Submitted:
13 September 2023
Posted:
14 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Sample Conditioning (Raw Material)
2.2. Physicochemical Analysis of the Raw Material
2.3. Growth Kinetics for A. niger. sp., Strains
2.4. Experimental Design Box Hunter & Hunter (Fermentation Process and Tratments)
2.5. Condensed and Hydrolyzable Tannins Determination
2.6. Antioxidant Activity
2.7. Antimicrobial and Antifungal Activity
2.8. HPLC-MS Analysis of Extracts Fermentation
3. Results
3.1. Physicochemical Analysis of the Raw Material
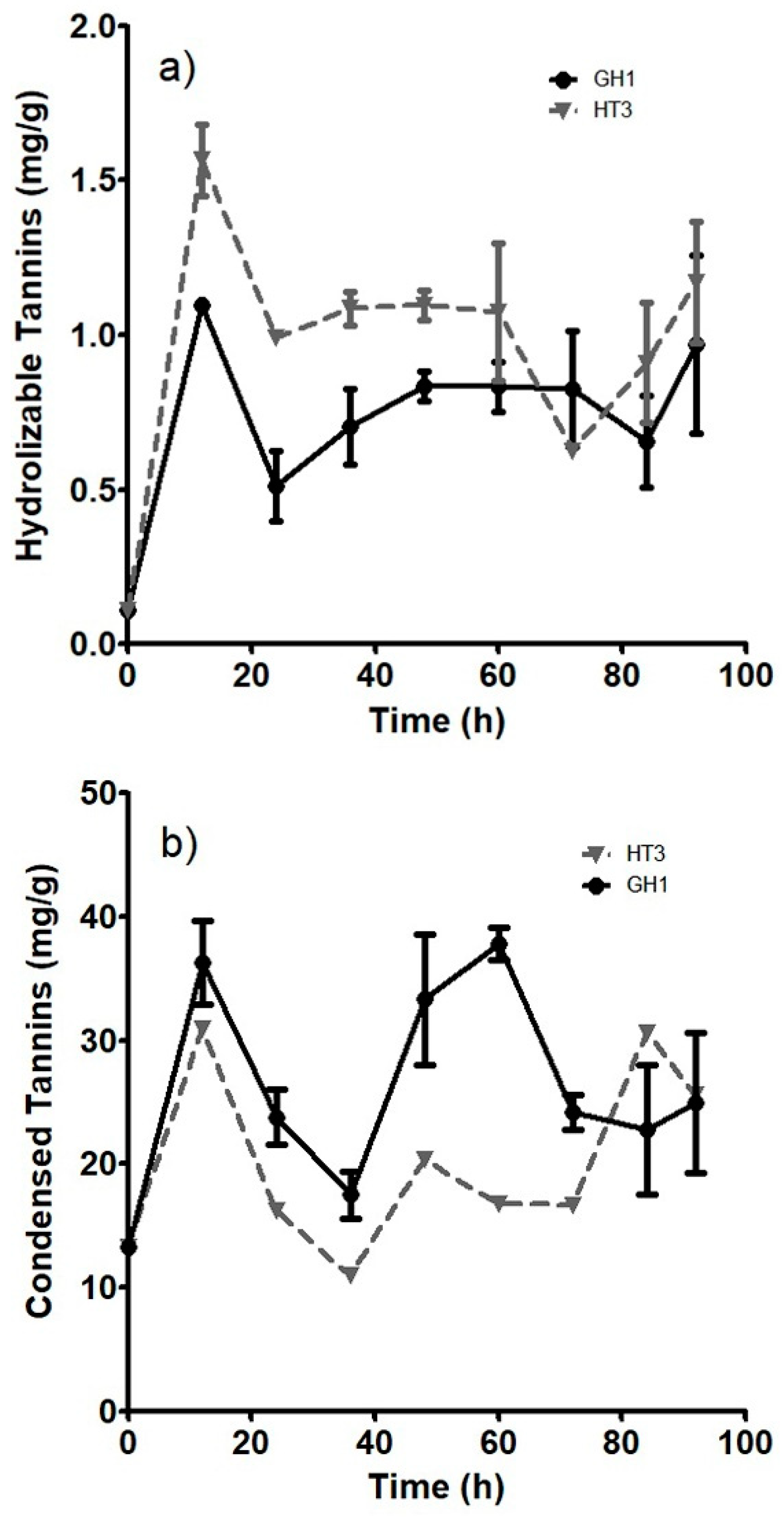
3.2. Growth Kinetics of A. niger. sp. Strains
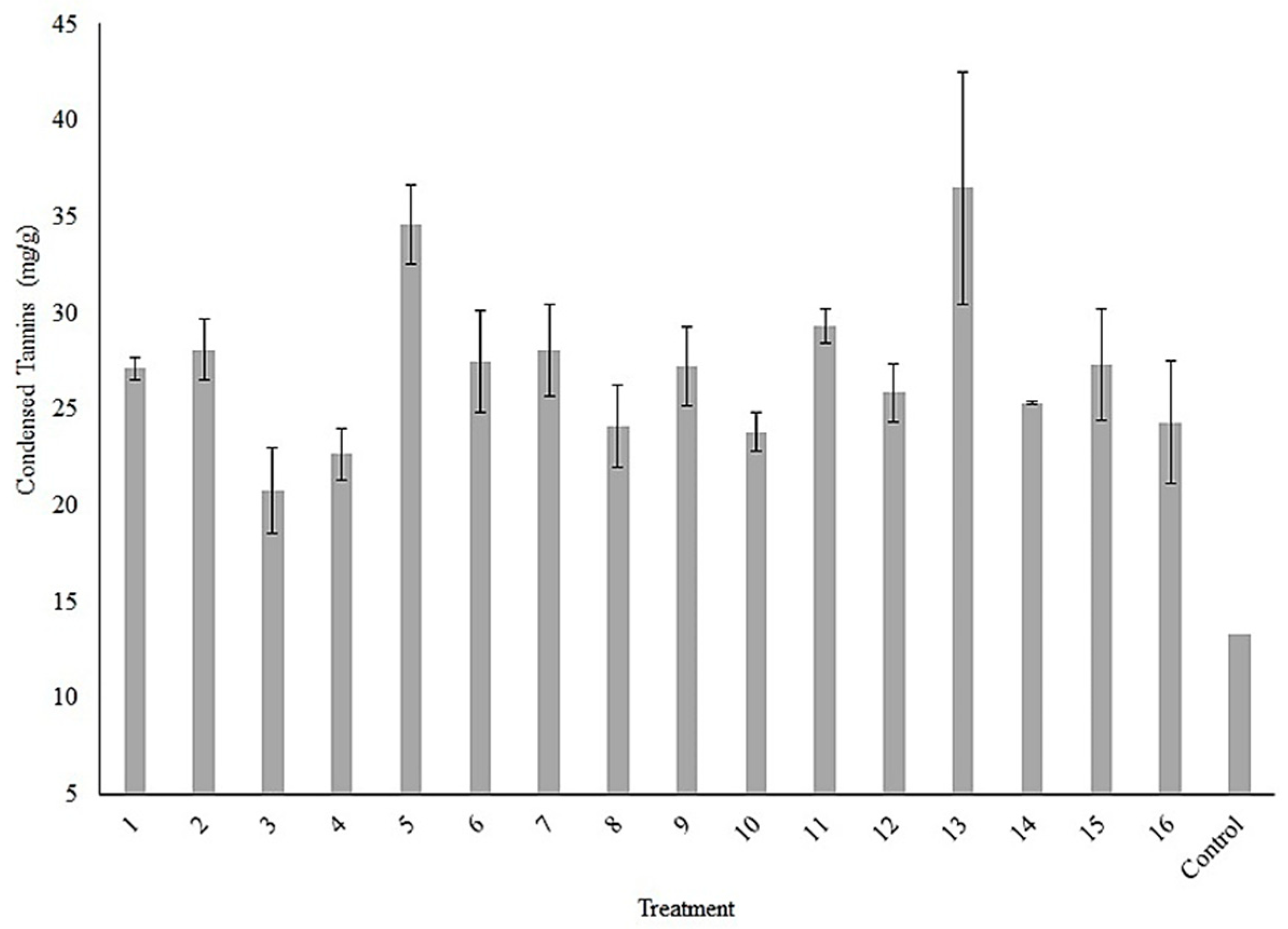
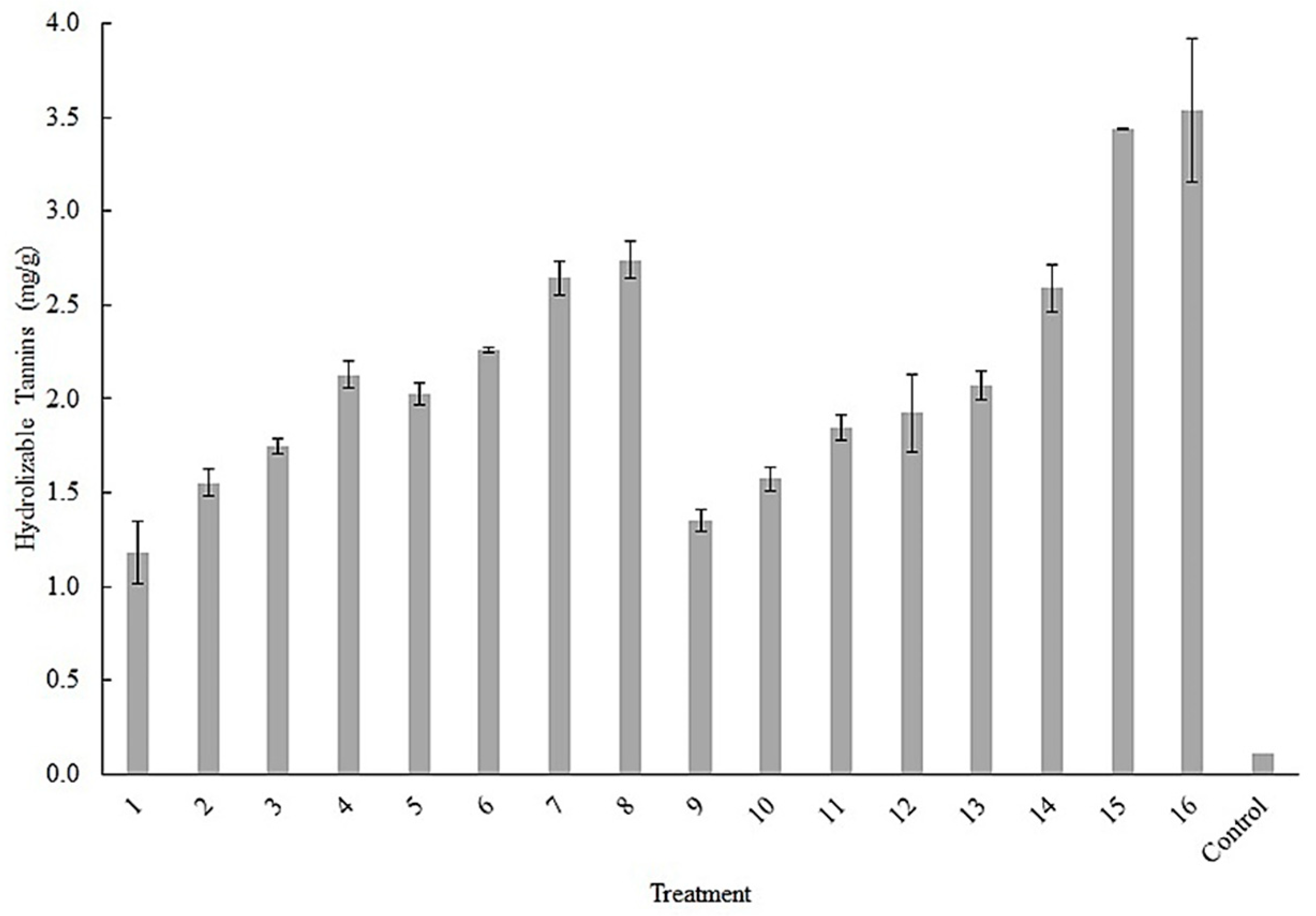
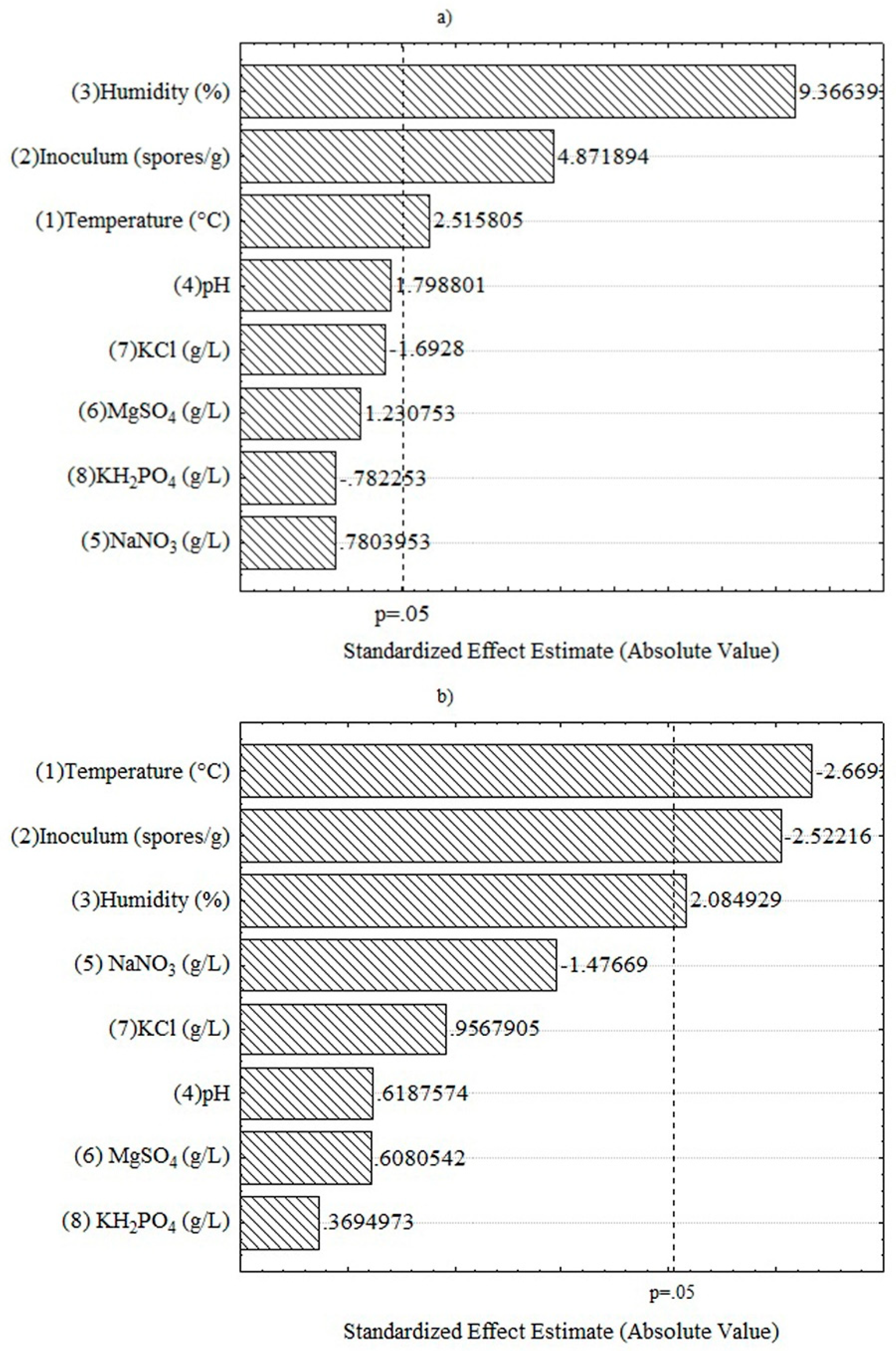
3.3. Evaluation of Box Hunter & Hunter Design and Response Factors
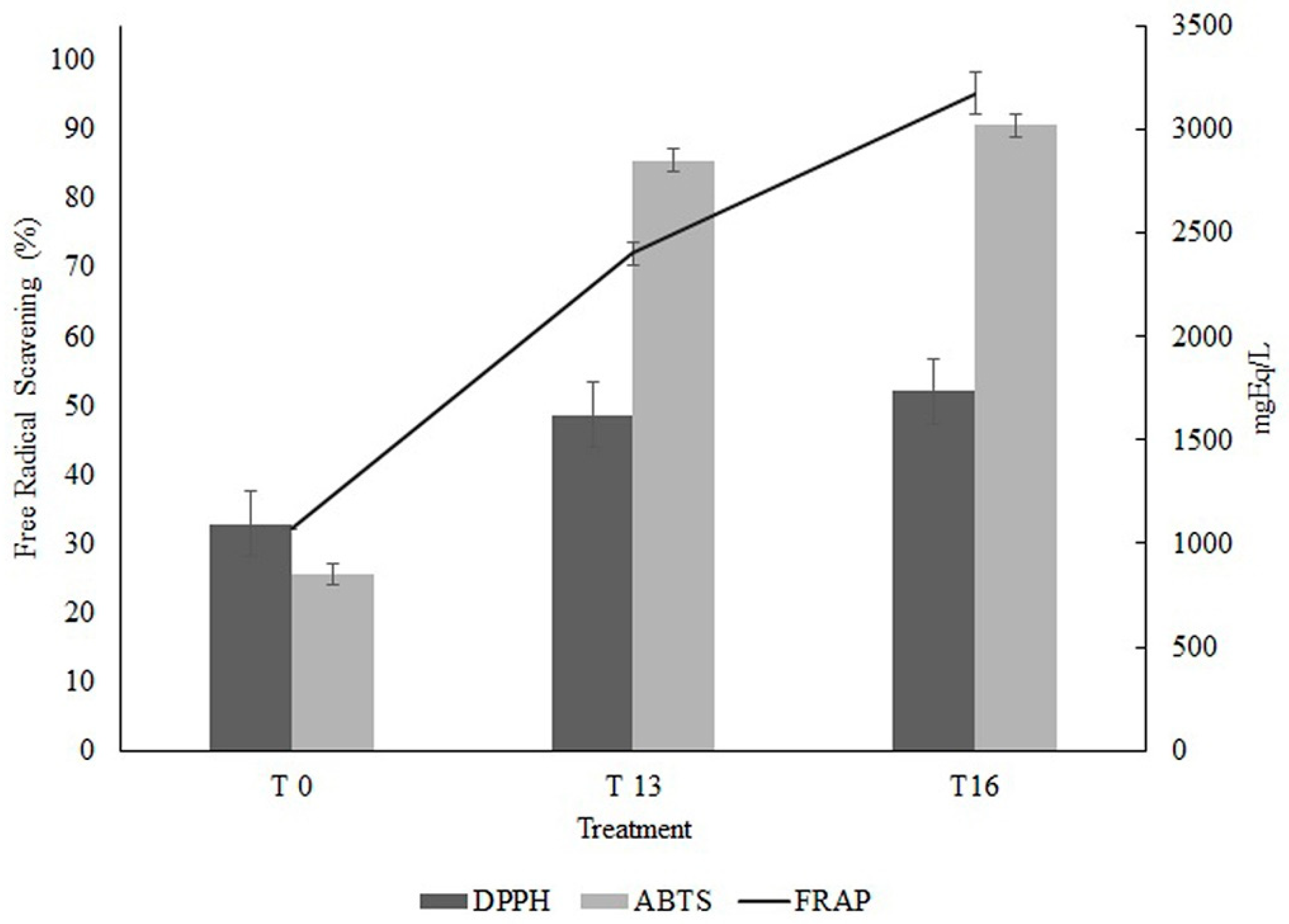
3.4. Antioxidant Activity of the Fermentation Extracts
3.5. Antimicrobial and Antifungal Activity of the Fermentation Extracts
3.6. HPLC-MS Analysis
4. Conclusion
Declaration of Competing Interest
Acknowledgments
References
- Amaral, D.T.; Bonatelli, I.A.S.; Romeiro-Brito, M.; Moraes, E.M.; Franco, F.F. Spatial Patterns of Evolutionary Diversity in Cactaceae Show Low Ecological Representation within Protected Areas. Biol Conserv 2022, 273, 109677. [Google Scholar] [CrossRef]
- de Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Underutilized Plants of the Cactaceae Family: Nutritional Aspects and Technological Applications. Food Chem 2021, 362, 130196. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cadena, R.; Espinosa-Solares, T.; Medina-Moreno, S.A.; Martínez, A.; Lizardi-Jiménez, M.A.; Téllez-Jurado, A. Effect of the Age of Opuntia ficus-indica Cladodes on the Release of Simple Sugars. Biocatal Agric Biotechnol 2021, 33, 102010. [Google Scholar] [CrossRef]
- di Bella, G.; Vecchio, G. lo; Albergamo, A.; Nava, V.; Bartolomeo, G.; Macrì, A.; Bacchetta, L.; lo Turco, V.; Potortì, A.G. Chemical Characterization of Sicilian Dried Nopal [Opuntia ficus-indica (L.) Mill.]. J Food Composit Anal 2022, 106, 104307. [Google Scholar] [CrossRef]
- Feugang, J.M.; Konarski, P.; Zou, D.; Stintzing, F.C.; Zou, C. Nutritional and Medicinal Use of Cactus Pear (Opuntia Spp.) Cladodes and Fruits. Front Biosci 2006, 2574–2589. [Google Scholar] [CrossRef]
- Vargas-Rodríguez, L.; Arroyo-Figueroa, G.; Herrera-Méndez, C.; Pérez-Nieto, A.; García-Vieyra, M.I.; Rodríguez-Núñez, J.R. Physical Properties of Mucilage Prickly Pear. Acta Univ 2016, 26. [Google Scholar] [CrossRef]
- Agostini-Costa, T. da S. Genetic and Environment Effects on Bioactive Compounds of Opuntia Cacti – A Review. J Food Composit Anal 2022, 109, 104514. [Google Scholar] [CrossRef]
- Yang, L.; Lu, M.; Carl, S.; Mayer, J.A.; Cushman, J.C.; Tian, E.; Lin, H. Biomass Characterization of Agave and Opuntia as Potential Biofuel Feedstocks. Biomass Bioenergy 2015, 76, 43–53. [Google Scholar] [CrossRef]
- Kuti, J.O. Antioxidant Compounds from Four Opuntia Cactus Pear Fruit Varieties. Food Chem 2004, 85, 527–533. [Google Scholar] [CrossRef]
- Ginestra, G.; Parker, M.L.; Bennett, R.N.; Robertson, J.; Mandalari, G.; Narbad, A.; lo Curto, R.B.; Bisignano, G.; Faulds, C.B.; Waldron, K.W. Anatomical, Chemical, and Biochemical Characterization of Cladodes from Prickly Pear [Opuntia ficus-indica (L.) Mill.]. J Agric Food Chem 2009, 57, 10323–10330. [Google Scholar] [CrossRef]
- Blando, F.; Russo, R.; Negro, C.; de Bellis, L.; Frassinetti, S. Antimicrobial and Antibiofilm Activity against Staphylococcus aureus of Opuntia ficus-indica (L.) Mill. Cladode Polyphenolic Extracts. Antioxidants 2019, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Brahmi, F.; Blando, F.; Sellami, R.; Mehdi, S.; de Bellis, L.; Negro, C.; Haddadi-Guemghar, H.; Madani, K.; Makhlouf-Boulekbache, L. Optimization of the Conditions for Ultrasound-Assisted Extraction of Phenolic Compounds from Opuntia ficus-indica [L.] Mill. Flowers and Comparison with Conventional Procedures. Ind Crops Prod 2022, 184, 114977. [Google Scholar] [CrossRef]
- Bakar, B.; Çakmak, M.; Ibrahim, M.S.; Özer, D.; Saydam, S.; Karatas, F. Investigation of Amounts of Vitamins, Lycopene, and Elements in the Fruits of Opuntia ficus-indica Subjected to Different Pretreatments. Biol Trace Elem Res 2020, 198, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Ouerghemmi, I.; Harbeoui, H.; Aidi-Wannes, W.; Bettaieb-Rebey, I.; Hammami, M.; Marzouk, B.; Saidani-Tounsi, M. Phytochemical Composition and Antioxidant Activity of Tunisian Cactus Pear (Opuntia ficus indica L.) Flower. J Food Biochem 2017, 41, e12390. [Google Scholar] [CrossRef]
- Messina, C.M.; Renda, G.; La Barbera, L.; Santulli, A. By-Products of Farmed European Sea Bass (Dicentrarchus labrax L.) as a Potential Source of n-3 PUFA. Biologia (Bratisl) 2013, 68, 288–293. [Google Scholar] [CrossRef]
- Messina, C.M.; Arena, R.; Morghese, M.; Santulli, A.; Liguori, G.; Inglese, P. Seasonal Characterization of Nutritional and Antioxidant Properties of Opuntia ficus-indica [(L.) Mill.] Mucilage. Food Hydrocoll 2021, 111, 106398. [Google Scholar] [CrossRef]
- Pizzi, A. Tannins Medical / Pharmacological and Related Applications: A Critical Review. Sustain Chem Pharm 2021, 22, 100481. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Rajasekar, N.; Sivanantham, A. Therapeutic Potential of Plant-Derived Tannins in Non-Malignant Respiratory Diseases. J Nutr Biochem 2021, 94, 108632. [Google Scholar] [CrossRef]
- de Hoyos-Martínez, P.L.; Merle, J.; Labidi, J.; Charrier – El Bouhtoury, F. Tannins Extraction: A Key Point for Their Valorization and Cleaner Production. J Clean Prod 2019, 206, 1138–1155. [Google Scholar] [CrossRef]
- Manan, M.A.; Webb, C. Modern Microbial Solid State Fermentation Technology for Future Biorefineries for the Production of Added-Value Products. Biofuel Res J 2017, 4, 730–740. [Google Scholar] [CrossRef]
- Mansor, A.; Ramli, M.S.; Abdul Rashid, N.Y.; Samat, N.; Lani, M.N.; Sharifudin, S.A.; Raseetha, S. Evaluation of Selected Agri-Industrial Residues as Potential Substrates for Enhanced Tannase Production via Solid-State Fermentation. Biocatal Agric Biotechnol 2019, 20, 101216. [Google Scholar] [CrossRef]
- Torres-León, C.; Ramírez-Guzmán, N.; Ascacio-Valdés, J.; Serna-Cock, L.; dos Santos Correia, M.T.; Contreras-Esquivel, J.C.; Aguilar, C.N. Solid-State Fermentation with Aspergillus niger to Enhance the Phenolic Contents and Antioxidative Activity of Mexican Mango Seed: A Promising Source of Natural Antioxidants. LWT 2019, 112, 108236. [Google Scholar] [CrossRef]
- Olukomaiya, O.O.; Adiamo, O.Q.; Fernando, W.C.; Mereddy, R.; Li, X.; Sultanbawa, Y. Effect of Solid-State Fermentation on Proximate Composition, Anti-Nutritional Factor, Microbiological and Functional Properties of Lupin Flour. Food Chem 2020, 315, 126238. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.C.; Liu, L.Y.; Yang, S.S. Protein Enrichment, Cellulase Production and in Vitro Digestion Improvement of Pangolagrass with Solid State Fermentation. J Microbiol Immunol Infect 2012, 45, 7–14. [Google Scholar] [CrossRef]
- Yafetto, L. Application of Solid-State Fermentation by Microbial Biotechnology for Bioprocessing of Agro-Industrial Wastes from 1970 to 2020: A Review and Bibliometric Analysis. Heliyon 2022, 8, e09173. [Google Scholar] [CrossRef]
- Munishamanna, K.B.; Suresha, K.B.; Veena, R.; Subramanya, S. Solid State Fermentation of Mango Peel and Mango Seed Waste by Different Yeasts and Bacteria for Nutritional Improvement. Int J Food Ferment Technol 2017, 7, 111. [Google Scholar] [CrossRef]
- Farinas, C.S. Advances in Cultivation Strategies of Aspergillus for Production of Enzymes Involved in the Saccharification of Lignocellulosic Feedstocks. In New and Future Developments in Microbial Biotechnology and Bioengineering: Aspergillus System Properties and Applications; Eds.; Elsevier: Chennai, India, 2016; pp. 141–154. ISBN 978-0-444-63505-1. [Google Scholar]
- Cano y Postigo, L.O.; Jacobo-Velázquez, D.A.; Guajardo-Flores, D.; Garcia Amezquita, L.E.; García-Cayuela, T. Solid-State Fermentation for Enhancing the Nutraceutical Content of Agrifood by-Products: Recent Advances and Its Industrial Feasibility. Food Biosci 2021, 41, 100926. [Google Scholar] [CrossRef]
- Cerda-Cejudo, N.D.; Buenrostro-Figueroa, J.J.; Sepúlveda, L.; Torres-Leon, C.; Chávez-González, M.L.; Ascacio-Valdés, J.A.; Aguilar, C.N. Recovery of Ellagic Acid from Mexican Rambutan Peel by Solid-State Fermentation-Assisted Extraction. Food Bioprod Process 2022, 134, 86–94. [Google Scholar] [CrossRef]
- Sepúlveda, L.; Wong-Paz, J.E.; Buenrostro-Figueroa, J.; Ascacio-Valdés, J.A.; Aguilera-Carbó, A.; Aguilar, C.N. Solid State Fermentation of Pomegranate Husk: Recovery of Ellagic Acid by SEC and Identification of Ellagitannins by HPLC/ESI/MS. Food Biosci 2018, 22, 99–104. [Google Scholar] [CrossRef]
- Araújo, L.F.; Medeiros, A.N.; Neto, A.P.; Oliveira, L.S.C.; da Silva, F.L.H. Protein Enrichment of Cactus Pear (Opuntia ficus - indica Mill) Using Saccharomyces cerevisiae in Solid-State Fermentation. Braz Arch Biol Technol 2005, 48. [Google Scholar] [CrossRef]
- Herrera, E.; Murillo, M.; Berumen, L.; Soto-Cruz, N.; Paez-Lerma, J. Protein enrichment of Opuntia ficus-indica using Kluyveromyces marxianus in solid-state fermentation. Cienc Inv Agr 2017, 44. [Google Scholar] [CrossRef]
- Ali, S.K.; Mahmoud, S.M.; El-Masry, S.S.; Alkhalifah, D.H.M.; Hozzein, W.N.; Aboel-Ainin, M.A. Phytochemical Screening and Characterization of the Antioxidant, Anti-Proliferative and Antibacterial Effects of Different Extracts of Opuntia ficus-indica Peel. J King Saud Univ Sci 2022, 34, 102216. [Google Scholar] [CrossRef]
- Kejla, L.; Schulzke, T.; Šimáček, P.; Auersvald, M. Anthrone Method Combined with Adsorption of Interferents as a New Approach towards Reliable Quantification of Total Carbohydrate Content in Pyrolysis Bio-Oils. J Anal Appl Pyrolysis 2023, 173, 106066. [Google Scholar] [CrossRef]
- Prasertsung, I.; Chutinate, P.; Watthanaphanit, A.; Saito, N.; Damrongsakkul, S. Conversion of Cellulose into Reducing Sugar by Solution Plasma Process (SPP). Carbohydr Polym 2017, 172, 230–236. [Google Scholar] [CrossRef]
- Gu, L.B.; Zhang, G.J.; Du, L.; Du, J.; Qi, K.; Zhu, X.L.; Zhang, X.Y.; Jiang, Z.H. Comparative Study on the Extraction of Xanthoceras sorbifolia Bunge (Yellow Horn) Seed Oil Using Subcritical n-Butane, Supercritical CO2, and the Soxhlet Method. LWT 2019, 111, 548–554. [Google Scholar] [CrossRef]
- Deepachandi, B.; Weerasinghe, S.; Andrahennadi, T.P.; Karunaweera, N.D.; Wickramarachchi, N.; Soysa, P.; Siriwardana, Y. Quantification of Soluble or Insoluble Fractions of Leishmania Parasite Proteins in Microvolume Applications: A Simplification to Standard Lowry Assay. Int J Anal Chem 2020, 1–8. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Rodríguez-Jasso, R.M.; Rodríguez, R.; Contreras-Esquivel, J.C.; Aguilar, C.N. Pectinase Production from Lemon Peel Pomace as Support and Carbon Source in Solid-State Fermentation Column-Tray Bioreactor. Biochem Eng J 2012, 65, 90–95. [Google Scholar] [CrossRef]
- Mitchell, D.A.; von Meien, O.F.; Krieger, N.; Dalsenter, F.D.H. A Review of Recent Developments in Modeling of Microbial Growth Kinetics and Intraparticle Phenomena in Solid-State Fermentation. Biochem Eng J 2004, 17, 15–26. [Google Scholar] [CrossRef]
- Palacios, C.E.; Nagai, A.; Torres, P.; Rodrigues, J.A.; Salatino, A. Contents of Tannins of Cultivars of Sorghum Cultivated in Brazil, as Determined by Four Quantification Methods. Food Chem 2021, 337, 127970. [Google Scholar] [CrossRef]
- Sawczuk, R.; Karpinska, J.; Filipowska, D.; Bajguz, A.; Hryniewicka, M. Evaluation of Total Phenols Content, Anti-DPPH Activity and the Content of Selected Antioxidants in the Honeybee Drone Brood Homogenate. Food Chem 2022, 368, 130745. [Google Scholar] [CrossRef]
- Sepúlveda, L.; Romaní, A.; Aguilar, C.N.; Teixeira, J. Valorization of Pineapple Waste for the Extraction of Bioactive Compounds and Glycosides Using Autohydrolysis. Innov Food Sci Emerg Technol 2018, 47, 38–45. [Google Scholar] [CrossRef]
- Sik, B.; Ajtony, Z.; Lakatos, E.; Székelyhidi, R. The Effects of Extraction Conditions on the Antioxidant Activities, Total Polyphenol and Monomer Anthocyanin Contents of Six Edible Fruits Growing Wild in Hungary. Heliyon 2022, 8, e12048. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, J.; Li, W.; Li, R.; Wang, X.; Qiao, H.; Sun, Q.; Zhang, H. Antibacterial Mechanism of the Polysaccharide Produced by Chaetomium globosum CGMCC 6882 against Staphylococcus aureus. Int J Biol Macromol 2020, 159, 231–235. [Google Scholar] [CrossRef]
- Aqueveque, P.; Céspedes, C.L.; Becerra, J.; Aranda, M.; Sterner, O. Antifungal Activities of Secondary Metabolites Isolated from Liquid Fermentations of Stereum hirsutum (Sh134-11) against Botrytis cinerea (Grey Mould Agent). Food Chem Toxicol 2017, 109, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, T.C.; Reis, N.D.S.; Silva, T.P.; Machado, F.P.P.; Bonomo, R.C.F.; Franco, M. Prickly Palm Cactus Husk as a Raw Material for Production of Ligninolytic Enzymes by Aspergillus niger. Food Sci Biotechnol 2016, 25, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Moßhammer, M.R.; Stintzing, F.C.; Carle, R. Cactus Pear Fruits (Opuntia Spp.): A Review of Processing Technologies and Current Uses. J Prof Assoc Cactus 2006, 8, 1–25. [Google Scholar] [CrossRef]
- Kuloyo, O.O.; du Preez, J.C.; García-Aparicio, M.P.; Kilian, S.G.; Steyn, L.; Görgens, J. Opuntia ficus-indica Cladodes as Feedstock for Ethanol Production by Kluyveromyces marxianus and Saccharomyces cerevisiae. World J Microbiol Biotechnol 2014, 30, 3173–3183. [Google Scholar] [CrossRef]
- Torres-León, C.; Ramírez-Guzmán, N.; Ascacio-Valdés, J.; Serna-Cock, L.; dos Santos Correia, M.T.; Contreras-Esquivel, J.C.; Aguilar, C.N. Solid-State Fermentation with Aspergillus niger to Enhance the Phenolic Contents and Antioxidative Activity of Mexican Mango Seed: A Promising Source of Natural Antioxidants. LWT 2019, 112, 108236. [Google Scholar] [CrossRef]
- Barba, F.J.; Putnik, P.; Bursać Kovačević, D.; Poojary, M.M.; Roohinejad, S.; Lorenzo, J.M.; Koubaa, M. Impact of Conventional and Non-Conventional Processing on Prickly Pear (Opuntia Spp.) and Their Derived Products: From Preservation of Beverages to Valorization of by-Products. Trends Food Sci Technol 2017, 67, 260–270. [Google Scholar] [CrossRef]
- Bouazizi, S.; Montevecchi, G.; Antonelli, A.; Hamdi, M. Effects of Prickly Pear (Opuntia ficus-indica L.) Peel Flour as an Innovative Ingredient in Biscuits Formulation. LWT 2020, 124, 109155. [Google Scholar] [CrossRef]
- Ali, S.K.; Mahmoud, S.M.; El-Masry, S.S.; Alkhalifah, D.H.M.; Hozzein, W.N.; Aboel-Ainin, M.A. Phytochemical Screening and Characterization of the Antioxidant, Anti-Proliferative and Antibacterial Effects of Different Extracts of Opuntia ficus-indica Peel. J King Saud Univ Sci 2022, 34, 102216. [Google Scholar] [CrossRef]
- Aruwa, C.E.; Amoo, S.O.; Kudanga, T. Extractable and Macromolecular Antioxidants of Opuntia ficus-indica Cladodes: Phytochemical Profiling, Antioxidant and Antibacterial Activities. S African J Bot 2019, 125, 402–410. [Google Scholar] [CrossRef]
- Ma, Y.; Ding, S.; Fei, Y.; Liu, G.; Jang, H.; Fang, J. Antimicrobial Activity of Anthocyanins and Catechins against Foodborne Pathogens Escherichia coli and Salmonella. Food Control 2019, 106, 106712. [Google Scholar] [CrossRef]
- Alqurashi, A.S.; Al Masoudi, L.M.; Hamdi, H.; Abu Zaid, A. Chemical Composition and Antioxidant, Antiviral, Antifungal, Antibacterial and Anticancer Potentials of Opuntia ficus-indica Seed Oil. Molecules 2022, 27, 5453. [Google Scholar] [CrossRef]
- Brahmi, F.; Haddad, S.; Bouamara, K.; Yalaoui-Guellal, D.; Prost-Camus, E.; de Barros, J.P.P.; Prost, M.; Atanasov, A.G.; Madani, K.; Boulekbache-Makhlouf, L.; Lizard, G. Comparison of Chemical Composition and Biological Activities of Algerian Seed Oils of Pistacia lentiscus L., Opuntia ficus indica (L.) Mill. and Argania spinosa L. Skeels. Ind Crops Prod 2020, 151, 112456. [Google Scholar] [CrossRef]
- Amel, R.; Hanen, F.; Ferid, L.; Riadh, K.; Chedly, A.; Aly, R. Upshot of the Ripening Time on Biological Activities, Phenol Content and Fatty Acid Composition of Tunisian Opuntia ficus-indica Fruit. Afr J Biotechnol 2013, 12, 5875–5885. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Sobeh, M.; Badr, W.K.; Abdelfattah, M.A.O.; Ali, Z.Y.; El-Tantawy, M.E.; Rabeh, M.A.; Wink, M. HPLC-PDA-MS/MS Profiling of Secondary Metabolites from Opuntia ficus-indica Cladode, Peel and Fruit Pulp Extracts and Their Antioxidant, Neuroprotective Effect in Rats with Aluminum Chloride Induced Neurotoxicity. Saudi J Biol Sci 2020, 27, 2829–2838. [Google Scholar] [CrossRef]
- Mena, P.; Tassotti, M.; Andreu, L.; Nuncio-Jáuregui, N.; Legua, P.; del Rio, D.; Hernández, F. Phytochemical Characterization of Different Prickly Pear (Opuntia ficus-indica (L.) Mill.) Cultivars and Botanical Parts: UHPLC-ESI-MSn Metabolomics Profiles and Their Chemometric Analysis. Food Res Int 2018, 108, 301–308. [Google Scholar] [CrossRef]
- Benayad, Z.; Martínez-Villaluenga, C.; Frias, J.; Gómez-Cordoves, C.; Es-Safi, N.E. Phenolic Composition, Antioxidant and Anti-Inflammatory Activities of Extracts from Moroccan Opuntia ficus-indica Flowers Obtained by Different Extraction Methods. Ind Crops Prod 2014, 62, 412–420. [Google Scholar] [CrossRef]
- Aruwa, C.E.; Amoo, S.; Kudanga, T. Phenolic Compound Profile and Biological Activities of Southern African Opuntia ficus-indica Fruit Pulp and Peels. LWT 2019, 111, 337–344. [Google Scholar] [CrossRef]
- Guevara-Figueroa, T.; Jiménez-Islas, H.; Reyes-Escogido, M.L.; Mortensen, A.G.; Laursen, B.B.; Lin, L. W.; de León-Rodríguez, A.; Fomsgaard, I.S.; Barba de la Rosa, A.P. Proximate Composition, Phenolic Acids, and Flavonoids Characterization of Commercial and Wild Nopal (Opuntia Spp.). J Food Compost Anal 2010, 23, 525–532. [Google Scholar] [CrossRef]
- de Albuquerque, J.G.; Escalona-Buendía, H.B.; de Magalhães, C.A.M.T.; dos Santos, L.M.; de Souza, A.J.; da Silva, V.M.A. Ultrasound Treatment for Improving the Bioactive Compounds and Quality Properties of a Brazilian Nopal (Opuntia ficus-indica) Beverage during Shelf-Life. LWT 2021, 149, 111814. [Google Scholar] [CrossRef]
- Antika, L.D.; Tasfiyati, A.N.; Hikmat, H.; Septama, A.W. Scopoletin: A Review of Its Source, Biosynthesis, Methods of Extraction, and Pharmacological Activities. Z Naturforsch C J Biosci 2022, 77, 303–316. [Google Scholar] [CrossRef]
- Elbatreek, M.H.; Mahdi, I.; Ouchari, W.; Mahmoud, M.F.; Sobeh, M. Current Advances on the Therapeutic Potential of Pinocembrin: An Updated Review. Biomed Pharmacother 2023, 157, 114032. [Google Scholar] [CrossRef]
- Amzad Hossain, M.; Ismail, Z. Quantification and Enrichment of Sinensetin in the Leaves of Orthosiphon stamineus. Arab J Chem 2016, 9, S1338–S1341. [Google Scholar] [CrossRef]
- Xu, D.; Qiao, F.; Xi, P.; Lin, Z.; Jiang, Z.; Romanazzi, G.; Gao, L. Efficacy of Pterostilbene Suppression of Postharvest Gray Mold in Table Grapes and Potential Mechanisms. Postharvest Biol Technol 2022, 183, 111745. [Google Scholar] [CrossRef]
- Paiva-Martins, F.; Fernandes, J.; Santos, V.; Silva, L.; Borges, F.; Rocha, S.; Belo, L.; Santos-Silva, A. Powerful Protective Role of 3,4-Dihydroxyphenylethanol−Elenolic Acid Dialdehyde against Erythrocyte Oxidative-Induced Hemolysis. J Agric Food Chem 2009, 58, 135–140. [Google Scholar] [CrossRef]
| Treatment | Temperature(°C) | Inoculum (spores/g) | Humidity (%) | pH | NaNO3 (g/L) | MgSO4 (g/L) | KCl (g/L) | KH2PO4 (g/L) |
| 1 | -1 | -1 | -1 | -1 | -1 | -1 | -1 | -1 |
| 2 | 1 | -1 | -1 | -1 | -1 | 1 | 1 | 1 |
| 3 | -1 | 1 | -1 | -1 | 1 | -1 | 1 | 1 |
| 4 | 1 | 1 | -1 | -1 | 1 | 1 | -1 | -1 |
| 5 | -1 | -1 | 1 | -1 | 1 | 1 | 1 | -1 |
| 6 | 1 | -1 | 1 | -1 | 1 | -1 | -1 | 1 |
| 7 | -1 | 1 | 1 | -1 | -1 | 1 | -1 | 1 |
| 8 | 1 | 1 | 1 | -1 | -1 | -1 | 1 | -1 |
| 9 | -1 | -1 | -1 | 1 | 1 | 1 | -1 | 1 |
| 10 | 1 | -1 | -1 | 1 | 1 | -1 | 1 | -1 |
| 11 | -1 | 1 | -1 | 1 | -1 | 1 | 1 | -1 |
| 12 | 1 | 1 | -1 | 1 | -1 | -1 | -1 | 1 |
| 13 | -1 | -1 | 1 | 1 | -1 | -1 | 1 | 1 |
| 14 | 1 | -1 | 1 | 1 | -1 | 1 | -1 | -1 |
| 15 | -1 | 1 | 1 | 1 | 1 | -1 | -1 | -1 |
| 16 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Factors | Levels | |||||||
| +1 | -1 | |||||||
| Temperature (°C) | 30 | 25 | ||||||
| Inoculum (spores/g) | 1x107 | 1x106 | ||||||
| Humidity (%) | 70 | 60 | ||||||
| pH | 7 | 6 | ||||||
| NaNO3 (g/L) | 6 | 3 | ||||||
| MgSO4 (g/L) | 0.52 | 0.26 | ||||||
| KCl (g/L) | 0.52 | 0.26 | ||||||
| KH2PO4 (g/L) | 1.52 | 0.52 | ||||||
| Putative compound | Unfermented sample | Treatment 13 | Treatment 16 |
| (+)-Catechin | - | - | + |
| 3,4-DHPEA-AC | - | + | + |
| Apigenin 7-O-diglucuronide | - | + | + |
| Caffeic acid 4-O-glucoside | + | - | - |
| Cyanidin | + | - | - |
| Dihydroquercetin | - | + | - |
| Feruloyl glucose | - | - | + |
| Isorhamnetin | + | + | + |
| Isorhamnetin 3-O-glucoside | - | + | - |
| p-Coumaric acid 4-O-glucoside | - | + | + |
| p-Coumaroyl glycolic acid | - | - | + |
| Pinocembrin | - | + | + |
| Pterostilbene | - | + | + |
| Quercetin | + | - | - |
| Rhamnetin | + | + | + |
| Scopoletin | - | + | + |
| Sinensetin | - | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





