1. Introduction
The global discourse concerning population growth rate (population dynamics) is rapidly intensifying, accompanied by a significant quandary: the capacity for production and equitable dissemination of food resources. These factors exert considerable pressure on enterprises to elevate the output of processed food items, necessitating more streamlined and proficient procedures that prioritize human well-being and consumer security. The issue of food contamination stands as a formidable challenge for the manufacturing sector, mandating stringent oversight of processes encompassing the procurement of raw materials to the final packaging stage. [
1,
2,
3].
This is the rationale behind the concerted efforts of directors, engineers, biologists, and scientists who have devised an array of protocols, documentation, and procedures dedicated to scrutinizing contamination throughout the food production cycle. Standardizing these protocols serves a twofold purpose: elucidating and preempting instances of cross-contamination across diverse facets including packaging, equipment, instruments, attire, hands, and personnel, ultimately safeguarding the welfare of consumers.
This constitutes a pivotal stride in ensuring the untainted nature of the process, rendering it amenable to evaluation by regulatory bodies such as ANVISA (Brazil’s National Health Surveillance Agency) or the FDA (Food and Drug Administration in the USA). Such assessments of quality and safety are indispensable for diverse food items, spanning the likes of broccoli, milk, meat, and coffee. [
1,
4].
This standardization establishes a foundational framework, compelling the entire industry to adhere to uniform parameters across every stage of production. This coherence not only fosters an elevation in quality but also, inextricably, safeguards human health and well-being. One pivotal facet demanding meticulous oversight within a company’s operations pertains to microbiological control.
Controlling contamination by bacteria, viruses, and fungi during the manufacturing process is achievable through the strategic management of several facets, facilitated by the implementation of the clean-in-place (CIP) protocol. This protocol entails the utilization of sanitizing agents such as hypochlorite, peracetic acid, and sodium hydroxide. These solutions serve the dual purpose of cleansing the equipment and governing the proliferation patterns of microorganisms across all operational junctures. [
5].
Presently, within vegetable production facilities, a chemical solution with a concentration of around 40-80 ppm is administered on an hourly basis throughout the manufacturing procedure. This solution is systematically applied to all components that interact with food items. Subsequently, to eliminate any residual chemical content, the equipment undergoes thorough rinsing with clean water. [
6].
These processes, owing to their sterilizing and hygienic attributes, significantly bolster manufacturing safety. Nonetheless, to guarantee the maintenance of quality standards and avert contamination, it is imperative that the water employed undergoes daily replacement. Regrettably, this engenders an environmental challenge, as the wastewater becomes laden with organic constituents, thereby yielding an elevated biochemical oxygen demand (BOD). Consequently, the water becomes unsuitable for reuse without undergoing appropriate treatment measures. [
6,
7].
The incapacity to recycle water accentuates operational expenses for the company during the product’s marketing phase. Moreover, this form of manufacturing procedure and framework does not provide an assured shield against the propagation of diseases, including but not limited to hepatitis A, botulism, bacterial and viral infections, diarrhea, and toxoplasmosis. To illustrate, within the Brazilian context, the regulatory agency ANVISA has intercepted chicken breast samples containing traces of the bacterium Listeria monocytogenes, recognized as a causative agent of meningitis. [
8]. Furthermore, it is an established fact that microorganisms can develop resistance over extended periods of exposure to chemical solutions. Consequently, it becomes imperative to progressively elevate the chemical concentration to ensure the sustenance of quality standards and to curtail the prevalence of biological entities in food products.
Apprehensions concerning this facet remain persistent and are mounting, a trend underscored by a noteworthy surge in the quantity of patents issued within these domains over the past half-decade, registering an approximate escalation of 500%. [
7].
This assertion gains further substantiation from the stance adopted by the FDA, particularly evident in its deliberation over the permissible threshold for the presence of chemical residues in sanitation agents employed within the realm of the food industry. Deviation from these stipulated benchmarks not only renders production unviable but also erects a formidable biological impediment within the industrial landscape.
Hence, the principal objective of this study is to introduce a novel technique capable of facilitating the expansion of the manufacturing process, concurrently with stringent control over plant contamination. This endeavor encompasses a thorough evaluation of fundamental factors including cost implications and heightened operational efficiency. [
9,
10,
11].
2. Materials and Methods
The used procedure was divided in construction of the pilot reactor and evaluation of measurement decreasing microorganism control.
2.1. Prototype of Circulation to Broccoli Decontamination
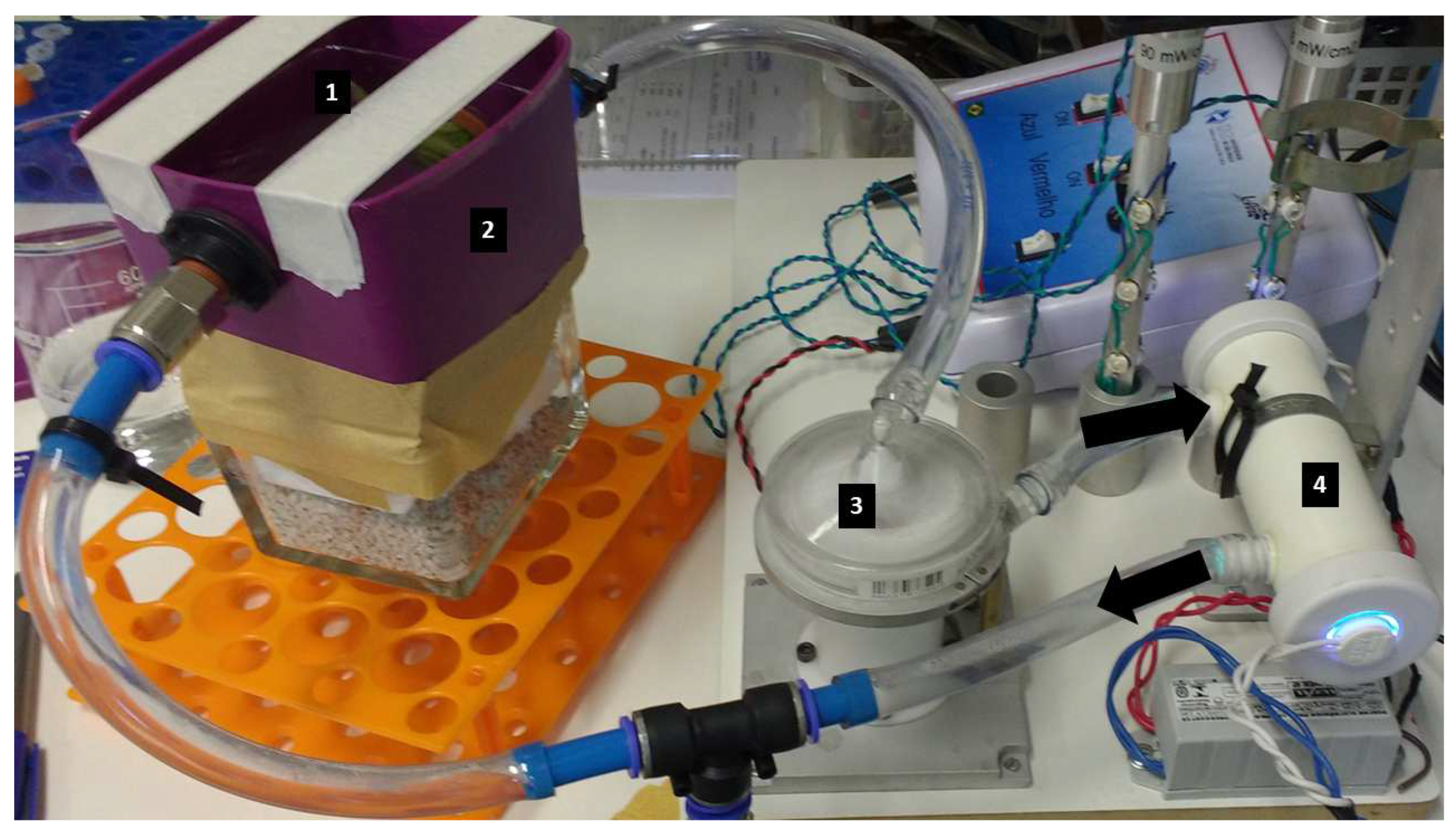
For this pilot study, it was developed in laboratory scale containing a flow, during which water is decontaminated in a constructed UV reactor. The following basic characteristics are present in
Table 1.
The flow is contained in a closed-loop system, consisting of a tank (1) where contaminated broccoli is inserted. In this tank, leaching takes place, removing the microorganisms through the water flow. The fluid circulation is facilitated by passing through the UV light reactor, the details of which will be provided later.
During circulation the irradiation is delivered to the water to inactivate the leaching microorganisms. This occurs for the entire content of the fluid as it passes through several passages within the reactor. The system operates with 10 circulations per minute, as shown in
Figure 1.
2.2. Reactors Geometry and total UVC delivery
For the characterization of the annular reactor light distribution, an optical fiber connected to the spectrometer (Ocean Optics USB 2000+UV-Vis) was used. The fiber is placed on the region of interested. From the measured intensity the delivered energy dose was calculated [
12].
2.3. Microbiological Method Analysis
To contaminate the vegetable broccoli, pieces of approximately 9.95 g were used and immersed in a solution with CFU/mL of E. Cole. After this step, the vegetable contaminated with microorganism were inserted into the circulation system. During the experiment, samples of the both the vegetable and water were taken, and the pour plate method was applied to analyze the presence of the microorganism.
2.4. Model of light delivery and photo inactivation
For this experiment the flow was characterized as non-turbulent while in contact with the UVC radiation. In this flow regime, well-behaved fluid movement is expected without the formation of bubbles and micro-bubbles, which could scatter the UVC radiation. Therefore, it was ensured that all the UVC light interacts effectively with the fluid content in
Figure 2.
Up to this point, the basic concepts for evaluating the flow profile and the mathematical influence on the radiation delivery in the water during the experimental process have been described [
13].
The first part explains the equation of the velocity profile. Subsequently, the Lambert-Beer law in the cylindrical coordinate system is used. [
4].
Therefore it will be described the general equation of the Lambert-Beer law for light distribution in the reactor present in this research [
14,
15].
where
I is the UVC radiation intensity traveling into the reactor to radial distance
r, the boundary condition
at
, is the initial intensity radiation and radial distance and
is absorption coefficient.
To determine the energy delivery in the fluid, it is necessary to use the relation of threshold energy in the solution that is described by [
4,
16]:
where
t is delivery time,
L is the length of radiation section and
v is velocity of fluid.
The principal influence on the kinetic equations is the n order of the equations (at first, it is suggested to be a first-order reaction). It is common to start the mathematical analysis by initially describing the following [
17,
18]:
where
and
is a variation of microorganism concentration, that variates with initial time to final, respectively, and
k is inactivation constant.
3. Results
Initially, emission data was quantified, yielding a value of 2.38 mW/cm², signifying the nominal irradiation within the annular reactor configuration. The water volume employed totaled 800 ml, while the internal capacity of the reactor amounted to 147 ml. These outcomes substantially contribute to the delineation of the flow profile.
Subsequently, the second segment of indispensable information required for assessing the system’s capacity to deactivate microorganisms involves establishing the requisite nominal dose for distinct microorganism types. Varying dosages for individual microorganisms are presented in the accompanying
Table 2
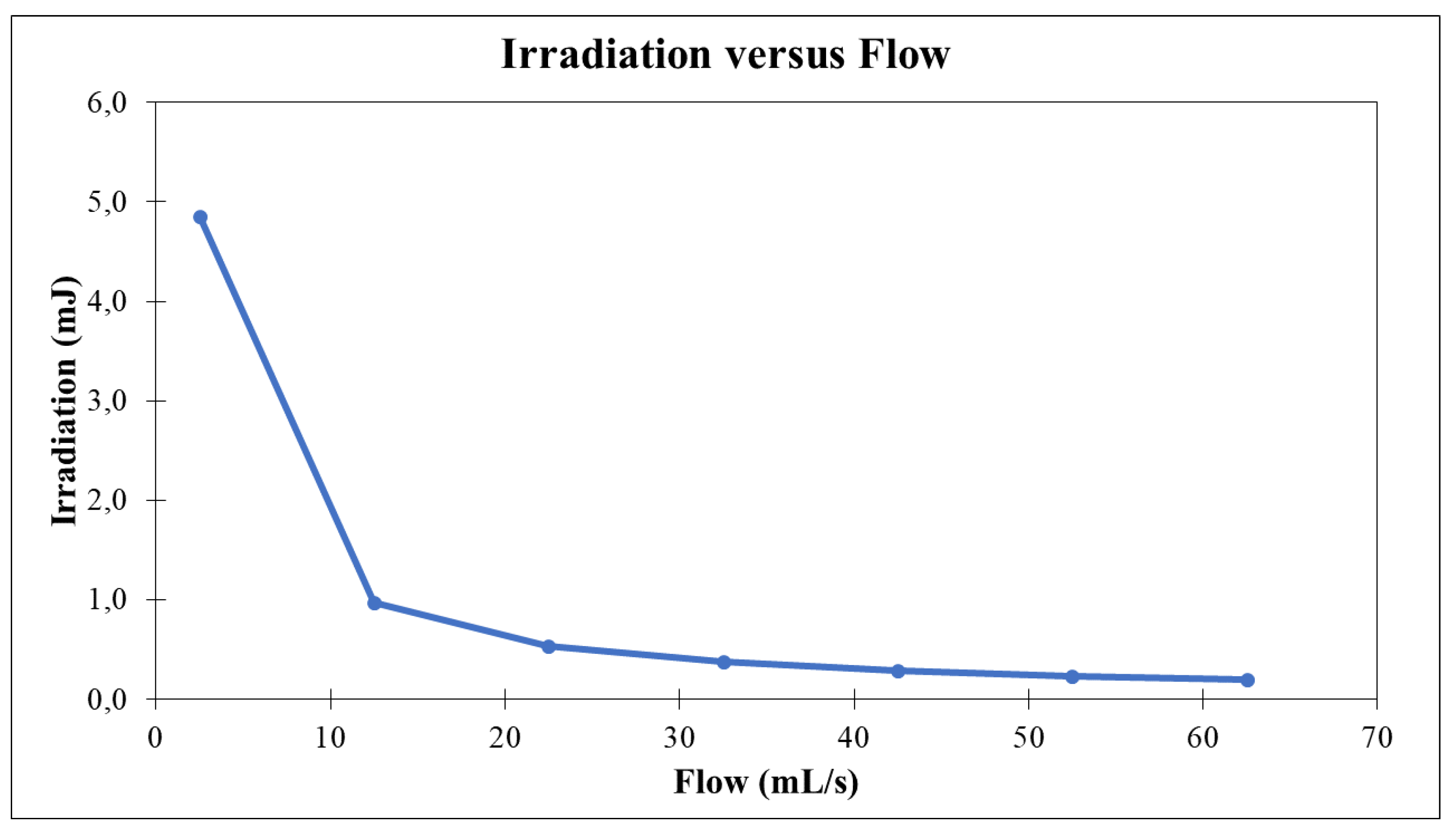
The capacity to illustrate the variance in nominal dosage administered to diverse microorganisms is feasible. Furthermore, an essential requirement involves approximating the direct delivery of irradiation within the flow and evaluating the reactor’s ability to render microorganisms inactive, as depicted in the accompanying
Figure 3.
The flow rate employed in this study is approximately 62.5 ml/s. The graph illustrates that each data point receives a quantified energy of 0.194 mJ. Nevertheless, the comprehensive fluency is derived through the incorporation of residence time, representing the interval during which the fluid undergoes irradiation across the experimental arrangement—essentially the throughput within the reactor. To accomplish this, it is essential to delineate the control volume (characterized as an infinitesimal unit) and apply equation 2.
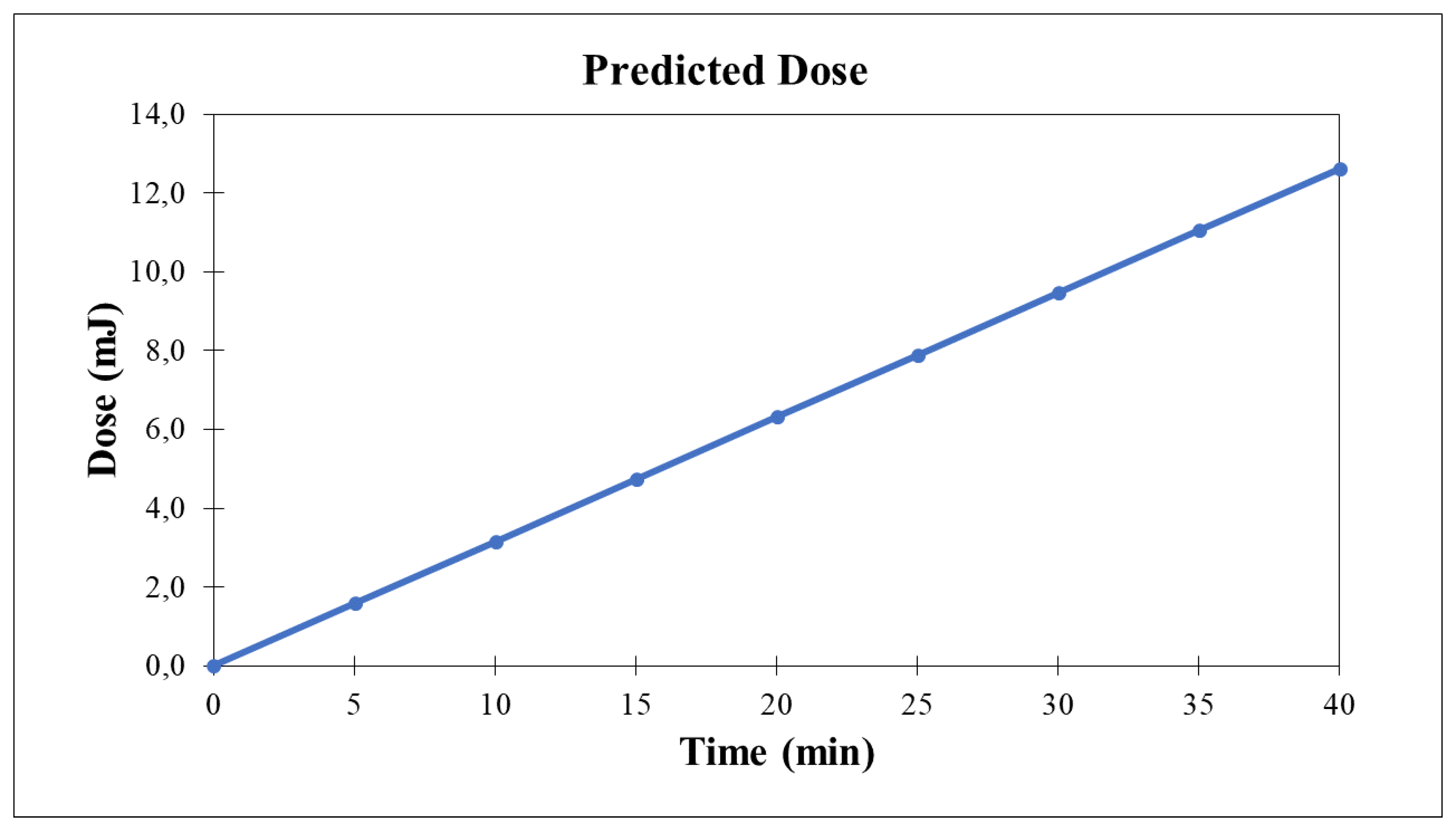
As a result, leveraging this data allowed for the estimation of the complete volume residing within the reactor at 147 ml, while the overall quantity of water employed amounted to 800 ml. The duration necessary for the entirety of the volume to traverse the system at least once is 70 seconds. These figures establish the correlative framework between dose and temporal variation, a dynamic that found application in the experimental depiction presented in the
Figure 4.
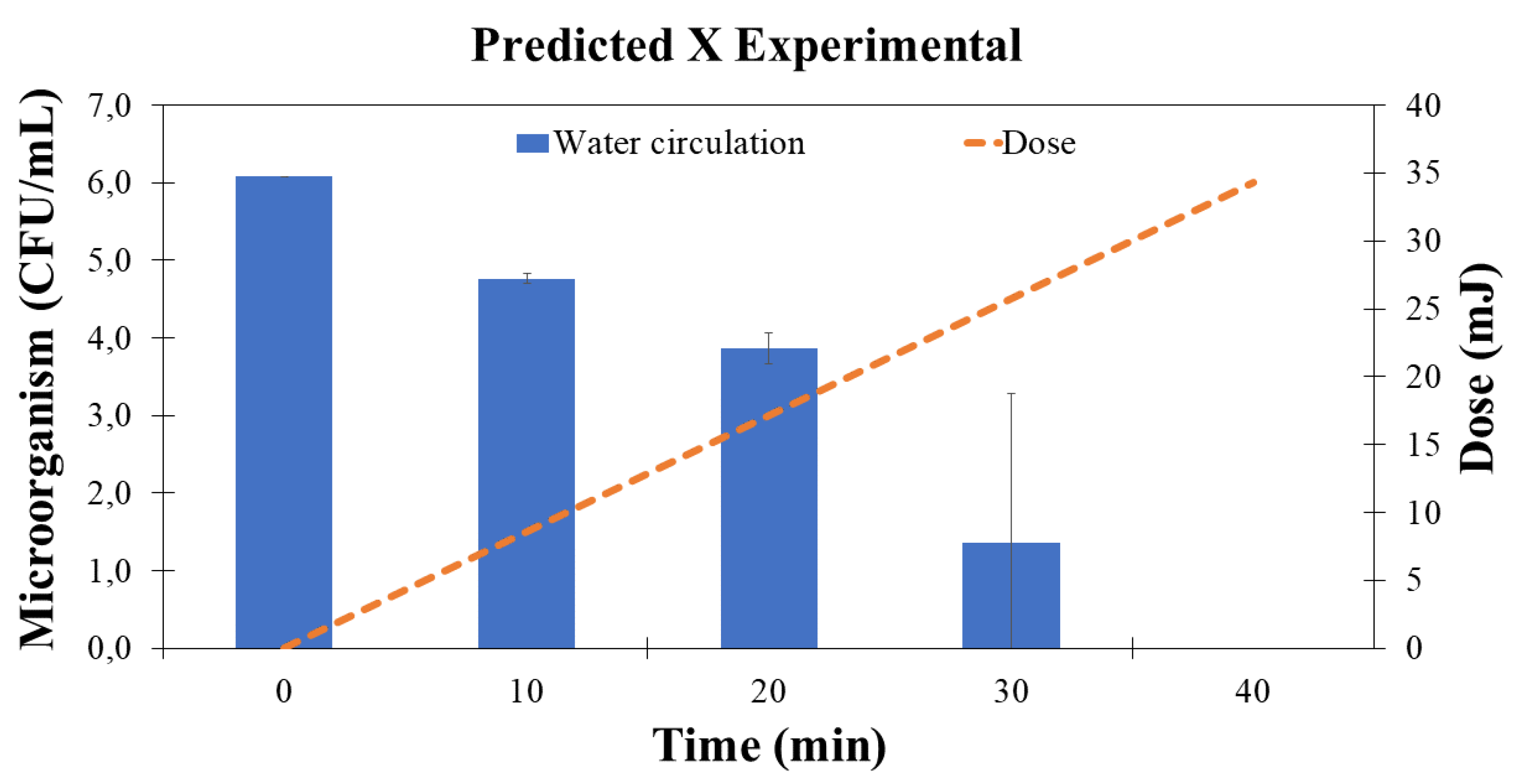
Having elucidated the dose dynamics and garnered endorsement for these mathematical outcomes, the research transitioned into the experimental phase, with a concentrated emphasis on microbiological considerations. The initial stride in this segment encompassed substantiating the viability of leaching vegetable constituents within this configured apparatus, operating under the stipulated physical property parameters.
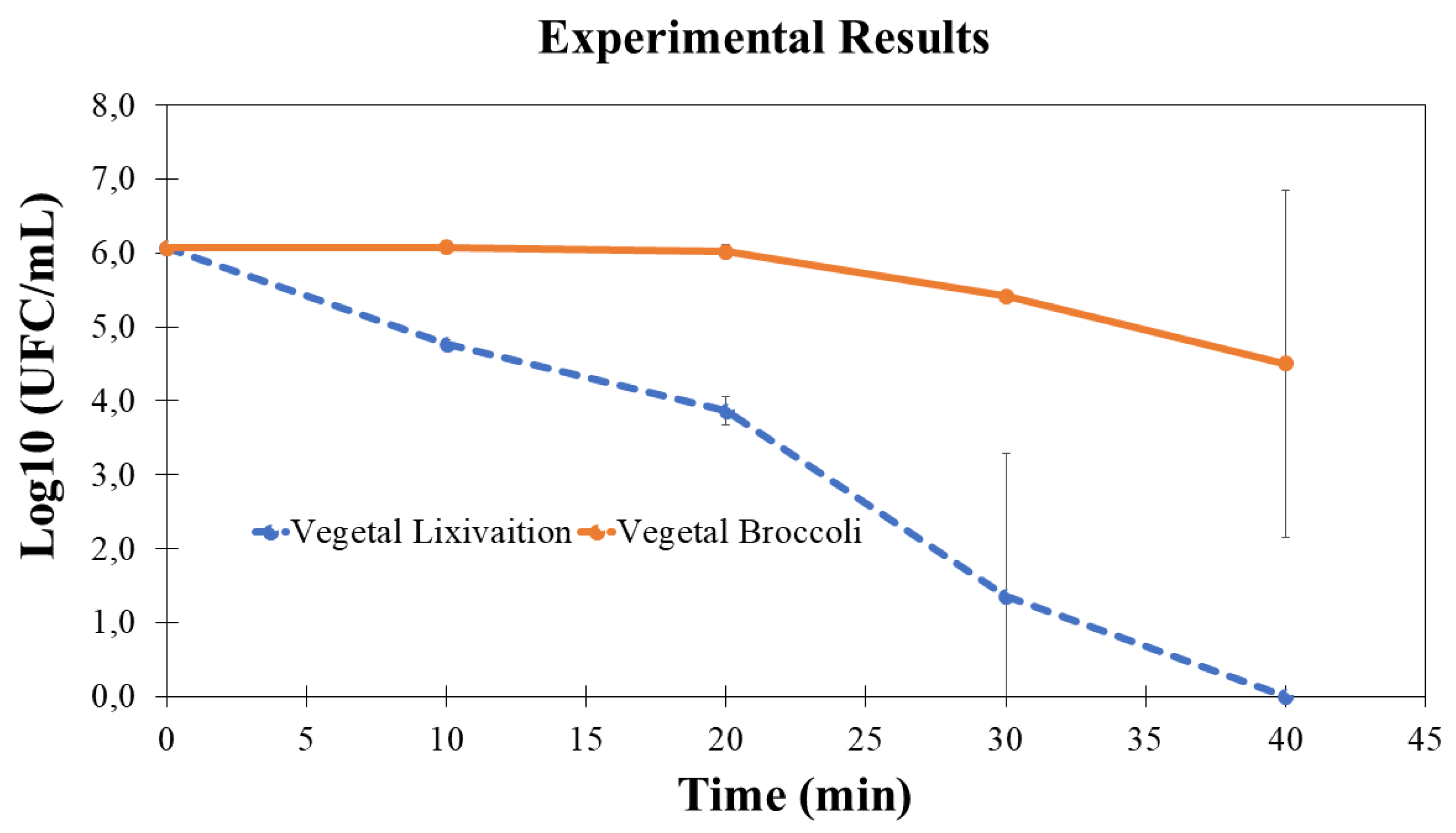
To achieve this objective, a controlled introduction of clean water was administered into the system, coupled with the incorporation of broccoli, known to harbor E. coli within its foliage. The initial concentration of the microorganism was set at
CFU/ml. The ensuing outcomes are graphically depicted in the accompanying
Figure 5.
Guided by the theoretical insights, it was ascertained that a duration of 35 to 40 minutes of fluid re-circulation was imperative for the deactivation of microorganisms within the solution. Following this procedural phase, the assessed outcomes pertaining to both the vegetable and the water within the system were graphically represented. This graphical representation juxtaposed the initial concentration of microorganisms against the temporal dimension (
Figure 5).
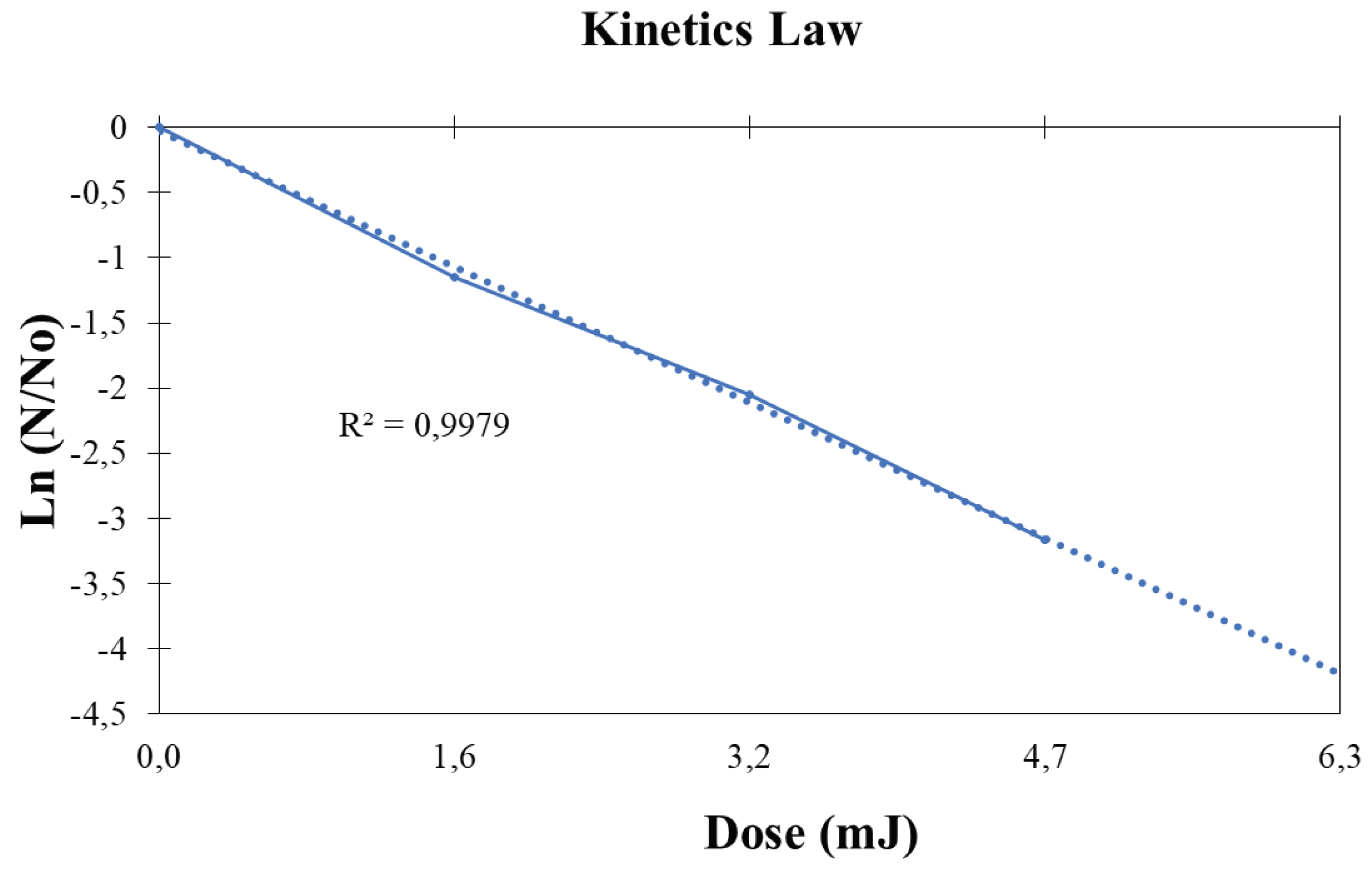
The final phase entailed the determination of kinetics law and the establishment of the mathematical order and dynamics governing microorganism inactivation. Initially, it was inferred that the process adheres to a first-order kinetics. Subsequently, a comparison was conducted between the experimental concentration rate over time and the mathematical kinetics law, with the outcomes succinctly presented in the
Figure 6.
4. Discussion
The imperative requirement for the advancement of novel technologies to bolster productivity, curtail expenditures, and concurrently alleviate environmental harm is progressively escalating.
The impact of ultraviolet radiation on DNA has been extensively documented in the scientific literature over a prolonged period. Depending on the wavelength of incident UV radiation, two distinct categories of damage manifest within the DNA structure. In the UVA range (320 - 400 nm), the predominant influence is that of indirect effects, owing to the lack of light absorption by nucleic acids within this domain. Nevertheless, this radiation has the potential to engender the production of reactive oxygen species (ROS)[
19,
20]
ROS emerges from the oxidative processes involving molecular oxygen within mitochondria and other molecules. This oxidative activity leads to the generation of superoxide (
), which subsequently has the potential to evolve into hydrogen peroxide (
) and molecular singlet oxygen (1
). These ROS possess the capability to induce the oxidation of nitrogenous bases, incite strand fractures, and even precipitate DNA-protein cross-linking[
21,
22]
Within the scientific literature, the documented minimal nominal dosage needed for the deactivation of E. coli ATCC 8739 is approximately 8.1 , whereas for E. coli 0157:H7 CCGU 29193, it rests around 3.5 . In the scope of the present study, the circulation system, operating at a flow rate of 62.5 ml/s, demonstrates the potential to administer a dosage of up to 12 mJ. This highlights that the system has the capability to provide fourfold the requisite energy for microorganism inactivation.
These findings signify a 99% and 99.999% diminishment of microorganisms, respectively, in comparison to their initial concentrations at time zero. The ensuing inquiries pertain to the correlation between time and microorganism inactivation, alongside the reactor’s efficacy in curtailing biological activity.
The employed technique achieved a reduction of 90% within 20 minutes, 99.9% within 30 minutes, and 99.999% within 40 minutes. This condition underscores the efficacy of microorganism inactivation and facilitated the formulation of a theoretical estimation, approximating that around 35 minutes are requisite for specific outcomes—a projection remarkably close to actuality.
Nevertheless, in the context of the vegetable, the outcome deviates from that observed in the case of water. This divergence can be attributed to the presence of a vortex induced during the leaching procedure of the broccoli within the tank. It becomes imperative to enhance mixing efficiency in this scenario. This objective could be accomplished by augmenting the separation between low-pressure and high-pressure regions, attainable through elevation of the flow rate or introduction of a barrier during the re-circulation phase [
23].
Microbiological inactivation by UVC radiation occurs initially through the absorption of radiation by DNA. In this process, two main excited states,
or
, are formed due to the absorption of photons by pyrimidine molecules present in the nitrogenous bases.[
24]
Consequently, the process triggers the formation of pyrimidine dimers, resulting in the generation of three distinct photo-products: 6-4 and 6-4 photoproducts (6-4 PPs), alongside Dewar dimers. Within this spectrum, it is the cyclobutane pyrimidine dimers (CPDs) and 6-4 photoproducts (6-4 PPs) that inflict the most substantial harm to DNA, accounting for 70-80% and 20-30%, respectively [
24].
The dosage administered to the microorganism facilitates the generation of these photo-products, subsequently culminating in the inactivation of the bacteria. Consequently, a reduced dosage administered to the microorganism possesses the inherent potential for regeneration, thereby facilitating the recovery of the microorganism.
5. Conclusions
In summary, the findings presented here highlight the viable prospect of leaching alongside the simultaneous attainment of efficient microorganism inactivation within the vegetable. Notably, the experimental investigation centered on broccoli, acknowledged for its intricate leaf structure that imparts intricacies to the leaching process.
What is particularly intriguing is the close concordance observed between mathematical predictions and empirical outcomes. This convergence robustly affirms the prospective success of microorganism inactivation within the circulatory framework, thereby enhancing the reliability of our conclusions.
As a result, this study makes a significant stride in the domain of food engineering, propelling the field of contamination control techniques to new heights. Importantly, it unequivocally underscores the possibility of circumventing reliance on auxiliary chemicals for microorganism inactivation. Instead, it underscores the paramount importance of implementing prudent measures to bolster production while concurrently mitigating environmental impact.
Funding
This research received no external funding of the grant number 2013/07276-1 (CEPOF-CEPID Program).
Acknowledgments
authors acknowledge the support provided by FAPESP (São Paulo Research Foundation),
Conflicts of Interest
The authors declare no conflict of interest.
References
- Oliveira, B.P.d.; Blanco, K.C.; Bagnato, V.S. Decontamination of circulating fluid with ultra-violet applied in foods. Book of abstracts, 2019.
- Oliveira, B.; Blanco, K.; Bagnato, V.S. Descontaminação de fluidos circulantes com ultra-violeta aplicados em alimentos. Livro de Resumos 2018. [Google Scholar]
- Blanco, K.C.; Corrêa, T.Q.; Perez, S.M.; Pereira, B.; de Oliveira, V.S. Optical Technologies for Improvement in Food Security. AND WASTE 2020, p. 98.
- Oliveira, B.P.d. Desenvolvimento de um sistema para inativação microbiológica em vegetais frescos por ação física através de líquidos circulantes. PhD thesis, Universidade de São Paulo, 2021.
- Oliveira, B.P.d.; Bagnato, V.S.; Inada, N.M.; Blanco, K.C. Dispositivo emissor de cortina de luz ultravioleta em autoclaves 2018.
- von Holy, A.; Holzapfel, W.H. The influence of extrinsic factors on the microbiological spoilage pattern of ground beef. International journal of food microbiology 1988, 6, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chemical Society Reviews 2008, 37, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.N.F.; Coelho, V.A.T.; Reis, L.H.G.; Cardoso, P.A.; Lacerda, L.G. Recall of food contaminated by bacteria and fungi. Revista Saúde Dos Vales-Rsv 2020, 2. [Google Scholar]
- Koutchma, T. Advances in ultraviolet light technology for non-thermal processing of liquid foods. Food and Bioprocess Technology 2009, 2, 138–155. [Google Scholar] [CrossRef]
- Oliveira, B.; Blanco, K.; Bagnato, V.S. Photonic decontamination of circulating fluids. Livro de Resumos 2017. [Google Scholar]
- Oliveira, B.P.d.; Blanco, K.C.; Bagnato, V.S. Contamination control in a portable-materials with photochemical process. International Journal of Chemistry 2019, 11, 86–94. [Google Scholar] [CrossRef]
- Harris, J.; Forney, L. POE Purifier for the Home with High Efficiency UV and Metal Removal. Disinfection and Reuse Symposium 2009. Water Environment Federation, 2009, pp. 909–932.
- Mathieu, J.; Scott, J. An introduction to turbulent flow; Cambridge University Press, 2000.
- Ye, Z.; Koutchma, T.; Parisi, B.; Larkin, J.; Forney, L. Ultraviolet inactivation kinetics of Escherichia coli and Yersinia pseudotuberculosis in annular reactors. Journal of Food Science 2007, 72, E271–E278. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z. UV disinfection between concentric cylinders. PhD thesis, 2006.
- Bird, R.B. Transport phenomena. Appl. Mech. Rev. 2002, 55, R1–R4. [Google Scholar] [CrossRef]
- Laidler, K.J.; Keith, J. ; others. Chemical kinetics; Vol. 2, McGraw-Hill New York, 1965.
- Frost, A.; Pearson, R. Kinetics and mechanism. The Journal of Physical Chemistry 1961, 65, 384–384. [Google Scholar] [CrossRef]
- Sinha, R.P.; Häder, D.P. UV-induced DNA damage and repair: a review. Photochemical & Photobiological Sciences 2002, 1, 225–236. [Google Scholar]
- Cannistraro, V.J.; Pondugula, S.; Song, Q.; Taylor, J.S. Rapid deamination of cyclobutane pyrimidine dimer photoproducts at TCG sites in a translationally and rotationally positioned nucleosome in vivo. Journal of Biological Chemistry 2015, 290, 26597–26609. [Google Scholar] [CrossRef]
- Féraud, G.; Berdakin, M.; Dedonder-Lardeux, C.; Jouvet, C.; Pino, G.A. Excited states of proton-bound DNA/RNA base homodimers: pyrimidines. The Journal of Physical Chemistry B 2015, 119, 2219–2228. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Fecko, C.J.; Nicewonger, R.B.; Webb, W.W.; Begley, T.P. DNA- protein cross-linking: Model systems for pyrimidine- aromatic amino acid cross-linking. Organic Letters 2006, 8, 681–683. [Google Scholar] [CrossRef]
- Oliveira, B.P.d. Reator utiliza luz ultravioleta para descontaminar vegetais.[Depoimento a Marcelo Canquerino]. Jornal da USP 2020, 20. [Google Scholar]
- Yokoyama, H.; Mizutani, R. Structural biology of DNA (6-4) photoproducts formed by ultraviolet radiation and interactions with their binding proteins. International journal of molecular sciences 2014, 15, 20321–20338. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).