-cementation due to its superior performance in terms of both quantity and quality of the precipitated , as well as the enhanced permeability and mechanical properties of the treated material. S. Pasteurii has a biological mechanism that enables it to remain in a dormant state for an extended duration until it is exposed to a favorable environment where it can resume its activity of precipitating calcium carbonate. Moreover, it exhibits the ability to endure severe alkaline environments with a pH level as high as 13.6, as well as temperatures of 55 °C for a duration of 4 hours, while persisting in the process of precipitation, although at a reduced rate.
- The results of the comparison between samples treated with Microbially Induced Calcium Carbonate Precipitation (MICP) and samples treated with Enzyme-Induced Calcium Carbonate Precipitation (EICP) by S. Pasteurii indicate that the EICP treatment yields superior mechanical and permeability properties. However, the MICP process is favored by a larger number of investigations due to its cost-effectiveness. One of the reasons for this outcome is attributed to the disparity in size between the bacterium (on the order of micrometers) and the enzyme (on the order of nanometers), which facilitates the migration of enzyme within nucleation sites, leading to improved homogeneity and enhanced favorable properties.
- The granularity of the treated particles is a crucial factor that greatly influences the homogeneity of bio-cementation and the treatment outcomes. An excessive amount of either fine or coarse grains will lead to an uneven treatment outcome, resulting in poor mechanical and impermeability properties. The homogeneity and outcome of bio-cementation are enhanced when a wide range and variation of particle sizes are used.
5.2. Future Reasearch Directions
- While there is a large body of literature dedicated to using bio-cementation for new construction materials, exploiting this phenomenon for remediation of existing damaged construction (existing concrete structures) to extend their service lives and avert demolition, thereby decreasing a portion of the construction waste, has not progressed as much. For example, there is a scarcity of research that has explored the potential application of bio-cementation for repairing cracks in concrete structures that do not already contain a bio-cementing agent as an admixture.
- The current literature has approached employing bio-cementation from a construction standpoint, while the complexity of the bio-cementation phenomenon emphasizes the necessity of a multidisciplinary approach to exploiting bio-cementation at the intersection of biology, material engineering, and building construction, which is essential for addressing numerous unresolved inquiries pertaining to the exploitation of bio-cementation. Which bacterial strains or enzymatic agents demonstrate compatibility and suitability for applications in soil stabilization or building construction? Does the soil or construction material offer an ideal environment for the proper activity of the bio-cementing agent? Does achieving the optimal condition for the bio-cementing agent necessarily result in the attainment of optimal material qualities that are suited for the purposes of soil stabilization and construction applications?
- A key step in the process of scaling up bio-cementation is conducting a comprehensive and systematic analysis of the biological composition of bacterial urease activity and the impact of various environmental factors on the bacterial precipitation quantity and quality to gain a better understanding of the influential factor for one specific bacterium. Being able to describe the kinetics of precipitation as a function of pH, temperature, and nutrition media provides a more comprehensive understanding of potential results and constraints. For example, conducting a comprehensive investigation on the impact of temperature on the activity of urease in S. Pasteurii, spanning from -5 °C to 55 °C at 5 °C intervals, can provide valuable insights into the consequences of freezing, the kinetics of precipitation in relation to temperature, and the temperature thresholds for bacterial viability and urease enzyme denaturation. In this context, using a multidisciplinary strategy in addressing this subject offers a broader and more extensive opportunity for experimental characterization. For instance, Optical Density (OD), which is a widely utilized method in microbiology for assessing cell viability, in the context of microbiologically induced calcium carbonate precipitation (MICP), can offer valuable insights into the performance of bacteria and the kinetics of precipitation as a function of temperature, pH, and nutrition media, paving the way for significant input information for future numerical models.
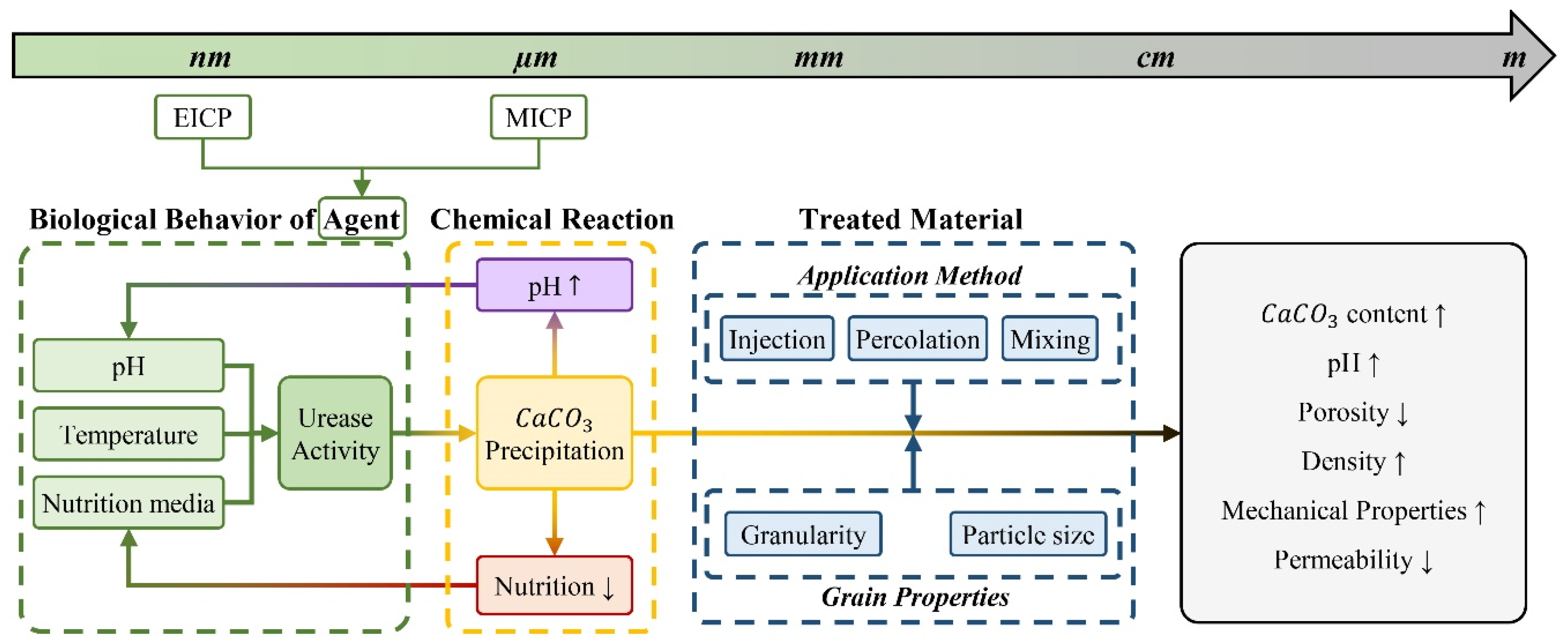
- Another underdeveloped aspect in the literature pertains to the numerical simulation models of bio-cementation. The significant constraints on studies are primarily attributed to the complex nature of the process, which occurs across different time and dimensional scales and entails interconnected biological, chemical, hydraulic and mechanical phenomena. The extremely limited research on this subject concentrated mostly on greater scales beginning with the pore in the treated material, without taking into account interrelated parameters [141,142]. Figure 5 presents a schematic illustration of the proposed flowchart by the author for the interrelated elements in bio-cementation that need to be taken into account as input for numerical modeling of this phenomenon, including pertinent disciplines and dimensional scales. The dimensional scale ranges from the nano or micro scale, depending on the use of EICP (nanometers) or MICP (micrometers), to the metric scale of treated construction material or soil. The multidisciplinary nature of bio-cementation involves the incorporation of diverse interrelated parameters from several disciplines at different stages as input. First, it is fundamental to establish the rate of urease activity from a biological perspective in relation to environmental conditions, including the initial pH, temperature, and nutrition media, to predict the rate and amount of precipitation. As stated in Section 2, the bio-cementation process produces ammonium as a byproduct, leading to a pH increase of 1 to 2 units (Figure 3). This pH alteration subsequently influences the rate of urease activity. Hence, the starting pH and pH variation as a function of time with calcium carbonate precipitation must be addressed when determining the urease activity rate. On the other hand, as precipitates, the availability of nutrition media for bio-cementing agents reduces with time, affecting urease activity once more. In parallel, parameters regarding treated material, such as application method and grain properties, that are specific for each application field, must be considered. For instance, in the case of soil stabilization by injection, the injection flow velocity and granularity of the treated soil are critical for fluid dynamic studies of injected solution propagation and bio-cementing agent migration. After taking into account all of these distinct characteristics from various disciplines on different dimensional scales, an accurate prediction of the bio-cementation process will be achievable.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Omoregie, A.I.; Palombo, E.A.; Ong, D.E.L.; Nissom, P.M. A Feasible Scale-up Production of Sporosarcina Pasteurii Using Custom-Built Stirred Tank Reactor for in-Situ Soil Biocementation. Biocatalysis and Agricultural Biotechnology 2020, 24, 101544. [Google Scholar] [CrossRef]
- Wani, K.M.N.S.; Mir, B.A. An Experimental Study on the Bio-Cementation and Bio-Clogging Effect of Bacteria in Improving Weak Dredged Soils. Geotech Geol Eng 2021, 39, 317–334. [Google Scholar] [CrossRef]
- Sharaky, A.M.; Mohamed, N.S.; Elmashad, M.E.; Shredah, N.M. Application of Microbial Biocementation to Improve the Physico-Mechanical Properties of Sandy Soil. Construction and Building Materials 2018, 190, 861–869. [Google Scholar] [CrossRef]
- Xu, K.; Huang, M.; Xu, C.; Zhen, J.; Jin, G.; Gong, H. Assessment of the Bio-Cementation Effect on Shale Soil Using Ultrasound Measurement. Soils and Foundations 2023, 63, 101249. [Google Scholar] [CrossRef]
- Dubey, A.A.; Ravi, K.; Mukherjee, A.; Sahoo, L.; Abiala, M.A.; Dhami, N.K. Biocementation Mediated by Native Microbes from Brahmaputra Riverbank for Mitigation of Soil Erodibility. Sci Rep 2021, 11, 15250. [Google Scholar] [CrossRef]
- Cheng, L.; Shahin, M.A.; Cord-Ruwisch, R. Bio-Cementation of Sandy Soil Using Microbially Induced Carbonate Precipitation for Marine Environments. Géotechnique 2014, 64, 1010–1013. [Google Scholar] [CrossRef]
- Abdel-Aleem, H.; Dishisha, T.; Saafan, A.; AbouKhadra, A.A.; Gaber, Y. Biocementation of Soil by Calcite/Aragonite Precipitation Using Pseudomonas Azotoformans and Citrobacter Freundii Derived Enzymes. RSC Adv. 2019, 9, 17601–17611. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wu, J.-X.; Zhang, K.; Shahin, M.A.; Cheng, L. Comparison between MICP-Based Bio-Cementation Versus Traditional Portland Cementation for Oil-Contaminated Soil Stabilisation. Sustainability 2022, 15, 434. [Google Scholar] [CrossRef]
- Venda Oliveira, P.J.; Neves, J.P.G. Effect of Organic Matter Content on Enzymatic Biocementation Process Applied to Coarse-Grained Soils. J. Mater. Civ. Eng. 2019, 31, 04019121. [Google Scholar] [CrossRef]
- Whiffin, V.S.; van Paassen, L.A.; Harkes, M.P. Microbial Carbonate Precipitation as a Soil Improvement Technique. Geomicrobiology Journal 2007, 24, 417–423. [Google Scholar] [CrossRef]
- Mujah, D.; Shahin, M.A.; Cheng, L. State-of-the-Art Review of Biocementation by Microbially Induced Calcite Precipitation (MICP) for Soil Stabilization. Geomicrobiology Journal 2017, 34, 524–537. [Google Scholar] [CrossRef]
- Omoregie, A.I.; Palombo, E.A.; Nissom, P.M. Bioprecipitation of Calcium Carbonate Mediated by Ureolysis: A Review. Environmental Engineering Research 2020, 26, 200379–0. [Google Scholar] [CrossRef]
- Iqbal, D.M.; Wong, L.S.; Kong, S.Y. Bio-Cementation in Construction Materials: A Review. Materials 2021, 14, 2175. [Google Scholar] [CrossRef]
- Huynh, N.N.T.; Imamoto, K.; Kiyohara, C. A Study on Biomineralization Using Bacillus Subtilis Natto for Repeatability of Self-Healing Concrete and Strength Improvement. ACT 2019, 17, 700–714. [Google Scholar] [CrossRef]
- Bagga, M.; Hamley-Bennett, C.; Alex, A.; Freeman, B.L.; Justo-Reinoso, I.; Mihai, I.C.; Gebhard, S.; Paine, K.; Jefferson, A.D.; Masoero, E.; et al. Advancements in Bacteria Based Self-Healing Concrete and the Promise of Modelling. Construction and Building Materials 2022, 358, 129412. [Google Scholar] [CrossRef]
- Huynh, N.N.T.; Phuong, N.M.; Toan, N.P.A.; Son, N.K. Bacillus Subtilis HU58 Immobilized in Micropores of Diatomite for Using in Self-Healing Concrete. Procedia Engineering 2017, 171, 598–605. [Google Scholar] [CrossRef]
- Huynh, N.N.T.; Imamoto, K.; Kiyohara, C. Biomineralization Analysis and Hydration Acceleration Effect in Self-Healing Concrete Using Bacillus Subtilis Natto. ACT 2022, 20, 609–623. [Google Scholar] [CrossRef]
- Huynh, N.; Imamoto, K.; Kiyohara, C. Compressive Strength Improvement and Water Permeability of Self-Healing Concrete Using Bacillus Subtilis Natto. In Proceedings of the XV International Conference on Durability of Building Materials and Components. eBook of Proceedings; CIMNE, 2020.
- Joshi, S.; Goyal, S.; Reddy, M.S. Corn Steep Liquor as a Nutritional Source for Biocementation and Its Impact on Concrete Structural Properties. Journal of Industrial Microbiology and Biotechnology 2018, 45, 657–667. [Google Scholar] [CrossRef]
- Wu, M.; Hu, X.; Zhang, Q.; Xue, D.; Zhao, Y. Growth Environment Optimization for Inducing Bacterial Mineralization and Its Application in Concrete Healing. Construction and Building Materials 2019, 209, 631–643. [Google Scholar] [CrossRef]
- Krishnapriya, S.; Venkatesh Babu, D.L.; G., P.A. Isolation and Identification of Bacteria to Improve the Strength of Concrete. Microbiological Research 2015, 174, 48–55. [Google Scholar] [CrossRef]
- Castro-Alonso, M.J.; Montañez-Hernandez, L.E.; Sanchez-Muñoz, M.A.; Macias Franco, M.R.; Narayanasamy, R.; Balagurusamy, N. Microbially Induced Calcium Carbonate Precipitation (MICP) and Its Potential in Bioconcrete: Microbiological and Molecular Concepts. Front. Mater. 2019, 6, 126. [Google Scholar] [CrossRef]
- Devrani, R.; Dubey, A.A.; Ravi, K.; Sahoo, L. Applications of Bio-Cementation and Bio-Polymerization for Aeolian Erosion Control. Journal of Arid Environments 2021, 187, 104433. [Google Scholar] [CrossRef]
- Fattahi, S.M.; Soroush, A.; Huang, N. Biocementation Control of Sand against Wind Erosion. J. Geotech. Geoenviron. Eng. 2020, 146, 04020045. [Google Scholar] [CrossRef]
- Dubey, A.A.; Devrani, R.; Ravi, K.; Dhami, N.K.; Mukherjee, A.; Sahoo, L. Experimental Investigation to Mitigate Aeolian Erosion via Biocementation Employed with a Novel Ureolytic Soil Isolate. Aeolian Research 2021, 52, 100727. [Google Scholar] [CrossRef]
- Zomorodian, S.M.A.; Ghaffari, H.; O’Kelly, B.C. Stabilisation of Crustal Sand Layer Using Biocementation Technique for Wind Erosion Control. Aeolian Research 2019, 40, 34–41. [Google Scholar] [CrossRef]
- Marvasi, M.; Mastromei, G.; Perito, B. Bacterial Calcium Carbonate Mineralization in Situ Strategies for Conservation of Stone Artworks: From Cell Components to Microbial Community. Front. Microbiol. 2020, 11, 1386. [Google Scholar] [CrossRef]
- Phillips, A.J.; Eldring, J. (Joe); Hiebert, R.; Lauchnor, E.; Mitchell, A.C.; Cunningham, A.; Spangler, L.; Gerlach, R. Design of a Meso-Scale High Pressure Vessel for the Laboratory Examination of Biogeochemical Subsurface Processes. Journal of Petroleum Science and Engineering 2015, 126, 55–62. [Google Scholar] [CrossRef]
- Cunningham, A.B.; Class, H.; Ebigbo, A.; Gerlach, R.; Phillips, A.J.; Hommel, J. Field-Scale Modeling of Microbially Induced Calcite Precipitation. Comput Geosci 2019, 23, 399–414. [Google Scholar] [CrossRef]
- Ariyanti, D. Feasibility of Using Microalgae for Biocement Production through Biocementation. J Bioproces Biotechniq 2012, 02. [Google Scholar] [CrossRef]
- Jansson, C.; Northen, T. Calcifying Cyanobacteria—the Potential of Biomineralization for Carbon Capture and Storage. Current Opinion in Biotechnology 2010, 21, 365–371. [Google Scholar] [CrossRef]
- Yu, X.; Chu, J.; Yang, Y.; Qian, C. Reduction of Ammonia Production in the Biocementation Process for Sand Using a New Biocement. Journal of Cleaner Production 2021, 286, 124928. [Google Scholar] [CrossRef]
- Porter, H.; Mukherjee, A.; Tuladhar, R.; Dhami, N.K. Life Cycle Assessment of Biocement: An Emerging Sustainable Solution? Sustainability 2021, 13, 13878. [Google Scholar] [CrossRef]
- Wu, C.; Chu, J.; Wu, S.; Hong, Y. 3D Characterization of Microbially Induced Carbonate Precipitation in Rock Fracture and the Resulted Permeability Reduction. Engineering Geology 2019, 249, 23–30. [Google Scholar] [CrossRef]
- Wang, Y.; Soga, K.; Dejong, J.T.; Kabla, A.J. Microscale Visualization of Microbial-Induced Calcium Carbonate Precipitation Processes. Journal of Geotechnical and Geoenvironmental Engineering 2019, 145. [Google Scholar] [CrossRef]
- Oktafiani, P.G.; Putra, H.; Erizal; Yanto, D.H.Y. Application of Technical Grade Reagent in Soybean-Crude Urease Calcite Precipitation (SCU-CP) Method for Soil Improvement Technique. Physics and Chemistry of the Earth, Parts A/B/C 2022, 128, 103292. [Google Scholar] [CrossRef]
- Omoregie, A.I.; Palombo, E.A.; Ong, D.E.L.; Nissom, P.M. Biocementation of Sand by Sporosarcina Pasteurii Strain and Technical-Grade Cementation Reagents through Surface Percolation Treatment Method. Construction and Building Materials 2019, 228, 116828. [Google Scholar] [CrossRef]
- Mutitu, K.D.; Munyao, M.O.; Wachira, M.J.; Mwirichia, R.; Thiong’o, K.J.; Marangu, M.J. Effects of Biocementation on Some Properties of Cement-Based Materials Incorporating Bacillus Species Bacteria – a Review. Journal of Sustainable Cement-Based Materials 2019, 8, 309–325. [Google Scholar] [CrossRef]
- Montaño-Salazar, S.M.; Lizarazo-Marriaga, J.; Brandão, P.F.B. Isolation and Potential Biocementation of Calcite Precipitation Inducing Bacteria from Colombian Buildings. Curr Microbiol 2018, 75, 256–265. [Google Scholar] [CrossRef]
- Perito, B.; Marvasi, M.; Barabesi, C.; Mastromei, G.; Bracci, S.; Vendrell, M.; Tiano, P. A Bacillus Subtilis Cell Fraction (BCF) Inducing Calcium Carbonate Precipitation: Biotechnological Perspectives for Monumental Stone Reinforcement. Journal of Cultural Heritage 2014, 15, 345–351. [Google Scholar] [CrossRef]
- Shu, S.; Yan, B.; Ge, B.; Li, S.; Meng, H. Factors Affecting Soybean Crude Urease Extraction and Biocementation via Enzyme-Induced Carbonate Precipitation (EICP) for Soil Improvement. Energies 2022, 15, 5566. [Google Scholar] [CrossRef]
- Rohy, H.; Arab, M.; Zeiada, W.; Omar, M.; Almajed, A.; Tahmaz, A. One Phase Soil Bio-Cementation with EICP-Soil Mixing.; April 2019.
- Zehner, J.; Røyne, A.; Sikorski, P. A Sample Cell for the Study of Enzyme-Induced Carbonate Precipitation at the Grain-Scale and Its Implications for Biocementation. Sci Rep 2021, 11, 13675. [Google Scholar] [CrossRef]
- Guan, D.; Zhou, Y.; Shahin, M.A.; Khodadadi Tirkolaei, H.; Cheng, L. Assessment of Urease Enzyme Extraction for Superior and Economic Bio-Cementation of Granular Materials Using Enzyme-Induced Carbonate Precipitation. Acta Geotech. 2023, 18, 2263–2279. [Google Scholar] [CrossRef]
- Miftah, A.; Khodadadi Tirkolaei, H.; Bilsel, H. Biocementation of Calcareous Beach Sand Using Enzymatic Calcium Carbonate Precipitation. Crystals 2020, 10, 888. [Google Scholar] [CrossRef]
- Hoang, T.; Alleman, J.; Cetin, B.; Choi, S.-G. Engineering Properties of Biocementation Coarse- and Fine-Grained Sand Catalyzed By Bacterial Cells and Bacterial Enzyme. J. Mater. Civ. Eng. 2020, 32, 04020030. [Google Scholar] [CrossRef]
- Murugan, R.; Suraishkumar, G.K.; Mukherjee, A.; Dhami, N.K. Influence of Native Ureolytic Microbial Community on Biocementation Potential of Sporosarcina Pasteurii. Sci Rep 2021, 11, 20856. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.; Pedreira, R.; Duarte, S.O.D.; Monteiro, G.A. About Calcium Carbonate Precipitation on Sand Biocementation. Engineering Geology 2020, 271, 105612. [Google Scholar] [CrossRef]
- Mahawish, A.; Bouazza, A.; Gates, W.P. Effect of Particle Size Distribution on the Bio-Cementation of Coarse Aggregates. Acta Geotech. 2018, 13, 1019–1025. [Google Scholar] [CrossRef]
- Cardoso, R.; Pires, I.; Duarte, S.O.D.; Monteiro, G.A. Effects of Clay’s Chemical Interactions on Biocementation. Applied Clay Science 2018, 156, 96–103. [Google Scholar] [CrossRef]
- Ashraf, M.S.; Hassan Shah, M.U.; Bokhari, A.; Hasan, M. Less Is More: Optimising the Biocementation of Coastal Sands by Reducing Influent Urea through Response Surface Method. Journal of Cleaner Production 2021, 315, 128208. [Google Scholar] [CrossRef]
- Graddy, C.M.R.; Gomez, M.G.; DeJong, J.T.; Nelson, D.C. Native Bacterial Community Convergence in Augmented and Stimulated Ureolytic MICP Biocementation. Environ. Sci. Technol. 2021, 55, 10784–10793. [Google Scholar] [CrossRef]
- Cui, M.-J.; Zheng, J.-J.; Zhang, R.-J.; Lai, H.-J. Soil Bio-Cementation Using an Improved 2-Step Injection Method. Arab J Geosci 2020, 13, 1270. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Wu, L.; Wang, C. Study of Magnesium Precipitation Based on Biocementation. Marine Georesources & Geotechnology 2019, 37, 1257–1266. [Google Scholar] [CrossRef]
- Mahawish, A.; Bouazza, A.; Gates, W.P. Unconfined Compressive Strength and Visualization of the Microstructure of Coarse Sand Subjected to Different Biocementation Levels. J. Geotech. Geoenviron. Eng. 2019, 145, 04019033. [Google Scholar] [CrossRef]
- Wani, K.M.N.S.; Mir, B.A. A Laboratory-Scale Study on the Bio-Cementation Potential of Distinct River Sediments Infused with Microbes. Transp. Infrastruct. Geotech. 2021, 8, 162–185. [Google Scholar] [CrossRef]
- Tri Huynh, N.N.; Quynh Nhu, N.; Khanh Son, N. Developing the Solution of Microbially Induced CaCO 3 Precipitation Coating for Cement Concrete. IOP Conf. Ser.: Mater. Sci. Eng. 2018, 431, 062006. [Google Scholar] [CrossRef]
- Rao, M.V.S.; Reddy, V.S.; Sasikala, Ch. Performance of Microbial Concrete Developed Using Bacillus Subtilus JC3. J. Inst. Eng. India Ser. A 2017, 98, 501–510. [Google Scholar] [CrossRef]
- Nguyen, H.N.T.; Nguyen, S.K. Use of Bio-Active Bacillus Subtilis Bacteria to Form Self-Healing Concrete. Sci. Tech. Dev. J. 2014, 17, 76–86. [Google Scholar] [CrossRef]
- Fang, C.; He, J.; Achal, V.; Plaza, G. Tofu Wastewater as Efficient Nutritional Source in Biocementation for Improved Mechanical Strength of Cement Mortars. Geomicrobiology Journal 2019, 36, 515–521. [Google Scholar] [CrossRef]
- Bhutange, S.P.; Latkar, M.V.; Chakrabarti, T. Studies on Efficacy of Biocementation of Cement Mortar Using Soil Extract. Journal of Cleaner Production 2020, 274, 122687. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Wu, L.; Chen, R. Improvement of Bio-Cementation at Low Temperature Based on Bacillus Megaterium. Appl Microbiol Biotechnol 2019, 103, 7191–7202. [Google Scholar] [CrossRef]
- Kaur, G.; Dhami, N.K.; Goyal, S.; Mukherjee, A.; Reddy, M.S. Utilization of Carbon Dioxide as an Alternative to Urea in Biocementation. Construction and Building Materials 2016, 123, 527–533. [Google Scholar] [CrossRef]
- Phua, Y.J.; Røyne, A. Bio-Cementation through Controlled Dissolution and Recrystallization of Calcium Carbonate. Construction and Building Materials 2018, 167, 657–668. [Google Scholar] [CrossRef]
- Wormser, G.P.; Stratton, C. Manual of Clinical Microbiology, 9th Edition Edited by Patrick R. Murray, Ellen Jo Baron, James H. Jorgensen, Marie Louise Landry, and Michael A. Pfaller Washington, DC: ASM Press, 2007 2488 Pp., Illustrated. $209.95 (Hardcover). Clinical Infectious Diseases 2008, 46, 153. [Google Scholar] [CrossRef]
- Bacterial Endospores | CALS. Available online: https://cals.cornell.edu/microbiology/research/active-research-labs/angert-lab/epulopiscium/bacterial-endospores (accessed on 4 August 2023).
- Gomez, M.G.; Graddy, C.M.R.; DeJong, J.T.; Nelson, D.C. Biogeochemical Changes During Bio-Cementation Mediated by Stimulated and Augmented Ureolytic Microorganisms. Sci Rep 2019, 9, 11517. [Google Scholar] [CrossRef]
- Behzadipour, H.; Sadrekarimi, A. Biochar-Assisted Bio-Cementation of a Sand Using Native Bacteria. Bull Eng Geol Environ 2021, 80, 4967–4984. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, L.; Li, C.; Zhang, H.; Amini, F. A Full Contact Flexible Mold for Preparing Samples Based on Microbial-Induced Calcite Precipitation Technology. Geotech. Test. J. 2014, 37, 917–921. [Google Scholar] [CrossRef]
- Wen, K.; Li, Y.; Liu, S.; Bu, C.; Li, L. Development of an Improved Immersing Method to Enhance Microbial Induced Calcite Precipitation Treated Sandy Soil through Multiple Treatments in Low Cementation Media Concentration. Geotech Geol Eng 2019, 37, 1015–1027. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Z.; Wang, J. Mechanical Properties of Bio-Cementation Materials in Pre-Precipitation Mixing Process. Environ Sci Pollut Res 2022, 29, 1314–1323. [Google Scholar] [CrossRef]
- Nething, C.; Smirnova, M.; Gröning, J.A.D.; Haase, W.; Stolz, A.; Sobek, W. A Method for 3D Printing Bio-Cemented Spatial Structures Using Sand and Urease Active Calcium Carbonate Powder. Materials & Design 2020, 195, 109032. [Google Scholar] [CrossRef]
- Erdmann, N.; Kästner, F.; de Payrebrune, K.; Strieth, D. Sporosarcina Pasteurii Can Be Used to Print a Layer of Calcium Carbonate. Engineering in Life Sciences 2022, 22, 760–768. [Google Scholar] [CrossRef]
- Hirsch, M.; Lucherini, L.; Zhao, R.; Clarà Saracho, A.; Amstad, E. 3D Printing of Living Structural Biocomposites. Materials Today 2023, 62, 21–32. [Google Scholar] [CrossRef]
- Tian, Z.; Tang, X.; Xiu, Z.; Xue, Z. The Spatial Distribution of Microbially Induced Carbonate Precipitation in Sand Column with Different Grouting Strategies. J. Mater. Civ. Eng. 2023, 35, 04022437. [Google Scholar] [CrossRef]
- van Paassen, L.A.; Ghose, R.; van der Linden, T.J.M.; van der Star, W.R.L.; van Loosdrecht, M.C.M. Quantifying Biomediated Ground Improvement by Ureolysis: Large-Scale Biogrout Experiment. ASCE J Soil Mech Found Div 2010, 136, 1721–1728. [Google Scholar] [CrossRef]
- Harkes, M.P.; van Paassen, L.A.; Booster, J.L.; Whiffin, V.S.; van Loosdrecht, M.C.M. Fixation and Distribution of Bacterial Activity in Sand to Induce Carbonate Precipitation for Ground Reinforcement. Ecological Engineering 2010, 36, 112–117. [Google Scholar] [CrossRef]
- Ginn, T.R.; Murphy, E.M.; Chilakapati, A.; Seeboonruang, U. Stochastic-Convective Transport with Nonlinear Reaction and Mixing: Application to Intermediate-Scale Experiments in Aerobic Biodegradation in Saturated Porous Media. Journal of Contaminant Hydrology 2001, 48, 121–149. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Chen, R. The Application of Bio-Cementation for Improvement in Collapsibility of Loess. Int. J. Environ. Sci. Technol. 2021, 18, 2607–2618. [Google Scholar] [CrossRef]
- Cheng, L.; Shahin, M.A.; Cord-Ruwisch, R. Surface Percolation for Soil Improvement by Biocementation Utilizing In Situ Enriched Indigenous Aerobic and Anaerobic Ureolytic Soil Microorganisms. Geomicrobiology Journal 2017, 34, 546–556. [Google Scholar] [CrossRef]
- Yang, Y.; Chu, J.; Liu, H.; Cheng, L. Construction of Water Pond Using Bioslurry-Induced Biocementation. J. Mater. Civ. Eng. 2022, 34, 06021009. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Wang, H.; Chen, R.; Wu, L. Bio-Cementation for the Mitigation of Surface Erosion in Loess Slopes Based on Simulation Experiment. J Soils Sediments 2022, 22, 1804–1818. [Google Scholar] [CrossRef]
- Cheng, L.; Cord-Ruwisch, R. Upscaling Effects of Soil Improvement by Microbially Induced Calcite Precipitation by Surface Percolation. Geomicrobiology Journal 2014, 31, 396–406. [Google Scholar] [CrossRef]
- Lai, H.-J.; Cui, M.-J.; Chu, J. Effect of PH on Soil Improvement Using One-Phase-Low-PH MICP or EICP Biocementation Method. Acta Geotech. 2022. [Google Scholar] [CrossRef]
- Joshi, S.; Goyal, S.; Reddy, M.S. Influence of Nutrient Components of Media on Structural Properties of Concrete during Biocementation. Construction and Building Materials 2018, 158, 601–613. [Google Scholar] [CrossRef]
- Williams, S.L.; Kirisits, M.J.; Ferron, R.D. Optimization of Growth Medium for Sporosarcina Pasteurii in Bio-Based Cement Pastes to Mitigate Delay in Hydration Kinetics. Journal of Industrial Microbiology and Biotechnology 2016, 43, 567–575. [Google Scholar] [CrossRef]
- Dhami, N.K.; Reddy, M.S.; Mukherjee, A. Significant Indicators for Biomineralisation in Sand of Varying Grain Sizes. Construction and Building Materials 2016, 104, 198–207. [Google Scholar] [CrossRef]
- Heveran, C.M.; Liang, L.; Nagarajan, A.; Hubler, M.H.; Gill, R.; Cameron, J.C.; Cook, S.M.; Srubar, W.V. Engineered Ureolytic Microorganisms Can Tailor the Morphology and Nanomechanical Properties of Microbial-Precipitated Calcium Carbonate. Sci Rep 2019, 9, 14721. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Soga, K.; DeJong, J.T.; Kabla, A.J. Microscale Investigations of Temperature-Dependent Microbially Induced Carbonate Precipitation (MICP) in the Temperature Range 4–50 °C. Acta Geotech. 2023, 18, 2239–2261. [Google Scholar] [CrossRef]
- Peterson, M.E.; Daniel, R.M.; Danson, M.J.; Eisenthal, R. The Dependence of Enzyme Activity on Temperature: Determination and Validation of Parameters. Biochem J 2007, 402, 331–337. [Google Scholar] [CrossRef]
- Aytekin, B.; Mardani, A.; Yazıcı, Ş. State-of-Art Review of Bacteria-Based Self-Healing Concrete: Biomineralization Process, Crack Healing, and Mechanical Properties. Construction and Building Materials 2023, 378, 131198. [Google Scholar] [CrossRef]
- Joshi, K.A.; Kumthekar, M.B.; Ghodake, V.P. Bacillus Subtilis Bacteria Impregnation in Concrete for Enhancement in Compressive Strength. 03.
- De Leeuw, N.H.; Parker, S.C. Surface Structure and Morphology of Calcium Carbonate Polymorphs Calcite, Aragonite, and Vaterite: An Atomistic Approach. Journal of Physical Chemistry B 1998, 102, 2914–2922. [Google Scholar] [CrossRef]
- Liu, L.; Liu, H.; Xiao, Y.; Chu, J.; Xiao, P.; Wang, Y. Biocementation of Calcareous Sand Using Soluble Calcium Derived from Calcareous Sand. Bull Eng Geol Environ 2018, 77, 1781–1791. [Google Scholar] [CrossRef]
- Choi, S.-G.; Park, S.-S.; Wu, S.; Chu, J. Methods for Calcium Carbonate Content Measurement of Biocemented Soils. Journal of Materials in Civil Engineering 2017, 29, 06017015. [Google Scholar] [CrossRef]
- ASTM D2166/D2166M-16: Standard Test Method for Unconfined Compressive Strength of Cohesive Soil.
- ASTM D1883-21: Standard Test Method for California Bearing Ratio (CBR) of Laboratory-Compacted Soils.
- ASTM D3441-16: Standard Test Method for Mechanical Cone Penetration Testing of Soils.
- Wu, M.; Hu, X.; Zhang, Q.; Xue, D.; Zhao, Y. Growth Environment Optimization for Inducing Bacterial Mineralization and Its Application in Concrete Healing. Construction and Building Materials 2019, 209, 631–643. [Google Scholar] [CrossRef]
- ASTM D2434-22: Standard Test Methods for Measurement of Hydraulic Conductivity of Coarse-Grained Soils.
- ASTM D2435/D2435M-11(2020): Standard Test Methods for One-Dimensional Consolidation Properties of Soils Using Incremental Loading.
- ASTM C1585-20: Standard Test Method for Measurement of Rate of Absorption of Water by Hydraulic-Cement Concretes.
- ASTM D5856-15: Standard Test Method for Measurement of Hydraulic Conductivity of Porous Material Using a Rigid-Wall, Compaction-Mold Permeameter.
- Xu, X.; Guo, H.; Li, M.; Deng, X. Bio-Cementation Improvement via CaCO3 Cementation Pattern and Crystal Polymorph: A Review. Construction and Building Materials 2021, 297, 123478. [Google Scholar] [CrossRef]
- Cheng, L.; Cord-Ruwisch, R. In Situ Soil Cementation with Ureolytic Bacteria by Surface Percolation. Ecological Engineering 2012, 42, 64–72. [Google Scholar] [CrossRef]
- Terzis, D.; Laloui, L. Cell-Free Soil Bio-Cementation with Strength, Dilatancy and Fabric Characterization. Acta Geotech. 2019, 14, 639–656. [Google Scholar] [CrossRef]
- Elmaloglou, A.; Terzis, D.; De Anna, P.; Laloui, L. Microfluidic Study in a Meter-Long Reactive Path Reveals How the Medium’s Structural Heterogeneity Shapes MICP-Induced Biocementation. Sci Rep 2022, 12, 19553. [Google Scholar] [CrossRef]
- Maleki-Kakelar, M.; Azarhoosh, M.J.; Golmohammadi Senji, S.; Aghaeinejad-Meybodi, A. Urease Production Using Corn Steep Liquor as a Low-Cost Nutrient Source by Sporosarcina Pasteurii: Biocementation and Process Optimization via Artificial Intelligence Approaches. Environ Sci Pollut Res 2022, 29, 13767–13781. [Google Scholar] [CrossRef]
- Mortazavi Bak, H.; Kariminia, T.; Shahbodagh, B.; Rowshanzamir, M.A.; Khoshghalb, A. Application of Bio-Cementation to Enhance Shear Strength Parameters of Soil-Steel Interface. Construction and Building Materials 2021, 294, 123470. [Google Scholar] [CrossRef]
- Yu, X.; Pan, X. Seawater Based Bio-Cementation for Calcareous Sand Improvement in Marine Environment. Marine Georesources & Geotechnology 2022, 1–10. [Google Scholar] [CrossRef]
- Bhaduri, S.; Debnath, N.; Mitra, S.; Liu, Y.; Kumar, A. Microbiologically Induced Calcite Precipitation Mediated by Sporosarcina Pasteurii. J Vis Exp 2016, 53253. [Google Scholar] [CrossRef]
- Henze, J.; Randall, D.G. Microbial Induced Calcium Carbonate Precipitation at Elevated PH Values (>11) Using Sporosarcina Pasteurii. Journal of Environmental Chemical Engineering 2018, 6, 5008–5013. [Google Scholar] [CrossRef]
- Optimizing the Use of Sporosarcina Pasteurii Bacteria for the Stiffening of Sand. Available online: http://www.envirobiotechjournals.com/article_abstract.php?aid=6965&iid=212&jid=1 (accessed on 4 August 2023).
- Onal Okyay, T.; Frigi Rodrigues, D. Optimized Carbonate Micro-Particle Production by Sporosarcina Pasteurii Using Response Surface Methodology. Ecological Engineering 2014, 62, 168–174. [Google Scholar] [CrossRef]
- Williams, S.L.; Kirisits, M.J.; Ferron, R.D. Influence of Concrete-Related Environmental Stressors on Biomineralizing Bacteria Used in Self-Healing Concrete. Construction and Building Materials 2017, 139, 611–618. [Google Scholar] [CrossRef]
- Dosier, G.K. Production of Masonry with Bacteria 2017.
- Biolith® Tile from Biomason | Build with Biolith. Available online: https://biomason.com/biolith (accessed on 30 March 2023).
- Lin, H.; Suleiman, M.T.; Brown, D.G.; Kavazanjian, E. Mechanical Behavior of Sands Treated by Microbially Induced Carbonate Precipitation. Journal of Geotechnical and Geoenvironmental Engineering 2016, 142, 04015066. [Google Scholar] [CrossRef]
- Filet, A.E.; Gadret, J.-P.; Loygue, M.; Borel, S. Biocalcis and Its Applications for the Consolidation of Sands. 2012, 1767–1780. [Google Scholar] [CrossRef]
- Gomez, M.G.; Martinez, B.C.; DeJong, J.T.; Hunt, C.E.; deVlaming, L.A.; Major, D.W.; Dworatzek, S.M. Field-Scale Bio-Cementation Tests to Improve Sands. Proceedings of the Institution of Civil Engineers - Ground Improvement 2015, 168, 206–216. [Google Scholar] [CrossRef]
- Phillips, A.J.; Troyer, E.; Hiebert, R.; Kirkland, C.; Gerlach, R.; Cunningham, A.B.; Spangler, L.; Kirksey, J.; Rowe, W.; Esposito, R. Enhancing Wellbore Cement Integrity with Microbially Induced Calcite Precipitation (MICP): A Field Scale Demonstration. Journal of Petroleum Science and Engineering 2018, 171, 1141–1148. [Google Scholar] [CrossRef]
- Van Paassen, L.A. Biogrout, Ground Improvement by Microbial Induced Carbonate Precipitation. 2009. [Google Scholar]
- Graddy, C.M.R.; Gomez, M.G.; Kline, L.M.; Morrill, S.R.; DeJong, J.T.; Nelson, D.C. Diversity of Sporosarcina -like Bacterial Strains Obtained from Meter-Scale Augmented and Stimulated Biocementation Experiments. Environ. Sci. Technol. 2018, 52, 3997–4005. [Google Scholar] [CrossRef]
- Lee, M.; Gomez, M.G.; El Kortbawi, M.; Ziotopoulou, K. Effect of Light Biocementation on the Liquefaction Triggering and Post-Triggering Behavior of Loose Sands. J. Geotech. Geoenviron. Eng. 2022, 148, 04021170. [Google Scholar] [CrossRef]
- Gomez, M.G.; Anderson, C.M.; Graddy, C.M.R.; DeJong, J.T.; Nelson, D.C.; Ginn, T.R. Large-Scale Comparison of Bioaugmentation and Biostimulation Approaches for Biocementation of Sands. J. Geotech. Geoenviron. Eng. 2017, 143, 04016124. [Google Scholar] [CrossRef]
- Hang, L.; Gao, Y.; van Paassen, L.A.; He, J.; Wang, L.; Li, C. Microbially Induced Carbonate Precipitation for Improving the Internal Stability of Silty Sand Slopes under Seepage Conditions. Acta Geotech. 2022. [Google Scholar] [CrossRef]
- Lai, H.-J.; Cui, M.-J.; Wu, S.-F.; Yang, Y.; Chu, J. Retarding Effect of Concentration of Cementation Solution on Biocementation of Soil. Acta Geotech. 2021, 16, 1457–1472. [Google Scholar] [CrossRef]
- Sohail, M.G.; Disi, Z.A.; Zouari, N.; Nuaimi, N.A.; Kahraman, R.; Gencturk, B.; Rodrigues, D.F.; Yildirim, Y. Bio Self-Healing Concrete Using MICP by an Indigenous Bacillus Cereus Strain Isolated from Qatari Soil. Construction and Building Materials 2022, 328, 126943. [Google Scholar] [CrossRef]
- Achal, V.; Mukherjee, A.; Basu, P.C.; Reddy, M.S. Lactose Mother Liquor as an Alternative Nutrient Source for Microbial Concrete Production by Sporosarcina Pasteurii. Journal of Industrial Microbiology and Biotechnology 2009, 36, 433–438. [Google Scholar] [CrossRef]
- Chidara, R.; Nagulagama, R.; Yadav, S. Achievement of Early Compressive Strength in Concrete Using Sporosarcina Pasteurii Bacteria as an Admixture. Advances in Civil Engineering 2014, 2014, e435948. [Google Scholar] [CrossRef]
- Mirshahmohammad, M.; Rahmani, H.; Maleki-Kakelar, M.; Bahari, A. Performance of Biological Methods on Self-Healing and Mechanical Properties of Concrete Using S. Pasteurii. Environ Sci Pollut Res 2023, 30, 2128–2144. [Google Scholar] [CrossRef]
- Kim, H.K.; Park, S.J.; Han, J.I.; Lee, H.K. Microbially Mediated Calcium Carbonate Precipitation on Normal and Lightweight Concrete. Construction and Building Materials 2013, 38, 1073–1082. [Google Scholar] [CrossRef]
- Chen, H.-J.; Peng, C.-F.; Tang, C.-W.; Chen, Y.-T. Self-Healing Concrete by Biological Substrate. Materials 2019, 12, 4099. [Google Scholar] [CrossRef]
- Chahal, N.; Siddique, R. Permeation Properties of Concrete Made with Fly Ash and Silica Fume: Influence of Ureolytic Bacteria. Construction and Building Materials 2013, 49, 161–174. [Google Scholar] [CrossRef]
- Chahal, N.; Siddique, R.; Rajor, A. Influence of Bacteria on the Compressive Strength, Water Absorption and Rapid Chloride Permeability of Concrete Incorporating Silica Fume. Construction and Building Materials 2012, 37, 645–651. [Google Scholar] [CrossRef]
- Zaerkabeh, R.; Sadeghi, A.M.; Afshin, H.; Majdani, R. Crack Healing and Mechanical Properties of Bacteria-Based Self-Healing Cement Mortar. Periodica Polytechnica Civil Engineering 2022, 66, 581–592. [Google Scholar] [CrossRef]
- Abo-El-Enein, S.A.; Ali, A.H.; Talkhan, F.N.; Abdel-Gawwad, H.A. Application of Microbial Biocementation to Improve the Physico-Mechanical Properties of Cement Mortar. HBRC Journal 2013, 9, 36–40. [Google Scholar] [CrossRef]
- Metwally, G.A.M.; Mahdy, M.; Abd El-Raheem, A.E.-R.H. Performance of Bio Concrete by Using Bacillus Pasteurii Bacteria. Civ Eng J 2020, 6, 1443–1456. [Google Scholar] [CrossRef]
- Nasser, A.A.; Sorour, N.M.; Saafan, M.A.; Abbas, R.N. Microbially-Induced-Calcite-Precipitation (MICP): A Biotechnological Approach to Enhance the Durability of Concrete Using Bacillus Pasteurii and Bacillus Sphaericus. Heliyon 2022, 8, e09879. [Google Scholar] [CrossRef]
- Xu, H.; Lian, J.; Gao, M.; Fu, D.; Yan, Y. Self-Healing Concrete Using Rubber Particles to Immobilize Bacterial Spores. Materials 2019, 12, 2313. [Google Scholar] [CrossRef]
- Qin, C.-Z.; Hassanizadeh, S.M.; Ebigbo, A. Pore-Scale Network Modeling of Microbially Induced Calcium Carbonate Precipitation: Insight into Scale Dependence of Biogeochemical Reaction Rates. Water Resources Research 2016, 52, 8794–8810. [Google Scholar] [CrossRef]
- Bagga, M.; Hamley-Bennett, C.; Alex, A.; Freeman, B.L.; Justo-Reinoso, I.; Mihai, I.C.; Gebhard, S.; Paine, K.; Jefferson, A.D.; Masoero, E.; et al. Advancements in Bacteria Based Self-Healing Concrete and the Promise of Modelling. Construction and Building Materials 2022, 358, 129412. [Google Scholar] [CrossRef]
- Xiao, P.; Liu, H.; Stuedlein, A.W.; Evans, T.M.; Xiao, Y. Effect of Relative Density and Biocementation on Cyclic Response of Calcareous Sand. Can. Geotech. J. 2019, 56, 1849–1862. [Google Scholar] [CrossRef]

 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





