Submitted:
07 September 2023
Posted:
12 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
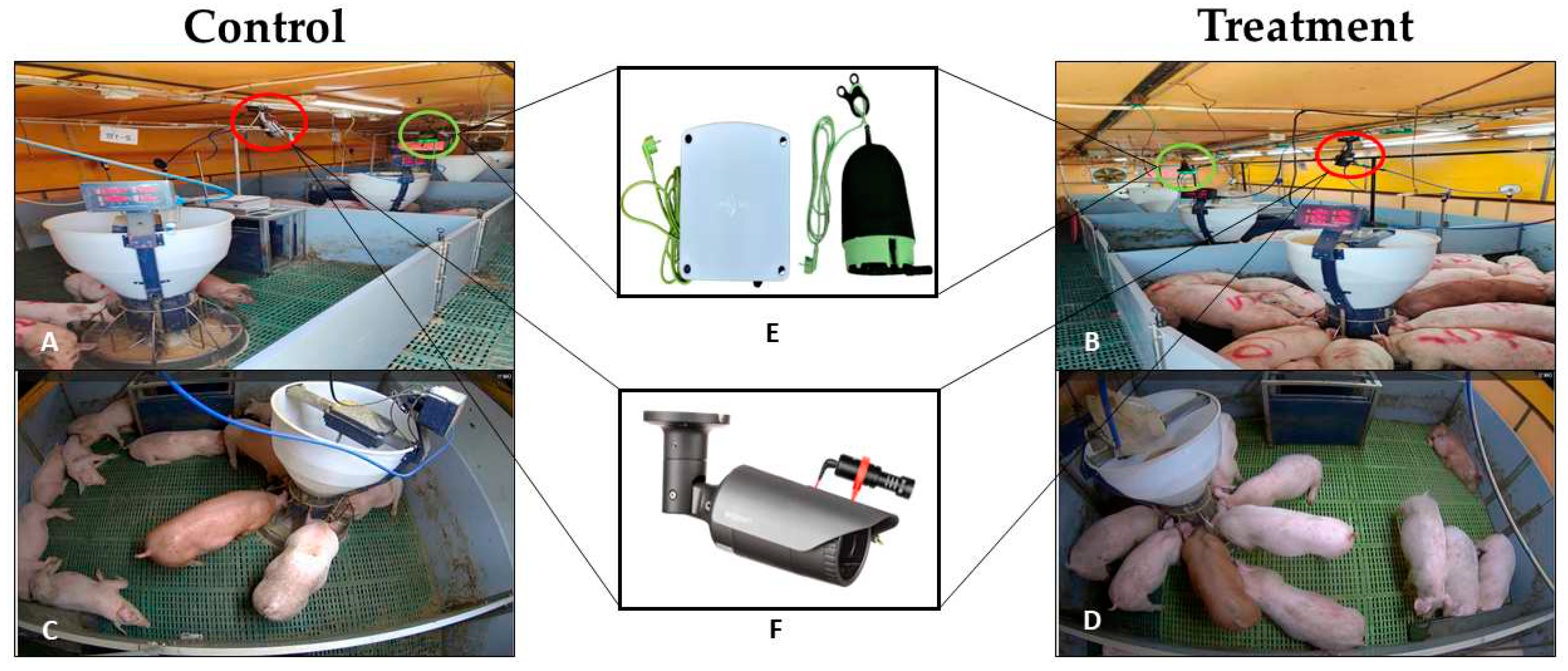
2.1. Animals and Housing
2.2. Environmental Conditions
2.3. Growth Performance
2.4. Pigs’ Behavior
2.4.1. Posture
2.4.2. Biting Behavior
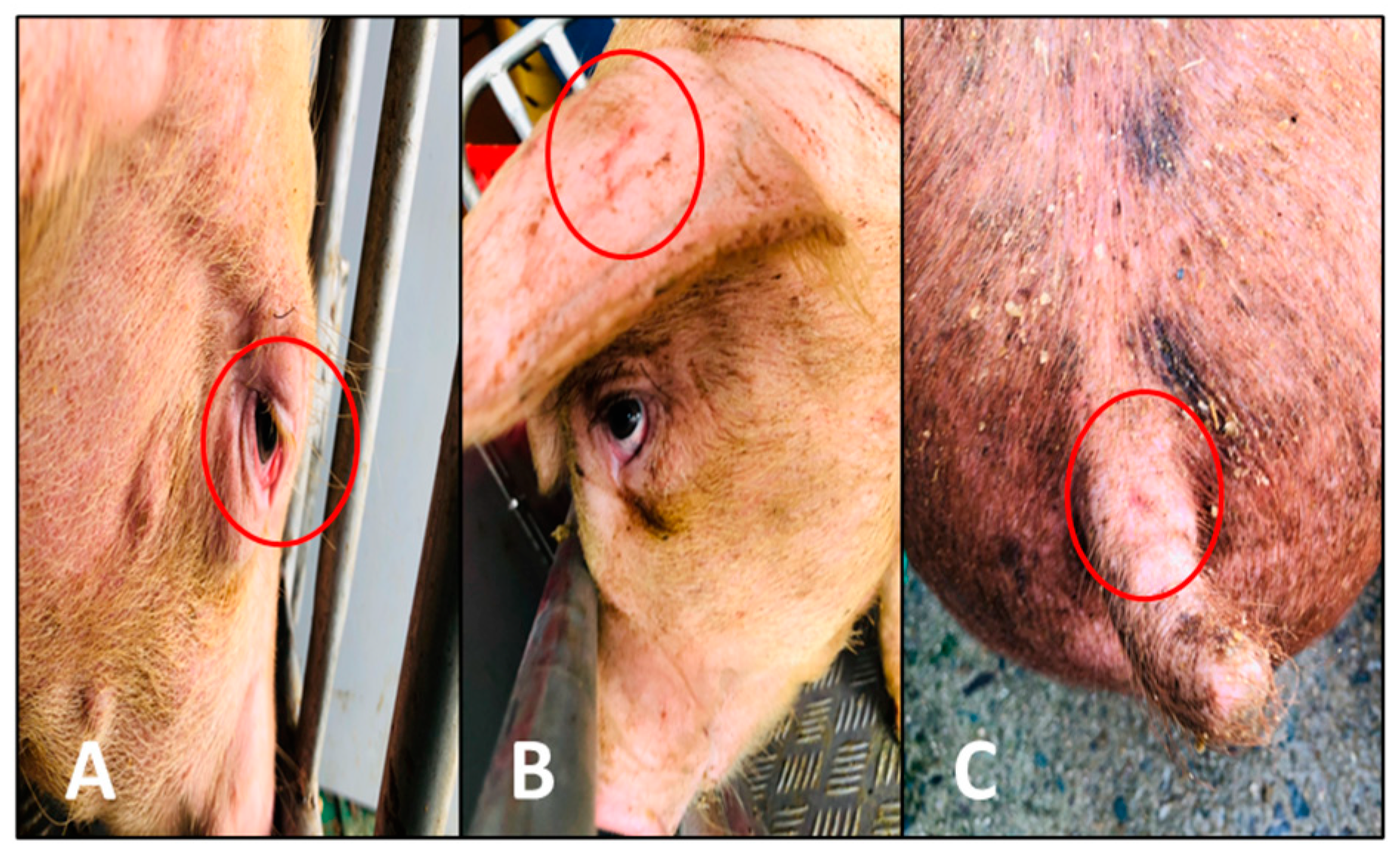
2.5. Health Conditions
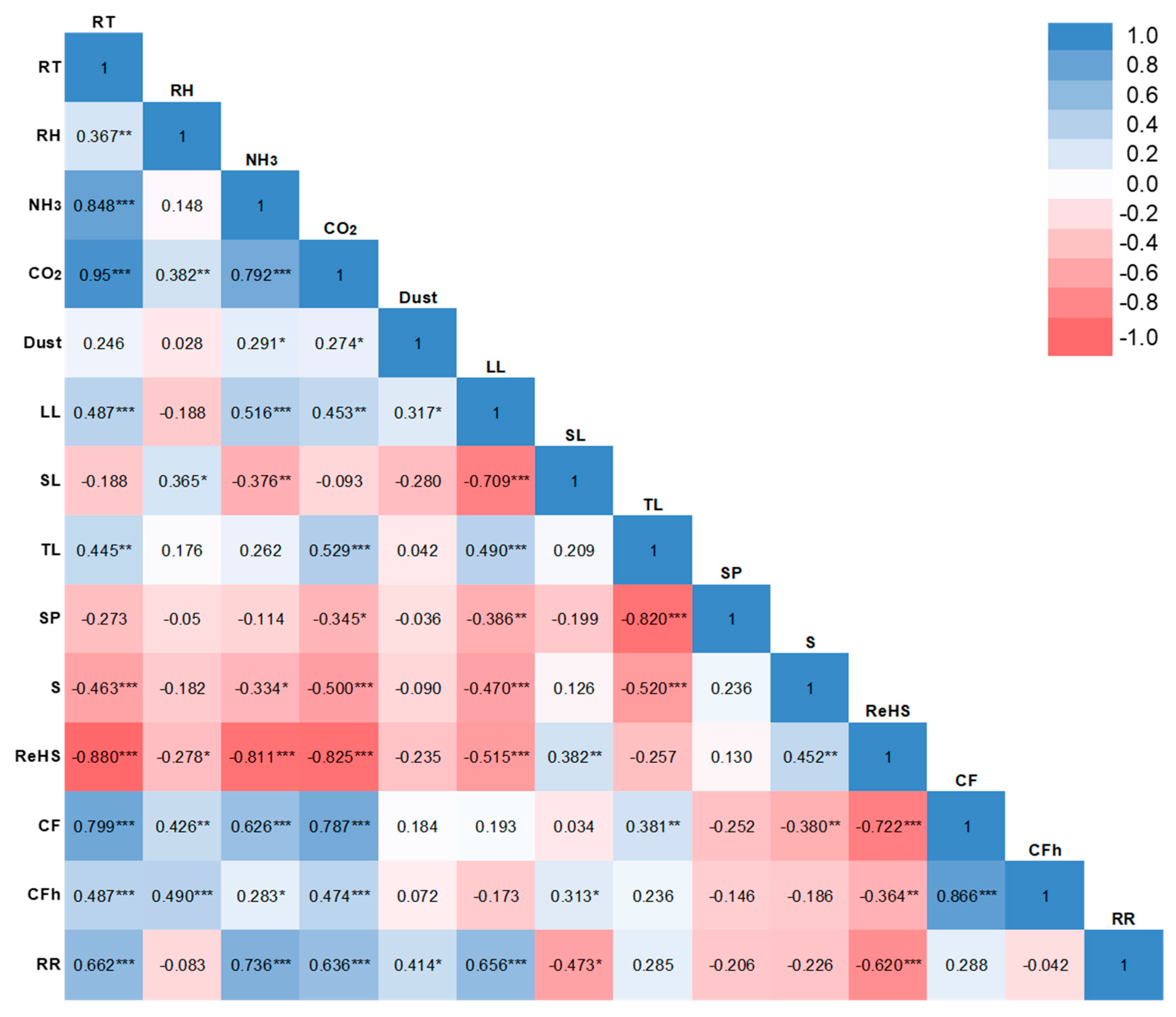
2.6. Statistical Analysis
3. Results
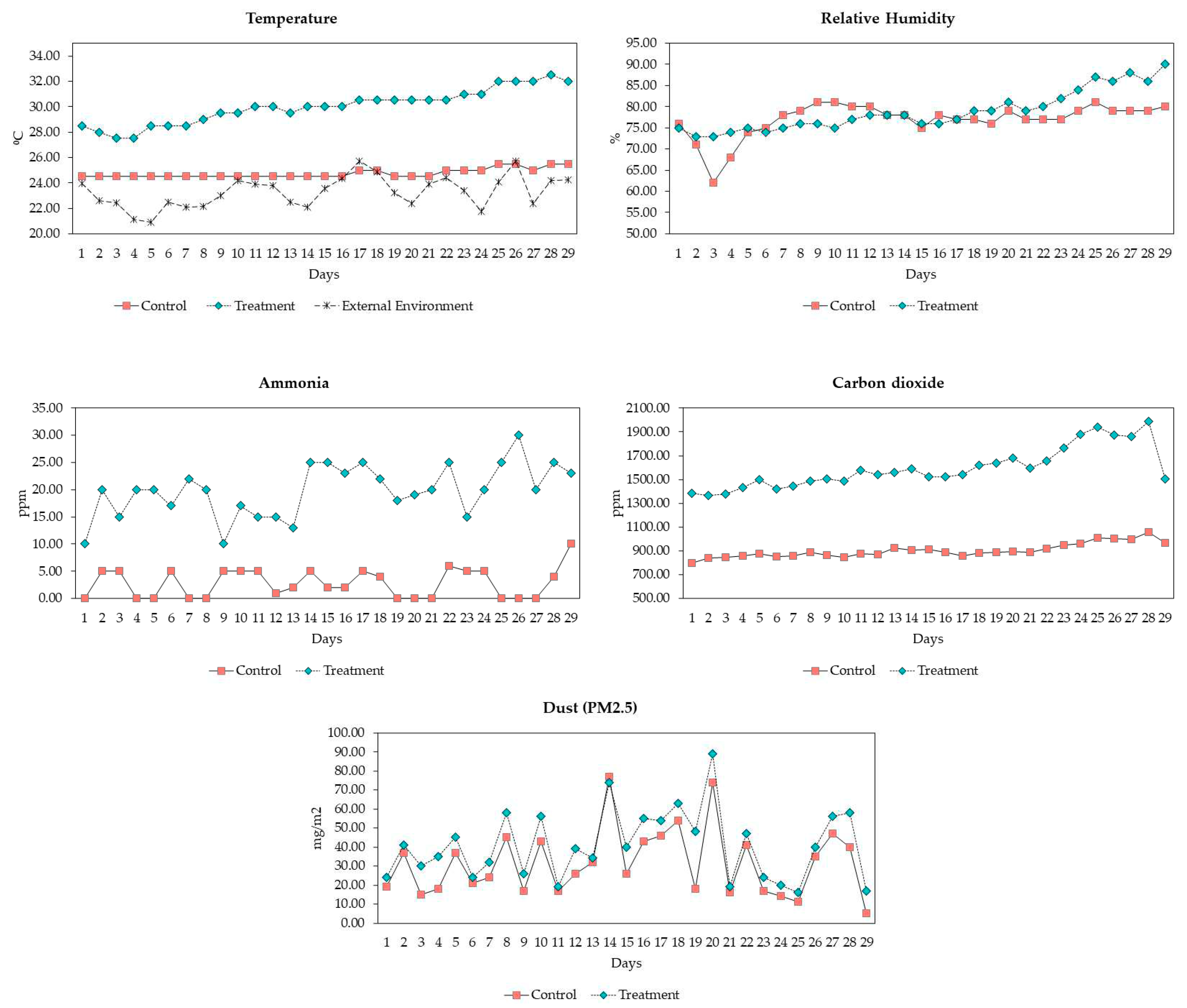
3.1. Environmental Conditions
3.2. Growth Performance
3.3. Behavioral Observations
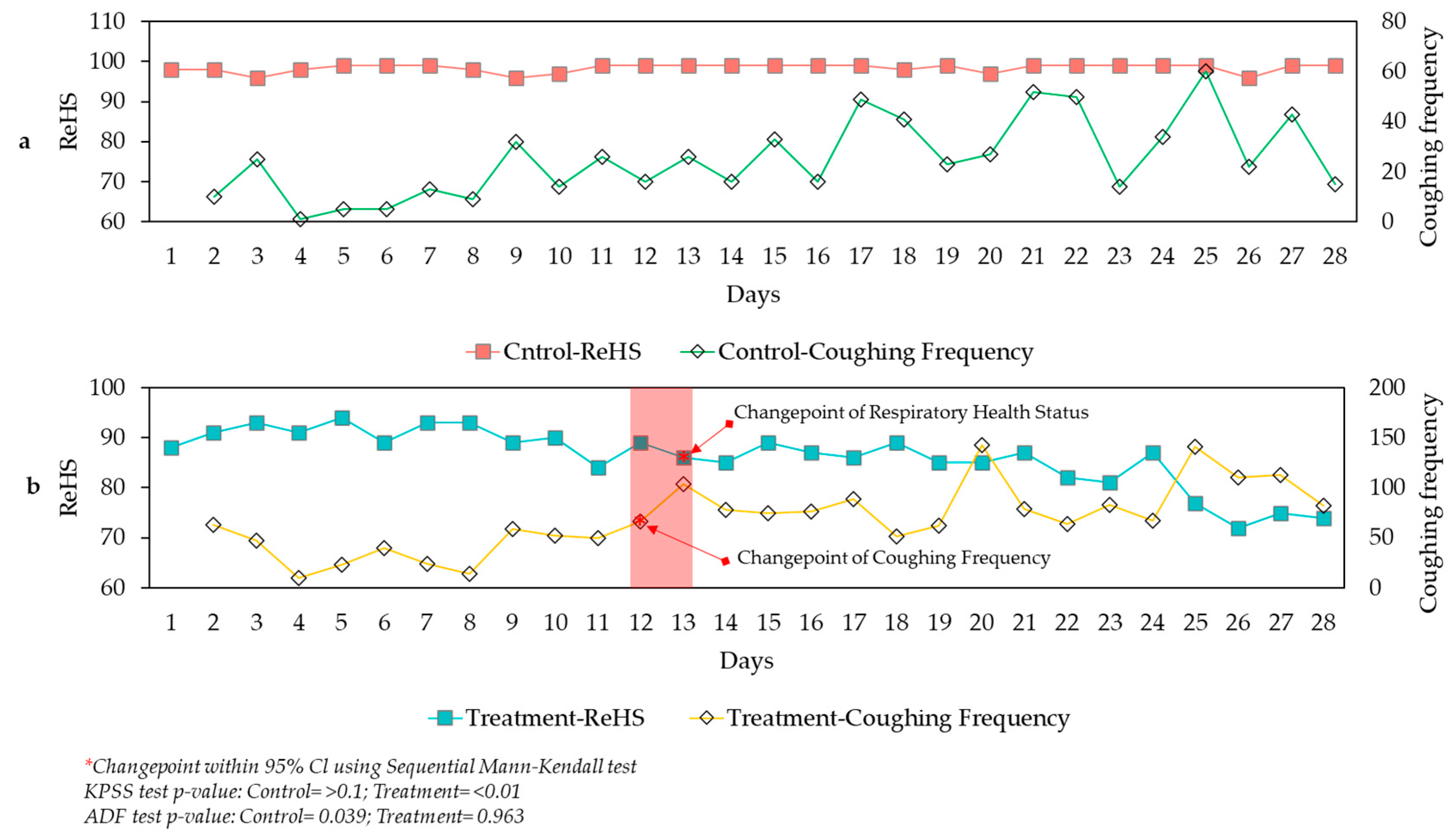
3.4. Health Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berton, M.P.; de Cássia Dourado, R.; de Lima, F.B.F.; Rodrigues, A.B.B.; Ferrari, F.B.; do Carmo Vieira, L.D.; de Souza, P.A.; Borba, H. Growing-Finishing Performance and Carcass Yield of Pigs Reared in a Climate-Controlled and Uncontrolled Environment. Int. J. Biometeorol. 2015, 59, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Costantino, A.; Fabrizio, E.; Calvet, S. The Role of Climate Control in Monogastric Animal Farming: The Effects on Animal Welfare, Air Emissions, Productivity, Health, and Energy Use. Appl. Sci. 2021, 11, 9549. [Google Scholar] [CrossRef]
- de Oliveira Júnior, G.M.; Ferreira, A.S.; Oliveira, R.F.M.; Silva, B.A.N.; de Figueiredo, E.M.; Santos, M. Behaviour and Performance of Lactating Sows Housed in Different Types of Farrowing Rooms during Summer. Livest. Sci. 2011, 141, 194–201. [Google Scholar] [CrossRef]
- Feng, K.; Wang, Y.; Hu, R.; Xiang, R. Continuous Measurement of Ammonia at an Intensive Pig Farm in Wuhan, China. Atmosphere 2022, 13, 442. [Google Scholar] [CrossRef]
- Haeussermann, A.; Hartung, E.; Gallmann, E.; Jungbluth, T. Influence of Season, Ventilation Strategy, and Slurry Removal on Methane Emissions from Pig Houses. Agric. Ecosyst. Environ. 2006, 112, 115–121. [Google Scholar] [CrossRef]
- Lee, S.; Lee, I.; Yeo, U.; Kim, J.; Kim, R. Machine Learning Approach to Predict Air Temperature and Relative Humidity inside Mechanically and Naturally Ventilated Duck Houses: Application of Recurrent Neural Network. Agriculture 2022, 12, 318. [Google Scholar] [CrossRef]
- Mun, H.-S.; Ampode, K.M.B.; Dilawar, M.A.; Mahfuz, S.; Chem, V.; Kim, Y.-H.; Moon, J.-P.; Yang, C.-J. Renewable Energy Sources: A Novel Technology for Eco-Friendly and Sustainable Pig Production. J. Biosyst. Eng. 2022, 47, 489–501. [Google Scholar] [CrossRef]
- Overmeyer, V.; Trimborn, M.; Clemens, J.; Hölscher, R.; Büscher, W. Acidification of Slurry to Reduce Ammonia and Methane Emissions: Deployment of a Retrofittable System in Fattening Pig Barns. J. Environ. Manage. 2023, 331, 117263. [Google Scholar] [CrossRef] [PubMed]
- Philippe, F.-X.; Nicks, B. Review on Greenhouse Gas Emissions from Pig Houses: Production of Carbon Dioxide, Methane and Nitrous Oxide by Animals and Manure. Agric. Ecosyst. Environ. 2015, 199, 10–25. [Google Scholar] [CrossRef]
- Rong, L. Effect of Partial Pit Exhaust Ventilation System on Ammonia Removal Ratio and Mass Transfer Coefficients from Different Emission Sources in Pig Houses. Energy Built Environ. 2020, 1, 343–350. [Google Scholar] [CrossRef]
- Tabase, R.K.; Millet, S.; Brusselman, E.; Ampe, B.; Sonck, B.; Demeyer, P. Effect of Ventilation Settings on Ammonia Emission in an Experimental Pig House Equipped with Artificial Pigs. Biosyst. Eng. 2018, 176, 125–139. [Google Scholar] [CrossRef]
- Zong, C.; Li, H.; Zhang, G. Ammonia and Greenhouse Gas Emissions from Fattening Pig House with Two Types of Partial Pit Ventilation Systems. Agric. Ecosyst. Environ. 2015, 208, 94–105. [Google Scholar] [CrossRef]
- Andersen, H.M.-L.; Dybkjær, L.; Herskin, M.S. Growing Pigs’ Drinking Behaviour: Number of Visits, Duration, Water Intake and Diurnal Variation. animal 2014, 8, 1881–1888. [Google Scholar] [CrossRef]
- Boyle, L.A.; Edwards, S.A.; Bolhuis, J.E.; Pol, F.; Šemrov, M.Z.; Schütze, S.; Nordgreen, J.; Bozakova, N.; Sossidou, E.N.; Valros, A. The Evidence for a Causal Link Between Disease and Damaging Behavior in Pigs. Front. Vet. Sci. 2022, 8. [Google Scholar] [CrossRef]
- Da Silva Cordeiro, A.F.; De Alencar Nääs, I.; Oliveira, S.R.M.; Violaro, F.; De Almeida, A.C.M.; Neves, D.P. Understanding Vocalization Might Help to Assess Stressful Conditions in Piglets. Animals 2013, 3, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Opderbeck, S.; Keßler, B.; Gordillio, W.; Schrade, H.; Piepho, H.-P.; Gallmann, E. Influence of A Cooled, Solid Lying Area on the Pen Fouling and Lying Behavior of Fattening Pigs. Agriculture 2020, 10, 307. [Google Scholar] [CrossRef]
- Rostagno, M.H.; Eicher, S.D.; Lay, D.C. Immunological, Physiological, and Behavioral Effects of Salmonella Enterica Carriage and Shedding in Experimentally Infected Finishing Pigs. Foodborne Pathog. Dis. 2011, 8, 623–630. [Google Scholar] [CrossRef]
- Alameer, A.; Buijs, S.; O’Connell, N.; Dalton, L.; Larsen, M.; Pedersen, L.; Kyriazakis, I. Automated Detection and Quantification of Contact Behaviour in Pigs Using Deep Learning. Biosyst. Eng. 2022, 224, 118–130. [Google Scholar] [CrossRef]
- Chung, Y.; Oh, S.; Lee, J.; Park, D.; Chang, H.-H.; Kim, S. Automatic Detection and Recognition of Pig Wasting Diseases Using Sound Data in Audio Surveillance Systems. Sensors 2013, 13, 12929–12942. [Google Scholar] [CrossRef]
- Hakansson, F.; Jensen, D.B. Automatic Monitoring and Detection of Tail-Biting Behavior in Groups of Pigs Using Video-Based Deep Learning Methods. Front. Vet. Sci. 2023, 9. [Google Scholar] [CrossRef] [PubMed]
- Matthews, S.G.; Miller, A.L.; Clapp, J.; Plötz, T.; Kyriazakis, I. Early Detection of Health and Welfare Compromises through Automated Detection of Behavioural Changes in Pigs. Vet. J. 2016, 217, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Matthews, S.G.; Miller, A.L.; PlÖtz, T.; Kyriazakis, I. Automated Tracking to Measure Behavioural Changes in Pigs for Health and Welfare Monitoring. Sci. Rep. 2017, 7, 17582. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Ji, N.; Yin, Y.; Dai, B.; Tu, D.; Sun, B.; Hou, H.; Kou, S.; Zhao, Y. Fusion of Acoustic and Deep Features for Pig Cough Sound Recognition. Comput. Electron. Agric. 2022, 197, 106994. [Google Scholar] [CrossRef]
- Tu, S.; Zeng, Q.; Liang, Y.; Liu, X.; Huang, L.; Weng, S.; Huang, Q. Automated Behavior Recognition and Tracking of Group-Housed Pigs with an Improved DeepSORT Method. Agriculture 2022, 12, 1907. [Google Scholar] [CrossRef]
- Lee, G.-T.; Nam, H.; Kim, S.-H.; Choi, S.-M.; Kim, Y.; Park, Y.-H. Deep Learning Based Cough Detection Camera Using Enhanced Features. Expert Syst. Appl. 2022, 206, 117811. [Google Scholar] [CrossRef]
- Lagua, E.B.; Mun, H.-S.; Ampode, K.M.B.; Chem, V.; Kim, Y.-H.; Yang, C.-J. Artificial Intelligence for Automatic Monitoring of Respiratory Health Conditions in Smart Swine Farming. Animals 2023, 13, 1860. [Google Scholar] [CrossRef]
- Clavijo, M.J.; Hu, D.; Krantz, S.; Cano, J.P.; Pereira Maróstica, T.; Henao-Diaz, A.; Poeta Silva, A.P.S.; Hemker, D.; Tapia, E.; Zimmerman, S.; et al. Mycoplasma Hyopneumoniae Surveillance in Pig Populations: Establishing Sampling Guidelines for Detection in Growing Pigs. J. Clin. Microbiol. 2021, 59, e03051–20. [Google Scholar] [CrossRef]
- Pessoa, J.; Rodrigues da Costa, M.; García Manzanilla, E.; Norton, T.; McAloon, C.; Boyle, L. Managing Respiratory Disease in Finisher Pigs: Combining Quantitative Assessments of Clinical Signs and the Prevalence of Lung Lesions at Slaughter. Prev. Vet. Med. 2021, 186, 105208. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, J.; Camp Montoro, J.; Pina Nunes, T.; Norton, T.; McAloon, C.; Garcia Manzanilla, E.; Boyle, L. Environmental Risk Factors Influence the Frequency of Coughing and Sneezing Episodes in Finisher Pigs on a Farm Free of Respiratory Disease. Animals 2022, 12, 982. [Google Scholar] [CrossRef]
- Silva, A.P.S.P.; Storino, G.Y.; Ferreyra, F.S.M.; Zhang, M.; Fano, E.; Polson, D.; Wang, C.; Derscheid, R.J.; Zimmerman, J.J.; Clavijo, M.J.; et al. Cough Associated with the Detection of Mycoplasma Hyopneumoniae DNA in Clinical and Environmental Specimens under Controlled Conditions. Porc. Health Manag. 2022, 8, 6. [Google Scholar] [CrossRef]
- Ko, H.-L.; Pardo, P.; Manteca, X.; Llonch, P. Correlations between Environmental Data with Activity Level and Respiratory Health by PLF Sensors.; September 6 2022.
- Jo, G.; Ha, T.; Jang, Y.N.; Hwang, O.; Seo, S.; Woo, S.E.; Lee, S.; Kim, D.; Jung, M. Ammonia Emission Characteristics of a Mechanically Ventilated Swine Finishing Facility in Korea. Atmosphere 2020, 11, 1088. [Google Scholar] [CrossRef]
- Mielcarek-Bocheńska, P.; Rzeźnik, W. Odors and Ammonia Emission from a Mechanically Ventilated Fattening Piggery on Deep Litter in Poland. Arch. Environ. Prot. 2022. [Google Scholar] [CrossRef]
- Zong, C.; Zhang, G.; Feng, Y.; Ni, J.-Q. Carbon Dioxide Production from a Fattening Pig Building with Partial Pit Ventilation System. Biosyst. Eng. 2014, 126, 56–68. [Google Scholar] [CrossRef]
- Ni, J.-Q.; Heber, A.J.; Diehl, C.A.; Lim, T.T. SE—Structures and Environment. J. Agric. Eng. Res. 2000, 77, 53–66. [Google Scholar] [CrossRef]
- Frøseth, R.B.; Bleken, M.A. Effect of Low Temperature and Soil Type on the Decomposition Rate of Soil Organic Carbon and Clover Leaves, and Related Priming Effect. Soil Biol. Biochem. 2015, 80, 156–166. [Google Scholar] [CrossRef]
- Misselbrook, T.; Hunt, J.; Perazzolo, F.; Provolo, G. Greenhouse Gas and Ammonia Emissions from Slurry Storage: Impacts of Temperature and Potential Mitigation through Covering (Pig Slurry) or Acidification (Cattle Slurry). J. Environ. Qual. 2016, 45, 1520–1530. [Google Scholar] [CrossRef]
- Thangarajan, R.; Bolan, N.S.; Naidu, R.; Surapaneni, A. Effects of Temperature and Amendments on Nitrogen Mineralization in Selected Australian Soils. Environ. Sci. Pollut. Res. Int. 2015, 22, 8843–8854. [Google Scholar] [CrossRef]
- Brown-Brandl, T.M.; Eigenberg, R.A.; Purswell, J.L. Using Thermal Imaging as a Method of Investigating Thermal Thresholds in Finishing Pigs. Biosyst. Eng. 2013, 114, 327–333. [Google Scholar] [CrossRef]
- Dziejowski, J.E.; Kazanowska, J. Heat Production During Thermophilic Decomposition of Municipal Wastes in the Dano-System Composting Plant. In Proceedings of the Microbiology of Composting; Insam, H., Riddech, N., Klammer, S., Eds.; Springer: Berlin, Heidelberg, 2002; pp. 111–118. [Google Scholar]
- Kil, D.Y.; Kim, B.G.; Stein, H.H. Feed Energy Evaluation for Growing Pigs*. Asian-Australas. J. Anim. Sci. 2013, 26, 1205–1217. [Google Scholar] [CrossRef]
- Mayorga, E.J.; Renaudeau, D.; Ramirez, B.C.; Ross, J.W.; Baumgard, L.H. Heat Stress Adaptations in Pigs. Anim. Front. 2019, 9, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Baumgard, L.H.; Rhoads, R.P. Effects of Heat Stress on Postabsorptive Metabolism and Energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef] [PubMed]
- Collin, A.; Milgen, J. van; Dubois, S.; Noblet, J. Effect of High Temperature on Feeding Behaviour and Heat Production in Group-Housed Young Pigs. Br. J. Nutr. 2001, 86, 63–70. [Google Scholar] [CrossRef]
- Pearce, S.C.; Gabler, N.K.; Ross, J.W.; Escobar, J.; Patience, J.F.; Rhoads, R.P.; Baumgard, L.H. The Effects of Heat Stress and Plane of Nutrition on Metabolism in Growing Pigs1. J. Anim. Sci. 2013, 91, 2108–2118. [Google Scholar] [CrossRef]
- Hoover, W.H.; Sawyer, M.S.; Apgar, W.P. Ovine Nutritional Responses to Elevated Ambient Carbon Dioxide. J. Nutr. 1971, 101, 1595–1600. [Google Scholar] [CrossRef]
- Drummond, J.G.; Curtis, S.E.; Simon, J.; Norton, H.W. Effects of Aerial Ammonia on Growth and Health of Young Pigs1. J. Anim. Sci. 1980, 50, 1085–1091. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, X.; He, Y.; Wang, K. Cough Sound Analysis to Assess Air Quality in Commercial Weaner Barns. Comput. Electron. Agric. 2019, 160, 8–13. [Google Scholar] [CrossRef]
- Cho, H.A.; Song, M.H.; Lee, J.H.; Oh, H.J.; Kim, Y.J.; An, J.W.; Chang, S.Y.; Go, Y.B.; Song, D.C.; Cho, S.Y.; et al. Effects of Different Stocking Density and Various Phytogenic Feed Additives Dosage Levels on Growing-Finishing Pigs. J. Anim. Sci. Technol. 2023, 65, 535–549. [Google Scholar] [CrossRef]
- Gomez, R.S.; Lewis, A.J.; Miller, P.S.; Chen, H.Y. Growth Performance and Digestive and Metabolic Responses of Gilts Penned Individually or in Groups of Four. J. Anim. Sci. 2000, 78, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Kim, H. Automatic Identification of a Coughing Animal Using Audio and Video Data. In Proceedings of the fourth International Conference on Information Science and Cloud Computing — PoS(ISCC2015); Sissa Medialab, Guangzhou, China, 9 February 2016; p. p. 008. [Google Scholar]
- Li, X.; Xiong, X.; Wu, X.; Liu, G.; Zhou, K.; Yin, Y. Effects of Stocking Density on Growth Performance, Blood Parameters and Immunity of Growing Pigs. Anim. Nutr. 2020, 6, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Ortega, A.D.S.; Babinszky, L.; Oriedo, O.; Csernus, B.; Ozsváth, X.; Czeglédi, L.; Oláh, J.; Szabo, C. Impact of Heat Stress Length and Dietary Antioxidant Supplementation on the Nutrient Digestibility, Metabolism and Immune Response of Fattening Pigs. Ann. Agric. Sci. 2023, 68, 87–96. [Google Scholar] [CrossRef]
- Ortega, A.D.S.; Szabo, C. Adverse Effects of Heat Stress on the Intestinal Integrity and Function of Pigs and the Mitigation Capacity of Dietary Antioxidants: A Review. Animals 2021, 11, 1135. [Google Scholar] [CrossRef]
- Olanrewaju, H.A.; Miller, W.W.; Maslin, W.R.; Thaxton, J.P.; Dozier, W.A.I.; Purswell, J.; Branton, S.L. Interactive Effects of Ammonia and Light Intensity on Ocular, Fear and Leg Health in Broiler Chickens. Int. J. Poult. Sci. 2007, 6, 762–769. [Google Scholar]
- Dalhamn, T. Mucous Flow and Ciliary Activity in the Trachea of Healthy Rats and Rats Exposed to Respiratory Irritant Gases (SO2, H3N, HCHO): A Functional and Morphologic (Light Microscopic and Electron Microscopic) Study, with Special Reference to Technique. Acta Physiol. Scand. Suppl. 1956, 36, 1–161. [Google Scholar] [PubMed]
- Wang, H.; Jiao, P.; Zhang, X.; Xing, H. Quantitative Proteomic Analysis of Trachea in Fatting Pig Exposed to Ammonia. J. Proteomics 2021, 247, 104330. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; He, Q.; Yao, W.; Shao, Y.; Li, J.; Huang, F. The Variation of Nasal Microbiota Caused by Low Levels of Gaseous Ammonia Exposure in Growing Pigs. Front. Microbiol. 2019, 10, 1083. [Google Scholar] [CrossRef] [PubMed]
- Stombaugh, D.P.; Teague, H.S.; Roller, W.L. Effects of Atmospheric Ammonia on the Pig. J. Anim. Sci. 1969, 28, 844–847. [Google Scholar] [CrossRef]
- Opriessnig, T.; Giménez-Lirola, L.G.; Halbur, P.G. Polymicrobial Respiratory Disease in Pigs. Anim. Health Res. Rev. 2011, 12, 133–148. [Google Scholar] [CrossRef]
- Wagner, J.; Kneucker, A.; Liebler-Tenorio, E.; Fachinger, V.; Glaser, M.; Pesch, S.; Murtaugh, M.P.; Reinhold, P. Respiratory Function and Pulmonary Lesions in Pigs Infected with Porcine Reproductive and Respiratory Syndrome Virus. Vet. J. Lond. Engl. 1997 2011, 187, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Wang, D.; Zhou, M.; Zhou, Y.; Guo, Y.; Chen, W. Potential Effects of Lung Function Reduction on Health-Related Quality of Life. Int. J. Environ. Res. Public. Health 2019, 16, 260. [Google Scholar] [CrossRef]
- Chen, X.; Wang, H.; Lyu, W.; Xu, R. The Mann-Kendall-Sneyers Test to Identify the Change Points of COVID-19 Time Series in the United States. BMC Med. Res. Methodol. 2022, 22, 233. [Google Scholar] [CrossRef]
- Nasirahmadi, A.; Hensel, O.; Edwards, S.A.; Sturm, B. A New Approach for Categorizing Pig Lying Behaviour Based on a Delaunay Triangulation Method. animal 2017, 11, 131–139. [Google Scholar] [CrossRef]
- Spoolder, H.A.M.; Aarnink, A.A.J.; Vermeer, H.M.; van Riel, J.; Edwards, S.A. Effect of Increasing Temperature on Space Requirements of Group Housed Finishing Pigs. Appl. Anim. Behav. Sci. 2012, 138, 229–239. [Google Scholar] [CrossRef]
- Muns, R.; Malmkvist, J.; Larsen, M.L.V.; Sørensen, D.; Pedersen, L.J. High Environmental Temperature around Farrowing Induced Heat Stress in Crated Sows. J. Anim. Sci. 2016, 94, 377–384. [Google Scholar] [CrossRef]
- Baert, S.; Aubé, L.; Haley, D.B.; Bergeron, R.; Devillers, N. The Protective Role of Wallowing against Heat Stress in Gestating and Lactating Sows Housed Outdoors. Physiol. Behav. 2022, 254, 113898. [Google Scholar] [CrossRef]
- Huynh, T.T.T.; Aarnink, A.J.A.; Gerrits, W.J.J.; Heetkamp, M.J.H.; Canh, T.T.; Spoolder, H.A.M.; Kemp, B.; Verstegen, M.W.A. Thermal Behaviour of Growing Pigs in Response to High Temperature and Humidity. Appl. Anim. Behav. Sci. 2005, 91, 1–16. [Google Scholar] [CrossRef]
- Mós, J.V. do N.; Nascimento, S.T.; Murata, L.S.; dos Santos, V.M.; Neto, A.J.S.; de Oliveira, E.M.; da Silva Lisboa, Á.; de Freitas Silva, L. Thermal Comfort of Sows in Free-Range System in Brazilian Savanna. J. Therm. Biol. 2020, 88, 102489. [Google Scholar] [CrossRef]
- Choi, H.L.; Han, S.H.; Albright, L.D.; Chang, W.K. The Correlation between Thermal and Noxious Gas Environments, Pig Productivity and Behavioral Responses of Growing Pigs. Int. J. Environ. Res. Public. Health 2011, 8, 3514. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Xing, H.; Bao, J. Selenium Mitigates Ammonia-Induced Neurotoxicity by Suppressing Apoptosis, Immune Imbalance, and Gut Microbiota-Driven Metabolic Disturbance in Fattening Pigs. Biol. Trace Elem. Res. 2023, 201, 3341–3355. [Google Scholar] [CrossRef]
- Sutherland, M.A.; Niekamp, S.R.; Johnson, R.W.; Van Alstine, W.G.; Salak-Johnson, J.L. Heat and Social Rank Impact Behavior and Physiology of PRRS-Virus-Infected Pigs. Physiol. Behav. 2007, 90, 73–81. [Google Scholar] [CrossRef]
- Escobar, J.; Van Alstine, W.G.; Baker, D.H.; Johnson, R.W. Behaviour of Pigs with Viral and Bacterial Pneumonia. Appl. Anim. Behav. Sci. 2007, 105, 42–50. [Google Scholar] [CrossRef]
- Abriel, M.; Jais, C. Influence of Housing Conditions on the Appearance of Cannibalism in Weaning Piglets. Landtechnik 2013, 6/2013. [Google Scholar]
- Brunberg, E.; Wallenbeck, A.; Keeling, L.J. Tail Biting in Fattening Pigs: Associations between Frequency of Tail Biting and Other Abnormal Behaviours. Appl. Anim. Behav. Sci. 2011, 133, 18–25. [Google Scholar] [CrossRef]
- Moinard, C.; Mendl, M.; Nicol, C.J.; Green, L.E. A Case Control Study of On-Farm Risk Factors for Tail Biting in Pigs. Appl. Anim. Behav. Sci. 2003, 81, 333–355. [Google Scholar] [CrossRef]
- Schrøder-Petersen, D.L.; Simonsen, H.B. Tail Biting in Pigs. Vet. J. 2001, 162, 196–210. [Google Scholar] [CrossRef]
- Taylor, N.R.; Main, D.C.J.; Mendl, M.; Edwards, S.A. Tail-Biting: A New Perspective. Vet. J. 2010, 186, 137–147. [Google Scholar] [CrossRef]
- D’Eath, R.B.; Jack, M.; Futro, A.; Talbot, D.; Zhu, Q.; Barclay, D.; Baxter, E.M. Automatic Early Warning of Tail Biting in Pigs: 3D Cameras Can Detect Lowered Tail Posture before an Outbreak. PLOS ONE 2018, 13, e0194524. [Google Scholar] [CrossRef]
- Odo, A.; Muns, R.; Boyle, L.; Kyriazakis, I. Video Analysis Using Deep Learning for Automated Quantification of Ear Biting in Pigs. IEEE Access 2023, 11, 59744–59757. [Google Scholar] [CrossRef]
- Ollagnier, C.; Kasper, C.; Wallenbeck, A.; Keeling, L.; Bee, G.; Bigdeli, S.A. Machine Learning Algorithms Can Predict Tail Biting Outbreaks in Pigs Using Feeding Behaviour Records. PLOS ONE 2023, 18, e0252002. [Google Scholar] [CrossRef]
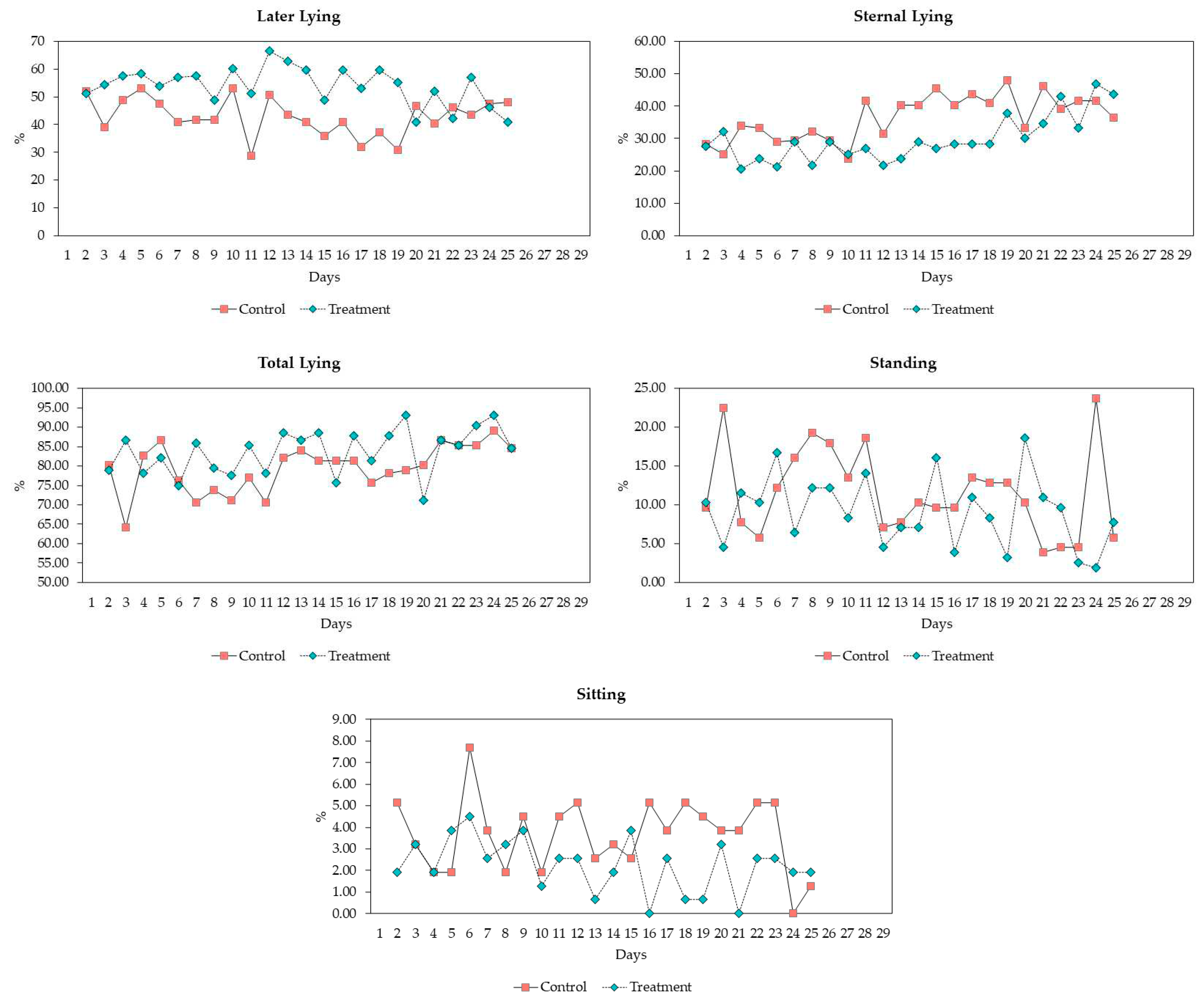
| Behavior | Definition |
|---|---|
| Lateral lying | The pig is lying on the side with limbs extended |
| Sternal lying | The pig is lying on its belly with at least two legs folded under the body |
| Total lying | Summation of lateral and sternal lying |
| Standing | Standing, walking, or running, the body is elevated and supported by 3 or 4 legs |
| Sitting | Hindquarters on floor with front legs straight |
| Resting at waterer | The pig is lying and the head is within a 0.5-meter radius of the water trough |
| Screaming | High-frequency content calls with a large amplitude often uttered in stressful and painful situations |
| Parameters | Control | Treatment | SEM | P-value | |
|---|---|---|---|---|---|
| Temperature (⁰C) | mean, SD min-max |
24.75 ±0.37 24.5-25.50 |
30.05 ±1.37 27.50-32.50 |
0.381 | <0.001 |
| Humidity (%) | mean, SD min-max |
76.96 ±4.14 62.00-81.00 |
79.00 ±4.79 73.00-90.00 |
0.609 | 0.095 |
| CO2 (ppm) | mean, SD min-max |
905.92 ±57.20 836.37-1,058.68 |
1,600.93 ±170.50 1,366.64-1,985.47 |
49.790 | <0.001 |
| NH3 (ppm) | mean, SD min-max |
2.89 ±2.70 0.00-10.00 |
20.14 ±4.50 10.00-30.00 |
1.263 | <0.001 |
| Dust (mg/m2) | mean, SD min-max |
32.00 ±17.92 50.00-77.00 |
41.39 ±18.33 16.00-89.00 |
2.482 | 0.058 |
| Bigger Pigs | Smaller Pigs | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | Treatment | Difference | Control | Treatment | Difference | |||
| N heads | 13 | 13 | 19 | 13 | 23 | |||
| SD (m2/head) | 0.45 | 0.45 | 0.32 | 0.45 | 0.27 | |||
| IW (kg) | 28.85 | 28.97 | 28.56 | 10.59 | 10.35 | |||
| FW (kg) | 55.79 | 48.32 | 43.58 | -13.40 | -21.89 | 30.05 | 22.61 | -24.76 |
| BWG (kg) | 26.94 | 19.35 | 15.02 | -28.18 | -44.26 | 19.46 | 12.27 | -36.98 |
| ADG (kg/day) | 0.962 | 0.691 | 0.536 | -28.18 | -44.26 | 0.695 | 0.438 | -36.98 |
| FI (kg) | 59.32 | 43.52 | 31.95 | -26.63 | -46.15 | 33.52 | 21.92 | -34.60 |
| FCR (FI/BWG) | 2.20 | 2.25 | 2.13 | 2.16 | -3.39 | 1.72 | 1.79 | 3.76 |
| Parameters | Control | Treatment | SEM | P-value | |
|---|---|---|---|---|---|
| Behavior | |||||
| Lateral Lying (%) | mean, SD min-max |
42.98 ±6.79 28.85-53.21 |
53.98 ±6.75 41.03-66.67 |
1.257 | <0.001 |
| Sternal Lying (%) | mean, SD min-max |
36.46 ±6.93 23.72-48.08 |
29.65 ±7.15 20.51-46.79 |
1.121 | 0.002 |
| Total Lying (%) | mean, SD min-max |
79.43 ±6.14 64.10-89.10 |
83.63 ±5.80 71.15-92.95 |
0.906 | 0.019 |
| Standing (%) | mean, SD min-max |
3.66 ±1.69 0.00-7.69 |
2.24 ±1.24 0.00-4.49 |
0.235 | 0.002 |
| Sitting (%) | mean, SD min-max |
11.62 ±5.66 3.85-23.72 |
9.11 ±4.53 1.92-23.72 |
0.755 | 0.097 |
| Resting at waterer (%) | mean, SD min-max |
11.70 ±1.05 10.73-12.82 |
16.56 ±1.03 15.38-17.31 |
1.151 | 0.005 |
| Screaming Frequency | mean, SD min-max |
38 ±6.24 31.00-43.00 |
65 ±4.58 60.00-69.00 |
6.360 | 0.005 |
| Health Conditions | |||||
| ReHS | mean, SD min-max |
98.37 ±1.04 96-99 |
86.04 ±5.93 72-94 |
1.023 | <0.001 |
| Coughing Frequency | mean, SD min-max |
25.07 ±15.85 1-60 |
69.07 ±33.80 10-143 |
4.668 | <0.001 |
| Coughing Frequency/head | mean, SD min-max |
0.96 ±0.61 0.04-2.31 |
1.26 ±0.61 0.18-2.60 |
0.085 | 0.086 |
| Respiration Rate | mean, SD min-max |
84.09 ±12.13 60.50-98.00 |
106.71 ±7.83 90.50-116.50 |
2.886 | <0.001 |
| Control | Treatment | ||||
|---|---|---|---|---|---|
| Weight Class | S | B | B | B | S |
| N heads | 13 | 13 | 19 | 13 | 23 |
| SD (m2/head) | 0.45 | 0.45 | 0.32 | 0.45 | 0.27 |
| Conjunctivitis (%) | 46.15 | 30.77 | 68.42 | 76.92 | 52.17 |
| Ear Wound (%) | 7.69 | - | 42.11 | 30.77 | 69.57 |
| Tail Wound (%) | - | - | 21.05 | 7.69 | 8.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





