Submitted:
08 September 2023
Posted:
11 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and discussion
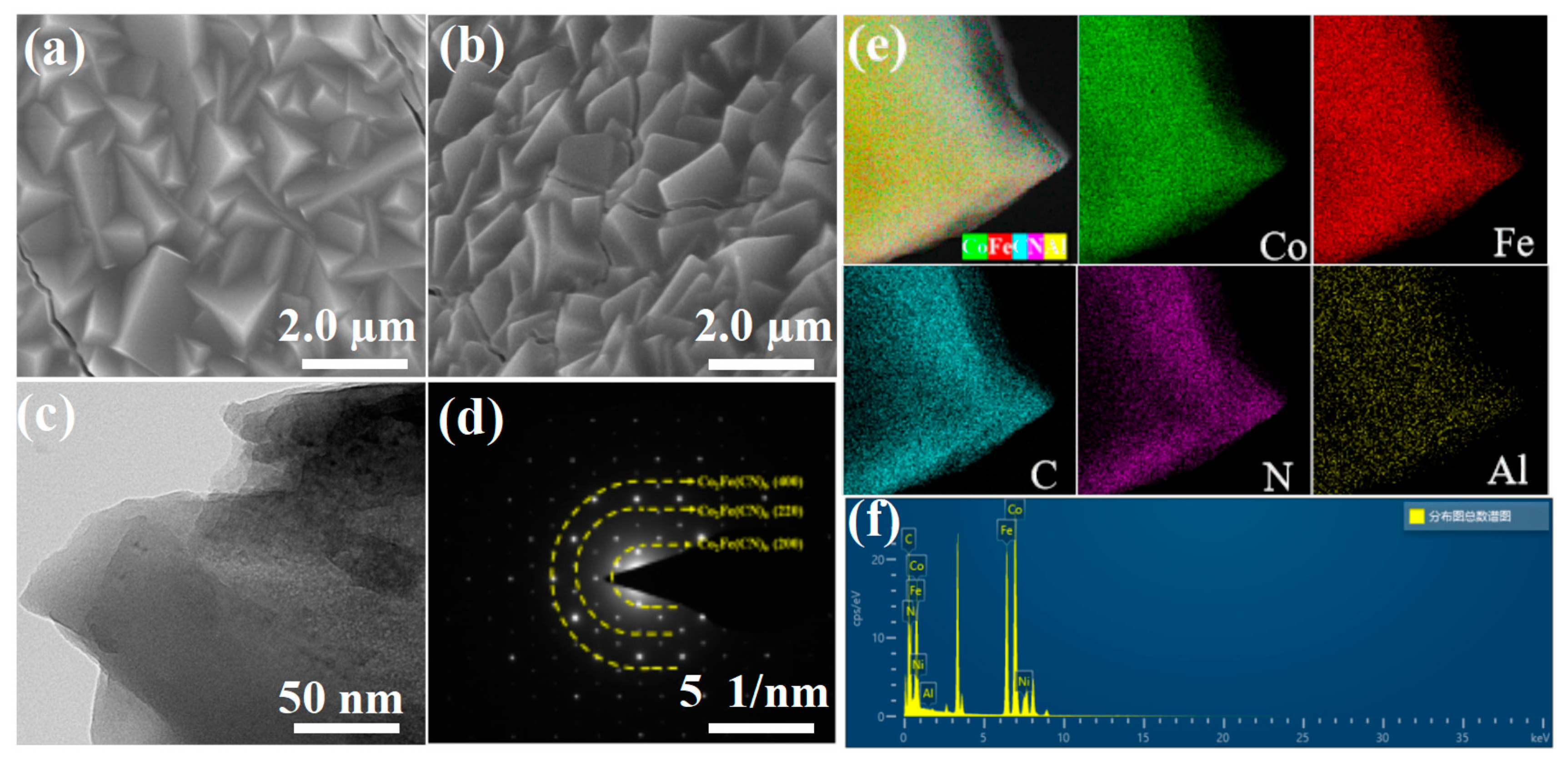
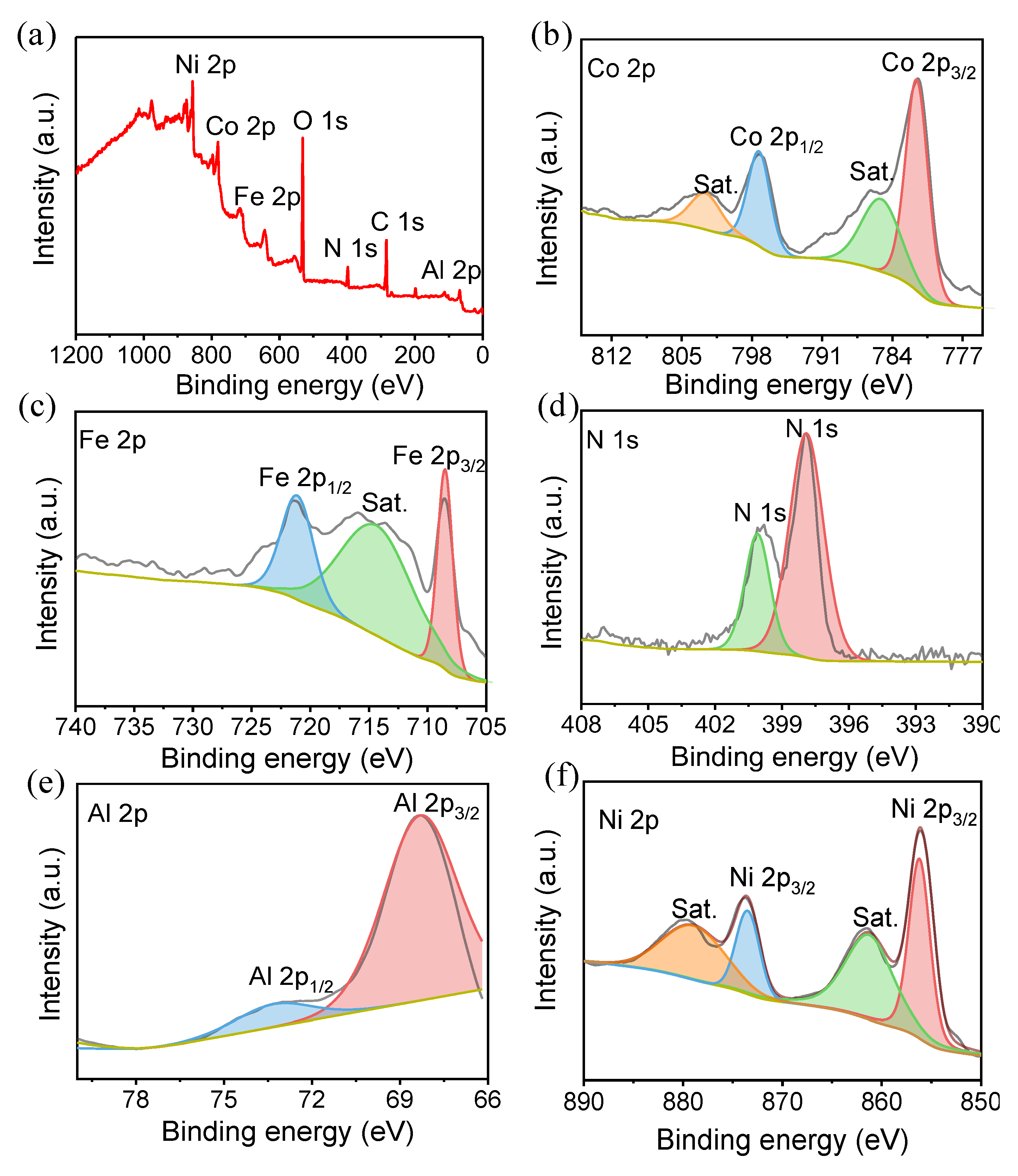
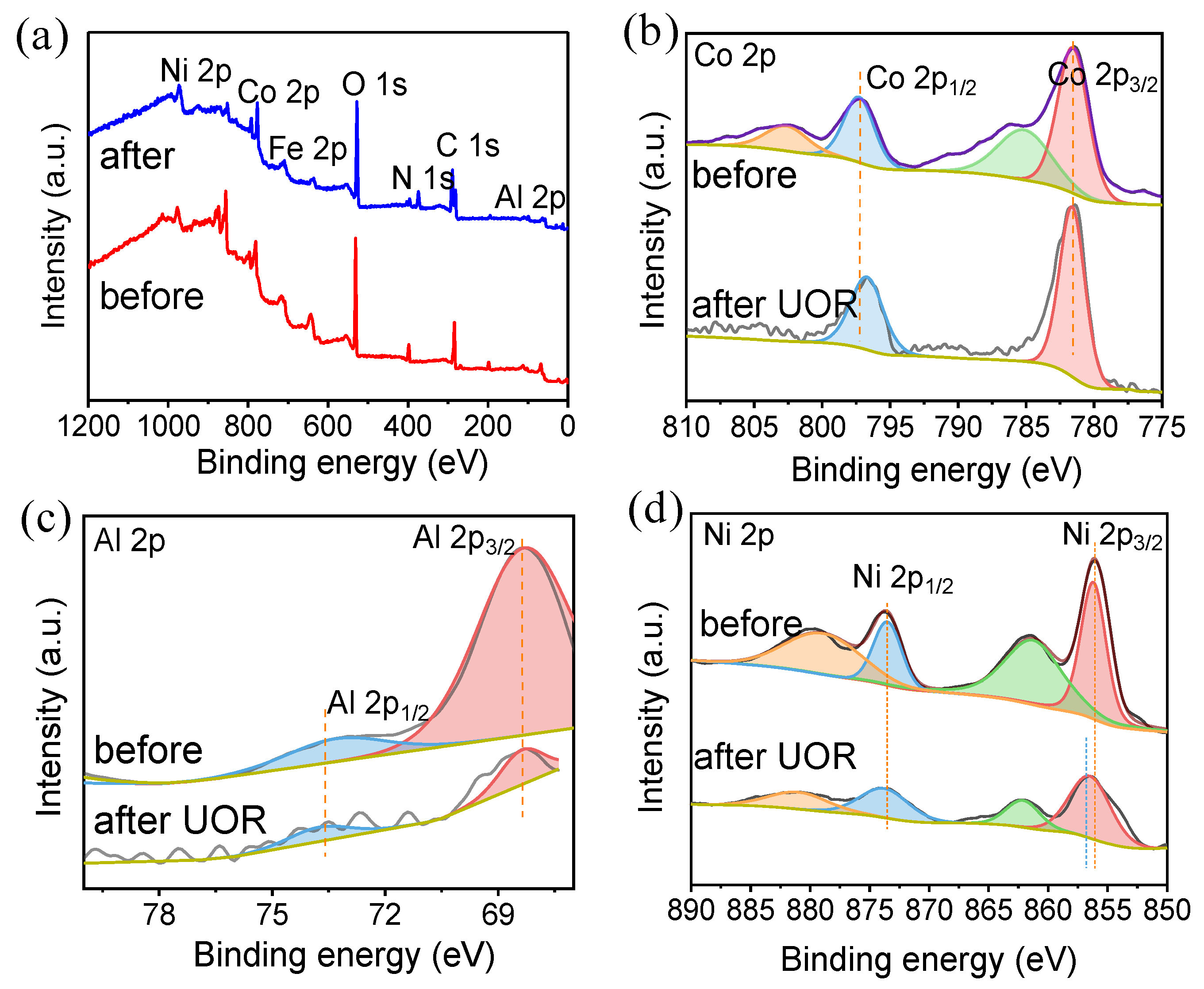
2.1. Characterizations
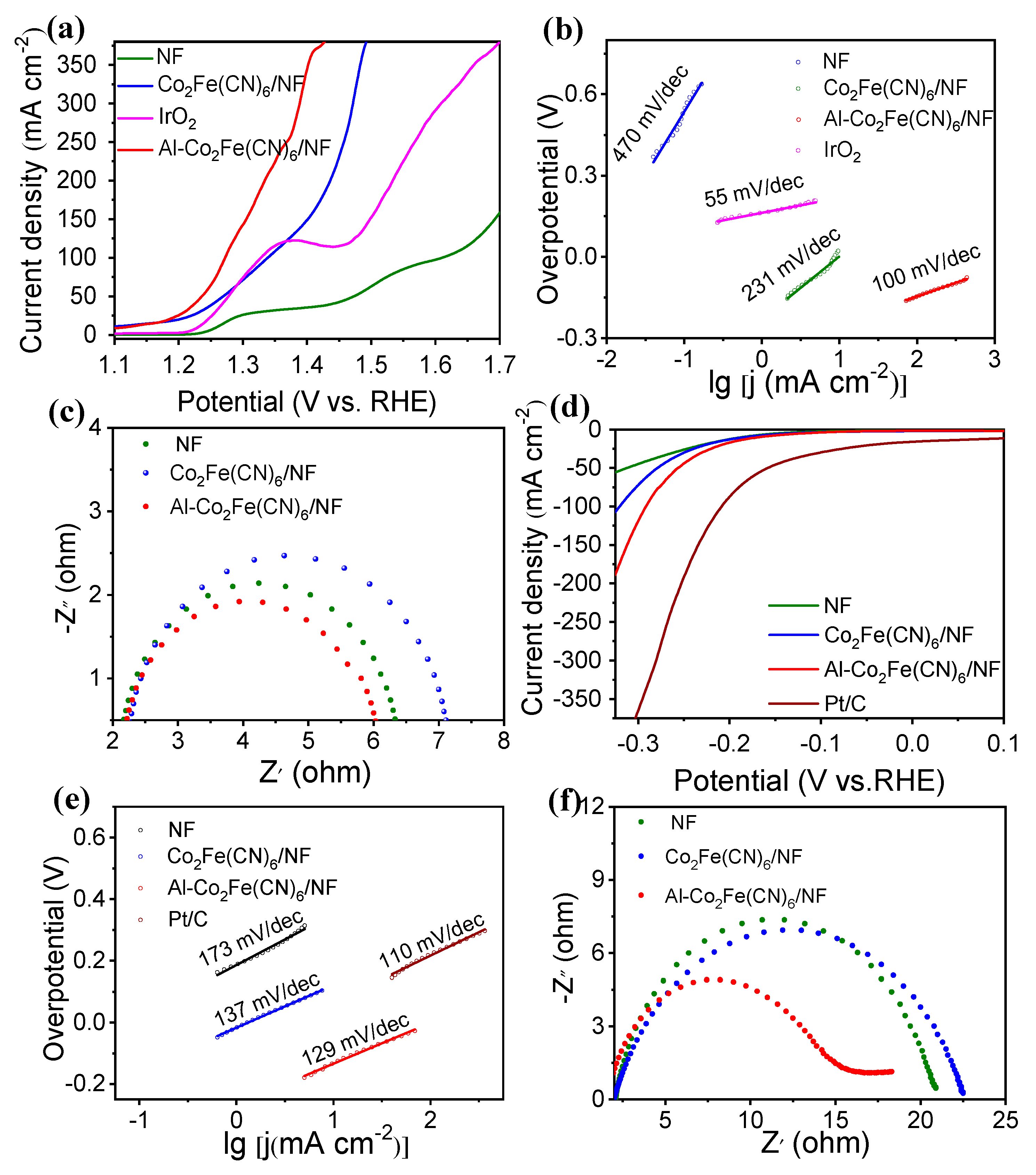
2.2. Electrochemical catalytic performances
2.3. Whole urea electrolysis
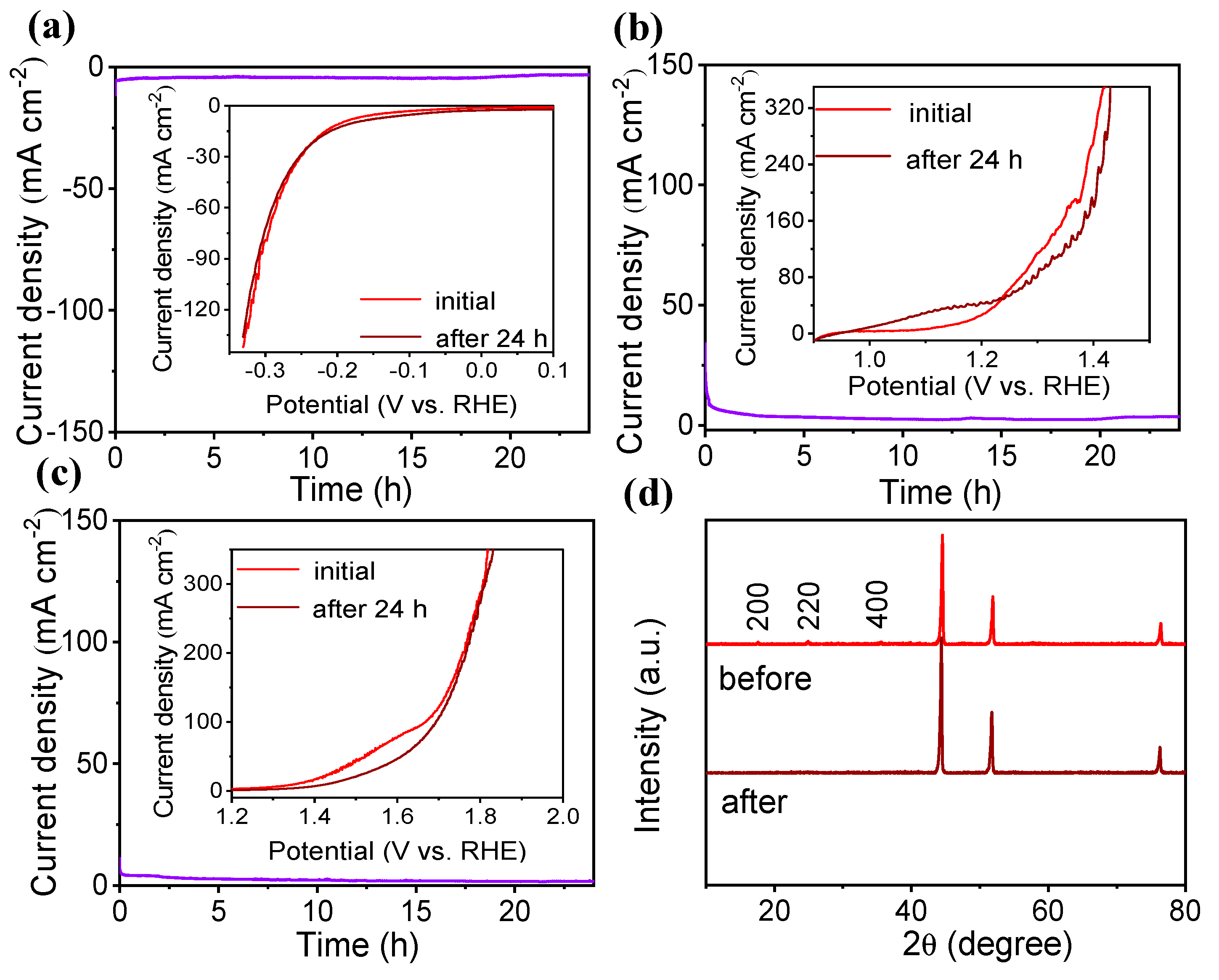
2.4. Stability of the catalyst
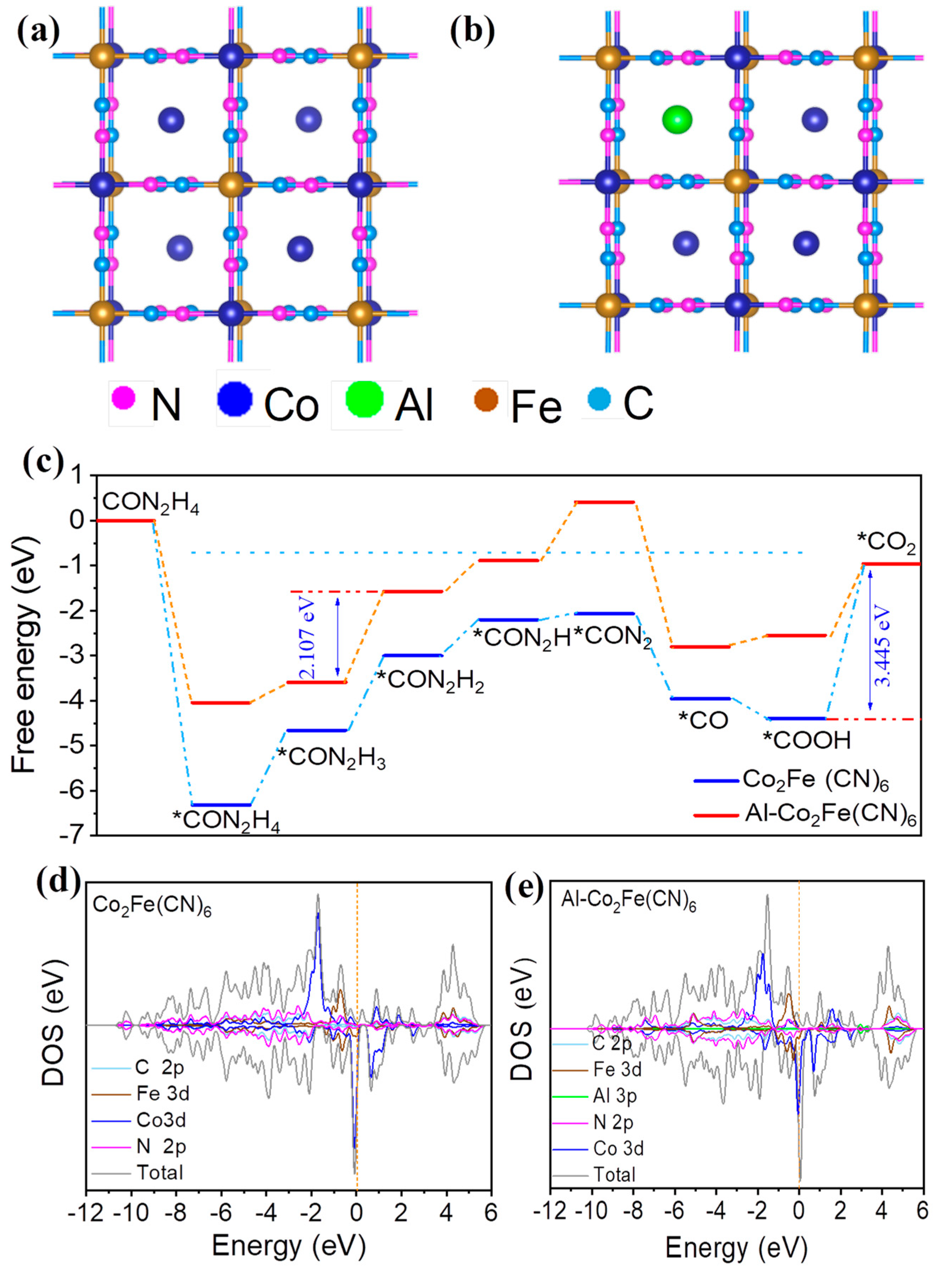
2.5. Catalytic Mechanisms
3. Experimental
3.1. Materials and chemicals
3.2. Preparation of the Al-Co2Fe(CN)6/NF electrode
3.3. Electrochemical measurements
3.4. DFT
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, P.; Li, L.; Nordlund, D.; Chen, H.; Fan, L.; Zhang, B.; Sheng, X.; Daniel, Q.; Sun, L. Dendritic core-shell nickel-iron-copper metal/metal oxide electrode for efficient electrocatalytic water oxidation. Nat. Commun. 2018, 9, 381. [Google Scholar] [CrossRef]
- Krausfeldt, L.E.; Farmer, A.T.; Gonzalez, H.F.C.; Zepernick, B.N.; Campagna, S.R.; Wilhelm, S.W. Urea Is Both a Carbon and Nitrogen Source for Microcystis aeruginosa: Tracking 13C Incorporation at Bloom pH Conditions. Front. Microbiol. 2019, 10, 1064. [Google Scholar] [CrossRef]
- Rollinson, A.N.; Jones, J.; Dupont, V.; Twigg, M.V. Urea as a hydrogencarrier: a perspective on its potential for safe, sustainable and long-term energy supply. Energy Environ. Sci. 2011, 4, 1216–1224. [Google Scholar]
- Wang, G.; Ling, Y.; Lu, X.; Wang, H.; Qian, F.; Tong, Y.; Li, Y. Solar driven hydrogen releasing from urea and human urine. Energy Environ. Sci. 2012, 5, 8215–8219. [Google Scholar] [CrossRef]
- Wei, S.; Wang, X.; Wang, J.; Sun, X.; Cui, L.; Yang, W.; Zheng, Y.; Liu, J. CoS2 nanoneedle array on Ti mesh: A stable and efficient bifunctional electrocatalyst for urea-assisted electrolytic hydrogen production. Electrochimica Acta 2017, 246, 776–782. [Google Scholar] [CrossRef]
- Boggs, B.K.; King, R.L.; Botte, G.G. Urea electrolysis: direct hydrogen production from urine. Chem. Commun. 2009, 4859–4861. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Wang, D.; Botte, G.G. Template-assisted synthesis of Ni–Co bimetallic nanowires for urea electrocatalytic oxidation. J. Appl. Electrochem. 2015, 45, 1217–1222. [Google Scholar] [CrossRef]
- Simka, W.; Piotrowski, J.; Robak, A.; Nawrat, G. Electrochemical treatment of aqueous solutions containing urea. J. Appl. Electrochem. 2009, 39, 1137–1143. [Google Scholar] [CrossRef]
- King, R.L.; Botte, G.G. Investigation of multi-metal catalysts for stable hydrogen production via urea electrolysis. J. Power Sources 2011, 196, 9579–9584. [Google Scholar] [CrossRef]
- Barbosa, J.R.; Paranhos, C.H.; Alves, O.C.; Checca, N.R.; Serna, J.P.; Rossi, A.L.; Silva, J.C.M. Low loading platinum dispersed on Ni/C nanoparticles as high active catalysts for urea electrooxidation reaction. Electrochimica Acta 2020, 355, 136752. [Google Scholar] [CrossRef]
- Yan, D.; Xia, C.; Zhang, W.; Hu, Q.; He, C.; Xia, B.Y.; Wang, S. Cation Defect Engineering of Transition Metal Electrocatalysts for Oxygen Evolution Reaction. Adv. Energy Mater. 2022, 12, 2202317. [Google Scholar] [CrossRef]
- Zhao, R.; Li, Q.; Jiang, X.; Huang, S.; Fu, G.; Lee, J.-M. Interface engineering in transition metal-based heterostructures for oxygen electrocatalysis. Mater. Chem. Front. 2021, 5, 1033–1059. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; He, T.; Wang, M.; Qi, R.; Yan, Y.; Dong, Z.; Liu, H.; Wang, H.; Xia, B.Y. Energy-saving hydrogen production coupling urea oxidation over a bifunctional nickel-molybdenum nanotube array. Nano Energy 2019, 60, 894–902. [Google Scholar] [CrossRef]
- Zhu, B.; Liang, Z.; Zou, R. Designing Advanced Catalysts for Energy Conversion Based on Urea Oxidation Reaction. Small 2020, 16, 1906133. [Google Scholar] [CrossRef]
- Ding, Y.; Li, Y.; Xue, Y.; Miao, B.; Li, S.; Jiang, Y.; Liu, X.; Chen, Y. Atomically thick Ni(OH)2 nanomeshes for urea electrooxidation. Nanoscale 2019, 11, 1058–1064. [Google Scholar] [CrossRef]
- Qin, H.; Ye, Y.; Li, J.; Jia, W.; Zheng, S.; Cao, X.; Lin, G.; Jiao, L. Synergistic Engineering of Doping and Vacancy in Ni(OH)2 to Boost Urea Electrooxidation. Adv. Funct. Mater. 2022, 33, 2209698. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, C.; Cui, S.; Lu, Z.; Ye, J.; Wen, Y.; Shi, W.; Huang, X.; Xue, L.; Bian, J.; et al. Multistep Dissolution of Lamellar Crystals Generates Superthin Amorphous Ni(OH)2 Catalyst for UOR. Adv. Mater. 2023, 35, e2301549. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Z.; Shen, C.; Liu, H.; Pang, X.; Gao, M.; Mu, J.; Cao, F.; Li, G. Ni/NiO heterostructures encapsulated in oxygen-doped graphene as multifunctional electrocatalysts for the HER, UOR and HMF oxidation reaction. Catal. Sci. Technol. 2021, 11, 2480–2490. [Google Scholar] [CrossRef]
- Zhao, Q.; Meng, C.; Kong, D.; Wang, Y.; Hu, H.; Chen, X.; Han, Y.; Chen, X.; Zhou, Y.; Lin, M.; et al. In Situ Construction of Nickel Sulfide Nano-Heterostructures for Highly Efficient Overall Urea Electrolysis. ACS Sustain. Chem. Eng. 2021, 9, 15582–15590. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, H.; Song, M.; Sun, L.; Song, R. Modulation of the morphology and electronic structure of Ni3S2 nano-forests via P and Mo co-doping in polyoxometalates to promote the urea oxidation reaction. J. Mater. Chem. A 2023, 11, 3584–3593. [Google Scholar] [CrossRef]
- Jia, X.; Kang, H.; Yang, X.; Li, Y.; Cui, K.; Wu, X.; Qin, W.; Wu, G. ,Jia, X.; Kang, H.; Yang, X.; Li, Y.; Cui, K.; Wu, X.; Qin, W.; Wu, G. Amorphous Ni(Ⅲ)-based sulfides as bifunctional water and urea oxidation anode electrocatalysts for hydrogen generation from urea-containing water. Appl. Catal. B Environ. 2022, 312, 121389. [Google Scholar] [CrossRef]
- Yuan, W.; Jiang, T.; Fang, X.; Fan, Y.; Qian, S.; Gao, Y.; Cheng, N.; Xue, H.; Tian, J. Interface engineering of S-doped Co2P@Ni2P core–shell heterostructures for efficient and energy-saving water splitting. Chem. Eng. J. 2022, 439, 135743. [Google Scholar] [CrossRef]
- Jiang, H.; Sun, M.; Wu, S.; Huang, B.; Lee, C.; Zhang, W. Oxygen-Incorporated NiMoP Nanotube Arrays as Efficient Bifunctional Electrocatalysts For Urea-Assisted Energy-Saving Hydrogen Production in Alkaline Electrolyte. Adv. Funct. Mater. 2021, 31, 2104951. [Google Scholar] [CrossRef]
- Tesfaye, R.M.; Das, G.; Park, B.J.; Kim, J.; Yoon, H.H. Ni-Co bimetal decorated carbon nanotube aerogel as an efficient anode catalyst in urea fuel cells. Sci. Rep. 2019, 9, 479. [Google Scholar] [CrossRef]
- Li, J.; Cui, H.; Du, X.; Zhang, X. The controlled synthesis of nitrogen and iron co-doped Ni3S2@NiP2 heterostructures for the oxygen evolution reaction and urea oxidation reaction. Dalton Trans. 2022, 51, 2444–2451. [Google Scholar] [CrossRef]
- Shen, J.; Li, Q.; Zhang, W.; Cai, Z.; Cui, L.; Liu, X.; Liu, J. Spherical Co3S4 grown directly on Ni–Fe sulfides as a porous nanoplate array on FeNi3 foam: a highly efficient and durable bifunctional catalyst for overall water splitting. J. Mater. Chem. A. 2022, 10, 5442–5451. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, H.; Yu, B.; Liu, Y.; Xu, W.; Wu, Z. An Unveiled Electrocatalysis Essence of NiCo Hydroxides through in Situ Raman Spectroscopy for Urea Oxidation. Energy Technol-GER. 2022, 10, 2101010. [Google Scholar] [CrossRef]
- Xu, Y.; Ren, T.; Ren, K.; Yu, S.; Liu, M.; Wang, Z.; Li, X.; Wang, L.; Wang, H. Metal-organic frameworks-derived Ru-doped Co2P/N-doped carbon composite nanosheet arrays as bifunctional electrocatalysts for hydrogen evolution and urea oxidation. Chem. Eng. J. 2021, 408, 127308. [Google Scholar] [CrossRef]
- Chen, F.; Yang, F.; Sheng, C.; Li, J.; Xu, H.; Qing, Y.; Chen, S.; Wu, Y.; Lu, X. Electronic structure modulation of nickel hydroxide porous nanowire arrays via manganese doping for urea-assisted energy-efficient hydrogen generation. J. Colloid Interface Sci. 2022, 626, 445–452. [Google Scholar] [CrossRef]
- Zhou, T.; Cao, Z.; Wang, H.; Gao, Z.; Li, L.; Ma, H.; Zhao, Y. Ultrathin Co–Fe hydroxide nanosheet arrays for improved oxygen evolution during water splitting. RSC Adv. 2017, 7, 22818–22824. [Google Scholar] [CrossRef]
- Man, I.C.; Su, H.-Y.; Calle-Vallejo, F.; Hansen, H.A.; Martínez, J.I.; Inoglu, N.G.; Kitchin, J.; Jaramillo, T.F.; Nørskov, J.K.; Rossmeisl, J. Universality in Oxygen Evolution Electrocatalysis on Oxide Surfaces. ChemCatChem 2011, 3, 1159–1165. [Google Scholar] [CrossRef]
- Sayed, E.T.; Eisa, T.; Mohamed, H.O.; Abdelkareem, M.A.; Allagui, A.; Alawadhi, H.; Chae, K.-J. Direct urea fuel cells: Challenges and opportunities. J. Power Sources 2019, 417, 159–175. [Google Scholar] [CrossRef]
- Yao, S.J.; Wolfson, S.K.; Ahn, B.K.; Liu, C.C. Anodic Oxidation of Urea and an Electrochemical Approach to De-ureation. Nature 1973, 241, 471–472. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J.J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B. 1996, 54, 11169. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Wang, G.; Ling, Y.; Lu, X.; Wang, H.; Qian, F.; Tong, Y.; Li, Y. Solar driven hydrogen releasing from urea and human urine. Energy Environ. Sci. 2012, 5, 8215–8219. [Google Scholar] [CrossRef]
- Min, S.; Zhao, C.; Zhang, Z.; Chen, G.; Qian, X.; Guo, Z.J. Synthesis of Ni(OH)2/RGO pseudocomposite on nickel foam for supercapacitors with superior performance. J. Mater. Chem. A. 2015, 3, 3641–3650. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Lin, H.; Liu, Y.; Ye, J.; Wen, Y.; Chen, A.; Wang, L.; Ni, F.; Zhou, Z.J. A. C. A lattice-oxygen-involved reaction pathway to boost urea oxidation. Nat Energy. 2019, 131, 16976–16981. [Google Scholar]
- Daramola, D.A.; Singh, D.; Botte, G.G. Dissociation Rates of Urea in the Presence of NiOOH Catalyst: A DFT Analysis. J. Phys. Chem. A 2010, 114, 11513–11521. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




