Submitted:
14 September 2023
Posted:
14 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Bone remodelling and homeostasis in health
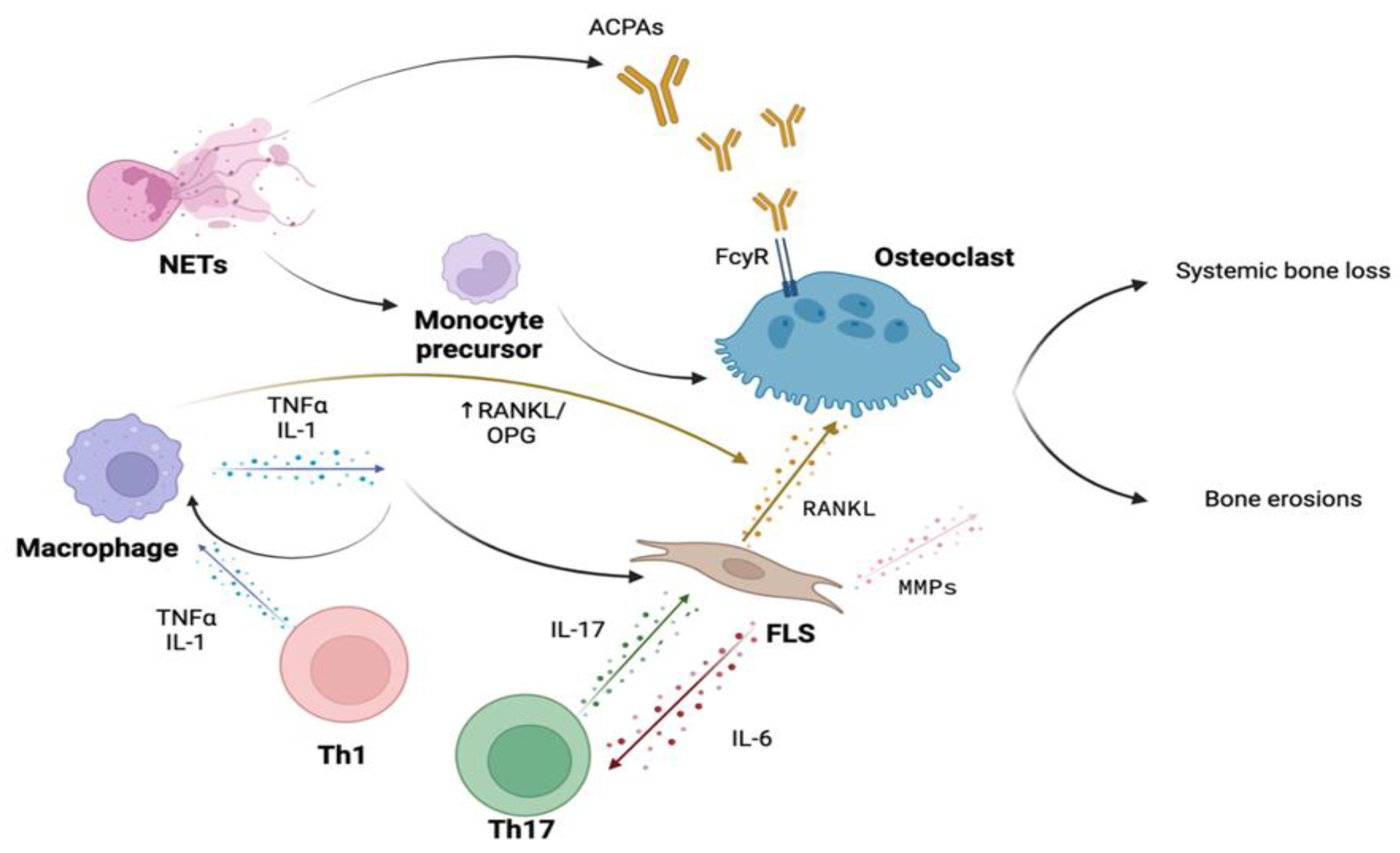
3. Bone involvement in Rheumatoid Arthritis
3.1. Immune cells
3.2. Cytokines
3.2.1. TNF- α
3.2.2. IL-6
3.2.3. OSCAR
3.3. Autoantibodies
3.4. Bone erosions in rheumatoid arthritis
3.5. Osteoporosis in rheumatoid arthritis
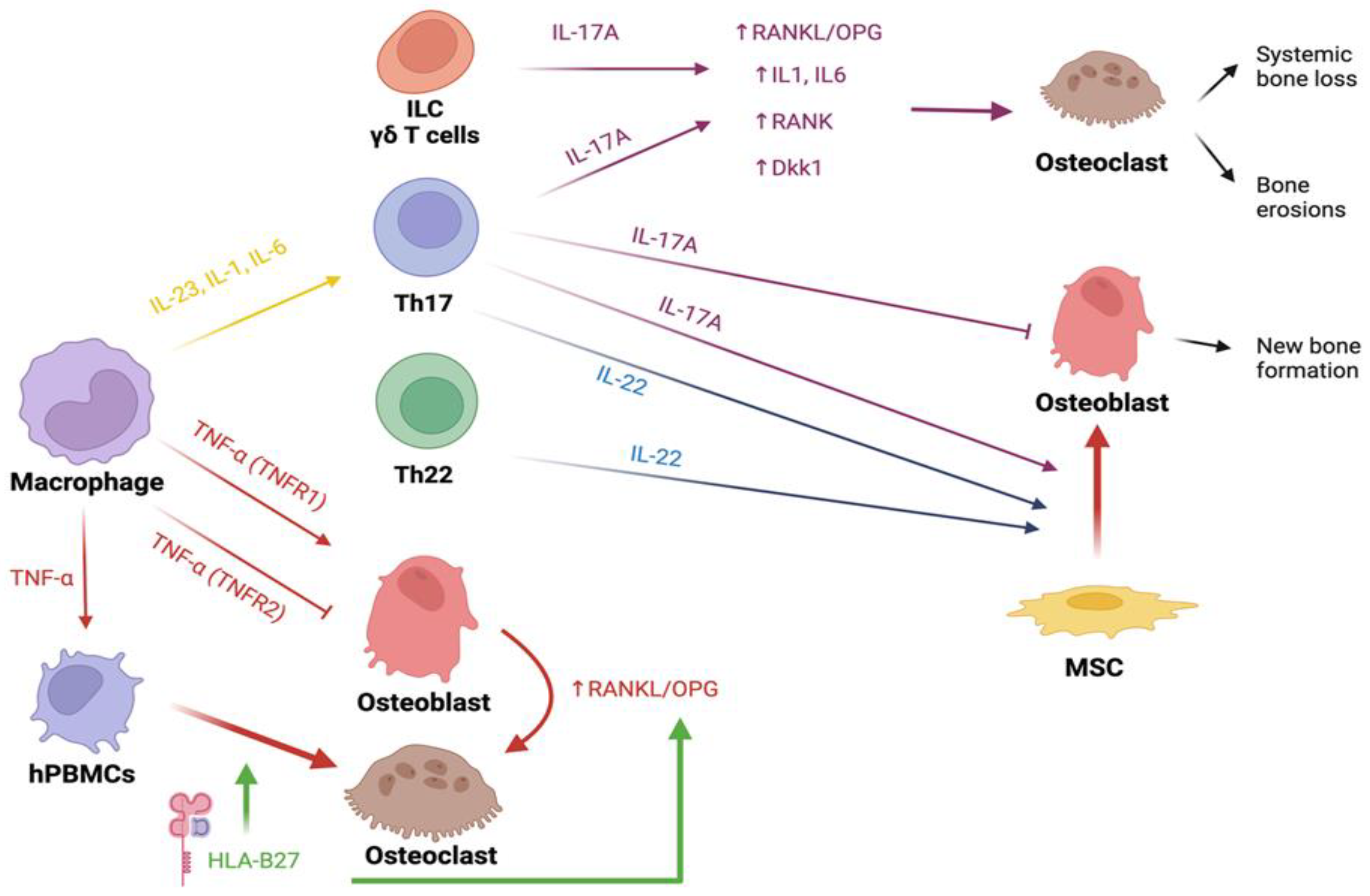
4. Bone involvement in spondyloarthritis
4.1. HLA-B27
4.2. Cytokines
4.2.1. IL-23 and IL-17
4.2.2. TNF-α
4.2.3. Other cytokines
4.3. Erosions in Spondyloarthritis
4.4. New Bone Formation in Spondyloarthritis
4.5. The enthesis milieu
4.6. New bone formation pathways
4.6.1. Wnt/ß-catenin
4.6.2. Cytokines
4.7. Osteoporosis in Spondyloarthritis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bolamperti, S.; Villa, I.; Rubinacci, A. Bone Remodeling: An Operational Process Ensuring Survival and Bone Mechanical Competence. Bone Res 2022, 10, 48. [CrossRef]
- Parfitt, A.M. Osteonal and Hemi-Osteonal Remodeling: The Spatial and Temporal Framework for Signal Traffic in Adult Human Bone. J Cell Biochem 1994, 55, 273–286. [CrossRef]
- Takayanagi, H. Osteoimmunology: Shared Mechanisms and Crosstalk between the Immune and Bone Systems. Nat Rev Immunol 2007, 7, 292–304. [CrossRef]
- Okamoto, K.; Nakashima, T.; Shinohara, M.; Negishi-Koga, T.; Komatsu, N.; Terashima, A.; Sawa, S.; Nitta, T.; Takayanagi, H. Osteoimmunology: The Conceptual Framework Unifying the Immune and Skeletal Systems. Physiol Rev 2017, 97, 1295–1349. [CrossRef]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and Activation of the Transcription Factor NFATc1 (NFAT2) Integrate RANKL Signaling in Terminal Differentiation of Osteoclasts. Dev Cell 2002, 3, 889–901. [CrossRef]
- Yeo, L.; Toellner, K.-M.; Salmon, M.; Filer, A.; Buckley, C.D.; Raza, K.; Scheel-Toellner, D. Cytokine MRNA Profiling Identifies B Cells as a Major Source of RANKL in Rheumatoid Arthritis. Ann Rheum Dis 2011, 70, 2022–2028. [CrossRef]
- Wehmeyer, C.; Pap, T.; Buckley, C.D.; Naylor, A.J. The Role of Stromal Cells in Inflammatory Bone Loss. Clin Exp Immunol 2017, 189, 1–11. [CrossRef]
- Silva, B.C.; Bilezikian, J.P. Parathyroid Hormone: Anabolic and Catabolic Actions on the Skeleton. Curr Opin Pharmacol 2015, 22, 41–50. [CrossRef]
- Nakashima, K.; de Crombrugghe, B. Transcriptional Mechanisms in Osteoblast Differentiation and Bone Formation. Trends Genet 2003, 19, 458–466. [CrossRef]
- Chen, G.; Deng, C.; Li, Y.-P. TGF-β and BMP Signaling in Osteoblast Differentiation and Bone Formation. Int J Biol Sci 2012, 8, 272–288. [CrossRef]
- Titorencu, I.; Pruna, V.; Jinga, V.V.; Simionescu, M. Osteoblast Ontogeny and Implications for Bone Pathology: An Overview. Cell Tissue Res 2014, 355, 23–33. [CrossRef]
- Kawane, T.; Qin, X.; Jiang, Q.; Miyazaki, T.; Komori, H.; Yoshida, C.A.; Matsuura-Kawata, V.K.D.S.; Sakane, C.; Matsuo, Y.; Nagai, K.; et al. Runx2 Is Required for the Proliferation of Osteoblast Progenitors and Induces Proliferation by Regulating Fgfr2 and Fgfr3. Sci Rep 2018, 8, 13551. [CrossRef]
- Cawley, K.M.; Bustamante-Gomez, N.C.; Guha, A.G.; MacLeod, R.S.; Xiong, J.; Gubrij, I.; Liu, Y.; Mulkey, R.; Palmieri, M.; Thostenson, J.D.; et al. Local Production of Osteoprotegerin by Osteoblasts Suppresses Bone Resorption. Cell Rep 2020, 32, 108052. [CrossRef]
- Westendorf, J.J.; Kahler, R.A.; Schroeder, T.M. Wnt Signaling in Osteoblasts and Bone Diseases. Gene 2004, 341, 19–39. [CrossRef]
- De Santis, M.; Di Matteo, B.; Chisari, E.; Cincinelli, G.; Angele, P.; Lattermann, C.; Filardo, G.; Vitale, N.D.; Selmi, C.; Kon, E. The Role of Wnt Pathway in the Pathogenesis of OA and Its Potential Therapeutic Implications in the Field of Regenerative Medicine. Biomed Res Int 2018, 2018, 7402947. [CrossRef]
- Bonewald, L.F. The Amazing Osteocyte. J Bone Miner Res 2011, 26, 229–238. [CrossRef]
- Bellido, T. Osteocyte-Driven Bone Remodeling. Calcif Tissue Int 2014, 94, 25–34. [CrossRef]
- Lorenzo, J.; Horowitz, M.; Choi, Y. Osteoimmunology: Interactions of the Bone and Immune System. Endocr Rev 2008, 29, 403–440. [CrossRef]
- Guder, C.; Gravius, S.; Burger, C.; Wirtz, D.C.; Schildberg, F.A. Osteoimmunology: A Current Update of the Interplay Between Bone and the Immune System. Front Immunol 2020, 11, 58. [CrossRef]
- Rivollier, A.; Mazzorana, M.; Tebib, J.; Piperno, M.; Aitsiselmi, T.; Rabourdin-Combe, C.; Jurdic, P.; Servet-Delprat, C. Immature Dendritic Cell Transdifferentiation into Osteoclasts: A Novel Pathway Sustained by the Rheumatoid Arthritis Microenvironment. Blood 2004, 104, 4029–4037. [CrossRef]
- Speziani, C.; Rivollier, A.; Gallois, A.; Coury, F.; Mazzorana, M.; Azocar, O.; Flacher, M.; Bella, C.; Tebib, J.; Jurdic, P.; et al. Murine Dendritic Cell Transdifferentiation into Osteoclasts Is Differentially Regulated by Innate and Adaptive Cytokines. Eur J Immunol 2007, 37, 747–757. [CrossRef]
- Maitra, R.; Follenzi, A.; Yaghoobian, A.; Montagna, C.; Merlin, S.; Cannizzo, E.S.; Hardin, J.A.; Cobelli, N.; Stanley, E.R.; Santambrogio, L. Dendritic Cell-Mediated in Vivo Bone Resorption. J Immunol 2010, 185, 1485–1491. [CrossRef]
- Wu, L.; Luo, Z.; Liu, Y.; Jia, L.; Jiang, Y.; Du, J.; Guo, L.; Bai, Y.; Liu, Y. Aspirin Inhibits RANKL-Induced Osteoclast Differentiation in Dendritic Cells by Suppressing NF-ΚB and NFATc1 Activation. Stem Cell Res Ther 2019, 10, 375. [CrossRef]
- Sato, K.; Suematsu, A.; Okamoto, K.; Yamaguchi, A.; Morishita, Y.; Kadono, Y.; Tanaka, S.; Kodama, T.; Akira, S.; Iwakura, Y.; et al. Th17 Functions as an Osteoclastogenic Helper T Cell Subset That Links T Cell Activation and Bone Destruction. J Exp Med 2006, 203, 2673–2682. [CrossRef]
- Zaiss, M.M.; Frey, B.; Hess, A.; Zwerina, J.; Luther, J.; Nimmerjahn, F.; Engelke, K.; Kollias, G.; Hünig, T.; Schett, G.; et al. Regulatory T Cells Protect from Local and Systemic Bone Destruction in Arthritis. J Immunol 2010, 184, 7238–7246. [CrossRef]
- Flores-Borja, F.; Jury, E.C.; Mauri, C.; Ehrenstein, M.R. Defects in CTLA-4 Are Associated with Abnormal Regulatory T Cell Function in Rheumatoid Arthritis. Proc Natl Acad Sci U S A 2008, 105, 19396–19401. [CrossRef]
- Moon, S.-J.; Ahn, I.E.; Jung, H.; Yi, H.; Kim, J.; Kim, Y.; Kwok, S.-K.; Park, K.-S.; Min, J.-K.; Park, S.-H.; et al. Temporal Differential Effects of Proinflammatory Cytokines on Osteoclastogenesis. Int J Mol Med 2013, 31, 769–777. [CrossRef]
- Wei, S.; Kitaura, H.; Zhou, P.; Ross, F.P.; Teitelbaum, S.L. IL-1 Mediates TNF-Induced Osteoclastogenesis. J Clin Invest 2005, 115, 282–290. [CrossRef]
- Marahleh, A.; Kitaura, H.; Ohori, F.; Kishikawa, A.; Ogawa, S.; Shen, W.-R.; Qi, J.; Noguchi, T.; Nara, Y.; Mizoguchi, I. TNF-α Directly Enhances Osteocyte RANKL Expression and Promotes Osteoclast Formation. Frontiers in Immunology 2019, 10.
- Diarra, D.; Stolina, M.; Polzer, K.; Zwerina, J.; Ominsky, M.S.; Dwyer, D.; Korb, A.; Smolen, J.; Hoffmann, M.; Scheinecker, C.; et al. Dickkopf-1 Is a Master Regulator of Joint Remodeling. Nature Medicine 2007 13:2 2007, 13, 156–163. [CrossRef]
- Zhang, L.; Ouyang, H.; Xie, Z.; Liang, Z.-H.; Wu, X.-W. Serum DKK-1 Level in the Development of Ankylosing Spondylitis and Rheumatic Arthritis: A Meta-Analysis. Exp Mol Med 2016, 48, e228. [CrossRef]
- Ma, Y.; Zhang, X.; Wang, M.; Xia, Q.; Yang, J.; Wu, M.; Han, R.; Chen, M.; Hu, X.; Yuan, Y.; et al. The Serum Level of Dickkopf-1 in Patients with Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Int Immunopharmacol 2018, 59, 227–232. [CrossRef]
- Courbon, G.; Lamarque, R.; Gerbaix, M.; Caire, R.; Linossier, M.-T.; Laroche, N.; Thomas, M.; Thomas, T.; Vico, L.; Marotte, H. Early Sclerostin Expression Explains Bone Formation Inhibition before Arthritis Onset in the Rat Adjuvant-Induced Arthritis Model. Sci Rep 2018, 8, 3492. [CrossRef]
- Mao, Y.-M.; Liao, T.; Ye, Q.-L.; Wu, G.-C.; Zhang, Q.; Tao, S.-S.; Zhao, C.-N.; Wu, Q.; Dan, Y.-L.; Pan, H.-F.; et al. Increased Circulating Sclerostin Levels in Rheumatoid Arthritis Patients: An Updated Meta-Analysis. Z Rheumatol 2023, 82, 51–58. [CrossRef]
- Wehmeyer, C.; Frank, S.; Beckmann, D.; Böttcher, M.; Cromme, C.; König, U.; Fennen, M.; Held, A.; Paruzel, P.; Hartmann, C.; et al. Sclerostin Inhibition Promotes TNF-Dependent Inflammatory Joint Destruction. Science Translational Medicine 2016, 8, 330ra35-330ra35. [CrossRef]
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A.; et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N Engl J Med 2016, 375, 1532–1543. [CrossRef]
- Singh, S.; Dutta, S.; Khasbage, S.; Kumar, T.; Sachin, J.; Sharma, J.; Varthya, S.B. A Systematic Review and Meta-Analysis of Efficacy and Safety of Romosozumab in Postmenopausal Osteoporosis. Osteoporos Int 2022, 33, 1–12. [CrossRef]
- Kobayakawa, T.; Miyazaki, A.; Kanayama, Y.; Hirano, Y.; Takahashi, J.; Suzuki, T.; Nakamura, Y. Comparable Efficacy of Denosumab and Romosozumab in Patients with Rheumatoid Arthritis Receiving Glucocorticoid Administration. Mod Rheumatol 2023, 33, 96–103. [CrossRef]
- Sims, N.A. Influences of the IL-6 Cytokine Family on Bone Structure and Function. Cytokine 2021, 146, 155655. [CrossRef]
- Shi, X.; Jiang, J.; Hong, R.; Xu, F.; Dai, S. Circulating IGFBP-3 and Interleukin 6 as Predictors of Osteoporosis in Postmenopausal Women: A Cross-Sectional Study. Mediators Inflamm 2023, 2023, 2613766. [CrossRef]
- Franchimont, N.; Wertz, S.; Malaise, M. Interleukin-6: An Osteotropic Factor Influencing Bone Formation? Bone 2005, 37, 601–606. [CrossRef]
- Li, Y.; Bäckesjö, C.-M.; Haldosén, L.-A.; Lindgren, U. IL-6 Receptor Expression and IL-6 Effects Change during Osteoblast Differentiation. Cytokine 2008, 43, 165–173. [CrossRef]
- Herman, S.; Müller, R.B.; Krönke, G.; Zwerina, J.; Redlich, K.; Hueber, A.J.; Gelse, H.; Neumann, E.; Müller-Ladner, U.; Schett, G. Induction of Osteoclast-Associated Receptor, a Key Osteoclast Costimulation Molecule, in Rheumatoid Arthritis. Arthritis Rheum 2008, 58, 3041–3050. [CrossRef]
- Crotti, T.N.; Dharmapatni, A.A.; Alias, E.; Zannettino, A.C.; Smith, M.D.; Haynes, D.R. The Immunoreceptor Tyrosine-Based Activation Motif (ITAM) -Related Factors Are Increased in Synovial Tissue and Vasculature of Rheumatoid Arthritic Joints. Arthritis Research & Therapy 2012, 14, R245. [CrossRef]
- Hecht, C.; Schett, G.; Finzel, S. The Impact of Rheumatoid Factor and ACPA on Bone Erosion in Rheumatoid Arthritis. Ann Rheum Dis 2015, 74, e4. [CrossRef]
- Harre, U.; Georgess, D.; Bang, H.; Bozec, A.; Axmann, R.; Ossipova, E.; Jakobsson, P.-J.; Baum, W.; Nimmerjahn, F.; Szarka, E.; et al. Induction of Osteoclastogenesis and Bone Loss by Human Autoantibodies against Citrullinated Vimentin. J Clin Invest 2012, 122, 1791–1802. [CrossRef]
- Kleyer, A.; Finzel, S.; Rech, J.; Manger, B.; Krieter, M.; Faustini, F.; Araujo, E.; Hueber, A.J.; Harre, U.; Engelke, K.; et al. Bone Loss before the Clinical Onset of Rheumatoid Arthritis in Subjects with Anticitrullinated Protein Antibodies. Annals of the Rheumatic Diseases 2014, 73, 854–860. [CrossRef]
- Seeling, M.; Hillenhoff, U.; David, J.P.; Schett, G.; Tuckermann, J.; Lux, A.; Nimmerjahn, F. Inflammatory Monocytes and Fcγ Receptor IV on Osteoclasts Are Critical for Bone Destruction during Inflammatory Arthritis in Mice. Proc Natl Acad Sci U S A 2013, 110, 10729–10734. [CrossRef]
- Mathsson, L.; Lampa, J.; Mullazehi, M.; Rönnelid, J. Immune Complexes from Rheumatoid Arthritis Synovial Fluid Induce FcgammaRIIa Dependent and Rheumatoid Factor Correlated Production of Tumour Necrosis Factor-Alpha by Peripheral Blood Mononuclear Cells. Arthritis Res Ther 2006, 8, R64. [CrossRef]
- Bugatti, S.; Bogliolo, L.; Vitolo, B.; Manzo, A.; Montecucco, C.; Caporali, R. Anti-Citrullinated Protein Antibodies and High Levels of Rheumatoid Factor Are Associated with Systemic Bone Loss in Patients with Early Untreated Rheumatoid Arthritis. Arthritis Res Ther 2016, 18, 226. [CrossRef]
- Amkreutz, J.A.M.P.; de Moel, E.C.; Theander, L.; Willim, M.; Heimans, L.; Nilsson, J.-Å.; Karlsson, M.K.; Huizinga, T.W.J.; Åkesson, K.E.; Jacobsson, L.T.H.; et al. Association Between Bone Mineral Density and Autoantibodies in Patients With Rheumatoid Arthritis. Arthritis Rheumatol 2021, 73, 921–930. [CrossRef]
- Shi, J.; Knevel, R.; Suwannalai, P.; van der Linden, M.P.; Janssen, G.M.C.; van Veelen, P.A.; Levarht, N.E.W.; van der Helm-van Mil, A.H.M.; Cerami, A.; Huizinga, T.W.J.; et al. Autoantibodies Recognizing Carbamylated Proteins Are Present in Sera of Patients with Rheumatoid Arthritis and Predict Joint Damage. Proceedings of the National Academy of Sciences 2011, 108, 17372–17377. [CrossRef]
- O’Neil, L.J.; Oliveira, C.B.; Sandoval-Heglund, D.; Barrera-Vargas, A.; Merayo-Chalico, J.; Aguirre-Aguilar, E.; Kaplan, M.J.; Carmona-Rivera, C. Anti-Carbamylated LL37 Antibodies Promote Pathogenic Bone Resorption in Rheumatoid Arthritis. Front Immunol 2021, 12, 715997. [CrossRef]
- Machold, K.P.; Stamm, T.A.; Nell, V.P.K.; Pflugbeil, S.; Aletaha, D.; Steiner, G.; Uffmann, M.; Smolen, J.S. Very Recent Onset Rheumatoid Arthritis: Clinical and Serological Patient Characteristics Associated with Radiographic Progression over the First Years of Disease. Rheumatology 2007, 46, 342–349. [CrossRef]
- Svensson, B.; Andersson, M.L.E.; Gjertsson, I.; Hafström, I.; Ajeganova, S.; Forslind, K. Erosion-Free Rheumatoid Arthritis: Clinical and Conceptional Implications—a BARFOT Study. BMC Rheumatology 2022, 6, 88. [CrossRef]
- Schett, G.; Gravallese, E. Bone Erosion in Rheumatoid Arthritis: Mechanisms, Diagnosis and Treatment. Nat Rev Rheumatol 2012, 8, 656–664. [CrossRef]
- Bromley, M.; Woolley, D.E. Histopathology of the Rheumatoid Lesion. Arthritis & Rheumatism 1984, 27, 857–863. [CrossRef]
- Gravallese, E.M.; Harada, Y.; Wang, J.T.; Gorn, A.H.; Thornhill, T.S.; Goldring, S.R. Identification of Cell Types Responsible for Bone Resorption in Rheumatoid Arthritis and Juvenile Rheumatoid Arthritis. Am J Pathol 1998, 152, 943–951.
- Kong, Y.-Y.; Feige, U.; Sarosi, I.; Bolon, B.; Tafuri, A.; Morony, S.; Capparelli, C.; Li, J.; Elliott, R.; McCabe, S.; et al. Activated T Cells Regulate Bone Loss and Joint Destruction in Adjuvant Arthritis through Osteoprotegerin Ligand. Nature 1999, 402, 43–47. [CrossRef]
- Berardi, S.; Corrado, A.; Maruotti, N.; Cici, D.; Cantatore, F.P. Osteoblast Role in the Pathogenesis of Rheumatoid Arthritis. Mol Biol Rep 2021, 48, 2843–2852. [CrossRef]
- Harre, U.; Schett, G. Cellular and Molecular Pathways of Structural Damage in Rheumatoid Arthritis. Semin Immunopathol 2017, 39, 355–363. [CrossRef]
- Bugatti, S.; Caporali, R.; Manzo, A.; Vitolo, B.; Pitzalis, C.; Montecucco, C. Involvement of Subchondral Bone Marrow in Rheumatoid Arthritis: Lymphoid Neogenesis and in Situ Relationship to Subchondral Bone Marrow Osteoclast Recruitment. Arthritis Rheum 2005, 52, 3448–3459. [CrossRef]
- Allard-Chamard, H.; Carrier, N.; Dufort, P.; Durand, M.; de Brum-Fernandes, A.J.; Boire, G.; Komarova, S.V.; Dixon, S.J.; Harrison, R.E.; Manolson, M.F.; et al. Osteoclasts and Their Circulating Precursors in Rheumatoid Arthritis: Relationships with Disease Activity and Bone Erosions. Bone Reports 2020, 12, 100282. [CrossRef]
- Crotti, T.N.; Flannery, M.; Walsh, N.C.; Fleming, J.D.; Goldring, S.R.; McHugh, K.P. NFATc1 Regulation of the Human Β3 Integrin Promoter in Osteoclast Differentiation. Gene 2006, 372, 92–102. [CrossRef]
- Nedeva, I.R.; Vitale, M.; Elson, A.; Hoyland, J.A.; Bella, J. Role of OSCAR Signaling in Osteoclastogenesis and Bone Disease. Frontiers in Cell and Developmental Biology 2021, 9.
- Chen, D.-Y.; Yao, L.; Chen, Y.-M.; Lin, C.-C.; Huang, K.-C.; Chen, S.-T.; Lan, J.-L.; Hsieh, S.-L.E. A Potential Role of Myeloid DAP12-Associating Lectin (MDL)-1 in the Regulation of Inflammation in Rheumatoid Arthritis Patients. PLOS ONE 2014, 9, e86105. [CrossRef]
- Jung, Y.-K.; Kang, Y.-M.; Han, S. Osteoclasts in the Inflammatory Arthritis: Implications for Pathologic Osteolysis. Immune Netw 2019, 19, e2. [CrossRef]
- Krishnamurthy, A.; Joshua, V.; Hensvold, A.H.; Jin, T.; Sun, M.; Vivar, N.; Ytterberg, A.J.; Engström, M.; Fernandes-Cerqueira, C.; Amara, K.; et al. Identification of a Novel Chemokine-Dependent Molecular Mechanism Underlying Rheumatoid Arthritis-Associated Autoantibody-Mediated Bone Loss. Annals of the Rheumatic Diseases 2016, 75, 721–729. [CrossRef]
- Negishi-Koga, T.; Gober, H.-J.; Sumiya, E.; Komatsu, N.; Okamoto, K.; Sawa, S.; Suematsu, A.; Suda, T.; Sato, K.; Takai, T.; et al. Immune Complexes Regulate Bone Metabolism through FcRγ Signalling. Nat Commun 2015, 6, 6637. [CrossRef]
- Edwards, J.C.; Cambridge, G. Rheumatoid Arthritis: The Predictable Effect of Small Immune Complexes in Which Antibody Is Also Antigen. Br J Rheumatol 1998, 37, 126–130. [CrossRef]
- Song, Y.W.; Kang, E.H. Autoantibodies in Rheumatoid Arthritis: Rheumatoid Factors and Anticitrullinated Protein Antibodies. QJM: An International Journal of Medicine 2010, 103, 139–146. [CrossRef]
- Hecht, C.; Englbrecht, M.; Rech, J.; Schmidt, S.; Araujo, E.; Engelke, K.; Finzel, S.; Schett, G. Additive Effect of Anti-Citrullinated Protein Antibodies and Rheumatoid Factor on Bone Erosions in Patients with RA. Annals of the Rheumatic Diseases 2015, 74, 2151–2156. [CrossRef]
- Buckley, C.D.; Ospelt, C.; Gay, S.; Midwood, K.S. Location, Location, Location: How the Tissue Microenvironment Affects Inflammation in RA. Nat Rev Rheumatol 2021, 17, 195–212. [CrossRef]
- Lu, M.-C.; Lai, N.-S.; Yu, H.-C.; Huang, H.-B.; Hsieh, S.-C.; Yu, C.-L. Anti-Citrullinated Protein Antibodies Bind Surface-Expressed Citrullinated Grp78 on Monocyte/Macrophages and Stimulate Tumor Necrosis Factor Alpha Production. Arthritis Rheum 2010, 62, 1213–1223. [CrossRef]
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid Arthritis. Nat Rev Dis Primers 2018, 4, 1–23. [CrossRef]
- Weitzmann, M.N.; Ofotokun, I. Physiological and Pathophysiological Bone Turnover — Role of the Immune System. Nat Rev Endocrinol 2016, 12, 518–532. [CrossRef]
- Luo, G.; Li, F.; Li, X.; Wang, Z.-G.; Zhang, B. TNF-α and RANKL Promote Osteoclastogenesis by Upregulating RANK via the NF-κB Pathway. Molecular Medicine Reports 2018, 17, 6605–6611. [CrossRef]
- Yao, Z.; Li, P.; Zhang, Q.; Schwarz, E.M.; Keng, P.; Arbini, A.; Boyce, B.F.; Xing, L. Tumor Necrosis Factor-α Increases Circulating Osteoclast Precursor Numbers by Promoting Their Proliferation and Differentiation in the Bone Marrow through Up-Regulation of c-Fms Expression *. Journal of Biological Chemistry 2006, 281, 11846–11855. [CrossRef]
- Gilbert, L.; He, X.; Farmer, P.; Rubin, J.; Drissi, H.; Wijnen, A.J. van; Lian, J.B.; Stein, G.S.; Nanes, M.S. Expression of the Osteoblast Differentiation Factor RUNX2 (Cbfa1/AML3/Pebp2αA) Is Inhibited by Tumor Necrosis Factor-α *. Journal of Biological Chemistry 2002, 277, 2695–2701. [CrossRef]
- Yeremenko, N.; Zwerina, K.; Rigter, G.; Pots, D.; Fonseca, J.E.; Zwerina, J.; Schett, G.; Baeten, D. Tumor Necrosis Factor and Interleukin-6 Differentially Regulate Dkk-1 in the Inflamed Arthritic Joint. Arthritis Rheumatol 2015, 67, 2071–2075. [CrossRef]
- Srirangan, S.; Choy, E.H. The Role of Interleukin 6 in the Pathophysiology of Rheumatoid Arthritis. Therapeutic Advances in Musculoskeletal 2010, 2, 247–256. [CrossRef]
- Chang, P.-Y.; Wu, H.-K.; Chen, Y.-H.; Hsu, Y.-P.; Cheng, M.-T.; Yu, C.-H.; Chen, S.-K. Interleukin-6 Transiently Promotes Proliferation of Osteoclast Precursors and Stimulates the Production of Inflammatory Mediators. Mol Biol Rep 2022, 49, 3927–3937. [CrossRef]
- Yoshitake, F.; Itoh, S.; Narita, H.; Ishihara, K.; Ebisu, S. Interleukin-6 Directly Inhibits Osteoclast Differentiation by Suppressing Receptor Activator of NF-ΚB Signaling Pathways *. Journal of Biological Chemistry 2008, 283, 11535–11540. [CrossRef]
- Takagawa, S.; Nakamura, F.; Kumagai, K.; Nagashima, Y.; Goshima, Y.; Saito, T. Decreased Semaphorin3A Expression Correlates with Disease Activity and Histological Features of Rheumatoid Arthritis. BMC Musculoskeletal Disorders 2013, 14, 40. [CrossRef]
- Akatsu, T.; Takahashi, N.; Udagawa, N.; Imamura, K.; Yamaguchi, A.; Sato, K.; Nagata, N.; Suda, T. Role of Prostaglandins in Interleukin-1-Induced Bone Resorption in Mice in Vitro. Journal of Bone and Mineral Research 1991, 6, 183–190. [CrossRef]
- Kim, J.H.; Jin, H.M.; Kim, K.; Song, I.; Youn, B.U.; Matsuo, K.; Kim, N. The Mechanism of Osteoclast Differentiation Induced by IL-11. The Journal of Immunology 2009, 183, 1862–1870. [CrossRef]
- Tanabe, N.; Maeno, M.; Suzuki, N.; Fujisaki, K.; Tanaka, H.; Ogiso, B.; Ito, K. IL-1α Stimulates the Formation of Osteoclast-like Cells by Increasing M-CSF and PGE2 Production and Decreasing OPG Production by Osteoblasts. Life Sciences 2005, 77, 615–626. [CrossRef]
- Jiang, Y.; Genant, H.K.; Watt, I.; Cobby, M.; Bresnihan, B.; Aitchison, R.; McCabe, D. A Multicenter, Double-Blind, Dose-Ranging, Randomized, Placebo-Controlled Study of Recombinant Human Interleukin-1 Receptor Antagonist in Patients with Rheumatoid Arthritis: Radiologic Progression and Correlation of Genant and Larsen Scores. Arthritis & Rheumatism 2000, 43, 1001–1009. [CrossRef]
- Gremese, E.; Tolusso, B.; Bruno, D.; Perniola, S.; Ferraccioli, G.; Alivernini, S. The Forgotten Key Players in Rheumatoid Arthritis: IL-8 and IL-17 – Unmet Needs and Therapeutic Perspectives. Frontiers in Medicine 2023, 10.
- Adamopoulos, I.E.; Suzuki, E.; Chao, C.-C.; Gorman, D.; Adda, S.; Maverakis, E.; Zarbalis, K.; Geissler, R.; Asio, A.; Blumenschein, W.M.; et al. IL-17A Gene Transfer Induces Bone Loss and Epidermal Hyperplasia Associated with Psoriatic Arthritis. Annals of the Rheumatic Diseases 2015, 74, 1284–1292. [CrossRef]
- Yago, T.; Nanke, Y.; Ichikawa, N.; Kobashigawa, T.; Mogi, M.; Kamatani, N.; Kotake, S. IL-17 Induces Osteoclastogenesis from Human Monocytes Alone in the Absence of Osteoblasts, Which Is Potently Inhibited by Anti-TNF-α Antibody: A Novel Mechanism of Osteoclastogenesis by IL-17. Journal of Cellular Biochemistry 2009, 108, 947–955. [CrossRef]
- Mills, K.H.G. IL-17 and IL-17-Producing Cells in Protection versus Pathology. Nat Rev Immunol 2023, 23, 38–54. [CrossRef]
- Le Goff, B.; Bouvard, B.; Lequerre, T.; Lespessailles, E.; Marotte, H.; Pers, Y.-M.; Cortet, B. Implication of IL-17 in Bone Loss and Structural Damage in Inflammatory Rheumatic Diseases. Mediators Inflamm 2019, 2019, 8659302. [CrossRef]
- Chemin, K.; Gerstner, C.; Malmström, V. Effector Functions of CD4+ T Cells at the Site of Local Autoimmune Inflammation—Lessons From Rheumatoid Arthritis. Frontiers in Immunology 2019, 10.
- Pfeifle, R.; Rothe, T.; Ipseiz, N.; Scherer, H.U.; Culemann, S.; Harre, U.; Ackermann, J.A.; Seefried, M.; Kleyer, A.; Uderhardt, S.; et al. Regulation of Autoantibody Activity by the IL-23–TH17 Axis Determines the Onset of Autoimmune Disease. Nat Immunol 2017, 18, 104–113. [CrossRef]
- Ciucci, T.; Ibáñez, L.; Boucoiran, A.; Birgy-Barelli, E.; Pène, J.; Abou-Ezzi, G.; Arab, N.; Rouleau, M.; Hébuterne, X.; Yssel, H.; et al. Bone Marrow Th17 TNFα Cells Induce Osteoclast Differentiation, and Link Bone Destruction to IBD. Gut 2015, 64, 1072–1081. [CrossRef]
- Vignali, D.A.A.; Collison, L.W.; Workman, C.J. How Regulatory T Cells Work. Nat Rev Immunol 2008, 8, 523–532. [CrossRef]
- Zaiss, M.M.; Axmann, R.; Zwerina, J.; Polzer, K.; Gückel, E.; Skapenko, A.; Schulze-Koops, H.; Horwood, N.; Cope, A.; Schett, G. Treg Cells Suppress Osteoclast Formation: A New Link between the Immune System and Bone. Arthritis Rheum 2007, 56, 4104–4112. [CrossRef]
- Fischer, L.; Herkner, C.; Kitte, R.; Dohnke, S.; Riewaldt, J.; Kretschmer, K.; Garbe, A.I. Foxp3+ Regulatory T Cells in Bone and Hematopoietic Homeostasis. Front Endocrinol (Lausanne) 2019, 10, 578. [CrossRef]
- Zhu, L.; Hua, F.; Ding, W.; Ding, K.; Zhang, Y.; Xu, C. The Correlation between the Th17/Treg Cell Balance and Bone Health. Immun Ageing 2020, 17, 30. [CrossRef]
- Komatsu, N.; Takayanagi, H. Mechanisms of Joint Destruction in Rheumatoid Arthritis — Immune Cell–Fibroblast–Bone Interactions. Nat Rev Rheumatol 2022, 18, 415–429. [CrossRef]
- Tsukasaki, M.; Takayanagi, H. Osteoimmunology: Evolving Concepts in Bone–Immune Interactions in Health and Disease. Nat Rev Immunol 2019, 19, 626–642. [CrossRef]
- Orr, C.; Vieira-Sousa, E.; Boyle, D.L.; Buch, M.H.; Buckley, C.D.; Cañete, J.D.; Catrina, A.I.; Choy, E.H.S.; Emery, P.; Fearon, U.; et al. Synovial Tissue Research: A State-of-the-Art Review. Nat Rev Rheumatol 2017, 13, 463–475. [CrossRef]
- Nygaard, G.; Firestein, G.S. Restoring Synovial Homeostasis in Rheumatoid Arthritis by Targeting Fibroblast-like Synoviocytes. Nat Rev Rheumatol 2020, 16, 316–333. [CrossRef]
- Brentano, F.; Schorr, O.; Ospelt, C.; Stanczyk, J.; Gay, R.E.; Gay, S.; Kyburz, D. Pre-B Cell Colony-Enhancing Factor/Visfatin, a New Marker of Inflammation in Rheumatoid Arthritis with Proinflammatory and Matrix-Degrading Activities. Arthritis Rheum 2007, 56, 2829–2839. [CrossRef]
- Tu, J.; Huang, W.; Zhang, W.; Mei, J.; Zhu, C. Two Main Cellular Components in Rheumatoid Arthritis: Communication Between T Cells and Fibroblast-Like Synoviocytes in the Joint Synovium. Frontiers in Immunology 2022, 13.
- Ouboussad, L.; Burska, A.N.; Melville, A.; Buch, M.H. Synovial Tissue Heterogeneity in Rheumatoid Arthritis and Changes With Biologic and Targeted Synthetic Therapies to Inform Stratified Therapy. Frontiers in Medicine 2019, 6.
- Buckley, C.; Filer, A. Fibroblasts and Fibroblastlike Synoviocytes. In Firestein and Kelley’s Textbook of Rheumatology; Elsevier, Inc.: Philadelphia, 2021; pp. 222–240.
- Wu, Z.; Ma, D.; Yang, H.; Gao, J.; Zhang, G.; Xu, K.; Zhang, L. Fibroblast-like Synoviocytes in Rheumatoid Arthritis: Surface Markers and Phenotypes. International Immunopharmacology 2021, 93, 107392. [CrossRef]
- Matsuda, K.; Shiba, N.; Hiraoka, K. New Insights into the Role of Synovial Fibroblasts Leading to Joint Destruction in Rheumatoid Arthritis. International Journal of Molecular Sciences 2023, 24, 5173. [CrossRef]
- Firestein, G.S. Evolving Concepts of Rheumatoid Arthritis. Nature 2003, 423, 356–361. [CrossRef]
- Grillet, B.; Pereira, R.V.S.; Van Damme, J.; Abu El-Asrar, A.; Proost, P.; Opdenakker, G. Matrix Metalloproteinases in Arthritis: Towards Precision Medicine. Nat Rev Rheumatol 2023, 19, 363–377. [CrossRef]
- Noss, E.H.; Chang, S.K.; Watts, G.F.M.; Brenner, M.B. Cadherin-11 Engagement Modulates Matrix Metalloproteinase Production by Rheumatoid Arthritis Synovial Fibroblasts. Arthritis Rheum 2011, 63, 3768–3778. [CrossRef]
- Danks, L.; Komatsu, N.; Guerrini, M.M.; Sawa, S.; Armaka, M.; Kollias, G.; Nakashima, T.; Takayanagi, H. RANKL Expressed on Synovial Fibroblasts Is Primarily Responsible for Bone Erosions during Joint Inflammation. Annals of the Rheumatic Diseases 2016, 75, 1187–1195. [CrossRef]
- Komatsu, N.; Win, S.; Yan, M.; Huynh, N.C.-N.; Sawa, S.; Tsukasaki, M.; Terashima, A.; Pluemsakunthai, W.; Kollias, G.; Nakashima, T.; et al. Plasma Cells Promote Osteoclastogenesis and Periarticular Bone Loss in Autoimmune Arthritis. J Clin Invest 2021, 131, e143060, 143060. [CrossRef]
- Juarez, M.; Toellner, D.S.; Karouzakis, E.; Hardy, R.; Yeo, L.; Bayley, R.; De Paz, B.; Raza, K.; Cooper, M.; Gay, S.; et al. Early Rheumatoid Arthritis and Resolving Fibroblasts Segregate According to Dickkopf Related Protein 1 Expression. The Lancet 2013, 381, S57. [CrossRef]
- Zheng, L.; Hu, F.; Bian, W.; Li, Y.; Zhang, L.; Shi, L.; Ma, X.; Liu, Y.; Zhang, X.; Li, Z. Dickkopf-1 Perpetuated Synovial Fibroblast Activation and Synovial Angiogenesis in Rheumatoid Arthritis. Clin Rheumatol 2021, 40, 4279–4288. [CrossRef]
- Barranco, C. Sclerostin: A Novel Role in TNF Arthritis? Nat Rev Rheumatol 2016, 12, 251–251. [CrossRef]
- Komatsu, N.; Takayanagi, H. Inflammation and Bone Destruction in Arthritis: Synergistic Activity of Immune and Mesenchymal Cells in Joints. Frontiers in Immunology 2012, 3.
- Chang, S.K.; Noss, E.H.; Chen, M.; Gu, Z.; Townsend, K.; Grenha, R.; Leon, L.; Lee, S.Y.; Lee, D.M.; Brenner, M.B. Cadherin-11 Regulates Fibroblast Inflammation. Proceedings of the National Academy of Sciences 2011, 108, 8402–8407. [CrossRef]
- Yellin, M.J.; Winikoff, S.; Fortune, S.M.; Baum, D.; Crow, M.K.; Lederman, S.; Chess, L. Ligation of CD40 on Fibroblasts Induces CD54 (ICAM-1) and CD106 (VCAM-1) up-Regulation and IL-6 Production and Proliferation. J Leukoc Biol 1995, 58, 209–216. [CrossRef]
- Ogura, H.; Murakami, M.; Okuyama, Y.; Tsuruoka, M.; Kitabayashi, C.; Kanamoto, M.; Nishihara, M.; Iwakura, Y.; Hirano, T. Interleukin-17 Promotes Autoimmunity by Triggering a Positive-Feedback Loop via Interleukin-6 Induction. Immunity 2008, 29, 628–636. [CrossRef]
- Van Seventer, G.A.; Shimizu, Y.; Horgan, K.J.; Shaw, S. The LFA-1 Ligand ICAM-1 Provides an Important Costimulatory Signal for T Cell Receptor-Mediated Activation of Resting T Cells. J Immunol 1990, 144, 4579–4586.
- Sato, H.; Muraoka, S.; Kusunoki, N.; Masuoka, S.; Yamada, S.; Ogasawara, H.; Imai, T.; Akasaka, Y.; Tochigi, N.; Takahashi, H.; et al. Resistin Upregulates Chemokine Production by Fibroblast-like Synoviocytes from Patients with Rheumatoid Arthritis. Arthritis Research & Therapy 2017, 19, 263. [CrossRef]
- Cambier, S.; Gouwy, M.; Proost, P. The Chemokines CXCL8 and CXCL12: Molecular and Functional Properties, Role in Disease and Efforts towards Pharmacological Intervention. Cell Mol Immunol 2023, 20, 217–251. [CrossRef]
- Lee, J.-H.; Kim, B.; Jin, W.J.; Kim, H.-H.; Ha, H.; Lee, Z.H. Pathogenic Roles of CXCL10 Signaling through CXCR3 and TLR4 in Macrophages and T Cells: Relevance for Arthritis. Arthritis Research & Therapy 2017, 19, 163. [CrossRef]
- Hirota, K.; Hashimoto, M.; Yoshitomi, H.; Tanaka, S.; Nomura, T.; Yamaguchi, T.; Iwakura, Y.; Sakaguchi, N.; Sakaguchi, S. T Cell Self-Reactivity Forms a Cytokine Milieu for Spontaneous Development of IL-17+ Th Cells That Cause Autoimmune Arthritis. J Exp Med 2007, 204, 41–47. [CrossRef]
- Burger, J.A.; Zvaifler, N.J.; Tsukada, N.; Firestein, G.S.; Kipps, T.J. Fibroblast-like Synoviocytes Support B-Cell Pseudoemperipolesis via a Stromal Cell–Derived Factor-1– and CD106 (VCAM-1)–Dependent Mechanism. J Clin Invest 2001, 107, 305–315. [CrossRef]
- Bombardieri, M.; Kam, N.-W.; Brentano, F.; Choi, K.; Filer, A.; Kyburz, D.; McInnes, I.B.; Gay, S.; Buckley, C.; Pitzalis, C. A BAFF/APRIL-Dependent TLR3-Stimulated Pathway Enhances the Capacity of Rheumatoid Synovial Fibroblasts to Induce AID Expression and Ig Class-Switching in B Cells. Ann Rheum Dis 2011, 70, 1857–1865. [CrossRef]
- Hasegawa, T.; Kikuta, J.; Sudo, T.; Matsuura, Y.; Matsui, T.; Simmons, S.; Ebina, K.; Hirao, M.; Okuzaki, D.; Yoshida, Y.; et al. Identification of a Novel Arthritis-Associated Osteoclast Precursor Macrophage Regulated by FoxM1. Nat Immunol 2019, 20, 1631–1643. [CrossRef]
- O’Neil, L.J.; Oliveira, C.B.; Wang, X.; Navarrete, M.; Barrera-Vargas, A.; Merayo-Chalico, J.; Aljahdali, R.; Aguirre-Aguilar, E.; Carlucci, P.; Kaplan, M.J.; et al. Neutrophil Extracellular Trap-Associated Carbamylation and Histones Trigger Osteoclast Formation in Rheumatoid Arthritis. Annals of the Rheumatic Diseases 2023, 82, 630–638. [CrossRef]
- Wigerblad, G.; Kaplan, M.J. Neutrophil Extracellular Traps in Systemic Autoimmune and Autoinflammatory Diseases. Nat Rev Immunol 2023, 23, 274–288. [CrossRef]
- Carmona-Rivera, C.; Carlucci, P.M.; Goel, R.R.; James, E.; Brooks, S.R.; Rims, C.; Hoffmann, V.; Fox, D.A.; Buckner, J.H.; Kaplan, M.J. Neutrophil Extracellular Traps Mediate Articular Cartilage Damage and Enhance Cartilage Component Immunogenicity in Rheumatoid Arthritis. JCI Insight 2020, 5, e139388, 139388. [CrossRef]
- Kim, T.S.; Silva, L.M.; Theofilou, V.I.; Greenwell-Wild, T.; Li, L.; Williams, D.W.; Ikeuchi, T.; Brenchley, L.; NIDCD/NIDCR Genomics and Computational Biology Core; Bugge, T.H.; et al. Neutrophil Extracellular Traps and Extracellular Histones Potentiate IL-17 Inflammation in Periodontitis. Journal of Experimental Medicine 2023, 220, e20221751. [CrossRef]
- Glaser, D.L.; Kaplan, F.S. Osteoporosis. Definition and Clinical Presentation. Spine (Phila Pa 1976) 1997, 22, 12S-16S. [CrossRef]
- Tong, J.-J.; Xu, S.-Q.; Zong, H.-X.; Pan, M.-J.; Teng, Y.-Z.; Xu, J.-H. Prevalence and Risk Factors Associated with Vertebral Osteoporotic Fractures in Patients with Rheumatoid Arthritis. Clin Rheumatol 2020, 39, 357–364. [CrossRef]
- Jin, S.; Hsieh, E.; Peng, L.; Yu, C.; Wang, Y.; Wu, C.; Wang, Q.; Li, M.; Zeng, X. Incidence of Fractures among Patients with Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Osteoporos Int 2018, 29, 1263–1275. [CrossRef]
- Conforti, A.; Di Cola, I.; Pavlych, V.; Ruscitti, P.; Berardicurti, O.; Ursini, F.; Giacomelli, R.; Cipriani, P. Beyond the Joints, the Extra-Articular Manifestations in Rheumatoid Arthritis. Autoimmun Rev 2021, 20, 102735. [CrossRef]
- Hacquard-Bouder, C.; Ittah, M.; Breban, M. Animal Models of HLA-B27-Associated Diseases: New Outcomes. Joint Bone Spine 2006, 73, 132–138. [CrossRef]
- Bowness, P. HLA-B27. Annu. Rev. Immunol. 2015, 33, 29–48. [CrossRef]
- Gui, L.; Gu, J. The Study of the Effect of HLA-B27 on THP-1 Monocytic Cells Survival and Its Mechanism. Int J Rheum Dis 2023, 26, 1474–1484. [CrossRef]
- Khan, M.A.; Yong, S.-B.; Wei, J.C.-C. Ankylosing Spondylitis: History, Epidemiology, and HLA-B27. Int J Rheum Dis 2023, 26, 413–414. [CrossRef]
- Kavadichanda, C.G.; Geng, J.; Bulusu, S.N.; Negi, V.S.; Raghavan, M. Spondyloarthritis and the Human Leukocyte Antigen (HLA)-B*27 Connection. Front. Immunol. 2021, 12, 601518. [CrossRef]
- Layh-Schmitt, G.; Yang, E.Y.; Kwon, G.; Colbert, R.A. HLA-B27 Alters the Response to Tumor Necrosis Factor α and Promotes Osteoclastogenesis in Bone Marrow Monocytes From HLA-B27-Transgenic Rats: Cellular Effects of HLA-B27 Misfolding in Transgenic Rats. Arthritis & Rheumatism 2013, 65, 2123–2131. [CrossRef]
- Papet, I.; Yousfi, M.E.; Godin, J.-P.; Mermoud, A.-F.; Davicco, M.-J.; Coxam, V.; Breuillé, D.; Obled, C. HLA-B27 Rats Develop Osteopaenia through Increased Bone Resorption without Any Change in Bone Formation.
- Rauner, M.; Stupphann, D.; Haas, M.; Fert, I.; Glatigny, S.; Sipos, W.; Breban, M.; Pietschmann, P. The HLA-B27 Transgenic Rat, a Model of Spondyloarthritis, Has Decreased Bone Mineral Density and Increased RANKL to Osteoprotegerin MRNA Ratio. J Rheumatol 2009, 36, 120–126. [CrossRef]
- Gamsjaeger, S.; Srivastava, A.K.; Wergedal, J.E.; Zwerina, J.; Klaushofer, K.; Paschalis, E.P.; Tatakis, D.N. Altered Bone Material Properties in HLA-B27 Rats Include Reduced Mineral to Matrix Ratio and Altered Collagen Cross-Links: BONE QUALITY IN HLA-B27 RATS. J Bone Miner Res 2014, 29, 2382–2391. [CrossRef]
- Rauner, M.; Thiele, S.; Fert, I.; Araujo, L.M.; Layh-Schmitt, G.; Colbert, R.A.; Hofbauer, C.; Bernhardt, R.; Bürki, A.; Schwiedrzik, J.; et al. Loss of Bone Strength in HLA-B27 Transgenic Rats Is Characterized by a High Bone Turnover and Is Mainly Osteoclast-Driven. Bone 2015, 75, 183–191. [CrossRef]
- Hauser, B.; Zhao, S.; Visconti, M.R.; Riches, P.L.; Fraser, W.D.; Piec, I.; Goodson, N.J.; Ralston, S.H. Autoantibodies to Osteoprotegerin Are Associated with Low Hip Bone Mineral Density and History of Fractures in Axial Spondyloarthritis: A Cross-Sectional Observational Study. Calcif Tissue Int 2017, 101, 375–383. [CrossRef]
- Kim, H.-R.; Kim, H.-Y.; Lee, S.-H. Elevated Serum Levels of Soluble Receptor Activator of Nuclear Factors- B Ligand (SRANKL) and Reduced Bone Mineral Density in Patients with Ankylosing Spondylitis (AS). Rheumatology 2006, 45, 1197–1200. [CrossRef]
- Tan, Z.Y.; Bealgey, K.W.; Fang, Y.; Gong, Y.M.; Bao, S. Interleukin-23: Immunological Roles and Clinical Implications. The International Journal of Biochemistry & Cell Biology 2009, 41, 733–735. [CrossRef]
- Ciccia, F.; Rizzo, A.; Triolo, G. Subclinical Gut Inflammation in Ankylosing Spondylitis. Current Opinion in Rheumatology 2016, 28, 89–96. [CrossRef]
- Yago, T.; Nanke, Y.; Kawamoto, M.; Furuya, T.; Kobashigawa, T.; Kamatani, N.; Kotake, S. IL-23 Induces Human Osteoclastogenesis via IL-17 in Vitro, and Anti-IL-23 Antibody Attenuates Collagen-Induced Arthritis in Rats. Arthritis Res Ther 2007, 9, R96. [CrossRef]
- Li, X.; Kim, K.-W.; Cho, M.-L.; Ju, J.-H.; Kang, C.-M.; Oh, H.-J.; Min, J.-K.; Lee, S.-H.; Park, S.-H.; Kim, H.-Y. IL-23 Induces Receptor Activator of NF-ΚB Ligand Expression in Fibroblast-like Synoviocytes via STAT3 and NF-ΚB Signal Pathways. Immunology Letters 2010, 127, 100–107. [CrossRef]
- Chisălău, B.; Crînguș, L.-I.; Vreju, F.; Pârvănescu, C.; Firulescu, S.; Dinescu, Ștefan; Ciobanu, D.; Tica, A.; Sandu, R.; Siloși, I.; et al. New Insights into IL-17/IL-23 Signaling in Ankylosing Spondylitis (Review). Exp Ther Med 2020. [CrossRef]
- Chalise, J.; Narendra, S.; Paudyal, B.; Magnusson, M. Interferon Alpha Inhibits Antigen-Specific Production of Proinflammatory Cytokines and Enhances Antigen-Specific Transforming Growth Factor Beta Production in Antigen-Induced Arthritis. Arthritis Res Ther 2013, 15, R143. [CrossRef]
- Tang, M.; Lu, L.; Yu, X. Interleukin-17A Interweaves the Skeletal and Immune Systems. Front. Immunol. 2021, 11, 625034. [CrossRef]
- Van Bezooijen, R.L.; Farih-Sips, H.C.M.; Papapoulos, S.E.; Löwik, C.W.G.M. Interleukin-17: A New Bone Acting Cytokine In Vitro. J Bone Miner Res 1999, 14, 1513–1521. [CrossRef]
- Uluçkan, Ö.; Jimenez, M.; Karbach, S.; Jeschke, A.; Graña, O.; Keller, J.; Busse, B.; Croxford, A.L.; Finzel, S.; Koenders, M.; et al. Chronic Skin Inflammation Leads to Bone Loss by IL-17-Mediated Inhibition of Wnt Signaling in Osteoblasts. Sci Transl Med 2016, 8, 330ra37. [CrossRef]
- Raimondo, A.; Lembo, S.; Di Caprio, R.; Donnarumma, G.; Monfrecola, G.; Balato, N.; Ayala, F.; Balato, A. Psoriatic Cutaneous Inflammation Promotes Human Monocyte Differentiation into Active Osteoclasts, Facilitating Bone Damage. European Journal of Immunology 2017, 47, 1062–1074. [CrossRef]
- Wu, D.; Li, C.; Zhang, S.; Wong, P.; Cao, Y.; Griffith, J.F.; Zhang, X.; Gu, J.; Tam, L.-S. Effect of Biologics on Radiographic Progression of Peripheral Joint in Patients with Psoriatic Arthritis: Meta-Analysis. Rheumatology 2020, 59, 3172–3180. [CrossRef]
- Osta, B.; Lavocat, F.; Eljaafari, A.; Miossec, P. Effects of Interleukin-17A on Osteogenic Differentiation of Isolated Human Mesenchymal Stem Cells. Frontiers in Immunology 2014, 5. [CrossRef]
- Kobayashi, K.; Takahashi, N.; Jimi, E.; Udagawa, N.; Takami, M.; Kotake, S.; Nakagawa, N.; Kinosaki, M.; Yamaguchi, K.; Shima, N.; et al. Tumor Necrosis Factor Alpha Stimulates Osteoclast Differentiation by a Mechanism Independent of the ODF/RANKL-RANK Interaction. J Exp Med 2000, 191, 275–286. [CrossRef]
- Li, P.; Schwarz, E.M. The TNF-Alpha Transgenic Mouse Model of Inflammatory Arthritis. Springer Semin Immunopathol 2003, 25, 19–33. [CrossRef]
- Ritchlin, C.T.; Haas-Smith, S.A.; Li, P.; Hicks, D.G.; Schwarz, E.M. Mechanisms of TNF-α– and RANKL-Mediated Osteoclastogenesis and Bone Resorption in Psoriatic Arthritis. J Clin Invest 2003, 111, 821–831. [CrossRef]
- Lambrecht, S.; Coudenys, J.; De Keyser, F.; Verbruggen, G.; Deforce, D.; Elewaut, D. Reduced Levels of the TGFb Family Member GDF15 in Spondyloarthritis versus Other Rheumatic Diseases. Annals of the Rheumatic Diseases 2011, 70, A88–A88. [CrossRef]
- Hinoi, E.; Ochi, H.; Takarada, T.; Nakatani, E.; Iezaki, T.; Nakajima, H.; Fujita, H.; Takahata, Y.; Hidano, S.; Kobayashi, T.; et al. Positive Regulation of Osteoclastic Differentiation by Growth Differentiation Factor 15 Upregulated in Osteocytic Cells under Hypoxia. J Bone Miner Res 2012, 27, 938–949. [CrossRef]
- Song, Y.; Cui, Y.; Zhang, X.; Lin, H.; Zhang, G.; Zeng, H.; Zeng, Y. Increased Serum Levels of MIC1/GDF15 Correlated with Bone Erosion in Spondyloarthritis: A Pilot Study. Medicine (Baltimore) 2018, 97, e13733. [CrossRef]
- De Martinis, M.; Sirufo, M.M.; Suppa, M.; Ginaldi, L. IL-33/IL-31 Axis in Osteoporosis. IJMS 2020, 21, 1239. [CrossRef]
- Perrigoue, J.G.; Li, J.; Zaph, C.; Goldschmidt, M.; Scott, P.; De Sauvage, F.J.; Pearce, E.J.; Ghilardi, N.; Artis, D. IL-31–IL-31R Interactions Negatively Regulate Type 2 Inflammation in the Lung. The Journal of Experimental Medicine 2007, 204, 481–487. [CrossRef]
- Perrigoue, J.G.; Zaph, C.; Guild, K.; Du, Y.; Artis, D. IL-31-IL-31R Interactions Limit the Magnitude of Th2 Cytokine-Dependent Immunity and Inflammation Following Intestinal Helminth Infection. The Journal of Immunology 2009, 182, 6088–6094. [CrossRef]
- Rosine, N.; Etcheto, A.; Hendel-Chavez, H.; Seror, R.; Briot, K.; Molto, A.; Chanson, P.; Taoufik, Y.; Wendling, D.; Lories, R.; et al. Increase In Il-31 Serum Levels Is Associated With Reduced Structural Damage In Early Axial Spondyloarthritis. Sci Rep 2018, 8, 7731. [CrossRef]
- Lems, W.; Miceli-Richard, C.; Haschka, J.; Giusti, A.; Chistensen, G.L.; Kocijan, R.; Rosine, N.; Jørgensen, N.R.; Bianchi, G.; Roux, C. Bone Involvement in Patients with Spondyloarthropathies. Calcif Tissue Int 2022, 110, 393–420. [CrossRef]
- Rossini, M.; Viapiana, O.; Idolazzi, L.; Ghellere, F.; Fracassi, E.; Troplini, S.; Povino, M.R.; Kunnathully, V.; Adami, S.; Gatti, D. Higher Level of Dickkopf-1 Is Associated with Low Bone Mineral Density and Higher Prevalence of Vertebral Fractures in Patients with Ankylosing Spondylitis. Calcif Tissue Int 2016, 98, 438–445. [CrossRef]
- Szentpetery, A.; Heffernan, E.; Haroon, M.; Kilbane, M.; Gallagher, P.; McKenna, M.J.; FitzGerald, O. Striking Difference of Periarticular Bone Density Change in Early Psoriatic Arthritis and Rheumatoid Arthritis Following Anti-Rheumatic Treatment as Measured by Digital X-Ray Radiogrammetry. Rheumatology 2016, 55, 891–896. [CrossRef]
- Haynes, D.R. Osteoprotegerin Expression in Synovial Tissue from Patients with Rheumatoid Arthritis, Spondyloarthropathies and Osteoarthritis and Normal Controls. Rheumatology 2003, 42, 123–134. [CrossRef]
- Kavanaugh, A.; Puig, L.; Gottlieb, A.B.; Ritchlin, C.; Li, S.; Wang, Y.; Mendelsohn, A.M.; Song, M.; Zhu, Y.; Rahman, P.; et al. Maintenance of Clinical Efficacy and Radiographic Benefit Through Two Years of Ustekinumab Therapy in Patients With Active Psoriatic Arthritis: Results From a Randomized, Placebo-Controlled Phase III Trial. Arthritis Care & Research 2015, 67, 1739–1749. [CrossRef]
- Van Der Heijde, D.; Landewé, R.B.; Mease, P.J.; McInnes, I.B.; Conaghan, P.G.; Pricop, L.; Ligozio, G.; Richards, H.B.; Mpofu, S. Brief Report: Secukinumab Provides Significant and Sustained Inhibition of Joint Structural Damage in a Phase III Study of Active Psoriatic Arthritis: INHIBITION OF JOINT STRUCTURAL DAMAGE WITH SECUKINUMAB. Arthritis & Rheumatology 2016, 68, 1914–1921. [CrossRef]
- Koenders, M.I.; Marijnissen, R.J.; Devesa, I.; Lubberts, E.; Joosten, L.A.B.; Roth, J.; Van Lent, P.L.E.M.; Van De Loo, F.A.; Van Den Berg, W.B. Tumor Necrosis Factor-Interleukin-17 Interplay Induces S100A8, Interleukin-1β, and Matrix Metalloproteinases, and Drives Irreversible Cartilage Destruction in Murine Arthritis: Rationale for Combination Treatment during Arthritis. Arthritis & Rheumatism 2011, 63, 2329–2339. [CrossRef]
- Dalbeth, N.; Pool, B.; Smith, T.; Callon, K.E.; Lobo, M.; Taylor, W.J.; Jones, P.B.; Cornish, J.; McQueen, F.M. Circulating Mediators of Bone Remodeling in Psoriatic Arthritis: Implications for Disordered Osteoclastogenesis and Bone Erosion. Arthritis research & therapy 2010, 12. [CrossRef]
- Chung, Y.; Li, Z.-C.; Sun, X.-L.; Liu, Y.-Y.; Shao, M.; Gan, Y.-Z.; Li, Y.-M.; Li, Y.-H.; Zhang, X.-W. Elevated Serum Dickkopf-1 Is a Biomarker for Bone Erosion in Patients with Psoriatic Arthritis. Chinese Medical Journal 2021, 134, 2583–2588. [CrossRef]
- Fassio, A.; Idolazzi, L.; Viapiana, O.; Benini, C.; Vantaggiato, E.; Bertoldo, F.; Rossini, M.; Gatti, D. In Psoriatic Arthritis Dkk-1 and PTH Are Lower than in Rheumatoid Arthritis and Healthy Controls. Clinical Rheumatology 2017, 36, 2377–2381. [CrossRef]
- Kragstrup, T.W.; Andersen, T.; Heftdal, L.D.; Hvid, M.; Gerwien, J.; Sivakumar, P.; Taylor, P.C.; Senolt, L.; Deleuran, B. The IL-20 Cytokine Family in Rheumatoid Arthritis and Spondyloarthritis. Front. Immunol. 2018, 9, 2226. [CrossRef]
- Marijnissen, R.J.; Koenders, M.I.; Smeets, R.L.; Stappers, M.H.T.; Nickerson-Nutter, C.; Joosten, L.A.B.; Boots, A.M.H.; Van Den Berg, W.B. Increased Expression of Interleukin-22 by Synovial Th17 Cells during Late Stages of Murine Experimental Arthritis Is Controlled by Interleukin-1 and Enhances Bone Degradation: IL-1-Driven Regulation of IL-22 in Chronic Arthritis. Arthritis & Rheumatism 2011, 63, 2939–2948. [CrossRef]
- Sagiv, M.; Adawi, M.; Awisat, A.; Shouval, A.; Peri, R.; Sabbah, F.; Rosner, I.; Kessel, A.; Slobodin, G. The Association between Elevated Serum Interleukin-22 and the Clinical Diagnosis of Axial Spondyloarthritis: A Retrospective Study. International Journal of Rheumatic Diseases 2022, 25, 56–60. [CrossRef]
- Poddubnyy, D.; Sieper, J. Mechanism of New Bone Formation in Axial Spondyloarthritis. Curr Rheumatol Rep 2017, 19, 55. [CrossRef]
- Sieper, J.; van der Heijde, D. Review: Nonradiographic Axial Spondyloarthritis: New Definition of an Old Disease? Arthritis Rheum 2013, 65, 543–551. [CrossRef]
- Neerinckx, B.; Lories, R. Mechanisms, Impact and Prevention of Pathological Bone Regeneration in Spondyloarthritis. Curr Opin Rheumatol 2017, 29, 287–292. [CrossRef]
- Gong, Y.; Zheng, N.; Chen, S.-B.; Xiao, Z.-Y.; Wu, M.-Y.; Liu, Y.; Zeng, Q.-Y. Ten Years’ Experience with Needle Biopsy in the Early Diagnosis of Sacroiliitis. Arthritis Rheum 2012, 64, 1399–1406. [CrossRef]
- Appel, H.; Loddenkemper, C.; Grozdanovic, Z.; Ebhardt, H.; Dreimann, M.; Hempfing, A.; Stein, H.; Metz-Stavenhagen, P.; Rudwaleit, M.; Sieper, J. Correlation of Histopathological Findings and Magnetic Resonance Imaging in the Spine of Patients with Ankylosing Spondylitis. Arthritis Research & Therapy 2006, 8, R143. [CrossRef]
- Baraliakos, X.; Haibel, H.; Listing, J.; Sieper, J.; Braun, J. Continuous Long-Term Anti-TNF Therapy Does Not Lead to an Increase in the Rate of New Bone Formation over 8 Years in Patients with Ankylosing Spondylitis. Annals of the rheumatic diseases 2014, 73, 710–715. [CrossRef]
- Braun, J.; Baraliakos, X.; Deodhar, A.; Baeten, D.; Sieper, J.; Emery, P.; Readie, A.; Martin, R.; Mpofu, S.; Richards, H.B. Effect of Secukinumab on Clinical and Radiographic Outcomes in Ankylosing Spondylitis: 2-Year Results from the Randomised Phase III MEASURE 1 Study.. [CrossRef]
- Ramiro, S.; Heijde, D.V.D.; Tubergen, A.V.; Stolwijk, C.; Dougados, M.; Bosch, F.V.D.; Landewé, R. Higher Disease Activity Leads to More Structural Damage in the Spine in Ankylosing Spondylitis: 12-Year Longitudinal Data from the OASIS Cohort.. [CrossRef]
- Heijde, D.V.D.; Landewé, R.; Einstein, S.; Ory, P.; Vosse, D.; Ni, L.; Lin, S.L.; Tsuji, W.; Davis, J.C. Radiographic Progression of Ankylosing Spondylitis after up to Two Years of Treatment with Etanercept. Arthritis and Rheumatism 2008, 58, 1324–1331. [CrossRef]
- van Duivenvoorde, L.M.; Dorris, M.L.; Satumtira, N.; van Tok, M.N.; Redlich, K.; Tak, P.P.; Taurog, J.D.; Baeten, D.L. Relationship between Inflammation, Bone Destruction, and Osteoproliferation in the HLA-B27/Human Β2 -Microglobulin-Transgenic Rat Model of Spondylarthritis. Arthritis Rheum 2012, 64, 3210–3219. [CrossRef]
- Llop, M.; Moreno, M.; Navarro-Compán, V.; Juanola, X.; de Miguel, E.; Almodóvar, R.; Quintana, E.C.; Sanz, J.S.; Beltrán, E.; Montesinos, M.D.R.; et al. Sustained Low Disease Activity Measured by ASDAS Slow Radiographic Spinal Progression in Axial Spondyloarthritis Patients Treated with TNF-Inhibitors: Data from REGISPONSERBIO. Arthritis Research & Therapy 2022, 24, 30. [CrossRef]
- van der Heijde, D.; Østergaard, M.; Reveille, J.D.; Baraliakos, X.; Kronbergs, A.; Sandoval, D.M.; Li, X.; Carlier, H.; Adams, D.H.; Maksymowych, W.P. Spinal Radiographic Progression and Predictors of Progression in Patients With Radiographic Axial Spondyloarthritis Receiving Ixekizumab Over 2 Years. J Rheumatol 2022, 49, 265–273. [CrossRef]
- Apostolakos, J.; Durant, T.J.; Dwyer, C.R.; Russell, R.P.; Weinreb, J.H.; Alaee, F.; Beitzel, K.; McCarthy, M.B.; Cote, M.P.; Mazzocca, A.D. The Enthesis: A Review of the Tendon-to-Bone Insertion. Muscles Ligaments Tendons J 2014, 4, 333–342.
- Russell, T.; Bridgewood, C.; Rowe, H.; Altaie, A.; Jones, E.; McGonagle, D. Cytokine “Fine Tuning” of Enthesis Tissue Homeostasis as a Pointer to Spondyloarthritis Pathogenesis with a Focus on Relevant TNF and IL-17 Targeted Therapies. Semin Immunopathol 2021, 43, 193–206. [CrossRef]
- Watad, A.; Rowe, H.; Russell, T.; Zhou, Q.; Anderson, L.K.; Khan, A.; Dunsmuir, R.; Loughenbury, P.; Borse, V.; Rao, A.; et al. Normal Human Enthesis Harbours Conventional CD4+ and CD8+ T Cells with Regulatory Features and Inducible IL-17A and TNF Expression. Ann Rheum Dis 2020, 79, 1044–1054. [CrossRef]
- McGonagle, D.; Wakefield, R.J.; Tan, A.L.; D’Agostino, M.A.; Toumi, H.; Hayashi, K.; Emery, P.; Benjamin, M. Distinct Topography of Erosion and New Bone Formation in Achilles Tendon Enthesitis: Implications for Understanding the Link between Inflammation and Bone Formation in Spondylarthritis. Arthritis Rheum 2008, 58, 2694–2699. [CrossRef]
- Jacques, P.; Lambrecht, S.; Verheugen, E.; Pauwels, E.; Kollias, G.; Armaka, M.; Verhoye, M.; Van der Linden, A.; Achten, R.; Lories, R.J.; et al. Proof of Concept: Enthesitis and New Bone Formation in Spondyloarthritis Are Driven by Mechanical Strain and Stromal Cells. Ann Rheum Dis 2014, 73, 437–445. [CrossRef]
- Gravallese, E.M.; Schett, G. Effects of the IL-23–IL-17 Pathway on Bone in Spondyloarthritis. Nat Rev Rheumatol 2018, 14, 631–640. [CrossRef]
- Zheng, G.; Xie, Z.; Wang, P.; Li, J.; Li, M.; Cen, S.; Tang, S.; Liu, W.; Ye, G.; Li, Y.; et al. Enhanced Osteogenic Differentiation of Mesenchymal Stem Cells in Ankylosing Spondylitis: A Study Based on a Three-Dimensional Biomimetic Environment. Cell Death Dis 2019, 10, 1–11. [CrossRef]
- Xie, Z.; Wang, P.; Li, Y.; Deng, W.; Zhang, X.; Su, H.; Li, D.; Wu, Y.; Shen, H. Imbalance Between Bone Morphogenetic Protein 2 and Noggin Induces Abnormal Osteogenic Differentiation of Mesenchymal Stem Cells in Ankylosing Spondylitis. Arthritis Rheumatol 2016, 68, 430–440. [CrossRef]
- Cui, H.; Li, Z.; Chen, S.; Li, X.; Chen, D.; Wang, J.; Li, Z.; Hao, W.; Zhong, F.; Zhang, K.; et al. CXCL12/CXCR4-Rac1–Mediated Migration of Osteogenic Precursor Cells Contributes to Pathological New Bone Formation in Ankylosing Spondylitis. Science Advances 2022, 8, eabl8054. [CrossRef]
- Daoussis, D.; Liossis, S.N.C.; Solomou, E.E.; Tsanaktsi, A.; Bounia, K.; Karampetsou, M.; Yiannopoulos, G.; Andonopoulos, A.P. Evidence That Dkk-1 Is Dysfunctional in Ankylosing Spondylitis. Arthritis and Rheumatism 2010, 62, 150–158. [CrossRef]
- Fang, X.; Chen, C.; Wang, Z.-X.; Zhao, Y.; Jiang, L.-Q.; Fang, Y.; Zhang, R.-D.; Pan, H.-F.; Tao, S.-S. Serum DKK-1 Level in Ankylosing Spondylitis: Insights from Meta-Analysis and Mendelian Randomization. Frontiers in Immunology 2023, 14. [CrossRef]
- Nocturne, G.; Pavy, S.; Boudaoud, S.; Seror, R.; Goupille, P.; Chanson, P.; Heijde, D.V.D.; Gaalen, F.V.; Berenbaum, F.; Mariette, X.; et al. Increase in Dickkopf-1 Serum Level in Recent Spondyloarthritis. Data from the DESIR Cohort. 2015. [CrossRef]
- Cortes, A.; Maksymowych, W.P.; Wordsworth, B.P.; Inman, R.D.; Danoy, P.; Rahman, P.; Stone, M.; Corr, M.; Gensler, L.S.; Gladman, D.; et al. Association Study of Genes Related to Bone Formation and Resorption and the Extent of Radiographic Change in Ankylosing Spondylitis. Annals of the Rheumatic Diseases 2015, 74, 1387–1393. [CrossRef]
- Hamid, H.S.A. el; Ibrahim, N.H.; Morsi, M.H.; Al-Tabbakh, A.-S.M.; EL-Melouk, M.S. Elevated Serum Dickkopf-1 Levels as a Biomarker for Disease Activity and Severity in Psoriatic Arthritis Patients. The Egyptian Journal of Hospital Medicine 2022, 89, 6445.
- Wahba, M.A.W.A.; El-Gazzar, N.M.; Elsharaby, R.M.; Tabra, S.A. DKK-1 in Psoriatic Arthritis: Correlation with Disease Activity and Enthesopathy. Reumatología Clínica 2023. [CrossRef]
- Aschermann, S.; Englbrecht, M.; Bergua, A.; Spriewald, B.M.; Said-Nahal, R.; Breban, M.; Schett, G.; Rech, J. Presence of HLA-B27 Is Associated with Changes of Serum Levels of Mediators of the Wnt and Hedgehog Pathway. Joint Bone Spine 2016, 83, 43–46. [CrossRef]
- Saad, C.G.S.; Ribeiro, A.C.M.; Moraes, J.C.B.; Takayama, L.; Goncalves, C.R.; Rodrigues, M.B.; de Oliveira, R.M.; Silva, C.A.; Bonfa, E.; Pereira, R.M.R. Low Sclerostin Levels: A Predictive Marker of Persistent Inflammation in Ankylosing Spondylitis during Anti-Tumor Necrosis Factor Therapy? Arthritis Res Ther 2012, 14, R216. [CrossRef]
- Appel, H.; Ruiz-Heiland, G.; Listing, J.; Zwerina, J.; Herrmann, M.; Mueller, R.; Haibel, H.; Baraliakos, X.; Hempfing, A.; Rudwaleit, M.; et al. Altered Skeletal Expression of Sclerostin and Its Link to Radiographic Progression in Ankylosing Spondylitis. Arthritis and Rheumatism 2009, 60, 3257–3262. [CrossRef]
- Luchetti, M.M.; Ciccia, F.; Avellini, C.; Benfaremo, D.; Guggino, G.; Farinelli, A.; Ciferri, M.; Rossini, M.; Svegliati, S.; Spadoni, T.; et al. Sclerostin and Antisclerostin Antibody Serum Levels Predict the Presence of Axial Spondyloarthritis in Patients with Inflammatory Bowel Disease. The Journal of Rheumatology 2018, 45, 630–637. [CrossRef]
- Sorour, N.E.; Abdel Hafeez, N.A.; Akl, E.M.; Mowafy, M.A.; Mohamed, A.A. Evaluation of Serum Level of Sclerostin in Patients with Psoriatic Arthritis. Benha Journal of Applied Sciences 2021, 6, 11–15. [CrossRef]
- Fayed, A.; Elgohary, R.; Fawzy, M. Evaluating the Role of Serum Sclerostin as an Indicator of Activity and Damage in Egyptian Patients with Rheumatoid Arthritis: University Hospital Experience. Clin Rheumatol 2020, 39, 1121–1130. [CrossRef]
- Ohba, S. Hedgehog Signaling in Skeletal Development: Roles of Indian Hedgehog and the Mode of Its Action. Int J Mol Sci 2020, 21, 6665. [CrossRef]
- Daoussis, D.; Filippopoulou, A.; Liossis, S.-N.; Sirinian, C.; Klavdianou, K.; Bouris, P.; Karamanos, N.K.; Andonopoulos, A.P. Anti-TNFα Treatment Decreases the Previously Increased Serum Indian Hedgehog Levels in Patients with Ankylosing Spondylitis and Affects the Expression of Functional Hedgehog Pathway Target Genes. Semin Arthritis Rheum 2015, 44, 646–651. [CrossRef]
- González-Chávez, S.A.; Quiñonez-Flores, C.M.; Pacheco-Tena, C. Molecular Mechanisms of Bone Formation in Spondyloarthritis. Joint Bone Spine 2016, 83, 394–400. [CrossRef]
- Lata, M.; Hettinghouse, A.S.; Liu, C. Targeting Tumor Necrosis Factor Receptors in Ankylosing Spondylitis. Ann N Y Acad Sci 2019, 1442, 5–16. [CrossRef]
- Haroon, N.; Inman, R.D.; Learch, T.J.; Weisman, M.H.; Lee, M.; Rahbar, M.H.; Ward, M.M.; Reveille, J.D.; Gensler, L.S. The Impact of TNF-Inhibitors on Radiographic Progression in Ankylosing Spondylitis. Arthritis Rheum 2013, 65, 2645–2654. [CrossRef]
- Koo, B.S.; Oh, J.S.; Park, S.Y.; Shin, J.H.; Ahn, G.Y.; Lee, S.; Joo, K.B.; Kim, T.-H. Tumour Necrosis Factor Inhibitors Slow Radiographic Progression in Patients with Ankylosing Spondylitis: 18-Year Real-World Evidence. Annals of the Rheumatic Diseases 2020, 79, 1327–1332. [CrossRef]
- Jo, S.; Wang, S.E.; Lee, Y.L.; Kang, S.; Lee, B.; Han, J.; Sung, I.-H.; Park, Y.-S.; Bae, S.-C.; Kim, T.-H. IL-17A Induces Osteoblast Differentiation by Activating JAK2/STAT3 in Ankylosing Spondylitis. Arthritis Research & Therapy 2018, 20, 115. [CrossRef]
- Ono, T.; Okamoto, K.; Nakashima, T.; Nitta, T.; Hori, S.; Iwakura, Y.; Takayanagi, H. IL-17-Producing Γδ T Cells Enhance Bone Regeneration. Nat Commun 2016, 7, 10928. [CrossRef]
- Kim, Y.-G.; Park, J.-W.; Lee, J.-M.; Suh, J.-Y.; Lee, J.-K.; Chang, B.-S.; Um, H.-S.; Kim, J.-Y.; Lee, Y. IL-17 Inhibits Osteoblast Differentiation and Bone Regeneration in Rat. Arch Oral Biol 2014, 59, 897–905. [CrossRef]
- Tok, M.N.; Duivenvoorde, L.M.; Kramer, I.; Ingold, P.; Pfister, S.; Roth, L.; Blijdorp, I.C.; Sande, M.G.H.; Taurog, J.D.; Kolbinger, F.; et al. Interleukin-17A Inhibition Diminishes Inflammation and New Bone Formation in Experimental Spondyloarthritis. Arthritis Rheumatol 2019, 71, 612–625. [CrossRef]
- Mease, P.J.; Landewé, R.; Rahman, P.; Tahir, H.; Singhal, A.; Boettcher, E.; Navarra, S.; Readie, A.; Mpofu, S.; Delicha, E.M.; et al. Secukinumab Provides Sustained Improvement in Signs and Symptoms and Low Radiographic Progression in Patients with Psoriatic Arthritis: 2-Year (End-of-Study) Results from the FUTURE 5 Study. RMD Open 2021, 7, e001600. [CrossRef]
- Deodhar, A.; Gensler, L.S.; Sieper, J.; Clark, M.; Calderon, C.; Wang, Y.; Zhou, Y.; Leu, J.H.; Campbell, K.; Sweet, K.; et al. Three Multicenter, Randomized, Double-Blind, Placebo-Controlled Studies Evaluating the Efficacy and Safety of Ustekinumab in Axial Spondyloarthritis. Arthritis Rheumatol 2019, 71, 258–270. [CrossRef]
- Baeten, D.; Østergaard, M.; Wei, J.C.-C.; Sieper, J.; Järvinen, P.; Tam, L.-S.; Salvarani, C.; Kim, T.-H.; Solinger, A.; Datsenko, Y.; et al. Risankizumab, an IL-23 Inhibitor, for Ankylosing Spondylitis: Results of a Randomised, Double-Blind, Placebo-Controlled, Proof-of-Concept, Dose-Finding Phase 2 Study. Ann Rheum Dis 2018, 77, 1295–1302. [CrossRef]
- McGonagle, D.; Watad, A.; Sharif, K.; Bridgewood, C. Why Inhibition of IL-23 Lacked Efficacy in Ankylosing Spondylitis. Frontiers in Immunology 2021, 12. [CrossRef]
- Dudakov, J.A.; Hanash, A.M.; van den Brink, M.R.M. Interleukin-22: Immunobiology and Pathology. Annu Rev Immunol 2015, 33, 747–785. [CrossRef]
- Sherlock, J.P.; Joyce-Shaikh, B.; Turner, S.P.; Chao, C.-C.; Sathe, M.; Grein, J.; Gorman, D.M.; Bowman, E.P.; McClanahan, T.K.; Yearley, J.H.; et al. IL-23 Induces Spondyloarthropathy by Acting on ROR-Γt+ CD3+CD4−CD8− Entheseal Resident T Cells. Nat Med 2012, 18, 1069–1076. [CrossRef]
- El-Zayadi, A.A.; Jones, E.A.; Churchman, S.M.; Baboolal, T.G.; Cuthbert, R.J.; El-Jawhari, J.J.; Badawy, A.M.; Alase, A.A.; El-Sherbiny, Y.M.; McGonagle, D. Interleukin-22 Drives the Proliferation, Migration and Osteogenic Differentiation of Mesenchymal Stem Cells: A Novel Cytokine That Could Contribute to New Bone Formation in Spondyloarthropathies. Rheumatology (Oxford) 2017, 56, 488–493. [CrossRef]
- Upadhyay, J.; Farr, O.M.; Mantzoros, C.S. The Role of Leptin in Regulating Bone Metabolism. Metabolism 2015, 64, 105–113. [CrossRef]
- Hartl, A.; Sieper, J.; Syrbe, U.; Listing, J.; Hermann, K.-G.; Rudwaleit, M.; Poddubnyy, D. Serum Levels of Leptin and High Molecular Weight Adiponectin Are Inversely Associated with Radiographic Spinal Progression in Patients with Ankylosing Spondylitis: Results from the ENRADAS Trial. Arthritis Res Ther 2017, 19, 140. [CrossRef]
- Karsenty, G.; Yadav, V.K. Regulation of Bone Mass by Serotonin: Molecular Biology and Therapeutic Implications. Annu Rev Med 2011, 62, 323–331. [CrossRef]
- Klavdianou, K.; Liossis, S.-N.; Papachristou, D.J.; Theocharis, G.; Sirinian, C.; Kottorou, A.; Filippopoulou, A.; Andonopoulos, A.P.; Daoussis, D. Decreased Serotonin Levels and Serotonin-Mediated Osteoblastic Inhibitory Signaling in Patients With Ankylosing Spondylitis. Journal of Bone and Mineral Research 2016, 31, 630–639. [CrossRef]
- Clunie, G.; Horwood, N. Loss and Gain of Bone in Spondyloarthritis: What Drives These Opposing Clinical Features? Ther Adv Musculoskelet Dis 2020, 12, 1759720X20969260. [CrossRef]
- Ghozlani, I.; Ghazi, M.; Nouijai, A.; Mounach, A.; Rezqi, A.; Achemlal, L.; Bezza, A.; El Maghraoui, A. Prevalence and Risk Factors of Osteoporosis and Vertebral Fractures in Patients with Ankylosing Spondylitis. Bone 2009, 44, 772–776. [CrossRef]
- Puche-Larrubia, M.Á.; Ladehesa-Pineda, L.; Font-Ugalde, P.; Escudero-Contreras, A.; Moltó, A.; López-Medina, C.; Collantes-Estévez, E. Distribution of Comorbidities in Spondyloarthritis with Regard to the Phenotype and Psoriasis: Data from the ASAS-COMOSPA Study. Therapeutic Advances in Musculoskeletal 2021, 13, 1759720X2110452. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




