1. Background
Urinary tract infections (UTIs) are one the most common human infections faced by clinicians working in the developing world and also, the most frequent bacterial infections in the human urinary system (Daudon et al., 2022). Most of these infections involve the lower urinary tract, namely the bladder and the urethra. Gram-negative bacteria cause 90% of UTI cases while gram-positive bacteria cause only 10% of the cases (Silago et al.,2022). The most frequent isolated uropathogen is Escherichia coli, accounting for 65%–90% of urinary tract infections , followed by Klebsiella pneumoniae, Staphylococcus saprophyticus, Enterococcus faecalis, Proteus mirabilis, and group B Streptococcus (GBS) (Ngong et al.,2021; Kline et al., 2017). Nowadays, UTI represents a serious public health problem and affect around 405 million people globally and nearly 0.23 million people died of UTIs, contributing to 5.2 million disability-adjusted life years (DALYs) in 2019 (Islam et al., 2022).
Women are at three times greater risk for UTI than men because of short, straight anatomy of the urinary tract, and the close proximity to the vagina and anus, along with the absence of bactericidal prostatic secretion and moist anal canal region., thus facilitating infection. Sexual intercourse also facilitates the ascent of bacteria into the bladder and the increase in the uterus weight as its grows and blocks the drainage of urine from the bladder, thus causing urinary stasis, which leads to infection of the urinary tract (Kline et al., 2017). Typical symptoms of UTIs are frequency of urination, dysuria, urgency, nocturia, suprapubic pain, haematuria, malaise, vague or mild abdominal pain, incontinence (Krishnaswamy et al.,2020). Also, several factors such as gender, age, race, HIV, diabetes, urinary catheter, genitourinary tract abnormalities (Odoki et al.,2019: Tula et al.,2020), pregnancy, infants, elderly (Odoki et al.,2019), and hospitalization status are associated to recurrent UTIs (Odoki et al.,2019). Recurrent Urinary tract infections and asymptomatic UTI in reproductive age female are associated with sexual dysfunction leading to deterioration of overall life quality, cystitis and pyelonephritis, Pelvic inflammatory disease (Fenta et al.,2020). Also, an untreated and repeated UTI infection in reproductive age females can affect the overall reproductive health including the ovulation process which is a serious health issue nowadays in our communities and it is associated to infertility as a result of pelvic inflammatory disease. To prevent the possibilities of evolving further complexity of UTI early detection and prompt treatment is very much crucial.
In Cameroon, routine culture and antimicrobial susceptibility testing of UTI are not frequently performed and the treatment is on an empirical basis. This may promote the overuse of antibiotics and the development of resistant microbial strains. The emergence of antibiotic resistance in the management of UTIs is a serious public health issue (Addis et al.,2021). Particularly in the developing world where there is high level of poverty, illiteracy and poor hygienic practices, there is also high prevalence of fake and spurious drugs of questionable quality in circulation. Reports from a study by Mouiche et al. (2019), revealed a prevalence of 68.2% of antimicrobial resistance among humans in Cameroon.
A similar study conducted in the Buea Health District by Ngong et al. 2021, reported 31% UTI prevalence among pregnant women. However, to our knowledge there is no published data on etiologic and antibiotic susceptibility of UTI among reproductive age female in Cameroon. Thus, the aim of this study is to determine the bacterial profile, antibiotic sensitivity and associated factors of UTI’s among reproductive age female attending the Logbaba District Hospital, Douala.
2. Materials and methods
2.1. Study area
This study was carryout at the Logbaba District Hospital, located in Douala, Wouri Division, Littoral region. Douala is a cosmopolitan town with a population over a million and the hospital received more than fifteen thousand patients per annum. This hospital consists of seven main units which are; Radiology, surgery, cardiology, pediatric, emergency and the medical laboratory.
2.2. Study design
This was a hospital based cross sectional study carried out between January 2023 to July 2023. At enrolment an informed consent and assent form was obtained from each participant. Data on the symptoms of UTIs reported, demographic characteristics, medical history and information on the risk factors tested were obtained using a questionnaire.
2.3. Study population
All reproductive age females attending the Logbaba District Hospital and which agreed to take part in the study by signing the inform consent were included in the study. Reproductive age females who were on antibiotics or had taken any within the previous weeks, as well as those who refused to give consent, were excluded from the study.
2.4. Sample Size Determination
Sample size of 259 was arrived by use of the Lorenz formula; n = z2p(1-p)/d2, where z= Z score for 95% confidence interval =1.96, p = prevalence, and d = acceptable error (5%). We used the prevalence of UTIs among non-pregnant women in Harar, Eastern Ethiopia, of 8.9% Abate et al. 2020
2.5. Ethical consideration
Ethical review and clearance was obtained from the Institutional review board (IRB/FHS UB) and administrative clearance from the regional delegation of public health and from the director of the Logbaba District Hospital.
2.6. Study procedure
2.6.1. Urine Specimen Collection for Culture
Midstream urine sample was collected in sterile clean leak proof bottles from each patient. To avoid contamination of the specimen, all participants were required to first clean the urethral area with a castile soap towelette. In addition, female participants were required to open widely the labia apart before sample collection. In patients with urinary catheters, urine specimens were collected from fresh catheters using a syringe and then transferred to a sterile specimen tube.
2.6.2. Laboratory analysis
Urinalysis
The color and consistency of freshly collected urine samples were recorded. A Combii 11 dipstick was used for urinalysis. The strip contained the following analytes; ascorbic acid, specific gravity, pH, ketone, glucose, urobilinogen, bilirubin, nitrite, leucocytes, blood and proteins. The reagent strip pad was completely immersed in fresh urine and observed for color changes. These were read against the color chart on the labeled test strip container and the results recorded.
Culture
CLED and Mueller Hilton were prepared according to the manufacturer instruction. Sterile urine samples were examined microscopically to detect significant pyuria on and were further inoculated on CLED under aseptic conditions, and incubated at 37°C for 24 hours. All positive cultures were then identified at species level by their colony characteristics, Gram-staining reaction and by biochemical testing using the standard microbiological technique. Gram positive cocci were identified using catalase and coagulase tests while for the gram-negative bacilli, oxidase test, catalase test and API20E were used for species identification.
Antibiotic susceptibility
Antimicrobial susceptibility of pure isolates to Vancomycin, Tetracycline (30µg), Imipenem, Gentamicin (20µg), Cefotaxime (30µg), Azithromycin, Ceftriaxone(30µg), Ciprofloxacin (5µg), Doxycycline, Ofloxacillin, trimethoprim-sulfamethoxazole (25µg), Amoxicillin, were performed by kirby-bauer disc diffusion method on Mueller-Hinton agar as described by Clinical and Laboratory Standards Institute guidelines (CLSI., 2019). The plates with the discs were incubated for 18–24 h at 35 ± 2℃ and the zone of inhibition was measured in millimeters. The zone of inhibition around the discs were measured with a transparent ruler and interpreted according into the NCCLS guidelines (CLSI., 2019).
Data analysis
Data was recorded on register and entered in a Microsoft excel 2010 data base in a secure computer and analysis was done with SPSS version 20. Data was statistically described in terms of percentage. the Chi-squared test was applied to analyze associations of UTI infections with sociodemographic factors. Multivariate analysis was applied to analyze risk factors associated with urinary tract infection. P-value less than 0.05 were considered statistically significant at 95% confidence interval.
3. Results
3.1. Sociodemographic factors of reproductive age females attending the Logbaba District Hospital
Two hundred and fifty-nine (259) reproductive aged females between 18 – 43 years were involved in this study. The mean age of the participants was 23.2 ± SD (6.56), the median age was 24. Majority (36.3%) of the participants falls within the age group 21-30 years and 31-40 years respectively, and age group ≤ 20 constituted 10.0 %. More than half (55.2%) of the participants were married. Majority (78.8%) of the participants ended their education in secondary school, 14.7 % in primary school and 6.6% in the university. Majority (41.3%) of the participants were housewife (
Table 1).
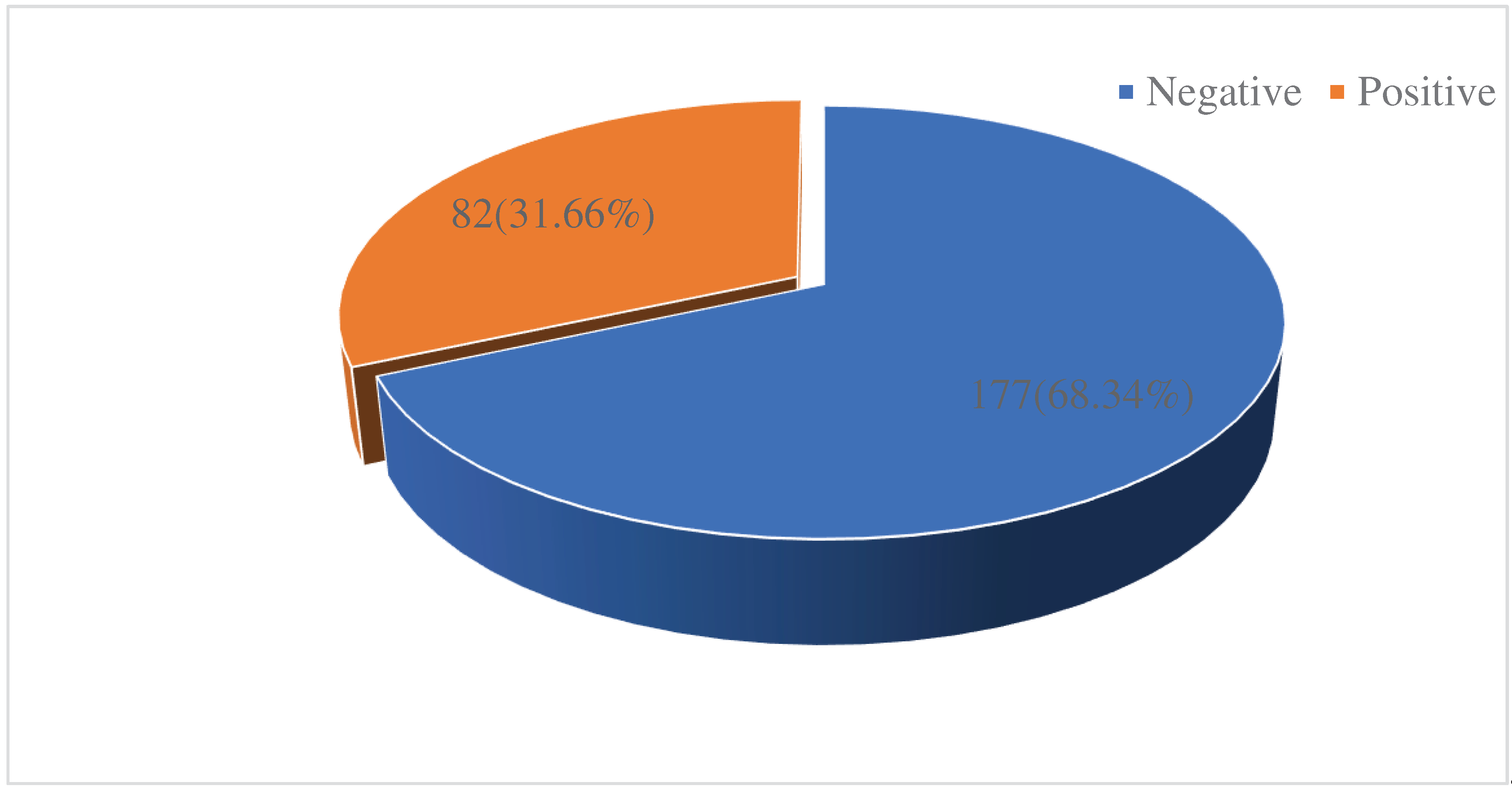
3.2. Prevalence of UTI’s among reproductive age women attending the Logbaba District Hospital.
Out of the 259 reproductive age women screened for UTI’s, 82 were positive for UTI’s given a prevalence of 31.66% (
Figure 1).
3.3. Distribution of the prevalence of UTI’s according to sociodemographic factors
Reproductive age females within the age range ≥20, 21–30 and 31–30 years recorded a decreasing UTI positivity of 12 (46.15%) ,28 (29.79%) and 28 (29.79%) respectively, and there was a significant association (p=0.04) between infection rate and age. Out of One hundred and fifty-six (156) participants with history of UTIs, 52 (28.7%) were positive for bacteriuria (
Table 2). However, history of UTI was significantly associated (p = 0.04) to the prevalence of UTI. Also, from the total number of subjects in each category who were diagnosed with UTIs 8(47.05%) attained tertiary level of education, 62(30.39%) secondary level and 12(31.58%) attained primary level education, and educational level was positively associated (p= 0.018) to the prevalence of UTI. On the other hand, type of toilet used (p=0.006) and Symptoms (p=0.02) were significantly related to the prevalence of UTI. Participant that used water cistern and those that have symptoms reported a positivity of 34 (34.34%) and 50 (41.32%), respectively
(Table 2).
3.4. Risk factors associated to Urinary tract infection among reproductive age females
In bivariable analysis, age range between 21-30 and 31 – 40 years, present of symptoms, urinary frequency, education (secondary), usage of water cistern toilets, previously infected with UTI and being a student were predictors to UTI (
Table 3). Variables with p value ≤.0.25
were subjected to multivariate analysis
. The multivariate analysis reveals that reproductive age females within the ages of 21-30 and 31 – 40 years were 2.53 (95% CI 1.32 – 4.43, p=0.006) and 0.19 (95% CI: 0.14 - 0.54; p <0.0001) times, respectively likely to suffer from UTI. Also, reproductive age female that were student were 1.8 times associated to UTI (95% CI: 1.21 - 2.75; p = 0.010). On the other hand, the odd of UTI was significantly five times higher in those previously infected with UTI compared to those without history of UTI (AOR= 5.34
, 95% CI = 1.86 - 18.15; p = 0.03). Subjects with symptoms were three times positively significantly associated with UTI (AOR = 2.86, 95% CI 1.78 - 4.67, p <0.0001) and participants showing symptoms of urinary frequency (AOR: 5.58, 95%CI: 2.13 - 13.30; p = 0.001) were found to have a statistically significant association with UTI. More significantly, those who use water cistern toilets were 3.52(1.38 - 9.38; p= 0.011) time more likely to get a UTI. Education (secondary) (AOR: 0.13, 95% CI 0.08 - 0.32; p<0.0001), was found to be
independent risk factors for UTI in reproductive age females (Table 3).
3.5. Frequency of bacteria identified and antibiotic susceptibility pattern of uti’s isolates
3.5.1. Frequency of UTI’s isolates identified
In the total 82 samples that yielded growth of bacteria causing UTI’s, single species were grown on all the samples.
Klebsiella Pneumoniae was the most frequent the species 27 (32.9%), followed by
E coli 22(26.8%),
Staphylococcus aureus 16 (19.5%), CONS 8 (9.8%),
Proteus species 6 (7.3 %) and
Pseudomonas aeruginosa 3(3.7%) (
Table 4). Gram-negative bacterial isolates were more prevalent than Gram-positive bacterial isolates (70.7% vs 29.3%)
3.5.2. The Antibiotic Sensitivity Pattern of UTI’s Isolates
We investigated the antibiotic susceptibility pattern of the 82 bacteria isolates as shown in Table 5. Majority of the isolates were sensitive to cefotaxime (87.80%), followed by imipenem (85.7%), vancomycin (79.27%), Ofloxacillin (76.83%), Ceftriaxone (75.61%), Ciprofloxacin (74.39%), Gentamicin (71.95%) and Doxycycline (69.51%). Amoxicillin (51.22%), cotrimoxazole (47.56%) Azithromycin (31.71%) and Tetracycline (21.95%) were the most resistant antibiotics, respectively. Amongst all the bacteria isolated, K pneumoniae, Staphylococcus aureus and E coli were more frequently resistant to all the antibiotics tested (Table 5).
4. Discussion
This study assessed the bacterial profile, antibiotic susceptibility pattern and associated factors of UTI among reproductive age females attending the Logbaba District Hospital. From our findings, the overall prevalence of UTI’s in reproductive age female in this study was 33.33%. Out of this bacterial UTI prevalence, symptomatic and asymptomatic patients reported a prevalence of 50/121 (41.32%) and 32/138 (23.19%), respectively. This is comparable to the prevalence of 31% of UTI reported in Buea, Cameroon (Ngong et al., 2021), and 32.2% reported in Bushenyi District, Uganda (Odoki et al.,2019). Our result is higher than the 20.4% reported in India (Muthulakshmi and Gopalakrishnan, 2017), Southern Ethiopia 7.8% (Tula et al.,2020), and in Zambia 16.5% (Mukosha et al.,2020). This difference in results could be due to variation in the environment, social habits of the community, and the standard of personal hygiene, sexual behaviour, limited healthcare infrastructure, diagnostic tools, the populations studied differ and education or may be due to the low economic status of the study subjects. The differences in design and methodologies might also affect comparison of prevalence in different surveys.
In this study, associated factors were also determined. the history of previous UTI and symptoms of UTI were independent risk factors for the acquisition of UTI. Recurrent urinary tract infections maybe linked to ascending of urethral microbiota to the bladder and/or untreated chronic/persistent bladder infection resulted from either ascending or bloodstream infections (Fenta et al.,2020). Also, it may be caused by the persistence of resistant strains from previous uropathogenic infections or by the recurrence of those illnesses. Age range between 21- 30 and 31 – 40 years were predictors to UTI’s. Our result is in line with Ngong et al. (2021), that revealed a significant association between UTI and advanced age. This is probably due to the fact that within this age groups, women are more often pregnant for the first or second time or involve in sexual activities, and thus more likely to have been exposed to obstetric situations and conditions than would make them pruned to UTIs. Participants who were using water cistern toilet had a nearly four-fold higher risk of UTIs as compared to the other participant using other toilets system. This is probably because water cistern toilet supports the growth of bacteria and predispose people that used such toilet. Reproductive age females with symptoms of urinary frequency were five times more likely to have a culture-positive result. This finding concurred with a study in Ethiopia by Mekonnen et al. (2023), that reported urine frequency as a predictor of UTI. Infections, injuries, diabetes or irritation of the bladder, as well as changes in the muscles and nerves that regulate bladder function, could all be associated to urine frequency. Significant bacteriuria was associated with secondary educational level. This might be due to insufficient knowledge of reproductive age female about the transmission and prevention of uropathogens.
In this study, Gram-negative bacterial isolates were more prevalent than Gram-positive bacterial isolates (70.7% vs 29.3%). Our finding is consistent with Fenta et al. (2020), who reported that gram negative were predominant bacteria in their finding. The possible explanation for the predominance of Gram-negative bacteria among isolated UTI aetiological agents may be because they are common members of the vaginal and rectal fora. Klebsiella Pneumoniae was the most frequent species 27 (32.9%), followed by E coli 22(26.8%), Staphylococcus aureus 16 (19.5%), CONS 8 (9.8%), Proteus mirabilis 6 (7.3 %) and Pseudomonas aeruginosa 3(3.7%). This finding is similar to a study carryout in Abakaliki, Nigeria, where Klebsiella spp. (24.5 %) was the most dominant isolate Muoneke et al. (2012), and contradict Ngong et al. (2020), and some studies in Ethiopia where E. coli is the most common organism identified in all UTI (Taye et al.,2018; Fenta et al.,2020). This disparity could be due to variations in specimen collection techniques and the existence of many virulence factors. K. pneumoniae was the predominant species may be because it is commonly found in wet areas especially in health institutions and can easily infect reproductive age females (Onyango et al., 2018). Another reason could be due to poor genital hygienic practices by reproductive age females who may find it difficult to clean properly after defecating or clean their genital after passing urine during their pregnancy (Vicar et al.,2023; Fenta et al.,2020). Also, adhesion structures like adhesin may help Klebsiella pneumoniae for progression to the bladder and capsule polysaccharide (CPS) of K. pneumoniae that promotes resistance to phagocytosis and serum bactericidal activity (Hao et al.,2021).
Antibiotic susceptibility test show that most isolates were susceptible to cefotaxime (87.80%), followed by Imipenem (85.7%), Vancomycin (79.27%), Ofloxacillin (76.83%), Ceftriaxone (75.61%), Ciprofloxacin (74.39%), Gentamicin (71.95%) and Doxycycline (69.51%). Our results, might be due to the fact that cefotaxime and Imipenem, are not widely available in the community, this minimized the chance to abuse them. Our findings partly agreed with Ngong et al. (2021), where Ciprofloxacin and Gentamicin (75.6%) were the most sensitive antibiotics and also partly in line with Fenta et al. (2020), which reported that Gentamicin and Meropenem were the most sensitive antibiotics. High resistance to Amoxicillin, Cotrimoxazole, Azithromycin and Tetracycline was detected. The irrational use and abuse of broad-spectrum antibiotics due to their affordability and easy access may be the reason for the high resistance to these drugs. 9(10.98%) isolates were resistant to imipenem may be a threat to antibiotic options available for the treatment of infections because carbapenems have the highest potency against bacteria. It is for this reason that they are reserved and used for more severe infections or as last-line drugs.
Furthermore, the overall MDR rate of isolates in our study was 100%, which is higher than the 66% MDR reported by Fanta et al. (2020), in Northwest Ethiopia. The MDR rates reported in this study and in the aforementioned countries threaten the effective treatment of UTI among reproductive age females as there are greater incidences of antibiotic resistance to popular antimicrobial drugs used to treat urinary tract infections. Additionally, this may result in a lengthier treatment period and hospitalization which can take a toll on family or caregivers (Taye et al.,2018). Untreated UTIs in reproductive age females can lead to pelvic inflammatory disease thus leading to infertility. Klebsiella pneumoniae, E. coli, and Staphylococcus aureus were the most resistant strains. Acquisition of resistance might be either mutational (changing the target site of a bacteria within its genetic material) or acquisition of new genetic material from other bacteria. Also, the alarming increase in resistance might be due various antibiotic resistance mechanism; preventing the uptake of antibiotic agents (turning off the production of porin channel proteins), modifying a drug target, inactivating a drug through the production of enzymes, and enhancing drug efflux pumps
5. Conclusion
In this study, the prevalence of UTIs among reproductive age females was 82/259 (31.66%). Klebsiella pneumoniae and Escherichia coli were the most predominant bacteria isolated. According to this study, Age range 21-30 years and 31 – 40 years, student, history of UTI, present of symptoms and secondary education were predictors to UTI. Majority of the isolates were sensitive to Cefotaxime, Imipenem and Vancomycin, and most of the bacterial isolates were resistant to Amoxicillin and Cotrimoxazole. All the isolates were multidrug drug resistant (100%) and Klebsiella Pneumoniae and E coli were the most resistant species. Therefore, there is a need to perform urine culture and antibiotic susceptibility before treatment of UTI. Since this will limit the progress of drug resistance.
Author Contributions
NFA conceived and designed the study: NFA and TPB implement the study: TPB and EDN supervised the study. NFA, TPB and EDN conducted data analysis: NFA, ECA, NNJ and EDN interpreted study results: NFA wrote the first draft of the manuscript, NFA, ECA, SBN and EDN reviewed and corrected the manuscript. All authors approved the final copy.
Acknowledgments
We are grateful to all who participated in this research.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Abbreviations
AOR: Adjusted odd ratio; CI: Confident interval; COR: Crude odd ratio; UTI: Urinary Tract Infection; MDR: Multiple Drug Resistant; OR: Odd ratio; WHO: World Health Organization; CONS: Coagulase Negative Staphylococcus.
References
- Abate, D., Marami, D. and Letta, S., 2020. Prevalence, antimicrobial susceptibility pattern, and associated factors of urinary tract infections among pregnant and nonpregnant women at public health facilities, Harar, Eastern Ethiopia: a comparative cross-sectional study. Canadian Journal of Infectious Diseases and Medical Microbiology, 2020. [CrossRef]
- Addis, T., Mekonnen, Y., Ayenew, Z., Fentaw, S. and Biazin, H., 2021. Bacterial uropathogens and burden of antimicrobial resistance pattern in urine specimens referred to Ethiopian Public Health Institute. PloS one, 16(11), p.e0259602. [CrossRef]
- Al-Rubeaan, K.A., Moharram, O., Al-Naqeb, D., Hassan, A. and Rafiullah, M.R.M., 2013. Prevalence of urinary tract infection and risk factors among Saudi patients with diabetes. World journal of urology, 31, pp.573-578. [CrossRef]
- Daudon, M., Petay, M., Vimont, S., Deniset, A., Tielens, F., Haymann, J.P., Letavernier, E., Frochot, V. and Bazin, D., 2022. Urinary tract infection inducing stones: Some clinical and chemical data. Comptes Rendus. Chimie, 25(S1), pp.315-334. [CrossRef]
- Fenta, A., Dagnew, M., Eshetie, S. and Belachew, T., 2020. Bacterial profile, antibiotic susceptibility pattern and associated risk factors of urinary tract infection among clinically suspected children attending at Felege-Hiwot comprehensive and specialized hospital, Northwest Ethiopia. A prospective study. BMC infectious diseases, 20, pp.1-10. [CrossRef]
- Kline, K.A. and Lewis, A.L., 2017. Gram-positive uropathogens, polymicrobial urinary tract infection, and the emerging microbiota of the urinary tract. Urinary tract infections: Molecular pathogenesis and clinical management, pp.459-502.
- Hao, G., Shu, R., Ding, L., Chen, X., Miao, Y., Wu, J., Zhou, H. and Wang, H., 2021. Bacteriophage SRD2021 recognizing capsular polysaccharide shows therapeutic potential in serotype K47 Klebsiella pneumoniae infections. Antibiotics, 10(8), p.894. [CrossRef]
- Islam, M.A., Islam, M.R., Khan, R., Amin, M.B., Rahman, M., Hossain, M.I., Ahmed, D., Asaduzzaman, M. and Riley, L.W., 2022. Prevalence, etiology and antibiotic resistance patterns of community-acquired urinary tract infections in Dhaka, Bangladesh. Plos one, 17(9), p.e0274423. [CrossRef]
- Mekonnen, S., Tesfa, T., Shume, T., Tebeje, F., Urgesa, K. and Weldegebreal, F., 2023. Bacterial profile, their antibiotic susceptibility pattern, and associated factors of urinary tract infections in children at Hiwot Fana Specialized University Hospital, Eastern Ethiopia. Plos one, 18(4), p.e0283637. [CrossRef]
- Krishnaswamy, P.H. and Basu, M., 2020. Urinary tract infection in gynaecology and obstetrics. Obstetrics, Gynaecology & Reproductive Medicine, 30(9), pp.276-282. [CrossRef]
- Mouiche, M.M.M., Moffo, F., Akoachere, J.F.T.K., Okah-Nnane, N.H., Mapiefou, N.P., Ndze, V.N., Wade, A., Djuikwo-Teukeng, F.F., Toghoua, D.G.T., Zambou, H.R. and Feussom, J.M.K., 2019. Antimicrobial resistance from a one health perspective in Cameroon: a systematic review and meta-analysis. BMC Public Health, 19, pp.1-20. [CrossRef]
- Mukosha, M., Nambela, L., Mwila, C., Chigunta, M., Kalungia, A.C., Lubeya, M.K. and Vwalika, B., 2020. Urinary tract infections and associated factors in HIV infected pregnant women at a tertiary hospital in Lusaka, Zambia. Pan African Medical Journal, 37(1). [CrossRef]
- Muoneke, V.U., Ibekwe, M.U. and Ibekwe, R.C., 2012. Childhood urinary tract infection in abakaliki: etiological organisms and antibiotic sensitivity pattern. Annals of medical and health sciences research, 2(1), pp.29-32. [CrossRef]
- Muthulakshmi, M. and Gopalakrishnan, S., 2017. Study on urinary tract infection among females of reproductive age group in a rural area of Kancheepuram district, Tamil Nadu. Int J Community Med Public Health, 4(10), pp.3915-21. [CrossRef]
- Ngong, I.N., Fru-Cho, J., Yung, M.A. and Akoachere, J.F.K.T., 2021. Prevalence, antimicrobial susceptibility pattern and associated risk factors for urinary tract infections in pregnant women attending ANC in some integrated health centers in the Buea Health District. BMC Pregnancy and Childbirth, 21, pp.1-10. [CrossRef]
- Nkwelle, C.E., Akoachere, J.F.T.K., Ndip, L.M., Nzang, F.A., Esemu, S.F. and Ndip, R.N., 2022. Asymptomatic Urinary Tract infection in Pregnant and Non-pregnant Women in the Limbe Health District of Cameroon: A Phenotypic and Biochemical analytic study.
- Onyango, H.A., Ngugi, C., Maina, J. and Kiiru, J., 2018. Urinary tract infection among pregnant women at Pumwani Maternity Hospital, Nairobi, Kenya: bacterial etiologic agents, antimicrobial susceptibility profiles and associated risk factors. Advances in microbiology, 8(03), p.175. [CrossRef]
- Odoki, M., Almustapha Aliero, A., Tibyangye, J., Nyabayo Maniga, J., Wampande, E., Drago Kato, C., Agwu, E. and Bazira, J., 2019. Prevalence of bacterial urinary tract infections and associated factors among patients attending hospitals in Bushenyi district, Uganda. International journal of microbiology, 2019. [CrossRef]
- Silago, V., Moremi, N., Mtebe, M., Komba, E., Masoud, S., Mgaya, F.X., Mirambo, M.M., Nyawale, H.A., Mshana, S.E. and Matee, M.I., 2022. Multidrug-resistant uropathogens causing community acquired urinary tract infections among patients attending health facilities in Mwanza and Dar es Salaam, Tanzania. Antibiotics, 11(12), p.1718. [CrossRef]
- Taye, S., Getachew, M., Desalegn, Z., Biratu, A. and Mubashir, K., 2018. Bacterial profile, antibiotic susceptibility pattern and associated factors among pregnant women with Urinary Tract Infection in Goba and Sinana Woredas, Bale Zone, Southeast Ethiopia. BMC research notes, 11(1), pp.1-7. [CrossRef]
- Tula, A., Mikru, A., Alemayehu, T. and Dobo, B., 2020. Bacterial profile and antibiotic susceptibility pattern of urinary tract infection among pregnant women attending antenatal care at a Tertiary Care Hospital in Southern Ethiopia. Canadian Journal of Infectious Diseases and Medical Microbiology, 2020. [CrossRef]
- Vicar E K., Samuel E. K. Acquah, Williams Wallana, Eugene D. Kuugbee, Emmanuel K. Osbutey, Abigail Aidoo, Emmanuel Acheampong, Gloria Ivy Mensah, "Urinary Tract Infection and Associated Factors among Pregnant Women Receiving Antenatal Care at a Primary Health Care Facility in the Northern Region of Ghana", International Journal of Microbiology, vol. 2023, Article ID 3727265, 10 pages, 2023. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).