1. Introduction
Dysphagia causes aspiration pneumonia, malnutrition, and difficulty in taking oral medications, as well as having a significant negative impact on patients’ quality of life [
1,
2]. Therefore, prevention or early detection of dysphagia is critical. Dysphagia is caused by structural lesions in the oral cavity, pharynx, esophagus, or other sites and physiologic pathologies such as neuromuscular diseases [
3]. Drug-induced dysphagia, is one of the causes of physiological dysfunction, and it is associated with skeletal muscle relaxants [
4], antipsychotic drugs [
5], antidementia drugs [
6], sedative-hypnotic/antianxiety drugs [
7], and antiepileptic drugs [
8]. However, the frequency of adverse drug events (ADEs), differences in onset risk between these drugs, and time from the start of administration to the onset of drug-induced dysphagia (hereafter referred to as time to onset) have not been thoroughly investigated. In addition, age [
9] has been reported to affect swallowing function [
10]. Therefore, drug-induced dysphagia must be evaluated with these factors in mind.
Drug use in clinical practice is extremely complex, and it differs significantly from well-designed clinical trials and epidemiological studies. When ADEs are rare or occur after a long period of time in pre-approval clinical drug trials, assessing them is difficult. In recent years, a large spontaneous reporting system (SRS) database has been used to detect unknown ADEs and assess safety in clinical practice [
11,
12,
13,
14,
15]. The Japanese Adverse Drug Event Report (JADER) database of the Pharmaceuticals and Medical Devices Agency (PMDA) is the largest and most well-known published database reflecting the realities of clinical practice in Japan. Approximately 700,000 adverse event reports were recorded in JADER from April 2004 to October 2021. JADER includes tables of patient demographics, such as gender and age; drug data tables, including generic name and administration start and end dates; adverse event tables, including adverse effects, outcomes; adverse event onset dates; and primary disease tables. An identification number can be used to connect tables. As a result, this database is often used for pharmacovigilance assessments. Large-scale spontaneous adverse event databases play an important role in epidemiologic research, where ADEs in clinical practice are collected over a long period of time, especially in drug safety assessment.
As a result, this study investigated the use of JADER to extract drugs associated with drug-induced dysphagia through signal detection. Furthermore, we investigated the profile of drug-induced dysphagia across drug classes as well as within each drug class.
2. Materials and Methods
2.1. Design and Data Source
We used PMDA’s JADER database as a large SRS database [
11,
12,
13,
14,
15] to determine the ADE profile of drug-induced dysphagia. The JADER database is freely accessible on the PMDA website from April 2004 to October 2021. The database contains four data tables: patient demographic information (“Demo”), drug information (“Drug”), ADEs (“Reac”), and primary disease (“Hist”). The Demo file contained demographic data stored at 10-year age intervals (e.g., 60–69 years). Data from other age classifications, such as first trimester, second trimester, third trimester, newborn, infant, pediatric, adolescent, adult, elderly, and unknown, were excluded because the actual age was unclear. Duplicate reports were also removed, and only the most recent list of ADE reports was extracted. ADEs in the Reac file were coded according to the medical dictionary terminology for regulatory activities [
16]. A drug information table was created in the Drug file with the following codes: suspected (higiyaku, in Japanese), interacting (sougosayou), and concomitant (heiyouyaku). However, “concomitant” referred to an unknown specific ADE causal relationship, and “interacting” was reported only for drugs that cause dysphagia, which was thought unlikely. Therefore, these categories were excluded and only “suspected” data were analyzed.
2.2. Adverse Events and Target Drugs
The names of adverse events in the JADER database are based on the High-Level Group Term, High-Level Term, and Preferred Term of the Medical Dictionary for Regulatory Activities–Japanese version (MedDRA/J). In this study, drug-induced dysphagia was defined using five terms from the MedDRA/J Ver.25.0 Preferred Terms section: excessive drooling, foreign body aspiration, aspiration, dysphagia, and swallowing pain. The analyzed adverse events were extracted from the JADER database, and the top 10 drugs with the most “drug-induced dysphagia” events were selected as the target drugs for analysis.
2.3. Outcomes and Analysis
In Japan, the reporting odds ratio (ROR) is an index used to assess the risk of ADEs in pharmacovigilance analyses, especially by the PMDA. The empirical Bayesian geometric mean [
17] is a similar index often used by the FDA, whereas the proportional reporting ratio [
18] is often used by the Medicines and Healthcare Products Regulatory Agency. Previous studies have reported RORs for some drugs and ADEs based on information from JADER [
11,
12,
13,
14,
15]. Therefore, RORs with 95% confidence intervals (CI) were calculated as shown in
Table 1 as follows: (a) number of cases of drug-induced dysphagia after use of target drugs, (b) number of cases of all other ADEs after use of target drugs, (c) number of cases of drug-induced dysphagia after use of all other drugs, and (d) number of cases of all other ADEs after use of all other drugs. Qualitative judgments were used for signal detection, which were based on signal indices exceeding predefined thresholds. ROR values <1 indicated no association between exposure and event, whereas estimates >1 indicated potentially relevant ADE safety signals for the exposure. Safety signals were considered significant if the ROR estimates and the lower limits of the corresponding 95% CI were greater than 1 [
19,
20]. Based on the information in the demo file, the age at which ADEs occurred was also extracted. The distribution ratio of the number of reports in each age group was calculated and divided by 10s–100s. The JADER database contains information on the start date of drug administration as well as the date of ADE occurrence. For each drug, the period from the start of a subject’s first prescription to the occurrence of the ADE was calculated. If the patient has multiple start dates, the data with the oldest start date was used as the start date. Furthermore, data that lacked a year, month, or day were excluded.
Abbreviations: ROR, Reporting Odds Ratios; 95% CI, 95% Confidence Interval
2.4. Statistical Analysis
All data analyses were conducted using JMP Pro® 16.0 (SAS Institute Inc., Cary, NC, USA).
2.5. Ethics
The Japanese Adverse Drug Event Report database, which was used in this study, is a public database published by the Japanese Ministry of Health, Labor and Welfare and is freely available on the internet. Therefore, the ethics committee of our hospital decided that no approval was required.
3. Results
3.1. Number of Data during the Study Period
From April 2004 to October 2021, there were 1,048,575 registered patients. Missing and duplicate data in the SRS are known to have an impact on the evaluation and analysis. In this study, only the most recent adverse event reports from the same patient were extracted, and 756,965 data were used for the final analysis after 287,951 missing data and 3,659 duplicate data were removed.
3.2. Comparison of ROR of Drug-Induced Dysphagia
Table 2 shows the ROR of drug-induced dysphagia for each drug. Signals were detected for all drugs analyzed. Signals were also detected for cevimeline, which acts similarly to acetylcholine. RORs were comparable for paroxetine, olanzapine, haloperidol, fluvoxamine, and risperidone. The RORs for milnacipran and aripiprazole were 41.8 and 23.7, respectively, slightly higher than the above drugs.
3.3. Distribution of Age at Onset of Drug-Induced Dysphagia
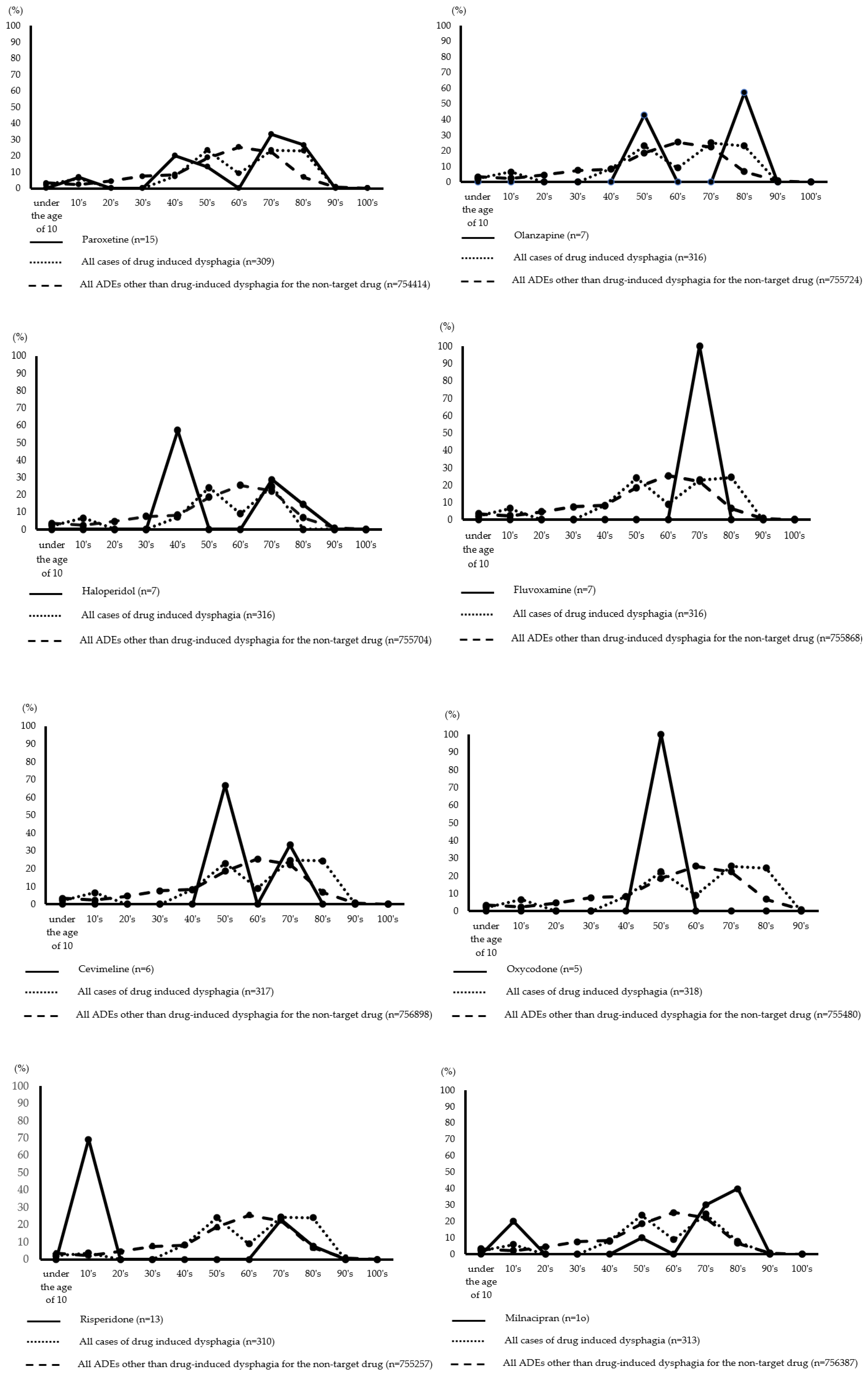
Figure 1 depicts the age distribution of drug-induced dysphagia by drug. The incidence of paroxetine and haloperidol was highest in patients in their 40s and 70s to 80s. Patients in their 50s and 80s were more likely to take olanzapine, while patients in their 70s were more likely to take fluvoxamine. Risperidone and aripiprazole had the highest incidence in patients between the ages of 10 and 70. Further, milnacipran had the highest incidence in patients between the ages of 10 and 50–90, while botulinum toxin type A had the highest incidence in patients between the ages of less than 10 and 40–70. For cevimeline, the highest expression rates were observed in the 50s and 70s. Further, oxycodone had the highest incidence in patients in their 50s.
3.4. Comparison of the Incidence of Drug-Induced Dysphagia for Each Drug
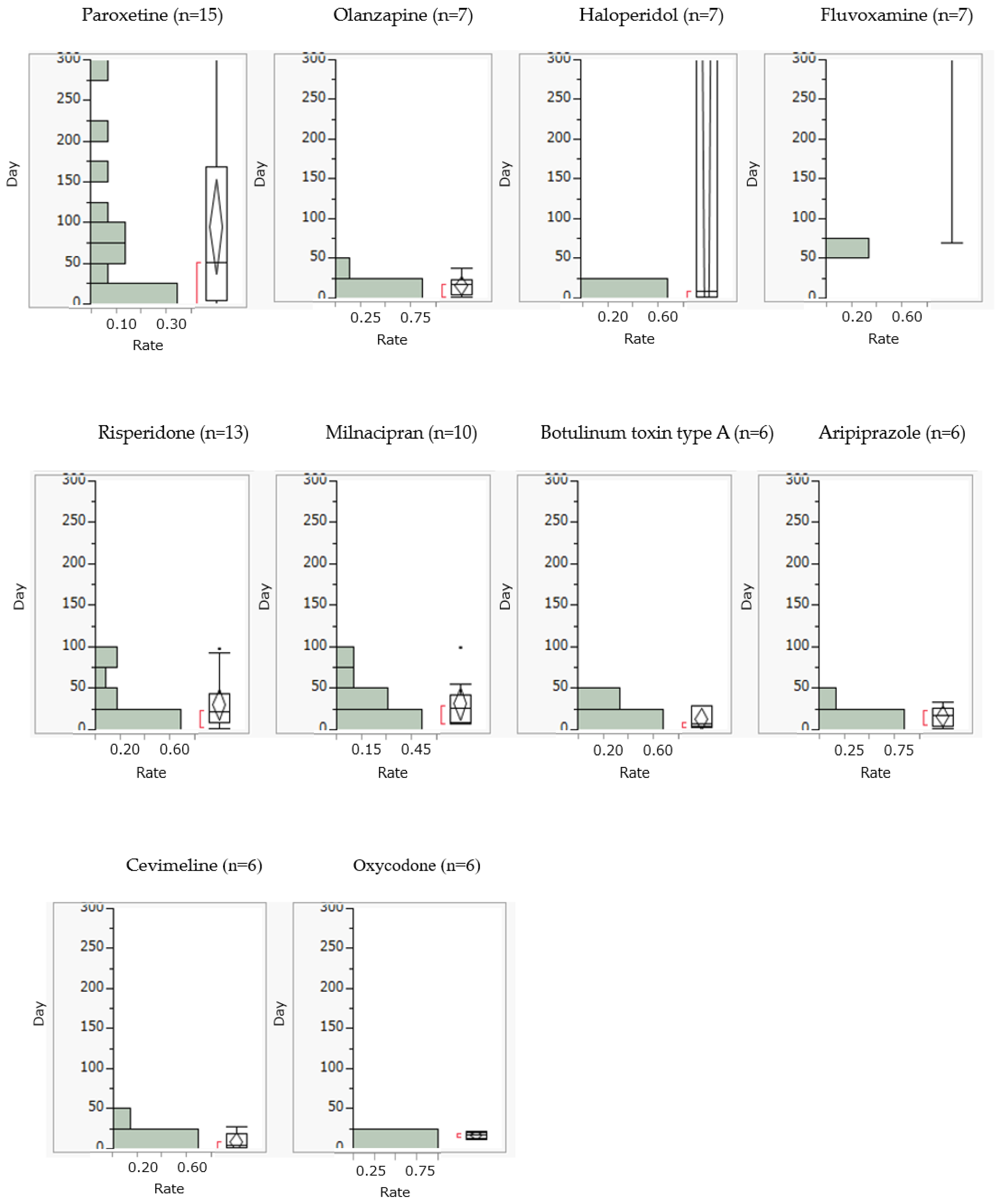
Drug-induced dysphagia generally occurred within 25 days of starting olanzapine, risperidone, milnacipran, botulinum toxin type A, aripiprazole, cevimeline, and oxycodone. On the other hand, paroxetine, haloperidol, and fluvoxamine caused drug-induced dysphagia after 25 days (
Figure 2).
4. Discussion
This study analyzed the incidence profile of drug-induced dysphagia using the JADER database. The most significant clinical finding of this study was that drug-induced dysphagia was observed for all target drugs studied. In particular, cevimeline was not found in clinical trials to cause dysphagia and was not listed as an adverse event in the package insert. This points to the presence of adverse events that were not detected during clinical trials. Second, and most importantly, drug-induced dysphagia was more common within approximately 25 days of taking the drug. Since there has never been a report of a comprehensive analysis of the incidence profile of drug-induced dysphagia using SRS, we believe that the findings of this study are completely novel.
All of the target drugs were associated with drug-induced dysphagia. Cevimeline is an acetylcholine-like drug that stimulates salivation and is used to treat xerostomia caused by Sjögren’s syndrome. Although the exact mechanism by which dysphagia develops is unknown, aspiration associated with salivary hypersecretion and nausea and vomiting, both of which are known side effects, are potential causes. The RORs of the antipsychotic drugs olanzapine, haloperidol, and risperidone were comparable, suggesting that drug-induced dysphagia may occur regardless of whether the drugs are classified as typical or atypical antipsychotics. This supported previously reported findings [
21]. Furthermore, the RORs for paroxetine and fluvoxamine were similar. However, the ROR of milnacipran was higher than these two drugs. Paroxetine and fluvoxamine are selective serotonin reuptake inhibitors, while milnacipran is a serotonin-norepinephrine reuptake inhibitor. The difference in ROR between antidepressants may be attributed to differences in mechanism of action. Antipsychotics and antidepressants both have anticholinergic effects and have been reported to be risk factors for the development of aspiration pneumonia [
22]. Therefore, patients taking these medications should be monitored for dysphagia. Since botulinum toxin type A is a topical drug, it was initially thought to be insignificant in the development of drug-induced dysphagia. However, administration to cervical-related muscles may cause some abnormality, resulting in excessive acetylcholine release and muscle contraction states, which may lead to the development of dysphagia. Among the target drugs, oxycodone had the lowest ROR. Pharmacologically, oxycodone is known to inhibit exocrine glands by blocking Ca ion channels, resulting in xerostomia. Thus, it may cause dysphagia due to xerostomia.
The age of onset of drug-induced dysphagia ranges between 50 and 80 years, suggesting that the incidence of drug-induced dysphagia increases with age. Skeletal muscles are known to undergo age-related changes from approximately 40 to 50 years of age [
23]. Therefore, age-related changes in swallowing-related muscles may affect the occurrence of drug-induced dysphagia, and caution should be exercised when medicating patients aged 50–80. On the other hand, risperidone and aripiprazole had the highest incidence of drug-induced dysphagia in the 10-year age group. This could be because both drugs are indicated for “childhood autism spectrum disorder” and are used frequently, resulting in a high number of prescriptions in the teenage age group.
A high percentage of drug-induced dysphagia occurred within approximately 25 days of taking the drug. According to a questionnaire survey of healthcare professionals involved in dysphagia [
24], 83 of 88 patients (76% of patients were >70 years old) developed dysphagia within 1 week of starting the medication. Risperidone was responsible for approximately 70% of the drug-induced dysphagia in the previous report. Although the setting and method of analysis differed from the present study, approximately 60% of drug-induced dysphagia due to risperidone was also observed within 2 weeks. Therefore, it was proposed that drug-induced dysphagia could occur early after drug administration. Drugs such as olanzapine, haloperidol, cevimeline, and oxycodone may also cause drug-induced dysphagia at an early stage. As the onset pattern is early, it is necessary to monitor dysphagia-related symptoms such as coughing while eating, difficulty swallowing pills, and changes in meal content using a questionnaire such as the EAT-10 [
25] from the initial phase of administration using the median time to onset as a guide. On the other hand, paroxetine and milnacipran were found to cause drug-induced dysphagia not only during the early stages of initiation but also during long-term use, suggesting the need to monitor for dysphagia over time regardless of when the drug is taken.
When interpreting the SRS results, it is critical to understand the limitations of the data. The SRS is not recommended for calculating true risk because it lacks direct information (i.e., the number of people who use drugs) to be the denominator. In addition, the SRS is known to have reporting bias (i.e., underreporting [
26,
27], safety information, and market trends), incorrect drug names or adverse event coding, a lack of basic information (i.e., age or gender) (Bate A, Evans SJ. 2009), and missing delayed ADEs [
11,
28]. However, the frequency of ADEs is low, and some drugs are discovered after a long period of time. According to the results of this study, there are unknown adverse events that cannot be detected in clinical trials [
29]. Prospective observational studies are needed to verify the hypothesis that there is an association between drugs and adverse events when the JADER database detected the ROR signal indicating the potential for an adverse event. Therefore, the SRS may be a valuable source of information for post-marketing investigations of ADEs.
5. Conclusions
The current study found that the age of onset or timing of drug-induced dysphagia varied among drugs. However, further studies are needed to determine whether our findings can be applied to early detection and response efforts in clinical settings.
Author Contributions
All listed authors comply with the Journal’s authorship policy. EK conceptualized and designed the study. EK and MK contributed to data collection. EK and MK analyzed the data. EK, MK, and NY produced the first draft of the manuscript. All authors contributed to subsequent drafts of the manuscript, including editing, and refining of the final manuscript. All authors approved the final version of the manuscript for submission.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the reason the Japanese Adverse Drug Event Report database, which was used in this study, is a public database published by the Japanese Ministry of Health, Labor and Welfare and is freely available on the internet.
Informed Consent Statement
The Japanese Adverse Drug Event Report database, which was used in this study, is a public database published by the Japanese Ministry of Health, Labor and Welfare and is freely available on the internet. Therefore, the ethics committee of our hospital decided that no approval was required.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morisaki, N.; Miura, H.; Hara, S.; Yamasaki, K. Relationship between decline of swallowing function and health-related QOL among frail elderly persons. J. J. Gerodont. 2013, 28, 20–26. [Google Scholar]
- Kose, E.; Yasuno, N. Drug-Induced Nutrient Deficiencies Among Older Adults. J Aging Sci. 2020, 8, 235. [Google Scholar]
- Aslam, M.; Vaezi, M.F. Dysphagia in the elderly. Gastroenterol. Hepatol. 2013, 9, 784–795. [Google Scholar]
- Blackie, J.D.; Lees, A.J. Botulinum toxin treatment in spasmodic torticollis. J. Neurol. Neurosurg. Psychiatry. 1990, 53, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.T. Dysphagia associated with risperidone therapy. Dysphagia. 2003, 18, 274–275. [Google Scholar] [CrossRef]
- Watanabe, T.; Shinagawa, S.; Sannomiya, M.; Ono. K.; Nakariya, K.; Nakayama, K. A case of extrapyramidal symptoms and delirium following administration of donepezil plus sulpiride. Clinical Psychiatry. 2010, 52, 405–458. [Google Scholar]
- Lim, H.C.; Nigro, M.A.; Beierwaltes, P.; Tolia, V.; Wishnow, R. Nitrazepam-induced cricopharyngeal dysphagia, abnormal esophageal peristalsis and associated bronchospasm. Brain Dev. 1992, 14, 309–394. [Google Scholar] [CrossRef]
- Jahromi, S.R.; Togha, M.; Fesharaki, S.H.; Najafi, M.; Moghadam, N.B.; Kheradmand, J.A.; Kazemi, H.; Gorji, A. Gastrointestinal adverse effects of antiepileptic drugs in intractable epileptic patients. Seizure. 2011, 20, 343–346. [Google Scholar] [CrossRef]
- Taniguchi, H.; Magara, J.; Inoue, M. Dysphagia in elderly people. J. Jpn. Soc. Parenter. Enteral nutr. 2013, 28, 1069–1074. [Google Scholar]
- Kose, E.; Hirai, T.; Seki, T.; Okudaira, M.; Yasuno, N. Anticholinergic Load Is Associated with Swallowing Dysfunction in Convalescent Older Patients after a Stroke. Nutrients 2022, 14, 2121. [Google Scholar] [CrossRef]
- Ohta, M. Causality assessment between reported fatal cerebral haemorrhage and suspected drugs: developing a new algorithm based on the analysis of the Japanese Adverse Event Report (JADER) database and literature review. Eur J Clin Pharmacol. 2021, 77, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Umetsu, R.; Abe, J.; Ueda, N.; Nakayama, Y.; Kinosada, Y.; Nakamura, M. Hyperglycemic adverse events following antipsychotic drug administration in spontaneous adverse event reports. J Pharm Health Care Sci. 2015, 1, 15. [Google Scholar] [CrossRef] [PubMed]
- Kose, E. Adverse drug event profile associated with pregabalin among patients with and without cancer: analysis of a spontaneous reporting database. J Clin Pharm Ther. 2018, 43, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Noda, A.; Sakai, T.; Obara, T.; Miyazaki, M.; Tsuchiya, M.; Oyanagi, G.; Murai. Y.; Mano, N. Characteristics of pediatric adverse drug reaction reports in the Japanese Adverse Drug Event Report Database. BMC Pharmacol Toxicol. 2020, 21, 36. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Obara, T.; Sakai, T.; Nomura, K.; Takamura, C.; Mano, N. Quality evaluation of the Japanese Adverse Drug Event Report database (JADER). Pharmacoepidemiol Drug Saf. 2020, 29, 173–181. [Google Scholar] [CrossRef]
- Szarfman, A.; Machado, S.G.; O’Neill, R.T. Use of screening algorithms and computer systems to efficiently signal higher-than-expected combinations of drugs and events in the US FDA’s spontaneous reports database. Drug Saf. 2002, 25, 381–392. [Google Scholar] [CrossRef]
- Evans, S.J.; Waller, P.C.; Davis, S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol. Drug Saf. 2001, 10, 483–486. [Google Scholar]
- Nomura, K.; Takahashi, K.; Hinomura, Y.; Kawaguchi, G.; Matsushita, Y.; Marui, H.; Anzai, T.; Hashiguchi, M.; Mochizuki, M. Effect of database profile variation on drug safety assessment: an analysis of spontaneous adverse event reports of Japanese cases. Drug Des Devel Ther. 2015, 9, 3031–3041. [Google Scholar] [CrossRef]
- Rothman, K.J.; Lanes, S.; Sacks, S.T. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. 2004, 13, 519–523. [Google Scholar] [CrossRef]
- van, Puijenbroek E. P.; Bate, A.; Leufkens, H.G.; Orre, R.; Egberts, A.C.G. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. 2002, 11, 3–10. [Google Scholar] [CrossRef]
- Kose, E.; Uno, K.; Hayashi, H. Evaluation of the Expression Profile of Extrapyramidal Symptoms Due to Antipsychotics by Data Mining of Japanese Adverse Drug Event Report (JADER) Database. Yakugaku Zasshi. 2017, 137, 111–120. [Google Scholar] [CrossRef]
- Kose, E.; Hirai, T.; Seki, T. Assessment of aspiration pneumonia using the Anticholinergic Risk Scale. Geriatr Gerontol Int. 2018, 18, 1230–1235. [Google Scholar] [CrossRef]
- Yamada, M.; Moriguch, Y.; Mitani, Y.; Aoyama, Y.; Arai, H. Age-dependent changes in skeletal muscle mass and visceral fat area in Japanese adults from 40 to 79 years-of-age. Geriatr Gerontol Int. 2014, 14, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, S.; Katsuragi, S. Investigation of actual survey and analysis of risk factors of drug-induced dysphagia [Translated from Japanese]. J. Sugiura Found. Dev. Community Care. 2014, 3, 30–33. [Google Scholar]
- Rothman, K.J.; Lanes, S.; Sacks, S.T. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. 2004, 13, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Sakaeda, T.; Tamon, A.; Kadoyama, K.; Okuno, Y. Data Mining of the Public Version of the FDA Event Reporting System. Int. J. Med. Sci. 2013, 10, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Bate, A.; Evans, S.J. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol. Drug. Saf. 2009, 18, 427–436. [Google Scholar] [CrossRef]
- Fujita, T. Signal Detection of Adverse Drug Reactions. Jpn J Pharmacoepidemiol. 2009, 14, 27–36. [Google Scholar] [CrossRef]
- Werner, F.M.; Coveñas, R. Safety of antipsychotic drugs: focus on therapeutic and adverse effects. Expert Opin Drug Saf. 2014, 3, 1031–1042. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).