Submitted:
09 September 2023
Posted:
14 September 2023
You are already at the latest version
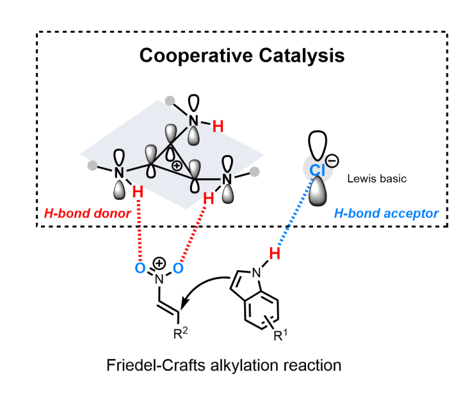
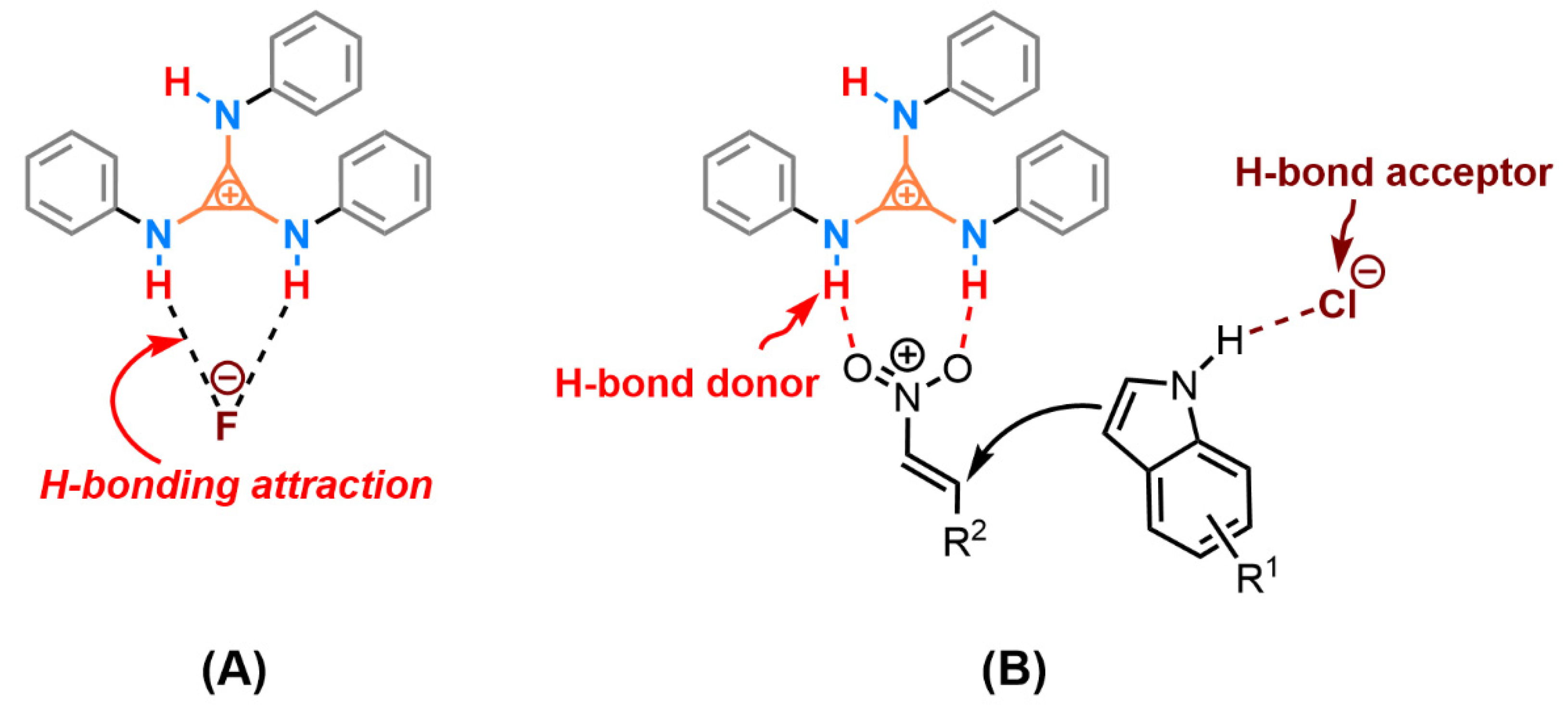
Abstract
Keywords:
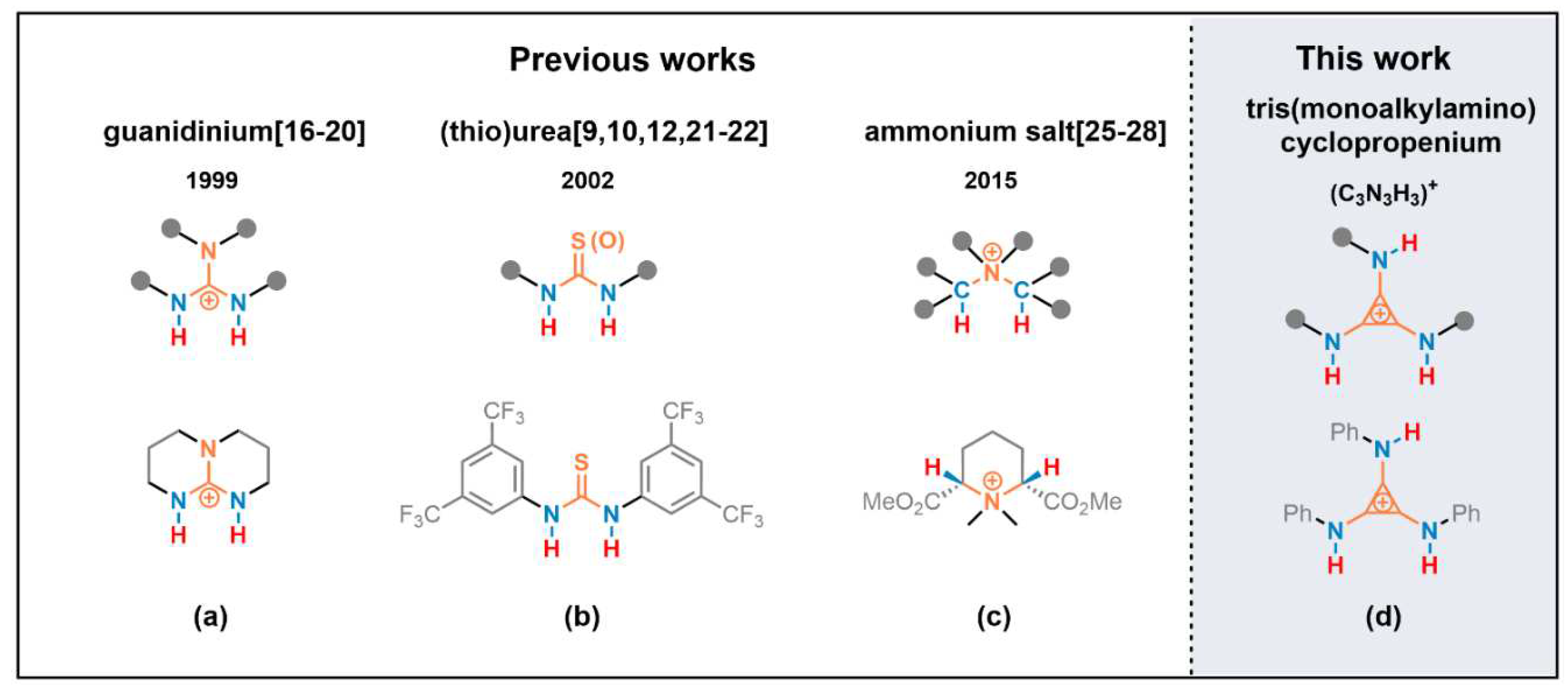
1. Introduction
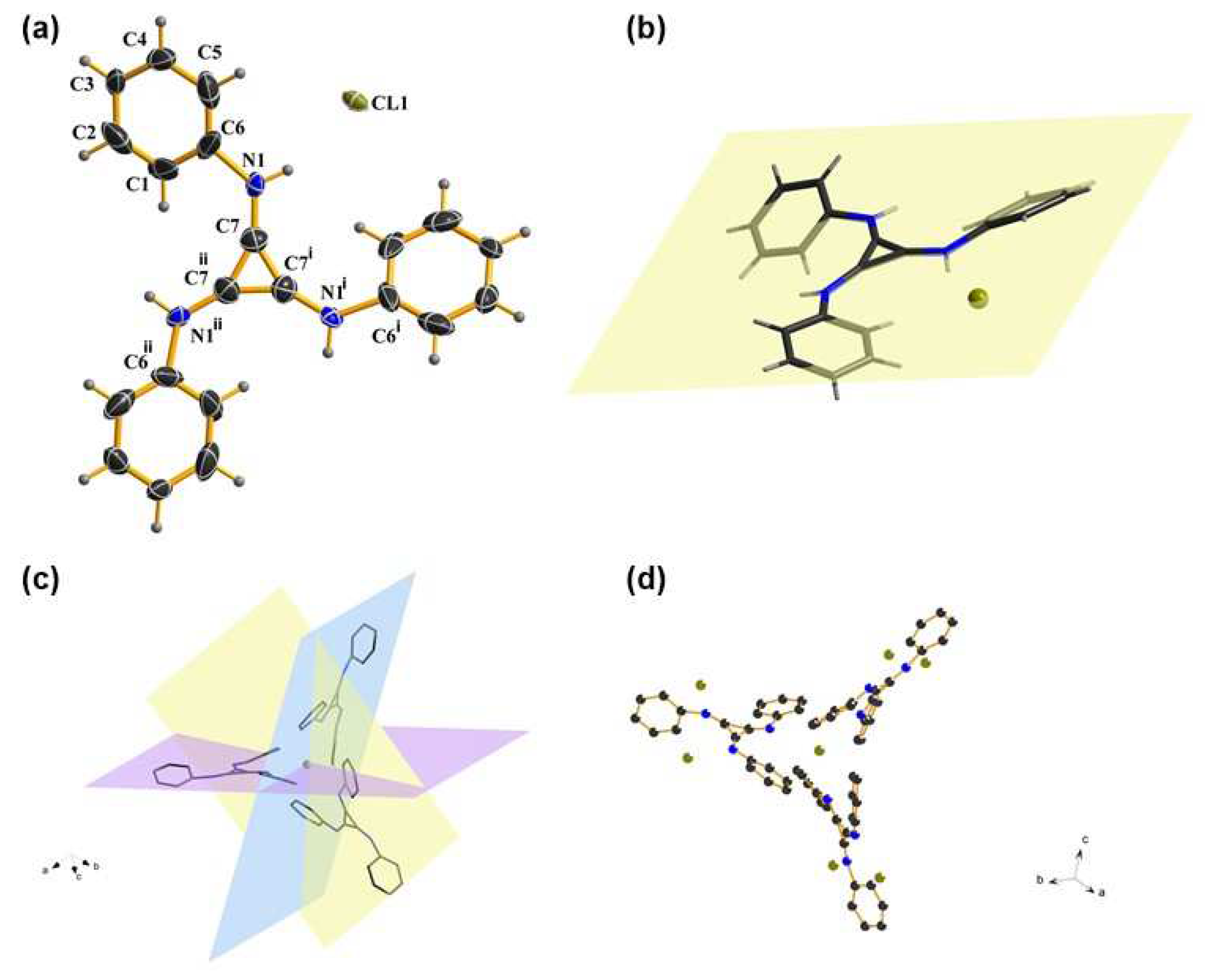
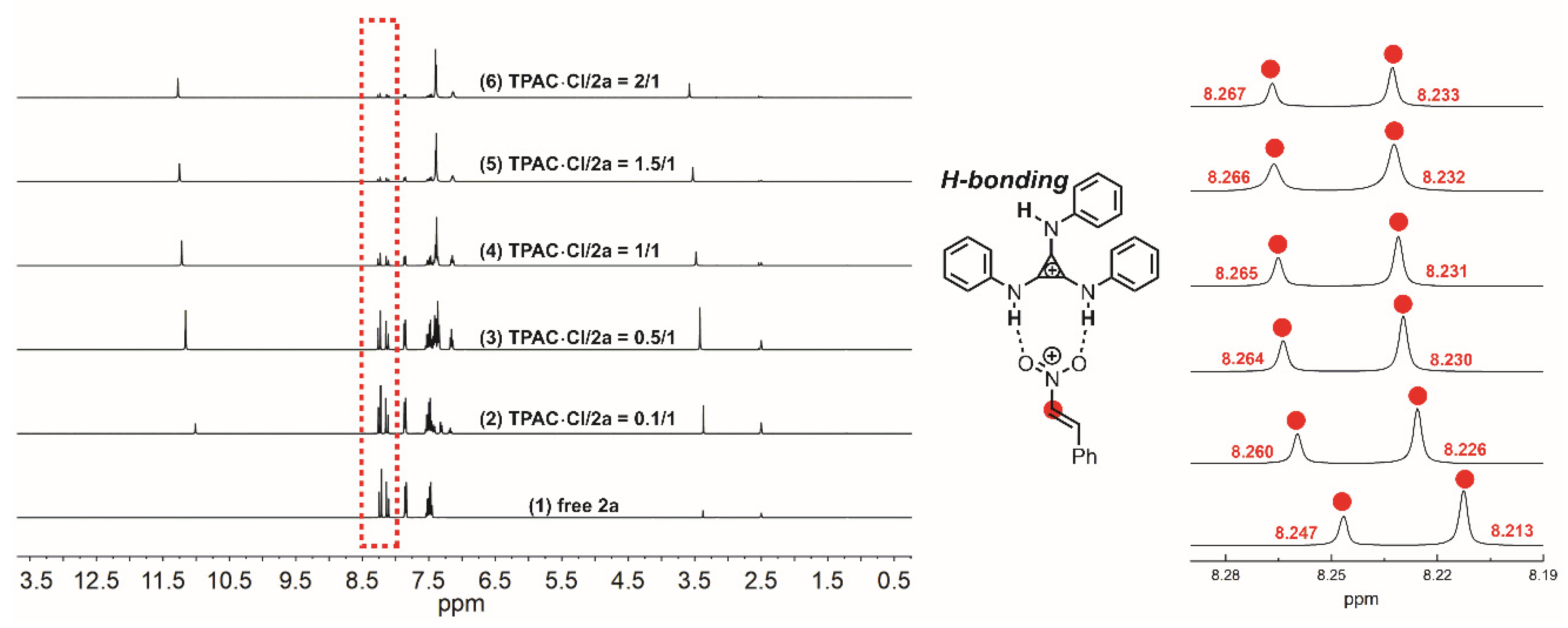
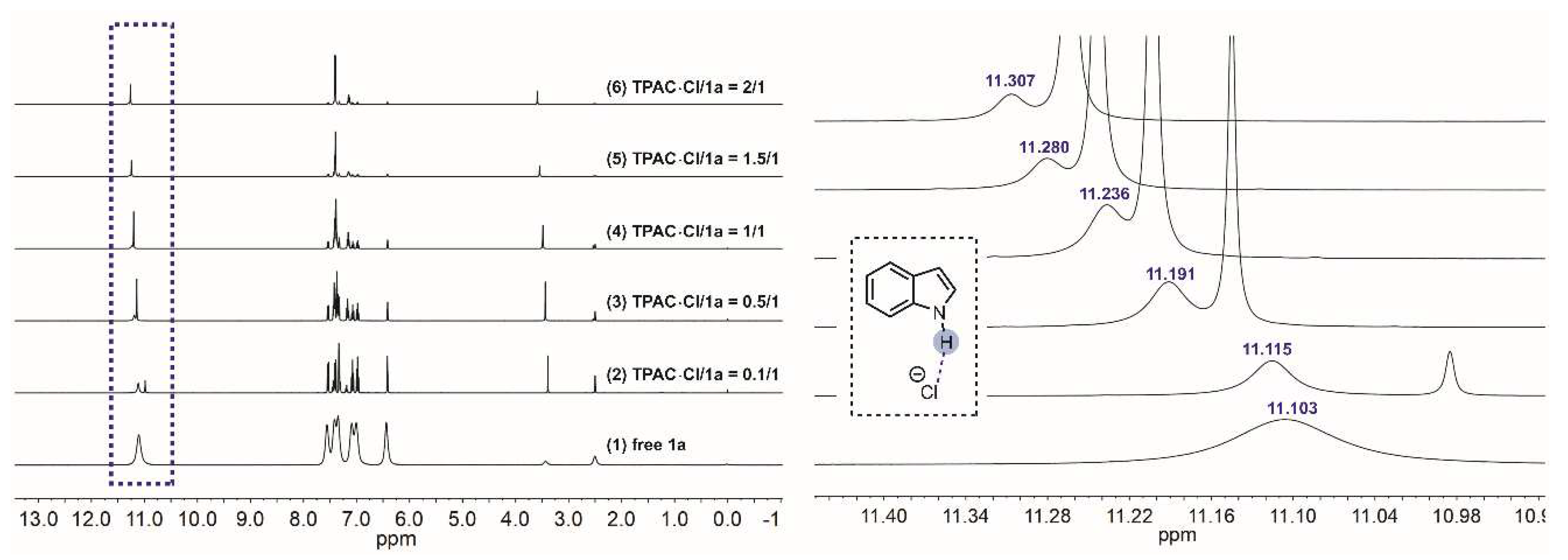
2. Results and Discussion
3. Materials and Methods
- Preparation of the catalyst TPAC·Cl [34]
- The general method for Friedel-Crafts Alkylation catalyzed by TPAC·Cl
4. Conclusions
Supplementary Materials
Author Contributions
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schreiner, P. R. , Metal-free organocatalysis through explicit hydrogen bonding interactions. Chem. Soc. Rev. 2003, 32, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Doyle, A. G.; Jacobsen, E. N. , Small-molecule H-bond donors in asymmetric catalysis. Chemical Reviews 2007, 107, 5713–5743. [Google Scholar] [CrossRef]
- Taylor, M. S.; Jacobsen, E. N. , Asymmetric catalysis by chiral hydrogen-bond donors. Angew. Chem. Int. Ed. 2006, 45, 1520–1543. [Google Scholar] [CrossRef]
- Malerich, J. P.; Hagihara, K.; Rawal, V. H. , Chiral Squaramide Derivatives are Excellent Hydrogen Bond Donor Catalysts. J. Am. Chem. Soc. 2008, 130, 14416–14417. [Google Scholar] [CrossRef] [PubMed]
- Phipps, R. J.; Hamilton, G. L.; Toste, F. D. , The progression of chiral anions from concepts to applications in asymmetric catalysis. Nat. Chem. 2012, 4, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Auvil, T. J.; Schafer, A. G.; Mattson, A. E. , Design Strategies for Enhanced Hydrogen- Bond Donor Catalysts. Eur. J. Org. Chem. 2014, 2014, 2633–2646. [Google Scholar] [CrossRef]
- Min, C.; Seidel, D. , Asymmetric Bronsted acid catalysis with chiral carboxylic acids. Chem. Soc. Rev. 2017, 46, 5889–5902. [Google Scholar] [CrossRef]
- Nishikawa, Y. , Recent topics in dual hydrogen bonding catalysis. Tetrahedron Lett. 2018, 59, 216–223. [Google Scholar] [CrossRef]
- Zhang, Z. G.; Schreiner, P. R. , (Thio)urea organocatalysis - What can be learnt from anion recognition? Chem. Soc. Rev. 2009, 38, 1187–1198. [Google Scholar] [CrossRef]
- Okino, T.; Hoashi, Y.; Takemoto, Y. , Enantioselective Michael Reaction of Malonates to Nitroolefins Catalyzed by Bifunctional Organocatalysts. J. Am. Chem. Soc. 2003, 125, 12672–12673. [Google Scholar] [CrossRef]
- Berkessel, A.; Cleemann, F.; Mukherjee, S.; Müller, T. N.; Lex, J. , Highly Efficient Dynamic Kinetic Resolution of Azlactones by Urea-Based Bifunctional Organocatalysts. Angew. Chem. Int. Ed. 2005, 44, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Connon, S. J. , Organocatalysis Mediated by (Thio)urea Derivatives. Chem. Eur. J. 2006, 12, 5418–5427. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K. H.; Sigman, M. S. , Systematically Probing the Effect of Catalyst Acidity in a Hydrogen-Bond-Catalyzed Enantioselective Reaction. Angew. Chem. Int. Ed. 2007, 46, 4748–4750. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K. H.; Sigman, M. S. , Evaluation of Catalyst Acidity and Substrate Electronic Effects in a Hydrogen Bond-Catalyzed Enantioselective Reaction. J. Org. Chem. 2010, 75, 7194–7201. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Deng, H.; Zhang, B.; Li, J.; Zhang, L.; Luo, S.; Cheng, J. P. , Physical Organic Study of Structure–Activity–Enantioselectivity Relationships in Asymmetric Bifunctional Thiourea Catalysis: Hints for the Design of New Organocatalysts. Chem. Eur. J. 2010, 16, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Corey, E. J.; Grogan, M. J. , Enantioselective synthesis of alpha-amino nitriles from N-benzhydryl imines and HCN with a chiral bicyclic guanidine as catalyst. Org. Lett. 1999, 1, 157–160. [Google Scholar] [CrossRef]
- Terada, M.; Nakano, M.; Ube, H. , Axially chiral guanidine as highly active and enantioselective catalyst for electrophilic amination of unsymmetrically substituted 1,3-dicarbonyl compounds. J. Am. Chem. Soc. 2006, 128, 16044–16045. [Google Scholar] [CrossRef]
- Uyeda, C.; Jacobsen, E. N. , Enantioselective Claisen rearrangements with a hydrogen-bond donor catalyst. J. Am. Chem. Soc. 2008, 130, 9228. [Google Scholar] [CrossRef]
- Selig, P. , Guanidine Organocatalysis. Synthesis 2013, 45, 703–718. [Google Scholar] [CrossRef]
- Fu, X.; Tan, C.-H. , Mechanistic considerations of guanidine-catalyzed reactions. Chem. Commun. 2011, 47, 8210–8222. [Google Scholar] [CrossRef]
- Wittkopp, A.; Schreiner, P. R. , Metal-Free, Noncovalent Catalysis of Diels–Alder Reactions by Neutral Hydrogen Bond Donors in Organic Solvents and in Water. Chem. Eur. J. 2003, 9, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T. P.; Jacobsen, E. N. , Highly enantioselective thiourea-catalyzed nitro-Mannich reactions. Angew. Chem. Int. Ed. 2005, 44, 466–468. [Google Scholar] [CrossRef] [PubMed]
- Malerich, J. P.; Hagihara, K.; Rawal, V. H. , Chiral Squaramide Derivatives are Excellent Hydrogen Bond Donor Catalysts. J. Am. Chem. Soc. 2008, 130, 14416–14417. [Google Scholar] [CrossRef] [PubMed]
- Aleman, J.; Parra, A.; Jiang, H.; Jorgensen, K. A. , Squaramides: Bridging from Molecular Recognition to Bifunctional Organocatalysis. Chem. Eur. J. 2011, 17, 6890–6899. [Google Scholar] [CrossRef]
- Reetz, M. T.; Huette, S.; Goddard, R. , Tetrabutylammonium salts of CH-acidic carbonyl compounds: real carbanions or supramolecules? J. Am. Chem. Soc. 1993, 115, 9339–9340. [Google Scholar] [CrossRef]
- Shirakawa, S.; Liu, S.; Kaneko, S.; Kumatabara, Y.; Fukuda, A.; Omagari, Y.; Maruoka, K. , Tetraalkylammonium Salts as Hydrogen-Bonding Catalysts. Angew. Chem. Int. Ed. 2015, 54, 15767–15770. [Google Scholar] [CrossRef]
- Kaneko, S.; Kumatabara, Y.; Shimizu, S.; Maruoka, K.; Shirakawa, S. , Hydrogen-bonding catalysis of sulfonium salts. Chem. Commun. 2017, 53, 119–122. [Google Scholar] [CrossRef]
- Kumatabara, Y.; Kaneko, S.; Nakata, S.; Shirakawa, S.; Maruoka, K. , Hydrogen-Bonding Catalysis of Tetraalkylammonium Salts in an Aza-Diels-Alder Reaction. Chem. Asian J. 2016, 11, 2126–2129. [Google Scholar] [CrossRef]
- Krebs, A. W. , Cyclopropenylium Compounds and Cyclopropenones. Angew. Chem. Int. Ed. 1965, 4, 10–22. [Google Scholar] [CrossRef]
- Breslow, R.; Groves, J. T.; Ryan, G. , Cyclopropenyl cation. J. Am. Chem. Soc. 1967, 89, 5048–5048. [Google Scholar] [CrossRef]
- Fuchter, M. J.; Smith, C. J.; Tsang, M. W. S.; Boyer, A.; Saubern, S.; Ryan, J. H.; Holmes, A. B. , Clean and efficient synthesis of O-silylcarbamates and ureas in supercritical carbon dioxide. Chem. Commun. 2008, 2152–2154. [Google Scholar] [CrossRef] [PubMed]
- Gaul, D. A.; Just, O.; Rees, W. S. , Synthesis and characterization of a series of zinc bis (alkyl)(trimethylsilyl)amide compounds. Inorg. Chem. 2000, 39, 5648–5654. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J. P.; Cumming, W. D. , Conformational preferences of the N-trimethylsilyl and O-trimethylsilyl groups. J. Am. Chem. Soc. 1971, 93, 928–932. [Google Scholar] [CrossRef]
- Weiss, R.; Hertel, M. , NITROGEN ANALOG OF DELTIC ACID. J. Chem. Soc., Chem. Commun. 1980, 223–224. [Google Scholar] [CrossRef]
- Bartoli, G.; Bencivenni, G.; Dalpozzo, R. , Organocatalytic strategies for the asymmetric functionalization of indoles. Chem. Soc. Rev. 2010, 39, 4449–4465. [Google Scholar] [CrossRef]
- Herrera, R. P.; Sgarzani, V.; Bernardi, L.; Ricci, A. , Catalytic enantioselective Friedel-Crafts alkylation of indoles with nitroalkenes by using a simple thiourea organocatalyst. Angew. Chem. Int. Ed. 2005, 44, 6576–6579. [Google Scholar] [CrossRef]
- Lancianesi, S.; Palmieri, A.; Petrini, M. , Synthetic Approaches to 3-(2-Nitroalkyl) Indoles and Their Use to Access Tryptamines and Related Bioactive Compounds. Chem. Rev. 2014, 114, 7108–7149. [Google Scholar] [CrossRef]
- Narumi, T.; Tsuzuki, S.; Tamamura, H. , Imidazolium Salt-Catalyzed Friedel-Crafts-Type Conjugate Addition of Indoles: Analysis of Indole/Imidazolium Complex by High Level ab Initio Calculations. Asian J. Org. Chem. 2014, 3, 497–503. [Google Scholar] [CrossRef]
- Zhuang, W.; Hazell, R. G.; Jorgensen, K. A. , Enantioselective Friedel-Crafts type addition of indoles to nitro-olefins using a chiral hydrogen-bonding catalyst - synthesis of optically active tetrahydro-[small beta]-carbolines. Org. Biomol. Chem. 2005, 3, 2566–2571. [Google Scholar] [CrossRef]
- Nickerson, D. M.; Mattson, A. E. , Transition Metal and Hydrogen Bond Donor Hybrids: Catalysts for the Activation of Alkylidene Malonates. Chem. Eur. J. 2012, 18, 8310–8314. [Google Scholar] [CrossRef]
- Boiocchi, M.; Del Boca, L.; Gómez, D. E.; Fabbrizzi, L.; Licchelli, M.; Monzani, E. , Nature of Urea−Fluoride Interaction: Incipient and Definitive Proton Transfer. J. Am. Chem. Soc. 2004, 126, 16507–16514. [Google Scholar] [CrossRef] [PubMed]
- Cametti, M.; Rissanen, K. , Recognition and sensing of fluoride anion. Chem. Commun. 2009, 2809–2829. [Google Scholar] [CrossRef] [PubMed]
- Lacour, J.; Moraleda, D. , Chiral anion-mediated asymmetric ion pairing chemistry. Chem. Commun. 2009, 7073–7089. [Google Scholar] [CrossRef]
- Komatsu, K.; Kitagawa, T. , Cyclopropenylium Cations, Cyclopropenones, and Heteroanalogues Recent Advances. Chem. Rev. 2003, 103, 1371–1428. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.; Schwab, O.; Hampel, F. , Ion-Pair Strain as the Driving Force for Hypervalent Adduct Formation between Iodide Ions and Substituted Iodobenzenes: Structural Alternatives to Meisenheimer Complexes. Chem. Eur. J. 1999, 5(3), 968–974. [Google Scholar] [CrossRef]
- Weiss, R.; Brenner, T.; Hampel, F.; Wolski, A. , The Consequences of an Electrostatic “Forced Marriage” between Two Electron-Rich Particles: Strained Ion Pairs. Angew. Chem. Int. Ed. 1995, 34, 439–441. [Google Scholar] [CrossRef]
- Weiss, R.; Rechinger, M.; Hampel, F.; Wolski, A. , Stable 1 : 1 Adducts from Iodoacetylenes and Iodide Ions: Ion Pair Strain as an Additional Driving Force? Angew. Chem. Int. Ed. 1995, 34, 441–443. [Google Scholar] [CrossRef]
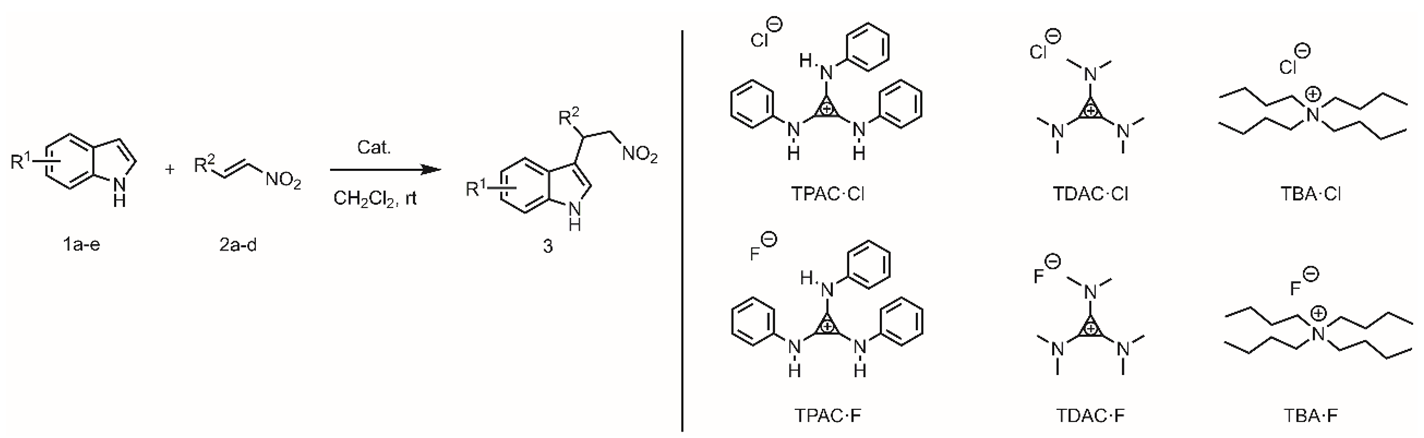 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Ion pair catalyst | Indole | R1 | R2 | Nitroalkene | product | Time / h | Yield / %b |
| 1 | - | 1a | H | Ph | 2a | 3aa | 24 | trace |
| 2 | TPAC·Cl | 1a | H | Ph | 2a | 3aa | 24 | 78 |
| 3 | TPAC·F | 1a | H | Ph | 2a | 3aa | 24 | trace |
| 4 | TDAC·Cl | 1a | H | Ph | 2a | 3aa | 24 | trace |
| 5 | TDAC·F | 1a | H | Ph | 2a | 3aa | 24 | trace |
| 6 | TBA·Cl | 1a | H | Ph | 2a | 3aa | 24 | trace |
| 7 | TBA·F | 1a | H | Ph | 2a | 3aa | 24 | trace |
| 8 | TPAC·Cl | 1b | 2-Me | Ph | 2a | 3ba | 24 | 86 |
| 9 | TPAC·Cl | 1c | 5-OMe | Ph | 2a | 3ca | 24 | 88 |
| 10 | TPAC·Cl | 1d | 5-Cl | Ph | 2a | 3da | 24(72)d | 16(52) |
| 11 | TPAC·Cl | 1e | 7-Me | Ph | 2a | 3ea | 24 | 57 |
| 12 | TPAC·Cl | 1a | H | 4-MeC6H4 | 2b | 3ab | 24 | 33 |
| 13 | TPAC·Cl | 1a | H | 4-MeOC6H4 | 2c | 3ac | 24 | 55 |
| 14 | TPAC·Cl | 1a | H | 2-thienyl | 2d | 3ad | 24 | 71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





