Submitted:
08 September 2023
Posted:
11 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. General Principles
- Diagnosis and indication
- Evaluation of reversible causes of hemodynamic instability
- Evaluation of contraindications for temporary MCS (tMCS)
- Treatment of PCCS using tMCS
- De-escalation and weaning from tMCS.
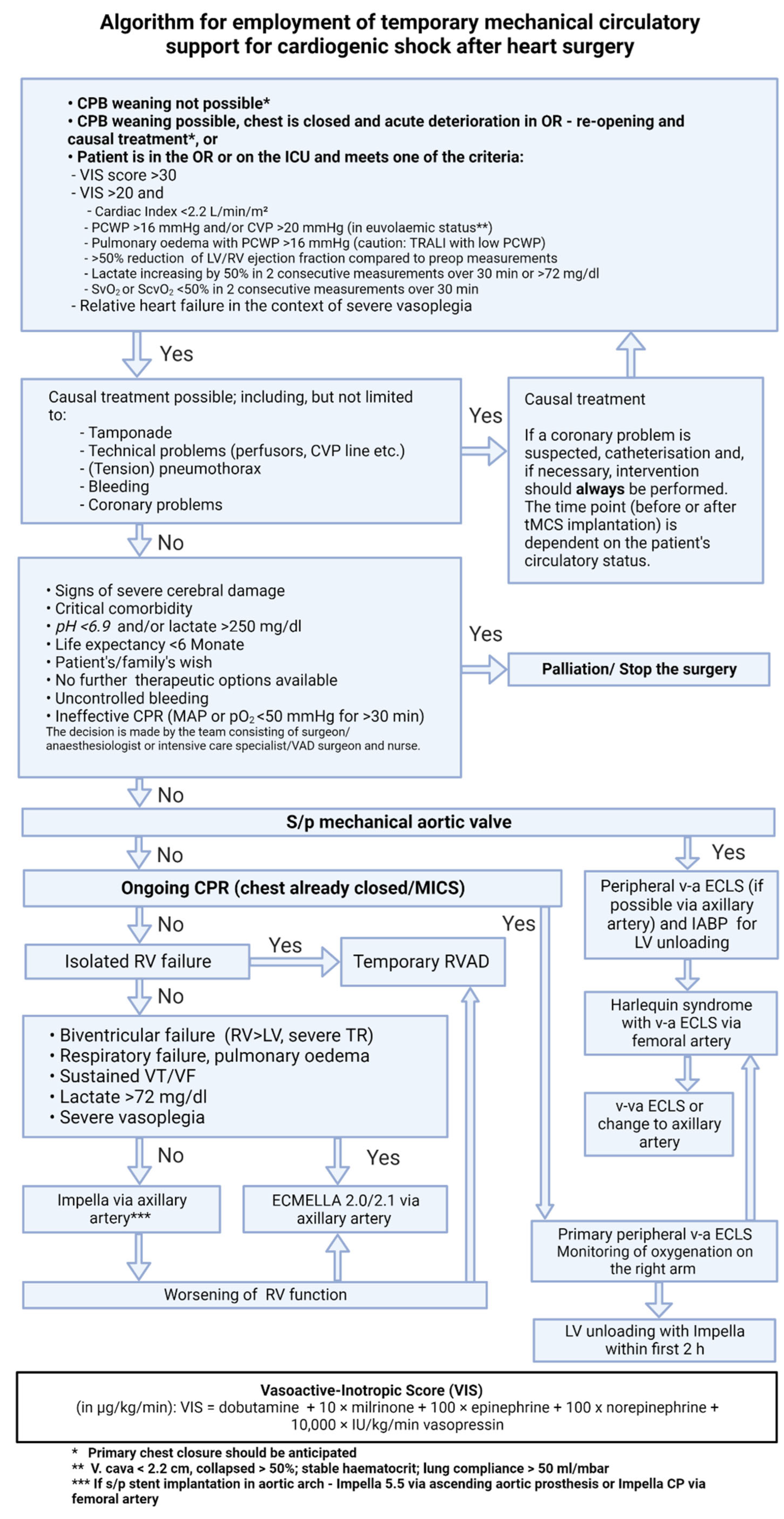
3. Diagnosis and Indication
- Criteria for postoperative CS in the ICU: Increasing catecholamine requirement or/and hemodynamic instability despite high-dose inotropic support.[11] Objective quantification of the catecholamine requirement should be performed using the vasoactive inotropic score (VIS score), which takes into account the cumulative drug support of the circulation (inotropes and vasopressors).[12]
3.1. Vasoactive Inotropic Score
4. Reversible Causes of Hemodynamic Instability
- Technical problems (ventilation, medication administration, measurement errors)
- (Tension) pneumothorax
- Bleeding
- Myocardial ischemia
- Iatrogenic dissection
- Pericardial tamponade
- Pulmonary embolism
5. Contraindications for Temporary MCS
- Signs of severe cerebral damage
- Critical comorbidities
- Malignancy with an anticipated life expectancy of less than 6 months
- Documented patient wishes/patient directives
- pH <6.9 mol/L
- Lactate >225 mg/dL (25 mmol/L)[14]
- No viable treatment options available
- Uncontrolled bleeding
- Ineffective resuscitation (MAP <50 mmHg or apO2 <50 mmHg for 30 minutes)
6. Temporary Mechanical Circulatory Support for PCCS Treatment
- Expected duration of support
- Anatomical characteristics of the patient (vascular access)
- Complication profiles of respective tMCS devices
- Availability of the tMCS devices
6.1. Isolated Left Ventricular (LV) Dysfunction
- In patients with a mechanical aortic valve prosthesis or a free-floating thrombus in the LV, a left ventricular Impella is contraindicated. In such cases, primary implantation of v-a ECLS has to be performed. Following this, LV unloading strategies (e.g., IABP, transseptal percutaneous venting, direct LV venting via the LV apex or pulmonary artery) should be discussed.
- The Impella CP (Abiomed, Danvers, MA, USA) is usually implanted percutaneously via femoral artery and can generate a flow of up to 3.5 L/min. For patients with an expected short tMCS duration, sole percutaneous Impella CP implantation can be considered.
- The Impella 5.5 can provide up to 5.8 L/min depending on afterload and has to be surgically implanted through a vascular prosthesis. Axillary artery is the preferred access route, as it allows for early postoperative mobilization of the patient and uncomplicated explantation.
- If the axillary artery is less than 7-mm in diameter, exhibits calcifications or anatomical peculiarities (such as arteria lusoria), implantation of an Impella CP via the axillary artery, possibly on the contralateral side, may alternatively be considered.
- Alternative access routes such as the ascending aorta (in open-chest patients) can be considered.
- We recommend performing surgical implantation via a 10 (8)-mm vascular prosthesis, which is anastomosed in end-to-side fashion to the target vessel.
6.2. Isolated Right Ventricular (RV) Dysfunction
- In patients with an open chest, cannulation of the pulmonary artery can be performed using a vascular prosthesis (10- or 8-mm diameter), which is tunneled out of the chest cavity allowing for thoracic closure and facilitating later explantation. For venous drainage, a cannula is usually placed in the right atrium via femoral or the right jugular vein. Alternatively, if no peripheral access is possible and extended support duration is anticipated, direct cannulation of the right atrium can be performed. In this case, a 14 mm graft is usually anastamosed to right atrium and exteriorized and a 24-26 Fr venous cannula is directly inserted and chest is closed.[9,16]
- In patients with a closed chest, percutaneous cannulation of the pulmonary artery can be performed via the right jugular vein. This can be achieved through two separate cannulas (long standard venous cannulas placed into the pulmonary artery and femoral vein), requiring two separate venous punctures.[16]
- Alternatively, the insertion of a double-lumen cannula (ProtekDuo by LivaNova PLC, London, UK) facilitated right ventricular support through a single puncture of the jugular vein. The tip of the catheter is placed in the main stem or upper right pulmonary artery under fluoroscopic and echocardiographic guidance. This method allows for early mobilization on ongoing right ventricular support.[17]
- The percutaneous Impella RP is inserted into the pulmonary artery via the femoral vein and generates a flow of up to 4.6 L/min. Disadvantages of the Impella RP are a limited support duration, impaired patient mobilization, and the lack of respiratory support.[5] Alternatively, the recently developed Impella RP Flex (Abiomed, Danvers, MA, USA) can be implanted through right jugular vein allowing mobilization of the patients.
6.3. Ongoing CPR
- In open-chest patients, cannulation of central vessels (ascending aorta, right atrium) can be considered.
- In patients with a closed thorax, peripheral v-a ECLS cannulation should be performed. Whether percutaneous or open surgical cannulation is chosen depends on the clinical situation, anatomical circumstances of the patient, and the surgeon’s preference.
- In patients with peripheral arterial occlusive disease, surgical exposure of the femoral vessels and surgical implantation should be performed as a primary approach.
- Placement of a peripheral perfusion cannula is recommended, but can be performed shortly after hemodynamic stabilization.
6.4. Severe Cardiogenic Shock
6.4.1. ECMELLA Approach
- ECMELLA combines the advantages of v-a ECLS and an Impella pump, namely: biventricular unloading through a simultaneous preload and afterload decrease, as well as pulmonary support with an oxygenator. Therapy on ECMELLA provides intensive mechanical circulatory support with high-volume flow, which is intended to ensure sufficient organ perfusion in phases of acute shock.[7]
- Another important advantage of the ECMELLA concept is the easy way of de-escalation once circulatory conditions have been stabilized. In this case, v-a ECLS weaning is usually performed first while continuing Impella therapy. This enables longer support with a lower risk of complications.[18]
- In this case, arterial vascular access is achieved via a Y-shaped vascular prosthesis, which is anastomosed to the axillary artery. One branch is used for Impella implantation, the other for the arterial cannula insertion of v-a ECLS. This method enables early postoperative mobilization (if venous drainage is performed via the right jugular vein) and bedside de-escalation on the ICU over time.[19,20]
6.4.2. Alternative Methods for Left Ventricular Unloading
Intra-aortic balloon pump (IABP)
- In our algorithm, the IABP is used only in combination with v-a ECLS for LV unloading in patients with absolute contraindications for an Impella pump. These include mechanical aortic valve prostheses or a floating thrombus in the left ventricle.[6,15] However, it should be noted that the degree of LV unloading achieved by an IABP depends strongly on the contractility of the left ventricle.[22]
Percutaneous venting
- The venting cannula of the TandemHeart system (LivaNova PLC, London, UK) enables unloading of the left ventricle. It is connected to the venous drainage.[16]
- The Bio-Medicus, NextGen two-stage cannula (Medtronic PLC, Dublin, Ireland) allows for simultaneous drainage of both atria.[17]
- A percutaneous cannula is placed under fluoroscopy and echocardiographic control.
- In specific cases (e.g., pre-operated patients or complex vascular status), percutaneous atrial septostomy can be performed as a last-resort option.
Surgical venting
- In patients with an open thorax an additional venous cannula can be placed through the upper right pulmonary vein into the LV.[16]
- Alternatively, a vent can be placed directly via the LV apex. This method does not necessarily require a median sternotomy. Apical LV venting can be performed through a left lateral mini-thoracotomy, taking into account potential complications such as coronary artery injury, ventricular perforation, and bleeding.[16]
- After placement, the cannulas for passive venting are tunneled outwards, fixated, and connected to the venous drainage of the v-a ECLS using a Y-shaped connector.
7. De-escalation and Weaning from tMCS
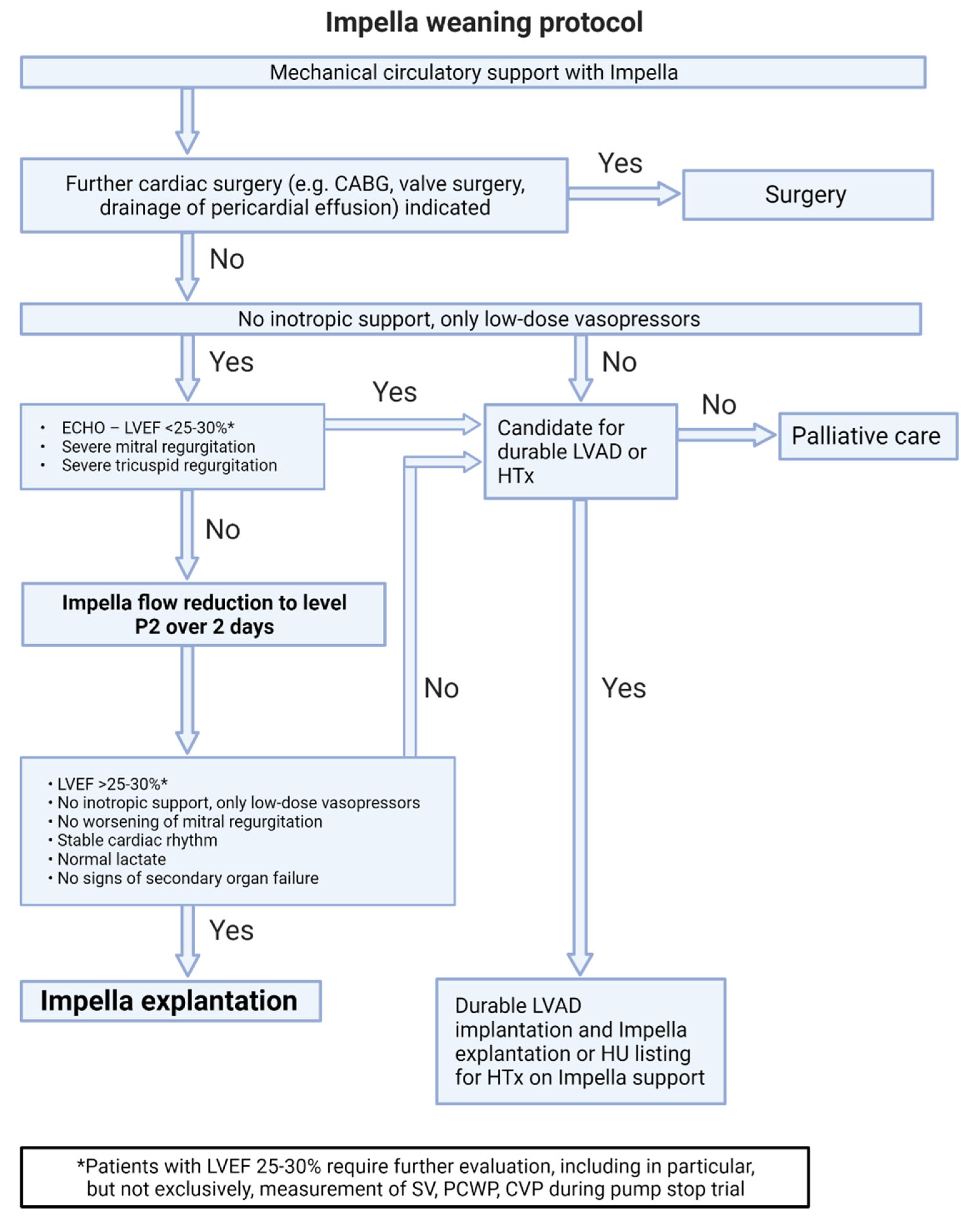
7.1. Discontinuation of Isolated Left Ventricular Impella Support (Figure 2)
- After hemodynamic stabilization and a significant reduction of inotropic medication (with only low-dose vasopressor therapy remaining), a stepwise reduction in support is performed until the P2 level is achieved. This is done gradually with a continuous re-evaluation of hemodynamics over at least 48 hours. Support should not be reduced below the P2 level, as this can cause retrograde flow through the pump into the left ventricle.
- If there are no severe valve pathologies, the LVEF is at least 25–30%, there is a stable rhythm, and no continuous inotropic treatment is needed while on support at the P2 level, the Impella can be removed.
- If this is not the case, alternative treatment concepts such as LVAD, heart transplantation, or palliation should be discussed.
- In case of increasing mitral regurgitation during a reduction of Impella flow, endovascular mitral valve reconstruction can be performed under Impella support.[23]
- If circulatory support is inadequate (increasing catecholamine demand and increasing arterial lactate despite maximum Impella therapy), v-a ECLS implantation for escalation to ECMELLA can be considered, but long-term LVAD therapy should be prioritized.[6]
- In the event of severe respiratory failure, veno-venous extracorporeal membrane oxygenation (v-v ECMO) can be implanted.
- If severe hemolysis, pump thrombosis, or Impella pump failure occur, a switch to a new Impella can be performed, but long-term LVAD therapy should be simultaneously discussed depending on the cardiac recovery potential.
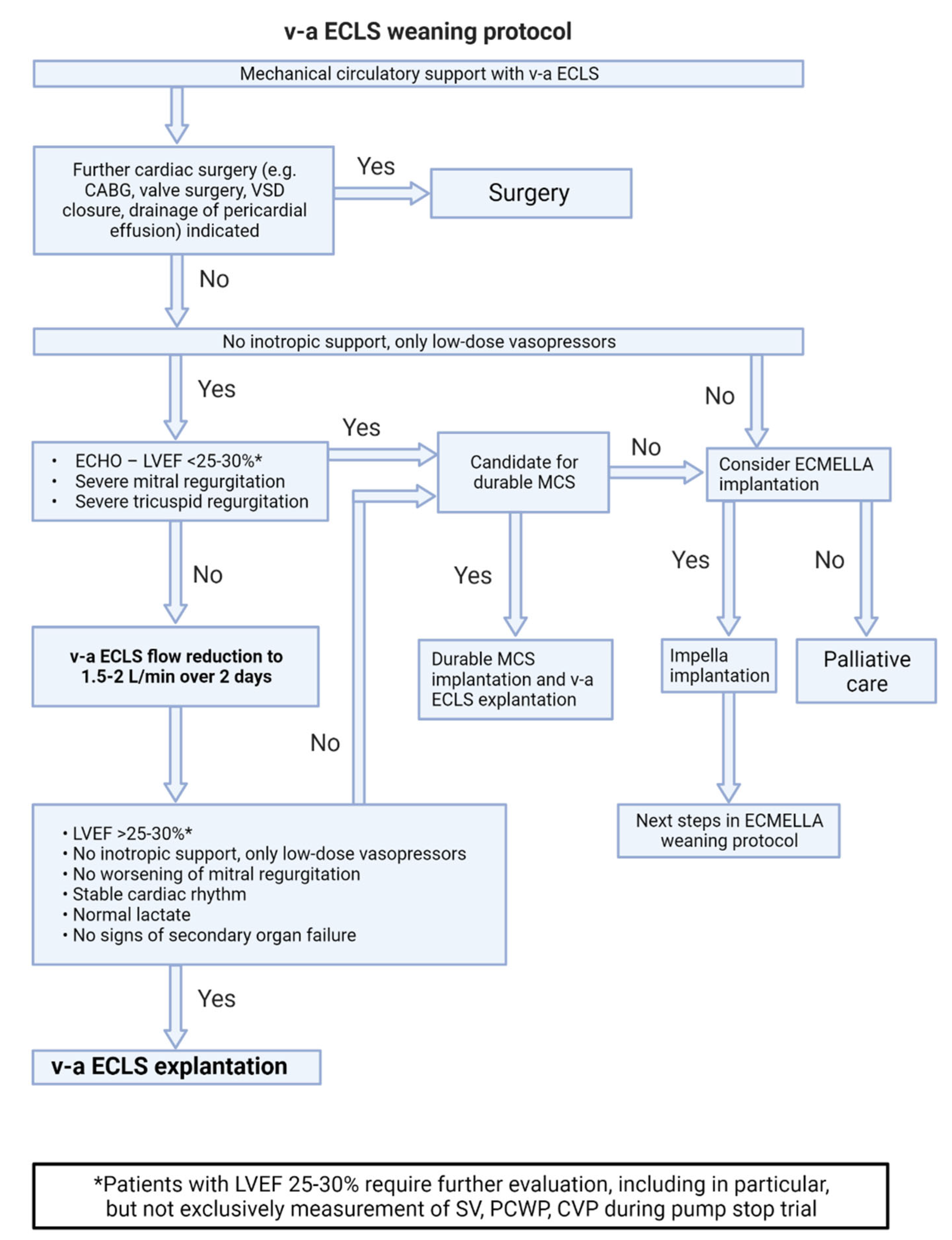
7.2. Weaning from v-a ECLS Support (Figure 3)
- The basic criteria for weaning from v-a ECLS are the same as for weaning from Impella support. After stabilization of the patient's hemodynamic condition, the v-a ECLS flow is gradually reduced to 1.5-2 L over at least 48 hours.
- If the general weaning criteria (no higher-grade valve pathologies, LVEF of at least 25–30%, stable rhythm, no inotropes) are met, v-a ECLS can be explanted.
- In patients on ECLS with unclear neurological status or severe complications, switch to Impella support in setting of bridge-to-decision therapy should be considered.[24]
- Harlequin syndrome (also known as differential hypoxemia) is a rare complication that can occur after onset of the unloading device or during myocardial recovery under v-a ECLS therapy. In severe respiratory failure, poorly oxygenated blood is ejected into the circulation, but remains in the supra-aortic vessels due to retrograde flow and a high afterload generated by v-a ECLS. In this situation, the therapy should be escalated to veno-veno-arterial ECLS. To do this, an additional arterial ECLS line is established in order to transport oxygenated blood to the right atrium. This counteracts differential hypoxemia and reduces the risk of cerebral and coronary hypoperfusion.[25]
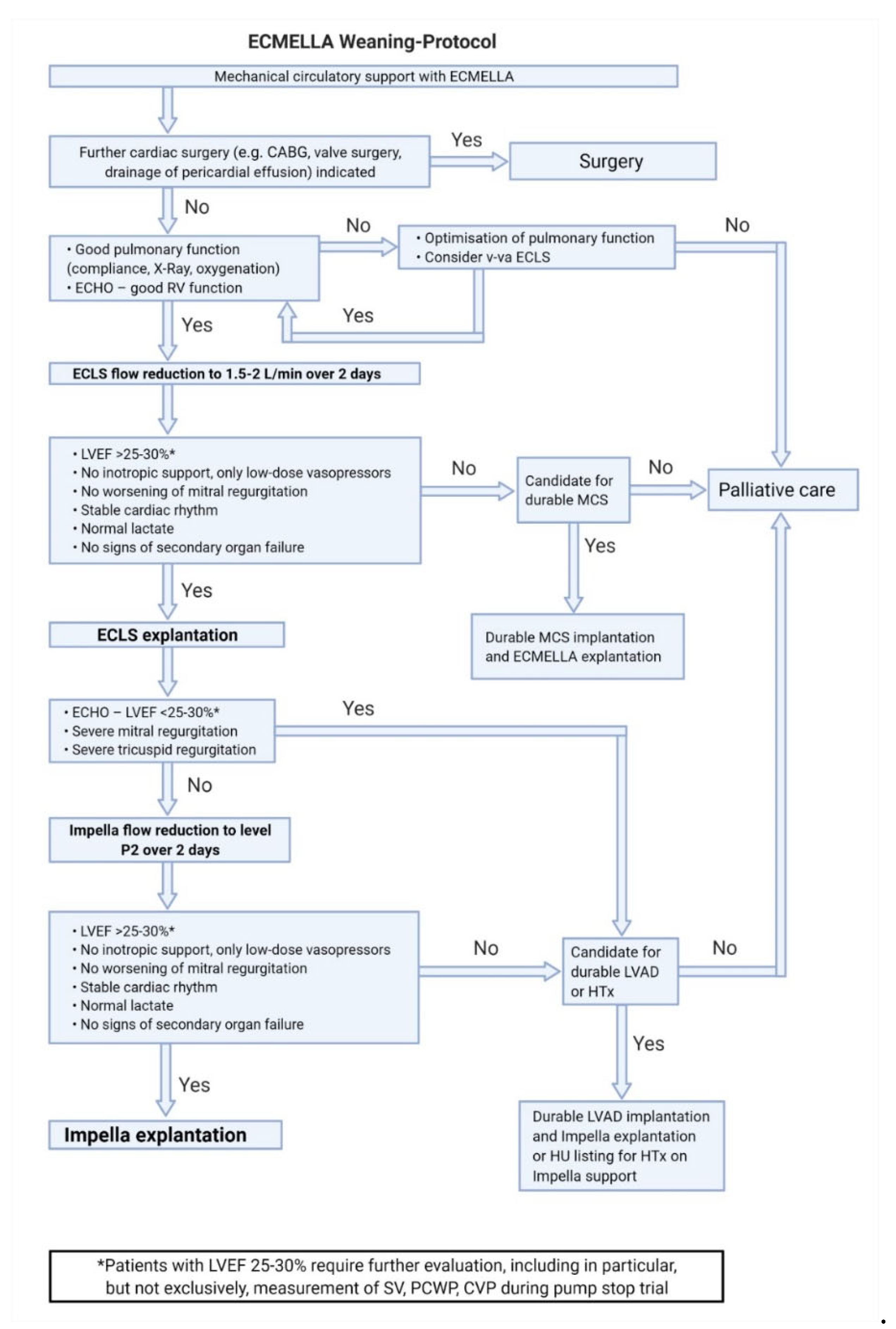
7.3. Weaning from ECMELLA Support (Figure 4)
- The basic criteria for ECMELLA weaning correspond to those for v-a ECLS and Impella weaning. Complications on v-a ECLS are common and increase with support duration. Therefore, the concept of ECMELLA weaning focuses primarily on the reduction of v-a ECLS support with potential explantation.
- ECMELLA therapy with femoral cannulation and simultaneous respiratory insufficiency also bears a risk of differential hypoxemia (Harlequin syndrome). In this case, escalating to vv-a ECMELLA should be considered.
- If de-escalation of ECMELLA therapy is not possible, long-term LVAD implantation on ECMELLA can be performed.
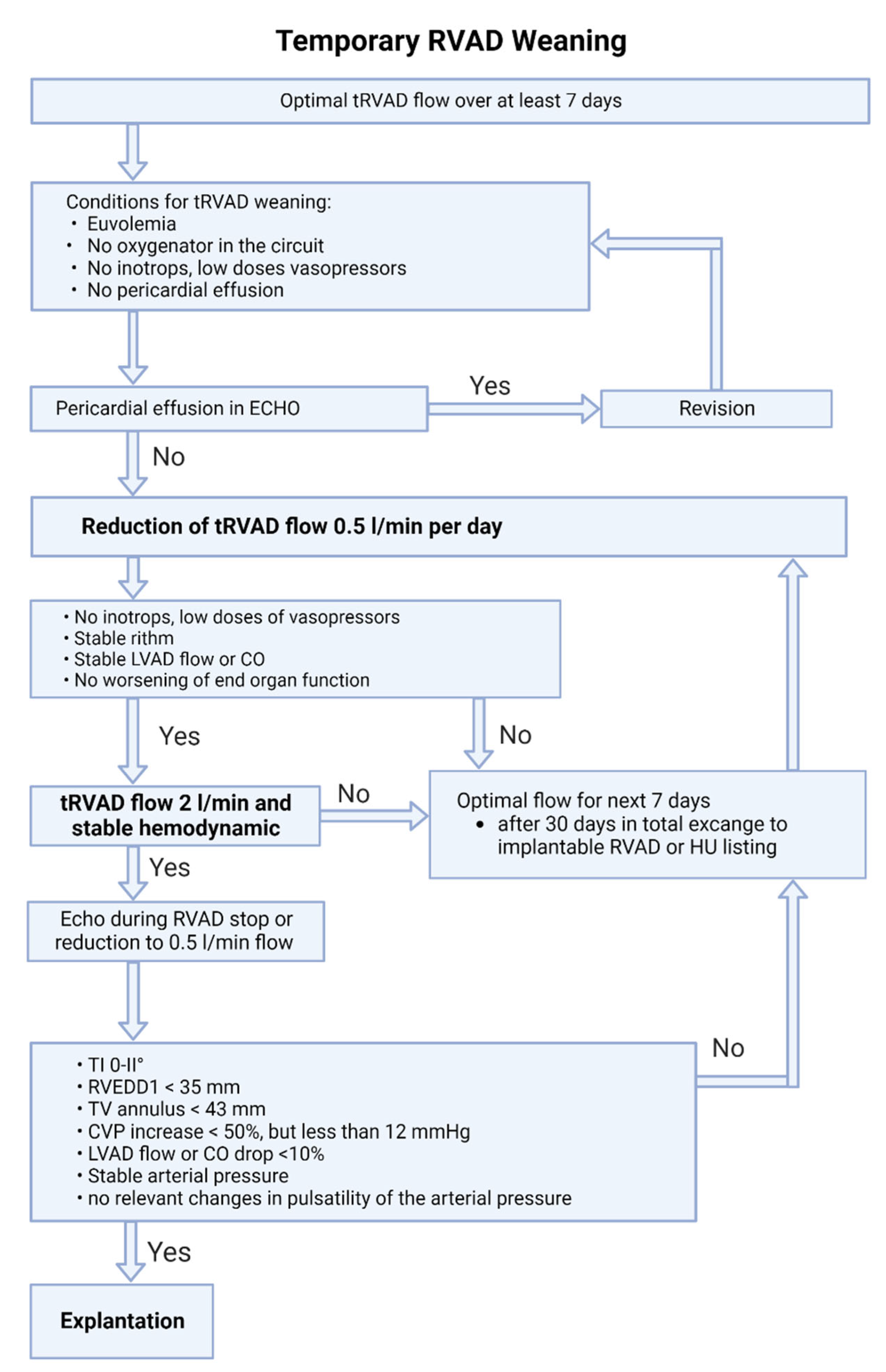
7.4. Weaning from Temporary RVAD Support (Figure 5)
- The right ventricle has a significant regenerative potential, but sometimes requires prolonged periods of support. Therefore, implantation of a permanent RVAD or heart transplantation should be considered only after longer periods of support (>30 days).[5] It should also be considered that implantation of a permanent RVAD is a complex off-label procedure with a significant potential for complications.[26]
- We recommend a stepwise flow reduction of the temporary RVAD by no more than 0.5 L/min per day until 2 L/min is reached.
- It is important to underline, that in case of temporary RV support with Impella RP device the flow should not be reduced below 1.5 L/min (P4 level), due to the iatrogenic tricuspid regurgitation caused by the device itself.
- If hemodynamics fail to stabilize at minimal flow, the optimal support level should be established and re-evaluation performed after seven days.
- If weaning from temporary RVAD support is not possible after a total of 30 days, switching to a permanent system and/or listing for heart transplantation should be considered.[10]
- If gas exchange is impaired during temporary RVAD support, the installation of an oxygenator in the extracorporeal circuit should be considered.
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stein, L.H. and S.C. Silvestry, Algorithmic management of postcardiotomy shock with mechanical support: Bring a map, a plan, and your parachute-and know how to use all three. JTCVS Open, 2021. 8: p. 55-65.
- Rustenbach, C.J. , et al., Risk factors associated with in-hospital mortality for patients with ECLS due to postcardiotomy cardiogenic shock after isolated coronary surgery. Artif Organs, 2022, 46, 1158–1164. [Google Scholar] [CrossRef]
- Fukuhara, S. , et al., Contemporary mechanical circulatory support therapy for postcardiotomy shock. Gen Thorac Cardiovasc Surg, 2016, 64, 183–91. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, R. , et al., 2020 EACTS/ELSO/STS/AATS expert consensus on post-cardiotomy extracorporeal life support in adult patients. J Thorac Cardiovasc Surg, 2021, 161, 1287–1331. [Google Scholar] [CrossRef]
- Nersesian, G. , et al., Temporary mechanical circulatory support for refractory heart failure: the German Heart Center Berlin experience. Ann Cardiothorac Surg, 2019, 8, 76–83. [Google Scholar] [CrossRef]
- Nersesian, G. , et al., Propensity score-based analysis of 30-day survival in cardiogenic shock patients supported with different microaxial left ventricular assist devices. J Card Surg, 2021, 36, 4141–4152. [Google Scholar] [CrossRef]
- Schrage, B. , et al., Left Ventricular Unloading Is Associated With Lower Mortality in Patients With Cardiogenic Shock Treated With Venoarterial Extracorporeal Membrane Oxygenation: Results From an International, Multicenter Cohort Study. Circulation, 2020, 142, 2095–2106. [Google Scholar] [CrossRef]
- Schrage, B. , et al., Timing of Active Left Ventricular Unloading in Patients on Venoarterial Extracorporeal Membrane Oxygenation Therapy. JACC Heart Fail, 2023, 11, 321–330. [Google Scholar] [CrossRef]
- Radakovic, D., et al., Left Ventricular Unloading in Patients on Venoarterial Extracorporeal Membrane Oxygenation Therapy in Cardiogenic Shock: Prophylactic Versus Bail-Out Strategy. Life (Basel), 2023. 13(2).
- Ott, S., et al., Improving Survival in Cardiogenic Shock-A Propensity Score-Matched Analysis of the Impact of an Institutional Allocation Protocol to Short-Term Mechanical Circulatory Support. Life (Basel), 2022. 12(11).
- Welker, C., J. Huang, and H. Ramakrishna, Analysis of the 2020 EACTS/ELSO/STS/AATS Expert Guidelines on the Management of Adult Postcardiotomy Extracorporeal Life Support. J Cardiothorac Vasc Anesth, 2022, 36, 2207–2219. [Google Scholar] [CrossRef]
- Belletti, A. , et al., Vasoactive-Inotropic Score: Evolution, Clinical Utility, and Pitfalls. J Cardiothorac Vasc Anesth, 2021, 35, 3067–3077. [Google Scholar] [CrossRef]
- Hyun, J. , et al., Vasoactive-Inotropic Score as a Determinant of Timely Initiation of Venoarterial Extracorporeal Membrane Oxygenation in Patients With Cardiogenic Shock. Circ J, 2022, 86, 687–694. [Google Scholar] [CrossRef]
- Rissel, R. , et al., Elevated lactate levels and impaired lactate clearance during extracorporeal life support (ECLS) are associated with poor outcome in cardiac surgery patients. PLoS One, 2022, 17, e0278139. [Google Scholar] [CrossRef] [PubMed]
- Nersesian, G. , et al., Prediction of survival of patients in cardiogenic shock treated by surgically implanted Impella 5+ short-term left ventricular assist device. Interact Cardiovasc Thorac Surg, 2020, 31, 475–482. [Google Scholar] [CrossRef]
- Brasseur, A. , et al., Hybrid extracorporeal membrane oxygenation. J Thorac Dis, 2018, 10 (Suppl 5), S707–S715. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Hernandez, E.J. , et al., Femoral venoarterial extracorporeal membrane oxygenation using a novel biatrial cannula for venous drainage and left ventricular venting. J Card Surg, 2020, 35, 3631–3633. [Google Scholar] [CrossRef]
- Bertoldi, L.F. , et al., Bridging INTERMACS 1 patients from VA-ECMO to LVAD via Impella 5.0: De-escalate and ambulate. J Crit Care, 2020, 57, 259–263. [Google Scholar] [CrossRef]
- Eulert-Grehn, J.J. , et al., ECMELLA 2.0: Single Arterial Access Technique for a Staged Approach in Cardiogenic Shock. Ann Thorac Surg, 2021, 111, e135–e137. [Google Scholar] [CrossRef]
- Montagner, M., et al., Single arterial access ECMELLA: A new concept and step-by-step procedure. Multimed Man Cardiothorac Surg, 2021. 2021.
- Thiele, H. , et al., Intraaortic Balloon Pump in Cardiogenic Shock Complicating Acute Myocardial Infarction: Long-Term 6-Year Outcome of the Randomized IABP-SHOCK II Trial. Circulation, 2019, 139, 395–403. [Google Scholar] [CrossRef]
- Gass, A. , et al., Peripheral venoarterial extracorporeal membrane oxygenation in combination with intra-aortic balloon counterpulsation in patients with cardiovascular compromise. Cardiology, 2014, 129, 137–43. [Google Scholar] [CrossRef] [PubMed]
- Nersesian, G. , et al., Percutaneous mitral valve repair assisted by a catheter-based circulatory support device in a heart transplant patient. J Card Surg, 2021, 36, 3905–3909. [Google Scholar] [CrossRef]
- Bernhardt, A.M. , et al., Impella 5.0 therapy as a bridge-to-decision option for patients on extracorporeal life support with unclear neurological outcomes. Eur J Cardiothorac Surg, 2019, 56, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Giunta, M. , et al., Management of harlequin syndrome under ECPELLA support: A report of two cases and a proposed approach. Ann Card Anaesth, 2023, 26, 97–101. [Google Scholar] [PubMed]
- Potapov, E. , et al., Mechanical circulatory support: Technical tips for the implantation of a right ventricular assist device. JTCVS Open, 2021, 8, 37–40. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




