Submitted:
06 September 2023
Posted:
11 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Pre-culture
2.2. Cultivation of cyanobacteria on various carrier
2.3. Determination of released growth-promoting substances by Luffa cylindrica
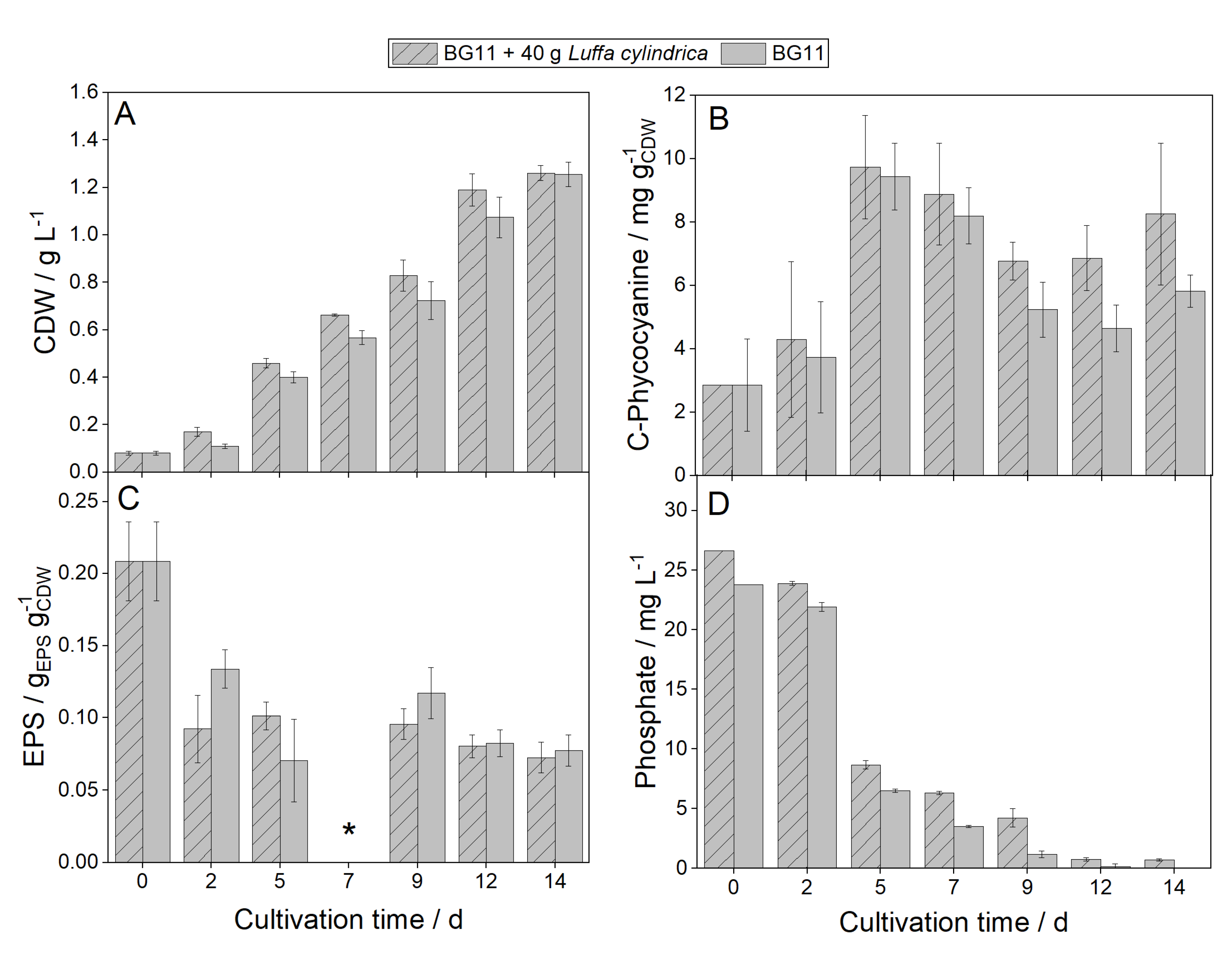
2.4. Cultivation of N. muscorum 1453-12a in BG11 and BG11 with soaked L. cylindrica
2.5. Determination of CDW, EPS, Phycobiliproteins, and Pigments
2.6. Analyzation of the cultivation medium
3. Results
3.1. Screening for biodegradable carriers as cultivation surface for phototrophic biofilms
| Carrier | Picture of Erlenmeyer Flask Filled with the Carrier before Cultivation | Picture of Erlenmeyer Flask Filled with the Carrier after Cultivation | Growth | Notes |
|---|---|---|---|---|
 | ||||

3.1.1. Influence of Luffa cylindrica on the growth of various Cyanobacteria
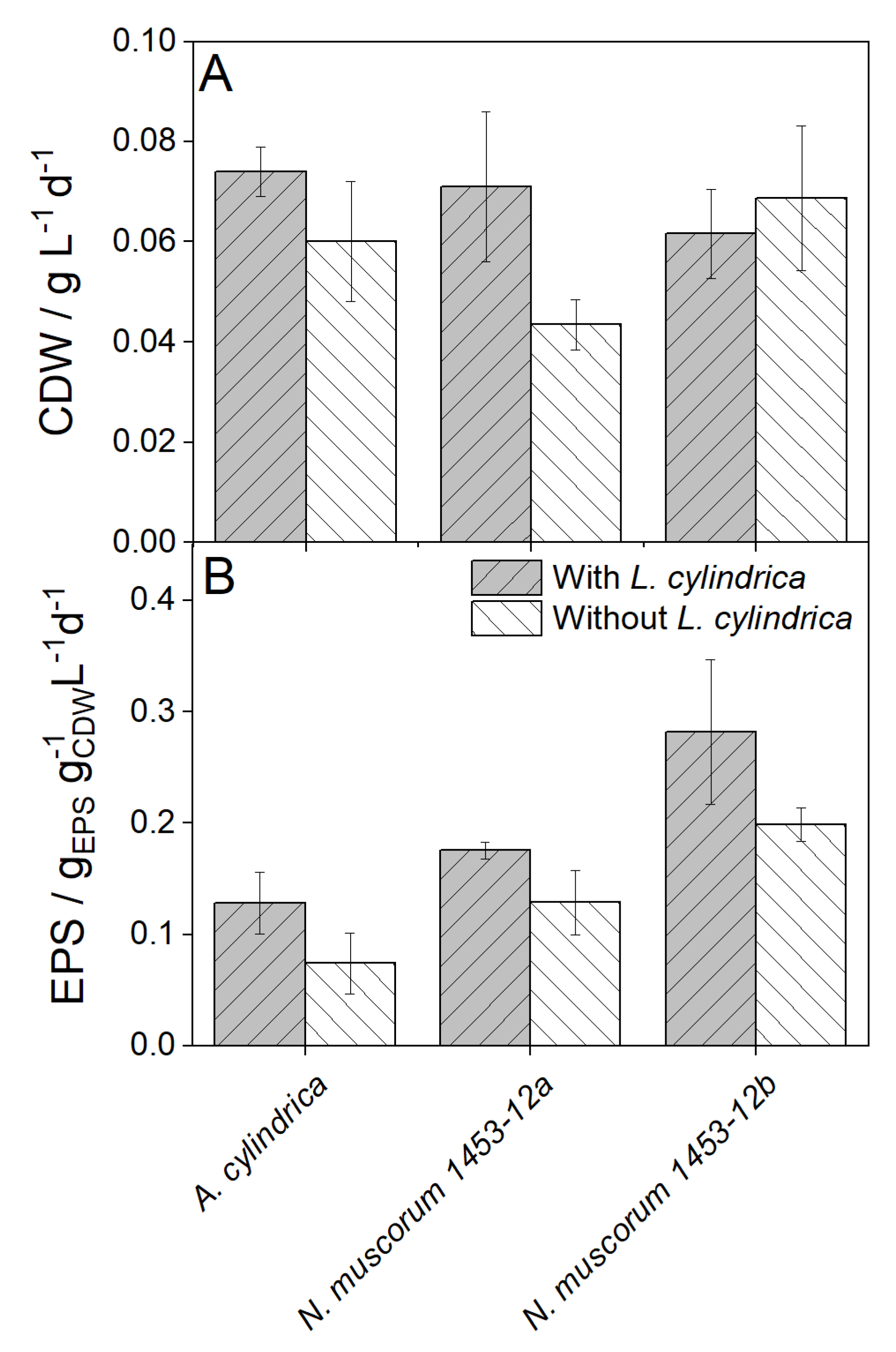
3.2. Growth-promoting substances released by Luffa cylindrica
| Anions/Cations | The concentration of Ions in distilled water soaked with 20 g L. cylindrica [mg/L] | The concentration of Ions in distilled water [mg/L] |
|---|---|---|
| Nitrate | 4.36 ± 3.93 | 1.84 ± 1.60 |
| Nitrite | 0.91 ± 0.29 | 0.65 ± 0.02 |
| Chloride | 4.31 ± 1.23 | 0.61 ± 0.01 |
| Phosphate | 13.22 ± 9.92 | 0 |
| Sulfate | 10.51 ± 3.34 | 3.06 ± 0.02 |
| Sugar | 0 | 0 |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whitton, B.A. Ecology of Cyanobacteria II: Their Diversity in Space and Time; Springer: Dordrecht, 2012; ISBN 9789400738553. [Google Scholar]
- Stanier, R.Y.; Cohen-Bazire, G. Phototrophic prokaryotes: the cyanobacteria. Annu. Rev. Microbiol. 1977, 31, 225–274. [Google Scholar] [CrossRef] [PubMed]
- Galetović, A.; Seura, F.; Gallardo, V.; Graves, R.; Cortés, J.; Valdivia, C.; Núñez, J.; Tapia, C.; Neira, I.; Sanzana, S.; et al. Use of Phycobiliproteins from Atacama Cyanobacteria as Food Colorants in a Dairy Beverage Prototype. Foods 2020, 9. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Menakha, M. Pharmaceutical applications of cyanobacteria—A review. Journal of Acute Medicine 2015, 5, 15–23. [Google Scholar] [CrossRef]
- Nozzi, N.E.; Oliver, J.W.K.; Atsumi, S. Cyanobacteria as a Platform for Biofuel Production. Front. Bioeng. Biotechnol. 2013, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, M.; Strieth, D. Terrestrial Microalgae: Novel Concepts for Biotechnology and Applications. In Progress in Botany Vol. 79; Cánovas, F.M., Lüttge, U., Matyssek, R., Eds.; Springer International Publishing; Imprint; Springer: Cham, 2018; pp. 269–312. ISBN 978-3-319-71412-7. [Google Scholar]
- Ennaceri, H.; Ishika, T.; Mkpuma, V.O.; Moheimani, N.R. Microalgal biofilms: Towards a sustainable biomass production. Algal Research 2023, 72, 103124. [Google Scholar] [CrossRef]
- Lau, N.-S.; Matsui, M.; Abdullah, A.A.-A. Cyanobacteria: Photoautotrophic Microbial Factories for the Sustainable Synthesis of Industrial Products. Biomed Res. Int. 2015, 2015, 754934. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Wang, J.; Chen, H.; Chen, D. Progress of marine biofouling and antifouling technologies. Chin. Sci. Bull. 2011, 56, 598–612. [Google Scholar] [CrossRef]
- Zhuang, L.-L.; Hu, H.-Y.; Wu, Y.-H.; Wang, T.; Zhang, T.-Y. A novel suspended-solid phase photobioreactor to improve biomass production and separation of microalgae. Bioresource Technology 2014, 153, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Kaewprasit, C. Relationships Between a Cotton Fiber’s Specific Surface Area and Its Physical Properties. Textile Research Journal 2004, 74, 730–734. [Google Scholar] [CrossRef]
- Chetsumon, A.; Maeda, I.; Umeda, F.; Yagi, K.; Miura, Y.; Mizoguchi, T. Antibiotic production by the immobilized cyanobacterium,Scytonema sp. TISTR 8208, in a seaweed-type photobioreactor. J Appl Phycol 1994, 6, 539–543. [Google Scholar] [CrossRef]
- Chetsumon, A.; Fujieda, K.; Hirata, K.; Yagi, K.; Miura, Y. Optimization of antibiotic production by the cyanobacteriumScytonema sp. TISTR 8208 immobilized on polyurethane foam. J Appl Phycol 1993, 5, 615–622. [Google Scholar] [CrossRef]
- Walther, J.; Erdmann, N.; Stoffel, M.; Wastian, K.; Schwarz, A.; Strieth, D.; Muffler, K.; Ulber, R. Passively immobilized cyanobacteria Nostoc species BB 92.2 in a moving bed photobioreactor (MBPBR): design, cultivation and characterization. Biotechnol. Bioeng. 2022. [Google Scholar] [CrossRef]
- Rippka, R.; Herdman, M.; Waterbury, J.B. Generic Assignments, Strain Histories and Properties of Pure Cultures of Cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Strieth, D.; Stiefelmaier, J.; Wrabl, B.; Schwing, J.; Schmeckebier, A.; Di Nonno, S.; Muffler, K.; Ulber, R. A new strategy for a combined isolation of EPS and pigments from cyanobacteria. J. Appl. Phycol. 2020, 58, 419. [Google Scholar] [CrossRef]
- Bennett, A.; Bogorad, L. Complementary chromatic adaptation in a filamentous blue-green alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochimica et Biophysica Acta (BBA)—Bioenergetics 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Chamovitz, D.; Sandmann, G.; Hirschberg, J. Molecular and biochemical characterization of herbicide-resistant mutants of cyanobacteria reveals that phytoene desaturation is a rate-limiting step in carotenoid biosynthesis. J. Biol. Chem. 1993, 268, 17348–17353. [Google Scholar] [CrossRef]
- Temraleeva, A.D.; Dronova, S.A.; Moskalenko, S.V.; Didovich, S.V. Modern methods for isolation, purification, and cultivation of soil cyanobacteria. Microbiology 2016, 85, 389–399. [Google Scholar] [CrossRef]
- Filippidis, A.; Moustaka-Gouni, M.; Papastergios, G.; Katsiapi, M.; Kantiranis, N. Cyanobacteria removal by Hellenic Natural Zeolite. Small Decentralized Water &.
- In-na, P.; Umar, A.A.; Wallace, A.D.; Flickinger, M.C.; Caldwell, G.S.; Lee, J.G. Loofah-based microalgae and cyanobacteria biocomposites for intensifying carbon dioxide capture. Journal of CO2 Utilization 2020, 42, 101348. [Google Scholar] [CrossRef]
- Akhtar, N.; Iqbal, J.; Iqbal, M. Removal and recovery of nickel(II) from aqueous solution by loofa sponge-immobilized biomass of Chlorella sorokiniana: characterization studies. J. Hazard. Mater. 2004, 108, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Edyvean, R.G.J. Loofa sponge immobilized fungal biosorbent: a robust system for cadmium and other dissolved metal removal from aqueous solution. Chemosphere 2005, 61, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Ogbonna, J.C.; Liu, Y.-C.; Liu, Y.-K.; Tanaka, H. Loofa (Luffa cylindrica) sponge as a carrier for microbial cell immobilization. Journal of Fermentation and Bioengineering 1994, 78, 437–442. [Google Scholar] [CrossRef]
- Pringle, J.H.; Fletcher, M. Influence of substratum wettability on attachment of freshwater bacteria to solid surfaces. Appl. Environ. Microbiol. 1983, 45, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Su, N.; Zhang, K.; Zhu, S.; Zhu, Z.; Qin, W.; Yang, Y.; Shi, Y.; Fan, S.; Wang, Z.; et al. Effect of fiber surface treatment on structure, moisture absorption and mechanical properties of luffa sponge fiber bundles. Industrial Crops and Products 2018, 123, 341–352. [Google Scholar] [CrossRef]
- Strieth, D. Produktive phototrophe Biofilme in Aerosolreaktoren, 1. Auflage; Verlag Dr. Hut: München, 2019; ISBN 978-3843939515. [Google Scholar]
- Characklis, W.G.; McFeters, G.A.; Marshall, K.C. Physiological ecology in biofilm systems; Wiley and sons: New York, 1990. [Google Scholar]
- Allaf, M.M.; Peerhossaini, H. Cyanobacteria: Model Microorganisms and Beyond. Microorganisms 2022, 10. [Google Scholar] [CrossRef]
- Ekelhof, A.; Melkonian, M. Microalgal cultivation in porous substrate bioreactor for extracellular polysaccharide production. J Appl Phycol 2017, 29, 1115–1122. [Google Scholar] [CrossRef]
- Schooling, S.R.; Beveridge, T.J. Membrane vesicles: an overlooked component of the matrices of biofilms. J. Bacteriol. 2006, 188, 5945–5957. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Hames, B.; Hyman, D.; Payne, C.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Wolfe, J. Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples: Laboratory Analytical Procedure (LAP). Technical Report NREL/TP-510-42621; National Renewable Energy Laboratory: Golden, CO, USA.
- Alhijazi, M.; Safaei, B.; Zeeshan, Q.; Asmael, M.; Eyvazian, A.; Qin, Z. Recent Developments in Luffa Natural Fiber Composites: Review. Sustainability 2020, 12, 7683. [Google Scholar] [CrossRef]
- Adeyanju, C.A.; Ogunniyi, S.; Ighalo, J.O.; Adeniyi, A.G.; Abdulkareem, S.A. A review on Luffa fibres and their polymer composites. J Mater Sci 2021, 56, 2797–2813. [Google Scholar] [CrossRef]
- Afreen, S.; Bano, F.; Ahmad, N.; Fatma, T. Screening and optimization of laccase from cyanobacteria with its potential in decolorization of anthraquinonic dye Remazol Brilliant Blue R. Biocatalysis and Agricultural Biotechnology 2017, 10, 403–410. [Google Scholar] [CrossRef]
- Büchs, J.; Zeols, B. Evaluation of Maximum to Specific Power Consumption Ratio in Shaking Bioreactors. J. Chem. Eng. Japan/JCEJ 2001, 34, 647–653. [Google Scholar] [CrossRef]
- Losen, M.; Frölich, B.; Pohl, M.; Büchs, J. Effect of oxygen limitation and medium composition on Escherichia coli fermentation in shake-flask cultures. Biotechnol Progress 2004, 20, 1062–1068. [Google Scholar] [CrossRef]
- Anderlei, T.; Büchs, J. Device for sterile online measurement of the oxygen transfer rate in shaking flasks. Biochemical Engineering Journal 2001, 7, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, N.T. Production of phycocyanin—a pigment with applications in biology, biotechnology, foods and medicine. Appl Microbiol Biotechnol 2008, 80, 1–14. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





