1. Introduction
The repair of maxillary bone defects is a challenge for many surgeons [
1]. Block grafting has become a surgical tool widely used in the treatment of mandibular bone defects resulting from traumatic injuries, resorption, tooth extraction, and birth defects [
2]. The objective of this procedure is to restore lost bone volume and has been frequently used in the field of implant dentistry as a way of enabling prosthetic rehabilitation of osseointegrated implants [
3,
4,
5]. Some types of grafts are recommended for reconstruction of atrophic alveolar ridges. Autogenous, xenogeneic, and allogeneic grafts are the most commonly used grafts [
6,
7,
8,
9].
Autogenous grafts are considered the “gold standard” because of their osteogenic and osteoconductive properties, which stimulate the process of bone incorporation [
10,
11,
12,
13,
14]. However, it has disadvantages such as surgical morbidity and a longer operative time [
15,
16,
17,
18,
19]. Autogenous grafting can be quite complex, and success depends on the size of the bone defect and availability of the donor site. This has led to the study and development of new biomaterials capable of restoring lost bone volume in quantity and quality [
20,
21,
22]. Studies have shown that block autogenous bone grafts may be more resistant to bone volume loss in the long term than particulate grafts [
23]. However, other studies have shown that block grafts are prone to resorption during repair [
24]. Thus, new materials and techniques have been developed to reduce the high rate of graft resorption and maintain bone volume [
23,
25].
As a viable alternative for replacing autogenous bone, the xenogeneic graft has been used in bone volume augmentation procedures [
26,
27,
28,
29,
30], as it presents clinical advantages such as a decrease in surgical morbidity, greater technical ease, and the ability to conform and adapt the block following the anatomy of the recipient bed [
26,
31,
32,
33]. To install the graft in the recipient bed, some surgical techniques can be used, such as bone grafts in onlay blocks, osteogenic distraction, and particulate bone grafts associated with titanium meshes or collagen membranes [
33]. In a clinical study [
34], the technique of onlay grafting of autogenous blocks collected from the iliac crest for restoration of lost maxillary bone volume was applied in 30 patients. During the bone incorporation period, extensive reabsorption of the total graft volume was observed.
In experimental studies [
35,
36], the influence of perforations of the recipient beds on grafting procedures using onlay blocks harvested from the iliac crest of rabbits was evaluated. It was shown that the blocks installed on perforated beds presented a smaller reabsorption during the repair time owing to greater blood perfusion from the bone matrix to the graft, which allowed a higher number of proteins associated with revascularization and osteogenesis.
Faced with the difficulty of establishing a grafting technique capable of providing adequate bone incorporation, less resorption during the repair period and maintenance of bone volume in the long term, this work aimed to comparatively evaluate the process of incorporation, resorption and remodeling of xenogeneic block bone grafts using two surgical techniques: onlay vs. inlay graft techniques. The conformation of the recipient site for an inlay graft presents an increased contact with the parent bone compared to the onlay graft. This might favor graft integration and bone formation within the inlay compared with onlay grafts. Hence, the objective of this study was to compare bone incorporation and remodeling process of xenogeneic en bloc grafts, installed using two bone grafting techniques, i.e., onlay vs. inlay.
2. Materials and Methods
2.1. Ethical statements
This research project was approved by the Committee on Ethics in the Use of Animals of the Faculty of Dentistry of Ribeirão Preto, University of São Paulo, Brazil, on September 25, 2019, protocol #2019.1.619.58.2. The proposed experimental procedures were carried out in accordance with legislation for animal experimentation in Brazil. The ARRIVE Checklist was used in this study.
2.2. Study design
In this prospective, randomized, split-mouth study (test and control sides in the same animal), two bone grafting techniques were comparatively evaluated on the lateral aspect of the rabbit mandibles. One side was prepared with perforations (onlay site), whereas the other side was prepared with trephines and drills to obtain a standardized recipient site (inlay site). A xenogeneic bone block was fixed in the center with a titanium screw in both sides of the mandible and covered with a collagen membrane. Two healing periods were applied in the study: 2 and 10 weeks of healing.
2.3. Experimental animals and sample size
Data from a study in which similar grafts were fixed to the bone plate of the lateral aspect of rabbit mandibles [
37]. In this study, the grafts were either treated with argon plasma or left untreated. A maximum standard deviation of 7.4 was found. In the present study, a 10% difference between the onlay and inlay was considered clinically relevant. Consequently, with α=0.05 and a power of 0.9, a sample size of eight pairs of animals was obtained to reject the null hypothesis that this response difference is zero (PS Power and Sample Size Calculations; by William D. Dupont and Walton D. Plummer). The sample size was increased to 10 for possible complications at various levels during the experiment. Hence, twenty adult male New Zealand White rabbits, 10 each period of healing, weighing 3.5–4.0 kg and aged between 5-6 months, were included in the study.
2.4. Randomization and allocation concealment
Randomization was performed electronically by an author who was not involved in the selection of animals or any surgical procedure (SPX). The treatment was maintained in a sealed opaque envelope that was opened immediately before the fixation of the first graft. The histological examinator was not informed of the time of healing and treatment allocation, and the histological slides were coded. However, the conformation of the sites could be recognized on the histological slides.
2.5. Biomaterials
SpBlock is a rigid dried block composed of collagenated cancellous equine bone. It is produced using an exclusive (Tecnoss, Giaveno, Italy) process that avoids the ceramization of hydroxyapatite crystals, thus accelerating physiological resorption.
Bio-Gide (Geistlich Biomaterials, Wolhusen, LU, Switzerland) is a porcine-derived resorbable membrane composed of types I and III collagen. It is composed of a bilayer structure with a smooth outer layer aimed at avoiding the progression of soft tissues within the region to be regenerated, and a porous inner layer that aims to favor bone cells and vessel growth [
38].
2.6. Anesthetic procedures
The surgical procedures were performed under general anesthesia with intramuscular acepromazine. (1.0 mg/kg; Acepran, Vetnil, Louveira, São Paulo), and xylazine (3.0 mg/Kg; Laboratórios Calier S/A, Barcelona, Spain) combined with ketamine I.M. (50.0 mg/Kg; União Química Farmacêutica Nacional S/A, Embuguaçú, São Paulo, Brazil) 15 min after acepromazine administration. When properly sedated, animals were subjected to prophylactic antibiotic therapy with oxytetracycline. (0.2 ml/Kg; Biovet; Vargem Grande Paulista, São Paulo, Brazil). The area to be operated was shaved, and antisepsis was performed by topical application of 1% polyvinylpyrrolidone iodine solution (Riodeíne Tintura, Rioquímica, São José do Rio Preto, São Paulo, Brazil). Local anesthesia was performed with 2% mepivacaine and 1:100,000 noradrenaline (Mepinor, Nova DFL, Rio de Janeiro, Brazil).
2.7. Surgical procedure
All surgeries were performed by a single operator (V.F.B.; see acknowledgments) who was experienced and qualified for the task.
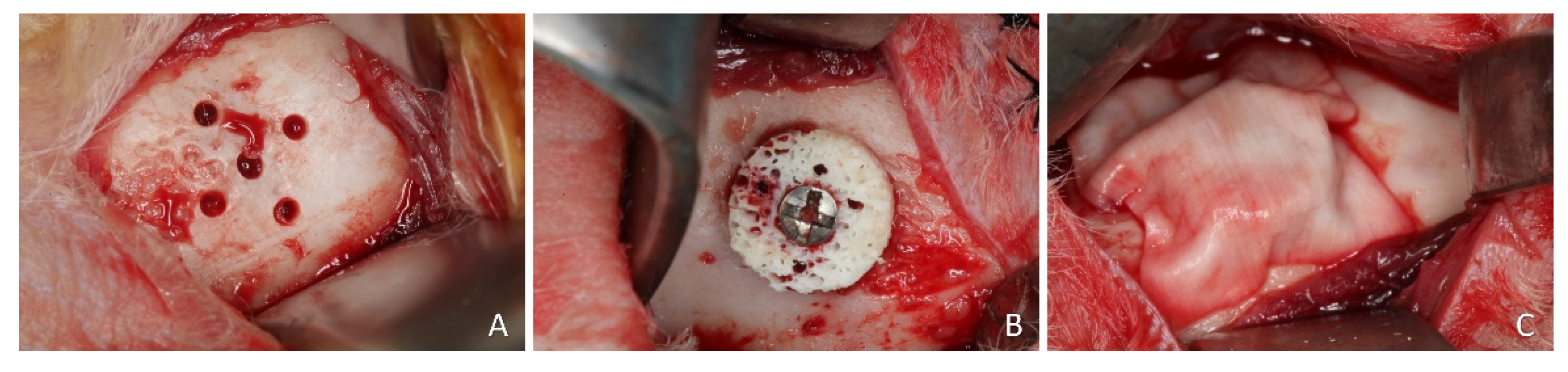
A linear incision of 2.5 to 3 cm was made bilaterally on the skin at the lower border of the mandible. Muscles and periosteum were reflected, and the flap was raised, exposing the convex bone of the buccal surface of the mandibular angle. At one site, a 1.0 mm truncated-conical drill coupled in a straight piece was used to perform five equidistant monocortical perforations in the entire diameter of the graft guided by a template made of stainless steel. These perforations reached the medullary portion of the recipient bed to promote blood and cell supply to the graft from the endosteum (
Figure 1A). The xenogeneic bone block, 7 mm in diameter and 3 mm in height, was appositionally fixed (onlay) using a 1.5 × 10 mm titanium screw (
Figure 1B) (Neodent, Curitiba, Brazil).
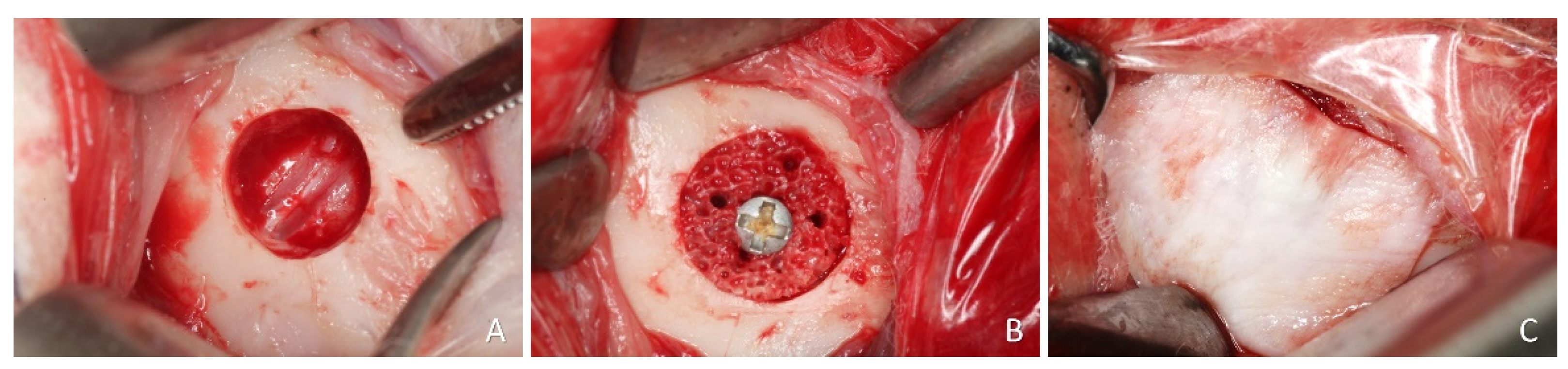
On the other side of the mandible a bone defect 7 mm wide and 3 mm deep was prepared using a trephine and refined with a diamond. (
Figure 2A). Subsequently, the xenogeneic bone block was positioned inside the defect, adapted to the level of the adjacent bone margin, and secured with a titanium screw (
Figure 2B). Collagen membranes (BioGuide®; Geistlich, Wolhusen, Switzerland) were used to cover both blocks (
Figure 1C and
Figure 2C). Wound closure was performed with Vicryl 4-0 on the muscular planes, and Nylon 4-0 on the skin with simple stitches.
2.8. Animal maintenance
All animals were medicated with ketoprofen (3.0 mg/kg, 12/12h, i.m., 10% Ketofen, Merial, Campinas, São Paulo, Brazil) and 2% tramadol hydrochloride (1.0 mg/kg, 12 /12h, subcutaneous., Cronidor, Agener União Saúde Animal, Apucarana, Paraná, Brazil) in the postoperative period and in the following 3 days.
Animals were housed in the Animal Facility of the Faculty of Dentistry of Ribeirão Preto, University of São Paulo. They were kept in individual metal cages (1 animal/4500 cm²) in an acclimatized room with split air conditioning, an exhaust fan (27 to 34 air changes/h), and automatic lighting control (12-hour light-dark cycle). All animals were fed with dedicated food and had access to water ad libitum.
A rigorous protocol for monitoring the animals was carried out throughout the experimental period, paying daily attention to the basic biological functions, feeding and excretion, behavioral signs in relation to postoperative pain, and monitoring of post-surgical infections and surgical wounds for suture care, bleeding, and/or signs of infection.
2.9. Euthanasia
The animals were euthanized by administering an overdose (2.0 mL) of intravenous thiopental 1.0 g (Thiopentax; Cristália, Itapira, São Paulo, Brazil) after 2 or 10 weeks, with 10 animals in each group. The experimental regions were dissected and reduced to individual blocks and maintained in 10% paraformaldehyde for fixation.
2.10. Histological processing
The specimens were taken to the FORP-USP hard tissue section laboratory for histological preparation. Initially, the specimens were washed with running water to completely remove the fixing agent, dehydrated in a gradual and increasing sequence of ethyl alcohol, changed every three days under constant agitation (60%, 80%, 96%, and absolute alcohol twice), and subsequently embedded in resin (LR WhiteTM HardGrid, London Resin Co Ltd, Berkshire, United Kingdom) for impregnation and subsequent polymerization in an oven at 60°C.
Once polymerization was complete, each block was cut following a transaxial plane in the center of the block guided by the fixation screw positioned at the center of the graft at the time of surgery.
Two sections of approximately 100 – 150 µm thickness were prepared using precision cutting/grinding equipment (Exakt, Apparatebau, Norderstedt, Germany) and ground to a thickness of approximately 60–80 µm. Histological sections were stained with either Toluidine Blue or with Stevenel’s Blue and Alizarin Red.
2.11. Histomorphometric evaluation
For histological and histomorphometric evaluation, an expert evaluator (K.A.A.A., see acknowledgements), who did not participate in the other stages of the study, was calibrated with another expert (D. B.) until the inter-rater agreement achieved a Cohen’s coefficient of k > 0.90.
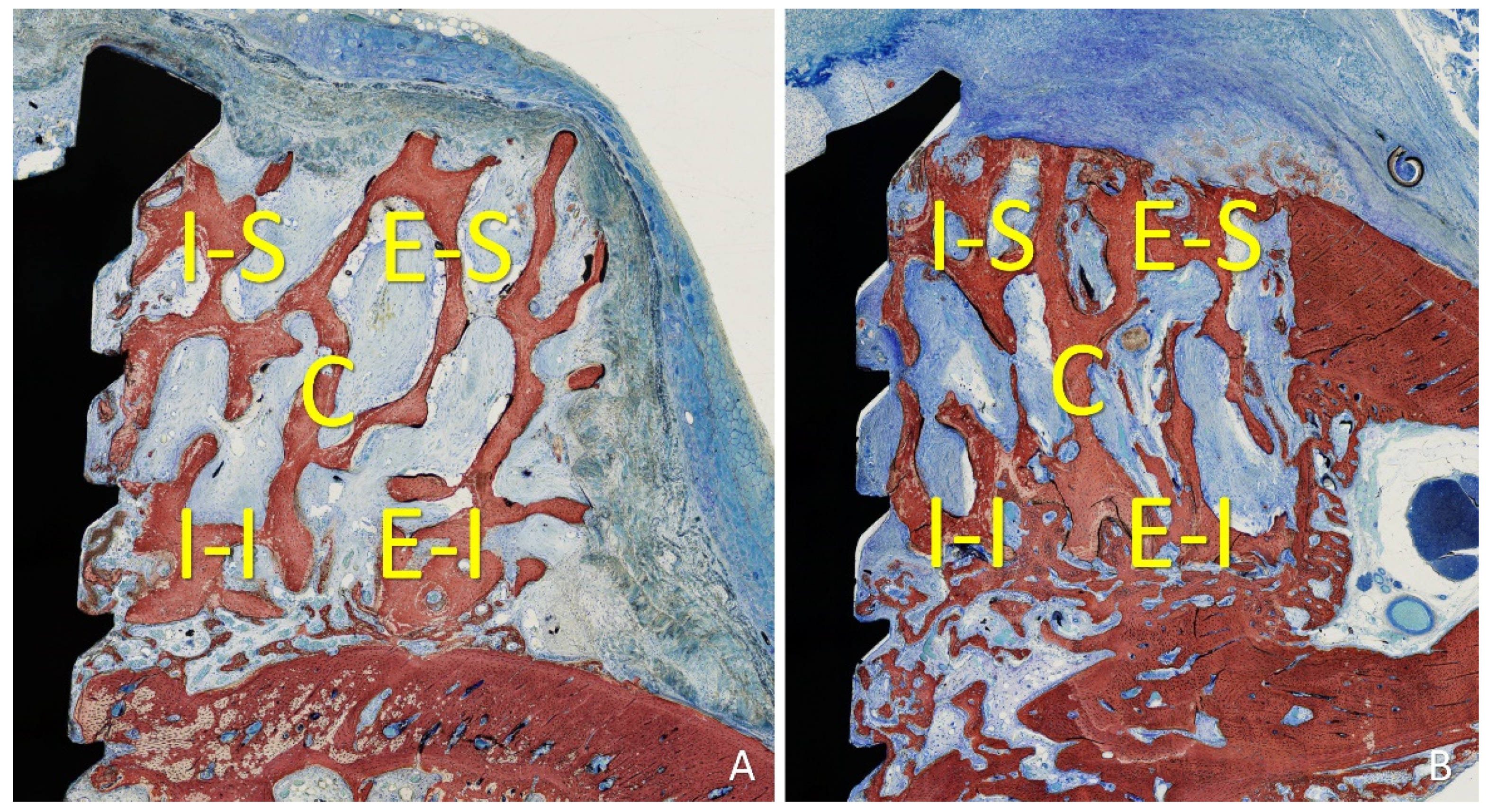
Five locations were evaluated within the grafted region both lateral to the fixation screw: inferior/internal (I-I), inferior/external (I-E), superior/internal (S-I), superior/external (S-E), and central (C). The following tissues were assessed: new bone, xenograft, soft tissues (marrow spaces, provisional matrix, dense and loose tissues, and connective tissue), vessels, inflammatory infiltrate, and osteoclastic zones (
Figure 3A,B). One grid containing 16 × 12 squares of 75 µm (1200 × 900 microns; 1.08 mm2) dimensions was superimposed onto the photomicrographs of each region using NIS-Elements software (Nikon, Tokyo, Japan).
2.12. Experimental outcomes and statistical methods.
The values obtained are expressed as the mean ± standard deviation. The primary variable was the mineralized new bone. The secondary variables were other tissues evaluated in the morphometric analysis. The Shapiro-Wilk test was used to determine the normality of data and, according to the results, the differences between the test and control sides were evaluated by a paired t-test or a Wilcoxon matched-pairs signed rank test. GraphPad Prism (version 10.0.2 for Windows, GraphPad Software, Boston, Massachusetts, USA,
www.graphpad.com) was used for the statistical analysis. The significance level was 5%.
3. Results
3.1. Clinical outcomes
The healing of the animals was uneventful. All histological slides were available for analysis, with n=10 for both periods.
3.2. Descriptive histological evaluation
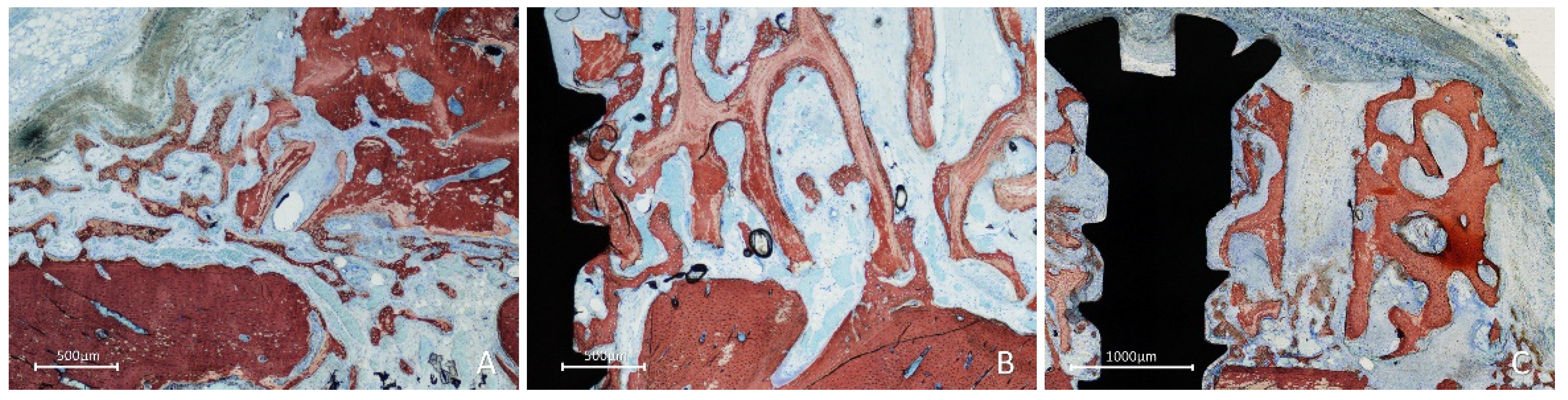
After two weeks of healing, in the onlay group, new bone was formed from the cortical layer of the lateral aspect of the mandible, interposed between the graft and parent bone, penetrating the cavities of the graft and lining onto the trabeculae (
Figure 4A). Active osteogenesis was observed through cortical perforations, which contributed to bone growth (
Figure 4B).
New bone was also observed forming laterally to the graft, progressing onto the outer surface of the graft (
Figure 5A), from the perforation at the fixation screw (Fig. 5B), and inside the perforations created in the grafts (
Figure 5C).
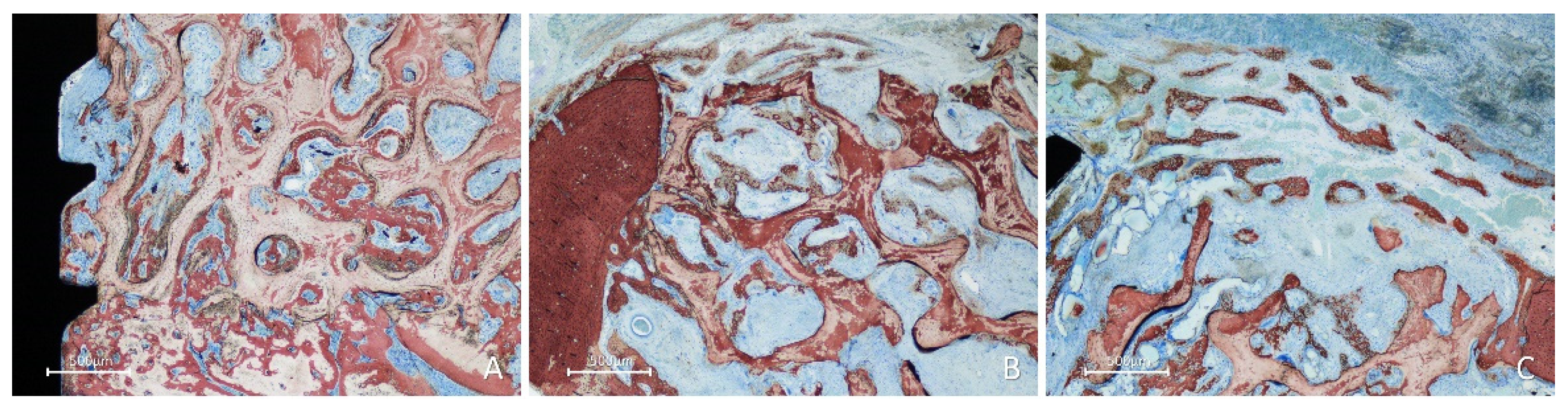
In the inlay group, after 2 weeks of healing, the position within the self-contained defect allowed the presence of multiple sources of new bone. Bone formation was observed in the cortex of the mandible, perforations of the fixation screw (
Figure 6A), and lateral aspects of the defect bone walls (
Figure 6B). At the periphery of the defect, new bone formed from the cortical layer, growing over the top of the graft and underneath the collagen membrane (
Figure 6B,C), closing the defect.
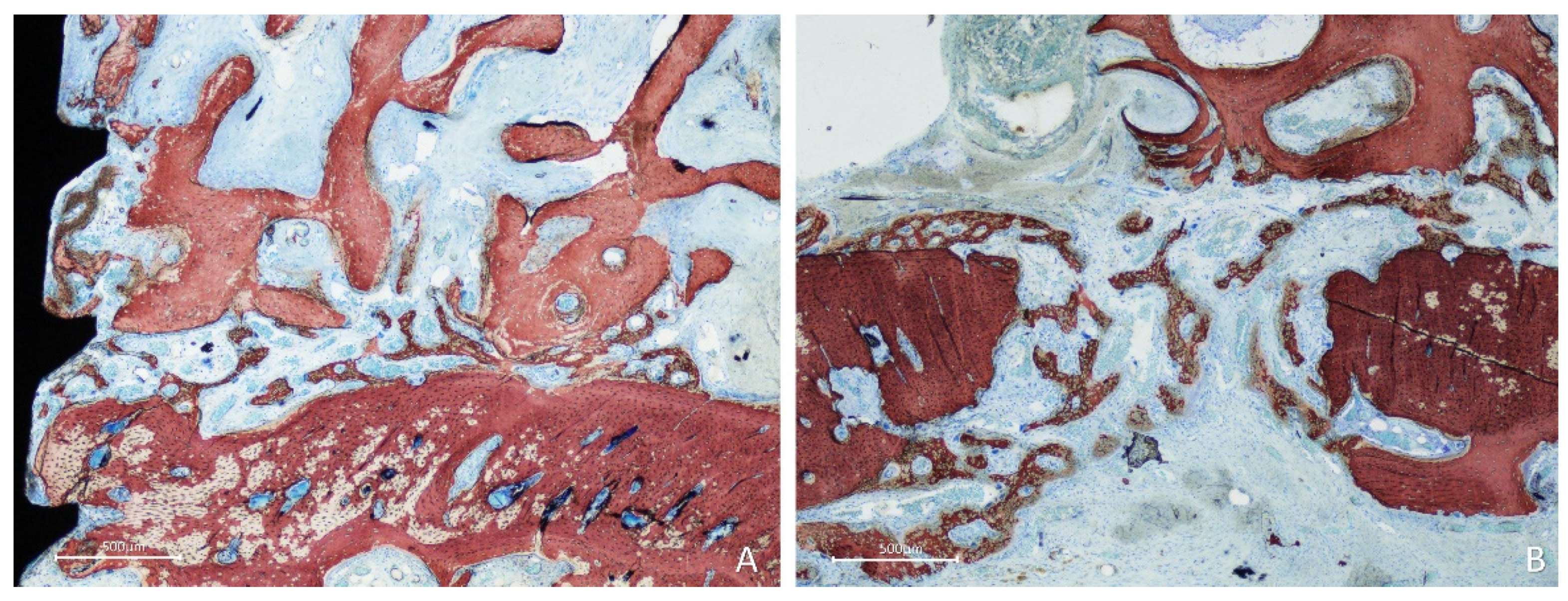
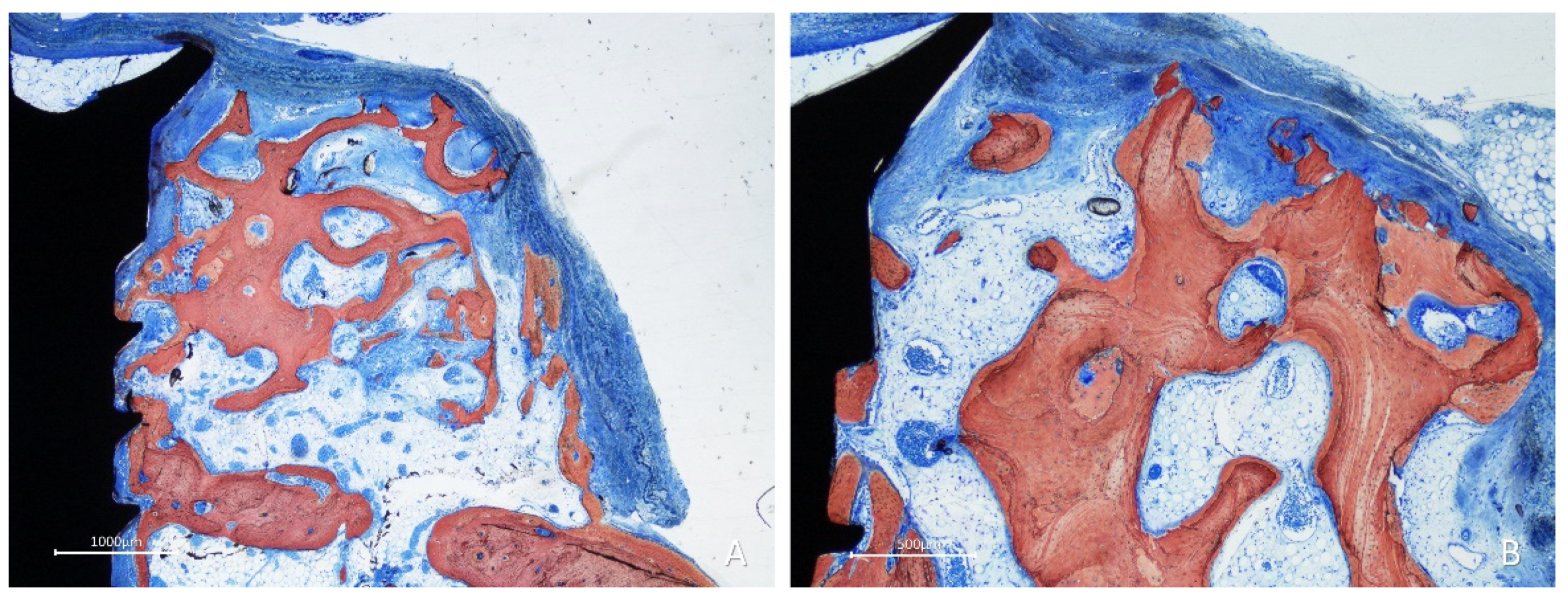
After 10 weeks of healing, bone was found lining the surface of the graft in the onlay group (
Figure 7A,B), while the space included among the trabeculae was filled with bone marrow and provisional matrix.
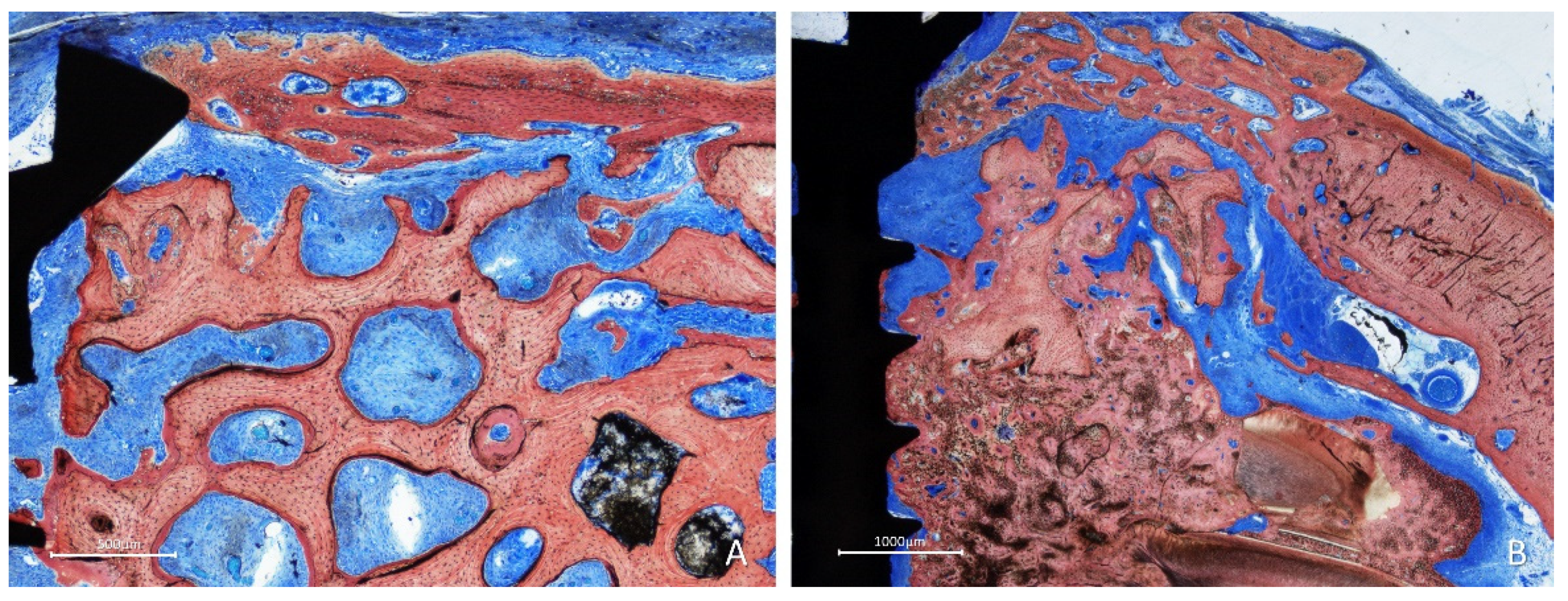
In most cases in the inlay group, the bone lined the trabeculae of the graft, and the spaces were filled with a dense provisional matrix or bone marrow (
Figure 8A). In some instances, dense bone occupied a large part of the graft (
Figure 8B); in several cases, the graft was covered by newly formed bone (
Figure 8A,B).
3.3. Histomorphometric assessments
After two weeks of healing, higher amounts of new bone were found in the inlay than in the onlay grafts. The differences were statistically significant for the mean total, I-I, and S-E regions. The region with the lowest amount of new bone was the S-I region in the inlay graft and the S-I and S-E regions in the onlay graft, that is, the regions farthest from the bone walls. The xenograft was found at a lower percentage in the inlay grafts than in the onlay grafts, and the difference was statistically significant only for the C region. The soft tissue percentage was similar in both groups, whereas that of the vessels was slightly higher in the onlay than in the inlay grafts in all regions. The difference was only statistically significant in the INF-EXT region. Small amounts of inflammatory infiltrates and osteoclast zones were also observed.
After 10 weeks of healing, the mean total new bone of both groups increased slightly compared with the previous period examined. While the inferior regions, closer to the bone walls, mostly presented a tendency to decrease the proportion of new bone, in the peripheral regions (superior), new bone increased considerably in percentage for both grafts. The percentage of xenografts decreased between the two evaluation periods. The difference was statistically significant only in the S-E direction for both the onlay and inlay groups. Between the two healing periods, the number of vessels decreased in the onlay graft group but increased in the inlay graft group. The percentage of inflammatory infiltrate increased only in the inlay group, while osteoclastic activity slightly increased in both grafts.
4. Discussion
The results from the present experiment showed that the mean total percentage of new bone was about the double in the inlay compared to the onlay grafts in both periods of healing. However, the difference was statistically significant only after two weeks (p=0.022) but not after 10 weeks of healing (p=0.080). This lack of significance may be related to the high variability among the animals observed in the results. However, statistically significant differences were found (p= 0.027) when the data for the superior regions (SI + SE) were merged. In the various regions evaluated within both grafts, the new bone proportion was always higher in the inlay grafts than in the onlay grafts. The reason for this difference is related to the placement of the inlay within a self-contained defect that presented multiple sites for bone formation. The defect was 7 mm wide and the dimensions may significantly affect healing.
In a study in rabbits, full-thickness bone defects 11 mm in diameter were created in the mandible [
39]. The defects were either filled with autogenous bone or biphasic calcium phosphate granules or left empty. The evaluations performed after 4 and 12 weeks revealed incomplete healing in all empty specimens. Conversely, both the autogenous bone and biphasic calcium phosphate groups showed better results, with the percentage of new bone being superior in the former compared to the latter. The defects used in the present study were slightly smaller and not full in thickness through the mandibular bone. This, in turn, means that healing was more favorable in the present model than that described in the study cited above, providing more bone walls from which bone is generated. This also resulted in more rapid formation of new bone within the inlay graft than in the onlay graft.
The tendency of circumferential defects to heal spontaneously, with [
40] or without implants [
41] might have also influenced the results. Nevertheless, the osteoconductive properties of the biomaterial used represent a key factor in obtaining proper healing.
In an experiment in dogs, a block graft composed of deproteinized bovine bone mineral (DBBM) and a block of autogenous bone was fixed to the lateral aspect of the mandible [
42]. After 6 months of healing, the autogenous block was found incorporated to the lateral aspect of the mandible while the DBBM block presented little newly formed bone at the base of the graft, close to the recipient site. In another experiment [
43,
44], standardized defects were created in the lateral aspect of the dog mandibles. After 3 months, either DBBM or autogenous blocks were placed and secured with fixation screws within the bone defects. Three months later, at all sites, one implant was placed in the region of the interface between the graft and recipient sites. The results after 3 months showed that all implants were integrated. However, the buccal aspects of the implant, that is, the aspect in contact with the graft, only presented integration in the autogenous group, whereas almost no integration was observed in the DBBM graft [
43]. Moreover, graft integration has been evaluated [
44]. While a vital autogenous block was well integrated into the parent bone, the DBBM block was mainly separated by the parent bone by a layer of connective tissue. In only a few instances, the bone occupied the graft in the basal zones.
In the present study, collagenated cancellous equine bone was used. New bone growing within the graft, lining onto the trabeculae at the basal aspect of the graft, was found already after 2 weeks of healing. After 10 weeks, new bone was formed within the graft toward the surface. This outcome agrees with that reported in another experiment in which similar graft blocks, either treated with plasma argon or left untreated, were fixed onto the lateral side of the mandible of rabbits [
37]. Similar to the present study, in that experiment, the bone grew within the graft onto the trabeculae up to the surface.
Analyses performed in different regions of the grafts allowed for further speculation. In both periods of healing, new bone was formed in high percentages in all but the superior regions of the inlay grafts. In practice, when lateral augmentation is performed on an alveolar bone with a flat surface (without a self-contained defect), the most lateral region of the graft might present very little bone, influencing the integration of implants in this zone.
New bone formation was observed between the base of the graft and recipient bone site. Owing to the convexity of the mandible in the region of interest, the adaptation of the graft onto that surface was not perfect, resulting in gaps. Nevertheless, these gaps were filled with newly formed bone, as it has already been described in another study mentioned above [
37]. The healing in the interface between gap and recipient site has been thoroughly described in a study that evaluate the healing of autogenous bone block grafts collected from the calvaria and secured either with a “position” or a “lag” screw technique [
45]. It was shown that, in this specific region, new bone that was present in a low percentage after 2 weeks, increased progressively so that, after 40 days, the graft was completely incorporated to the parent bone of the mandible. In another rabbit experiment [
46], autogenous bone blocks collected from the calvaria were glued with cyanoacrylate. Despite the optimal fixation of the graft, no incorporation of the graft onto the bone mandible was observed after 40 days of healing. Glue and connective tissue were found interposed between the graft and recipient bed.
In the present study, perforations produced at the recipient sites showed active osteogenesis. This activity has been described in detail in other studies [
45,
46]. The influence of perforations at the recipient sites was evaluated in an experimental study on 36 rabbits [
35]. Autogenous blocks harvested from the iliac crest were grafted to the lateral aspect of the mandible. One side was drilled while the control site was left intact. The healing was studied after 3, 5, 7, 10, 20, and 60 days. The authors concluded that the perforations induced angiogenesis earlier than in the control sites, allowing for larger remodeling and higher bone graft density.
The biomaterial was reduced by approximately 1/3 between weeks 2 and 10. However, it was still present at a high percentage after 10 weeks in both grafts. The slightly increased number of osteoclasts in the 12- compared to the 2-week healing period substantiates the speculation that graft resorption was still in progress. These results are similar to those reported in a similar study [
37]. In that study, the percentage of xenograft decreased by approximately 1/3 between weeks 2 and 10. However, in that study, the pick of osteoclast presence was seen after 6 weeks.
The percentage of soft tissues increased between 2 and 10 weeks. This was partly due to the remodeling of the newly formed bone and the formation of immature and mature bone marrow, as well as the invasion of the external regions of the graft by connective tissue.
As limitations of the present study should be mentioned the model used, the position of the graft, far away from the alveolar bone, and the faster healing of rabbits compared humans [
47]. Longer periods of healing should be analyzed. Based on the results from the present study, clinical study should be performed to evaluate the healing in humans.
5. Conclusions
The percentage of new bone increased faster and was higher in the inlay compared to the onlay grafts. The composition of the grafts allowed new bone to reach the most peripheral regions in both graft groups, although it was higher in the inlay group. Marginal closure of the defects by newly formed bone was observed in the inlay group.
Author Contributions
Conceptualization, R.S., S.P.X., K.M., and D.B.; methodology, R.S., S.P.X., and D.B.; validation, K.M., Y.N., S.B.; formal analysis, R.S., E.R.S., and D.B.; investigation, S.P.X. and E.R.S.; resources, S.P.X., D.B. and Y.N.; data curation, R.S. and D.B.; writing—original draft preparation, R.S. and D.B; writing—review and editing, R.S., S.P.X., D.B. and S.B.; visualization, K.M. and Y.N.; supervision, S.P.X., D.B., and S.B.; project administration, S.P.X., D.B. and Y.N.; funding acquisition, S.P.X, D.B., and Y.N. All authors have read and agreed to the published version of the manuscript.
Funding
The experiment was financially supported by ARDEC Academy, Rimini, Italy.
Institutional Review Board Statement
This research project was approved by the Committee on Ethics in the Use of Animals of the Faculty of Dentistry of Ribeirão Preto, University of São Paulo, Brazil, on September 25, 2019, protocol #2019.1.619.58.2.
Data Availability Statement
The data is available following a reasonable request.
Acknowledgments
We thank Dr. Vitor Ferreira Balan for the surgical procedures and Mr Sebastiao Blanco (University of São Paulo, Faculty of Dentistry of Ribeirão Preto) for processing the histological slides. The scientific contribution in the histological assessment by Dr Karol Alí Apaza Alccayhuaman (Department of Oral Biology, Medical University of Vienna, 1090 Vienna, Austria) was greatly appreciated.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Marx, R.E. Bone and bone graft healing. Oral Maxillofac Surg Clin North Am. 2007, 19, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Habal, M.B. Bone grafting in craniofacial surgery. Clin Plast Surg. 1994, 21, 349–363. [Google Scholar] [CrossRef]
- Adell, R.; Lekholm, U.; Rockler, B.; Brånemark, P.I. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surg. 1981, 10, 387–416. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Dahl, E.; Enbom, L.; Engevall, S.; Engquist, B.; Eriksson, A.R.; Feldmann, G.; Freiberg, N.; Glantz, P.O.; Kjellman, O.; et al. Osseointegrated oral implants. A Swedish multicenter study of 8139 consecutively inserted Nobelpharma implants. J Periodontol. 1988, 59, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Jemt, T.; Lekholm, U.; Gröndahl, K. 3-year followup study of early single implant restorations ad modum Brånemark. Int J Periodontics Restorative Dent. 1990, 10, 340–349. [Google Scholar]
- Misch, C.M.; Misch, C.E.; Resnik, R.R.; Ismail, Y.H. Reconstruction of maxillary alveolar defects with mandibular symphysis grafts for dental implants: a preliminary procedural report. Int J Oral Maxillofac Implants. 1992, 7, 360–366. [Google Scholar]
- Misch, C.E. Divisions of available bone in implant dentistry. Int J Oral Implantol. 1990, 7, 9–17. [Google Scholar]
- Salvato, G.; Agliardi, E. Calvarial bone grafts in severe maxillary atrophy: preprosthetic surgery with sedation. Implant Dent. 2007, 16, 356–361. [Google Scholar] [CrossRef]
- Le, B.; Burstein, J.; Sedghizadeh, P.P. Cortical tenting grafting technique in the severely atrophic alveolar ridge for implant site preparation. Implant Dent. 2008, 17, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Donovan, M.G.; Dickerson, N.C.; Mitchell, J.C. Calvarial bone harvest and grafting techniques for maxillary and mandibular implant surgery. Atlas Oral Maxillofac Surg Clin North Am. 1994, 2, 109–122. [Google Scholar] [CrossRef]
- von Arx, T.; Cochran, D.L.; Hermann, J.S.; Schenk, R.K.; Higginbottom, F.L.; Buser, D. Lateral ridge augmentation and implant placement: an experimental study evaluating implant osseointegration in different augmentation materials in the canine mandible. Int J Oral Maxillofac Implants 2001, 16, 343–354. [Google Scholar] [PubMed]
- von Arx, T.; Cochran, D.L.; Hermann, J.S.; Schenk, R.K.; Buser, D. Lateral ridge augmentation using different bone fillers and barrier membrane application. A histologic and histomorphometric pilot study in the canine mandible. Clin Oral Implants Res. 2001, 12, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Chiriac, G.; Herten, M.; Schwarz, F.; Rothamel, D.; Becker, J. Autogenous bone chips: influence of a new piezoelectric device (Piezosurgery) on chip morphology, cell viability and differentiation. J Clin Periodontol. 2005, 32, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Nowzari, H.; Aalam, A.A. Mandibular cortical bone graft part 2: surgical technique, applications, and morbidity. Compend Contin Educ Dent. 2007, 28, 274–280. [Google Scholar]
- Chiapasco, M.; Zaniboni, M.; Boisco, M. Augmentation procedures for the rehabilitation of deficient edentulous ridges with oral implants. Clin Oral Implants Res. 2006, 17 Suppl 2, 136–159. [Google Scholar] [CrossRef]
- Nkenke, E.; Weisbach, V.; Winckler, E.; Kessler, P.; Schultze-Mosgau, S.; Wiltfang, J.; Neukam, F.W. Morbidity of harvesting of bone grafts from the iliac crest for preprosthetic augmentation procedures: a prospective study. Int J Oral Maxillofac Surg. 2004, 33, 157–163. [Google Scholar] [CrossRef]
- Nkenke, E.; Schultze-Mosgau, S.; Radespiel-Tröger, M.; Kloss, F.; Neukam, F.W. Morbidity of harvesting of chin grafts: a prospective study. Clin Oral Implants Res. 2001, 12, 495–502. [Google Scholar] [CrossRef]
- von Arx, T.; Häfliger, J.; Chappuis, V. Neurosensory disturbances following bone harvesting in the symphysis: a prospective clinical study. Clin Oral Implants Res. 2005, 16, 432–439. [Google Scholar] [CrossRef]
- McAllister, B.S.; Haghighat, K. Bone augmentation techniques. J Periodontol. 2007, 78, 377–396. [Google Scholar] [CrossRef]
- Buser, D.; Chappuis, V.; Belser, U.C.; Chen, S. Implant placement post extraction in esthetic single tooth sites: when immediate, when early, when late? Periodontol 2000. 2017, 73, 84–102. [Google Scholar] [CrossRef]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable Materials for Bone Repair and Tissue Engineering Applications. Materials (Basel). 2015, 8, 5744–5794. [Google Scholar] [CrossRef] [PubMed]
- Cicciù, M.; Cervino, G.; Herford, A.S.; Famà, F.; Bramanti, E.; Fiorillo, L.; Lauritano, F.; Sambataro, S.; Troiano, G.; Laino, L. Facial Bone Reconstruction Using both Marine or Non-Marine Bone Substitutes: Evaluation of Current Outcomes in a Systematic Literature Review. Mar Drugs. 2018, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Dasmah, A.; Thor, A.; Ekestubbe, A.; Sennerby, L.; Rasmusson, L. Particulate vs. block bone grafts: three-dimensional changes in graft volume after reconstruction of the atrophic maxilla, a 2-year radiographic follow-up. J Craniomaxillofac Surg. 2012, 40, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Nyström, E.; Ahlqvist, J.; Legrell, P.E.; Kahnberg, K.E. Bone graft remodelling and implant success rate in the treatment of the severely resorbed maxilla: a 5-year longitudinal study. Int J Oral Maxillofac Surg. 2002, 31, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Gordh, M.; Alberius, P. Some basic factors essential to autogeneic nonvascularized onlay bone grafting to the craniofacial skeleton. Scand J Plast Reconstr Surg Hand Surg. 1999, 33, 129–146. [Google Scholar] [CrossRef]
- Pistilli, R.; Felice, P.; Piatelli, M.; Nisii, A.; Barausse, C.; Esposito, M. Blocks of autogenous bone versus xenografts for the rehabilitation of atrophic jaws with dental implants: preliminary data from a pilot randomised controlled trial. Eur J Oral Implantol. 2014, 7, 153–171. [Google Scholar]
- Al Ruhaimi, K.A. Bone graft substitutes: a comparative qualitative histologic review of current osteoconductive grafting materials. Int J Oral Maxillofac Implants. 2001, 16, 105–114. [Google Scholar] [PubMed]
- Troeltzsch, M.; Troeltzsch, M.; Kauffmann, P.; Gruber, R.; Brockmeyer, P.; Moser, N.; Rau, A.; Schliephake, H. Clinical efficacy of grafting materials in alveolar ridge augmentation: A systematic review. J Craniomaxillofac Surg. 2016, 44, 1618–1629. [Google Scholar] [CrossRef]
- Silva, E.R.; Balan, V.F.; Botticelli, D.; Soldini, C.; Okamoto, R.; Xavier, S.P. Histomorphometric, Immunohistochemical and Microtomographic Comparison between Autogenous and Xenogenous Bone Blocks for Mandibular Lateral Augmentation in Rabbits. Materials (Basel). 2021, 14, 6049. [Google Scholar] [CrossRef]
- Silva, E.R.; Chaushu, L.; Balan, V.F.; Botticelli, D.; Xavier, S.P. Fixation screw minimizes bone graft loss following autogenous lateral block graft augmentation: An experimental in vivo study. J Stomatol Oral Maxillofac Surg. 2022, 123, 395–400. [Google Scholar] [CrossRef]
- Kolk, A.; Handschel, J.; Drescher, W.; Rothamel, D.; Kloss, F.; Blessmann, M.; Heiland, M.; Wolff, K.D.; Smeets, R. Current trends and future perspectives of bone substitute materials - from space holders to innovative biomaterials. J Craniomaxillofac Surg. 2012, 40, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Grusovin, M.G.; Felice, P.; Karatzopoulos, G.; Worthington, H.V.; Coulthard, P. The efficacy of horizontal and vertical bone augmentation procedures for dental implants - a Cochrane systematic review. Eur J Oral Implantol. 2009, 2, 167–184. [Google Scholar] [PubMed]
- Xuan, F.; Lee, C.U.; Son, J.S.; Fang, Y.; Jeong, S.M.; Choi, B.H. Vertical ridge augmentation using xenogenous bone blocks: a comparison between the flap and tunneling procedures. J Oral Maxillofac Surg. 2014, 72, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Nyström, E.; Legrell, P.E.; Forssell, A.; Kahnberg, K.E. Combined use of bone grafts and implants in the severely resorbed maxilla. Postoperative evaluation by computed tomography. Int J Oral Maxillofac Surg. 1995, 24, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Faria, P.E.; Okamoto, R.; Bonilha-Neto, R.M.; Xavier, S.P.; Santos, A.C.; Salata, L.A. Immunohistochemical, tomographic and histological study on onlay iliac grafts remodeling. Clin Oral Implants Res. 2008, 19, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, W.F., Jr.; Okamoto, R.; Faria, P.E.; Arnez, M.F.; Xavier, S.P.; Salata, L.A. Immunohistochemical, tomographic and histological study on onlay bone graft remodeling. Part II: calvarial bone. Clin Oral Implants Res. 2009, 20, 1254–1264. [Google Scholar] [CrossRef]
- Kanayama, M.; Botticelli, D.; Apaza Alccayhuaman, K.A.; Yonezawa, D.; Silva, E.R.; Xavier, S.P. The Impact on the Healing of Bioactivation with Argon Plasma of a Xenogeneic Graft with Adequate Fixation but Poor Adaptation to the Recipient Site: An Experimental Study in Rabbits. Int J Oral Maxillofac Implants. 2021, 36, 703–714. [Google Scholar] [CrossRef]
- Schwarz, F.; Sager, M.; Ferrari, D.; Mihatovic, I.; Becker, J. Influence of recombinant human platelet-derived growth factor on lateral ridge augmentation using biphasic calcium phosphate and guided bone regeneration: a histomorphometric study in dogs. J Periodontol. 2009, 80, 1315–1323. [Google Scholar] [CrossRef]
- Kotagudda Ranganath, S.; Schlund, M.; Delattre, J.; Ferri, J.; Chai, F. Bilateral double site (calvarial and mandibular) critical-size bone defect model in rabbits for evaluation of a craniofacial tissue engineering constructs. Mater Today Bio. 2022, 14, 100267. [Google Scholar] [CrossRef]
- Carmagnola, D.; Berglundh, T.; Lindhe, J. The effect of a fibrin glue on the integration of Bio-Oss with bone tissue. A experimental study in labrador dogs. J Clin Periodontol. 2002, 29, 377–383. [Google Scholar] [CrossRef]
- Botticelli, D.; Berglundh, T.; Buser, D.; Lindhe, J. The jumping distance revisited: An experimental study in the dog. Clin Oral Implants Res. 2003, 14, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.G.; Sonohara, M.; Hayacibara, R.; Cardaropoli, G.; Lindhe, J. Lateral ridge augmentation by the use of grafts comprised of autologous bone or a biomaterial. An experiment in the dog. J Clin Periodontol. 2002, 29, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- De Santis, E.; Lang, N.P.; Scala, A.; Viganò, P.; Salata, L.A.; Botticelli, D. Healing outcomes at implants installed in grafted sites: an experimental study in dogs. Clin Oral Implants Res. 2012, 23, 340–350. [Google Scholar] [CrossRef]
- De Santis, E.; Lang, N.P.; Favero, G.; Beolchini, M.; Morelli, F.; Botticelli, D. Healing at mandibular block-grafted sites. An experimental study in dogs. Clin Oral Implants Res. 2015, 26, 516–522. [Google Scholar] [CrossRef]
- Caneva, M.; Botticelli, D.; Carneiro Martins, E.N.; Caneva, M.; Lang, N.P.; Xavier, S.P. Healing at the interface between recipient sites and autologous block bone grafts affixed by either position or lag screw methods: a histomorphometric study in rabbits. Clin Oral Implants Res. 2017, 28, 1484–1491. [Google Scholar] [CrossRef]
- De Santis, E.; Silva, E.R.; Martins, E.N.C.; Favero, R.; Botticelli, D.; Xavier, S.P. Healing at the Interface Between Autologous Block Bone Grafts and Recipient Sites Using n-Butyl-2-Cyanoacrylate Adhesive as Fixation: Histomorphometric Study in Rabbits. J Oral Implantol. 2017, 43, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Botticelli, D.; Lang, N.P. Dynamics of osseointegration in various human and animal models - a comparative analysis. Clin Oral Implants Res. 2017, 28, 742–748. [Google Scholar] [CrossRef]
Figure 1.
Clinical photographs of the onlay procedures. A, perforations of the cortical layer. B, block graft secured with a screw. Note the perforations of the block. C, collagen membrane on the top of the block.
Figure 1.
Clinical photographs of the onlay procedures. A, perforations of the cortical layer. B, block graft secured with a screw. Note the perforations of the block. C, collagen membrane on the top of the block.
Figure 2.
Clinical photographs of the inlay procedures. A, preparation of a calibrated defect, 7 mm wide and 3 mm deep. B, block graft inserted into the defect and secured with a screw. Note the perforations of the block. C, collagen membrane on the top of the block.
Figure 2.
Clinical photographs of the inlay procedures. A, preparation of a calibrated defect, 7 mm wide and 3 mm deep. B, block graft inserted into the defect and secured with a screw. Note the perforations of the block. C, collagen membrane on the top of the block.
Figure 3.
Five locations were evaluated within the grafted region both lateral to the fixation screw: inferior/internal (I-I), inferior/external (I-E), superior/internal (S-I), superior/external (S-E), and central (C).
Figure 3.
Five locations were evaluated within the grafted region both lateral to the fixation screw: inferior/internal (I-I), inferior/external (I-E), superior/internal (S-I), superior/external (S-E), and central (C).
Figure 4.
Photomicrographs of ground sections showing healing aspects at onlay graft sites after 2 weeks. A, new bone formed from the recipient site and from the perforation of the fixation screw. B, active osteogenesis through cortical perforations. Stevenel’s blue and alizarin red stain.
Figure 4.
Photomicrographs of ground sections showing healing aspects at onlay graft sites after 2 weeks. A, new bone formed from the recipient site and from the perforation of the fixation screw. B, active osteogenesis through cortical perforations. Stevenel’s blue and alizarin red stain.
Figure 5.
Photomicrographs of ground sections showing healing aspects at onlay graft sites after 2 weeks. New bone formed: A, laterally to the graft and from the perforations of the cortical layer; B, from the fixation screw site; C, inside a perforation created in the graft. Stevenel’s blue and alizarin red stain.
Figure 5.
Photomicrographs of ground sections showing healing aspects at onlay graft sites after 2 weeks. New bone formed: A, laterally to the graft and from the perforations of the cortical layer; B, from the fixation screw site; C, inside a perforation created in the graft. Stevenel’s blue and alizarin red stain.
Figure 6.
Photomicrographs of ground sections showing healing aspects at inlay graft sites after 2 weeks. Bone formation was observed: A, from the cortex of the mandible and the perforations of the fixation screw; B, from the lateral walls of the defect; C, from the lateral walls of the defect growing over the top of the graft and underneath the collagen membrane. Stevenel’s blue and alizarin red stain.
Figure 6.
Photomicrographs of ground sections showing healing aspects at inlay graft sites after 2 weeks. Bone formation was observed: A, from the cortex of the mandible and the perforations of the fixation screw; B, from the lateral walls of the defect; C, from the lateral walls of the defect growing over the top of the graft and underneath the collagen membrane. Stevenel’s blue and alizarin red stain.
Figure 7.
Photomicrographs of ground sections showing healing aspects at onlay graft sites after 10 weeks. A, the space included among the trabeculae was filled with bone marrow and provisional matrix. B, note new bone deposed onto the trabeculae. Stevenel’s blue and alizarin red stain.
Figure 7.
Photomicrographs of ground sections showing healing aspects at onlay graft sites after 10 weeks. A, the space included among the trabeculae was filled with bone marrow and provisional matrix. B, note new bone deposed onto the trabeculae. Stevenel’s blue and alizarin red stain.
Figure 8.
Figure 6. Photomicrographs of ground sections showing healing aspects at inlay graft sites after 10 weeks. A, In some instances, dense bone occupied a large part of the graft. B, in several cases, the graft was covered by newly formed bone. Stevenel’s blue and alizarin red stain.
Figure 8.
Figure 6. Photomicrographs of ground sections showing healing aspects at inlay graft sites after 10 weeks. A, In some instances, dense bone occupied a large part of the graft. B, in several cases, the graft was covered by newly formed bone. Stevenel’s blue and alizarin red stain.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).