Submitted:
10 September 2023
Posted:
12 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
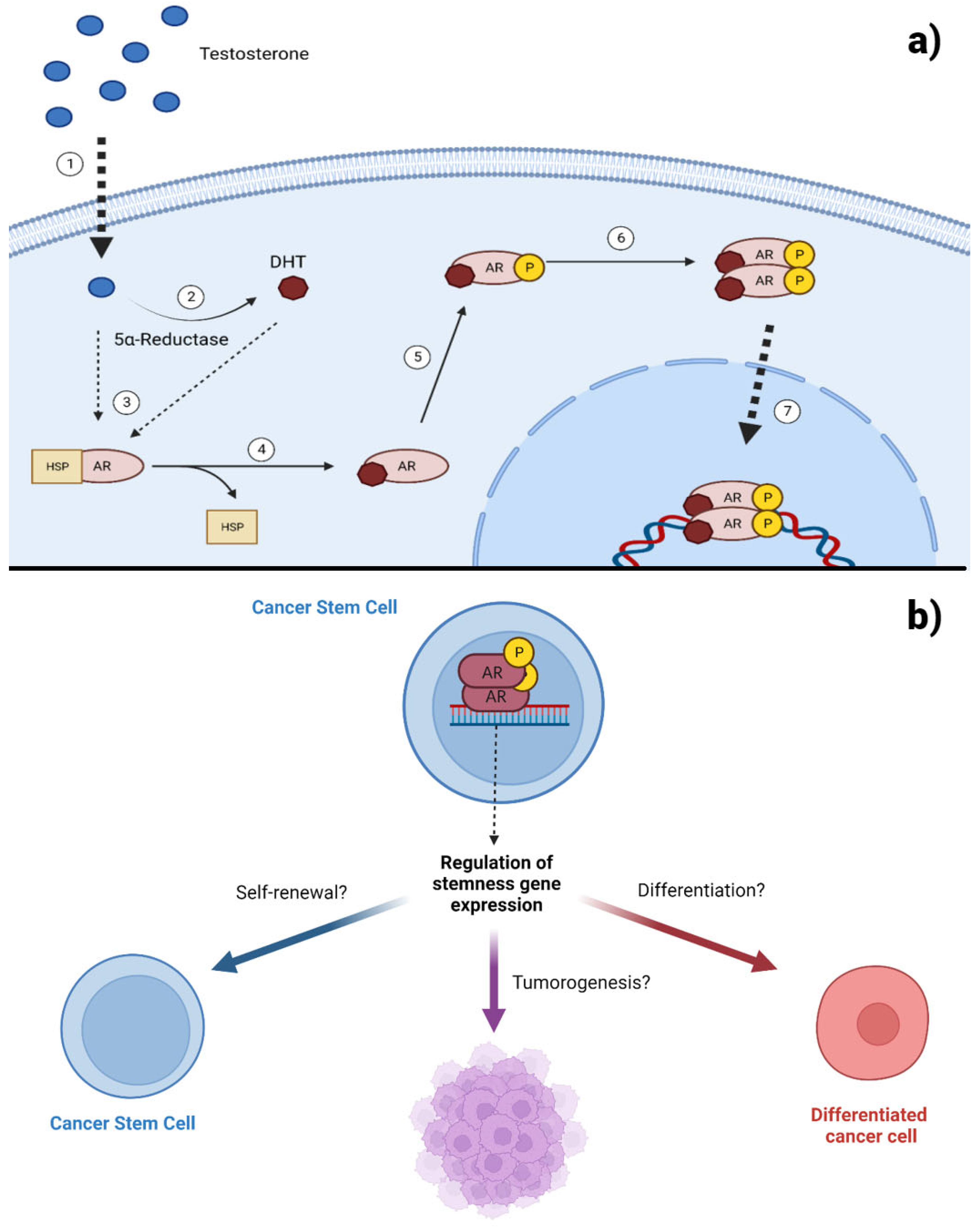
2. AR Signaling in Prostate Cancer
2.1. The Role of AR in PC Tumor Growth
2.2. AR Expression in Prostate Cancer Stem Cells
3. AR Signaling in Breast Cancer
3.1. Action of Androgens in ERα- and ERα+ Tumors
3.2. Regulation of Breast Cancer Stem Cells by AR
4. AR Signaling in Glioblastoma
4.1. AR Activity in the Maintenance of GSCs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006, 441, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Jaenisch R, Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008, 132, 567–582. [Google Scholar] [CrossRef] [PubMed]
- Rossi F, Noren H, Jove R, Beljanski V, Grinnemo KH. Differences and similarities between cancer and somatic stem cells: Therapeutic implications. Stem Cell Res Ther. 2020, 11, 489. [Google Scholar] [CrossRef]
- Walcher L, Kistenmacher AK, Suo H, Kitte R, Dluczek S, Strauß A, Blaudszun AR, Yevsa T, Fricke S, Kossatz-Boehlert U. Cancer Stem Cells-Origins and Biomarkers: Perspectives for Targeted Personalized Therapies. Front Immunol. 2020, 11, 1280. [Google Scholar] [CrossRef]
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997, 3, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 2003, 100, 3983–3988. [Google Scholar] [CrossRef]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumor-initiating cells. Nature. 2004, 432, 396–401. [Google Scholar] [CrossRef]
- Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG. The side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2- cancer cells are similarly tumorigenic. Cancer Res. 2005, 65, 6207–6219. [Google Scholar] [CrossRef]
- O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumor growth in immunodeficient mice. Nature. 2007, 445, 106–110. [Google Scholar] [CrossRef]
- Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003, 3, 895–902. [Google Scholar] [CrossRef]
- Borlongan MC, Wang H. Profiling and targeting cancer stem cell signaling pathways for cancer therapeutics. Front Cell Dev Biol. 2023, 11, 1125174. [Google Scholar]
- Lee J, Troike K, Fodor R, Lathia JD. Unexplored Functions of Sex Hormones in Glioblastoma Cancer Stem Cells. Endocrinology. 2022, 163, bqac002. [Google Scholar] [CrossRef] [PubMed]
- Banerjee PP, Banerjee S, Brown TR, Zirkin BR. Androgen action in prostate function and disease. Am J Clin Exp Urol. 2018, 6, 62–77. [Google Scholar]
- Batista RL, Mendonca BB. Integrative and Analytical Review of the 5-Alpha-Reductase Type 2 Deficiency Worldwide. Appl Clin Genet. 2020, 13, 83–96. [Google Scholar] [CrossRef]
- Jin HJ, Kim J, Yu J. Androgen receptor genomic regulation. Transl Androl Urol. 2013, 2, 157–177. [Google Scholar]
- Ling K, Jiang L, Liang S, Kwong J, Yang L, Li Y, PingYin, Deng Q, Liang Z. Nanog interaction with the androgen receptor signaling axis induce ovarian cancer stem cell regulation: Studies based on the CRISPR/Cas9 system. J Ovarian Res. 2018, 11, 36.
- Riaz N, Idress R, Habib S, Azam I, Lalani EM. Expression of Androgen Receptor and Cancer Stem Cell Markers (CD44+/CD24- and ALDH1+): Prognostic Implications in Invasive Breast Cancer. Transl Oncol. 2018, 11, 920–929. [Google Scholar] [CrossRef]
- Zhao N, Wang F, Ahmed S, Liu K, Zhang C, Cathcart SJ, DiMaio DJ, Punsoni M, Guan B, Zhou P, Wang S, Batra SK, Bronich T, Hei TK, Lin C, Zhang C. Androgen Receptor, Although Not a Specific Marker For, Is a Novel Target to Suppress Glioma Stem Cells as a Therapeutic Strategy for Glioblastoma. Front Oncol. 2021, 21, 616625. [Google Scholar]
- Verma S, Shankar E, Kalayci FNC, Mukunda A, Alassfar M, Singh V, Chan ER, MacLennan GT, Gupta S. Androgen Deprivation Induces Transcriptional Reprogramming in Prostate Cancer Cells to Develop Stem Cell-Like Characteristics. Int J Mol Sci. 2020, 21, 9568. [Google Scholar] [CrossRef]
- Bergengren O, Pekala KR, Matsoukas K, Fainberg J, Mungovan SF, Bratt O, Bray F, Brawley O, Luckenbaugh AN, Mucci L, Morgan TM, Carlsson SV. 2022 Update on Prostate Cancer Epidemiology and Risk Factors-A Systematic Review. Eur Urol. 2023, 84, 191–206. [Google Scholar] [CrossRef]
- Yu EM, Aragon-Ching JB. Advances with androgen deprivation therapy for prostate cancer. Expert Opin Pharmacother. 2022, 23, 1015–1033. [Google Scholar] [CrossRef] [PubMed]
- Morote J, Aguilar A, Planas J, Trilla E. Definition of Castrate Resistant Prostate Cancer: New Insights. Biomedicines. 2022, 10, 689. [Google Scholar]
- Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Chen Y, Fedor HL, Lotan TL, Zheng Q, De Marzo AM, Isaacs JT, Isaacs WB, Nadal R, Paller CJ, Denmeade SR, Carducci MA, Eisenberger MA, Luo J. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014, 371, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Fujita K, Nonomura N. Role of Androgen Receptor in Prostate Cancer: A Review. World J Mens Health. 2019, 37, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Luo, J. Development of AR-V7 as a putative treatment selection marker for metastatic castration-resistant prostate cancer. Asian J Androl. 2016, 18, 580–585. [Google Scholar] [CrossRef]
- Konda P, Viswanathan SR. How splicing confers treatment resistance in prostate cancer. Elife. 2022, 11, e82070. [Google Scholar] [CrossRef]
- Baron S, Manin M, Beaudoin C, Leotoing L, Communal Y, Veyssiere G, Morel L. Androgen receptor mediates non-genomic activation of phosphatidylinositol 3-OH kinase in androgen-sensitive epithelial cells. J Biol Chem. 2004, 279, 14579–14586. [Google Scholar] [CrossRef]
- Dahiya V, Bagchi G. Non-canonical androgen signaling pathways and implications in prostate cancer. Biochim Biophys Acta Mol Cell Res. 2022, 1869, 119357. [Google Scholar]
- Wen Y, Hu MC, Makino K, Spohn B, Bartholomeusz G, Yan DH, Hung MC. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res. 2000, 60, 6841–6845. [Google Scholar]
- Guo Z, Dai B, Jiang T, Xu K, Xie Y, Kim O, Nesheiwat I, Kong X, Melamed J, Handratta VD, Njar VC, Brodie AM, Yu LR, Veenstra TD, Chen H, Qiu Y. Regulation of androgen receptor activity by tyrosine phosphorylation. Cancer Cell. 2006, 10, 309–319. [Google Scholar] [CrossRef]
- Cornforth AN, Davis JS, Khanifar E, Nastiuk KL, Krolewski JJ. FOXO3a mediates the androgen-dependent regulation of FLIP and contributes to TRAIL-induced apoptosis of LNCaP cells. Oncogene. 2008, 27, 4422–4433. [Google Scholar] [CrossRef] [PubMed]
- Brodin G, ten Dijke P, Funa K, Heldin CH, Landström M. Increased smad expression and activation are associated with apoptosis in normal and malignant prostate after castration. Cancer Res. 1999, 59, 2731–2738. [Google Scholar]
- Wang H, Song K, Sponseller TL, Danielpour D. Novel function of androgen receptor-associated protein 55/Hic-5 as a negative regulator of Smad3 signaling. J Biol Chem. 2005, 280, 5154–5162. [Google Scholar] [CrossRef]
- Song K, Wang H, Krebs TL, Wang B, Kelley TJ, Danielpour D. DHT selectively reverses Smad3-mediated/TGF-beta-induced responses through transcriptional down-regulation of Smad3 in prostate epithelial cells. Mol Endocrinol. 2010, 24, 2019–2029. [Google Scholar] [CrossRef] [PubMed]
- Liao X, Thrasher JB, Pelling J, Holzbeierlein J, Sang QX, Li B. Androgen stimulates matrix metalloproteinase-2 expression in human prostate cancer. Endocrinology. 2003, 144, 1656–1663. [Google Scholar] [CrossRef]
- Chuan YC, Pang ST, Cedazo-Minguez A, Norstedt G, Pousette A, Flores-Morales A. Androgen induction of prostate cancer cell invasion is mediated by ezrin. J Biol Chem. 2006, 281, 29938–29948. [Google Scholar] [CrossRef]
- Lin CY, Jan YJ, Kuo LK, Wang BJ, Huo C, Jiang SS, Chen SC, Kuo YY, Chang CR, Chuu CP. Elevation of androgen receptor promotes prostate cancer metastasis by induction of epithelial-mesenchymal transition and reduction of KAT5. Cancer Sci. 2018, 109, 3564–3574. [Google Scholar] [CrossRef]
- Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005, 65, 10946–10951. [Google Scholar] [CrossRef]
- Patrawala L, Calhoun-Davis T, Schneider-Broussard R, Tang DG. Hierarchical organization of prostate cancer cells in xenograft tumors: The CD44+alpha2beta1+ cell population is enriched in tumor-initiating cells. Cancer Res. 2007, 67, 6796–6805. [Google Scholar] [CrossRef]
- Losada-García A, Salido-Guadarrama I, Cortes-Ramirez SA, Cruz-Burgos M, Morales-Pacheco M, Vazquez-Santillan K, Rodriguez-Martinez G, González-Ramírez I, Gonzalez-Covarrubias V, Perez-Plascencia C, Rodríguez-Dorantes M. SFRP1 induces a stem cell phenotype in prostate cancer cells. Front Cell Dev Biol. 2023, 11, 1096923. [Google Scholar]
- Gu G, Yuan J, Wills M, Kasper S. Prostate cancer cells with stem cell characteristics reconstitute the original human tumor in vivo. Cancer Res. 2007, 67, 4807–4815. [Google Scholar] [CrossRef] [PubMed]
- Qin J, Liu X, Laffin B, Chen X, Choy G, Jeter CR, Calhoun-Davis T, Li H, Palapattu GS, Pang S, Lin K, Huang J, Ivanov I, Li W, Suraneni MV, Tang DG. The PSA(-/lo) prostate cancer cell population harbors self-renewing long-term tumor-propagating cells that resist castration. Cell Stem Cell. 2012, 10, 556–569. [Google Scholar] [CrossRef] [PubMed]
- Seiler D, Zheng J, Liu G, Wang S, Yamashiro J, Reiter RE, Huang J, Zeng G. Enrichment of putative prostate cancer stem cells after androgen deprivation: Upregulation of pluripotency transactivators concurs with resistance to androgen deprivation in LNCaP cell lines. Prostate. 2013, 73, 1378–1390. [Google Scholar] [CrossRef]
- Lee SO, Ma Z, Yeh CR, Luo J, Lin TH, Lai KP, Yamashita S, Liang L, Tian J, Li L, Jiang Q, Huang CK, Niu Y, Yeh S, Chang C. New therapy targeting differential androgen receptor signaling in prostate cancer stem/progenitor vs. non-stem/progenitor cells. J Mol Cell Biol. 2013, 5, 14–26. [Google Scholar] [CrossRef]
- Schroeder A, Herrmann A, Cherryholmes G, Kowolik C, Buettner R, Pal S, Yu H, Müller-Newen G, Jove R. Loss of androgen receptor expression promotes a stem-like cell phenotype in prostate cancer through STAT3 signaling. Cancer Res. 2014, 74, 1227–1237. [Google Scholar] [CrossRef]
- Vummidi Giridhar P, Williams K, VonHandorf AP, Deford PL, Kasper S. Constant Degradation of the Androgen Receptor by MDM2 Conserves Prostate Cancer Stem Cell Integrity. Cancer Res. 2019, 79, 1124–1137.
- Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, Stanisławek A. Breast Cancer-Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies-An Updated Review. Cancers (Basel). 2021, 13, 4287. [Google Scholar] [CrossRef] [PubMed]
- Kensler KH, Regan MM, Heng YJ, Baker GM, Pyle ME, Schnitt SJ, Hazra A, Kammler R, Thürlimann B, Colleoni M, Viale G, Brown M, Tamimi RM. Prognostic and predictive value of androgen receptor expression in postmenopausal women with estrogen receptor-positive breast cancer: Results from the Breast International Group Trial 1-98. Breast Cancer Res. 2019, 21, 30. [Google Scholar] [CrossRef] [PubMed]
- Hwang KT, Kim YA, Kim J, Park JH, Choi IS, Hwang KR, Chai YJ, Park JH. Influence of Androgen Receptor on the Prognosis of Breast Cancer. J Clin Med. 2020, 9, 1083. [Google Scholar] [CrossRef]
- Cochrane DR, Bernales S, Jacobsen BM, Cittelly DM, Howe EN, D'Amato NC, Spoelstra NS, Edgerton SM, Jean A, Guerrero J, Gómez F, Medicherla S, Alfaro IE, McCullagh E, Jedlicka P, Torkko KC, Thor AD, Elias AD, Protter AA, Richer JK. Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res. 2014, 22, R7. [Google Scholar]
- He L, Du Z, Xiong X, Ma H, Zhu Z, Gao H, Cao J, Li T, Li H, Yang K, Chen G, Richer JK, Gu H. Targeting Androgen Receptor in Treating HER2 Positive Breast Cancer. Sci Rep. 2017, 7, 14584. [Google Scholar] [CrossRef] [PubMed]
- Huang R, Han J, Liang X, Sun S, Jiang Y, Xia B, Niu M, Li D, Zhang J, Wang S, Wei W, Liu Q, Zheng W, Zhang G, Song Y, Panga D. Androgen Receptor Expression and Bicalutamide Antagonize Androgen Receptor Inhibit β-Catenin Transcription Complex in Estrogen Receptor-Negative Breast Cancer. Cell Physiol Biochem. 2017, 43, 12–2225. [Google Scholar]
- Ni M, Chen Y, Lim E, Wimberly H, Bailey ST, Imai Y, Rimm DL, Liu XS, Brown M. Targeting androgen receptor in estrogen receptor-negative breast cancer. Cancer Cell. 2011, 20, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Claus J, Patel G, Autore F, Colomba A, Weitsman G, Soliman TN, Roberts S, Zanetti-Domingues LC, Hirsch M, Collu F, George R, Ortiz-Zapater E, Barber PR, Vojnovic B, Yarden Y, Martin-Fernandez ML, Cameron A, Fraternali F, Ng T, Parker PJ. Inhibitor-induced HER2-HER3 heterodimerisation promotes proliferation through a novel dimer interface. Elife. 2018, 7, e32271. [Google Scholar] [CrossRef]
- Cuenca-López MD, Montero JC, Morales JC, Prat A, Pandiella A, Ocana A. Phospho-kinase profile of triple negative breast cancer and androgen receptor signaling. BMC Cancer. 2014, 14, 302. [Google Scholar]
- Chen ZJ, Wei W, Jiang GM, Liu H, Wei WD, Yang X, Wu YM, Liu H, Wong CK, Du J, Wang HS. Activation of GPER suppresses epithelial mesenchymal transition of triple negative breast cancer cells via NF-κB signals. Mol Oncol. 2016, 10, 775–788. [Google Scholar] [CrossRef]
- Shen Y, Yang F, Zhang W, Song W, Liu Y, Guan X. The Androgen Receptor Promotes Cellular Proliferation by Suppression of G-Protein Coupled Estrogen Receptor Signaling in Triple-Negative Breast Cancer. Cell Physiol Biochem. 2017, 43, 2047–2061. [Google Scholar] [CrossRef]
- Giovannelli P, Di Donato M, Auricchio F, Castoria G, Migliaccio A. Androgens Induce Invasiveness of Triple Negative Breast Cancer Cells Through AR/Src/PI3-K Complex Assembly. Sci Rep. 2019, 9, 4490. [Google Scholar] [CrossRef]
- Lanzino M, Maris P, Sirianni R, Barone I, Casaburi I, Chimento A, Giordano C, Morelli C, Sisci D, Rizza P, Bonofiglio D, Catalano S, Andò S. DAX-1, as an androgen-target gene, inhibits aromatase expression: A novel mechanism blocking estrogen-dependent breast cancer cell proliferation. Cell Death Dis. 2013, 4, e724. [Google Scholar] [CrossRef]
- Lanzino M, Sisci D, Morelli C, Garofalo C, Catalano S, Casaburi I, Capparelli C, Giordano C, Giordano F, Maggiolini M, Andò S. Inhibition of cyclin D1 expression by androgen receptor in breast cancer cells--identification of a novel androgen response element. Nucleic Acids Res. 2010, 38, 5351–5365. [Google Scholar] [CrossRef]
- Peters AA, Buchanan G, Ricciardelli C, Bianco-Miotto T, Centenera MM, Harris JM, Jindal S, Segara D, Jia L, Moore NL, Henshall SM, Birrell SN, Coetzee GA, Sutherland RL, Butler LM, Tilley WD. Androgen receptor inhibits estrogen receptor-alpha activity and is prognostic in breast cancer. Cancer Res. 2009, 69, 6131–6140. [Google Scholar] [CrossRef] [PubMed]
- Anestis A, Sarantis P, Theocharis S, Zoi I, Tryfonopoulos D, Korogiannos A, Koumarianou A, Xingi E, Thomaidou D, Kontos M, Papavassiliou AG, Karamouzis MV. Estrogen receptor beta increases sensitivity to enzalutamide in androgen receptor-positive triple-negative breast cancer. J Cancer Res Clin Oncol. 2019, 145, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Barton VN, D'Amato NC, Gordon MA, Lind HT, Spoelstra NS, Babbs BL, Heinz RE, Elias A, Jedlicka P, Jacobsen BM, Richer JK. Multiple molecular subtypes of triple-negative breast cancer critically rely on androgen receptor and respond to enzalutamide in vivo. Mol Cancer Ther. 2015, 14, 769–778. [Google Scholar] [CrossRef]
- Barton VN, Christenson JL, Gordon MA, Greene LI, Rogers TJ, Butterfield K, Babbs B, Spoelstra NS, D'Amato NC, Elias A, Richer JK. Androgen Receptor Supports an Anchorage-Independent, Cancer Stem Cell-like Population in Triple-Negative Breast Cancer. Cancer Res. 2017, 77, 3455–3466. [Google Scholar] [CrossRef] [PubMed]
- Fernández NB, Sosa SM, Roberts JT, Recouvreux MS, Rocha-Viegas L, Christenson JL, Spoelstra NS, Couto FL, Raimondi AR, Richer JK, Rubinstein N. RUNX1 Is Regulated by Androgen Receptor to Promote Cancer Stem Markers and Chemotherapy Resistance in Triple Negative Breast Cancer. Cells. 2023, 12, 444. [Google Scholar] [CrossRef] [PubMed]
- 66. Rosas E, Roberts JT, O'Neill KI, Christenson JL, Williams MM, Hanamura T, Spoelstra NS, Vahrenkamp JM, Gertz J, Richer JK. A Positive Feedback Loop Between TGFβ and Androgen Receptor Supports Triple-negative Breast Cancer Anoikis Resistance. Endocrinology, 2021; 162, bqaa226.
- Wen PY, Weller M, Lee EQ, Alexander BM, Barnholtz-Sloan JS, Barthel FP, Batchelor TT, Bindra RS, Chang SM, Chiocca EA, Cloughesy TF, DeGroot JF, Galanis E, Gilbert MR, Hegi ME, Horbinski C, Huang RY, Lassman AB, Le Rhun E, Lim M, Mehta MP, Mellinghoff IK, Minniti G, Nathanson D, Platten M, Preusser M, Roth P, Sanson M, Schiff D, Short SC, Taphoorn MJB, Tonn JC, Tsang J, Verhaak RGW, von Deimling A, Wick W, Zadeh G, Reardon DA, Aldape KD, van den Bent MJ. Glioblastoma in adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020, 22, 1073–1113. [Google Scholar] [CrossRef]
- Bao D, Cheng C, Lan X, Xing R, Chen Z, Zhao H, Sun J, Wang Y, Niu C, Zhang B, Fang S. Regulation of p53wt glioma cell proliferation by androgen receptor-mediated inhibition of small VCP/p97-interacting protein expression. Oncotarget. 2017, 8, 23142–23154. [Google Scholar] [CrossRef]
- Yu X, Jiang Y, Wei W, Cong P, Ding Y, Xiang L, Wu K. Androgen receptor signaling regulates growth of glioblastoma multiforme in men. Tumour Biol. 2015, 36, 67–72.
- Rodríguez-Lozano DC, Piña-Medina AG, Hansberg-Pastor V, Bello-Alvarez C, Camacho-Arroyo I. Testosterone Promotes Glioblastoma Cell Proliferation, Migration, and Invasion Through Androgen Receptor Activation. Front Endocrinol. 2019, 10, 16. [Google Scholar] [CrossRef]
- Rodríguez-Lozano DC, Velázquez-Vázquez DE, Del Moral-Morales A, Camacho-Arroyo I. Dihydrotestosterone Induces Proliferation, Migration, and Invasion of Human Glioblastoma Cell Lines. Onco Targets Ther. 2020, 13, 8813–8823. [Google Scholar] [CrossRef]
- Zalcman N, Gutreiman M, Shahar T, Weller M, Lavon I. Androgen Receptor Activation in Glioblastoma Can Be Achieved by Ligand-Independent Signaling through EGFR-A Potential Therapeutic Target. Int J Mol Sci. 2021, 22, 10954. [Google Scholar] [CrossRef] [PubMed]
- Zalcman N, Canello T, Ovadia H, Charbit H, Zelikovitch B, Mordechai A, Fellig Y, Rabani S, Shahar T, Lossos A, Lavon I. Androgen receptor: A potential therapeutic target for glioblastoma. Oncotarget. 2018, 9, 19980–19993. [Google Scholar] [CrossRef] [PubMed]
- Read TA, Fogarty MP, Markant SL, McLendon RE, Wei Z, Ellison DW, Febbo PG, Wechsler-Reya RJ. Identification of CD15 as a marker for tumor-propagating cells in a mouse model of medulloblastoma. Cancer Cell. 2009, 15, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Lathia JD, Gallagher J, Heddleston JM, Wang J, Eyler CE, Macswords J, Wu Q, Vasanji A, McLendon RE, Hjelmeland AB, Rich JN. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell. 2010, 6, 421–432. [Google Scholar] [CrossRef]
- Alonso MM, Diez-Valle R, Manterola L, Rubio A, Liu D, Cortes-Santiago N, Urquiza L, Jauregi P, Lopez de Munain A, Sampson N, Aramburu A, Tejada-Solís S, Vicente C, Odero MD, Bandrés E, García-Foncillas J, Idoate MA, Lang FF, Fueyo J, Gomez-Manzano C. Genetic and epigenetic modifications of Sox2 contribute to the invasive phenotype of malignant gliomas. PLoS ONE. 2011, 6, e26740. [Google Scholar]
- Du Z, Jia D, Liu S, Wang F, Li G, Zhang Y, Cao X, Ling EA, Hao A. Oct4 is expressed in human gliomas and promotes colony formation in glioma cells. Glia. 2009, 57, 724–733. [Google Scholar] [CrossRef]
- Niu CS, Li DX, Liu YH, Fu XM, Tang SF, Li J. Expression of NANOG in human gliomas and its relationship with undifferentiated glioma cells. Oncol Rep. 2011, 26, 593–601. [Google Scholar]
- Rasper M, Schäfer A, Piontek G, Teufel J, Brockhoff G, Ringel F, Heindl S, Zimmer C, Schlegel J. Aldehyde dehydrogenase 1 positive glioblastoma cells show brain tumor stem cell capacity. Neuro Oncol. 2010, 12, 1024–1033. [Google Scholar] [CrossRef]
- Loras A, Gonzalez-Bonet LG, Gutierrez-Arroyo JL, Martinez-Cadenas C, Marques-Torrejon MA. Neural Stem Cells as Potential Glioblastoma Cells of Origin. Life (Basel). 2023, 13, 905. [Google Scholar]
- Matsubara S, Matsuda T, Nakashima K. Regulation of Adult Mammalian Neural Stem Cells and Neurogenesis by Cell Extrinsic and Intrinsic Factors. Cells. 2021, 10, 1145. [Google Scholar] [CrossRef]
- Kelava I, Chiaradia I, Pellegrini L, Kalinka AT, Lancaster MA. Androgens increase excitatory neurogenic potential in human brain organoids. Nature. 2022, 602, 112–116. [Google Scholar] [CrossRef] [PubMed]
- La Rosa P, Bartoli G, Farioli Vecchioli S, Cesari E, Pagliarini V, Sette C. Androgen Receptor signaling promotes the neural progenitor cell pool in the developing cortex. J Neurochem. 2021, 157, 1153–1166. [Google Scholar] [CrossRef] [PubMed]
- Kim HJ, Kim TJ, Kim YG, Seong C, Cho JH, Kim W, Lee KH, Kim DY. Antioxidant and Antiproliferative Activity of Finasteride against Glioblastoma Cells. Pharmaceutics. 2021, 13, 1410. [Google Scholar] [CrossRef] [PubMed]
| Cancer Stem Cell | AR effect on stemness | References |
|---|---|---|
| Prostate Cancer Stem Cell | Decreases self-renewal capacity Decreases expression of stemness factors Increases cell differentiation |
[42,44,45] |
| Breast Cancer Stem Cell | Promotes self-renewal Promotes expression of stemness factors |
[63,64,65] |
| Glioma Stem Cell | Promotes self-renewal Promotes expression of stemness factors |
[18,84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





