INTRODUCTION
Salmonella enterica is a Gram-negative bacterium that is rod-shaped, noncapsulated, facultatively anaerobic, and nonsporulating. It belongs to the Enterobacteriaceae family. It can be found in water, soil, and animal faeces. It is also a foodborne pathogen that has emerged as the leading cause of food-borne bacterial infection (Eng et al., 2015). Salmonellosis is a disease caused by typhoidal and nontyphoidal Salmonella serovars that were primarily responsible for food poisoning in the twentieth century. Its infection has become a major public health issue in the United States, causing an estimated 1.4 million illnesses and 600 deaths each year (Roth et al., 2018). Salmonella is the most common causative agent of gastroenteritis in Klang Valley, Malaysia, according to Nor et al. (2023). Many studies have been conducted over the last few decades to control food poisoning and other microbial infections caused by Salmonella. Biofilms of Salmonella have been shown to adhere to surfaces such as stainless steel, polyester, plastic, and aluminium (Merino et al. 2019).
Biofilms are communities of microbial cells that are adhered to a living or inert surface and encased in a self-produced extracellular polymeric matrix (Yaacob et al. 2021). Bacterial attachment initiates biofilm formation, which is followed by microcolony formation, biofilm maturation, and finally biofilm dispersion. Biofilms can be found everywhere such as restrooms, hotels, food stalls, labs, and hospitals. They aid in drug, chemical, and physical stress resistance, as well as the host immune system. Antibiotics, antifungals, and natural products are examples of potential biofilm control measures (Zawawi et al. 2020; Johari et al. 2020; Isa et al. 2022). MADIO PRO+, a multipurpose disinfectant with a novel formulation, was tested for antibiofilm efficacy against S. Typhimurium biofilm in this study.
METHODOLOGY
Microorganism
Salmonella enterica serovar Typhimurium ATCC14028 was grown at 37 °C in nutrient broth. Culture purity was regularly confirmed by Gram staining and biochemical test. The bacterial inoculum was adjusted to an optical density (OD) of 0.7 at 600 nm before crystal violet assay.
Disinfectants
Commercial disinfectants used in this study are MADIO PRO+, chloroxylenol, sodium dodecyl benzene sulfonate, benzalkonium chloride, and sodium hypochlorite. They were tested at 25% (v/v).
Crystal violet assay
The effect of S. Typhimurium biofilm biomass following exposure to disinfectants was evaluated in a 96-wells microplate. Overnight inoculum (150 μL) and test solution (50 μL) were added to the microplate wells. An equal volume of fresh broth was added as negative control. The microplate was incubated overnight at 37 °C for 24 h. After discarding the medium, the biofilm fractions were rinsed with distilled water twice, heat-fixed at 60 °C for 30 min, stained with 0.5% (w/v) Crystal violet for five min, de-stained with sterile distilled water thrice, let to dry at room temperature, solubilized with 200 μL of 95% (v/v) ethanol for 10 min, and measured at 600 nm using ThermoFisher Scientific microplate reader.
Microplate biofilm assay for FTIR spectroscopy
Salmonella Typhimurium biofilm was grown in a 6-well microplate. Overnight inoculum (4 mL) was added to the microplate wells. Then, a volume of 1 mL of fresh nutrient medium was added. The microplate was incubated overnight at 37 °C. After 24 h period at 37 °C incubation, the content of the microplate was discarded while the microplate wells were rinsed with distilled water twice and the biofilm fraction was scrapped from the wall of the well after being suspended with phosphate-buffered saline. The suspension was then transferred into 1.5 mL centrifuge tubes and vortexed for 3 min. Then, they were centrifuged at 4000 rpm for 15 min at 4 °C to obtain the pellet. The resulting pellets were dried in the oven at 60 °C for at least 2 h.
FTIR spectroscopy
The biochemical composition of biofilm was determined using Perkin Elmer Spectrum One FTIR spectrometer. The dried cell pellets were positioned in direct contact with the diamond crystal, scanned in a range between 3000 cm−1 and 600 cm−1 with 4 cm-1 spectral resolution, and ratioed against a background spectrum previously collected from the clean sampling surface. Spectral data analysis, visualization, and processing were performed by using Perkin Elmer Applications Spectrum software.
Statistical analysis
Experimental data generated from crystal violet assay was expressed as mean ± standard deviation with n=3. A significant difference between control and test groups (p<0.05) was determined using an independent T-test.
RESULTS AND DISCUSSION
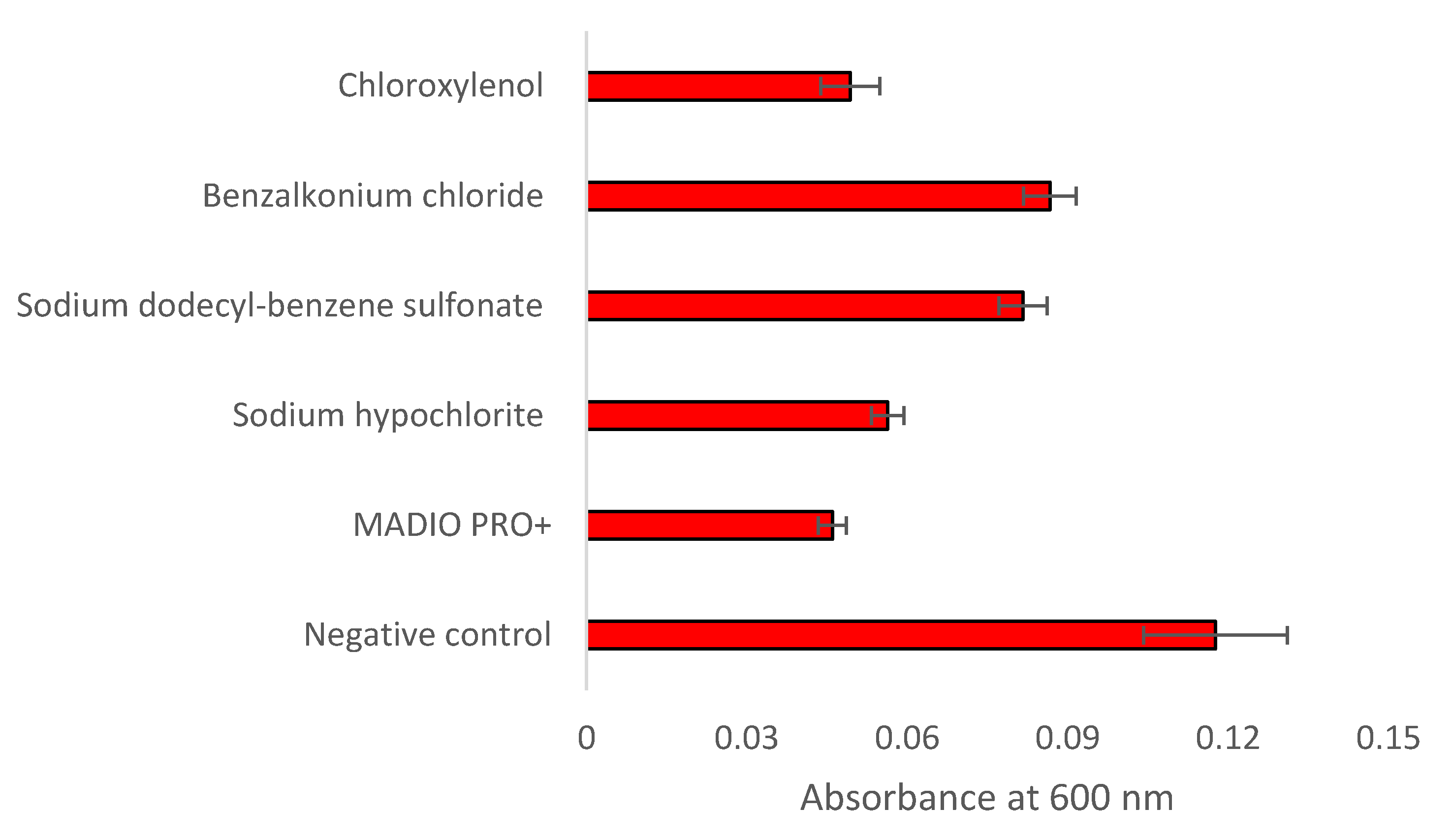
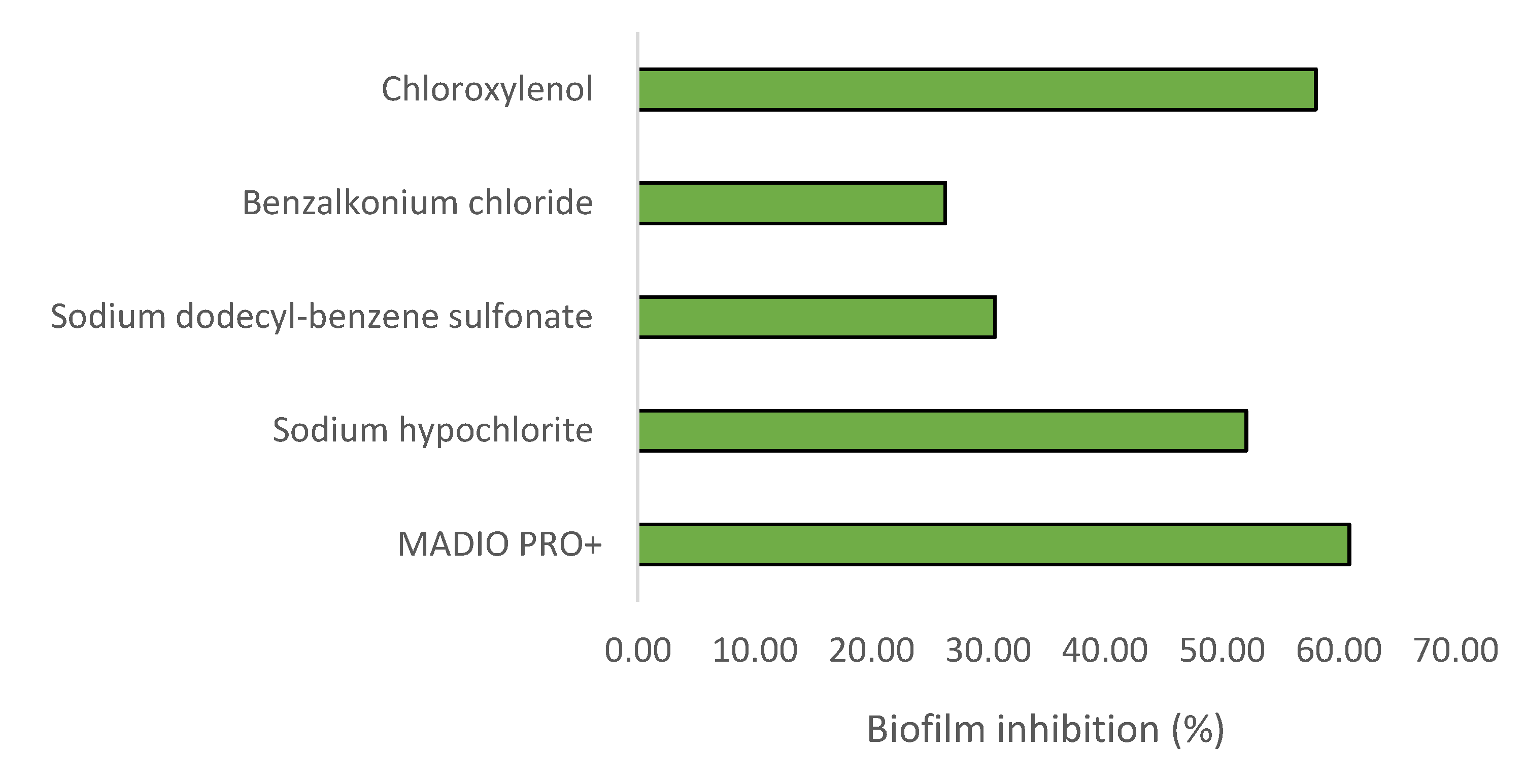
Figure 1 and
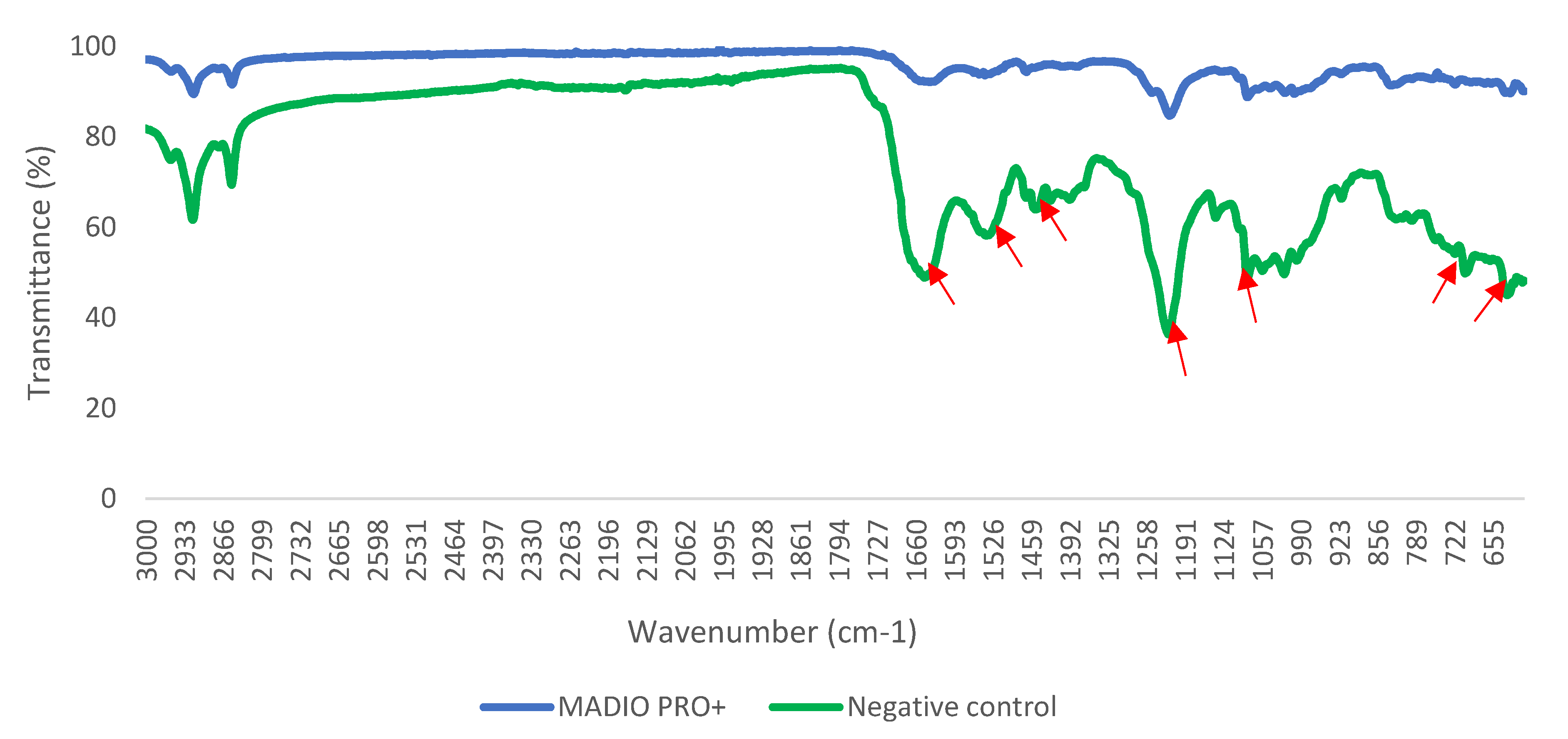
Figure 2 show the result of crystal violet assay. Treatment with all disinfectant significantly (p<0.05) reduced biomass of S. Typhimurium biofilm. Biofilm inhibition shown by MADIO PRO+, sodium hypochlorite, sodium dodecyl-benzene sulfonate, benzalkonium chloride and chloroxylenol were found to be 60.91%, 52.12%, 30.59%, 26.35%, and 58.07%, respectively. Treatment with MADIO PRO+ caused changes in FTIR spectral peaks associated with lipid (1460 cm
-1), protein (630 cm
-1, 702 cm
-1, 1550 cm
-1, 1650 cm
-1), and nucleic acid (1080 cm
-1, 1229 cm
-1). The biochemical modifications of S. Typhimurium biofilm were consistent with the inhibitory effects as shown by the crystal violet assay.
Chloroxylenol, sodium dodecyl-benzene sulfonate, benzalkonium chloride, and sodium hypochlorite are disinfectants commonly used for disinfecting surfaces and cleaning equipments. They generally function by disrupting the bacterial cell membrane and interfering with cellular metabolism (Bhathal et al. 2018; Capita et al. 2019; Wang et al. 2016). In this study, MADIO PRO+ disinfectant effectively inhibited S. Typhimurium biofilm by modifying its structure. It is understood that changes in FTIR spectroscopic fingerprint, peak position, and peak intensity indicate the changes in the structure of biological molecules (DeQueiroz and Day 2007; Yahya et al. 2018; Kamaruzzaman et al. 2022).
CONCLUSION
The multipurpose disinfectant MADIO PRO+ has an advantage in controlling Salmonella biofilm. The structural changes in the Salmonella biofilm mediate its antibiofilm efficacy. This discovery could help the food and health care industries to plan better microbial intervention strategies.
References
- Bhathal, M.K.; Kukreja, U.; Kukreja, N. Evaluation of Efficacy of Different Denture Disinfectants on Biofilms Formed on Acrylic Resin. Dent. J. Adv. Stud. 2018, 06, 020–027. [Google Scholar] [CrossRef]
- Capita, R.; Fernández-Pérez, S.; Buzón-Durán, L.; Alonso-Calleja, C. Effect of Sodium Hypochlorite and Benzalkonium Chloride on the Structural Parameters of the Biofilms Formed by Ten Salmonella enterica Serotypes. Pathogens 2019, 8, 154. [Google Scholar] [CrossRef] [PubMed]
- DeQueiroz, G.A.; Day, D.F. Antimicrobial activity and effectiveness of a combination of sodium hypochlorite and hydrogen peroxide in killing and removing Pseudomonas aeruginosa biofilms from surfaces. Journal of Applied Microbiology 2007, 103, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Eng, S.K.; Pusparajah, P.; Ab Mutalib, N.S.; Ser, H.L.; Chan, K.G.; Lee, L.H. Salmonella: a review on pathogenesis, epidemiology and antibiotic resistance. Frontiers in Life Science 2015, 8, 284–293. [Google Scholar] [CrossRef]
- Isa, S.F.M.; Hamid, U.M.A.; Yahya, M.F.Z.R. Treatment with the combined antimicrobials triggers proteomic changes in P. aeruginosa-C. albicans polyspecies biofilms. ScienceAsia 2022, 48, 215–222. [Google Scholar] [CrossRef]
- Johari, N.A.; Amran, S.S.D.; Kamaruzzaman, A.N.A.; Man, C.A.I.C.; Yahya MF, Z.R. Anti-biofilm potential and mode of action of Malaysian plant species: A review. Science Letters 2020, 14, 34–46. [Google Scholar]
- Kamaruzzaman, A.N.A.; Mulok, T.E.T.Z.; Nor, N.H.M.; Yahya, M.F.Z.R. FTIR SPECTRAL CHANGES IN Candida albicans BIOFILM FOLLOWING EXPOSURE TO ANTIFUNGALS. Malays. Appl. Biol. 2022, 51, 57–66. [Google Scholar] [CrossRef]
- Merino, L.; Procura, F.; Trejo, F.M.; Bueno, D.J.; Golowczyc, M.A. Biofilm formation by Salmonella sp. in the poultry industry: Detection, control and eradication strategies. Food Res. Int. 2019, 119, 530–540. [Google Scholar] [CrossRef]
- Nor, F.M.; Aazmi, S.; Anuar, T.S.; Muslim, A.; Aziz, M.N.; Ibrahim, N.; Yahya, M.F.Z.R.; Zainuri, S.N.; Yusof, F.Z.M. A Laboratory Perspective on an Epidemiological Pattern of Infectious Gastroenteritis: A Five-year Surveillance between 2016 to 2020 from Established Private Healthcare Centers within Klang Valley in Malaysia. J. Pure Appl. Microbiol. 2023, 17, 180–192. [Google Scholar] [CrossRef]
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, H.; Xing, T.; Wu, N.; Xu, X.; Zhou, G. Removal of Salmonella biofilm formed under meat processing environment by surfactant in combination with bio-enzyme. Lwt 2016, 66, 298–304. [Google Scholar] [CrossRef]
- Yaacob, M.F.; Murata, A.; Nor, N.H.M.; Jesse, F.F.A.; Yahya, M.F.Z.R. Biochemical composition, morphology and antimicrobial susceptibility pattern of Corynebacterium pseudotuberculosis biofilm. J. King Saud Univ. - Sci. 2020, 33, 101225. [Google Scholar] [CrossRef]
- Yahya, M.F.Z.R.; Alias, Z.; Karsani, S.A. Antibiofilm activity and mode of action of DMSO alone and its combination with afatinib against Gram-negative pathogens. Folia Microbiol. 2017, 63, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Zawawi, W.M.A.W.M.; Ibrahim, M.S.A.; Rahmad, N.; Hamid, U.M.A.; Yahya, M.F.Z.R. Proteomic analysis of Pseudomonas aeruginosa biofilm treated with Chromolaena odorata extracts. Malays. J. Microbiol. 2020, 16, 124–133. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).