1. Introduction
The pyrolysis and gasification processes are thermal treatment methods that take place in successive stages from drying to subsequent devolatilization, gasification of the coke, and finishing with partial oxidation. There is no clear distinction between these phases from the studies carried out so far, so they can be run simultaneously over specific temperature ranges in real processes. The process of devolatilization shall be carried out in the absence of oxygen in the temperature range 350 to 850 °C. Depending on the heating rate, the stationary time in the chemical reaction area, and the feed content, the resulting gasification products can be classified as follows: (a) solids and mainly coke; (b) liquids consisting of heavy hydrocarbons, water, different types of oils and tar; And c) gaseous components such as H
2, CO, CO
2, C
xH
y, H
2O, etc. After that, secondary reactions may occur in which the resulting volatiles participate in the formation of the various products [
1,
2,
3,
4,
5].
After the pyrolysis process, the gasification process takes place. Using a gasification agent (steam, air, or oxygen) allows the conversion of larger molecules into stable gases such as CO, CO
2, C
xHy, H
2, water, tar, and ash [
6,
7]. The gasification process takes place at higher temperatures, often exceeding 1200 °C. Following the two pyro-gasification processes, the resulting product is a synthetic gas with a temperature of not more than 1000 °C and a composition based on gases such as CO, H
2, CO
2, C
xH
y, and other gases inert gases generated according to the gasification agent used [
8]. Synthesis gas can be utilised in different types of energy installations, such as internal combustion engines or thermal engines, of which gas turbines are most known for electricity generation [
9,
10]. In addition, several processes can be integrated to improve the quality of synthesis gas, such as a water gas shift reactor (WGS) used for the conversion of carbon monoxide into hydrogen or carbon dioxide capture processes [
11].
The mixture's compound has a crucial contribution in determining the composition of the synthesis gas and determining the optimal process parameters. The gasification agent also plays a significant role in setting operational parameters. In this study, the gasification agent used was air, in which case the optimal ER (ratio equivalent) ratio was established.
Given the sharp increase in plastic waste resulting from anthropogenic activities, sustainable reduction has become a global goal. This waste can be recovered by its gasification for producing a synthesis gas with improved properties (e.g. H
2/CO ratio) [
12]. The present study analysed the mix of plastic (PP) and wood biomass poplar (P) in various proportions. The syngas was used as fuel in a gas turbine with a power installed of 5 MW for electricity production.
2. Methods
2.1. Gasification process description
The gasification process has the role of transforming different solid components from the feed-in gaseous compounds by using a gasification agent; in this case, the air was used as a gasification agent. The equations presented below describe the stages that occur during the gasification process [
9].
In the current study, a mix between plastic – PP and wood – poplar was used. The feedstock composition, in dry basis (db), for each case is presented in
Table 1 [
13,
14]. The lower heating value (LHV) in kJ/kg was given by equation 9.
The cases studied are the following:
Case 1. P-PP mix gasification without CO2 capture process;
Case 2. P-PP mix gasification with pre-combustion CO2 capture process;
Case 3. P-PP mix gasification with post-combustion CO2 capture process.
For all three cases, the a-f cases are considered, in which the poplar and plastic content varies in the feedstock mix: a. P 95% + PP 5%; b. 90% + PP 10%; c. P 85% + PP 15%; d. P 80% + PP 20%; e. P 75% + PP 25%; f. P 70% + PP 30%.
All described processes were simulated in the ChemCAD software to determine the energy and mass balances and technical effects of CO2 capture process integration as well.
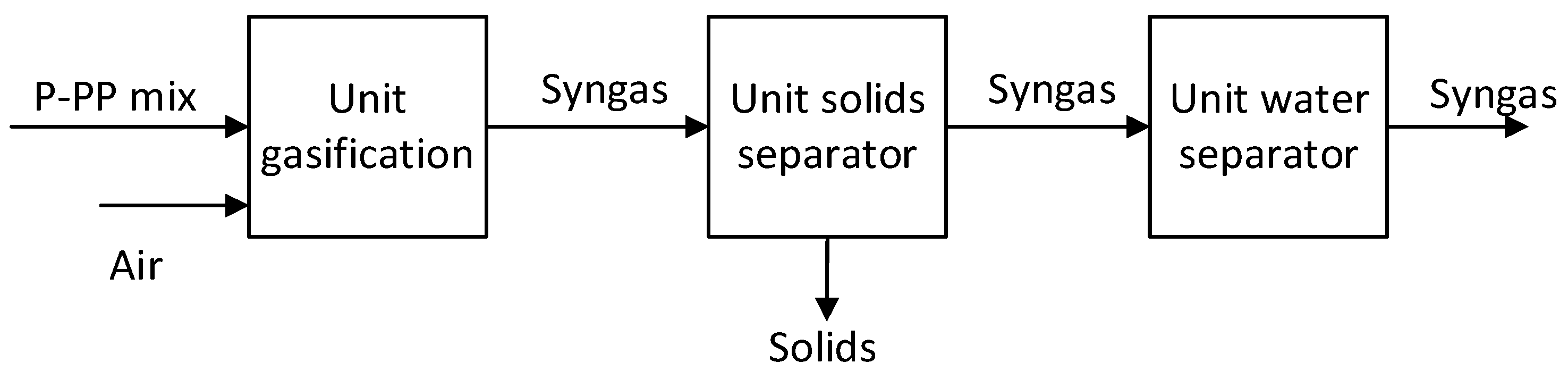
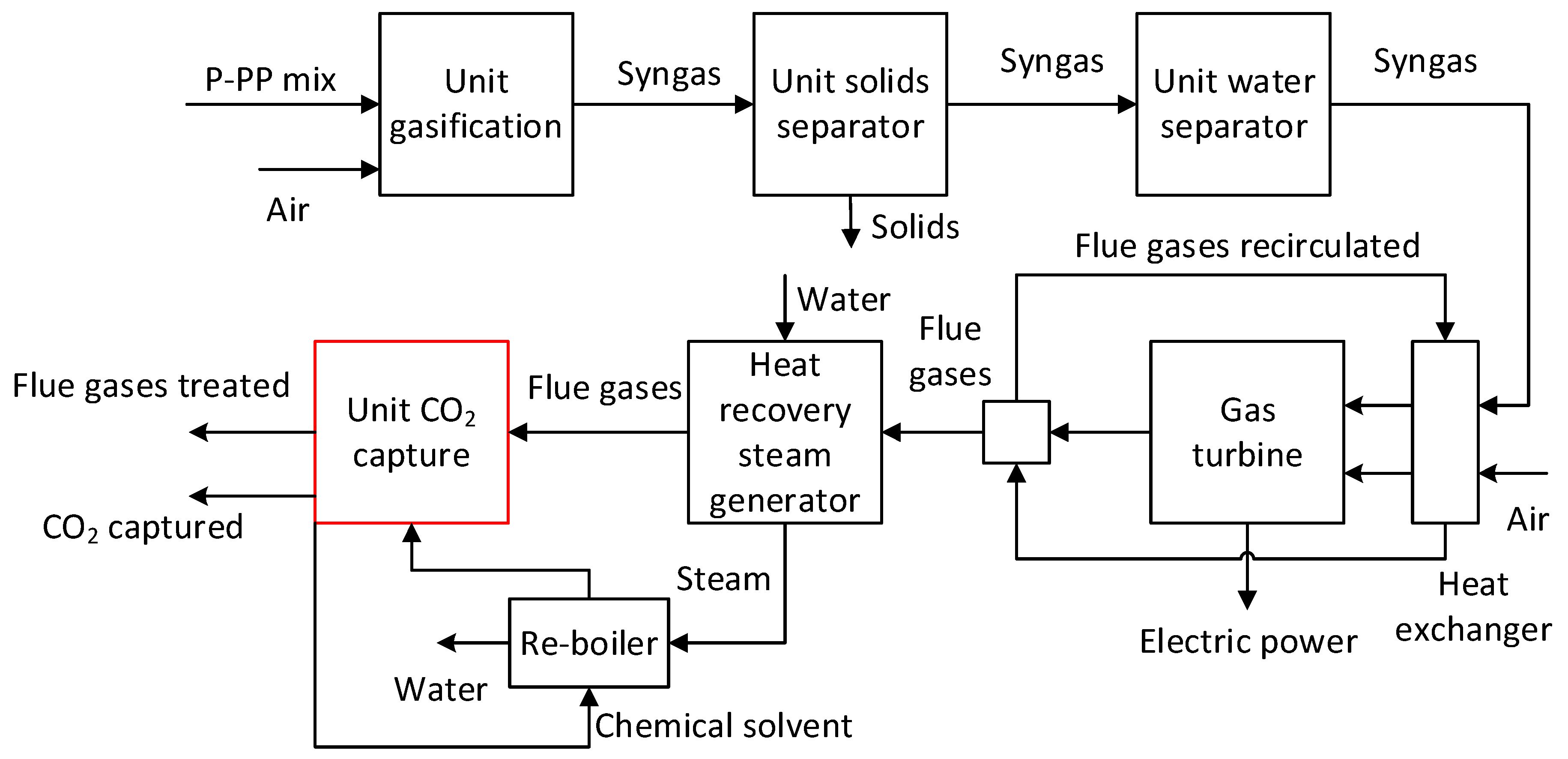
The composition of the synthetic gas was determined after different stages: after gasification unit, after solid separator unit, and after water separator facility. The illustrative chart of the gasification process is presented in
Figure 1, and the essential information of the process simulation are posted in
Table 2.
For establishing the optimum ER (equation 10), the cold gas efficiency (CGE) was calculated based on equation 11.
and
represent the syngas and feedstock flow, in kg/h, while
and
represent the LHV, in kJ/kg for syngas and feedstock.
where the
and
represent the real and stoichiometric air flow, in kg/h.
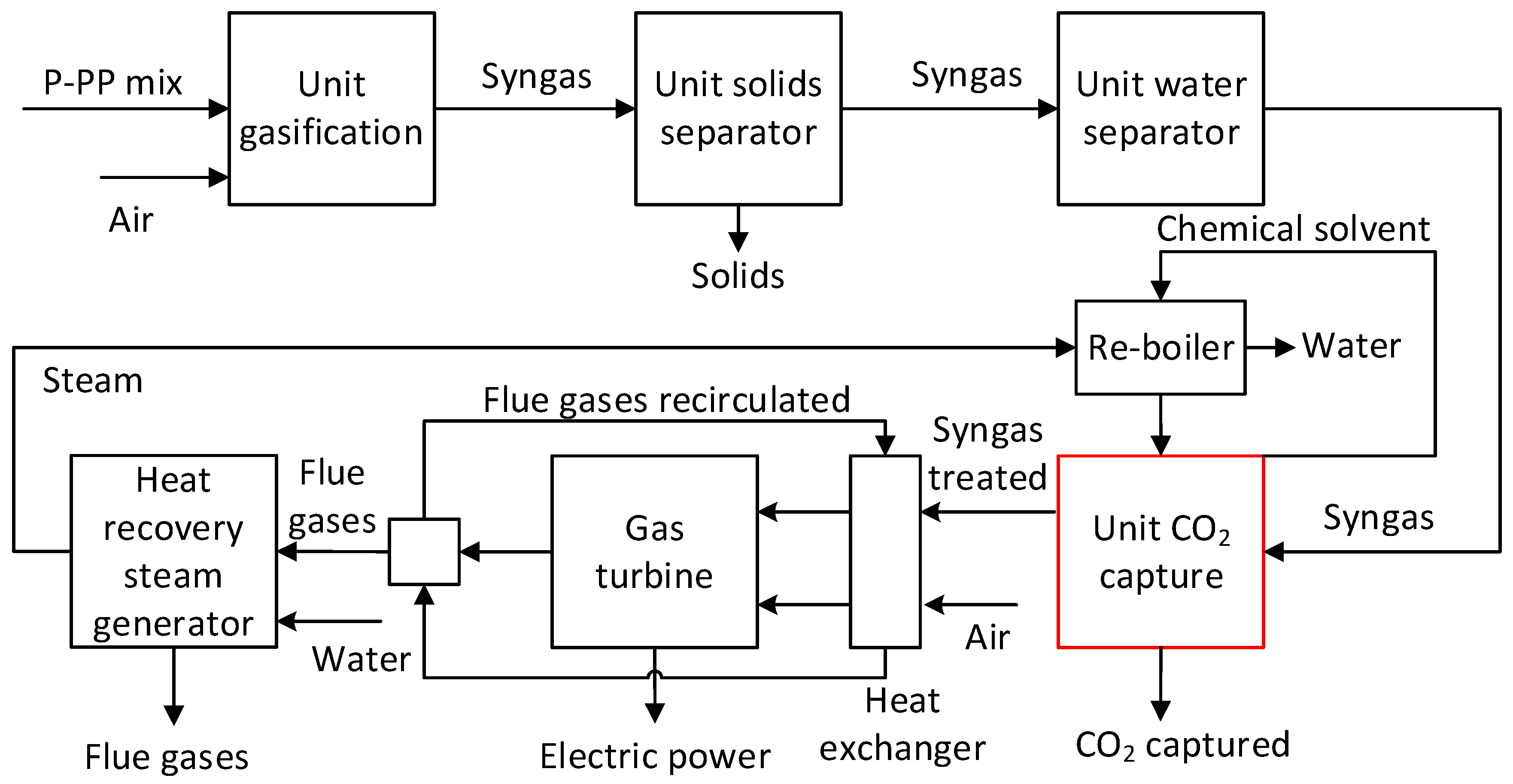
2.2. Syngas decarbonization
To improve the quality of the synthetic gas, the CAP was integrated pre-, and post-combustion. In the pre-combustion variant, the CAP for removing the CO
2 was integrated after the gasification process and the water separation unit. In the post-combustion variant, the CO
2 capture process was integrated after the syngas combustion. The results were obtained in the ChemCAD tool using the Peng Robinson thermodynamic model [
15,
16].
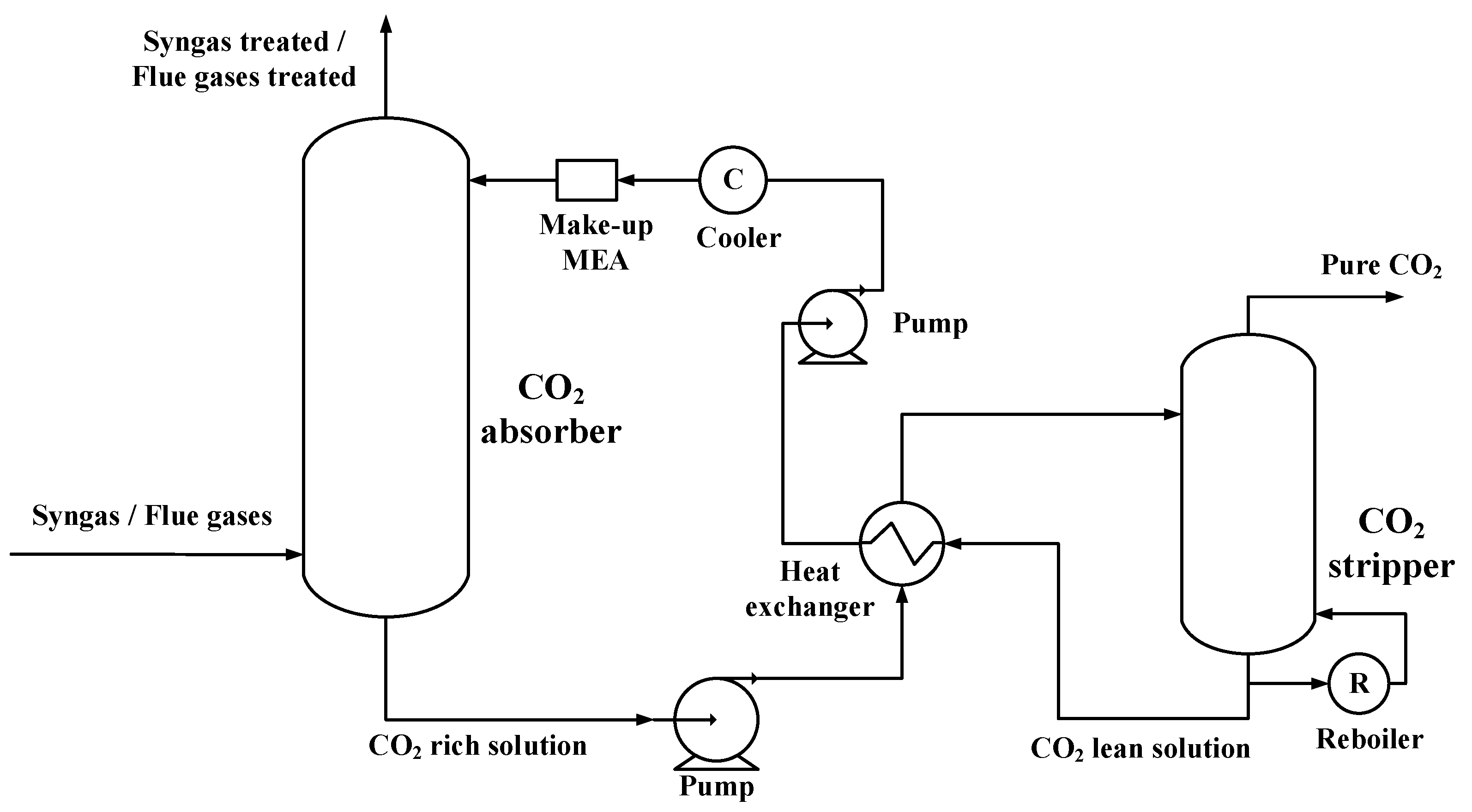
Figure 2 presents the chemical absorption process that could be used both in pre-, or post-combustion cases [
17,
18]. Ethanolamine (MEA) in a weight content of 30 % was used as an alkanolamines solution for CO
2 removal. The efficiency of CO
2 removal was considered 90 %, and the lean loading solvent (γ
lean) was considered 0.21 kmol
CO2/kmol
MEA for all cases analysed [
19,
20].
The CO
2 capture process is characterised by the L/G ratio, which is the ratio of the flow rate of chemical solvent used to the flow rate of the syngas/flue gases [
20,
21]. It is also characterized by the amount of specific heat energy required in the chemical solvent regeneration process [
22], which is calculated with the following equation:
where:
- represents the heat requested in the regeneration process, in MJ/h;
- represents the CO
2 flow captured, in kg/h.
In this study, the heat requested in the regeneration process was obtained by cooling the flue gases at the outlet of the gas turbine from 540 °C to 120°C. The available heat flow recovered from flue gases to obtain a steam (p = 5 bar and T = 424.25 K) was 9 209 MJ/h (
Figure 4 and
Figure 5). However, the net heat flow used directly in the solvent regeneration was lower than the heat flow available, 434 – 776 MJ/h for pre-combustion and 5 318 – 6 102 MJ/h post-combustion case. Therefore, the heat available after the solvent regeneration was used for air and syngas heating before the combustion chamber.
2.3. Syngas conversion in electricity
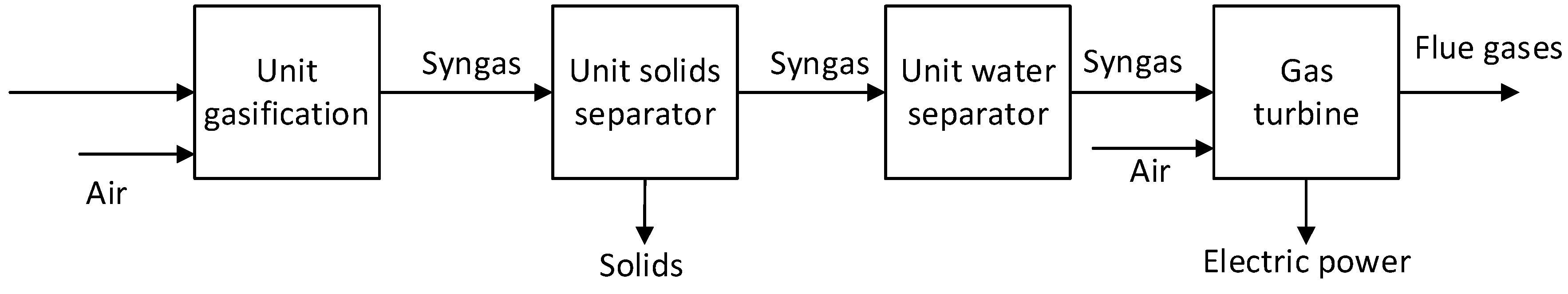
A gas turbine type SGT-100, which can be used in simple or combined cycles, with a power of 5 MW, was used to valorize the produced syngas. Its characteristics are shown in
Table 3 [
23]. Schematic diagrams for the three cases analysed are shown in
Figure 3,
Figure 4 and
Figure 5. The net plant efficiency was determined in 2 variants: a) including the gas turbine only, and considering as input the chemical heat from syngas (
), equation 13; and b) for the whole process including the gasification process, and considering as input the chemical heat of mix feedstock flow (
), equation 14. The CO
2 emission factor was determined with equation 15.
where:
- represents the gas turbine power, in MW;
- represents the compressor power, in MW;
- represents the required heat recovered from the flue gas, in MW.
where:
- represents the CO
2 amount generated, in kg/year;
- represents the electricity produced, in MWh/year.
3. Results and discussion
3.1. Influence of ER ratio on the gasification process
The quantity of air injected into the gasifier unit significantly impacts the reaction products. Thus, the influence of the ER ratio on the syngas content produced, the LHV of the syngas, and the CGE was analysed for all mix cases considered.
In
Table 4, the syngas composition is shown by gasification, solid separation, and water separation units for Case 1a.
The H
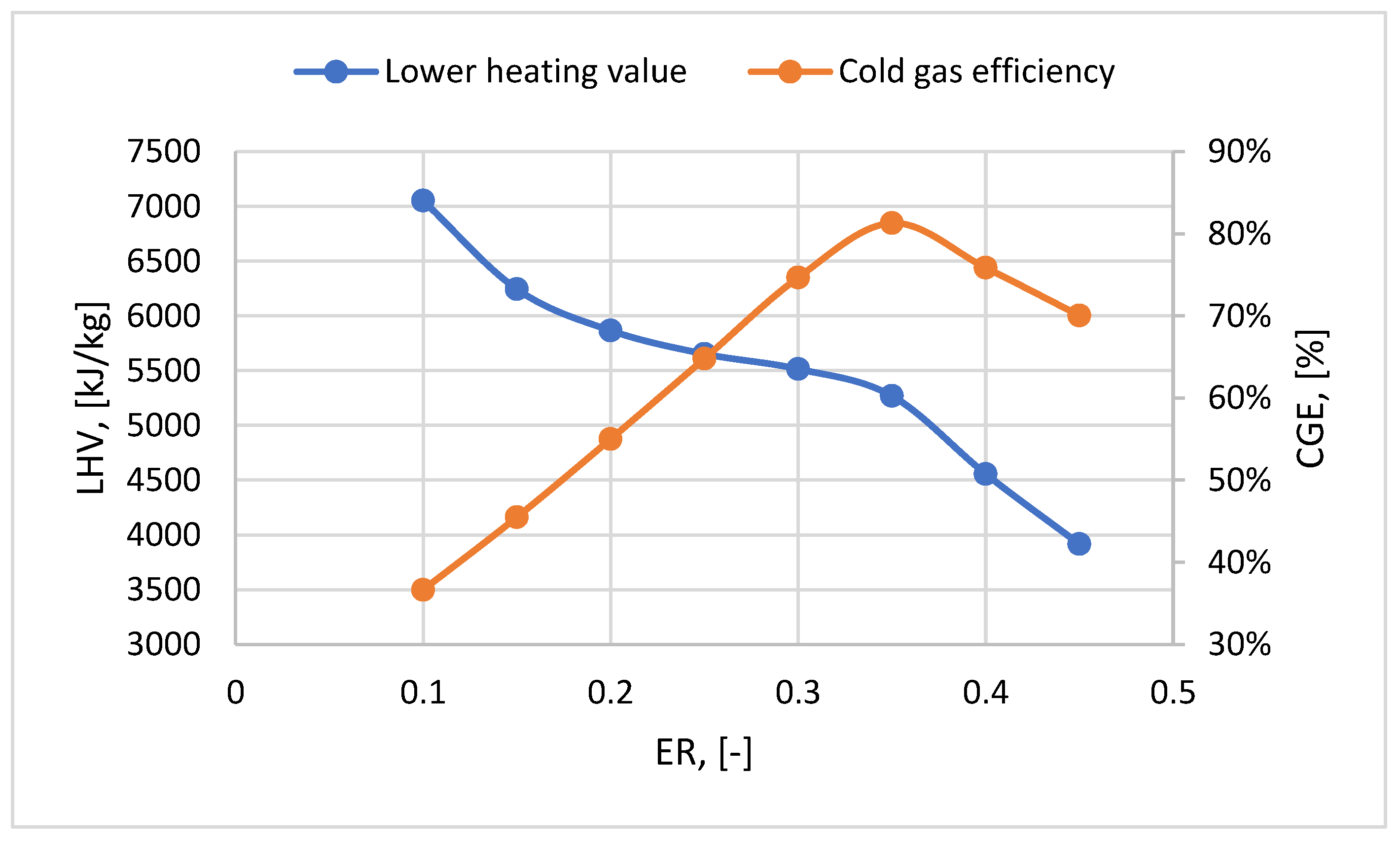
2 and CO concentrations in the syngas increased up to an ER value of 0.35 and decreased tendency after a higher ER ratio. Consequently, the efficiency of the syngas has the best value for this ER ratio after the gasification process, even though the LHV of the syngas decreases with ER ratio increases (
Figure 6).
Considering that for the other mix cases analysed (Case 1b-f) the same interpretations of the results were obtained for the syngas composition; they are presented in
Table 4 only for Case 1a.
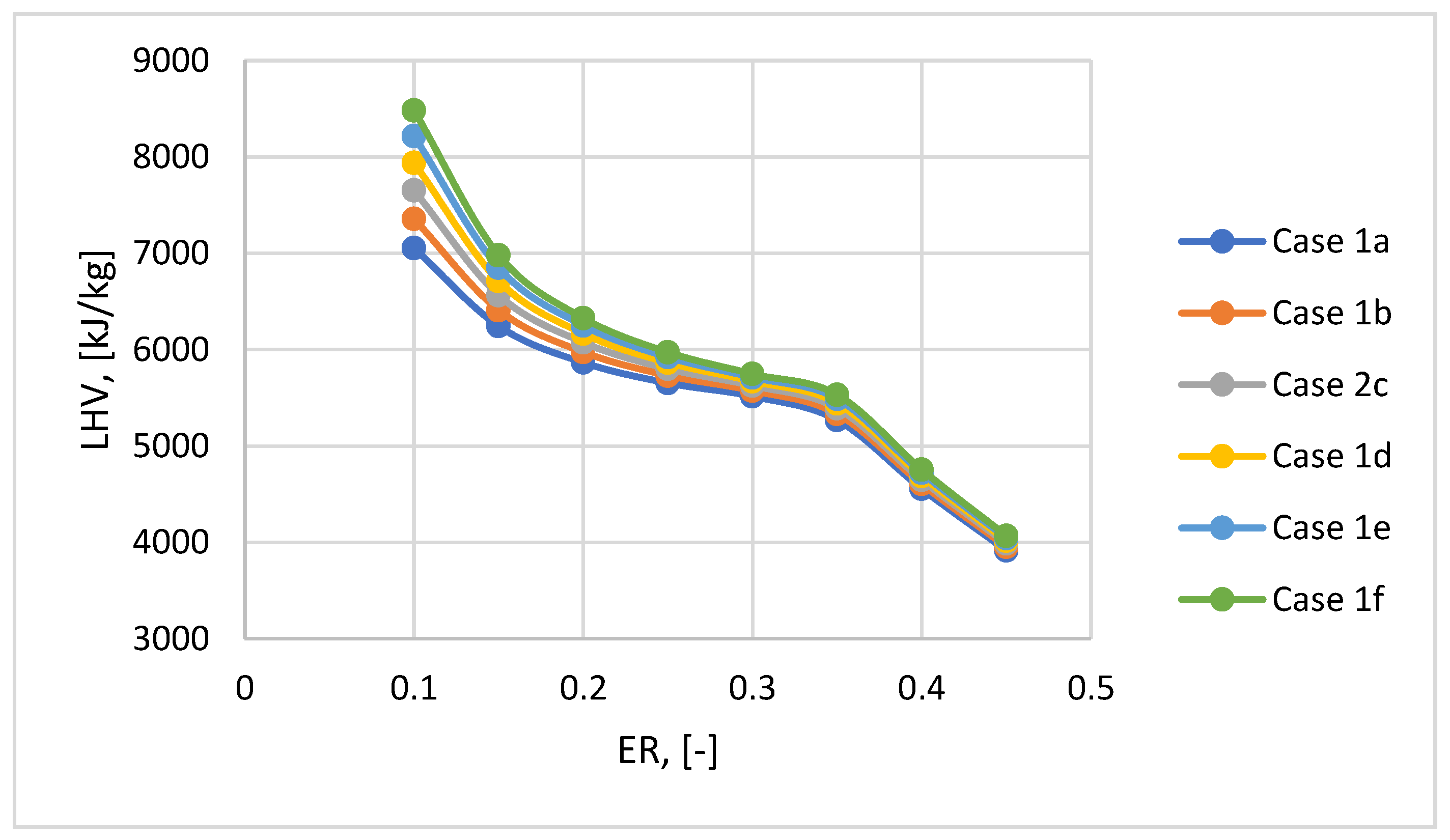
The LHV ranges from 3 900-8 500 kJ/kg depending on the ER ratio and the raw material mix used in the gasification process. It is observed that (
Figure 7) LHV decreases with the introduction of more air into the gasification reactor due to the lower concentration of H
2 in the syngas produced, even if the CO concentration is increasing. Increasing the content of plastic in the mix has a positive impact on the LHV value (with a percentage increase between 1-5%), obtaining the highest value at a plastic content in the mix of 30% regardless of the ER ratio.
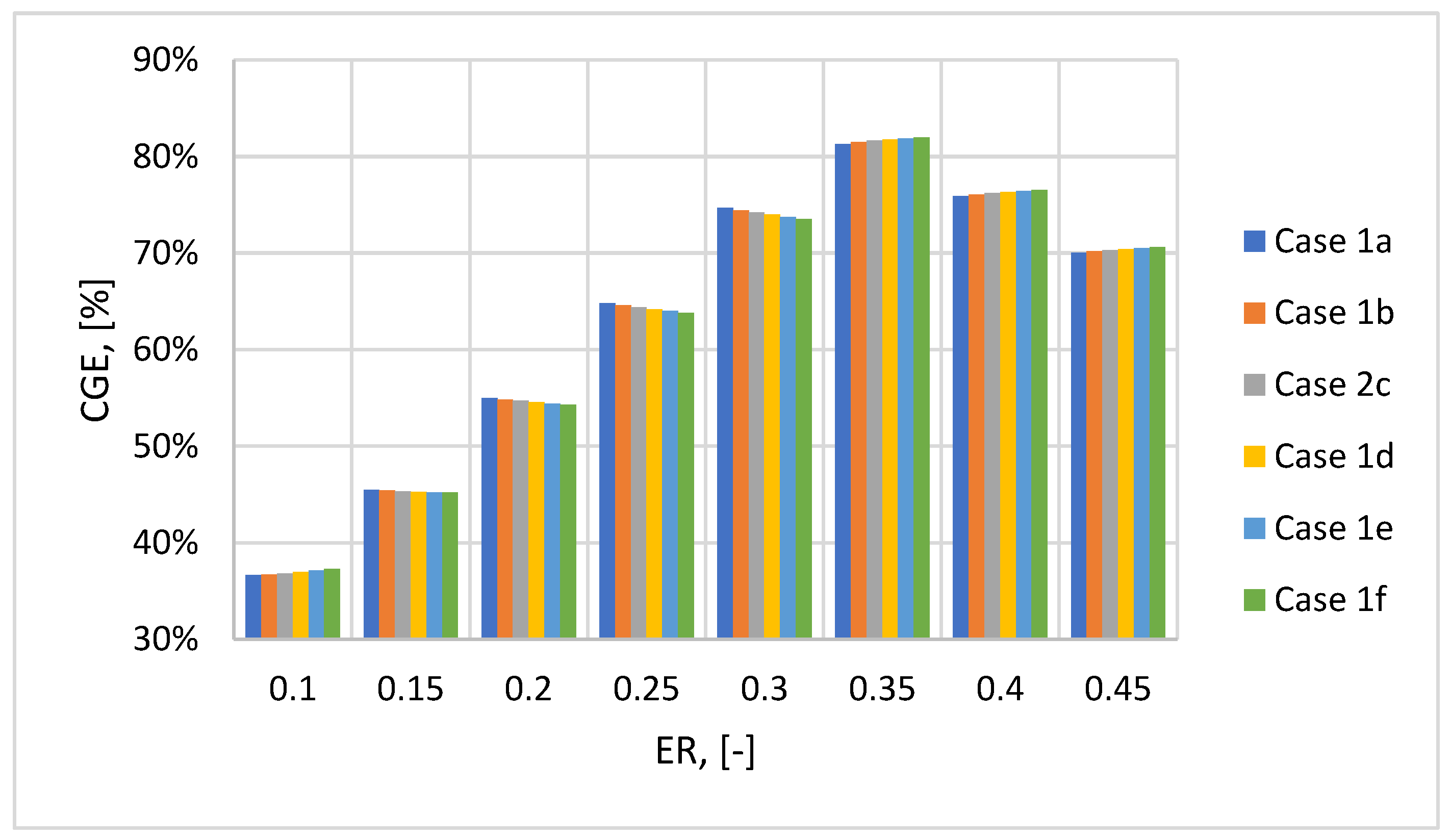
Figure 8 shows the CGE variation. The best results are obtained at the ER ratio of 0.35. For this ratio, the CGE is between 81.3-82%. The CGE value starts to decrease after an ER ratio of 0.35 due to the more drastic decrease of LHV (LHV at an ER ratio of 0.4 is 14% lower than LHV at an ER ratio of 0.35), even though the flow rate of syngas generated is higher. Case 1f showed the best values in terms of CGE, as in the LHV case.
H
2/CO is the ratio of the number of moles of hydrogen to the number of moles of carbon monoxide in the syngas, with values up to 0.73 for Case 1f. It means that the concentration value for H
2 is the highest in this case, and the value for CO is the lowest. On the other hand, the CO
2 concentration decreases as the concentration of plastic increases in the mixture. Considering that the CGE was obtained at an ER ratio of 0.35, the optimal ER ratio is considered in the following analyses.
Table 5 shows the results obtained after the gasification process for all the mix cases analysed for this ER ratio.
3.2. Energetic valorisation of syngas in a gas turbine
After the gasification process and syngas treatment (removal of solid particles, water), the syngas was used to produce electricity in a gas turbine with a power of 5 MW for all the mix cases studied (a-f). The flue gases temperature at the turbine inlet was about 1 200 °C and at the outlet about 540 °C. The ratio between the flow rate required for combustion and the flow rate of the syngas was between 4.28-4.55 kg
air/kg
syngas. Thus, to have the same gas turbine power, with increasing plastic content in the mix, the required syngas flow rate is lower due to better LHV and CGE in this Case (1f). Therefore, just to exemplify, the net plant efficiency is the highest in Case 1f, 41.06% or 33.7%, with the lowest emission factor of 907.44 kgCO
2/MWh (
Table 6).
Table 7 and
Table 8 show the results obtained for L/G ratio and the quantity of heat energy to regenerate the solvent. The capture efficiency in both cases (Case 2 and 3) was 90%. The L/G ratio did not exceed 1 kgsolvent/kgsyngas or kgsolvent/kgflue_gases and the specific heat duty was varied between 2.521-2.636 GJ/tCO
2.
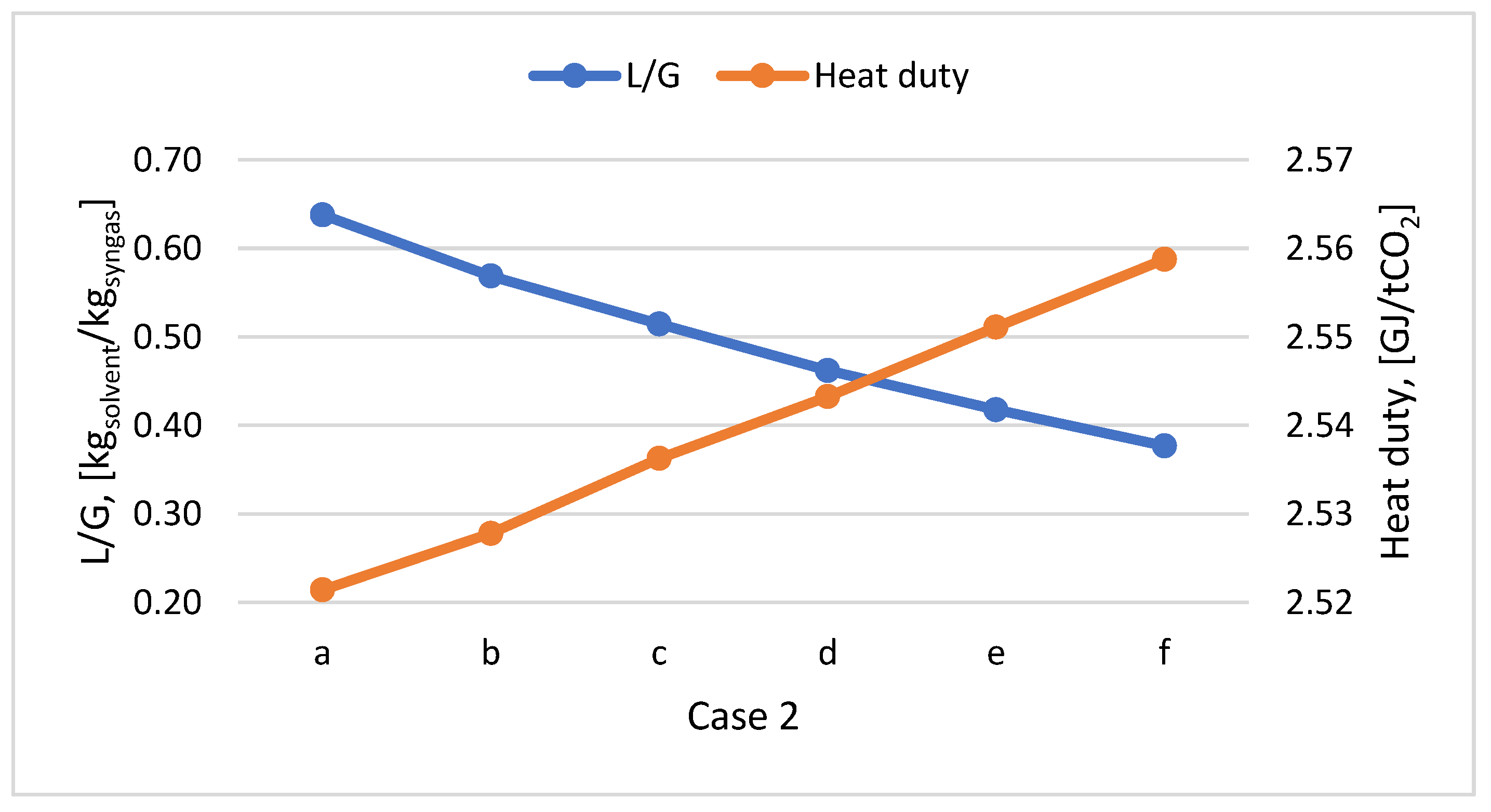
Considering that the CO
2 concentration in synthetic gas decreased as the plastic content increased in the feedstock, the L/G ratio decreased (
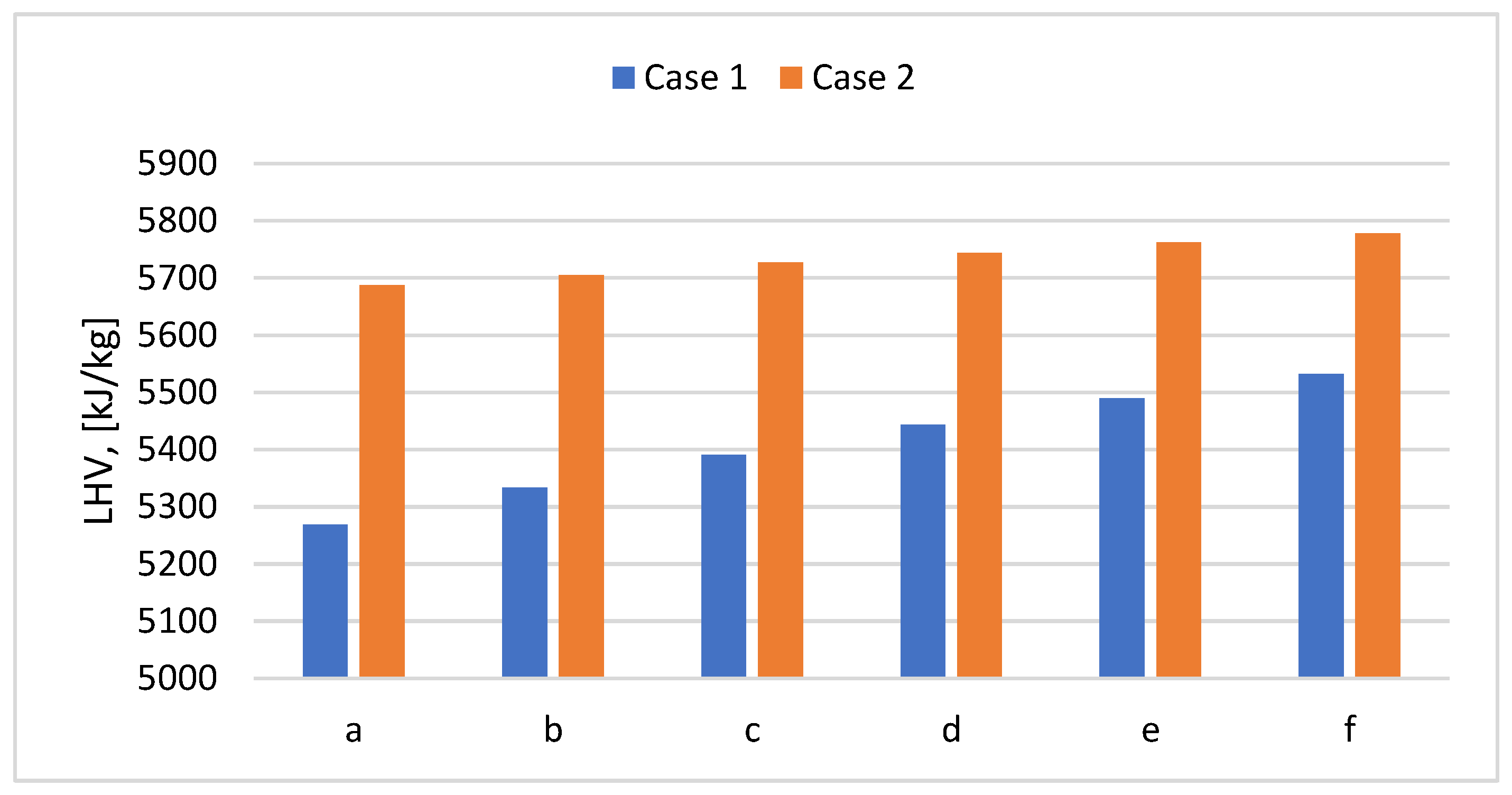
Figure 9). The specific heat required for solvent regeneration is approximately the same for all cases. Thus, the plastic PP content in the feedstock mix doesn’t influence the heat consumption for solvent regeneration. As it can see in
Figure 10, the LHV has increased from 5 269-5 532 kJ/kg in Case 1 to 5 687-5 778 kJ/kg in Case 2.
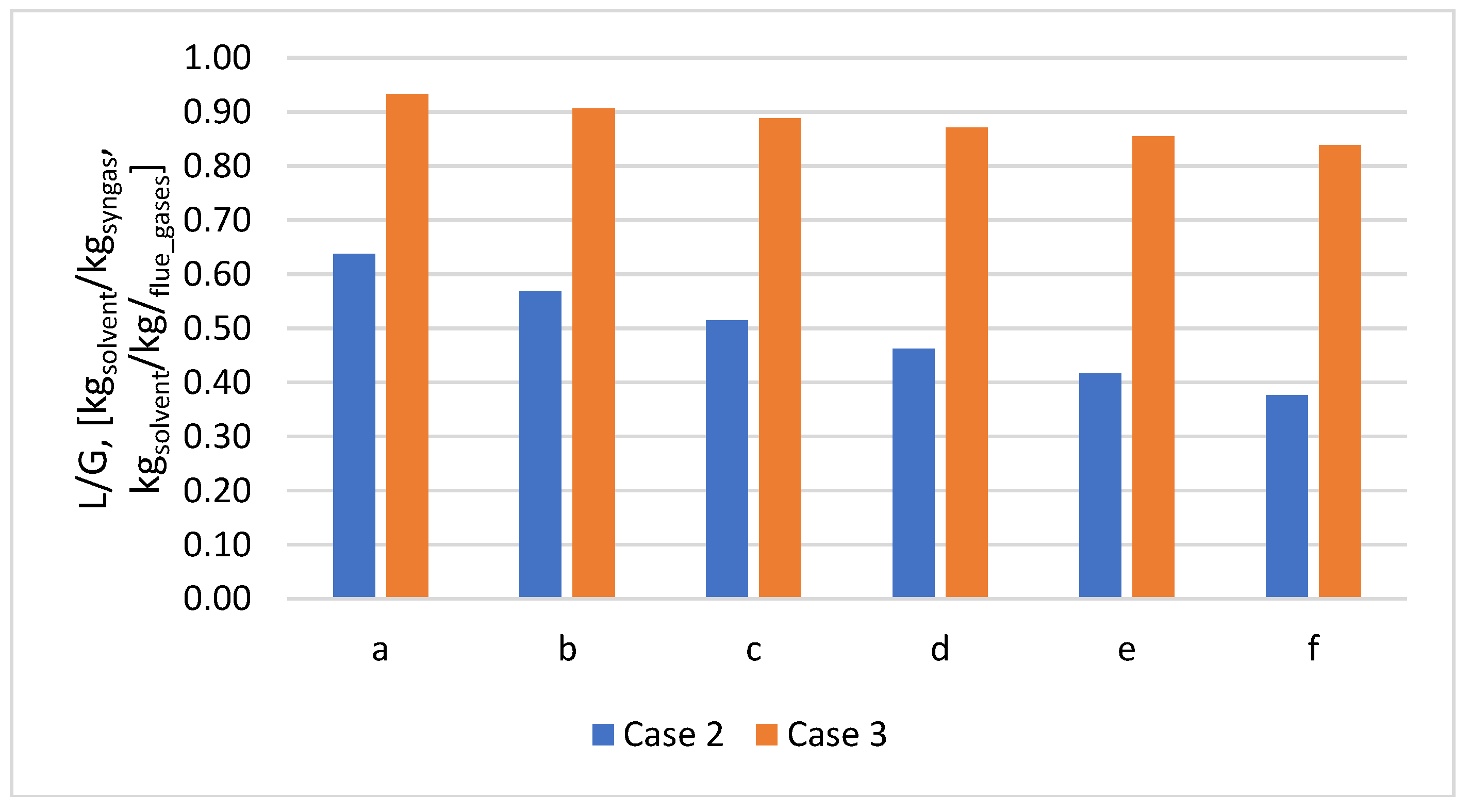
The amount of solvent is significantly lower in the pre-combustion case (Case 2) than in the post-combustion case (Case 3) for the same CO
2 capture efficiency (90%) due to a lower amount of CO
2 in the stream. For cases a-f, the L/G ratio decreases with increasing plastic concentration in the mix for both cases studied (
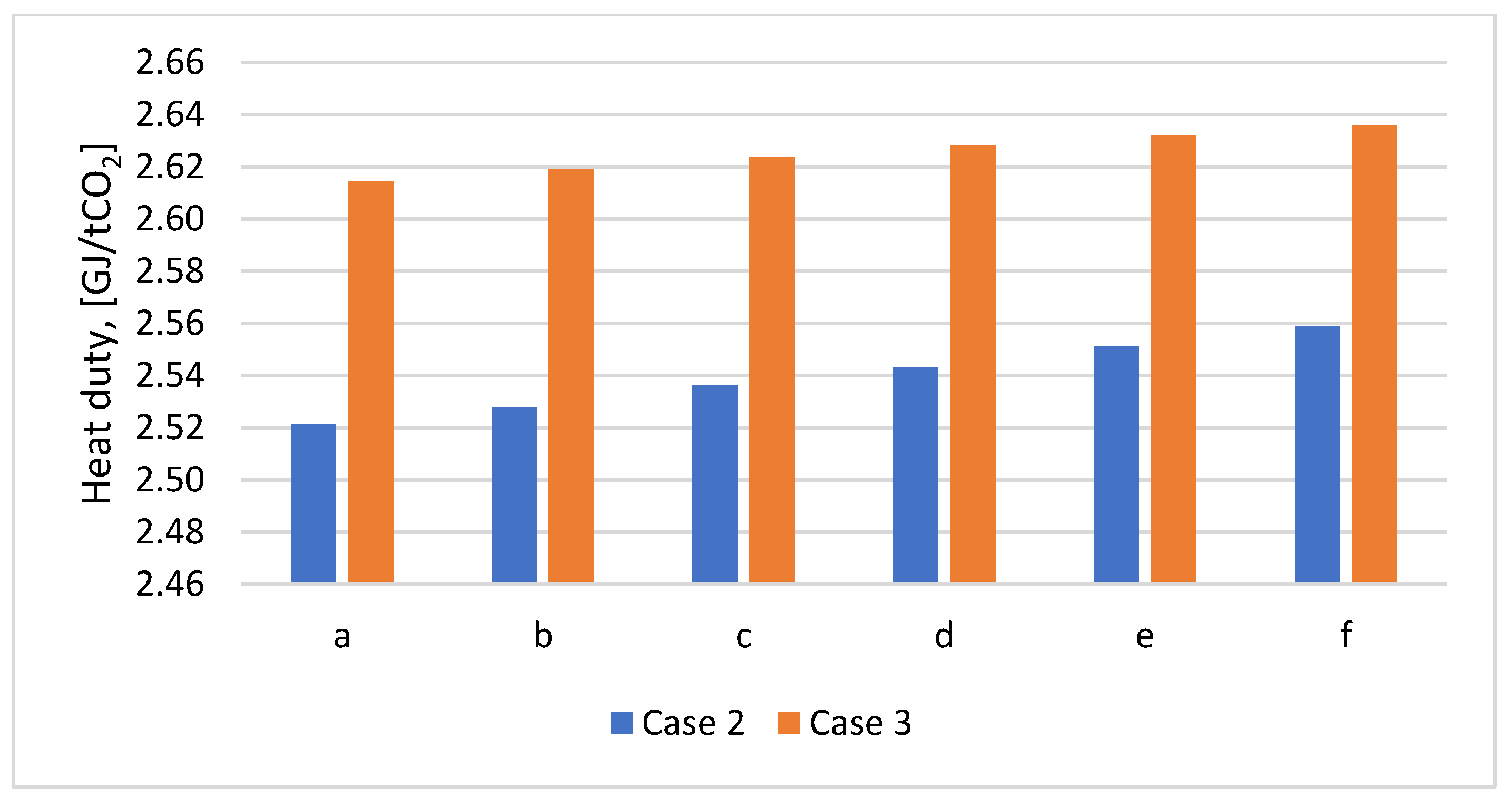
Figure 11). Specific heat duty does not differ significantly, regardless of how the CO
2 capture process is integrated. For example, for Case 2a an amount of 2.521 GJ/tCO
2 is needed, and in Case 2b an amount of 2.614 is needed, with an increase of 3.69%. Specific heat duty increases with increasing plastic content in the mix (
Figure 12).
The water consumption for the CO2 capture process varies between 3 442.72-6 241.96 kgH2O/year in Case 2. The lowest water consumption value corresponds to Case 2f when the plastic content in the mix is 30% due to the lower amount of CO2 captured per year because less feedstock mix is needed to produce the same power. In Case 2, the water consumption is significantly lower than in Case 3 (42 099.81-47 332.05 kgH2O/year) due to the lower gas stream debit treated in the CO2 capture unit. In both cases, the water footprint is approximately 3 kgH2O/tCO2_captured.
The results obtained for the energy valorization of syngas in a gas turbine, Case 2 and 3, are also presented in
Table 7 and
Table 8. Due to the higher LHV obtained from syngas in Case 2, after the gasification process and the capture process, the amount of syngas needed to have a power of 5 MW is lower than in Case 1, by 3.85-7.28% depending on the plastic content in the mix.
After integration of the capture process, the net plant efficiency decreases due to the use of part of the energy produced in the regeneration process of the chemical solvent. In Case 2, the net plant efficiency penalty (
) is between 1.13-2.05%, and in Case 3, the
is between 16.42-18.22%. The significant difference in the cycle efficiency penalty between the two cases analysed (Case 2 and 3) is due to the amount of CO
2 that is removed from the treated gas stream; in Case 3, this amount is much higher.
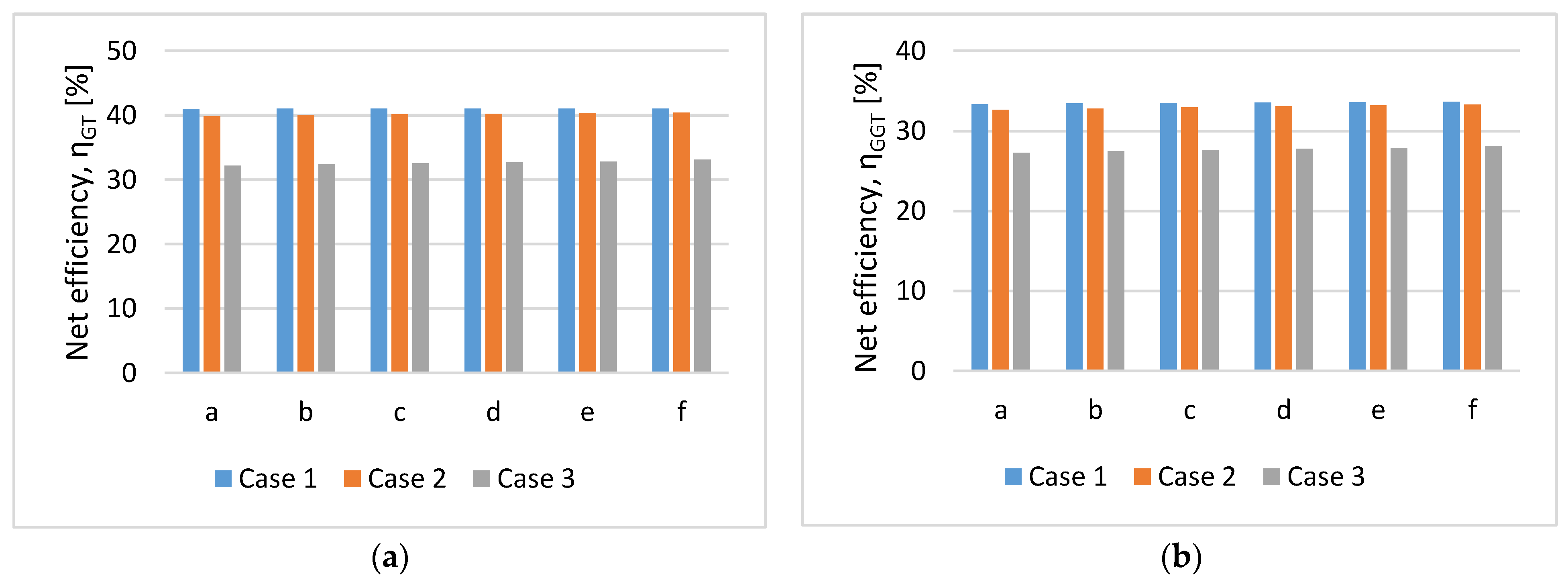
Figure 13 shows the comparative net plant efficiency (
and
) for the three cases studied in cases a-f.
- left)/ (- right).
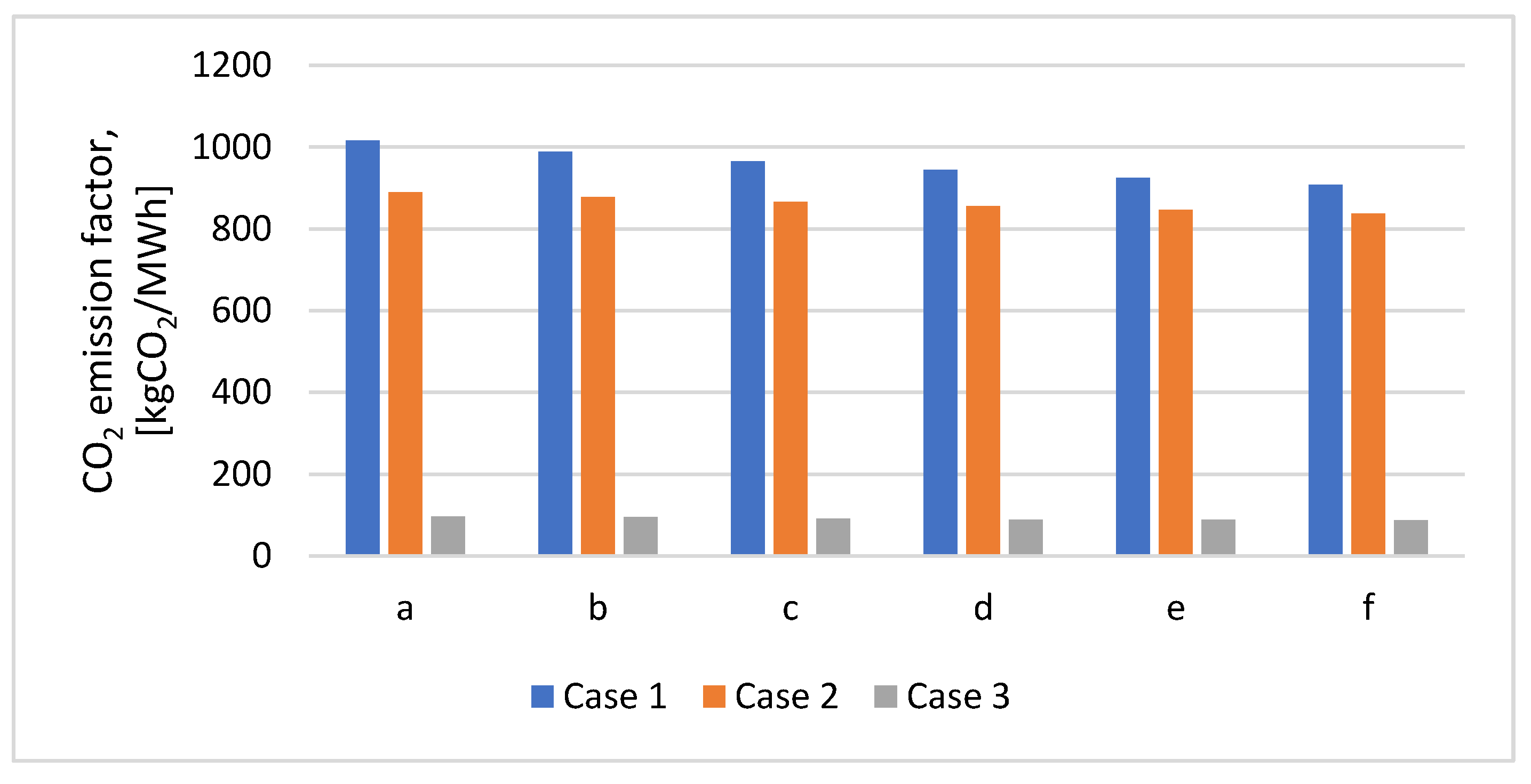
Figure 14 shows the CO
2 emission factor for the three cases in cases a-f. For Case 2, a CO
2 emission factor varied between 837.13-889.28 kgCO
2/MWh was obtained, with 7.74-12.51% lower than in Case 1 because the syngas CO
2 content is negligible as comparing with post-combustion case. As for the CO
2 emission factor in Case 3, CO
2 emission factor decreases by about 90% compared to Case 1 due to the high amount of CO
2 content captured from flue gas. In Case 3, the main disadvantage is the quantity of heat energy necessary in the solvent regeneration process, which is much higher than in Case 2 due to the more considerable CO
2 gas stream flow treated.
3.3. Negative CO2 emissions
Biomass, a renewable energy source, is considered CO
2 neutral due to the CO
2 absorption in the growth process (photosynthesis process). Therefore, the CO
2 emissions generated during gasification and combustion processes according to poplar utilization are not considered in the CO
2 emission factor determination. Thus, the CO
2 emission factors for all three cases studied were recalculated, considering only the CO
2 emissions generated from plastic utilisation. The recalculated CO
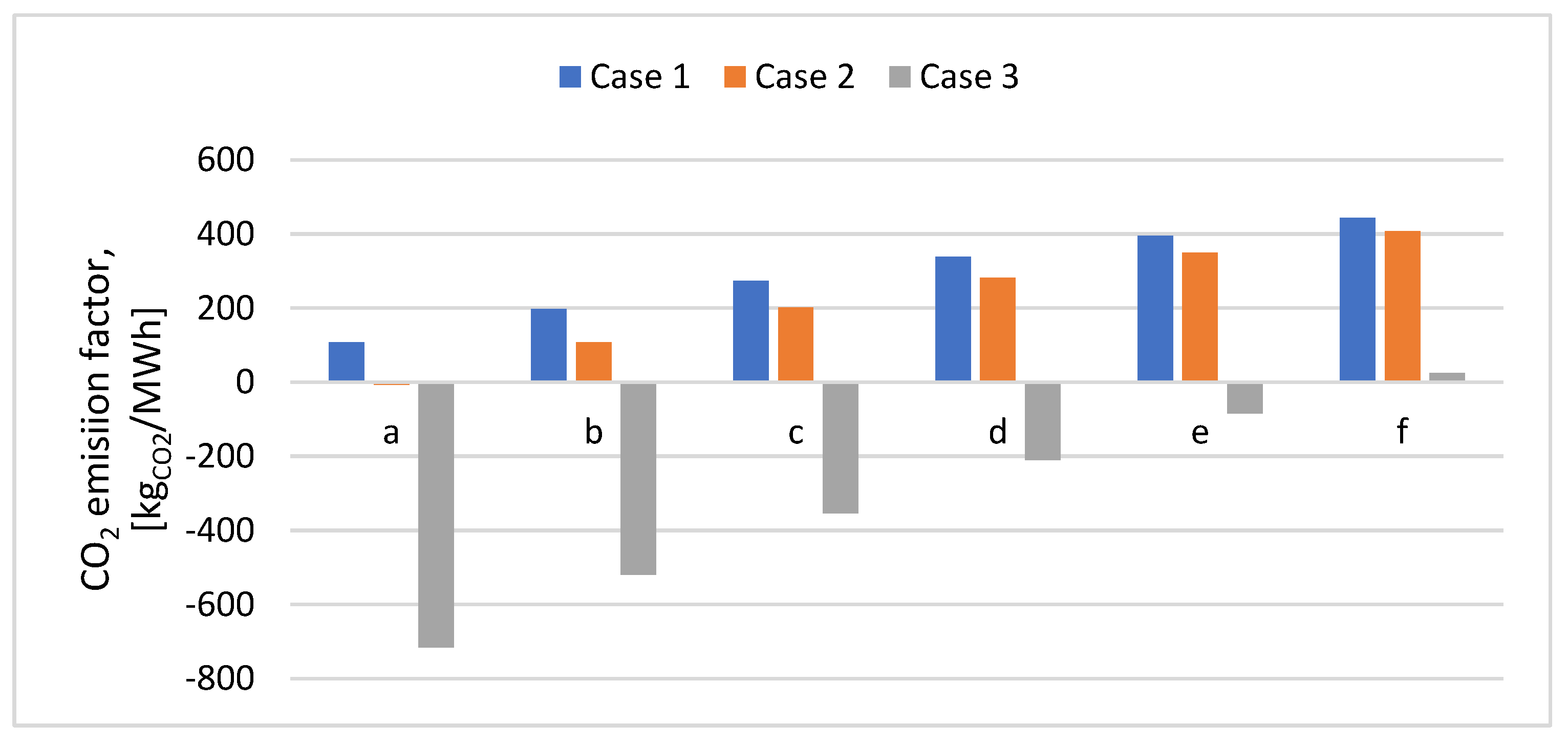
2 emission factor was determined with equation 16, and the results are shown in
Figure 15.
where:
- represents the CO
2 emission factor for plastic, in kgCO
2/MWh;
- represents the CO
2 emission factor for poplar, in kgCO
2/MWh.
As compared to the initial assessment when all CO2 emissions were taken into consideration, in Case 1, without CO2 capture technology, the CO2 emission factor was lower, 89.47%, for a 95% poplar content in the mix and 51.08% for a 70% poplar content in the mix. Further, by decreasing the poplar content in the mix, the CO2 emissions increased because more plastic was used. In Case 2, only when 95% poplar was used in the mix a negative CO2 emission of -6 kgCO2/MWh was obtained. In Case 3, when CAP post-combustion was integrated, for a poplar content in the mix between 75-95%, the CO2 emission factor was negative varied between -84.94 and -716.16 kgCO2/MWh. However, for 70% poplar content in the mix, the CO2 emissions have slightly increased, being positive.
4. Conclusions
The optimal ER ratio considered was 0.35 for all cases studied. The proportion of PP in the feedstock mix varied between 5-30 % wt.. Considering that the CO2 content ranged between 2.8-11.5% in syngas, the CAP integration's influence on the gas turbine energy system was studied. Monoethanolamine was used in a mass concentration of 30%, and the CO2 capture efficiency considered was 90%. As expected, an increase in the LHV of the mixture was observed after the pre-combustion CO2 capture process integration, as the proportion of plastic increased. The LHV varies between 5 269-5 532 kJ/kg (without CO2 capture process) and 5 687-5 778 kJ/kg (with pre-combustion CO2 capture process). Also, an increase in the H2/CO ratio from 0.63 to 0.73 was observed as an increase of the plastic mass content in the mixture. The net plant efficiency was around 41%, with a CO2 emission factor between 907.44-1 016.55 kgCO2/MWh without the CO2 capture process according to the PP content in the mix feed. By integrating the pre-combustion capture process, the net plant efficiency () decreases by 1.13-2.05% and the CO2 emission factor by 7.74-12.51%. When post-combustion capture is integrated, net plant efficiency () decreases by 16.42-18.22% and the CO2 emission factor by about 90%.
Author Contributions
Conceptualization, N.S and C.D.; methodology, N.S and C.D.; software, N.S and C.D.; validation, N.S and C.D.; formal analysis, N.S and C.D.; investigation, N.S and C.D.; resources, N.S and C.D.; data curation, N.S and C.D.; writing—original draft preparation, N.S and C.D.; writing—review and editing, N.S and C.D.; visualization, N.S and C.D.; supervision, N.S and C.D.; project administration, N.S and C.D.; funding acquisition, N.S and C.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by EU Horizon 2020 (InNoPlastic), GA no. 10100061, and by the UEFISCDI within the National Project number 106PTE/2022. Additionally, the results presented in this article have been funded by the Ministry of Investments and European Projects through the Human Capital Sectoral Operational Program 2014-2020, Contract no. 62461/03.06.2022, SMIS code 153735.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
N.S. acknowledges Academy of Romanian Scientists.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Antal, M. J.; Grønli, M. The art, science, and technology of charcoal production. Ind. Eng. Chem. Res. 2003, 42.8, 1619–1640. [Google Scholar] [CrossRef]
- Vasile, C.; Brebu, M. A. Thermal valorisation of biomass and of synthetic polymer waste. Upgrading of pyrolysis oils. Cellul. Chem. Technol. 2006, 40.7, 489–512. [Google Scholar]
- Abnisa, F.; Daud, W. M. A. W. A review on co-pyrolysis of biomass: an optional technique to obtain a high-grade pyrolysis oil. Energy Convers. Manag. 2014, 87, 71–85. [Google Scholar] [CrossRef]
- Varhegyi, G.; Antal Jr, M. J.; Szekely, T.; Szabo, P. Kinetics of the thermal decomposition of cellulose, hemicellulose, and sugarcane bagasse. Energy Fuels. 1989, 3.3, 329–335. [Google Scholar] [CrossRef]
- Couhert, C.; Commandre, J. M.; Salvador, S. Is it possible to predict gas yields of any biomass after rapid pyrolysis at high temperature from its composition in cellulose, hemicellulose and lignin? Fuel. 2009, 88.3, 408–417. [Google Scholar] [CrossRef]
- Kumar, R. A review on the modelling of hydrothermal liquefaction of biomass and waste feedstocks. Energy Nexus. 2022, 5, 100042. [Google Scholar] [CrossRef]
- Jalili, M.; Ghasempour, R.; Ahmadi, M. H.; Chitsaz, A.; Holagh, S. G. An integrated CCHP system based on biomass and natural gas co-firing: Exergetic and thermo-economic assessments in the framework of energy nexus. Energy Nexus. 2022, 5, 100016. [Google Scholar] [CrossRef]
- Ciuffi, B.; Chiaramonti, D.; Rizzo, A. M.; Frediani, M.; Rosi, L. A critical review of SCWG in the context of available gasification technologies for plastic waste. Appl. Sci. 2020, 10.18, 6307. [Google Scholar] [CrossRef]
- Badea, A. A.; Vodă, I.; Dincă, C. F. Comparative analysis of coal, natural gas and nuclear fuel life cycles by chains of electrical energy production. U.P.B. Sci. Bull. Ser. C. 2010, 72, 221–238. Available online: https://www.scientificbulletin.upb.ro/rev_docs_arhiva/full8515.pdf.
- Dinca, C.; Badea, A. A.; Apostol, T.; Lazaroiu, G. GHG emissions evaluation from fossil fuel with CCS. Environ. Eng. Manag. J. 2009, 8.1, 81–89. [Google Scholar] [CrossRef]
- Dinca, C.; Slavu, N.; Cormoş, C. C.; Badea, A. CO2 capture from syngas generated by a biomass gasification power plant with chemical absorption process. Energy. 2018, 149, 925–936. [Google Scholar] [CrossRef]
- Block, C.; Ephraim, A.; Weiss-Hortala, E.; Minh, D. P.; Nzihou, A.; Vandecasteele, C. Co-pyrogasification of plastics and biomass, a review. Waste Biomass Valor. 2019, 10.3, 483–509. [Google Scholar] [CrossRef]
- Sannigrahi, P.; Ragauskas, A. J.; Tuskan, G. A. Poplar as a feedstock for biofuels: a review of compositional characteristics. Biofuels, Bioprod, Bioref. 2010, 4, 209–226. [Google Scholar] [CrossRef]
- Yao, D.; Yang, H.; Chen, H.; Williams, P. T. Co-precipitation, impregnation and so-gel preparation of Ni catalysts for pyrolysis-catalytic steam reforming of waste plastics. Appl. Catal. B. 2018, 239, 565–577. [Google Scholar] [CrossRef]
- Cormos, C. C.; Dinca, C. Techno-economic and environmental implications of decarbonization process applied for Romanian fossil-based power generation sector. Energy. 2021, 119734. [Google Scholar] [CrossRef]
- Ababneh, H.; AlNouss, A.; Al-Muhtaseb, S.A. Carbon Capture from Post-Combustion Flue Gas Using a State-Of-The-Art, Anti-Sublimation, Solid–Vapor Separation Unit. Processes 2022, 10, 2406. [Google Scholar] [CrossRef]
- Dinca, C.; Badea, A.; Stoica, L.; Pascu, A. Absorber design for the improvement of the efficiency of post-combustion CO2 capture. J. Energy Inst. 2015, 88.3, 304–313. [Google Scholar] [CrossRef]
- Sifat, N.S.; Haseli, Y. A Critical Review of CO2 Capture Technologies and Prospects for Clean Power Generation. Energies 2019, 12, 4143. [Google Scholar] [CrossRef]
- Slavu, N.; Badea, A.; Dinca, C. Technical and Economical Assessment of CO2 Capture-Based Ammonia Aqueous. Processes 2022, 10, 859. [Google Scholar] [CrossRef]
- Pascu, A.; Stoica, L.; Dinca, C.; Badea, A. (2016). The package type influence on the performance of the CO2 capture process by chemical absorption. U.P.B. Sci. Bull. Ser. C. 2016, 78, 259–270. Available online: https://www.scientificbulletin.upb.ro/rev_docs_arhiva/full409_910116.pdf.
- Peu, S.D.; Das, A.; Hossain, M.S.; Akanda, M.A.M.; Akanda, M.M.H.; Rahman, M.; Miah, M.N.; Das, B.K.; Islam, A.R.M.T.; Salah, M.M. A Comprehensive Review on Recent Advancements in Absorption-Based Post Combustion Carbon Capture Technologies to Obtain a Sustainable Energy Sector with Clean Environment. Sustainability 2023, 15, 5827. [Google Scholar] [CrossRef]
- Rajiman, V.; Hairul, N.A.H.; Shariff, A.M. Effect of CO2 Concentration and Liquid to Gas Ratio on CO2 Absorption from Simulated Biogas Using Monoethanolamine Solution. IOP Conf. Ser.: Mater. Sci. Eng. 2020, 991, 012133. [Google Scholar] [CrossRef]
- SGT-100 | Industrial Gas Turbine | Gas Turbines | Manufacturer | Siemens Energy Global. Available online: https://www.siemens-energy.com/global/en/offerings/power-generation/gas-turbines/sgt-100.html (accessed on 12 July 2023).
Figure 1.
Diagram of the gasification process.
Figure 1.
Diagram of the gasification process.
Figure 2.
CO2 capture process.
Figure 2.
CO2 capture process.
Figure 3.
Diagram of the syngas conversion in electricity without CO2 capture process.
Figure 3.
Diagram of the syngas conversion in electricity without CO2 capture process.
Figure 4.
Diagram of the syngas conversion in electricity with pre-combustion CO2 capture.
Figure 4.
Diagram of the syngas conversion in electricity with pre-combustion CO2 capture.
Figure 5.
Diagram of the syngas conversion in electricity with post-combustion CO2 capture process.
Figure 5.
Diagram of the syngas conversion in electricity with post-combustion CO2 capture process.
Figure 6.
LHV and CGE variation according to ER for Case 1a.
Figure 6.
LHV and CGE variation according to ER for Case 1a.
Figure 7.
LHV variation according to ER.
Figure 7.
LHV variation according to ER.
Figure 8.
CGE variation according to ER.
Figure 8.
CGE variation according to ER.
Figure 9.
L/G ratio and heat duty variation according to PP content in the mix for Case 2.
Figure 9.
L/G ratio and heat duty variation according to PP content in the mix for Case 2.
Figure 10.
LHV according to PP content in the mix for Cases 1 and 2.
Figure 10.
LHV according to PP content in the mix for Cases 1 and 2.
Figure 11.
Variation of the L/G ratio according to PP content in the mix for Cases 2 and 3.
Figure 11.
Variation of the L/G ratio according to PP content in the mix for Cases 2 and 3.
Figure 12.
Variation of the heat duty according to PP content in the mix for Cases 2 and 3.
Figure 12.
Variation of the heat duty according to PP content in the mix for Cases 2 and 3.
Figure 13.
Net plant efficiency depending on the mix used (ηGT - left)/ (ηGGT - right).
Figure 13.
Net plant efficiency depending on the mix used (ηGT - left)/ (ηGGT - right).
Figure 14.
CO2 emission factor depending on the mix used.
Figure 14.
CO2 emission factor depending on the mix used.
Figure 15.
CO2 emission factor depending on the mix used (poplar CO2 neutral).
Figure 15.
CO2 emission factor depending on the mix used (poplar CO2 neutral).
Table 1.
Main data for feedstock compositiondb*.
Table 1.
Main data for feedstock compositiondb*.
| Composition |
Biomass |
Plastic |
Mix poplar with plastic (polypropylene) P-PP, % wt. |
| P** |
PP*** |
95-5 |
90-10 |
85-15 |
80-20 |
75-25 |
70-30 |
| C – Carbon, wt.% |
50.02 |
83.74 |
51.71 |
53.39 |
55.08 |
56.76 |
58.45 |
60.14 |
| H – Hydrogen, wt.% |
6.28 |
13.71 |
6.65 |
7.02 |
7.39 |
7.77 |
8.14 |
8.51 |
| O – Oxygen, wt.% |
42.17 |
0.98 |
40.11 |
38.05 |
35.99 |
33.93 |
31.87 |
29.81 |
| N – Nitrogen, wt.% |
0.19 |
0.02 |
0.18 |
0.17 |
0.16 |
0.16 |
0.15 |
0.14 |
| S – Sulphur, wt.% |
0.02 |
0.08 |
0.02 |
0.03 |
0.03 |
0.03 |
0.04 |
0.04 |
| A – Ash, wt.% |
1.32 |
1.47 |
1.33 |
1.34 |
1.35 |
1.35 |
1.35 |
1.36 |
| LHV, MJ/kg |
18.95 |
42.34 |
20.12 |
21.29 |
22.46 |
23.63 |
24.80 |
25.97 |
Table 2.
Main data for gasification process simulation.
Table 2.
Main data for gasification process simulation.
| Process type |
Adiabatic |
| Oxidizing agent |
Air |
| P-PP flow mix, kg/h |
1 |
| Temperature, °C |
600-1200 |
| Pressure, bar |
1.013 |
| ER, [-] |
0.1; 0.15; 0.2; 0.25; 0.3; 0.35; 0.4; 0.45 |
Table 3.
Main data for gasification process simulation.
Table 3.
Main data for gasification process simulation.
| Type |
SGT-100 |
| Power, MW |
5 |
| Speed, rpm |
17,384 |
| Pressure ratio, - |
14 |
| Flue gases temperature at the gas turbine inlet, °C |
~ 544 |
| Flue gases flow, kg/s |
up to 19.5 |
Table 4.
Syngas composition after gasification, solid separation, and water separation units for Case 1a.
Table 4.
Syngas composition after gasification, solid separation, and water separation units for Case 1a.
| ER |
0.1 |
0.15 |
0.2 |
0.25 |
0.3 |
0.35 |
0.4 |
0.45 |
| Syngas composition after gasification unit, mol fraction |
| H2
|
0.1677 |
0.1761 |
0.1781 |
0.1772 |
0.1746 |
0.1672 |
0.1294 |
0.0942 |
| CH4
|
0.0206 |
0.0135 |
0.0096 |
0.0072 |
0.0055 |
0.0021 |
0.0000 |
0.0000 |
| N2
|
0.1987 |
0.2681 |
0.3262 |
0.3757 |
0.4183 |
0.4534 |
0.4847 |
0.5141 |
| CO |
0.0851 |
0.1319 |
0.1747 |
0.2127 |
0.2463 |
0.2670 |
0.2526 |
0.2357 |
| CO2
|
0.0657 |
0.0627 |
0.0578 |
0.0521 |
0.0464 |
0.0407 |
0.0373 |
0.0377 |
| H2O |
0.1544 |
0.1241 |
0.1014 |
0.0839 |
0.0699 |
0.0660 |
0.0927 |
0.1153 |
| H2S |
0.0001 |
0.0001 |
0.0001 |
0.0001 |
0.0001 |
0.0001 |
0.0000 |
0.0000 |
| Char |
0.3026 |
0.2188 |
0.1478 |
0.0873 |
0.0353 |
0.0000 |
0.0000 |
0.0000 |
| SiO2
|
0.0052 |
0.0047 |
0.0043 |
0.0039 |
0.0037 |
0.0034 |
0.0032 |
0.0030 |
| Syngas composition after solids separator unit, mol fraction |
| H2
|
0.2423 |
0.2267 |
0.2101 |
0.1950 |
0.1817 |
0.1678 |
0.1298 |
0.0945 |
| CH4
|
0.0297 |
0.0174 |
0.0113 |
0.0079 |
0.0057 |
0.0021 |
0.0000 |
0.0000 |
| N2
|
0.2870 |
0.3453 |
0.3848 |
0.4134 |
0.4352 |
0.4550 |
0.4863 |
0.5157 |
| CO |
0.1230 |
0.1699 |
0.2060 |
0.2341 |
0.2563 |
0.2679 |
0.2534 |
0.2364 |
| CO2
|
0.0949 |
0.0808 |
0.0681 |
0.0573 |
0.0483 |
0.0409 |
0.0374 |
0.0378 |
| H2O |
0.2230 |
0.1598 |
0.1196 |
0.0923 |
0.0728 |
0.0662 |
0.0930 |
0.1156 |
| H2S |
0.0001 |
0.0001 |
0.0001 |
0.0001 |
0.0001 |
0.0001 |
0.0000 |
0.0000 |
| Syngas composition after solids separator unit, mol fraction |
| H2
|
0.3119 |
0.2699 |
0.2386 |
0.2148 |
0.1959 |
0.1797 |
0.1431 |
0.1068 |
| CH4
|
0.0382 |
0.0207 |
0.0129 |
0.0087 |
0.0062 |
0.0023 |
0.0000 |
0.0000 |
| N2
|
0.3694 |
0.4109 |
0.4370 |
0.4554 |
0.4694 |
0.4872 |
0.5362 |
0.5831 |
| CO |
0.1583 |
0.2022 |
0.2340 |
0.2579 |
0.2764 |
0.2869 |
0.2794 |
0.2673 |
| CO2
|
0.1221 |
0.0962 |
0.0774 |
0.0632 |
0.0521 |
0.0438 |
0.0413 |
0.0427 |
| H2S |
0.0001 |
0.0001 |
0.0001 |
0.0001 |
0.0001 |
0.0001 |
0.0001 |
0.0001 |
Table 5.
Results of gasification process for ER = 0.35 – Case 1.
Table 5.
Results of gasification process for ER = 0.35 – Case 1.
| Case |
1a |
1b |
1c |
1d |
1e |
1f |
| ER, - |
0.35 |
0.35 |
0.35 |
0.35 |
0.35 |
0.35 |
| Gas temperature, °C |
40 |
40 |
40 |
40 |
40 |
40 |
| Syngas flow |
3.10 |
3.25 |
3.40 |
3.55 |
3.70 |
3.85 |
| LHV, kJ/kg |
5 269 |
5 333 |
5 391 |
5 443 |
5 490 |
5 532 |
| H2/CO, - |
0.63 |
0.65 |
0.67 |
0.69 |
0.71 |
0.73 |
| CGE, % |
81.30 |
81.47 |
81.63 |
81.77 |
81.89 |
81.98 |
| Syngas composition, mole % |
| H2
|
17.97 |
18.42 |
18.83 |
19.21 |
19.55 |
19.87 |
| CH4
|
0.23 |
0.25 |
0.28 |
0.30 |
0.32 |
0.34 |
| N2
|
48.72 |
49.03 |
49.29 |
49.53 |
49.74 |
49.94 |
| CO |
28.69 |
28.41 |
28.14 |
27.88 |
27.63 |
27.38 |
| CO2
|
4.38 |
3.88 |
3.45 |
3.08 |
2.75 |
2.46 |
| H2S |
0.01 |
0.01 |
0.01 |
0.01 |
0.01 |
0.01 |
Table 6.
Results of syngas use in a gas turbine – Case 1.
Table 6.
Results of syngas use in a gas turbine – Case 1.
| Case |
1a |
1b |
1c |
1d |
1e |
1f |
| Gas turbine power, MW |
5 |
5 |
5 |
5 |
5 |
5 |
| Syngas flow, kg/h |
4 226.19 |
4 166.87 |
4 122.22 |
4 079.09 |
4 046.16 |
4 011.36 |
| Air flow, kg/h |
18 105.73 |
18 166.44 |
18 181.29 |
18 213.95 |
18 214.20 |
18 243.19 |
| Combustion chamber temperature, °C |
1 202.88 |
1 201.25 |
1 201.68 |
1 201.06 |
1 201.89 |
1 201.19 |
| Flue gases temperature at the gas turbine outlet, °C |
543.71 |
542.15 |
541.99 |
541.20 |
541.38 |
540.62 |
| Flue gases flow, kg/h |
22 331.94 |
22 333.32 |
22 303.52 |
22 293.05 |
22 260.38 |
22 254.56 |
| ηGT, % |
41.01 |
41.04 |
41.04 |
41.05 |
41.05 |
41.06 |
| ηGGT, % |
33.34 |
33.44 |
33.51 |
33.57 |
33.61 |
33.66 |
| CO2 emission factor, kgCO2/MWh |
1 016.55 |
989.18 |
965.43 |
943.92 |
924.98 |
907.44 |
| Flue gases composition, wt. % |
| H2
|
0.02 |
0.02 |
0.02 |
0.02 |
0.02 |
0.02 |
| O2
|
13.03 |
13.10 |
13.12 |
13.16 |
13.17 |
13.21 |
| N2
|
72.95 |
73.16 |
73.33 |
73.49 |
73.62 |
73.75 |
| CO2
|
11.55 |
11.22 |
10.97 |
10.72 |
10.52 |
10.32 |
| H2O |
2.45 |
2.50 |
2.56 |
2.61 |
2.66 |
2.70 |
Table 7.
Results of syngas use in a gas turbine – Case 2.
Table 7.
Results of syngas use in a gas turbine – Case 2.
| Case |
2a |
2b |
2c |
2d |
2e |
2f |
| Gas turbine power, MW |
5 |
5 |
5 |
5 |
5 |
5 |
| Syngas flow, kg/h |
3 918.41 |
3 906.34 |
3 889.38 |
3 880.25 |
3 868.40 |
3 856.88 |
| Air flow, kg/h |
18 374.05 |
18 359.46 |
18 371.19 |
18 357.30 |
18 357.26 |
18 363.23 |
| Combustion chamber temperature, °C |
1 201.21 |
1 201.80 |
1 201.12 |
1 201.61 |
1 201.50 |
1 201.02 |
| Flue gases temperature at the gas turbine outlet, °C |
539.97 |
540.18 |
539.57 |
539.75 |
539.54 |
539.11 |
| Flue gases flow, kg/h |
22 292.47 |
22 265.81 |
22 260.58 |
22 237.57 |
22 225.08 |
22 220.13 |
| L/G, [kgsolvent/kgsyngas] |
0.64 |
0.57 |
0.51 |
0.46 |
0.42 |
0.38 |
| Heat duty, GJ/tCO2
|
2.521 |
2.528 |
2.536 |
2.543 |
2.551 |
2.559 |
| Heat flow used for solvent regeneration, MJ/h |
776 |
683 |
610 |
542 |
485 |
434 |
| Water consumption CO2 capture, kg/year |
6 241.96 |
5 480.06 |
4 882.46 |
4 327.74 |
3 863.26 |
3 442.72 |
| ηGT, % |
39.89 |
40.03 |
40.16 |
40.26 |
40.35 |
40.44 |
| ηGGT, % |
32.7 |
32.8 |
32.96 |
33.08 |
33.19 |
33.28 |
| Penalty efficiency, % |
2.05 |
1.87 |
1.63 |
1.47 |
1.28 |
1.13 |
| CO2 emission factor, kgCO2/MWh |
889.28 |
878.06 |
866.01 |
856.04 |
845.93 |
837.13 |
| Flue gases composition, wt. % |
| H2
|
0.02 |
0.02 |
0.02 |
0.02 |
0.02 |
0.02 |
| O2
|
13.32 |
13.32 |
133.3 |
13.32 |
13.32 |
13.33 |
| N2
|
74.00 |
74.07 |
74.14 |
74.20 |
74.27 |
74.32 |
| CO2
|
10.19 |
10.07 |
9.93 |
9.83 |
9.72 |
9.61 |
| H2O |
2.46 |
2.52 |
2.57 |
2.63 |
2.67 |
2.72 |
Table 8.
Results of syngas use in a gas turbine – Case 3.
Table 8.
Results of syngas use in a gas turbine – Case 3.
| Case |
3a |
3b |
3c |
3d |
3e |
3f |
| Gas turbine power, MW |
5 |
5 |
5 |
5 |
5 |
5 |
| Syngas flow, kg/h |
4 226.19 |
4 166.87 |
4 122.22 |
4 079.09 |
4 046.16 |
4 011.36 |
| Air flow, kg/h |
18 105.73 |
18 166.44 |
18 181.29 |
18 213.95 |
18 214.20 |
18 243.19 |
| Combustion chamber temperature, °C |
1 202.88 |
1 201.25 |
1 201.68 |
1 201.06 |
1 201.89 |
1 201.19 |
| Flue gases temperature at the gas turbine outlet, °C |
543.71 |
542.15 |
541.99 |
541.20 |
541.38 |
540.62 |
| Flue gases flow, kg/h |
21 849.09 |
21 863.24 |
21 840.66 |
21 837.35 |
21 811.32 |
21 812.57 |
| L/G, [kgsolvent/kgflue_gases] |
0.93 |
0.91 |
0.89 |
0.87 |
0.86 |
0.84 |
| Heat duty, GJ/tCO2
|
2.614 |
2.619 |
2.624 |
2.628 |
2.632 |
2.636 |
| Heat flow used for solvent regeneration, MJ/h |
6 102 |
5 930 |
5 807 |
5 692 |
5 580 |
5 317 |
| Water consumption CO2 capture, kg/year |
47 332.05 |
45 925.13 |
44 893.42 |
43 926.56 |
42 998.89 |
42 099.81 |
| ηGT, % |
32.19 |
32.40 |
32.54 |
32.68 |
32.81 |
33.13 |
| ηGGT, % |
27.27 |
27.47 |
27.62 |
27.75 |
27.88 |
28.14 |
| Penalty efficiency, % |
18.22 |
17.86 |
17.58 |
17.33 |
17.06 |
16.42 |
| CO2 emission factor, kgCO2/MWh |
96.63 |
95.41 |
91.73 |
88.49 |
87.88 |
87.33 |
| Flue gases composition, wt. % |
| H2
|
0.02 |
0.02 |
0.02 |
0.02 |
0.02 |
0.02 |
| O2
|
13.32 |
13.38 |
13.40 |
13.44 |
13.44 |
13.47 |
| N2
|
74.56 |
74.73 |
74.88 |
75.02 |
75.14 |
75.24 |
| CO2
|
1.12 |
1.11 |
1.06 |
1.03 |
1.02 |
1.01 |
| H2O |
10.93 |
10.72 |
10.58 |
10.45 |
10.33 |
10.20 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).