1. Introduction
Zinc (Zn) is one of the most important micronutrients in plants. It catalyzes several metabolic enzyme reactions (Saravanan et al., 2007) and promotes photosynthesis, plant resistance to environmental stresses, formation of pollen and proteins, metabolism of nitrogen and carbohydrates and production of antioxidants (Hussain et al., 2020). Amongst the micronutrients, soil Zn deficiency, reaching about one-third of arable soils is the most frequent challenge globally (Cakmak et al., 2017), affecting crop and animal health and productivity (Cakmak, 2008). Zn deficiency has been linked to hidden hunger (Kihara et al., 2020), manifested through malnutrition associated disorders.
Zinc exists in soil as both water-soluble and insoluble complexes, with major portion being non-bioavailable for plants uptake (Sadeghzadeh, 2013). Bioavailability of zinc depends on soil physicochemical conditions such as concentration of zinc in solution, its interactions with other nutrients and ion speciation, plant roots exudates and microbial activities (Loneragan & Webb, 1993; Li et al., 2003; Gupta et al., 2016; Hacisalihoglu, 2020). Increasing soil P availability, as influenced by long-term fertilization, may compromise Zn uptake by plants due to the antagonistic nature of the two nutrients, with potential adverse effects on crop yield and nutritional quality (Zhang et al., 2012). On the other hand, application of nitrogen fertilizer can increase plant uptake of P and Zn from soil (Pasley et al., 2019). Zinc deficiency results in poor plant growth, yields and nutritional quality arising from depressed zinc-associated metabolic processes (Sadeghzadeh, 2013). Remedying soil zinc deficiency and promoting crop productivity calls for agronomic interventions with potentials of enhancing zinc bioavailability, both at lower costs and ecofriendly manner. The conventional application of synthetic Zn-based fertilizers is not only less-efficient and non-ecofriendly, but also associated with high fertilizer costs (Kushwaha et al., 2020).
In the recent past, a lot of attention has been directed towards the potential of zinc solubilization by soil microbial communities (Khanghahi et al., 2018; Kushwaha et al., 2020; Saini et al., 2021) to remedy soil zinc deficiency. The soil microbes that are capable of solubilizing zinc from the insoluble sources are generally referred to as zinc solubilizing microbes (ZSMs) (Kushwaha et al., 2020; Rani et al., 2020). In addition to zinc solubilization, ZSMs can also enhance the capabilities of plants to uptake Zn from the soil, thus improving enrichment of Zn in the edible plant parts (Khanghahi et al., 2018; Kushwaha et al., 2020; Rani et al., 2020; Saini et al., 2021). The ZSMs, existing either in bacteria or fungal nature, may increase bioavailability of Zn to plants from the insoluble sources (Dhaked et al., 2017; Hussain et al., 2018) by employing different biological processes involving chelation, chemical transformation and production of organic acids (Fasim et al., 2002; Saravan et al., 2004; Mumtaz et al., 2017). This makes ZSMs be deemed as potential alternatives for enhancing Zn bioavailability and supplementation for plants in a sustainable, eco-friendly and cost-effective manner (Kushwaha et al., 2020; Rani et al., 2020). Fungal strains belonging to the genera Penicillium, Glomus, Aspergillus, Trichoderma and Beauveria have been identified as prominent fungal ZSMs (Khande et al., 2017; Vidyashree et al., 2018; Kushwaha et al., 2020) whereas the most promising bacterial strains have been identified in the genera Azospirillum, Burkholderia, Serratia, Agrobacterium, Bacillus, Rhizobium, Thiobacillus, Gluconacetobacter and Pseudomonas among others (Saravan et al., 2007; Anuradha et al., 2015; Rani et al., 2020).
Besides ZSMs, addition of micronutrient-based fertilizers, breeding zinc-efficient germplasm, seed biofortification, introducing beneficial soil microorganisms, correcting soil alkalinity alongside adopting crop rotation practices can also alleviate soil zinc deficiency (Rengel et al., 1999; White & Broadley, 2011; Rawat et al., 2013). Addressing soil-based Zn deficiencies can reduce food insecurity and hidden hunger that affects at least 30% of the global human population (Welch, 2002; Klassen-Wigger et al., 2018).
Regenerative agriculture practices involving crop rotation, application of organic manure and reduced use of inorganic fertilizers, have been widely promoted to improve soil health, fertility and sustain agricultural productivity (Kearney et al., 2022; Jama et al., 2000; Cai et al., 2019). Application of manure, either alone or in combination with inorganic fertilizers, is an agronomic practice with a great potential to improve Zn availability in the soil. Manure use is a traditional soil fertility management practice employed by many smallholder farmers in sub-Saharan Africa, especially those with mixed crop-livestock system in place (Mugwe et al., 2009; Mucheru-Muna et al., 2014). If manure is properly managed, manure application can improve soil fertility, crop yields and soil biological properties through provision of multiple nutrients (Kihanda et al., 2006; Gautam et al., 2020; Tang et al., 2020).
However, despite the benefits of such agricultural management practices on improving soil fertility and crop yields, little is known on their effects on zinc solubilizing microbial species, especially in tropical soils. In this study, we assessed how regenerative agriculture practices such as manure and crop residue applications, with or without fertilizer applications, affect the abundance of zinc solubilizing microbial species in a tropical agricultural soil.
2. Materials and Methods
2.1. Study site
This research was carried out in October 2019 (during short rains period) in a long-term agronomic trial named INM3 in Madeya, Siaya County, Kenya. The trial was established in 2003 by International Center for Tropical Agriculture (CIAT) and lies between 0.14 °N and 34.40 °E, under a sub-humid climate with biannual rainfall (1200–1600 mm) and average temperature of 23.2 ± 1.5 °C (Kihara, 2009). Soils in the trial site are Ferralsols, characterized by low pH (5.1 ± 0.3); with sand:silt:clay ratio of 15:21:64 and extractable inorganic phosphorus (Olsen) content of 2.99 ± 2.09 mg kg−1 (Kihara, 2009; Paul et al., 2013). Crop production in the region is mainly for subsistence, rain-fed and mostly practiced under conventional tillage in smallholder farms (mostly less than 1 ha), with maize being the dominant staple food crop grown mostly as intercrop with common beans. Plant residue retention is less common as big proportion is grazed on after harvest, and only few farmers incorporate manure in soil, but in considerably low quantities and at inconsistent intervals.
2.2. Experimental design
The experiment was initiated in 2003 under a split plot design with 48 treatments replicated four times. The main plots were two farmyard manure rates (0 and 4 t ha-1 FYM application), sub-plots were three cropping systems [Maize and Tephrosia (i.e., Tephrosia vogelii) rotation, maize and soybean intercropping and continuous maize], sub-sub plot were two residue application rates (with or without 2 t ha−1 residue application), and sub-sub-sub plots were four rates of N fertilization (0, 30, 60 and 90 kg N ha−1) as urea, two rates of P (0 and 45 kg P ha−1 as TSP), and blanket application of K (at 60 kg ha−1) as muriate of potash during planting). The whole experiment has 192 plots each measuring 4.5 m by 6 m.
Within the trial, maize and tephrosia were planted at a spacing of 25 cm by 75 cm and in a rotation system, with 2 seeds placed per hill and later thinned to one. Soybean was intercropped with maize, at a spacing of 5 cm by 75 cm. Urea was applied in two splits; whereby ⅓ was applied during planting and ⅔ applied during topdressing. Farmyard manure was incorporated in the field during planting. Hand ploughing and weeding using hoes were restricted to 15 cm depth.
2.3. Selection of treatments
Eleven (11) treatments representing different management practices under conventional tillage were selected for the study (
Table 1). The 11 treatments were selected to confer the effects of certain input combinations embedded in different management practices (under either continuous maize cropping or maize-tephrosia rotation) on putative zinc solubilizing microbes.
2.4. Soil sampling and analysis
Soil samples were collected, 8 weeks after planting, at 0–15 cm depth in October 2019 during the short rains period. Maize was the main crop during sampling. Samples were taken from five spots per plot within each treatment following a “W” shaped pattern, using an auger. The samples were transferred in a bucket, thoroughly mixed, representative samples packed in labeled zip-lock bags, kept in cool box with frozen ice packs and transported to the laboratory for processing and further analyses. The samples for biological assessments (except microbial biomass C) were sieved (2mm) and stored frozen (-20 °C) until extraction. The samples for various chemical analyses were air-dried before grinding and sieving. Samples for microbial biomass C assessment were immediately sieved (2mm) and analysed right after sampling from the field.
2.5. Soil chemical analyses
Soil organic carbon and total N were determined by Carbon Nitrogen (CN) Elemental Analyzer. Soil pH was determined in water (soil: water; 1:2), while the exchangeable cations (Mehlich 3 P, Ca, Mg, K, Zn, S, Fe, B, Mn, Na and Al) were extracted following Mehlich (1984) procedure; and concentrations determined by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES).
2.6. Determination of microbial biomass carbon
Microbial biomass C was determined using MicroBiometer Soil Test kit (
https://microbiometer.com). Briefly, fresh soil samples were sifted. Using a calibrated syringe, 1ml of samples were taken, compacted to 0.5 ml and the excess soil removed from the tip of the syringe. One sachet of the provided powder from the Kit was transferred into clean tube; water added and briefly mixed using a whisker. Soil samples were added, briefly mixed with the contents, whisked for 30 seconds to fully mix with the fluid and allowed to settle for 20 minutes. After every five minutes, the contents were briefly tapped to allow the floating debris to settle down the tube. After 20 minutes settling time, the samples were extracted using pipette and 3 drops carefully applied to the reading card without wetting the surrounding of the card. The readings for microbial biomass carbon were thereafter taken by imaging the card using MicroBiometer App from Google Play Store.
2.7. Deoxyribonucleic acid (DNA) extraction from soil
Total DNA was extracted from 0.2 g fresh soil samples using Phenol-Chloroform Isoamyl (PCI) Alcohol as described in Orwa et al., (2020). Briefly, 0.2 g soil were suspended in 200 µl of solution A (containing 100ul Tris-HCl (pH 8.0), 100 mM EDTA (pH 8.0); vigorously vortexed, 5 µl of Lysozyme (20mg/ml solution) added and mixture incubated in a water bath (370C) for 30 minutes. 400 µl of lysis buffer (containing 400 mM Tris-HCl (pH 8.0), 60 mM EDTA (pH 8.0), 150 mM NaCl and 1% sodium dodecyl sulfate) was added, incubated at room temperature for 10 minutes. Thereafter, 10 µl of Guanidinium thiocyanate (GITC; for protein denaturation) was added and the mix incubated (65 °C) in a water bath for 2 hours. An equal volume of phenol chloroform isoamyl was added, mixed briefly and at 13,200 rpm for 15 minutes at 4 °C and the supernatant containing the crude DNA transferred to a new tube. To the supernatant was added 150 µl of sodium acetate and 600 µl isopropyl alcohol (2-propanol), mixed briefly by inversion and left at room temperature for 10 minutes. This was followed by centrifugation at 13,200 rpm for 10 minutes to pellet the DNA. The supernatant was discarded, and resultant DNA pellets washed in 300 µl of 70% ethanol two times. The DNA pellets were air-dried and thereafter dissolved in 30 µl of PRC water. DNA quality and quality was checked on 1% agarose gel electrophoresis against a 1Kb marker. The DNA pellets were lyophilized and shipped to MRDNA labs (
www.mrdnalab.com, Shallowater, TX, USA) for amplicon generation and sequencing.
2.7.1. Soil DNA sequencing, bioinformatics sequence processing and taxonomic identification
The PCR amplification of the 16S rRNA gene V4 variable region was carried out from extracted DNA generated from rRNA, using barcoded bacteria/archaeal primers 515/806 with barcode on the forward end (i.e., 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′)) as previously described by Caporaso et al. (2012). Briefly, PCR amplicons were generated using HotStarTaq Plus Master Mix Kit (Qiagen, USA) under the following conditions: 94 °C for 3 min, followed by 28 cycles of 94 °C for 30 s, 53 °C for 40 s and 72 °C for 1 min; final elongation (72 °C for 5 min). Illumina DNA library preparation and sequencing was done following the manufacturer’s guidelines. The raw reads were preprocessed using a proprietary pipeline by the sequencing facility. Briefly, the sequences were joined, followed by removal of barcodes, sequences with ambiguous base calls and those with less than 150 base pairs. This was followed by denoising, generation of operational taxonomic units (OTUs), removal of chimeras and clustering of OTUS at 3% divergence (97% similarity). Finally, the OTUs were taxonomically classified using BLASTn against a curated database derived from RDPII and NCBI (
www.ncbi.nlm.nih.gov, http://rdp.cme.msu.edu).
2.7.2. Identification of the potential zinc solubilizing microbial species
Since the study majorly aimed at understanding the general overview of the agronomic management effects on potential zinc solubilizing microbes, we conducted in-depth literature review to identify and list the potential soil microbial genera known to solubilize zinc (ZSM). The search was done in Google Scholar and Web of Science search engines, with the timeline restricted to return outcomes only up to the year 2022. The resultant files were reviewed and the potential zinc solubilizing microbes reported in the articles listed. Using this list of potential zinc solubilizers, we then identified and selected those microbes from the already available Illumina data. Thus, the potential ZSM genera reported herein are those most commonly documented by several previous literatures. We further acknowledge that some of the potential ZSM genera reported herein although have the potentials of solubilizing zinc, may also perform other functions in the soil.
2.8. Statistical data analysis
Microbial diversity analysis was conducted using Vegan package in R Project (R Development Core Team, 2016). Alpha diversity (microbial diversity within samples) was estimated using Shannon and Chao 1 diversity indices to test the significant differences between the different agronomic management systems. Beta diversity (ᵦ diversity; diversity between samples ) was used to test the significant differences between samples. This was determined by computing the Principal component analysis (PCA) of the ZSM versus the treatments using PAST software (v.4.05) (Hammer et al., 2001). The data were Logarithm10 transformed before performing PCA to meet the assumptions of parametric tests (Koorem et al., 2014). Determination of Bray-Curtis similarities and Principal Component Analysis (PCA) were also carried out using PAST software (v.4.05).
Hierarchical cluster analysis (HCA), Analysis of variance (ANOVA), Canonical correspondence analysis (CCA) and Non-metric dimensional scaling (NMDS) were performed using R project (R Development Core Team, 2016). ANOVA was performed on square root transformed data. Mean separation was done using Tukey’s HSD at P≤0.05. Canonical correspondence analysis (CCA) was used to assess the relationship between the soil microbial community richness and soil chemical parameters. The CCA analysis was performed using Anacor library and cca function in Vegan library in R, overall significance was tested using anova function, and step function used to determine significant variables with permutation test at 999-maximum permutations.
3. Results
3.1. Effects of management practices on soil chemical and physical characteristics in INM3 site
Agronomic management practices significantly affected the soil chemical and physical parameters. Soil pH, SOC, total N, Mg, Zn, Fe and CEC were significantly (
P ≤ 0.05) higher under sole application of FYM (inm4) compared to no input application (Inm1) or application of inorganic fertilizer only (inm2;
Table 2). Application of inorganic fertilizer only (Inm2) significantly increased (
P ≤ 0.05) soil P (Olsen) and S, but significantly reduced (
P ≤ 0.05) soil pH relative to no input application (Inm1). The highest pH was in treatment with combined application of FYM and crop residues followed by all other FYM treatments while the no input and inorganic fertilizer treatments had the lowest pH.
Simultaneous application of FYM, NP fertilizers and residue (inm6) significantly increased (
P ≤ 0.05) soil pH, SOC, total N, Mg, Zn, Fe and CEC relative to combined application of NP fertilizers and residue only (inm5). Combined application of P and FYM (Inm10) significantly increased (
P ≤ 0.05) soil pH, total N, Mg, B and CEC relative to sole application of P (inm9) under continuous maize system. Integrating both P and 90 kgN ha
-1 (Inm11) fertilizers in a continuous maize system (
Table 2) significantly reduced soil pH compared to sole application of P (Inm9). This is perhaps due to the increases in soil acidity effect associated with urea nitrogen application.
All the management systems without FYM application had zinc concentrations ranges (1.0-1.84 mg kg-1) below the critical soil zinc deficiency threshold (0.6-2 mg kg-1; Kihara et al., 2020). However, all the systems where FYM was added solely or in combination with other nutrients had zinc concentration ranges (2.61-3.57 mg kg-1) above the critical deficiency threshold of 0.6-2.0 mg kg-1. Highest zinc concentrations (3.29-3.57 mg kg-1) were obtained in systems where FYM was added in combination with full NPK fertilization.
Olsen P negatively correlated with total N, SOC and K; but positively correlated with S, Fe and EC. Zn, Cu, B and C.E.C positively correlated with total N, SOC, pH, K, Ca and Mg (
Table 3). C.E.C positively significantly correlated with the soil characteristics. Total N positively correlated with SOC, pH, K, Ca, Mg, Cu, B, Zn and C.E.C; and a similar correlation (as that of total N) true for the variables relating to SOC, pH, K and Ca and Mg. These correlations indicate that the management systems such as application of FYM, that increase SOC, also (concomitantly) increase several soil chemical parameters involving the soil pH, N, K, Ca, Mg, Cu, B, Zn and C.E.C.
3.2. Overall microbial abundance and ZSM species abundance in INM3
Overall, 2,113,815 and 2,219,424 high quality reads for bacteria and fungi, respectively, were obtained. The 2,113,815 high quality bacterial sequences were clustered into 691 OTUs at 97% genetic distance; that were further assigned to 36 phyla, 87 classes, 161 orders and 298 families. Similarly, the 2,219,424 high quality fungal sequences were clustered into 721 OTUs at 97% genetic distance, and were further assigned to 27 phyla, 79 classes, 191 orders and 379 families.
The ten most dominant bacterial phylotypes at phylum level were Proteobacteria, Actinobacteria, Firmicutes, Acidobacteria, Gemmatimonadetes, Chloroflexi, Planctomycetes, Verrucomicrobia, Bacteriodetes and Nitrospirae whereas the most abundant fungal phylotypes (in order from highest to lowest genera) were
Mortierella,
Fusarium,
Penicillium,
Gymnochlora,
Sclerostagonospora, Rhizophlyctis, Alternaria,
Myceliophthora,
Cochliobolus,
Humicola and
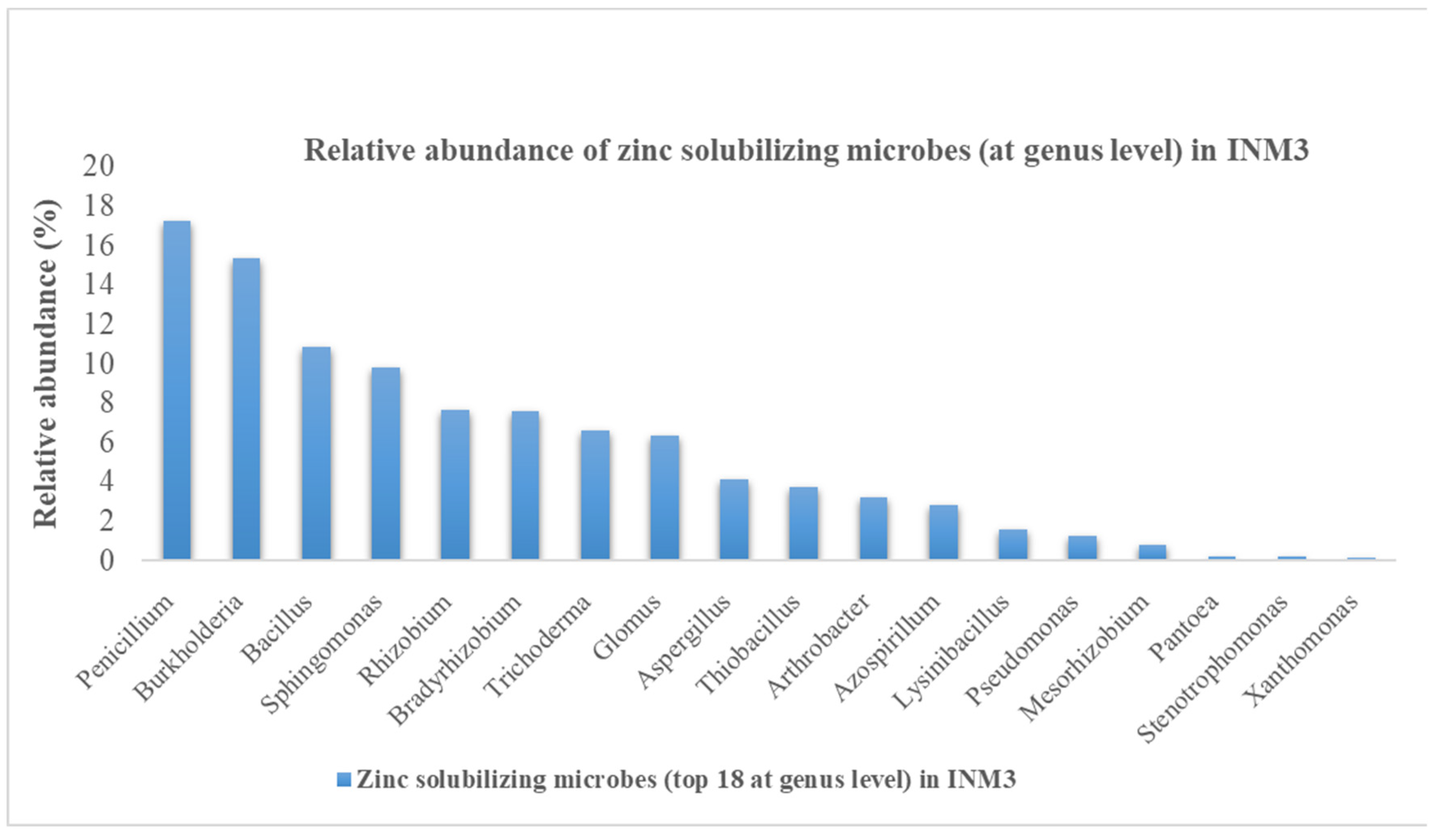
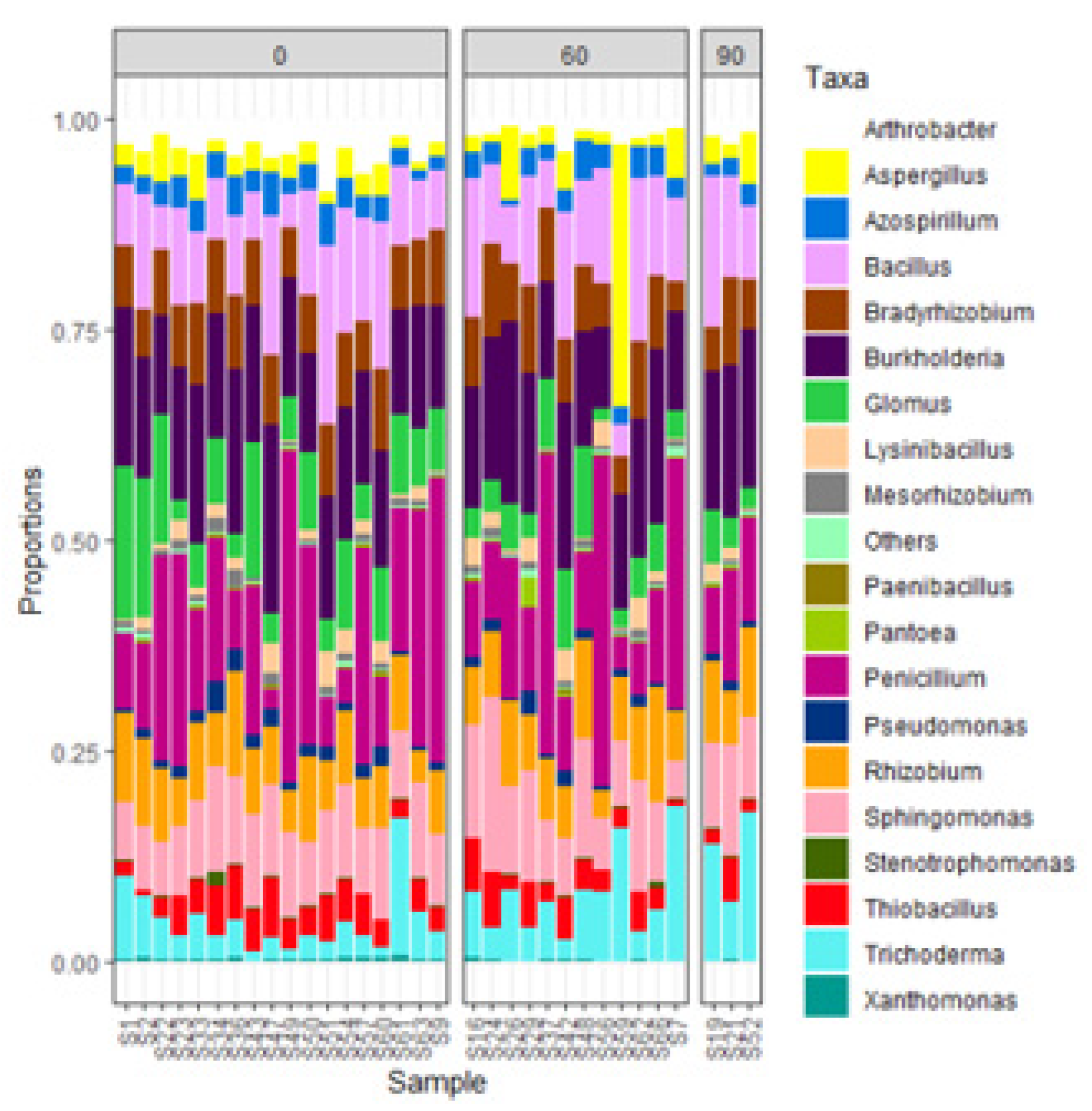
Pseudofavolus. Out of 691 bacterial and 721 fungal phylotypes (at genus level), 31 potential zinc solubilizing microbial genera were identified from the Illumina data based on the outcomes ZSM genera obtained from the literature reviews. Fifteen (15) out of the 31 potential ZSM genera identified in INM3 site (
Figure 1) had relative abundance greater than 0.5%. The 15 potential ZSM genera comprised of
Penicillium spp,
Burkholderia spp,
Bacillus spp.,
Sphingomonas spp.,
Rhizobium spp.,
Bradyrhizobium spp.,
Trichoderma spp.,
Glomus spp.,
Aspergillus spp.,
Thiobacillus spp.,
Arthrobacter spp.,
Azospirillum spp.,
Lysinibacillus spp.,
Pseudomonas spp. and
Mesorhizobium spp in INM3.
3.3. Effect of agronomic management practices on ZSM species
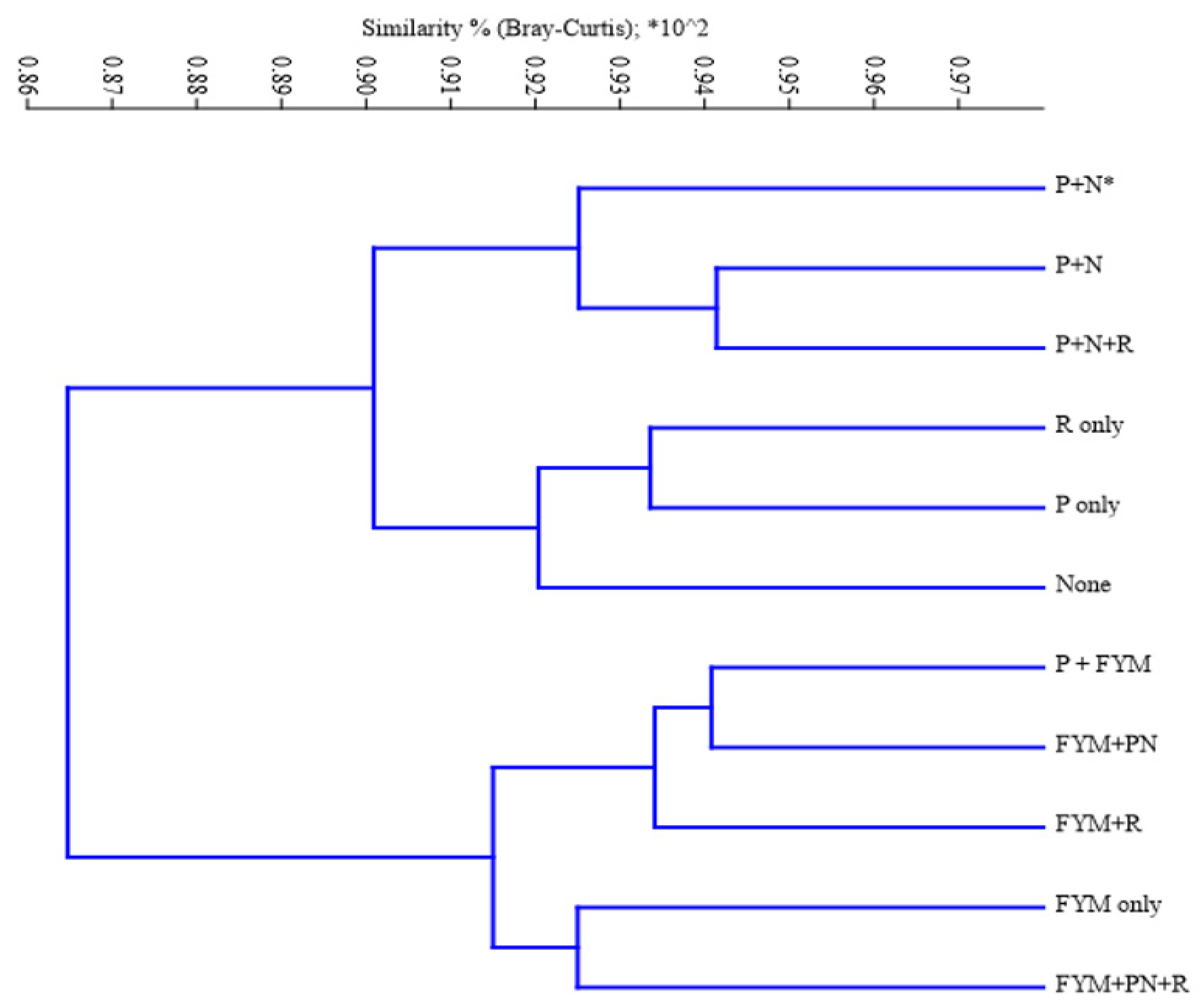
Bray-Curtis similarity cluster analysis revealed distinct grouping of treatments based on the management practices implemented. The results in the dendrogram (
Figure 2) showed that the ZSM species were clustered in two major clades based on either application or no application of farmyard manure (i.e., management practices having FYM addition were grouped in one major clade and those without FYM in a different clade (
Figure 2: HCA results)). The subsequent clades emanating from each of the two major clades showed that the species in the different management practices were not quite homogeneous, although either application of, or lack of, FYM similarly affected them.
The clustering of the management practices with FYM addition had almost 91.4% similarity index (Bray-Curtis) while those lacking FYM addition had 90.0% (
Figure 2). Management practices with combined application of P, N and residue were similarly grouped (92.5% similarity index); and the same case was observed under the treatments with no input application, sole application of either residue or P (all under 92.3% similarity index).
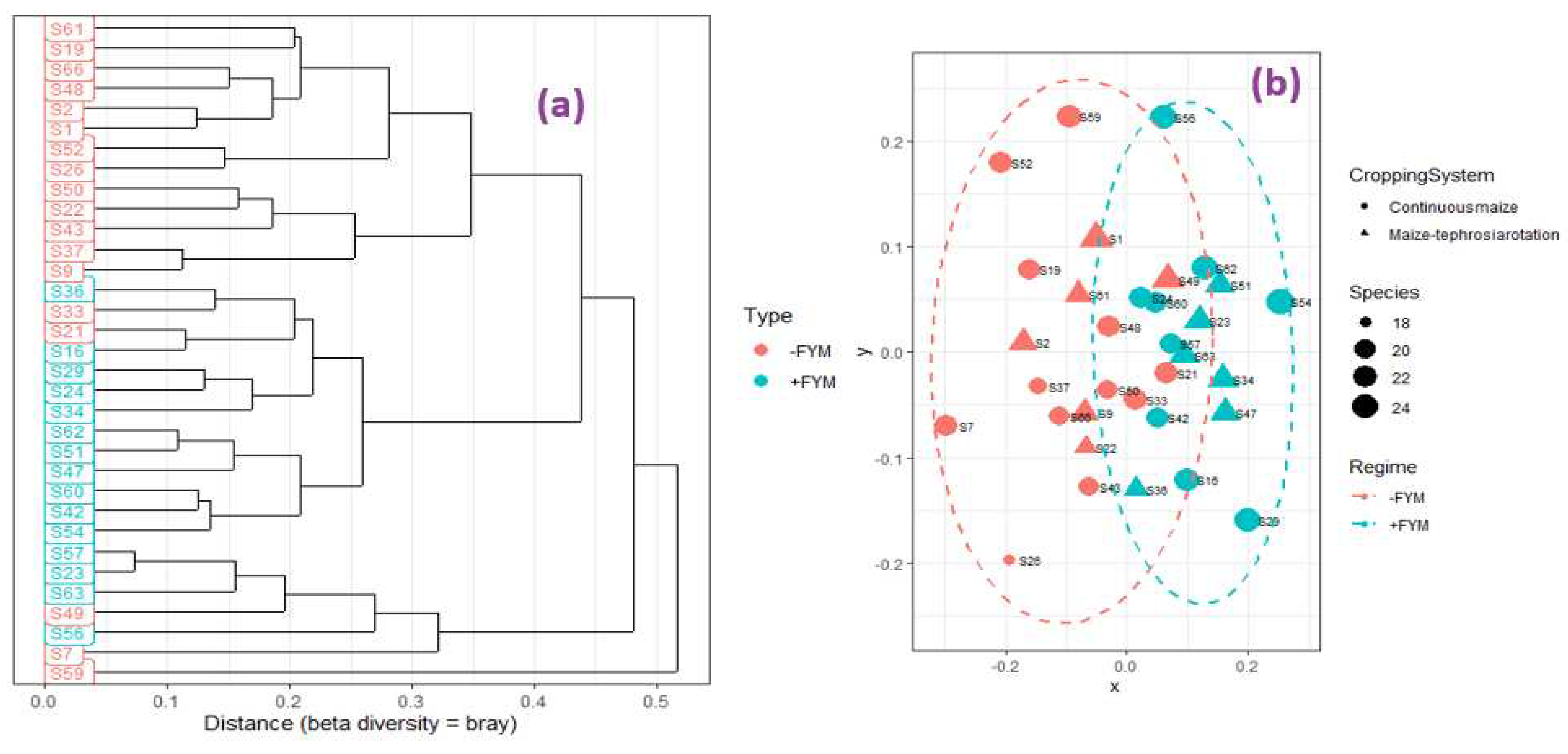
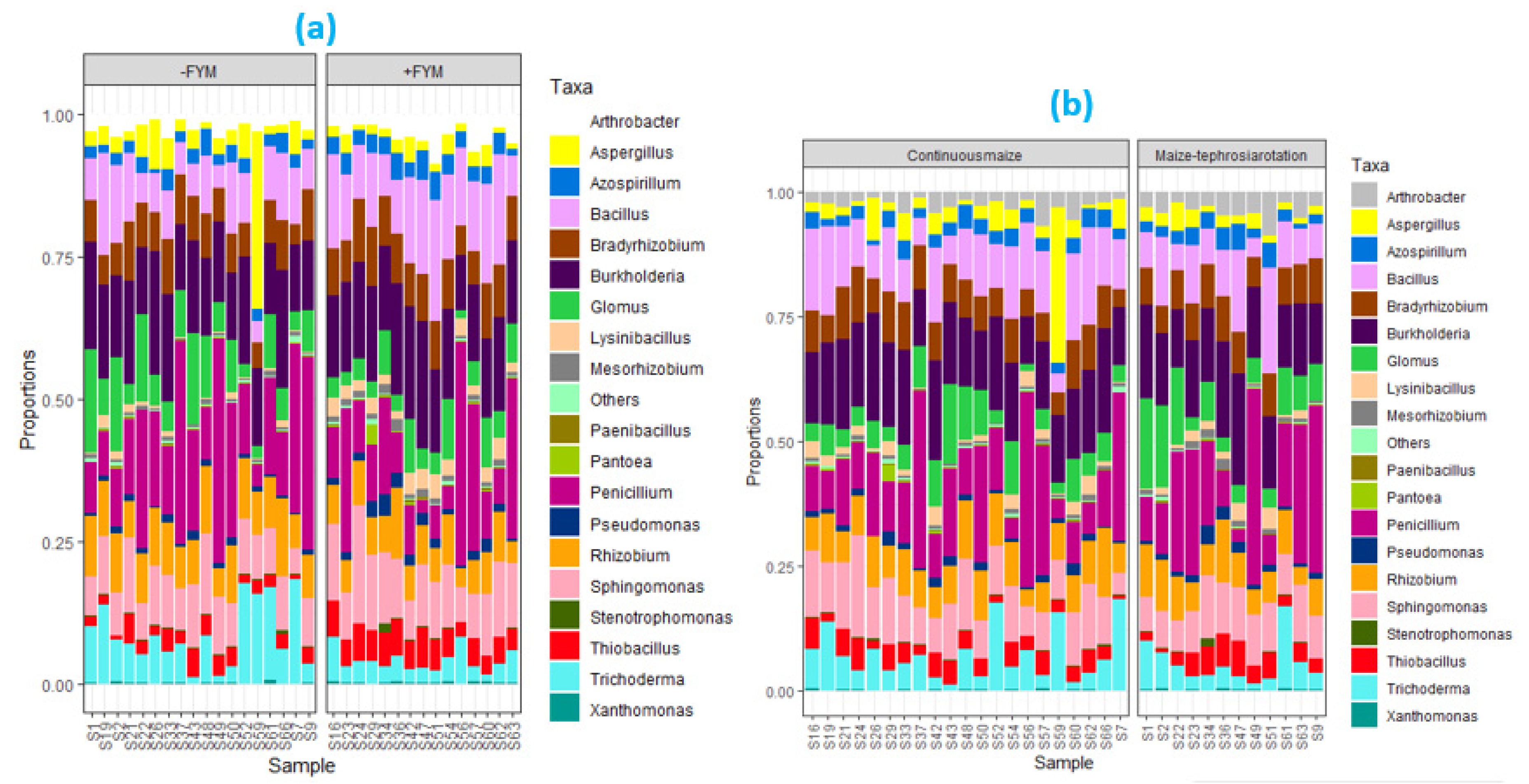
A shift in the distribution of ZSM was observed in the integrated soil fertility management under practice with application of FYM relative to practices lacking FYM addition (
Figure 3 a, b and
Figure 4, a,b). However, cropping systems had negligible influence on ZSM species distribution (
Figure 3 a, b and
Figure 4, a,b).
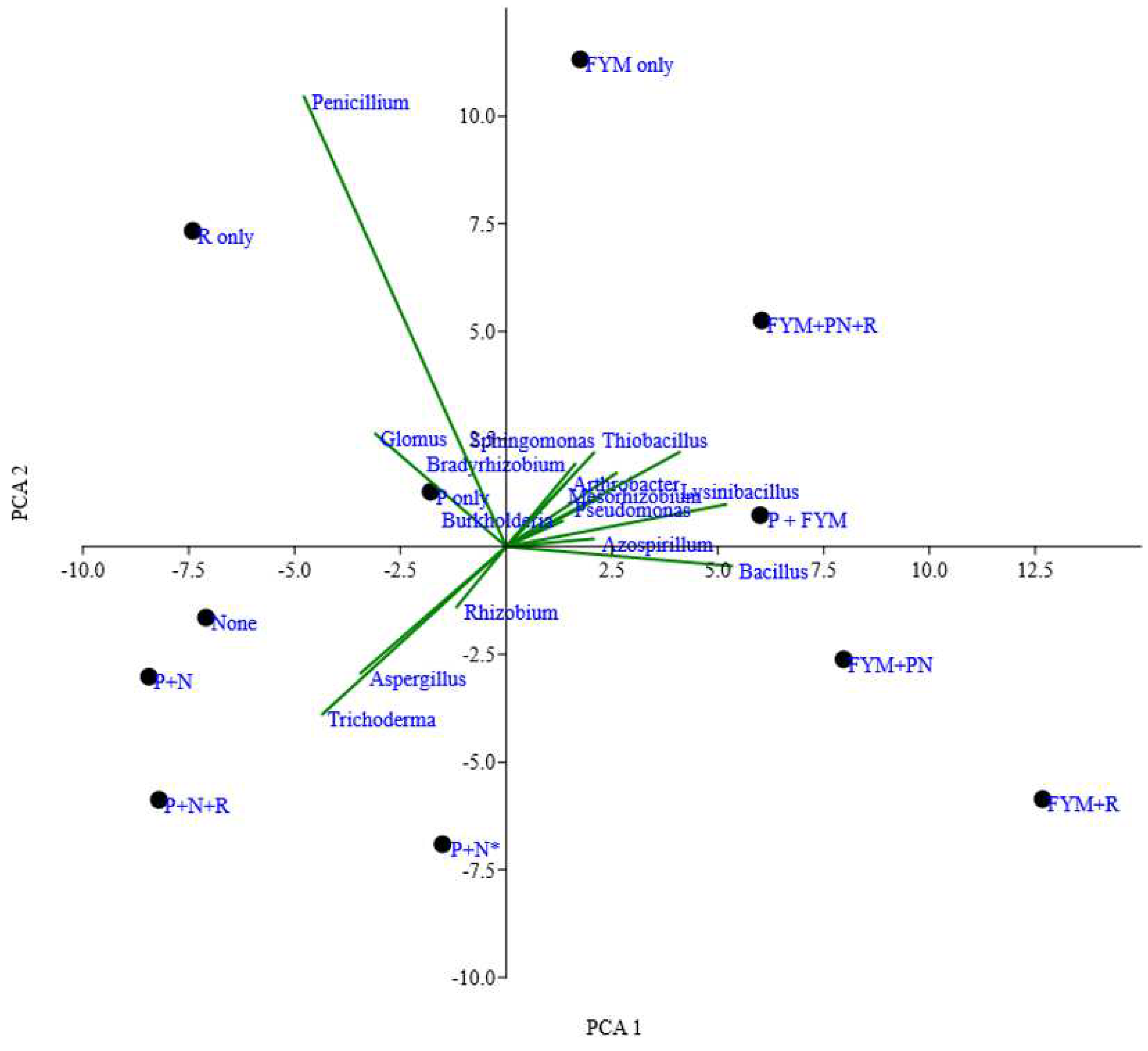
The management practices with FYM manure addition had two-times more ZSM species (with relative abundance > 0.5%) compared to those lacking FYM (
Figure 5; PCA Results).
Penicillium spp.,
Glomus spp.,
Rhizobium spp.,
Sphingomonas spp.,
Aspergillus spp. and
Trichoderma spp. were grouped in the management systems lacking FYM while the remaining ZSM species (i.e.,
Bacillus spp.,
Azospirilium spp.,
Burkholderia spp.,
Pseudomonas spp.,
Thiobacillus spp.,
Arthrobacter spp.,
Mesorhizobium spp.,
Bradyrhizobium spp., and
Lysinibacillus spp.) were grouped in management practices that received either sole FYM or combined application of FYM with other inputs (
Figure 5).
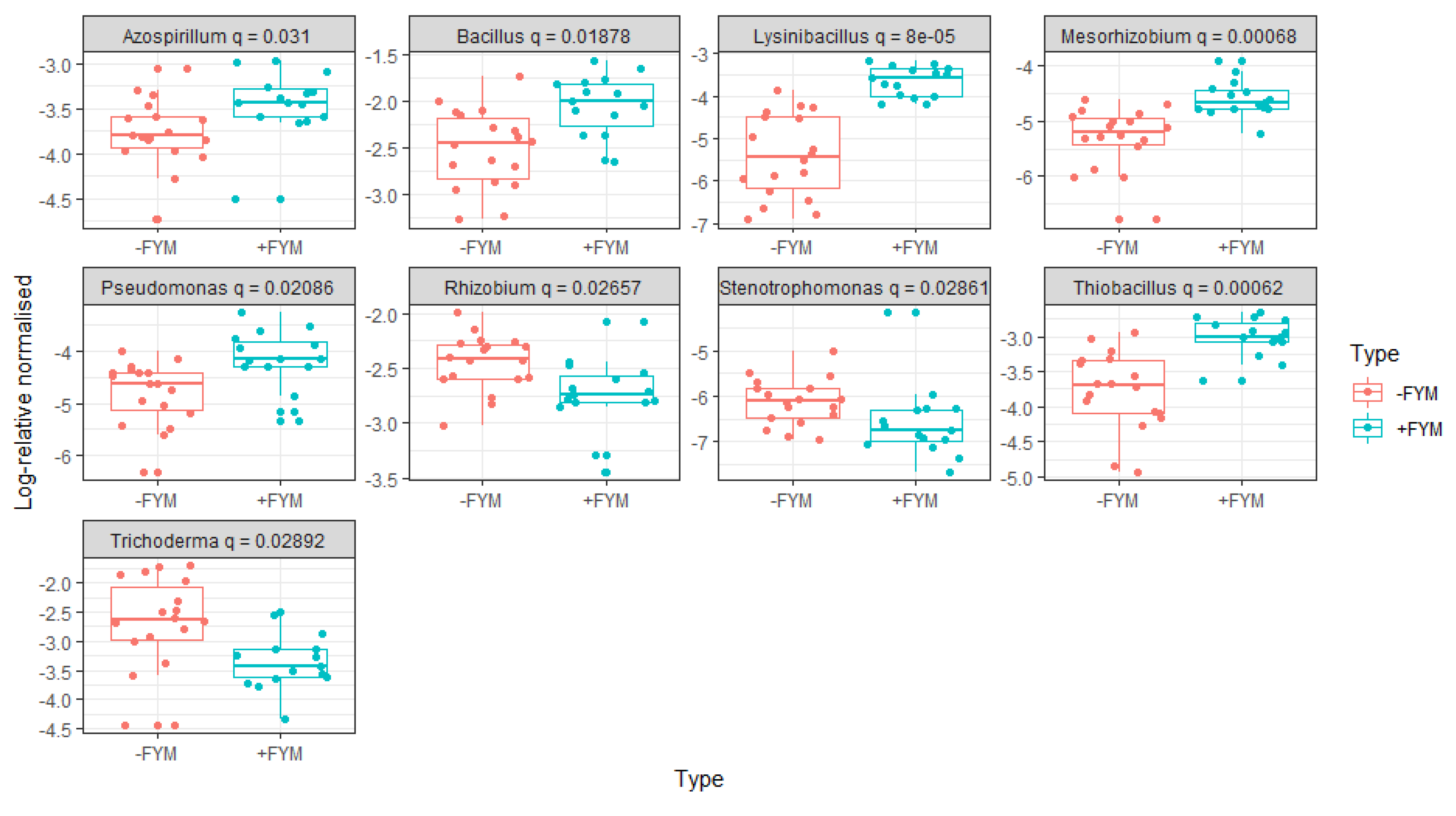
Eleven (11) ZSM species comprising of
Azospirillum spp.,
Bacillus spp.,
Lysinibacillus spp.,
Mesorhizobium spp.
Pseudomonas spp.,
Rhizobium spp.,
Stenotrophomonas spp.,
Thiobacillus spp. and
Trichoderma spp. were significantly (P<0.05) affected by the effects of FYM application (
Figure 6).
Application of inorganic N fertilizer (
Figure 7) alone or in combination with FYM and crop residues influenced the proportions of specific ZSM.
Glomus spp.,
Penicillium spp. and
Thiobacillus spp. proportions were depressed under systems with nitrogen applied at 90 kgN/ha compared to no-application (0 kgN ha
-1). The proportions of ZSM were not significantly influenced by P (data not shown). Also, application of phosphorus, nitrogen and residue did not significantly affect the overall abundance and diversity ZSM species (
Table 4), although species richness and diversity were slightly higher in systems with, relative to without, P applied. Still, application of FYM (at 4 t ha
-1) significantly increased ZSM abundance and diversity; and soil bio-chemical parameters involving SOC, total N, Zn and Fe.
3.3. Relationship between soil chemical characteristics and ZSM abundance
The ZSM microbial species richness and diversity significantly correlated positively with SOC, pH, Ca, Mg, Zn, Fe and C.E.C (
Table 5).
Amongst the individual ZSM species, soil total N, SOC, pH, Ca, Mg and C.E.C positively correlated with
Bradyrhizobium spp.,
Arthrobacter spp.,
Thiobacillus spp.,
Pseudomonas spp.,
Azospirillum spp.,
Lysinibacillus spp., and
Mesorhizobium spp (
Table 6).
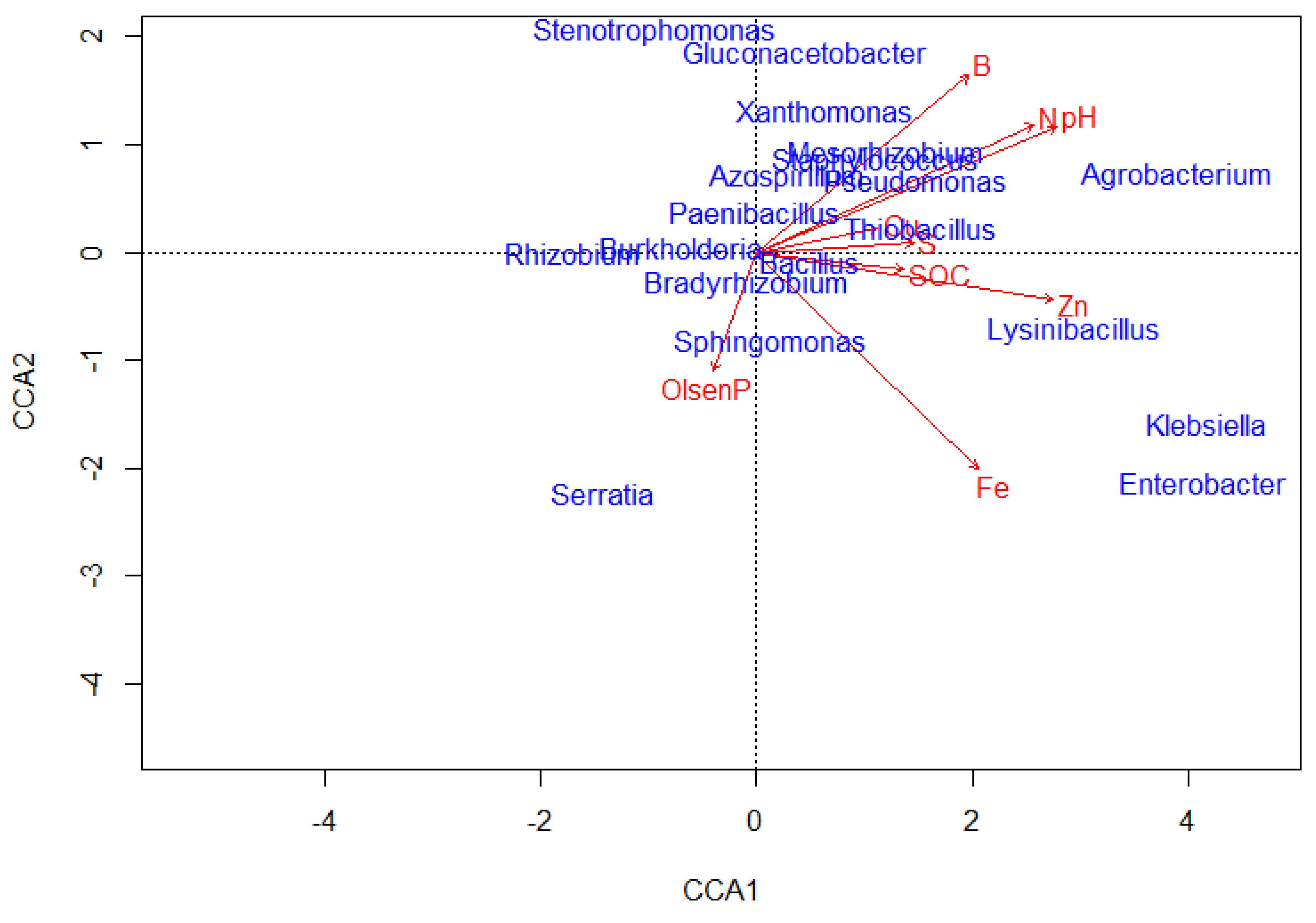
Canonical correspondence analysis (CCA) results showed that soil chemical parameters involving SOC, Olsen P, total N, Fe and Zn significantly affected the distribution of ZSM species (
Table 7).
The distribution of seventeen (17) SZM species (at genus level) involving,
Agrobacterium spp.,
Arthrobacter spp.,
Aspergillus spp.,
Azospirillum spp
Bacillus spp.,
Bradyrhizobium spp.,
Glomus spp.,
Lysinibacillus spp.,
Mesorhizobium spp.,
Pantoea spp.,
Penicillium spp.,
Pseudomonas spp.,
Rhizobium spp.,
Sphingomonas spp.,
Thiobacillus spp.,
Trichoderma spp. and
Xanthomonas spp were significantly affected by the soil chemical characteristics (
Figure 8;
Table 7).
Lysinibacillus spp. strongly correlated with soil available Zn;
Sphingomonas spp. and Serratia spp. with Olsen P; while
Thiobacillus spp. strongly correlated with S and SOC (
Figure 8).
4. Discussion
The ability of soil microbes to solubilize zinc is of interest since it can enhance availability of zinc for plant growth and ultimately stimulate deposition in edible plant tissues. Nitrogen, phosphorus, potassium and zinc are among the major limiting nutrients for sustainable crop productivity; whose availability may be influenced by both environmental conditions and/or agronomic management practices. Some of these essential plant nutrients may be susceptible to leaching, slow solubility, poor mobility and fixation in insoluble compounds; diminishing their availability for crop uptake, hence deficiency which not only affects crop production but also nutritional quality.
4.1. Influence of soil chemical properties on zinc solubilizing microbial species
In this study, we hypothesized that the soil chemical and biological properties are critical in determining soil microbial species abundance, distribution and diversity; and thus, would influence the zinc solubilizing microbial species abundance and distribution. The results from correlations and CCA showed that the soil chemical and biological parameters were relevant to the distribution and abundance of the zinc solubilizing microbes. These results corroborate recent findings on effects of different soil nutrients on related soil microbial properties in the same site (Kihara et al., 2012; 2018; Margenot et al., 2017; Bolo et al., 2021) and elsewhere in Australia (Xue et al., 2018) and China (Niu et al., 2021). The significant effects of SOC, macronutrients (Olsen P and total N) and micronutrients (Fe and Zn) on zinc solubilizing microbial species abundance and distribution indicates the important role of nutrition in driving the microbial community distribution and abundance. Previous reports (Kihara et al., 2012, Koorem et al., 2014; Vukicevich et al., 2016; Lian et al., 2019) linked certain soil microbial parameters to nutrient enrichment and soil organic carbon accumulation, providing food and energy for microbial growth and development. At various scales, the soil microbial community often displays heterogeneous distribution, and this is majorly due to the influences of prevailing soil conditions involving soil fertility (nutrient supply) status, physico-chemical and biological properties. Besides microbial distribution, the dynamics of microbial abundance is also influenced by the soil fertility status and physico-chemical conditions (Bolo et al., 2021). Thus, in most instances, the microbial abundance can increase with increasing nutrient availability (i.e., soil fertility) and decrease with declining nutrient availability (Koorem et al., 2014), although the reverse may be true in some instances involving specific microbial groups. This explains the significant positive correlations observed between numerous soil chemical parameters and zinc solubilizing microbial species.
We also observed slight negative effect of soil N (i.e., when N was applied at 90 kgN ha-1) on the proportions of certain ZSM populations (Glomus, Penicillium and Thiobacillus) and this indicates either limited access to soil available N or low N demand by some of these ZSMs. Nitrogen occurs mostly in soils mostly in complex organic forms and this limits its access by microbes that lack saprotrophic nutrition capabilities like Glomus (Hodge & Fitter, 2010). In addition, the few observed ZSM species that negatively correlated with some soil characteristics suggest the likelihood of resource (nutrient) competition existing between them and the other species that had positive correlations. Thiobacillus are not only zinc solubilizers but also sulfur oxidizers (Zhi-Hui et al., 2010), thus explaining their positive correlation with soil S. SOC provides food that releases vital energy required by microbes for growth and development. Influences of different soil chemical (and biological) parameters on soil microbial structure have been previously observed, with some increasing, decreasing or having no change in response (Koorem et al., 2014; Bolo et al., 2021).
There was no major shifts in microbial species proportions under the two cropping systems (i.e., maize and tephrosia rotation and continuous maize systems), and this demonstrates high similarity of such systems in terms of ZSM colonization. This also points to the possibility of small-scale soil microbial heterogeneity within the two cropping systems, perhaps contributed by the sampling location (plot level identity). In a previous study, Koorem et al. (2014) reported that the abundance of the microbial groups tested depended on the sampling location, with small-scale biotic and abiotic heterogeneity contributing a great deal in influencing microbial species coexistence. Similarly, in a study conducted in long-term no-till systems in Tennessee, crop rotation sequence did not influence bacterial richness, diversity and community structure (Ashworth et al., 2017). This also corroborates previous observations in a study conducted on a Brazilian Oxisol where rotation with different crops did not influence related microbial parameters (Balota et al., 2004). However, the findings contradict previous observations by Bolo et al. (2021) where related microbial functional groups associated with phosphorus solubilization were significantly affected by cropping systems perhaps due to the systems that were under comparison. In the study, Bolo et al. (2021) compared maize soybean intercropping versus rotation under conservation tillage as opposed to the current study that compared maize-tephrosia rotation versus sole maize under a continuously tilled integrated soil fertility management system.
4.2. Stimulation of zinc solubilizing microbial species with FYM application
Regenerative agriculture practices involving incorporation of organic matter (farmyard manure application and residue retention) among others (Giller et al., 2021), can strongly influence the soil physicochemical parameters, biological properties and enhance soil fertility by stimulating microbial abundance, nutrient cycling and regulating SOC availability (Bolo et al., 2021). Application of organic matter (i.e., FYM) has been shown to improve soil physiochemical conditions (Mucheru-Muna, et al., 2007) alongside stimulating positive microbial link between nutrient availability (Ayaga et al., 2006), regulation of SOC and moisture. In our study, application of farmyard manure not only influenced the distribution, but also increased the abundance and diversity of several zinc solubilizing microbial species in INM3 site.
The HCA revealed a systematic grouping, where the ZSMs were clustered in two major clades, depicting the effect of either application of (or no addition of) organic matter (FYM) on the ZSM. FYM is important in improving nutrient availability, soil pH, moderating temperature, moisture retention, enhancing SOC availability (Kihanda et al., 2006; Mugwe et al., 2009; Mucheru-Muna et al., 2014), and this creates favorable and enabling conditions that stimulate microbial proliferation and activities (Gautam et al., 2020; Tang et al., 2020). Some of the ZSM species are copiotrophic, preferring nutrient and carbon rich environments (Lladó & Baldrian, 2017; Babin et al., 2019), and the addition of FYM could enhance soil nutrients and carbon availability, thereby stimulating the copiotrophic ZSM species. However, the clustering of the treatments without FYM application (but with other inputs) in a different major clade depicts that not all ZSM species are copiotrophic, but rather, some would likely prefer certain environmental conditions not influenced by availability of FYM. Besides, the differences in similarity indices of different management systems in different subclades (sub-clusters;
Figure 4) under each major clade perhaps depict that the ZSM species in the different management system pairs were not quite homogeneous, and this echoes the previous suggestion by Tomsone et al. (2012).
The clustering of the ZSM species within a particular management system (i.e., with either application or no application of farmyard manure) observed in this study demonstrates strong similarity of microbial groups within each of those management systems. The slight shift in clustering of the microbial species in one management system distinct from the other management system as observed indicates heterogeneity between the two particular systems. This heterogeneity can be attributed to the effects of the specific management systems on soil physiochemical and biological conditions that would, consequently and concomitantly, either directly or indirectly influence the different microbial parameters. This involves influences on soil pH, SOC, moisture, temperature, nutrient availability/fertility and numerous biological characteristics involving the effects on certain microbial structures that can also alter microbial community composition in a given system. These assertions strongly relate to the observed ZSM microbial species responses to application of FYM. In this study, besides the ZSM microbial species being distinctly grouped with either application or no application of FYM, the systems with FYM added had more ZSM species abundance with majority being significantly elevated following addition of FYM. Yet, ZSM species richness and diversity (Shannon); SOC and soil pH and nutrient supply (macro- and micronutrients) was elevated under systems with FYM application compared to those lacking FYM, implying the beneficial influences of FYM on soil biochemical conditions.
Among other observations, the increase in ZSM species richness (abundance) and diversity following addition of FYM relative to no application could also be attributed to the effect of FYM in increasing SOC and nutrient availability, and creation of microclimate that would favor microbial proliferation, as observed the known copiotrophic ZSM genera involving Pseudomonas spp., and Bacillus spp., Rhizobium spp. (Fierer & Jackson, 2006; Kamran, et al., 2017) that thrive in nutrient rich environments. Consistent with the results of this study, previous studies (Kumar, et al., 2017;Wang et al., 2019) have reported increased microbial species abundance and diversity following farmard manure application.
The significant positive correlation between ZSM species richness/diversity with soil pH, zinc and other nutrients denotes microbial nutrient (especially Zn) demand, and this may culminate to variation in the intensity of solubilization activities. Besides, the significant correlation between total soil zinc content with ZSMs abundance also points to increased microbial solubilization and/or mineralization of different pools of zinc. In the study site, available soil zinc concentrations ranged between 1.00 mg/kg to 3.57 mg/kg; which is within the critical soil zinc deficiency threshold (0.6-2.0 mg/kg; Kihara et al., 2020), implying that zinc deficiency is an issue of concern in this site. However, it is interesting to point out that the highest values of Zinc (above the critical threshold of 2.0 mg/kg) were only recorded in the systems with either sole application of FYM or combined application of FYM with other inputs (full fertilization); with the ZSM species abundance significantly correlating with zinc availability. This denotes the potential of FYM application in not only increasing zinc solubilising microbial abundance, but also the available zinc concentrations beyond the deficiency threshold limits through stimulating ZSM solubilization activities. The positive capacity of manure to provide multiple nutrients and stimulate biological parameters was recently acknowledged (Kihanda et al., 2006; Gautam et al., 2020; Tang et al., 2020). The increase in soil zinc concentrations (above critical thresholds) and also ZSM abundance and diversity following application of FYM, either sole or in combination with other nutrients, is an indication that FYM, upon mineralization, can significantly influence micronutrient availability; and that combining FYM with other inorganic nutrients at appropriate rates can further provide more environmental benefits by increasing zinc availability but also stimulating zinc solubilizing microbial species abundance, diversity and activities.
Some of the ZSM species also perform different functions other than zinc solubilization in the soil, and could be affected by other parameters directly linked to FYM application. Recent studies have shown that some of the ZSM species (i.e.,
Penicillium spp.,
Glomus spp.,
Rhizobium spp.,
Sphingomonas spp.,
Aspergillus spp. and
Trichoderma spp.) that were distinctively grouped in the management practices lacking FYM are also functional phosphorus solubilizing microbes that are negatively affected by increasing P availability (Alori et al., 2017; Kalayu, 2019; Bolo et al., 2021). Addition of FYM could stimulate soil P availability upon decomposition and mineralization thereby suppressing some of the phosphorus solubilization-linked strains of ZSM species. In the study, application of FYM significantly increased pH relative to no application, and this contributed to 31.4% increase in soil P (
Table 2) and that could influence the P-solubilization associated ZSM species.
5. Conclusions
This study revealed that application of FYM increases ZSM abundance and diversity. This is important in the quest for sustainable agricultural production through enhancing provision and availability of multiple soil nutrients for a balanced plant (and human) nutrition. Besides macronutrients, application of FYM improved soil zinc availability, attributable to increased microbial (especially the ZSM) abundance and activities. The study also demonstrated that application of inorganic N fertilizer, either alone or in combination with FYM and crop residues, influenced the proportions of specific ZSM. Furthermore, the improvement of key soil chemical parameters with application of FYM stimulated ZSM species richness and abundance. These positive ZSM responses with manure application indicate the potential for biologically mediated soil Zn enrichment, as an alternative to Zn-based fertilizer application, to enhance sustainable, eco-friendly and cost-effective Zn bioavailability and supplementation for plants.
However, credible information on the relationship between zinc solubilizers’ abundance and diversity with plant nutrition (zinc uptake) is largely missing and requires further research. In addition, it is still unclear what the crop yields trends/patterns would be, relevant to the quantities of Zn available from microbial solubilization compared to application of Zn-based fertilizers; and whether the quantities of Zn solubilized would be dependable for sustainable crop yields. Still, the quantities of Zn that can be potentially solubilized by these microbes from the different pools lies widely unknown. Moreover, a dearth of knowledge still exists on whether the nutrient (Zn) quantities solubilized by the microbes can be in measures that could sustainably offset the soil deficiency thresholds, and the right combination of systems to optimize the realization of microbial driven nutrient (Zn) solubilization. Furthermore, besides Zn-based fertilizers being costly, there is scanty knowledge on the potential economic benefits that can accrue from microbial derived nutrient (Zn) mineralization versus the use of inorganic zinc-based fertilizers in enhancing crop production.
Acknowledgments
This work received financial support from the German Federal Ministry for Economic Cooperation and Development (BMZ) commissioned and administered through the Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) Fund for International Agricultural Research (FIA), grant number: 81232393. The long-term trials where the research was conducted, and the staff time for some of the co-authors, was supported through the CGIAR Research Program on Water, Land and Ecosystems (WLE) with support from CGIAR Fund Donors including: the Australian Center for International Agricultural Research (ACIAR); Bill and Melinda Gates Foundation; Netherlands Directorate General for International Cooperation (DGIS); Swedish International Development Cooperation Agency (Sida); Swiss Agency for Development Cooperation (SDC); and the UK Department of International Development (DIFD). In the One CGIAR, the work is closely aligned to the Excellence in Agronomy (EIA) Initiative.
References
- Alori, E. T., Glick, B. R., & Babalola, O. O. (2017). Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Frontiers in microbiology, 8, 971. [CrossRef]
- Anuradha, P., Syed, I., Swati, M., & Patil, V. D. (2015). Solubilization of insoluble zinc compounds by different microbial isolates in vitro condition. International Journal of Tropical Agriculture, 33(2 (Part II)), 865-869.
- Ashworth, A. J., DeBruyn, J. M., Allen, F. L., Radosevich, M., & Owens, P. R. (2017). Microbial community structure is affected by cropping sequences and poultry litter under long-term no-tillage. Soil Biology and Biochemistry, 114, 210-219. [CrossRef]
- Ayaga, G., Todd, A., & Brookes, P. C. (2006). Enhanced biological cycling of phosphorus increases its availability to crops in low-input sub-Saharan farming systems. Soil Biology and Biochemistry, 38(1), 81-90. [CrossRef]
- Babin, D., Deubel, A., Jacquiod, S., Sørensen, S. J., Geistlinger, J., Grosch, R., & Smalla, K. (2019). Impact of long-term agricultural management practices on soil prokaryotic communities. Soil Biology and Biochemistry, 129, 17-28. [CrossRef]
- Balota, E. L., Colozzi Filho, A., Andrade, D. S., & Dick, R. P. (2004). Long-term tillage and crop rotation effects on microbial biomass and C and N mineralization in a Brazilian Oxisol. Soil and Tillage Research, 77(2), 137-145. [CrossRef]
- Bolo, P., Kihara, J., Mucheru-Muna, M., Njeru, E. M., Kinyua, M., & Sommer, R. (2021). Application of residue, inorganic fertilizer and lime affect phosphorus solubilizing microorganisms and microbial biomass under different tillage and cropping systems in a Ferralsol. Geoderma, 390, 114962. [CrossRef]
- Cai, A., Xu, M., Wang, B., Zhang, W., Liang, G., Hou, E., & Luo, Y. (2019). Manure acts as a better fertilizer for increasing crop yields than synthetic fertilizer does by improving soil fertility. Soil and Tillage Research, 189, 168-175. [CrossRef]
- Cakmak, I. (2008). Enrichment of cereal grains with zinc: agronomic or genetic biofortification?. Plant and soil, 302(1), 1-17. [CrossRef]
- Cakmak, I., McLaughlin, M. J., & White, P. (2017). Zinc for better crop production and human health. Plant and Soil, 411, 1–4. [CrossRef]
- Caporaso, J. G., Lauber, C. L., Walters, W. A., Berg-Lyons, D., Huntley, J., Fierer, N., Owens, S.M., Betley, J., Fraser, L., Bauer, M., Gormley, N, Gilbert, J.A, Smith, G., & Knight, R. (2012). Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. The ISME journal, 6(8), 1621-1624. [CrossRef]
- Dhaked, B. S., Triveni, S., Reddy, R. S., & Padmaja, G. (2017). Isolation and screening of potassium and zinc solubilizing bacteria from different rhizosphere soil. Int. J. Curr. Microbiol. App. Sci, 6(8), 1271-1281. [CrossRef]
- Fasim, F., Ahmed, N., Parsons, R., & Gadd, G. M. (2002). Solubilization of zinc salts by a bacterium isolated from the air environment of a tannery. FEMS microbiology letters, 213(1), 1-6. [CrossRef]
- Gautam, A., Sekaran, U., Guzman, J., Kovács, P., Hernandez, J. L. G., & Kumar, S. (2020). Responses of soil microbial community structure and enzymatic activities to long-term application of mineral fertilizer and beef manure. Environmental and Sustainability Indicators, 8, 100073. [CrossRef]
- Giller, K. E., Hijbeek, R., Andersson, J. A., & Sumberg, J. (2021). Regenerative Agriculture: An agronomic perspective. Outlook on Agriculture, 50(1), 13-25. [CrossRef]
- Gupta, N., Ram, H., & Kumar, B. (2016). Mechanism of Zinc absorption in plants: uptake, transport, translocation and accumulation. Reviews in Environmental Science and Bio/Technology, 15(1), 89-109. [CrossRef]
- Hacisalihoglu, G. (2020). Zinc (Zn): The last nutrient in the alphabet and shedding light on zn efficiency for the future of crop production under suboptimal zn. Plants, 9(11), 1471. [CrossRef]
- Hammer, Ø., Harper, D. A., & Ryan, P. D. (2001). PAST: Paleontological statistics software package for education and data analysis. Palaeontologia electronica, 4(1), 9.
- Hodge, A., & Fitter, A. H. (2010). Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proceedings of the National Academy of Sciences, 107(31), 13754-13759. [CrossRef]
- Hussain, A., Zahir, Z. A., Asghar, H. N., Ahmad, M., Jamil, M., Naveed, M., & Akhtar, M. F. U. Z. (2018). Zinc solubilizing bacteria for zinc biofortification in cereals: a step toward sustainable nutritional security. In Role of rhizospheric microbes in soil (pp. 203-227). Springer, Singapore. [CrossRef]
- Hussain, A., Zahir, Z.A., Ditta, A., Tahir, M.U., Ahmad, M., Mumtaz, M.Z., Hayat, K. & Hussain, S. (2020). Production and implication of bio-activated organic fertilizer enriched with zinc-solubilizing bacteria to boost up maize (Zea mays L.) production and biofortification under two cropping seasons. Agronomy, 10(1), p.39. [CrossRef]
- Jama, B., Palm, C. A., Buresh, R. J., Niang, A., Gachengo, C., Nziguheba, G., & Amadalo, B. (2000). Tithonia diversifolia as a green manure for soil fertility improvement in western Kenya: a review. Agroforestry systems, 49(2), 201-221. [CrossRef]
- Kalayu, G. (2019). Phosphate solubilizing microorganisms: promising approach as biofertilizers. International Journal of Agronomy, 2019. [CrossRef]
- Kearney, S. G., Carwardine, J., Reside, A.E., Adams, V.M., Nelson, R., Coggan, A., Spindler, R. & Watson, J. E. (2022). Saving species beyond the protected area fence: Threats must be managed across multiple land tenure types to secure Australia’s endangered species. Conservation Science and Practice, e617. [CrossRef]
- Khande, R., Sharma, S. K., Ramesh, A., & Sharma, M. P. (2017). Zinc solubilizing Bacillus strains that modulate growth, yield and zinc biofortification of soybean and wheat. Rhizosphere, 4, 126-138. [CrossRef]
- Khanghahi, M. Y., Ricciuti, P., Allegretta, I., Terzano, R., & Crecchio, C. (2018). Solubilization of insoluble zinc compounds by zinc solubilizing bacteria (ZSB) and optimization of their growth conditions. Environmental Science and Pollution Research, 25(26), 25862-25868. [CrossRef]
- Kihanda, F. M., Warren, G. P. & Micheni, A. N. (2006). Effect of manure application on crop yield and soil chemical properties in a long-term field trial of semi-arid Kenya. Nutrient Cycling in Agroecosystems 76:341–354. [CrossRef]
- Kihara, J. M. (2009). Conservation tillage in Kenya: the biophysical processes affecting its effectiveness. Center for Development Research, PHD (2009), p. 141.
- Kihara, J., Bolo, P., Kinyua, M., Rurinda, J., & Piikki, K. (2020). Micronutrient deficiencies in African soils and the human nutritional nexus: opportunities with staple crops. Environmental geochemistry and health, 1-19. [CrossRef]
- Kihara, J., Martius, C., Bationo, A., Thuita, M., Lesueur, D., Herrmann, L., Amelung, W. & Vlek, P. L. (2012). Soil aggregation and total diversity of bacteria and fungi in various tillage systems of sub-humid and semi-arid Kenya. Applied Soil Ecology, 58, 12-20. [CrossRef]
- Klassen-Wigger, P., Geraets, M., Messier, M. C., Detzel, P., Lenoble, H. P., & Barclay, D. V. (2018). Micronutrient fortification of bouillon cubes in Central and West Africa. In Food Fortification in a Globalized World (pp. 363-372). Academic Press. [CrossRef]
- Koorem, K., Gazol, A., Öpik, M., Moora, M., Saks, Ü., Uibopuu, A., Sõber, V., & Zobel, M. (2014). Soil nutrient content influences the abundance of soil microbes but not plant biomass at the small-scale. PLoS One, 9(3), e91998. [CrossRef]
- Kushwaha, P., Kashyap, P. L., Pandiyan, K., & Bhardwaj, A. K. (2020). Zinc-Solubilizing Microbes for Sustainable Crop Production: Current Understanding, Opportunities, and Challenges. In Phytobiomes: Current Insights and Future Vistas (pp. 281-298). Springer, Singapore. [CrossRef]
- Li, H. Y., Zhu, Y. G., Smith, S. E., & Smith, F. A. (2003). Phosphorus–zinc interactions in two barley cultivars differing in phosphorus and zinc efficiencies. Journal of plant nutrition, 26(5), 1085-1099. [CrossRef]
- Lian, T., Mu, Y., Jin, J., Ma, Q., Cheng, Y., Cai, Z., & Nian, H. (2019). Impact of intercropping on the coupling between soil microbial community structure, activity, and nutrient-use efficiencies. PeerJ, 7, e6412. [CrossRef]
- Lladó S., & Baldrian P (2017) Community-level physiological profiling analyses show potential to identify the copiotrophic bacteria present in soil environments. PLoS One 12:e0171638. [CrossRef]
- Loneragan, J. F., & Webb, M. J. (1993). Interactions between zinc and other nutrients affecting the growth of plants. In Zinc in soils and plants (pp. 119-134). Springer, Dordrecht. [CrossRef]
- Margenot, A. J., Sommer, R., Mukalama, J., & Parikh, S. J. (2017). Biological P cycling is influenced by the form of P fertilizer in an Oxisol. Biology and Fertility of Soils, 53(8), 899-909. [CrossRef]
- Mucheru-Muna, M., Mugendi, D., Pypers, P., Mugwe, J., Kung’u, J., Vanlauwe, B., & Merckx, R. (2014). Enhancing maize productivity and profitability using organic inputs and mineral fertilizer in central Kenya small-hold farms. Experimental Agriculture, 50(2), 250-269. [CrossRef]
- Mugwe, J., Mugendi, D., Mucheru-Muna, M., Odee, D., & Mairura, F. (2009). Effect of selected organic materials and inorganic fertilizer on the soil fertility of a Humic Nitisol in the central highlands of Kenya. Soil Use and Management, 25(4), 434-440. [CrossRef]
- Mumtaz, M. Z., Ahmad, M., Jamil, M., & Hussain, T. (2017). Zinc solubilizing Bacillus spp. potential candidates for biofortification in maize. Microbiological research, 202, 51-60. [CrossRef]
- Niu, H., Pang, Z., Fallah, N., Zhou, Y., Zhang, C., Hu, C., Lin, W. & Yuan, Z. (2021). Diversity of microbial communities and soil nutrients in sugarcane rhizosphere soil under water soluble fertilizer. Plos one, 16(1), e0245626. [CrossRef]
- Orwa, P., Mugambi, G., Wekesa, V., & Mwirichia, R. (2020). Isolation of haloalkaliphilic fungi from Lake Magadi in Kenya. Heliyon, 6(1), e02823. [CrossRef]
- Pasley, H. R., Cairns, J. E., Camberato, J. J., & Vyn, T. J. (2019). Nitrogen fertilizer rate increases plant uptake and soil availability of essential nutrients in continuous maize production in Kenya and Zimbabwe. Nutrient cycling in agroecosystems, 115(3), 373-389. [CrossRef]
- Paul, B. K., Vanlauwe, B., Ayuke, F., Gassner, A., Hoogmoed, M., Hurisso, T.T., Koala, S., Lelei, D., Ndabamenye, T., Six, J. and Pulleman, M.M. (2013). Medium-term impact of tillage and residue management on soil aggregate stability, soil carbon and crop productivity. Agriculture, ecosystems & environment, 164, 14-22. [CrossRef]
- Prasad, R., Shivay, Y. S., & Kumar, D. (2016). Interactions of zinc with other nutrients in soils and plants-A Review. Indian Journal of Fertilisers, 12(5), 16-26.
- R Development Core Team R: A Language and Environment for Statistical Computing R Foundation for Statistical Computing, Vienna (2016).
- Rani, N., Kaur, R., & Kaur, S. (2020). Zinc solubilising bacteria to augment soil fertility–a comprehensive review. Int J AgriSci Vet Med, 8(1), 38-44.
- Rawat, N., Neelam, K., Tiwari, V. K., & Dhaliwal, H. S. (2013). Biofortification of cereals to overcome hidden hunger. Plant Breeding, 132(5), 437-445. [CrossRef]
- Rengel, Z., Batten, G. D., & Crowley, D. D. (1999). Agronomic approaches for improving the micronutrient density in edible portions of field crops. Field crops research, 60(1-2), 27-40. [CrossRef]
- Rezaeiniko, B., Enayatizamir, N., & Norouzi Masir, M. (2019). The Effect of Zinc Solubilizing Bacteria on Zinc Uptake and Some Properties of Wheat in the Greenhouse. JWSS-Isfahan University of Technology, 22(4), 249-260. [CrossRef]
- Sadeghzadeh, B. (2013). A review of zinc nutrition and plant breeding. Journal of soil science and plant nutrition, 13(4), 905-927. [CrossRef]
- Saini, P., Nagpal, S., Saini, P., Kumar, A., & Gani, M. (2021). Microbial Mediated Zinc Solubilization in Legumes for Sustainable Agriculture. Phytomicrobiome Interactions and Sustainable Agriculture, 254-276. [CrossRef]
- Saravanan, V. S., Madhaiyan, M., & Thangaraju, M. (2007). Solubilization of zinc compounds by the diazotrophic, plant growth promoting bacterium Gluconacetobacter diazotrophicus. Chemosphere, 66(9), 1794-1798. [CrossRef]
- Tang, H., Li, C., Xiao, X., Shi, L., Cheng, K., Wen, L., & Li, W. (2020). Effects of short-term manure nitrogen input on soil microbial community structure and diversity in a double-cropping paddy field of southern China. Scientific Reports, 10(1), 1-9. [CrossRef]
- Tomsone, L., Kruma, Z., Alsina, I., & Lepse, L. (2012). The application of hierarchical cluster analysis for clasifying horseradish genotypes (Armoracia rusticana L.) roots. Chemical Technology, 62(4), 52-56. [CrossRef]
- Vidyashree, D. N., Muthuraju, R., & Panneerselvam, P. (2018). Evaluation of zinc solubilizing bacterial (ZSB) strains on growth, yield and quality of tomato (Lycopersicon esculentum). Int. J. Curr. Microbiol. Appl. Sci, 7(04), 2018. [CrossRef]
- Vukicevich, E., Lowery, T., Bowen, P., Úrbez-Torres, J. R., & Hart, M. (2016). Cover crops to increase soil microbial diversity and mitigate decline in perennial agriculture. A review. Agronomy for Sustainable Development, 36(3), 1-14. [CrossRef]
- Welch, R. M. (2002). The impact of mineral nutrients in food crops on global human health. Plant and Soil, 247(1), 83-90. [CrossRef]
- White, P. J., & Broadley, M. R. (2011). Physiological limits to zinc biofortification of edible crops. Frontiers in plant science, 2, 80. [CrossRef]
- Xue, P. P., Carrillo, Y., Pino, V., Minasny, B., & McBratney, A. (2018). Soil properties drive microbial community structure in a large scale transect in South Eastern Australia. Scientific reports, 8(1), 1-11. [CrossRef]
- Zhang, Y. Q., Deng, Y., Chen, R.Y., Cui, Z.L., Chen, X.P., Yost, R., Zhang, F.S. & Zou, C. Q. (2012). The reduction in zinc concentration of wheat grain upon increased phosphorus-fertilization and its mitigation by foliar zinc application. Plant and Soil, 361(1), 143-152. [CrossRef]
- Zhi-Hui, Y. A. N. G., Stöven, K., Haneklaus, S., Singh, B. R., & Schnug, E. (2010). Elemental sulfur oxidation by Thiobacillus spp. and aerobic heterotrophic sulfur-oxidizing bacteria. Pedosphere, 20(1), 71-79. [CrossRef]
Figure 1.
Relative abundance (>0.5%) of zinc solubilizing microbial species in INM3 site in the year 2019.
Figure 1.
Relative abundance (>0.5%) of zinc solubilizing microbial species in INM3 site in the year 2019.
Figure 2.
Bray-Curtis similarity based on hierarchical clustering of zinc solubilizing microbial species based on different soil fertility management systems in INM3 Madeya long-term trial, in the year 2019.
Figure 2.
Bray-Curtis similarity based on hierarchical clustering of zinc solubilizing microbial species based on different soil fertility management systems in INM3 Madeya long-term trial, in the year 2019.
Figure 3.
Dendrogram (a) and non-metric multidimensional scaling plot of Zinc solubilizing microbial species (genus level) distribution in INM3 long-term trial, Western Kenya, in year 2019.
Figure 3.
Dendrogram (a) and non-metric multidimensional scaling plot of Zinc solubilizing microbial species (genus level) distribution in INM3 long-term trial, Western Kenya, in year 2019.
Figure 4.
Proportions of ZSM species under the influence of FYM application (a) and cropping systems (b) in INM3 trial in the year 2019.
Figure 4.
Proportions of ZSM species under the influence of FYM application (a) and cropping systems (b) in INM3 trial in the year 2019.
Figure 5.
Effect of soil fertility management practices on zinc solubilizing microbes (at genus level with relative abundance >0.5%), as defined by principal components analysis (PCA) in INM3- Madeya long-term trial in the year 2019.
Figure 5.
Effect of soil fertility management practices on zinc solubilizing microbes (at genus level with relative abundance >0.5%), as defined by principal components analysis (PCA) in INM3- Madeya long-term trial in the year 2019.
Figure 6.
Zinc solubilizing microbial species (genus level) significantly influenced by management practices in INM3 Madeya long-term trial in the year 2019.
Figure 6.
Zinc solubilizing microbial species (genus level) significantly influenced by management practices in INM3 Madeya long-term trial in the year 2019.
Figure 7.
Proportions of ZSM species under the influence of nitrogen fertilizer application at 0, 60 and 90 kgN ha-1 in INM3 trial in the year 2019.
Figure 7.
Proportions of ZSM species under the influence of nitrogen fertilizer application at 0, 60 and 90 kgN ha-1 in INM3 trial in the year 2019.
Figure 8.
Canonical correspondence analysis of the relationship between soil chemical characteristics and zinc solubilizing microbial species distribution in INM3 site in year 2019.
Figure 8.
Canonical correspondence analysis of the relationship between soil chemical characteristics and zinc solubilizing microbial species distribution in INM3 site in year 2019.
Table 1.
Description of the treatments selected for the study in INM3 Madeya experimental site.
Table 1.
Description of the treatments selected for the study in INM3 Madeya experimental site.
| Code |
Treatment
abbreviation |
Treatment description |
Cropping
system |
FYM |
Res |
N
(Kg ha-1) |
P
(Kg ha-1) |
| Inm1 |
None |
No input |
MT |
- |
- |
0 |
0 |
| Inm2 |
P+N |
NP fertilizer only |
MM |
- |
- |
60 |
45 |
| Inm3 |
R only |
Residue only |
MT |
- |
+ |
0 |
0 |
| Inm4 |
FYM only |
FYM only |
MT |
+ |
- |
0 |
0 |
| Inm5 |
P+N+R |
NP fertilizer + residue only |
MM |
- |
+ |
60 |
45 |
| Inm6 |
FYM+PN+R |
NP fertilizer + residue +FYM |
MM |
+ |
+ |
60 |
45 |
| Inm7 |
FYM+PN |
NP fertilizer + FYM only |
MT |
+ |
- |
60 |
45 |
| Inm8 |
FYM+R |
Residue + FYM only |
MM |
+ |
+ |
0 |
0 |
| Inm9 |
P only |
P fertilizer only |
MM |
- |
- |
0 |
45 |
| Inm10 |
P+FYM |
P + FYM only |
MM |
+ |
- |
0 |
45 |
| Inm11 |
P+N* |
90N+P fertilizer only |
MM |
- |
- |
90 |
45 |
Table 2.
Effects of management systems on soil chemical and physical characteristics in INM3 site in the year 2019.
Table 2.
Effects of management systems on soil chemical and physical characteristics in INM3 site in the year 2019.
| Treatment |
pH |
SOC (%) |
N (%) |
P
(mg kg-1) |
Mg
(mg kg-1) |
Mn
(mg kg-1) |
S
(mg kg-1) |
Cu
(mg kg-1) |
B
(mg kg-1) |
Zn
(mg kg-1) |
Fe
(mg kg-1) |
EC |
CEC |
| Inm1 |
4.84de
|
1.70cd
|
0.13ef
|
18.1e
|
37.5d
|
394ab
|
17.1d
|
5.26c
|
0.13cd
|
1.00c
|
118c
|
31.7b
|
6.54d
|
| Inm2 |
4.51f
|
1.64d
|
0.12f
|
79.0a
|
37.5d
|
431ab
|
22.1bc
|
5.51bc
|
0.17bcd
|
1.30c
|
128bc
|
43.6ab
|
6.84d
|
| Inm3 |
4.88de
|
1.83bcd
|
0.14def
|
13.0e
|
45.3d
|
400ab
|
19.0cd
|
5.62abc
|
0.18abcd
|
1.23c
|
117c
|
32.0b
|
6.64d
|
| Inm4 |
5.16abc
|
2.05a
|
0.17ab
|
23.8de
|
122.3ab
|
396ab
|
19.8cd
|
5.92abc
|
0.23abc
|
2.61ab
|
143b
|
42.0ab
|
11.8ab
|
| Inm5 |
4.66ef
|
1.68cd
|
0.13f
|
71.2ab
|
36.7d
|
441a
|
23.3ab
|
5.65abc
|
0.12cd
|
1.28c
|
133bc
|
44.0ab
|
6.71d
|
| Inm6 |
5.06bcd
|
1.96ab
|
0.16bcd
|
65.6ab
|
138.7a
|
397ab
|
21.1bc
|
6.29ab
|
0.19abcd
|
3.57a
|
169a
|
52.2a
|
12.9a
|
| Inm7 |
4.95cd
|
1.86abc
|
0.15cde
|
55.9bc
|
126.7ab
|
366b
|
23.7ab
|
6.12abc
|
0.17bcd
|
3.29a
|
174a
|
50.4a
|
12.6a
|
| Inm8 |
5.43a
|
2.07a
|
0.18a
|
20.5e
|
152.3a
|
420ab
|
21.8bc
|
6.37a
|
0.29a
|
3.46a
|
126bc
|
42.5ab
|
12.1ab
|
| Inm9 |
4.98cd
|
1.70cd
|
0.13ef
|
56.7bc
|
89.8bc
|
389ab
|
24.0ab
|
5.85abc
|
0.17bcd
|
1.84bc
|
126bc
|
42.7ab
|
10.1bc
|
| Inm10 |
5.32ab
|
1.83bcd
|
0.16bc
|
59.2abc
|
161.5a
|
405ab
|
26.2a
|
6.23ab
|
0.27ab
|
2.58ab
|
139bc
|
47.8a
|
13.0a
|
| Inm11 |
4.54f
|
1.74cd
|
0.14ef
|
42.5cd
|
55.4cd
|
425ab
|
24.3ab
|
5.77abc
|
0.12d
|
1.61bc
|
129bc
|
48.2a
|
8.62cd
|
Table 3.
Correlation between different soil chemical characteristics in INM3 site in the year 2019.
Table 3.
Correlation between different soil chemical characteristics in INM3 site in the year 2019.
| |
P† |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| P† |
1 |
N% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| N (%) |
-.43*
|
1 |
SOC% |
|
|
|
|
|
|
|
|
|
|
|
|
|
| SOC (%) |
-.43*
|
.85**
|
1 |
pH |
|
|
|
|
|
|
|
|
|
|
|
|
| pH (1:2) ₩ |
-.34 |
.76**
|
.70**
|
1 |
K† |
|
|
|
|
|
|
|
|
|
|
|
| K† |
-.40*
|
.76**
|
.72**
|
.80**
|
1 |
Ca† |
|
|
|
|
|
|
|
|
|
|
| Ca† |
-.10 |
.71**
|
.54**
|
.82**
|
.58**
|
1 |
Mg† |
|
|
|
|
|
|
|
|
|
| Mg† |
-.10 |
.70**
|
.73**
|
.85**
|
.70**
|
.87**
|
1 |
Mn† |
|
|
|
|
|
|
|
|
| Mn† |
.07 |
.15 |
.07 |
-.02 |
-.11 |
-.04 |
-.07 |
1 |
S† |
|
|
|
|
|
|
|
| S† |
.45*
|
-.02 |
-.18 |
-.01 |
-.16 |
.32 |
.25 |
-.11 |
1 |
Cu† |
|
|
|
|
|
|
| Cu† |
-.07 |
.44*
|
.54**
|
.49**
|
.46**
|
.56**
|
.65**
|
.01 |
.26 |
1 |
B† |
|
|
|
|
|
| B† |
-.30 |
.73**
|
.60**
|
.72**
|
.54**
|
.69**
|
.63**
|
.23 |
.11 |
.43*
|
1 |
Zn† |
|
|
|
|
| Zn† |
-.02 |
.73**
|
.70**
|
.65**
|
.62**
|
.67**
|
.84**
|
-.04 |
.23 |
.54**
|
.42*
|
1 |
Fe† |
|
|
|
| Fe† |
.36*
|
.19 |
.30 |
.14 |
.21 |
.29 |
.41*
|
-.14 |
.10 |
.28 |
-.09 |
.56**
|
1 |
Na† |
|
|
| Na† |
.13 |
-.01 |
.24 |
.21 |
-.05 |
.38*
|
.41*
|
-.04 |
.18 |
.38*
|
.08 |
.18 |
.26 |
1 |
EC |
|
| EC |
.43*
|
.18 |
-.05 |
-.10 |
-.02 |
.23 |
.19 |
-.22 |
.47**
|
.28 |
-.04 |
.40*
|
.47**
|
.09 |
1 |
C.E.C |
| C.E.C |
.06 |
.67**
|
.60**
|
.67**
|
.45**
|
.85**
|
.82**
|
.08 |
.28 |
.60**
|
.53**
|
.74**
|
.55**
|
.45*
|
.41*
|
1 |
Table 4.
Zinc solubilizing microbial species richness and diversity and soil chemical parameters under different agronomic inputs in 2019.
Table 4.
Zinc solubilizing microbial species richness and diversity and soil chemical parameters under different agronomic inputs in 2019.
| Inputs |
Diversity
(Shannon) |
Richness |
pH |
SOC
(%) |
N
(%) |
Olsen P
(mg kg-1) |
K
(mg kg-1) |
Zn
(mg kg-1) |
Fe
(mg kg-1) |
Nitrogen (kg ha-1)
0 |
2.33 ± 0.15a
|
21.2 ± 1.44a
|
5.10 ± 0.26a
|
1.85 ± 0.17a
|
0.15 ± 0.02a
|
31.9 ± 22.0b |
343±123a
|
2.12 ± 1.02a
|
128 ± 15.5a
|
| 60 |
2.27 ± 0.19a
|
21.3 ± 2.10a
|
4.80 ± 0.27a
|
1.79 ± 0.18a
|
0.14 ± 0.02a
|
66.6 ± 15.8a |
251±94a
|
2.36 ± 1.27a
|
151 ± 25.2a
|
| 90 |
2.32 ± 0.07a
|
21.0 ± 1.00a
|
4.54 ± 0.13a
|
1.75 ± 0.10a
|
0.14 ± 0.01a
|
42.5 ± 16.7ab |
162±39a
|
1.61 ± 0.50a
|
129 ± 7.8a
|
Phosphorus (kg ha-1)
0 |
2.30 ± 0.16a
|
21.1 ± 1.24a
|
5.08 ± 0.26a
|
1.90 ± 0.19a
|
0.16 ± 0.02a
|
18.9 ± 5.65b |
381±124a
|
2.07 ± 1.18a
|
126 ± 14.6a
|
| 45 |
2.31 ± 0.16a
|
21.3 ± 1.85a
|
4.86 ± 0.32a
|
1.77 ± 0.15a
|
0.14 ± 0.02a
|
60.7 ± 18.0a |
243±89.3a
|
2.21 ± 1.04a
|
143± 23.1a
|
FYM ( t ha-1)
0 |
2.25 ± 0.15b |
20.44 ± 1.42b |
4.74 ± 0.21b |
1.72 ± 0.11b |
0.13 ± 0.01b |
46.8 ± 27.0a
|
212±60.5b
|
1.38 ± 0.45b |
125 ± 9.8b |
| 4 |
2.38 ± 0.15a |
22.2 ± 1.37a |
5.19 ± 0.24a |
1.94 ± 0.15a |
0.16 ± 0.02a |
43.9 ± 23.5a
|
390±104a
|
3.10 ± 0.82a |
150 ± 24.3a |
Residue (t ha-1)
0 |
2.35 ± 0.12a
|
21.2 ± 1.61a
|
4.9 ± 0.32a
|
1.79 ± 0.15a
|
0.14 ± 0.02a
|
47.1 ± 23.1a
|
257±103a
|
2.03 ± 0.84a
|
137 ± 21.1a
|
| 2 |
2.23 ± 0.20a
|
21.3 ± 1.76a
|
5.01 ± 0.31a
|
1.87 ± 0.20a
|
0.15 ± 0.02a
|
42.6 ± 29.1a
|
357±130a
|
2.38 ± 1.42a
|
136 ± 23.8a
|
Table 5.
Relationship between ZSM microbial diversity indices and soil chemical parameters in INM3 study site in 2019.
Table 5.
Relationship between ZSM microbial diversity indices and soil chemical parameters in INM3 study site in 2019.
| Microbial Indices |
Olsen P
(mg kg-1) |
N
(%) |
SOC
(%) |
pH |
K
(mg kg-1) |
Ca
(mg kg-1) |
Mg
(mg kg-1) |
Zn
(mg kg-1) |
Fe
(mg kg-1) |
C.E.C
(meq 100g-1) |
| Diversity (Shannon) |
-.137 |
.286 |
.167 |
.375* |
.312 |
.498** |
.480** |
.468** |
.283 |
.350* |
| Species richness |
.105 |
.252 |
.390* |
.272 |
.300 |
.382* |
.422* |
.348* |
.642** |
.514** |
Table 6.
Relationship between soil chemical characteristics and ZSMs abundance (counts) in INM3 Madeya in year 2019.
Table 6.
Relationship between soil chemical characteristics and ZSMs abundance (counts) in INM3 Madeya in year 2019.
| Variables |
Burkholderia spp |
Bacillus spp |
Trichoderma spp |
Rhizobium spp |
Sphingomonas spp |
Bradyrhizobium spp |
Glomus spp |
Aspergillus spp |
Arthrobacter spp |
Thiobacillus spp |
Pseudomonas spp |
Azospirillum spp |
Lysinibacillus spp |
Mesorhizobium spp |
Paenibacillus spp |
Pantoea spp |
Xanthomonas spp |
Staphylococcus spp |
Mucor spp |
Serratia spp |
Agrobacterium spp |
Micrococcus spp |
Sporosarcina spp |
| P (ppm) |
0.01 |
0.17 |
.37* |
0.24 |
0.02 |
-0.06 |
-0.23 |
0.26 |
-0.33 |
-0.19 |
-0.24 |
0.08 |
0.09 |
-0.24 |
0.19 |
0.08 |
-0.33 |
0.25 |
0.01 |
0.25 |
-0.08 |
-0.02 |
0.10 |
| N (%) |
.41* |
.37* |
-.35* |
-0.21 |
.41* |
.47** |
-0.30 |
-0.19 |
.64** |
.77** |
.57** |
.52** |
.49** |
.78** |
0.17 |
-0.02 |
.46** |
0.12 |
-0.30 |
-0.17 |
.35* |
0.16 |
0.07 |
| SOC (%) |
.38* |
.37* |
-0.31 |
-.44* |
.60** |
.57** |
-0.18 |
-0.12 |
.47** |
.74** |
.54** |
.45* |
.52** |
.71** |
0.05 |
-0.03 |
0.21 |
0.18 |
-0.25 |
-0.12 |
.37* |
0.30 |
0.20 |
| pH |
0.11 |
0.30 |
-.53** |
-.35* |
0.24 |
.48** |
-0.04 |
-0.15 |
.65** |
.65** |
.60** |
.51** |
.47** |
.65** |
0.06 |
0.03 |
.42* |
0.14 |
-.42* |
-0.09 |
.37* |
0.19 |
-0.08 |
| K (ppm) |
0.17 |
0.33 |
-.48** |
-0.30 |
0.23 |
.44* |
-0.08 |
-0.27 |
.46** |
.57** |
.55** |
.49** |
.54** |
.69** |
-0.09 |
0.09 |
0.22 |
-0.01 |
-0.29 |
-0.24 |
.48** |
0.22 |
-0.04 |
| Ca (ppm) |
0.20 |
.52** |
-.42* |
-0.33 |
0.24 |
.39* |
-0.27 |
-0.14 |
.60** |
.71** |
.66** |
.57** |
.61** |
.58** |
0.29 |
0.12 |
.50** |
0.18 |
-.45** |
0.07 |
0.32 |
0.16 |
0.19 |
| Mg (ppm) |
0.24 |
.50** |
-.45** |
-.37* |
.41* |
.55** |
-0.30 |
-0.16 |
.42* |
.71** |
.60** |
.53** |
.66** |
.60** |
0.14 |
0.11 |
0.30 |
0.21 |
-.41* |
0.06 |
0.28 |
0.23 |
0.19 |
| Mn (ppm) |
.34* |
-0.19 |
.40* |
.62** |
0.18 |
-0.10 |
-0.22 |
.49** |
0.17 |
-0.03 |
0.06 |
0.23 |
-0.34 |
0.22 |
-0.07 |
-0.13 |
-0.11 |
0.26 |
0.28 |
-0.21 |
-0.11 |
0.21 |
-.36* |
| S (ppm) |
-0.04 |
0.30 |
-0.12 |
0.07 |
-0.12 |
-0.08 |
-.40* |
-0.05 |
-0.08 |
0.05 |
0.04 |
0.06 |
0.17 |
-0.19 |
.40* |
0.03 |
0.05 |
0.08 |
-0.26 |
.37* |
-0.09 |
-0.15 |
0.06 |
| Cu (ppm) |
0.09 |
.37* |
-0.30 |
-0.09 |
.41* |
0.21 |
-0.21 |
-0.01 |
0.26 |
.45** |
.51** |
0.31 |
.45** |
0.30 |
0.23 |
-0.02 |
0.05 |
0.16 |
-0.15 |
-0.09 |
0.18 |
.46** |
0.21 |
| B (ppm) |
0.15 |
0.28 |
-0.26 |
-0.31 |
0.11 |
0.14 |
-0.2 |
-0.10 |
.62** |
.47** |
.47** |
.43* |
0.26 |
.60** |
0.27 |
-0.12 |
.38* |
0.14 |
-0.31 |
-0.12 |
0.14 |
0.11 |
-0.03 |
| Zn (ppm) |
0.29 |
.48** |
-0.32 |
-0.20 |
.52** |
.59** |
-.43* |
-0.21 |
0.28 |
.71** |
.48** |
.46** |
.65** |
.59** |
0.11 |
0.21 |
0.30 |
0.13 |
-0.34 |
0.11 |
0.21 |
0.14 |
0.23 |
| Fe (ppm) |
0.29 |
.37* |
-0.05 |
-0.16 |
.61** |
.55** |
-0.21 |
-0.01 |
-0.08 |
.52** |
0.33 |
0.25 |
.54** |
0.33 |
0.19 |
.50** |
0.05 |
0.14 |
-0.26 |
0.02 |
0.34 |
0.10 |
.36* |
| Na (ppm) |
0.25 |
-0.02 |
-0.23 |
-0.25 |
.45** |
0.27 |
-0.10 |
0.11 |
0.02 |
.37* |
0.33 |
0.01 |
0.10 |
0.04 |
0.05 |
0.09 |
-0.03 |
0.05 |
-0.30 |
0.16 |
0.11 |
0.15 |
0.22 |
| EC |
0.21 |
0.32 |
0.10 |
0.01 |
0.23 |
0.03 |
-0.33 |
0.01 |
-0.02 |
0.23 |
-0.05 |
-0.03 |
.36* |
0.02 |
.48** |
-0.08 |
0.14 |
0.03 |
-0.02 |
0.21 |
0.07 |
-0.05 |
.41* |
| C.E.C |
.41* |
.51** |
-0.18 |
-0.19 |
.54** |
.51** |
-.42* |
0.18 |
.50** |
.82** |
.64** |
.57** |
.63** |
.62** |
0.30 |
0.15 |
.37* |
.37* |
-0.34 |
0.01 |
0.25 |
0.23 |
0.32 |
Table 7.
Bi-plot CCA analysis scores for constraining zinc solubilizing microbial species and soil chemical properties in INM3 site in year 2019.
Table 7.
Bi-plot CCA analysis scores for constraining zinc solubilizing microbial species and soil chemical properties in INM3 site in year 2019.
| |
CCA1 |
CCA2 |
R2 |
P-value |
Zinc solubilizing microbial genera
Agrobacterium
|
0.91 |
0.42 |
0.27 |
0.02 |
| Arthrobacter |
0.64 |
0.76 |
0.66 |
0.001 |
| Aspergillus |
0.98 |
-0.21 |
0.51 |
0.001 |
| Azospirillum |
0.96 |
0.26 |
0.39 |
0.001 |
| Bacillus |
1.00 |
0.03 |
0.57 |
0.001 |
| Bradyrhizobium |
0.90 |
-0.43 |
0.42 |
0.001 |
| Burkholderia |
1.00 |
-0.01 |
0.03 |
0.67 |
| Enterobacter |
1.00 |
0.10 |
0.02 |
0.73 |
| Glomus |
0.23 |
-0.97 |
0.19 |
0.05 |
| Gluconacetobacter |
0.62 |
0.79 |
0.02 |
0.71 |
| Klebsiella |
0.99 |
0.12 |
0.02 |
0.68 |
| Lysinibacillus |
1.00 |
-0.05 |
0.76 |
0.001 |
| Mesorhizobium |
0.92 |
0.40 |
0.33 |
0.01 |
| Paenibacillus |
0.81 |
0.58 |
0.07 |
0.38 |
| Pantoea |
0.33 |
-0.94 |
0.69 |
0.004 |
| Penicillium |
0.24 |
0.97 |
0.84 |
0.001 |
| Pseudomonas |
0.98 |
-0.22 |
0.32 |
0.03 |
| Rhizobium |
-1.00 |
-0.04 |
0.38 |
0.002 |
| Serratia |
-0.09 |
-1.00 |
0.02 |
0.64 |
| Sphingomonas |
0.75 |
-0.66 |
0.26 |
0.03 |
| Staphylococcus |
0.67 |
0.74 |
0.06 |
0.47 |
| Stenotrophomonas |
-0.31 |
0.95 |
0.01 |
0.84 |
| Thiobacillus |
1.00 |
0.04 |
0.59 |
0.001 |
| Trichoderma |
1.00 |
-0.01 |
0.67 |
0.001 |
| Xanthomonas |
0.71 |
0.70 |
0.22 |
0.05 |
Soil chemical properties
Olsen P |
-0.11 |
-0.41 |
|
0.04 |
| N |
0.71 |
0.45 |
|
0.001 |
| Zn |
0.76 |
-0.17 |
|
0.02 |
| pH |
0.77 |
0.44 |
|
0.13 |
| SOC |
0.38 |
-0.06 |
|
0.05 |
| S |
0.4 |
0.03 |
|
0.22 |
| B |
0.55 |
0.62 |
|
0.69 |
| Fe |
0.57 |
-0.76 |
|
0.03 |
| Cu |
0.31 |
0.09 |
|
0.56 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).