Introduction
As was explained in the introductory physiology paper on water and salt metabolism in this issue (N. Lameire), the vast majority of dysnatremias, including hyponatremia arise from a primary imbalance in electrolyte-free water intake and loss, and less frequently from changes in the salt content of the body. Thus, the perturbation in water balance rather than any change in salt content is the main problem.
Hyponatremia is the most frequent electrolyte abnormality and is defined as a serum sodium concentration of less than 135 mEq/L [
1]. A disproportion between total body water (TBW) and sodium content [
2] below 120 mEq/L is considered as severe hyponatremia [
3]. The highest prevalence may be found among elderly ICU hospitalized patients (20-35%) with an increased mortality risk due to multiple comorbidities and medications [
4], and in marathon runners around 10% [
3,
5].
The causes of hyponatremia can be divided based on tonicity to hypotonic (decreased concentration of solute), isotonic, and hypertonic (falsely low sodium) [
6]; and according to the volume of the extracellular fluid (ECF) as hypovolemic, euvolemic, or hypervolemic hyponatremia [
7]. In addition, hyponatremia may develop rapidly as acute (<48 h) with usually severe symptoms, or slowly as chronic hyponatremia, usually as asymptomatic or with mild symptoms.
Nephrologists are very familiar with hyperosmolality in their uremic patients due to their elevated blood urea levels. However, urea is a non-effective osmole since it freely diffuses between the intra and extracellular compartment due to the constitutive presence of aquaporins. Tonicity, which is potentially confused with osmolality, denotes the concentration of osmoles - also known as effective osmoles - that do not freely cross cell membranes. Hypotonic plasma causes movement of water intracellularly, leading to cellular swelling. Conversely, hypertonic plasma causes cellular shrinkage. Sodium and glucose (in the presence of insulin lack) as obligate extracellular solutes are thus effective osmoles and contribute to both osmolality and tonicity, whereas membrane-permeable urea contributes to osmolality without affecting tonicity.
Hypertonic hyponatremia
An increased serum tonicity (>290 mOsm/kg) in hypertonic hyponatremia may be caused by hyperglycemia and use of mannitol. In case of hyperglycemia a conversion factor [plasma sodium (PNa) decreases by 1.6 mEq/L for every 100 mg/dL of plasma glucose over 100 mg/dL] should be used [
8].
Isotonic Hyponatremia(275-290 mOsm/kg) is also known as pseudo-hyponatremia and is associated with hypertriglyceridemia and hyperproteinemia. This creates a laboratory artifact. Ninety-three percent of plasma is water, and 7% is proteins and lipids. Sodium is distributed in the aqueous fraction. When PNa is measured in total serum or plasma using an indirect ion-selective electrode (ISE), a dilution step is required. When levels of plasma triglycerides, cholesterol or proteins are increased, the volume of the nonaqueous fraction also increases and displaces the water fraction so that the total plasma now contains less water per unit volume (and hence less sodium per unit volume). Under these circumstances, measuring plasma Na
+ will lead to a dilution error, causing a falsely reduced plasma sodium. Direct ISE (eg, blood gas analyzer) avoids this error. In addition, isotonic hyponatremia can also be found with the use of irrigant solutions (mannitol, sorbitol or glycine) in urological (transurethral resection of the prostate), colonoscopy in gastroenterology and some gynecological procedures [
9].
Hypotonic hyponatremia
As explained in the physiology introduction, a normal plasma sodium (135-145 mmol/l) and plasma osmolality/tonicity (275-290 mOsm/kg) is maintained by interplay between thirst and ADH secretion, balancing water intake with urinary water excretion.
Hypotonic hyponatremia or true hyponatremia (serum osmolality <275 mOsm/kg) is the most common type of hyponatremia and may be found in cases of: a) free water excess caused by either intake of large volume exceeding the kidney diluting capacity or in cases of reduced kidney water excretion with inappropriately high non-osmotically stimulated ADH level. The latter can be caused by a decreased effective arterial blood volume because of diarrhea, vomiting, heart failure with low cardiac output, or vasodilation in sepsis or cirrhosis), syndrome of inappropriate antidiuresis hormone (SIADH) with autonomous ADH secretion (brain or lung disorder, certain classes of drugs and other conditions like nausea and pain), and decreased cortisol release and lack of the inhibitory effect on ADH secretion [
10]; b) reduced glomerular filtration rate (GFR) and thus kidneys ultrafiltration capacity (acute/chronic and end-stage kidney disease); c) decreased solute daily consumption (<600 mOsm) and hence, reduced solute excretion and urine volume (beer potomania, excessive tea consumption without sufficient nutritional osmolar intake). Hence, the beer potomania is considered as an uncommon etiology of hyponatremia, reported solely through case presentations. The mechanism of water retention by the kidney in the presence of a broad range of urine osmolality, seems to involve a dynamic course of vasopressin secretion during the development of hyponatremia, though the status of vasopressin release is rarely investigated. Thus, a detailed fluid balance studies, sequential observations of changes in urine and plasma osmolality would advance the understanding of its pathophysiology [
11].
Based on the Edelman equation, hypotonic hyponatremia will occur when the ratio between the sum of total body exchangeable sodium (NaE) and total body exchangeable potassium (KE) and total body water (TBW) decreases. There are 4 different scenarios where this can occur leading to hypotonic hyponatremia: (1) normal NaE and KE with increased TBW, eg, primary polydipsia; (2) increased NaE and KE and increased TBW but with a proportionally higher increase in TBW, eg, cirrhosis and heart failure; (3) decreased NaE and KE and decreased TBW but with a proportionally higher decrease in NaE + KE, eg, hypovolemia; and (4) decreased NaE and KE with increased TBW, eg. SIADH.
An important concept based on this equation is thus that hypotonic hyponatremia is always caused by electrolyte-free water (EFW) excess, i.e., TBW is proportionally higher to the sum of NaE and KE.
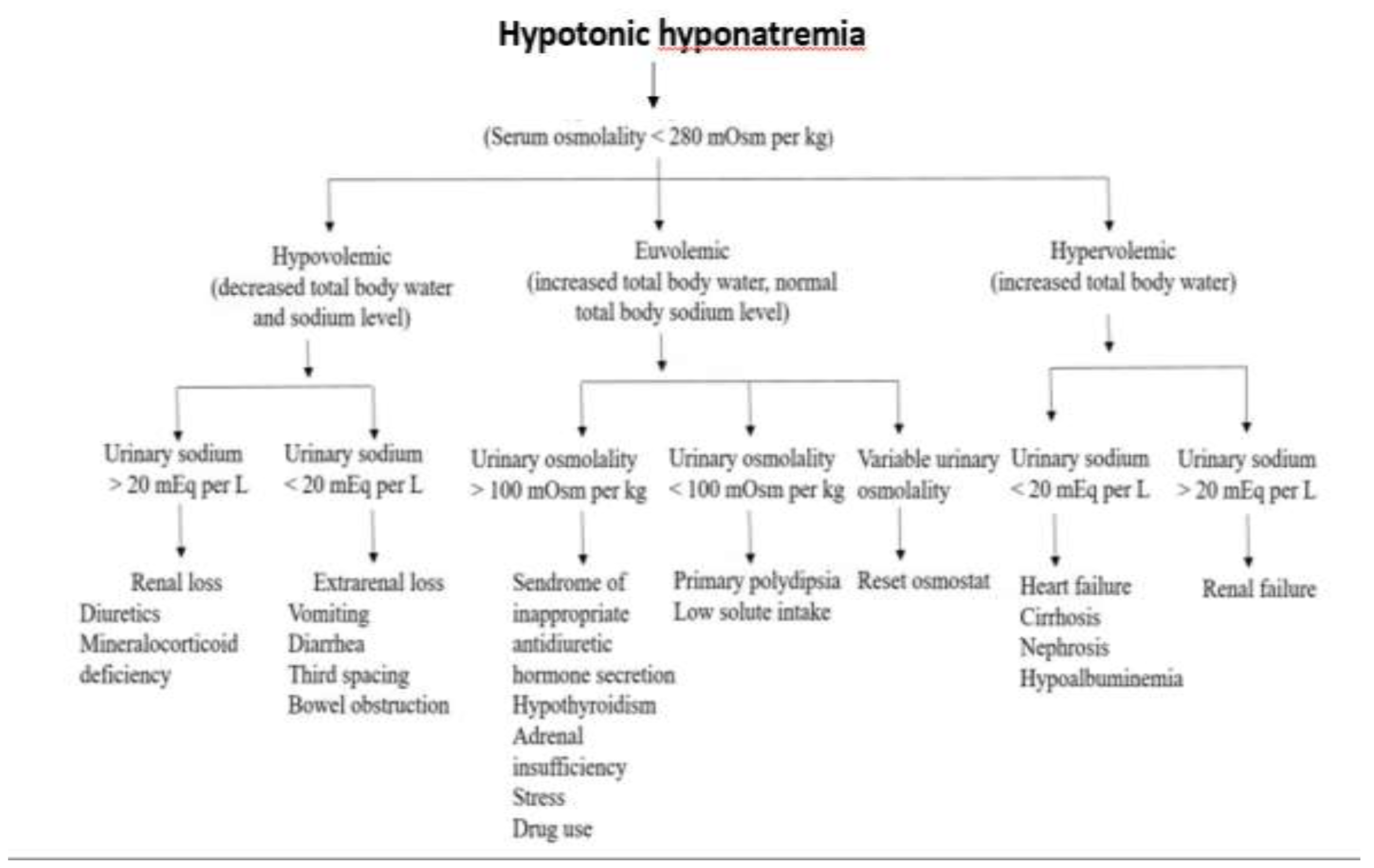
Clinical history and physical examination provide essential information for the diagnosis of hypotonic hyponatremia and should allow classification of patients with hypovolemic, euvolemic, or hypervolemic hyponatremia. It should be noted in
Figure 1 that the assessment of the urinary sodium excretion is important to differentiate the hypo- and hypervolemic forms associated hyponatremia while to diagnose the different causes of euvolemic hyponatremia, measuring the urinary osmolality should be helpful.
Hyperglycemia and other causes of non-hypotonic hyponatremia must be excluded to confirm hypotonic hyponatremia. Initial laboratory tests and imaging studies combined with a history and physical examination are usually sufficient to arrive at the cause of hyponatremia.
An extensive list of causes of hypotonic hyponatremia can be consulted in the review by Adrogue et al. [
12].
Hypovolemic hypotonic hyponatremia
The most frequent category hypotonic hypovolemic hyponatremia (greater decrease of TBW relative to the total body sodium and potassium decrease) may be caused by the use of diuretics with volume depletion and stimulated antidiuretic hormone (ADH) release [
6], fluid loss (vomiting, diarrhea, sweating) [
2], loss of fluids in the third space most frequently associated with hypoalbuminemia, cerebral salt-wasting condition (increased brain natriuretic peptide), combined glucocorticoid and mineralocorticoid deficiency [
6], and exercise-associated hyponatremia (EAH) [
13]. A clinically important, although relatively rare cause of hyponatremia is treatment with diuretics, particularly known as thiazides induced hyponatremia (TIH). Besides of the increased sodium excretion, there is a complex mechanism of thiazide induced hyponatremia interfering with the diluting mechanisms of the urine by their action on the cortical diluting nephron segments (see physiology introduction), by stimulation of non-osmotic antidiuretic hormone release, and by their induction of chronic potassium depletion [
14]. Here, the hypokalemia shifts sodium intracellularly and enhances vasopressin release worsening hyponatremia. The role of the hypokalemia induced hyponatremia is important to understand the adequate treatment of diuretic induced hyponatremia (see below the discussion on therapy of hyponatremia). Hypertensive old women that are with low body mass and hypokalemic are especially susceptible to thiazide-induced hyponatremia, particularly when they have a preexisting defect in renal capacity to excrete free water. However, they rarely develop severe hyponatremia and TIH resolve at two weeks after drug withdrawal [
15].
Euvolemic Hyponatremia(stable total body sodium with increased TBW) in presence of excessive (nausea, severe pain), or inappropriate ADH secretion known as SIADH [
16]. It may be divided into cases with diluted urine (adrenal insufficiency, hypothyroidism, and excessive intake of water - polydipsia or beer - potomania), or concentrated urine – true SIADH [
17]. SIADH has been characterized by inappropriate ADH secretion regardless of an increased plasma volume reducing urine output and leading to hyponatremia [
18]. The diagnosis is based on the exclusion of other causes since any unique test or sign is unavailable [
19]. It may be caused by any central nervous system (CNS) disorder, ectopic ADH production (small cell carcinoma of the lung), pneumonia or tuberculosis, and in patients with various postoperative anti-pain medication. Treatment of the underlying causes of SIADH [
12] together with reduction of fluid intake and vasopressin 2 receptor inhibitors use may be helpful for treatment of the hyponatremia [
20].
Euvolemic hyponatremia may also be caused by various drugs (antidepressants, opioids and antipsychotics [
21], thiazides, some anti-cancer drugs, nonsteroidal anti-inflammatory drugs) [
22], and recreational drugs (ecstasy) - or excessive fluid use during transurethral resection (TUR) syndrome, colonoscopy or cardiac catheterization intervention [
9], and cerebral salt wasting [
23].
Ecstasy-associated hyponatremia is complicated and due to large water intake, often secondary to ecstasy mediated hyperthermia with loss of water and electrolytes, and non-osmotic ADH-secretion mediated by ecstasy and its metabolites. Women are overrepresented (85%) in the population with this complication [
24].
Hyponatremia associated with disorders in cerebral pathology such as contusion, intracranial hemorrhage, CNS tumors, meningitis and encephalitis is most commonly due to SIADH or cerebral salt wasting (CSW), especially in those with subarachnoid hemorrhage [
25]. CSW may be caused by either decreased sympathetic nervous system function or secretion of a circulating factor that decreases renal sodium reabsorption. It is characterized by low serum sodium with low plasma osmolality and high urine osmolality (> 100 mOsm/L [mmol/L] and frequently > 300). Urine sodium is usually > 40 mmol/L and serum uric acid is low. CSW may be improved with isotonic saline administration, as main difference from SIADH that does not.
Hypervolemic Hyponatremia(greater increase of TBW compared to the increase of total body sodium) can develop in the presence of acute / chronic renal failure or nephrotic syndrome) or by extrarenal causes such as congestive heart failure, liver cirrhosis or excessive fluid intake [
26]. In this category there is a decreased effective circulating volume stimulating non-osmotically induced antidiuretic hormone (ADH) release resulting in water retention and generalized edema.
Clinical picture
Patient’s medical history is important as it could point to a certain diagnostic direction. Clinical symptoms and signs correspond to the chronicity and degree of hyponatremia. In patients with a gradual decrease in sodium diagnosed as mild to moderate hyponatremia (>120 mEq/L) and developed over more than 48 the symptoms may be absent or minimal.
As hyponatremia develops, all cells take up water and swell. This is problematic for the brain in the non-distensible cranium. Astrocytes are particularly sensitive to osmotic stress, though expulsion of potassium and electrolytes occurs over 6 to 12 hours and organic osmolytes including glutamine and taurine over a 24- to 48-hour period. The decreased intracellular solute content mitigates swelling with hypotonic hyponatremia. Taking into account the time course of brain compensation, acute hyponatremia is therefore defined by expert opinion as developing over less than 48 hours.
Acutely developed severe hyponatremia (<48 hrs, <120 mEq/L) causes thus cerebral edema and the symptomatology may vary between either mild (headaches, vomiting, nausea, fatigue, anorexia, muscle cramps [
3], impaired cognition, reduced attention, altered posture and gait with increased falls symptoms [
27]) or severe symptomatology due to hyponatremic encephalopathy presenting as confusion, agitation, seizures, or even coma and death [
28,
29]. Chronic hyponatremia may also interfere with the bone metabolism and be associated with an increased risk of osteoporosis and bone fracture [
30]. Nevertheless, an urgent evaluation of the volume and neurological status is essential for prevention of neurological damages [
31].
Therapeutical approach
The treatment of hyponatremia should be based on the underlying cause [
2,
6], the duration and degree of hyponatremia, the observed symptoms, and volume status of the patient. The speed of correction depends on the severity of the symptoms [
6]. The type of fluids and the speed of administration as cornerstone of the initial management are calculated based on the equation for sodium deficit = (140 – serum sodium) x total body water, where total body water = kilograms of body weight x 0.6 [
5,
6].
Of note, rapid hyponatremia correction may lead to complications [
33]. In severely symptomatic acute hyponatremia rapid correction with 3% sodium chloride and 100 mL intravenous (IV) bolus [
30] is either repeated based on the persistence of symptoms [
26], or given up to 5 mmol/L sodium increase over 1-4 hours is reached [
6]. In cases of mild to moderate hyponatremia with symptoms slow infusion of 3% sodium chloride should be administered, after calculation of the sodium deficit and frequently monitored [
26].
One of the few recent studies prospectively investigating whether hypertonic saline is best administered as slow continuous infusion (SCI) therapy or rapid intermittent bolus (RIB) therapy for symptomatic severe hyponatremia is the SALSA study [
34]. The primary outcome was “overcorrection” at any given period, defined as increase in the serum Na
+ level by > 12 or 18 mmol/L within 24 or 48 hours, respectively. The results showed that both RIB and SIC therapies of hypertonic saline for treating hyponatremia were effective and safe, with no difference in the need for overcorrection. However, RIB had a lower incidence of therapeutic re-lowering treatment and tended to have a better efficacy in achieving serum Na
+ within 1 hour compared to SCI. Overall, the results showed that rapid intermittent administration of hypertonic saline is the preferred treatment of symptomatic hyponatremia, which is consistent with the current consensus guidelines.
Less aggressive treatment is certainly administered in asymptomatic chronic hyponatremia: if hypovolemic, an isotonic sodium chloride fluid without diuretics should be administered and if euvolemic it should be restricted up to 1-liter daily fluid. In hypervolemia the underlying cause should be treated restricting salt and fluids, and diuretics should be regularly used.
Within therapies for chronic non-severe hyponatremia, oral urea as an oral osmotic diuretic that increases urinary water excretion appears as very effective and safe treatment for prevention of possibly associated morbidity and mortality [
35]. Since, it has been tolerated poorly due to its taste, there are newer proven safe and efficient oral American formulations enhancing its palatability [
36]. The dose of oral urea is 0.25–0.5 g/kg/ day and it should be increased in cases of a higher urine osmolality, usually > 30 g/day [
37].
However, in severe chronic hyponatremia <120 mEq/L immediate 3% IV saline (15-30 mL/hour) should be administered even in asymptomatic patients [
38].
In specific patients with hypokalemia, alcoholism, malnutrition, liver disease [
39], and high-risk for osmotic demyelination syndrome (ODS) sodium correction should be limited up to 8 mEq/L, in the averaged-risk patients up to 10 mEq/L and up to 12 mEq/L for 24 hours in others. Serum sodium should be measured every 4-6 hours, and if the increase has reached 8 mEq/L (8 mmol/L) in the first 12 hours, measures to prevent a further increase should be instituted by matching urine output with 5% dextrose in water. When desmopressin (dDAVP) is used to avoid overly rapid correction, the recommended dose is 2 - 4 μg parenterally every 6 to 8 hours. If inadvertent overcorrection has occurred, there is a window of opportunity to again decrease serum sodium levels using dDAVP to prevent brain lesions [
12].
Particularly in case of combined hyponatremia and hypokalemia it should be remembered that the primary mechanism of the hyponatremia in that situation is that potassium depletion results in a shift of sodium into the cell with a commensurate exit of potassium from the cell into extracellular fluid. The reverse process occurs during potassium repletion. Treating simultaneously the hyponatremia with saline and the hypokalemia with potassium infusions risks an “overshooting“ of the plasma sodium increase with dangerous hypernatremia as a consequence and risk of ODS [
40]
.
In patients with normovolemic hypotonic hyponatremia fluids should be restricted. Malnourished SIADH patients should be put on high protein diet, taking into account the solute load and consequently more free water renal removal. SIADH hyponatremia patients usually have sodium levels <135 mEq/L with serum osmolality <280 mOsm/kg, urinary sodium levels >20 mMol/L and urine osmolality >100 mOsm/L [
41]. Here, the underlying cause of SIADH should be corrected and fluids restricted, while in hypervolemic hyponatremia fluid restriction as well as low salt diet should be prescribed [
42].
Mild and asymptomatic hyponatremia is treated with adequate solute intake (salt and protein) and initial fluid restriction of 500 mL/d with adjustments based on serum sodium levels. Prolonged fluid restriction of 1200-1800 mL/d is usually maintaining the patient asymptomatic. Moderate and/or mild symptomatic hyponatremia is treated with hypertonic saline 3% raising the serum sodium up to 8 mmol/L during the first day and correcting sodium and potassium losses with 0.9% saline and furosemide use. Severe hyponatremia or severe symptoms are treated with hypertonic saline (3%) 1–2 mL/kg IV in 3–4 h. In cases with hypovolemic hyponatremia due to the diuretic use and with low blood potassium levels, simultaneous correction of potassium can assist the correction of hyponatremia [
33].
Concerning the prognosis, it depends from the severity and underlying cause of hyponatremia and is poor in acute, severe hyponatremia, particularly in older population [
43]. The consequences of untreated or inadequately treated hyponatremia are altered cognitive status, seizures, rhabdomyolysis, coma and death. Rapid correction of chronic hyponatremia may cause ODS, previously recognized as central pontine myelinolysis [
44], and should be treated with dDAVP [
45].
Specific treatment with selective vasopressin 2 receptor antagonists (vaptans) should be considered in either euvolemic or hypervolemic patients with high ADH activity [
46,
47]. These drugs increase renal free water excretion while remaining sodium unaffected, thus increasing the serum sodium level. However, American and European guidelines did not reach the same conclusion regarding these medications [
48]. In addition to common use in SIADH patients [
33], the US unlike the EU guidelines recommend their use in patients with cirrhosis, or heart failure who fail limiting fluid intake [
48]. The evidence suggests that vaptans may slightly be more effective than fluid restriction in hyper or euvolemic hyponatremia, but should not be used in people with hypovolemia [
49].
Finally, hyponatremia frequently present (~30%) in nursing homes and in approximately 30% of people who are depressed and use selective serotonin reuptake inhibitors [
32]. People with hyponatremia require longer hospitalizations, with increased medicare costs and a higher number of readmissions [
50].