1. Introduction
In the 1990s, a concept of fetal programming emerged as a risk of developing type 2 diabetes, hypertension and dyslipidemia in patients born small for gestational age (SGA) [
1]. In the same year, Barker put forward a hypothesis, suggesting that poor intrauterine growth caused by fetal malnutrition was associated with an increased incidence of cardiovascular diseases (CVD) in adulthood. Barker argued that individuals starved in utero are more likely to become overweight as adults, and that they are more likely to suffer from diseases associated with obesity including cardiovascular problems and diabetes [
2]. The fetal origin hypothesis has also expanded the conventional focus on adult health behaviors, which include smoking, appropriate levels of physical activity and type of diet, to include earlier environmental factors that may affect fetal well-being. Barker’s hypothesis has inspired scientists to study the phenomenon of fetal programming in a more profound way. Birth weight is strongly correlated with the gender of the newborn, maternal and paternal anthropometric parameters, gestational age and also maternal comorbidities, i.e., diabetes, hypertension or smoking [
3].
Low birth weight (LBW) remains a significant public health problem worldwide. Birth weight among predictors of child mortality and morbidity. LBW newborns, as defined by the World Health Organization (WHO), are those born less than 2500g (up to and including 2499g) [
4]. The term LBW refers to an absolute weight of <2500 g independent of gestational age.
SGA is defined as birth weight below 10 centile for gestational age and concerns about 5 to 10 % of neonates in developed countries [
5]. It has been reported that 52% of unexplained stillbirths are associated with SGA, which is also a cause of 10 % perinatal mortality. In addition, even 72% of unexplained fetal deaths are associated with low birth weight [
6]. The “thrifty phenotype” hypothesis suggests that early life metabolic adaptations promote survival of an organism by selecting an appropriate growth trajectory in response to adverse intrauterine environmental cues [
7]. The global prevalence of low birth weight is estimated to be between 15% and 20% of all births, representing more than 20 million births per year [
8].
Children born as small for gestational age can present two types of intrauterine growth restriction – symmetrical and asymmetrical. The first type accounts for approximately 20–25% of all cases. The disturbances occur during organogenesis, in the first or second trimester of pregnancy. In this type all fetal dimensions are decreased regarding anthropometrical dimensions as well as internal organs, which is usually accompanied by a permanent reduction in growth potential. The second type (asymmetrical IUGR) accounts for the majority of cases, i.e., about 75-80%. This type is formed at the end of the second and third trimesters of pregnancy due to abnormal cell growth not their amount. In asymmetrical type neonates born with low birth weight while other anthropometric parameters such as body length, head and chest circumference remain normal. For this reason, ponderal index [PI =birth weight × 100/length3 (g/cm3)] in this type is lower than in symmetrical IUGR [
9,
10,
11]. According to nomenclature, terms: intrauterine growth restriction and fetal growth restriction are used interchangeably. Both terms refer to prenatal diagnosis of growth restriction. Moreover, these both terms (IUGR and fetal growth restriction) are often used conversely with small for gestational age (SGA) [
12]. For assessing fetal growth, Roth’s ponderal index (PI) as a ratio of body weight to length has been used. In newborns with IUGR, where the accumulation of adipose tissue and muscle mass is reduced, ponderal index is reduced [
13].
LBW is associated with a number of long-term complications. Numerous epidemiological studies show that low birth weight, regardless of other risk factors which appear later in life, can lead to cardiovascular diseases (especially hypertension and hyperlipidemia), stroke and increased mortality in adulthood [
14,
15]. Babies born as LBW may be burdened with long-term neurological disabilities, impaired language development, poorer academic performance and increased risk of chronic diseases, including the previously mentioned cardiovascular diseases and diabetes [
16]. In general, the development of LBW can be caused by fetal, placental and maternal factors. Despite their different etiologies, these causes often share a common final pathway of inadequate utero-placental perfusion and fetal nutrition [
17]. Most children with SGA compensate for limited intrauterine growth by catching up early [
18].
Catch-up growth (especially in terms of body weight) has been shown to increase the occurrence of some cardiometabolic risk factors, including resistance to insulin, overweight and obesity, already in childhood [
19]. The metabolic syndrome is a cluster of cardiovascular risk factors, including obesity, alterations in glucose-insulin metabolism, hypertension and dyslipidemia [
18]. The effect of birth weight on lipid levels in childhood is somewhat controversial. Low birth weight appears to be associated with unfavorable lipid levels [
20]. It is considered that poor catch-up growth in height may increase the risk of high total cholesterol [
21].
A positive correlation between elevated levels of total cholesterol, LDL cholesterol and apolipoprotein B in adults born as SGA and a small abdominal circumference observed in the newborn after birth was also revealed [
22]. There are relatively few reports referring to research on lipids and/or lipoproteins in the blood serum of SGA children [
21,
23,
24,
25,
26,
27,
28,
29]. Although their results vary depending on the studied population, it is worth emphasizing that the assessment of the lipid profile in SGA children is recommended in certain regions [
30].
Cardiovascular disease prevention is defined as a coordinated set of actions taken at the population level or targeted at individuals to eliminate or minimize the consequences of CVD and related disabilities [
31]. The effectiveness of such efforts at prevention has been proven in several population-based studies, including the CARDIA study [
32].
This study is a retrospective work aiming to determine whether children from the Polish population born as small for gestational age are associated with early disturbances in the lipid profile.
2. Materials and Methods
The study included 93 children (48 girls, 45 boys) aged 5-11 years, randomly selected from an outpatient clinic, born on time small for gestational age, i.e., birth weight below 10 centile according to gestational age (SGA – small for gestational age) detected prenatally by fetal size measurements during an obstetric ultrasonography. The control group included 47 healthy subjects (24 girls, 23 boys), born with proper birth weight (appropriate for gestational age - AGA), age- and sex-matched to the analysed group. Gestational age was calculated due to mothers’ last menstrual period.
The small for gestational age group was divided into two subgroups such as symmetrical (n = 43) and asymmetrical IUGR (n = 50). The Ponderal index was calculated from the formula: birth weight × 100/length3 (g/cm3).
All children were examined at the Pediatric Cardiology and Rheumatology Department of the Medical University of Lodz. At the time of the study they were in good condition, without chronic illnesses or drugs taking history.
The exclusion criteria in mothers were: multiple gestation, gestational hypertension/pre-eclampsia, pre-existing (before pregnancy) diabetes, gestational diabetes, intrahepatic cholestasis of pregnancy, maternal infection with syphilis or HIV, systemic diseases (e.g., hypertension, diabetes, rheumatological diseases, thyroid diseases, chronic kidney diseases, obesity), and taking certain drugs (e.g., antiarrhythmic, epileptic, anti-inflammatory drugs, insulin). Also, illicit drug use during pregnancy, maternal smoking or alcohol use were exclusion criteria for participation in the study. The exclusion criteria in children were: evidence for chromosomal or infectious etiology of SGA, hypothyroidism, systemic or acute disease (e.g., kidney or liver dysfunction, hypertension, gastrointestinal, nephrological, neurological, or cardiac diseases). We did not exclude children who had histories of taking antibiotics or other symptomatic drugs used for infections. Children with other pharmacological treatment were excluded. Consent was acquired from all children’s parents. All patients underwent physical examination when demographic and anthropometric data were acquired. The data involved information about the birth weight and gestational age. The current weight, height and body mass index (BMI) were measured. The body weight was measured in children bare-footed with a calibrated scale. BMI was defined as weight [kg] / height [m]2. Body weight was measured in subjects wearing light clothes and bare-footed with a calibrated scale. All patients were collected blood samples for fasting glucose and lipid profile including total cholesterol, triglycerides, HDL-cholesterol (high density lipoprotein-cholesterol), LDL-cholesterol (low density lipoprotein-cholesterol). Blood samples were collected in the morning in children who had fasted for 12 hours.
The study was approved by the Medical Ethical Committee of the Health Sciences Faculty of Lodz University (No: RNN/150/09/KB).
3. Results
The medical history analysis showed a statistically significant difference in birth weight (SGA group – 2545.22 209.07g versus control group - 3370.29 +/- 503.11 g; p<0.001), while there was no significant difference between the groups with regards to gestational age. The physical examination did not confirm any statistically significant differences between mean values of weight and body mass index (BMI), whereas children in the SGA group were significantly smaller compared to control subjects (
Table 1).
SGA patients were divided into 2 subgroups –symmetrical (n=43) and asymmetrical (N=50) types of intrauterine growth restriction. The significant difference in Ponderal index (PI) between the symmetrical (mean 2.017 ± 0.23 g/cm3) and asymmetrical (mean 1.75 ± 0.16 g/cm3) group was observed (p < 0.00001).
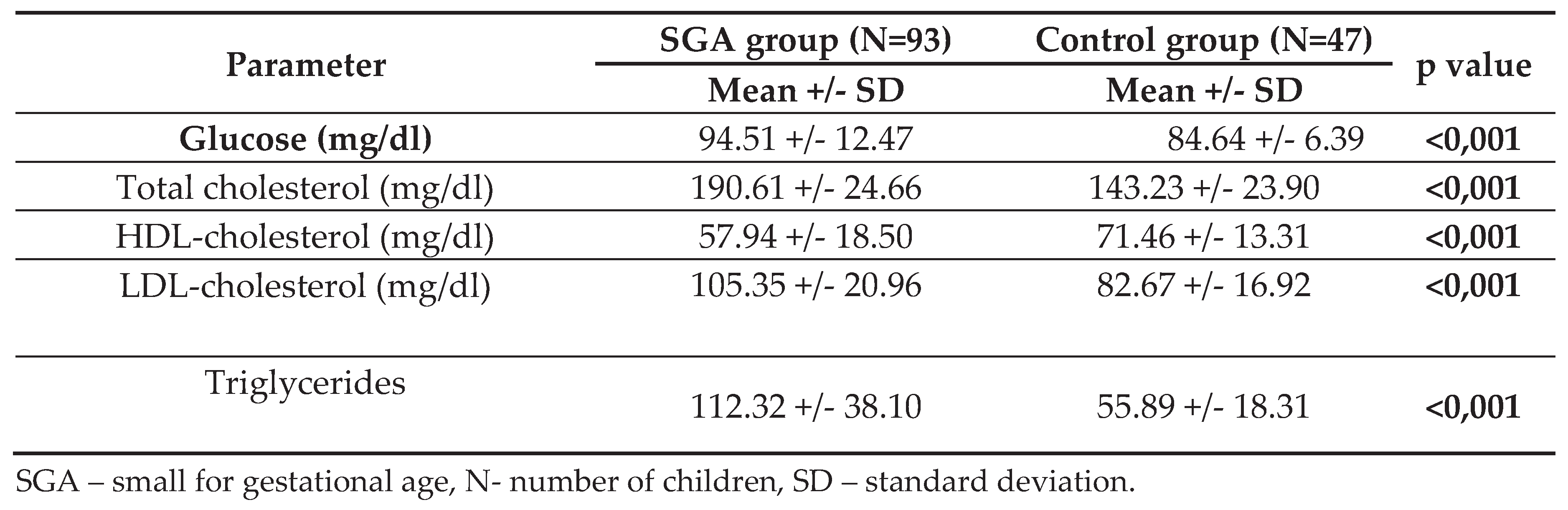
The analysis of blood samples revealed statistically significant higher levels of fasting glucose (p<0.001) in the SGA group. Similar findings were observed for average total cholesterol levels (SGA group 190.61 24.66 mg/dl vs control subjects 143.23 23.90; p<0.001). The analysis of particular fractions of cholesterol showed significantly higher mean values of triglycerides, LDL-cholesterol as well as lower mean values of HDL-cholesterol in children born SGA (
Table 2).
Analysing the differences regarding to metabolic factors between the subgroup of children with asymmetric and symmetrical IUGR, we obtained a statistically significant difference only in fasting glucose concentration (mean glucose level for asymmetric IUGR =90.56 ± 10.21 versus symmetrical IUGR = 98.95 ± 14.79; p<0.001). Total cholesterol, HDL-cholesterol, LDL-cholesterol and triglycerides were not statistically significantly different in IUGR subgroups.
We studied numerous correlations in both groups of patients. Surprisingly few correlations achieved statistical significance.
In children from SGA group there was negative correlation between birth weight and fasting glucose levels (r=-0.17; p=0.033), total cholesterol levels (r=-0.01; p=0.921), LDL-cholesterol levels (r=-0.02; p=0.848), triglycerides levels (r=-0.04; p=0.734) and positive correlation between birth weight and HDL-cholesterol levels (r=0.09; p=0.415). None of these correlations were statistically significant. In the AGA group, the correlation results were slightly different. There were positive correlation between birth weight and fasting glucose levels (r=0.33; p=0.054), total cholesterol levels (r=0.03; p=0.861), HDL-cholesterol levels (r=0.16; p=0.352), triglycerides levels (r=-0.02; p=0.894) and negative correlation between birth weight and LDL-cholesterol levels (r=-0.01; p=0.946). In this group, the correlations did not reach statistical significance either.
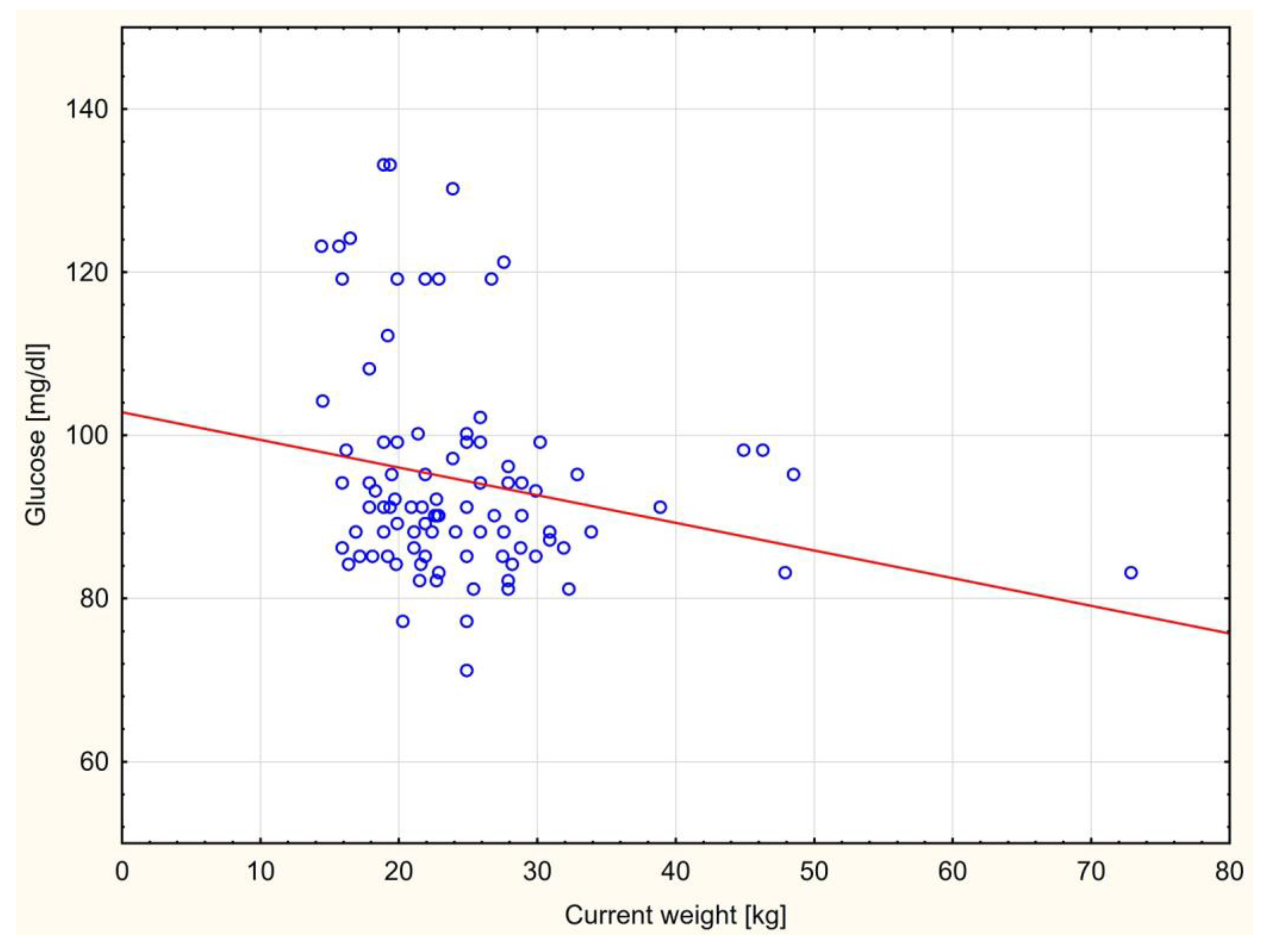
There was only a statistically significant negative correlation between the fasting glucose level and current body weight in children born small for gestational age group (r=-0.22; p=0.034) (
Figure 1).
In AGA group this correlation was positive but without statistical meaning (r=0.18; p=0.305). Contrary to expectations, we found a negative correlation between total cholesterol and current body weight as well as BMI in SGA group but not statistically significant (r=-0.01; p=0.922 and r=-0.09; p=0.393 respectively). In AGA group these correlations were different – we observed positive not significant correlation according to total cholesterol levels and actual patient’s weight (r=0.05; p=0.785) and BMI (r=0.04; p=0.839).
In SGA group current body weight correlated also negatively with LDL-cholesterol (r=-0.03; p=0.811), HDL-cholesterol levels (r=-0.002; p=0.979) whereas with triglycerides levels the correlation was positive (r=0.08; p=0.462). These correlations were without statistical meaning.
According to Body Mass Index in SGA group also for fasting glucose level (r=-0.16; p=0.118), LDL-cholesterol (r=-0.08; p=0.448) and HDL-cholesterol levels (r=-0.06; p=0.545) we found negative not statistically significant correlations, while for triglycerides positive correlation was observed (r=0.10; p=0.320). In the AGA group, we observed a negative correlation with BMI for HDL cholesterol (r=-0.381; p=0.023) and, contrary to the SGA group, also a negative correlation for triglycerides (r=-0.17; p=0.329). Positive correlation was found in AGA group between BMI and fasting glucose (r=0.10; p=0.577) as well as LDL-cholesterol levels (0.06; p=0.746). All mentioned above correlations was not statistically significant.
With regard to the correlation between metabolic parameters and the subgroup of SGA patients, we obtained the following results. We found a statistically significant negative correlation in patients with asymmetric type of IUGR between birth length and fasting glucose concentration (r=-0.27; p=0.05). For both types of IUGR we observed negative correlation between birth and current body weight (r=-0.06; p=0.66 and r=-0.008; p=0.59), but without statistical meaning. BMI of patients correlated positive with glucose level in patients with asymmetrical IUGR and negatively in patients with symmetrical type (r=0.03; p=0.84 and r=-0.15; p=0.33). These correlations were not statistically significant. According to total cholesterol levels, HDL-cholesterol, LDL-cholesterol and triglycerides concentration there were no statistically significant correlations found regarding to BMI, birth and actual weight in two IUGR subgroups.
4. Discussion
Our results show that there were significant differences in the lipid profile between the SGA group and the AGA controls. The prenatal environment also plays a role in shaping CVD outcomes, and the prenatal and postnatal growth exposures may result in different combinations with different effects on downstream lipid levels [
33].
Since the 1990s, several controversial studies have supported the hypothesis that low birth weight can lead to metabolic changes in this group in later life.
Human observational studies shown associations between postnatal growth and cholesterol levels. Results from a prospective cohort study (ALSPAC, Avon Longitudinal Study of Study of Parents and Children) in the UK, which began in 1991, suggest positive associations between ponderal index (kg/m3 ) during infancy and LDL-C and TG levels at 15 years of age [
34], with a negative association for HDL-C. Another prospective birth cohort, the Amsterdam Born Children and their Development (ABCD) study [
35] found a negative association for HDL-C and a positive association for TG at 5-6 years of age, when weight or weight-for-length change between one and three months was used as the exposure.
According to ponderal index, similar to other researchers, we found significantly lower value of PI in patients with asymmetrical type of IUGR [
9,
10,
11].
However, some of the previous works are not directly comparable with our paper because of differences in age, weight and height of the examined children.
Our results correspond to reports of Donker et al., who showed a correlation between low birth weight and increased serum triglyceride levels in children in a similar age group (7 to 11 years old) [
23]. Prevalence rates in the upper decile of serum lipid concentrations for children born with low birth weight (±2, 500 g) compared to children with birth weight >2, 500 g were calculated for each race-gender group. American researchers have shown that among white boys with low birth weight, higher than expected percentages of subjects were in the highest decile group of triglycerides concentrations (0 01 ± p ± 0.05) [
23]. This was the first study to find an association between low birth weight and elevated triglycerides in later childhood.
In turn, Antal et al., analysing 14 to 16-year-olds, did not find differences in lipid levels between children with low birth weight and children with normal birth weight. It should be mentioned that the examined children differ according to fetal age, including children born prematurely [
24]. Findings similar to ours were presented by Koklu et al. in a group of different age (infants with SGA) than our group. The authors observed significantly higher levels of triglycerides, than in the AGA group. They also evaluated the aortic intima-media thickness showing higher values in the SGA group and a positive correlation with the level of triglycerides [
36]. The relationship between carotid IMT complex thickness and lipid disorders has been increasingly reported in scientific studies [
37].
Spanish researchers studied one hundred and thirty-five newborns with intrauterine growth retardation and 116 newborns born at term, between 38 and 41 weeks’ gestation.
They concluded that triglyceride concentrations were higher in the study group compared to neonates born on time (45 +/- 27 and 36 +/- 19 mg/dl, respectively, p < 0.001). Differences in serum triglyceride levels compared to controls were observed in both male and female neonates with asymmetric growth retardation. However, they did not note differences between the SGA and AGA groups in terms of total cholesterol, LDL- and HDL-cholesterol [
38]. In another work, some Chinese researchers evaluated metabolic changes in young rats born as SGA, and obtained results which corresponded to our findings, i.e., they showed that rats born with low birth weight demonstrated significantly higher levels of total cholesterol, triglycerides and LDL-cholesterol [
39]. A team of Spanish researches - Ibanez et al. conducted a study in which they evaluated girls with precocious puberty, aged 5 - 18 years old. They noted a relationship between occurrence of dyslipidemia and low birth weight, suggesting that detected metabolic disorders originate from the prenatal period [
25]. Tenhola et al., in a Finnish study on the population at the age of 5 to 12 years old, born SGA, did not reveal a relationship between body mass index (BMI) and serum lipid concentrations, but nearly half of the SGA children (47.3%) were in the highest quartile for serum total cholesterol which was appropriate for gestational age children (p=0.038) . Interestingly, no body mass but weak dynamics of catch-up height in SGA children, increased the risk of elevated triglycerides levels at the age of 12 years almost 14 times more comparing to SGA children who had a good catch-up growth in height [
21]. Krochik et al. evaluated early risk factors of metabolic syndrome occurrence in SGA children in the period prior to puberty. Although the study group showed significantly higher basal insulin, basal cortisol and uric acid levels, there was no statistically significant difference between the examined groups in LDL- and HDL-cholesterol levels [
27]. Also Umer et al. noticed that low birth weight is related to higher LDL, non-HDL, and triglycerides, and lower HDL levels at 11 year-old children [
28]. Huang et al. studied a population of prepubertal short children (at the age of 6 years) born SGA. In children with SGA serum concentrations of total cholesterol, triglycerides, Apo B and Apo B/ApoA-I were significantly higher. It was observed that more than 33% of short SGA children had hypercholesterolemia and 23% hypertriglyceridemia compared do short AGA group [
40].
In contrast to the results of the present study, Evangelidou et al. [
41] did not note significant differences in the serum total cholesterol, LDL-cholesterol, triglycerides , Apo-A-1, Apo-B, Lp(a) between the SGA group and the AGA controls. However, within the SGA group , Lp(a) levels were significantly higher in the subgroup SGA <3rd centile than in the subgroup SGA 3rd – 10 th percentile (p<0.05). In our work, we compared glucose levels between SGA and AGA group, demonstrating a significantly higher level in the SGA group, and a negative correlation with the current body weight.
Italian researchers made similar observations to those that we did [
42]. Long-term studies related to children born as SGA in two age groups (respectively 8.4 + -1.4 years and 13.3 + - 1.8 years) evaluated lipids and glucose levels. With regards to a glucose level, the results that we obtained correspond to those received by the above authors, i.e., glucose levels were significantly higher in both the SGA groups compared to the AGA group. The lipid profile between the groups showed no changes.
Swedish researchers conducted a prospective, longitudinal cohort study that enrolled two hundred and eighty-five Swedish children born to LBW and 95 children with normal birth weight ( 2501-4500 g). At ages 3.5 and 7 years, blood samples were evaluated and compared between groups for glucose, insulin, homeostatic model assessment of insulin resistance (HOMA-IR), cholesterol, triglycerides, high and low density lipoproteins (HDL and LDL), apolipoprotein B (ApoB) and apolipoprotein A1 (ApoA1). They proved that the SGA group demonstrated a significantly higher level of the mean fasting glucose than the control group. But there were no considerable differences in insulin, HOMA-IR (homeostatic model assessment for insulin resistance), blood lipid levels between SGA and AGA groups [
29].
On the other hand, Hong Zu Deng et al. [
43] in their work showed no differences between SGA and AGA levels of glucose and insulin but this study was conducted on a younger pediatric group.
However, attention should be paid to the limitations regarding the interpretation of our paper results. The was a single-center study. Hence, it was carried out on a relatively small group of patients, which was its major limitation. Besides, the study design was based on collected retrospective data, which was another limitation.
The above limitations might suggest that the obtained results are not easily applicable to the whole population of pre-pubertal SGA children.
5. Conclusions
We have made one more contribution to professional literature by observing that children born SGA, with no differences in weight and BMI, have an abnormal lipid profile compared to AGA children. This may be a risk factor for cardiovascular diseases in adulthood. This group should be closely monitored by of a team of specialists - pediatricians, cardiologists, pediatric cardiologists, endocrinologists and pediatric nutritionists in order to prevent cardiovascular complications.
Author Contributions
Conceptualization: J.Z. and K.N-J.; methodology, J.Z. and K. N-J.; software, A.W.; validation, J.Z., K. N-J. and A.W.; formal analysis, J.Z., K. N-J and A.W. ; investigation, J.Z. and K. N-J..; resources, M.G., B.K.; data curation, J.Z. and K. N-J.; writing—original draft preparation, J.Z., K. N-J., B.K. and M.G.; writing—review and editing, J.Z. and M.G.; visualization, M.G.; supervision, E.S.; project administration, E.S. All authors have read and agreed to the published version of the manuscript. We, the authors, are the only persons responsible for the content of the article.
Funding
This work was supported by grant from the Medical University of Lodz Poland No: 503/1-000-01/503-11-001. We gratefully acknowledge contribution of the children whose data were used in this analysis, their caregivers and the team from the Immunopathology and Genetics Laboratory at the University Pediatric Centre Medical University of Lodz, Poland.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the the Medical Ethical Committee of the Health Sciences Faculty of Lodz University (No: RNN/150/09/KB).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data used to support the findings of this study are included within the article.
Acknowledgments
We thank all the participants who contributed to this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hong YH, Chung S. Small for gestational age and obesity related comorbidities. Ann Pediatr Endocrinol Metab. 2018; 23:4-8. [CrossRef]
- Barker D J. The fetal and infant origins of adult disease. British Medical Journal 1990; 301 :1111. [CrossRef]
- Thomas P, Peabody J, Turnier V, Clark RH. A new look at intrauterine growth and the impact of race, altitude, and gender. Pediatrics. 2000;106(2):E21. [CrossRef]
- Organization, WH. International statistical classification of diseases and related health problems, tenth revision, 2nd ed. World Health Organization; 2004.
- Figueras F, Eixarch E, Gratacos E, et al. Predictiveness of antenatal umbilical artery Doppler for adverse pregnancy outcome in small-for-gestational-age babies according to customised birthweight centiles: population-based study. BJOG. 2008;115:590-594. [CrossRef]
- Alisi A, Panera N, Agostoni C, et al. Intrauterine growth retardation and nonalcoholic Fatty liver disease in children. Int J Endocrinol. 2011; 2011:269853. [CrossRef]
- Hofman PL, Cutfield WS, Robinson EM, et al. Insulin resistance in short children with intrauterine growth retardation. J Clin Endocrinol Metab. 1997; 82:402–406. [CrossRef]
- Desta M, Tadese M, Kassie B, Gedefaw M. Determinants and adverse perinatal outcomes of low birth weight newborns delivered in Hawassa University Comprehensive Specialized Hospital, Ethiopia: a cohort study. BMC research notes. 2019;12(1):1–7.
- Lubchenco LO, Hansman C, Boyd E. Intrauterine growth in length and head circumference as estimated
from live births at gestational ages from 26 to 42 weeks. Pediatrics 1966; 37: 403–408.
- Suhag A, Berghella V. Intrauterine growth restriction (IUGR): etiology and diagnosis. Curr Obstet Gynecol Rep. 2013;2(2):102–111.
- Sharma D, Shastri S, Sharma P. Intrauterine growth restriction: antenatal and postnatal aspects. Clin Med
Insights Pediatr. 2016;10:67–83.
- Bakketeig LS. Current growth standards, definitions, diagnosis and classification of fetal growth
retardation. Eur J Clin Nutr. 1998;52(Suppl 1):S1–S4.
- Hwang JK, Kang HN, Ahn JH, Lee HJ, Park HK, Kim CR. Effects of Ponderal Index on Neonatal Mortality and Morbidities in Extremely Premature Infants. J Korean Med Sci. 2022;37(24):e198. Published 2022 Jun 20. [CrossRef]
- Mohseni R, Mohammed SH, Safabakhsh M, et al. Birth Weight and Risk of Cardiovascular Disease Incidence in Adulthood: a Dose-Response Meta-analysis. Curr Atheroscler Rep. 2020;22:12. [CrossRef]
- Wang T, Tang Z, Yu X, et al. Birth Weight and Stroke in Adult Life: Genetic Correlation and Causal Inference With Genome-Wide Association Data Sets. Front Neurosci. 2020;14:479. [CrossRef]
- Zerbeto AB, Cortelo FM, C Filho ÉB. Association between gestational age and birth weight on the language development of Brazilian children: a systematic review. J Pediatr (Rio J). 2015;91(4):326-332. [CrossRef]
- Cutland CL, Lackritz EM, Mallett-Moore T, et al. Low birth weight: Case definition & guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine. 2017;35(48 Pt A):6492-6500. [CrossRef]
- Nordman H, Jääskeläinen J, Voutilainen R. Birth Size as a Determinant of Cardiometabolic Risk Factors in Children. Horm Res Paediatr. 2020; 93 (3): 144–153. [CrossRef]
- Lurbe E, Aguilar F, Álvarez J, Redon P, Torró MI, Redon J. Determinants of Cardiometabolic Risk Factors in the First Decade of Life: A Longitudinal Study Starting at Birth. Hypertension. 2018; 71(3):437-443. [CrossRef]
- Mullett MD, Cottrell L, Lilly C, et al. Association between birth characteristics and coronary disease risk factors among fifth graders. J Pediatr. 2014;164(1):78-82. [CrossRef]
- Tenhola S, Martikainen A, Rahiala E, et al. Serum lipid concentrations and growth characteristics in 12-year-old children born small for gestational age. Pediatr Res. 2000; 48:623-628. [CrossRef]
- Barker DJP, Martyn CN, Osmond C, et al. Growth in utero and serum cholesterol concentrations in adult life. BMJ. 1993; 307:1524–1527. [CrossRef]
- Donker G, Labarthe D, Harrist R, et al. Low birth weight and serum lipid concentrations at age 7–11 years in a biracial sample. Am J Epidemiol. 1997; 145:398-407. [CrossRef]
- Antal M, Agfalvi R, Nagy K, et al. Lipid status in adolescents born with low birth weight. Z Ernahrungswiss. 1998; 37 (Suppl 1):S131-S133.
- Ibanez L, Potau N, de Zegher F. Precocious pubarche, dyslipidemia, and low IGF binding protein-1 in girls: relation to reduced prenatal growth. Pediatr Res. 1999; 46:320–322. [CrossRef]
- Okosun IS, Dever GE, Choi ST. Low birth weight is associated with elevated serum lipoprotein(a) in white and black American children ages 5-11 y. Public Health. 2002; 116:33-38. [CrossRef]
- Krochik AG, Chaler EA, Maceiras M, et al. Presence of early risk markers of metabolic syndrome in prepubertal children with a history of intrauterine growth restriction. Arch Argent Pediatr. 2010; 108:10-16. [CrossRef]
- Umer A, Hamilton C, Cottrell L, et al. Association between birth weight and childhood cardiovascular disease risk factors in West Virginia. J Dev Orig Health Dis. 2020;11:86-95. [CrossRef]
- Starnberg J, Norman M, Westrup B, et al. Cardiometabolic risk factors in children born with marginally low birth weight: A longitudinal cohort study up to 7 years-of-age. PLoS One. 2019;14:e0215866. [CrossRef]
- Boguszewski MC, Mericq V, Bergada I, et al. Latin American consensus: children born small for gestational age. BMC Pediatr. 2011; 11:66. [CrossRef]
- Porta, M. (Ed.) A Dictionary of Epidemiology, 6th ed.; Oxford University Press: New York, NY, USA, 2014;
pp. 33–225
- Liu K, Daviglus ML, Loria CM, et al. Healthy lifestyle through young adulthood and the presence of low cardiovascular disease risk profile in middle age: the Coronary Artery Risk Development in (Young) Adults (CARDIA) study. Circulation. 2012;125(8):996-1004. [CrossRef]
- Zouridis A, Manousopoulou A, Potiris A, et al. Impact of Maternal Food Restriction on Heart Proteome in Appropriately Grown and Growth-Restricted Wistar-Rat Offspring. Nutrients. 2021;13(2):466. Published 2021 Jan 30. [CrossRef]
- Howe LD, Tilling K, Benfield L, Logue J, Sattar N, Ness AR, Smith GD, Lawlor DA. Changes in ponderal index and body mass index across childhood and their associations with fat mass and cardiovascular risk factors at age 15. PLoS One. 2010 Dec 8;5(12):e15186.
- Oostvogels AJ, Stronks K, Roseboom TJ, van der Post JA, van Eijsden M, Vrijkotte TG. Maternal prepregnancy BMI, offspring’s early postnatal growth, and metabolic profile at age 5-6 years: the ABCD Study. J Clin Endocrinol Metab. 2014;99(10):3845-3854. [CrossRef]
- Koklu E, Kurtoglu S, Akcakus M, et al. Increased aortic intima-media thickness is related to lipid profile in newborns with intrauterine growth restriction. Horm Res. 2006; 65:269-275. [CrossRef]
- Guo HJ, Li CC, Bian XY, Hao Q. Correlation study on the relationship between dyslipidemia and carotid intima-media thickness in patients with diabetes mellitus. Pak J Med Sci. 2023;39(3):875-879.
- Molina M, Casanueva V, Cid X, et al. Lipid profile in newborns with intrauterine growth retardation. Rev Med Chil. 2000; 128:741-748.
- Zheng RD, Wang WJ, Ying YQ, et al. Effects of intrauterine growth retardation with catch-up growth on sugar-lipid metabolism and adipocyte function in young rats. Chinese Journal of Contemporary Pediatrics. 2015;17:1124-1130.
- Huang Y, Li Y, Chen Q, et al. Low serum adiponectin levels are associated with reduced insulin sensitivity and lipid disturbances in short children born small for gestational age. Clin Endocrinol (Oxf). 2015;83:78-84. [CrossRef]
- Evagelidou EN, Giapros VI, Challa AS, et al. Serum adiponectin levels, insulin resistance, and lipid profile in children born small for gestational age are affected by the severity of growth retardation at birth. Eur J Endocrinol. 2007;156:271-277. [CrossRef]
- Chiavaroli V, Marcovecchio ML, De Giorgis T, et al. Progression of cardio-metabolic risk factors in subjects born small to karge for gestational age. PLoS One. 2014;9:e104278. [CrossRef]
- Deng HZ, Deng H, Su Z, et al. Insulin resistance and adiponectin levels are associated with height catch-up growth in pre-pubertal Chinese individuals born small for gestational age. Nutr Metab (Lond). 2012;9:107. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).