1. Introduction
The brown planthopper, Nilaparvata lugens (Stål) (Hemiptera: Delphacidae), is an economically-important pest that damages Asian rice, Oryza sativa L., crops by direct injury and by transmitting rice virus disease. Brown planthopper is distributed over approximately half of the rice-growing area of China and causes annual losses of approximately 1 to 1.5 million tons of rice (Cheng and Lou 2003).
Effective control of brown planthopper has been seriously compromised in recent years by the widespread appearance of resistance to imidacloprid, a neonicotinoid insecticide that has been used as the primary control tactic for outbreaks of brown planthopper in eastern and southeastern Asia since the mid-1990s. Imidacloprid resistance was first reported in Thailand, but has since been reported in other countries, including China (Matsumura et al. 2008, Wang et al. 2008, Cheng et al. 2011).
Microbial symbionts benefit their insect hosts (Gibson and Hunter 2010) by impacting nutrition (Douglas 2009, Hosokawa et al. 2010), reproduction (Wilkinson et al. 2001), virulence regulation (Lu et al. 2004), and detoxification (Dowd and Shen 1990, Xu et al. 2009). Kikuchi (2012) showed that fenitrothion-degrading Burkholderia strains established a symbiosis with the hemipteran Riptortus pedestris (F.) conferring resistance to fenitrothion.
Bacteria and yeast-like symbiotes are important symbionts of the brown planthopper that provide their hosts with cholesterol, vitamins, and essential amino acids that the host cannot produce (Sasaki et al. 1996). Exposure of neonates to high temperature, antibiotics, lysozyme via injections, and insecticides reduced yeast-like symbiont abundance and, thus, influenced host growth and development (Raguraman et al.1988, Shankar and Baskaran 1988). These symbionts also play crucial roles in changes in the virulence of brown planthopper populations to resistant rice varieties (Lu et al. 2004), because the rate of change in endosymbiotic genes is much more rapid than occurs in host genes (Shikawa and Yamaji 1985, Ishikawa et al. 1986, Campbell 1990). The symbiont-related mechanisms underlying high resistance to imidacloprid in brown planthopper have received relatively little attention. Analysis of strains identified a single point mutation (Y151S) in two nAChR subunits that were associated with a dramatic reduction in binding to imidacloprid (Liu et al. 2005). Enhanced detoxification of imidacloprid by carboxyesterase, glutathione-S-transferase, and cytochrome P450 monooxygenase appears to be the predominant mechanism of resistance in field-selected populations (Wen et al. 2009, Puinean et al. 2010).
Zhang et al. (2013) studied the mid-gut bacterial communities of the larvae of the striped rice stem borer (Chilo suppressalis [Walker]) using PCR-DGGE. Hou et al. (2013) analyzed yeast-like symbiont diversity in N. lugens with PCR-DGGE and found several previously detected, undetected, and uncultured fungi. Xu et al. (2014) reported the structures of bacterial communities in N. lugens from different geographic and resistant virulent populations using DGGE. In this study, we used PCR-DGGE to assess bacterial and yeast-like symbiont communities in imidacloprid-resistant and imidacloprid-susceptible brown planthopper populations, with the goal of revealing the possible functions of these endosymbionts in the development of imidacloprid resistance.
2. Materials and Methods
2.1. Source of test materials
Rice plants. Rice varieties, including TN1 (brown planthopper susceptible), were planted in clay pots (15 cm diameter) in the greenhouse of the Zhejiang Academy of Agricultural Sciences in Hangzhou, China. The experiments described below were conducted with 45-day-old rice plants.
Insects. The susceptible N. lugens population provided by the Zhejiang Research Institute of Chemical Industry had not been exposed to any insecticide for at least 10 years before the study. The resistant N. lugens population was selected by spraying imidacloprid (LC50) for more than 50 generations at the Zhejiang Academy of Agricultural Science. The resistance ratio of the resistant N. lugens population was nearly 400 times greater than that of the susceptible population. Female adult N. lugens from the imidacloprid-susceptible and imidacloprid-resistant populations were collected and used for tests.
2.2. Methods
Total DNA extraction. DNA was extracted from 50 female adults from each brown planthopper population, after which the samples were surface-sterilized with 75% ethanol for 1 min. Genomic DNA was extracted using a Bacterial DNA Kit (Omega Bio-Tek Company Ltd, Guangzhou China) or a Yeast DNA Kit (Omega, Guangzhou China). DNA purity and concentration were measured by a protein nucleic acid spectrophotometer (DU800, Beckman Instruments Inc., California USA).
PCR amplification. All primers used in this study are shown in
Table 1 and were synthesized by Shanghai Shenggong Bioengineering Company, Ltd., China. For analysis of bacterial diversity, PCR amplification of the 16S rRNA gene was conducted using bacteria-specific primer set 49f-1525r (Muyzer et al. 1993, Henckel et al. 1999). For bacterial diversity analysis, PCR amplification of the 16S rRNA gene was performed using the arch341f-534r primer set (Nakagawa and Fukui 2003). For analysis of yeast diversity, PCR amplification of the 26S rRNA gene was performed as described previously (Prakitchaiwattana et al. 2004) with initial amplification of the D1/D2 region using eukaryotic universal primers NL-1 and NL-4 (Taylor et al. 2002), followed by nested PCR using primers GCNL-1 and LS2 (Cocolin et al. 2002). DNA from each sample was subjected to DGGE following PCR amplification with each primer set (
Table 1). All PCR amplification was conducted in a final volume of 25 µL containing 0.5 µL (50 ng/µL) template, 1 μL of template DNA, 0.5 μL of primer NL-1 and primer NL-4 (10 μM), 21.5 μL of Platinum PCR Supermix High Fidelity, and 1.5 μL of sterile double-distilled water. Reactions were performed in a PTC-220 DNA Engine Dyad MJ Research thermocycler.
DGGE analysis. DGGE was performed using the D Code Universal Mutation System (Bio-Rad Laboratories, California USA) for separation of PCR products. PCR products were applied directly onto 8% (w/v) polyacrylamide gels in a running buffer containing 1× TAE (20 mM Tris, 10 mM acetate, 0.5 mM EDTA [pH 8.3]) and a denaturing gradient of 30–60% urea and formamide (for bacteria and yeast) or 35–55% urea and formamide (for Archaea, where 100% denaturant contains 7 M urea and 40% formamide). Electrophoresis was performed at 80 V for 14 h at a constant temperature of 60 °C. After electrophoresis, the gels were stained using SYBR Green I nucleic acid stain (ThermoFisher Scientific, Massachusetts, USA) and photographed under UV transillumination. Sterile blades were used to excise bands from the gels, after which each band was mixed with 20 µL of 0.1× TE buffer solution, incubated overnight at 4 °C, and used for PCR amplification with the appropriate primer set.
Data analysis. DGGE profiles were analyzed using quantity BIO-1D software (Bio-Rad Laboratories, California USA) to determine the position and intensity of each band. The Shannon-Weaver index (H), an expression of the proportional abundance of species in a community, The Shannon-Weaver was calculated using the formula:
where Pi is the ratio of the DNA quantity of the ith band to the total DNA quantity of all the bands of the sample, S is the number of DGGE bands in the sample, N is the quantity of the amplified DNA of all bands in the DGGE lanes, and Ni is the quantity of the amplified DNA of the ith band).
The evenness index (E), an expression of the similarity in the number of individuals of multiple species in an environment, was calculated using the formula:
where S is the total number of species in the sample. Sorenson’s pairwise similarity coefficient (Cs) is used to compare the presence or absence of species in different populations and was calculated using the formula:
where a and b are the number of bands in the DNA DGGE figures of two different samples and j is the number of bands in the two DGGE lanes.
The significance of differences between the two insect populations werr analyzed using a one-way ANOVA with a significance threshold of P < 0.05.
3. Results
DGGE analysis. The Shannon-Weaver diversity index and evenness index suggest that the quantity of symbiotic microorganisms, dominant species of symbiotic microorganism, and relative abundance of symbiotic microorganisms in imidacloprid-resistant and imidacloprid-susceptible N. lugens populations were similar. The similarity coefficient of the bacterial communities of the imidacloprid-resistant and imidacloprid-susceptible populations was 0.53, while that of the yeast-like symbiont communities was 0.56 (
Table 2). These results suggest that there were differences in the microbial community structures of the imidacloprid-resistant and imidacloprid-susceptible populations of N. lugens. Thus, the development of insecticide resistance by N. lugens might be related to the distribution of the population of yeast-like symbionts; whereas, resistance seemed to have no relationship with the richness of the symbiotic bacterial population.
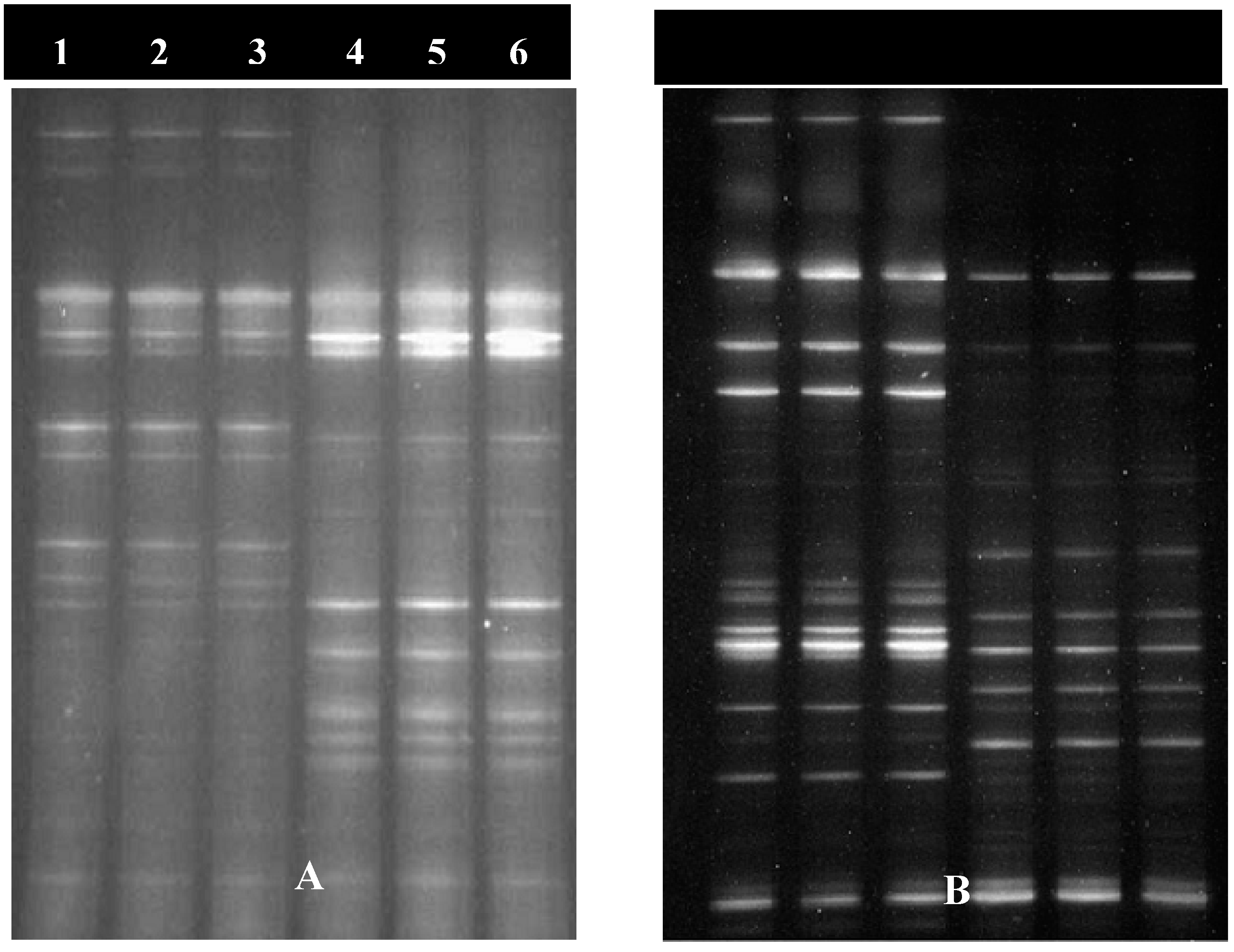
As shown in
Figure 1, 15 bacterial bands and 12 yeast-like bands were selected for partial sequencing. Sequence comparison analysis was conducted based on sequences in the NCBI GenBank database. All selected clones were closely related (≥97% sequence identity) to the reported species in the NCBI GenBank. The numbers of bacterial species in the imidacloprid-susceptible and imidacloprid-resistant populations were not significantly different. The bacteria in the susceptible population were members of the Enterobacteriaceae and Moraxellaceae families. The bacteria in the resistant population belonged to more families, Oxalobacteriaceae, Rhodobacteriaceae and Sphingomonadaceae as well as Enterobacteriaceae (
Table 3). Yeast-like fungi Cryptococcus luteolus, Pseudozyma aphidis, Pseudozyma antarctica, Capnodiales sp., and Cladosporiumper angustum were identified for the first time in N. lugens. The imidacloprid-susceptible population contained more yeast-like symbiont species than did the imidacloprid-resistant population. Cryptococcus luteolus, Pseudozyma aphidis, and Pseudozyma antarctica were detected only in the susceptible population, while Cladosporium perangustum was detected only in the resistant population; Capnodiales sp. and some unknown species existed in both populations (
Table 4).
4. Discussion
Insects harbor symbionts that enhance fitness by contributing to digestion, nutrition, reproduction, and resistance to xenobiotics (Douglas 2011). However, few studies on the contributions of symbionts to resistance to insecticides have been performed because of the difficulty of separating symbionts from host insects. The evolution of insecticide resistance is accompanied by a series of physiological changes in the host (Klepzig et al. 2009), which alter the structure and function of the microorganism community (Gimonneau et al. 2014). In this study, PCR-DGGE assays indicated that there were no significant differences in the banding abundance, Shannon-Weaver diversity index, or evenness index of the imidacloprid-susceptible and imidacloprid-resistant populations. These findings could indicate that the development of insecticide resistance by N. lugens was not closely related to the richness of the symbiotic microorganism population; instead, it seemed to be related to the distribution of the yeast-like symbiont population. Sequencing analysis showed that common bacteria (Arsenophonus nasoniae, Enterobacter asburiae) existed in the resistant and susceptible populations; whereas, Herbaspirillum sp., Sphingomonas sp., and Amaricoccus sp. were detected only in the imidacloprid-resistant population. We speculate that aromatic compound degradation (Baraniecki and Aislabie 2002, Singleton et al.2008, Lafortune et al. 2009, Bacosa and Suto 2010) resulted in these species transitioning from secondary bacteria species to dominant species under imidacloprid exposure, perhaps enhancing imidacloprid resistance. Herbaspirillum sp. and Amaricoccus sp. also function in nitrogen fixation (Elbeltagy et al. 2001, Valverde et al. 2003) and intracellular storage of synthesized polymers (Falvo et al. 2001, Lemos et al. 2008); these strains could contribute to raw material storage by synthesizing amino acids and proteins.
In this study, the yeast Cryptococcus luteolus, Pseudozyma aphidis, Pseudozyma antarctica, Capnodiales sp., and Cladosporium perangustum were found in N. lugens for the first time. The study revealed that the yeast-like symbiont population of N. lugens was a mixture of many types of yeast and showed that the microbial species in imidacloprid-susceptible and imidacloprid-resistant populations varied. Capnodiales sp. was detected in 2 populations and might have been carried into the body when N. lugens fed on infected rice plants. Cladosporium perangustum was detected in the resistant population; other 3 yeasts (Cryptococcus luteolus, Pseudozyma aphidis, and Pseudozyma antarctica) only exist in susceptible population.
Cladosporium perangustum is abundant in the air, from which the organism can be absorbed by insects (Hsu et al. 2012). Basidiomycetous yeast Cryptococcus luteolus produces polysaccharides (Vorotynskaya et al. 1992), while Pseudozyma antarctica are an excellent source of edible single-cell protein and facilitate utilization of waste glycerol (Morita et al. 2007). Pseudozyma aphidis has biocontrol activity and provides a natural barrier against some plant pathogens (Avis and Belanger 2001, Urquhart and Punja 2002). The manner in which differences in the microorganism distributions in the populations are related to insecticide resistance merits further study.
The results reported here indicate that there are significant differences in the microbial community structures of imidacloprid-susceptible and imidacloprid-resistant brown planthopper populations. More evidence is required to assess whether changes in microbial community structure are related to insecticide resistance.
Author Contributions
We confirm that all authors have approved the manuscript and agree with submission to the Journal. Zhang Juefeng carried out the experiments and drafted the manuscript. Li Fang, Zhong Haiying helped with experimental procedures and manuscript preparation. Chen Jianming designed the study and critically revised the manuscript. All authors read and approved the final manuscript.
Funding
This research was funded by the Zhejiang Provincial Programs for Science and Technology Development (2023C02030), National Nature Science Foundation of China (2020R17A33B03).
Acknowledgments
We thank Xin Wang from Institute of Plant Protection and Microbiology, Zhejiang Academy of Agricultural Sciences for his help in analyzing the data, and we are grateful to Guangjie Liu from AgriLife Research and Extension Center, Texas A & M University, USA for the suggestion for improving this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Avis, T.J.; and R.R. Belanger. Specificity and mode of action of the antifungal fatty acid cis-9-hcptadcccnoic acid produced by Psedozyma flocculosa. Appl. Environ. Microbiol. 2001, 67, 956–960. [CrossRef]
- Baraniecki, C.A.; J. Aislabie. Characterization of Sphingomonas sp. Ant 17, an aromatic hydrocarbon-degrading bacterium isolated from Antarctic soil. Micro. Ecol. 2002, 43, 44–54. [CrossRef] [PubMed]
- Bacosa, H.; K. Suto. Preferential degradation of aromatic hydrocarbons in kerosene by a microbial consortium. Intern. Biodeter. Biodegrad. 2010, 64, 702–710. [CrossRef]
- Campbell, B.C. On the role of microbial symbiotes in herbivorous insects. In: Bernays EA (ed) Insect-plant interactions, Florida CRC Press Inc.1990; vol. I 1-44.
- Cheng, J.A.; Y.G. Lou. Basic research on the outbreak mechanism and sustainable management of rice planthoppers. Chinese J. Appl. Entomol. 2011, 48, 231–238. [CrossRef]
- Cocolin, L.D.; M. Manzano. An application of PCR-DGGE analysis to profile the yeast populations in raw milk. Intern. Dairy. J. 2002, 12, 407–411. [CrossRef]
- Douglas, A.E. Lessons from studying insect symbioses. Cell Host Microbe. 2011, 10, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A E. The microbial dimension in insect nutritional ecology. Funct. Ecol. 2009, 23, 38–47. [CrossRef]
- Dowd,P.F.; S.K. Shen. The contribution of symbiotic yeast to toxin resistance of the cigarette beetle (Lasioderma serricorne). Entomol. Exp. Appl. 1990, 56, 24. [CrossRef]
- Elbeltagy, A.; K. Nishioka.; T. Sato.; H. Suzuki, B. Ye.; T. Hamada.; T. Isawa.; H. Mitsui. Endophytic colonization and in planta nitrogen fixation by a Herbaspirillum sp. isolated from wild rice species. Appl. Environ. Microb. 2001, 67, 5285–5293. [CrossRef]
- Falvo, A.; C. Levantesi; S. Rossetti; R. J. Seviour; V. Tandoi. Synthesis of intracellular storage polymers by Amaricoccus kaplicensis, a tetrad forming bacterium present in activated sludge. J. Appl. Microbiol. 2001, 91, 299–305. [CrossRef]
- Fischer, S.G.; L. S. Lerman. DNA fragments differing by single base-pair substitutions are separated in denaturing gradient gels: correspondence with melting theory. P. Natl. Acad. Sci. USA. 1983, 80, 1579–1583. [CrossRef] [PubMed]
- Gibson, C.M. and M. S. Hunter. Extraordinarily widespread and fantastically complex: comparative biology of endosymbiotic bacterial and fungal mutualists of insects. Ecol.Lett. 2010, 13, 223–234. [CrossRef]
- Gimonneau, G.; M.T. Tchioffo.; L. Abate.; A. Boissiere.; I. Morlais. Composition of Anopheles coluzzii and Anopheles gambiae microbiota from larval to adult stages. Infect. Genet. Evol. 2014, 28, 715–724. [CrossRef]
- Henckel, T., M. Friedrich, R. Conrad. Molecular analyses of the methane-oxidizing microbial community in rice field soil by targeting the genes of the 16S rRNA, particulate methane monooxygenase, and methanol dehydrogenase. Appl. Environ. Microb. 1999, 65, 1980–1990. [CrossRef] [PubMed]
- Hosokawa, T.R. Koga, Y. Kikuchi. Wolbachia as a bacteriocyte-associated nutritional mutualist. P. Natl. Acad. Sci. USA. 2010, 107, 769–774. [Google Scholar] [CrossRef]
- Hou, Y.; Ma, Z.; Dong, S.; Yu, X.P. Analysis of yeast-like symbiote diversity in the brown planthopper (BPH), Nilaparvata lugens Stål, using a novel nested PCR-DGGE protocol. Curr. Microbiol. 2013, 67, 263–270. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Kung, P.Y.; Wu, T.N.; Shen, Y.H. Characterization of indoor-air bioaerosols in Southern Taiwan. Aerosol. Air. Qual. Res. 2012, 12, 651–661. [Google Scholar] [CrossRef]
- Ishikawa, M.; T. Meshi; F. Motoyoshi; N. Takamatsu; Y. Okada. In vitro mutagenesis of the putative replicase genes of tobacco mosaic virus. Nucleic Acids Res. 1986, 14, 8291–8305. [CrossRef]
- Ishikawa, H.; M. Yamaji. Symbionin, an aphid endosymbiont-specific protein—I: Production of insects deficient in symbiont. Insect Biochem. 1985, 15, 155–163. [CrossRef]
- Kikuchi, Y.; M. Hayatsu; T. Hosokawa. Symbiont-mediated insecticide resistance. P. Natl. Acad. Sci. USA. 2012, 109, 8618–8622. [CrossRef]
- Klepzig. K. D.; Adams, A.S.; Handelsman, J.; Raffa, K.F. Symbioses: a key driver of insect physiological processes, ecological interactions, evolutionary diversification, and impacts on humans. Environ. Entomol. 2009, 38, 67–77. [CrossRef]
- Lafortune. I.; Juteau, P.; Déziel, E.; Lépine, F.; Beaudet, R.; Villemu, R. Bacterial diversity of a consortium degrading high-molecular-weight polycyclic aromatic hydrocarbons in a two-liquid phase biosystem. Microb.Ecol. 2009, 57, 455–468. [CrossRef] [PubMed]
- Lerman.L. S.; Fischer,S. G.; Hurley, I.; Silverstein, K.; Lumelsky, N. Sequence-determined DNA separations. Annu Rev Biophys. 1984, 13, 399–423. [CrossRef]
- Liu, Z.; Williamson,M. S.; Lansdell, S.J. A nicotinic acetylcholine receptor mutation conferring target-site resistance to imidacloprid in Nilaparvata lugens (brown planthopper). P. Natl. Acad. Sci. USA. 2005, 102, 8420–8425. [CrossRef] [PubMed]
- Lu. Z. X., Yu, X.P.; Chen, J.M.; Zheng, X.S. Dynamics of yeast-like symbiote and its relationship with the virulence of brown planthopper, Nilaparvata lugens Stål, to resistant rice varieties. J. Asia-Pac. Entomol. 2004, 7, 317–323. [CrossRef]
- Lemos. P. C.; Levantesi, C.; Serafim, L.S.; Rossetti, S.; Maria, A.M. Microbial characterisation of polyhydroxyalkanoates storing populations selected under different operating conditions using a cell-sorting RT-PCR approach. Appl.microbiol.biot. 2008, 78, 351–360. [CrossRef]
- Matsumura, M.; Takeuchi, H.; Satoh, M.; Thanh, D.V. Species-specific insecticide resistance to imidacloprid and fipronil in the rice planthoppers Nilaparvata lugens and Sogatella furcifera in East and South-east Asia. Pest. Manag. Sci. 2008, 64, 1115–1121. [Google Scholar] [CrossRef]
- Muyzer, G.; Waal, E.C. De.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ.Microb. 1993, 59, 695–700. [Google Scholar] [CrossRef]
- Morita. T.; Konishi, M.; T. Fukuoka, T.; Imura, D. Kitamoto. Microbial conversion of glycerol into glycolipid biosurfactants, mannosylerythritol lipids, by a basidiomycete yeast, Pseudozyma antarctica JCM 10317T. J.Biosci.Bioeng. 2007, 104, 78–81. [CrossRef]
- Nakagawa.T.; Fukui, M. Molecular characterization of community structures and sulfur metabolism within microbial streamers in Japanese hot springs. Appl. Environ. Microb. 2003, 69, 7044–7057. [CrossRef]
- Prakitchaiwattana.C.J.; Fleet, G.H.; Heard, G.M. Application and evaluation of denaturing gradient gel electrophoresis to analyze the yeast ecology of wine grapes. FEMS. Yeast. Res. 2004, 4, 865–877. [CrossRef] [PubMed]
- Puinean. A. M.; Denholm, I.; Millar, N.S.; Williamson, M. Characterisation of imidacloprid resistance mechanisms in the brown planthopper, Nilaparvata lugens Stål (Hemiptera: Delphacidae). Pestic. Biochem. Phys. 2010, 97, 129–132. [CrossRef]
- Raguraman., S.; Jayaraj, S.; Saxena, R.C. Effect of neem on yeast-like symbionts (YLS) harbored by brown planthopper (BPH). International Rice Research Newsletter (Philippines). 1988.vol. 13.
- Singleton. D. R.; Richardson, S.D.; Aitken, M.D. Effects of enrichment with phthalate on polycyclic aromatic hydrocarbon biodegradation in contaminated soil. Biodegradation. 2008, 19, 577–587. [CrossRef] [PubMed]
- Sasaki, T.; Kawamura, M.; Ishikawa, H. Nitrogen recycling in the brown planthopper, Nilaparvata lugens: involvement of yeast-like endosymbionts in uric acid metabolism. J. Insect Physiol. 1996, 42, 125–129. [Google Scholar] [CrossRef]
- Shankar, G.; Baskaran, P. Impact of the presence of Parasites on the population of resident endosymbiotes in the brown planthopper, Nilaparvata lugens. Curr.Sci. 1988, 57, 213. [Google Scholar]
- Taylor.J. P.; Wilson, B.; Mills, M.S.; Burns, R.G. Comparison of microbial numbers and enzymatic activities in surface soils and subsoils using various techniques. Soil.Biol.Biochem. 2002, 34, 387–401. [CrossRef]
- Urquhart. E. J.; Punja, Z.K. Hydrolytic enzymes and antifungal compounds produced by Tilletiopsis species, phyllosphere yeasts that are antagonists of powdery mildew fungi. Can. J. Microbiol. 2002, 48, 219–229. [CrossRef]
- Vorotynskaya, S.L.; Vitovskaya, G.A.; Ananjeva, E.P. Studies on the properties of polysaccharides produced by the yeasts Cryptococcus luteolus (Saito) skinner. Mikol.Fitopatol. 1992, 26, 367–371. [Google Scholar] [CrossRef]
- Valverde. A.; Velázquez, E.; Gutiérrez, C.E.; Cervantes, A. Ventosa, Igual1, J.M. Herbaspirillum lusitanum sp. nov., a novel nitrogen-fixing bacterium associated with root nodules of Phaseolus vulgaris. Int.J. Syst.Evol.Micr. 2003, 53, 1979–1983. [CrossRef]
- Wang, Y.H.; Gao, C.F.; Zhu, Y.C.; Chen, J.; Li, W.H.; Zhuang, Y.L.; Dai, D.J.; Zhou, W.J.C.; Ma, Y.; Shen, J.L. Imidacloprid susceptibility survey and selection risk assessment in field populations of Nilaparvata lugens (Homoptera: Delphacidae). J. Econ. Entomol. 2008, 101, 515–522. [Google Scholar] [CrossRef]
- Wen, Y.; Liu, Z.; Bao, H.; Han, Z.J. Imidacloprid resistance and its mechanisms in field populations of brown planthopper, Nilaparvata lugens Stål in China. Pestic. Biochem.Phys. 2009, 94, 36–42. [Google Scholar] [CrossRef]
- Wilkinson, T.L.; Adams, D.; Minto, L.B.; Douglas, A.E. The impact of host plant on the abundance and function of symbiotic bacteria in an aphid. J.Exp.Biol. 2001, 204, 3027–3038. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.X.; Zheng, X.S.; Liu, S.P.; Lv, Z.X. The role of endosymbionts in insect host resistance against adverse factors. Chinese Bulletin of Entomology. 2009, 46, 350–354. (in chinese). [Google Scholar]
- Xu, H.X.; Zheng, X.S.; Yang Y., J.; Lv, Z.X. Bacterial community in different populations of rice brown planthopper Nilaparvata lugens (Stål). Rice Science. 2014, 21, 59–64. [Google Scholar] [CrossRef]
- Zhang, J.F.; Chen, J.M.; He, Y.P. Diversity analysis of bacterial community in midguts of larvae of the striped stem borer, Chilo suppressalis (Lepidoptera: Crambidae), with different levels of resistance to insecticides. Acta Entomol. Sin. 2013, 56, 1075–1082. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).