1. Introduction
Atractylodes macrocephala (Bai-Zhu
aka. in Chinese) is the dried rhizome of
Atractylodes macrocephala Koidz, which is widely used as a tonic herbal in eastern Asia. [
1] It belongs to the homologous resource of medicine and food in China. Its components include hydrophobic lactones, volatile oils, and hydrophilic carbohydrates. Among the hydrophobic components of atractylodes, the contents of atractylenolide I, II, III and atractylon are relatively high, which is an important active component of Bai-Zhu, and plays an important role in anti-tumor, anti-inflammation, anti-depression, anti-hypertension etc. [
2,
3] The hydrophilic carbohydrate compounds in Bai-Zhu are also one kind of important components.[
4,
5] Therefore, it is significant to develop a simple and rapid analytical method for simultaneous detection of hydrophobic atractylenolide, atractylon, and hydrophilic carbohydrates in Bai-Zhu for its quality control.
Normally, the analyses of atractylenolide I, II, III, atractylon and sugars in Bai-Zhu were completed by employing reversed-phase liquid chromatography (RP-HPLC) [
6,
7] and hydrophilic interaction liquid chromatography (HILIC)[
8], respectively. It was complicated and time-consuming. Because of the excellent orthogonality of RP-HPLC and HILIC, two-dimension HPLC (2D-HPLC) methods were always employed to analyze non-polar amd polar components simultaneously. [
9,
10,
11] Although 2D-HPLC method owes high separation resolution, the instrument system is highly complex. In order to make use of the characteristics of the two separation modes simultaneously on a simple instrument, column tandem technique were employed.[
12,
13] Zhu et al. connected the C18 column with the hydrophilic interaction column in series, and used ultra-high performance liquid chromatography mass spectrometry to simultaneously separate and determine acrylamide, caprolactam, benzidine and aniline.[
14] Granafei et al. established a method of HILIC column in tandem with RP-LC column to separate the phospholipid mixture in soybean.[
15] Falasca et al. used C18 column and HILIC column to separate a variety of quaternary ammonium compounds with great differences in hydrophobicity, including acetylcholine, choline, carnitine, acetylcarnitine and seven other low-polarity acylcarnitines.[
16] The RP-LC column in tandem with HILIC column is beneficial to the separation of polar and non-polar substances in the mixture samples, and has been successfully applied to the analysis of phenols in wine [
17], mouse serum metabolites [
18], drugs [
19] etc. Therefore, the series of RP-LC column and HILIC column, which mobile phase (acetonitrile-H
2O system) is also compatible, is easy to achieve and can be used for the analysis of polar and non-polar compounds.
In this paper, an analytical method was established by HPLC-DAD-ELSD with C18-NH2 column tandem for the simultaneous determination of hydrophobic components atractylenolide I, II, III, atractylone and hydrophilic compounds glucose, fructose and sucrose in Bai-Zhu. The tandem of DAD and ELSD can simultaneously detected of sugar with no UV absorption and atractylenolide I, II, III, and atractylone. It provided a reference method for the quality control of Bai-Zhu.
2. Materials and Methods
2.1. Materials and Instrumentation
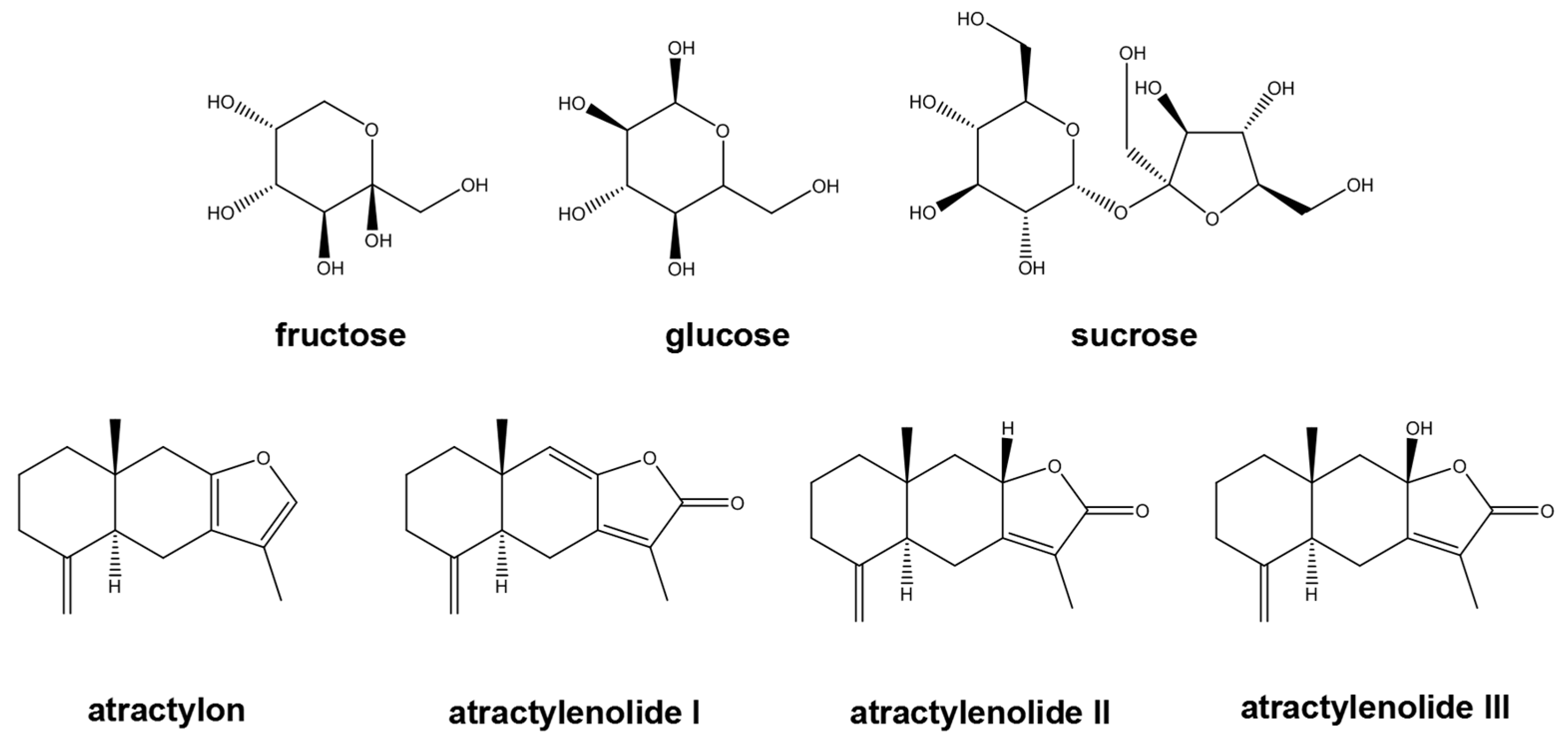
The standards of atractylenolide I, II, III (purchased form Shanghai Yuanye Biotechnology Co. P. R. China), atractylone (purchased form Sichuan Chengdu Aifa Biotechnology Co., Ltd. P. R. China) were >98% purifity (HPLC). Fructose, glucose, and sucrose (>99%) were from Shanghai Maclean's Biochemical Technology Co., Ltd (Shanghai, P. R. China).(
Figure 1). Other chemicals were analytical purity. All medicinal slice of Bai-Zhu samples were purchased in local pharmacy (Changsha, P. R. China).
Shimadzu 8040 HPLC system (Shimadzu, Japan) was employed which containing SPD-M20A diode array detector (DAD) and ELSD-LT II low temperature-evaporative light scattering detector (ELSD). The separation was accomplished on InertsilTM NH2 column (250 mm × 4.6 mm, 5 µm) (Shimadzu, Japan) and Diamonsil C18 column (250mm × 4.6 mm, 5 µm) (Dikma, P. R. China).
2.2. Preparation of Standard Solutions
To construct the calibration curves, 0.50 mL, 1.0 mL, 4.0 mL, and 8.0 mL of a mixed standard solution with concentrations of 1.0 mg/mL, 1.0 mg/mL, 1.0 mg/mL, 0.025 mg/mL, 0.025 mg/mL, 0.025 mg/mL, 0.025 mg/mL for fructose, glucose, sucrose, atractylon, atractylenolide I, II, III were diluted with methanol to 10.0 mL. A series of mixed standard working solutions were obtained. 20 μL of the solution was injected in HPLC, respectively. Linear regression was calculated by using the method of least squares. All the above solutions are stored in a 4 ◦C refrigerator.
2.3. Sample Preparation
The medicinal slice of Bai-Zhu was crushed into powder (20 mesh). 0.20 g of the powder was extracted in 5 mL of methanol in an ultrasonic bath for 45 minutes. After centrifuging at 5000 rpm for 5 minutes, the supernatant was filtered from 0.22 µm filter membrane. The filtrate was injected into HPLC.
2.4. HPLC Conditions
HPLC-DAD-ELSD conditions are as follows: an InertsilTM NH2 column (250 mm × 4.6 mm, 5 µm) was directly connected to a Diamonsil C18 column (250mm × 4.6 mm, 5 µm) in series through a PEEK pipeline (30 mm in length). A gradient elution (solvent A: H2O, solvent B: acetonitrile (ACN)) was employed: 0-5 min 15% A, 5-12 min 15 to 20% A, 12-17 min 20 to 22% A, 17-32 min 22 to 30% A, 32-45 min 30 to 35% A, 45-65 min 35 to 40% A. The flow rate was 1 mL/min. UV detection wavelengths for quantitative analysis was set at 274 nm for atractylenolide I and 218 nm for atractylon, atractylenolide II, III, respectively. ELSD conditions for sugars detection are as follows: detector drift tube temperature was 50 ◦C, gas flow 1.5 L/min and signal gain 8. Injection volume of sample solutions was 10 μL.
3. Results and Discussion
3.1. Selection of Detectors
Except big difference in polarity, there are also difference in spectral characteristic between sugars and terpenoids. It is well known that sugars have not UV absorption and cannot be analyzed using DAD. Because atractylon, atractylenolide I, II, III have high volatility and low content, it is difficult to be detected by ELSD sensitively. Therefore the selection and combination of detectors is important for the simultaneous analysis of sugars and atractylon, atractylenolide I, II, III in Bai-Zhu.
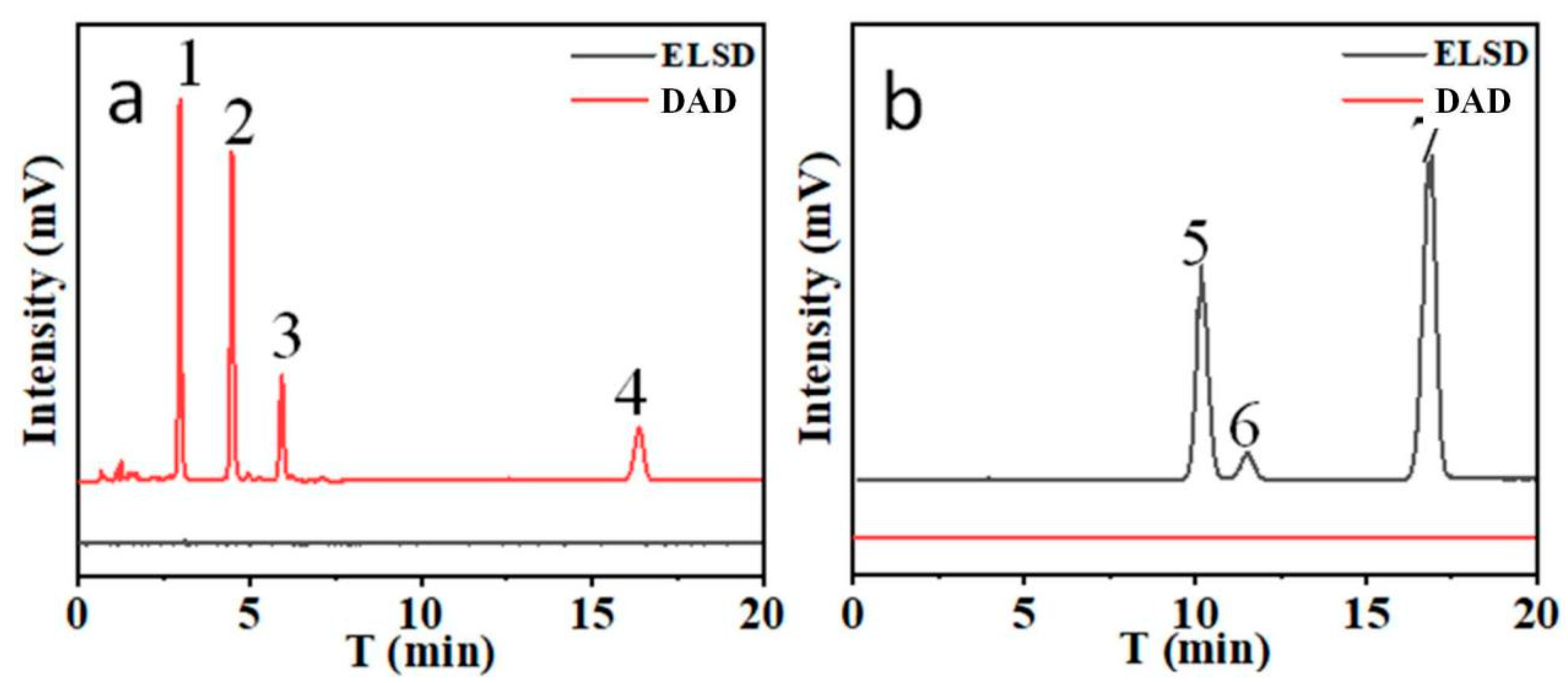
In this method, DAD and ELSD tandem was employed for the analysis of Bai-Zhu. As shown in
Figure 2, two kind of compounds have very different response in DAD and ELSD.
It can be seen that both atractylenolide and atractylon have good responses on DAD, but there is no response on ELSD. On the contrary, sugars respond good on ELSD and have no response on DAD. Therefore, the combination of DAD and ELSD is a good choice for the simultaneous detection of two kinds of compounds. In addition, the response difference on difference detector can also increase the analytical selectivity due to incomplete separation.
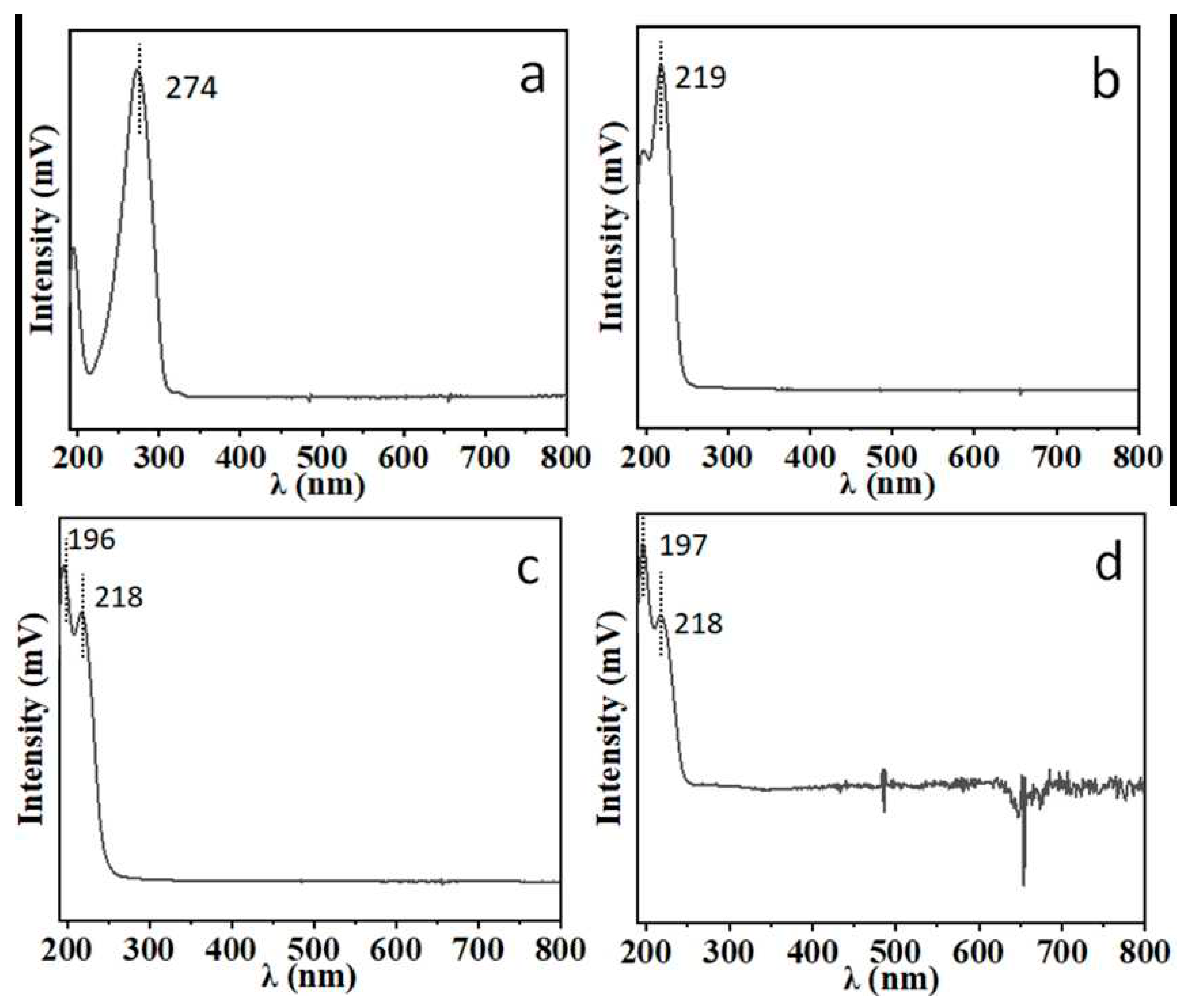
For the quantitative analysis of atractylenolide I, II, III, and atractylenolide, the optimal UV detection wavelength was investigated. The UV absorption spectrum for each compound is shown in
Figure 3. Characteristic absorption wavelength of atractylenolide I is 274 nm, one of atractylenolide II, III and atractylon is 218-219 nm. To obtain optimal sensitivity and anti-interference ability, 274 nm was selected as the optimal detection wavelength for the analysis of atractylenolide I, 218 nm was selected as the optimal detection wavelength for atractylenolide II, III and atractylon.
3.2. Optimization of Separation Conditions
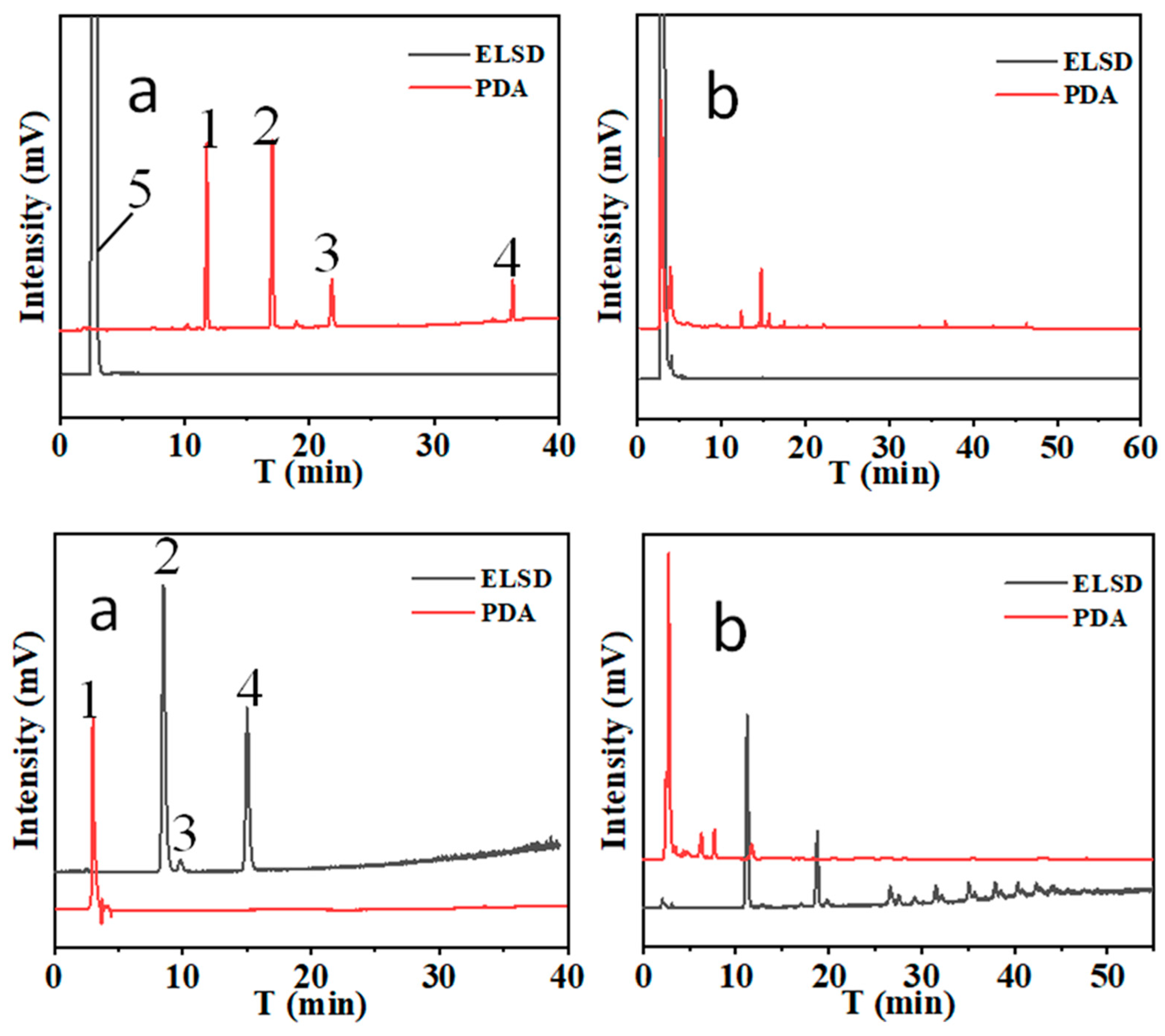
On single column mode for the separation, hydrophobic atractylenolide I, II, III, and atractylone can be separated on C
18 column based on hydrophobic interaction (revised phase separation mode) (
Figure 4. upper) and hydrophilic sugars can be separated on -NH
2 column based on hydrophilic interaction separation mode (
Figure 4. lower).
Sugars can not be separated completely on -C18 column, and atractylenolide I, II, III and atractylone can not be separated completely on -NH2 column based on H2O-ACN mobile phase system.
When -C
18 column in series with -NH
2 column, two types of compounds can be separated completed. The results are shown in
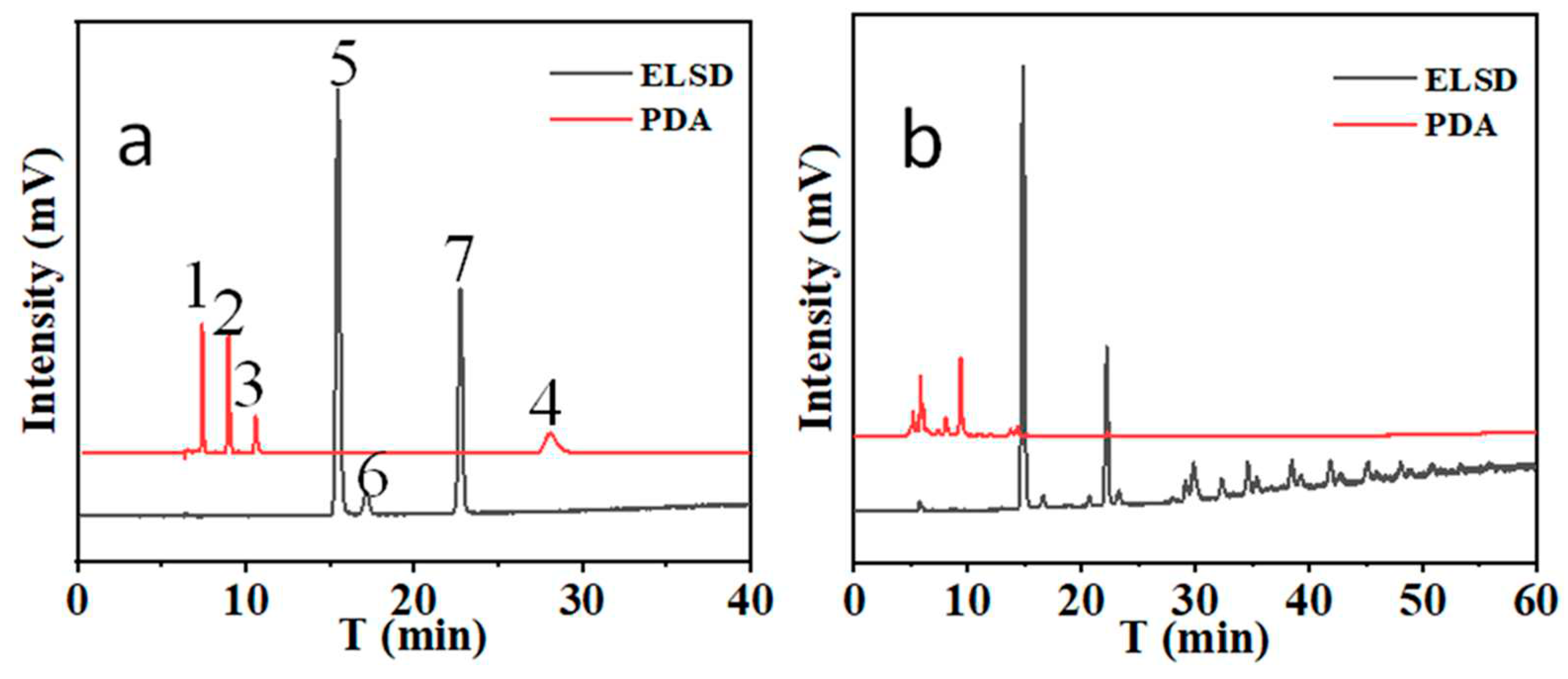
Figure 5.
In addition, to investigating whether the connection order of chromatographic columns in column series affect the separation and response of the compounds, two modes of C18 columns in front of NH2 columns and NH2 columns in front of C18 columns on the simultaneous separation of atractylenolide I, atractylenolide II, atractylenolide III, atractylone, fructose, glucose, and sucrose were tested. The results shown that there was not significant different on separation and response between two modes.
3.3. Calibration Curves, LOD, and LOQ
To construct the calibration curves, 0.50 mL, 1.0 mL, 4.0 mL, and 8.0 mL of a mixed standard solution with concentrations of 1.0 mg/mL, 1.0 mg/mL, 1.0 mg/mL, 0.025 mg/mL, 0.025 mg/mL, 0.025 mg/mL, 0.025 mg/mL for fructose, glucose, sucrose, atractylon, atractylenolide I, II, III were diluted with methanol to 10.0 mL. A series of mixed standard working solutions were obtained. 20 μL of the solution was injected in HPLC, respectively.Linear regression was calculated by using the method of least squares. The LOD for seven compounds was 0.1-5.0 μg/mL (S/N=3), and the LOQ was 1.20-50.0 μg/mL. The results are shown in
Table 1.
3.4. Accuracy
Weighing 0.20 g of Bai-Zhu powder from the same batch with known content, parallel 6 portions, and adding a certain amount of reference solutions. The three spiked levels were 0.035, 0.35, and 0.625 mg/g for atractylenolide I, II, III, and atractylone in samples. The three spiked levels were 1.25, 12.5, 25.0 mg/g for glucose, fructose, and sucrose, respectively. Then obtaining the extraction solution according to the preparation method described in
Section 2.3, and analyzing by HPLC according to the chromatographic conditions described in
Section 2.4. The average recoveries of atractylenolide I, II, III, atractylone, glucose, fructose, and sucrose were in the range of 97.8-101.2%, 98.7-102.2%, 97.5-103.1%, 97.1-100.7%, 96.5-99.6%, 97.1-101.2%, and 96.7-100.1%, respectively. And all RSDs were within acceptable ranges. The results are shown in
Table 2.
3.5. Stability
To investigate the sample solution stability, a same one was analyzed at 0, 2, 4, 6, 8, 10, 12, and 24 hours, respectively. The results shown that RSDs of atractylenolide I, II, III, atractylone, glucose, fructose, and sucrose are 1.5%, 1.1%, 1.0%, 1.3%, 2.7%, 2.5% and 1.1%, respectively, It indicates that the extract of Bai-Zhu has good stability within 24 hours.
3.6. Application
17 batches of Bai-Zhu sample were analyzed by using proposed C1
8-NH
2 column tandem HPLC-DAD-ELSD method. The results were also compared with that obtained from single column methods such as RP-HPLC-DAD and HILIC-ELSD. The results obtained by C1
8-NH
2 column tandem HPLC-DAD-ELSD method are listed in
Table 3.
From the results, it can be seen that: (1) there are no significant differences on results between that obtained from C
18-NH
2 column tandem HPLC-DAD-ELSD and RP-HPLC-DAD (the results are listed) and HILIC-ELSD (the results are listed). (2) Chinese medicine must be processed before it can be used as medicine, which is one of the characteristics of Chinese medicine. Traditional Chinese medicine processing is a pharmaceutical technology adopted according to the theory of traditional Chinese medicine, according to the needs of syndrome differentiation and the nature of drugs, as well as the different requirements of dispensing and preparation. For Bai-Zhu, heating is a common processing method. Compared with raw materials, the content of atractylon in heating prepared products decreased to different degrees (almost lower 10 times), the contents of atractylenolide I, II, and III significantly increased. The reason is that atractylon will be transformed under heating conditions, mainly into atractylenolides. Therefore, the heating processing is very important for the Bai-Zhu quality control.[
20,
21,
22] (3) It can been found that The main low molecular weight sugar in Bai-Zhu is fructose. Moreover, oligosaccharides are abundant (
Figure 5(b)). Therefore, the sugars in processed Bai-Zhu are higher that raw materials due to the hydrolysis of oligosaccharides. In addition, the extract from Bai-Zhu can regulate the disorders of glucose and lipid metabolism in mice with type 2 diabetes mellitus, enhance insulin sensitivity, improve the level of inflammation, and alleviate liver injury and lipid deposition. Its mechanism may be related to the up-regulation of the protein expression of GLP-1R.[
23] Because glucose intake is very dangerous for people with type 2 diabetes. The sugars monitoring in Bai-Zhu is very important when Bai-Zhu is used for the treatment of type 2 diabetics.
Funding
This research was funded by the National Natural Science Foundation of China (22276049, 22276050), the China Scholarship Council 2020 International Cooperation Training Program for Innovative Talents, the Aid Program for S&T innovation research team in higher education institutions, the construction program of key disciplines of Hunan Province (2015JC1001), and the Hunan Province 100 experts project.
Conflicts of Interest
There are no conflicts to declare.
References
- Zhu, B.; Zhang, Q.L.; Hua, J.W.; Cheng, W.L.; Qin, L.P. The traditional uses,phytochemistry, and pharmacology of Atractylodes macrocephala Koidz.: A review. J. Ethnopharmacol. 2018, 226, 143–167. [Google Scholar] [CrossRef]
- Hoang, Le.S.; Tran, M.H.; Lee, J.S.; Ngo, Q.M.T.; Woo, M.H.; Min, B.S. Inflammatory inhibitory activity of sesquiterpenoids from Atractylodes macrocephala Rhizomes. Chem. Pharm. Bull. 2016, 64, 507–511. [Google Scholar] [CrossRef]
- Deng, M.; Chen, H.J.; Long, J.Y.; Song, J.W.; Xie, L.; Li, X.F. Atractylenolides (I, II, and III): a review of their pharmacology and pharmacokinetics. Arch. Pharm. Res. 2021, 44, 633–654. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.S.; Li, Y.X.; Jiang, S.L.; Zheng, F.; Qiao, W. Isolation,purification,and structural characterization of polysaccharides from Atractylodis Macrocephalae Rhizoma and their immunostimulatory activity in RAW264.7 cells. Int. J. Biol. Macromol. 2020, 163, 270–278. [Google Scholar] [CrossRef]
- Feng, Y.Y.; Ji, H.Y.; Dong, X.D. ; Polysaccharide extracted from atractylodes macrocephala koidz (PAMK) induce apoptosis in transplanted H22 cells in mice. Int. J. Biol. Macromol. 2019, 137, 604–611. [Google Scholar] [CrossRef]
- Yan, H.; Sun, Y.Y.; Zhang, Q.L.; Yang, M.J.; Wang, X.R. Simultaneous determination and pharmacokinetic study of Atractylenolide I, II and III in rat plasma after intragastric administration of Baizhufuling extract and Atractylodis extract by UPLC-MS/MS. J. Chromatogr. B, 2015; 993–994, 86–92. [Google Scholar]
- Zheng, L.J.; Zhang, M.; Qin, K.M.; Cai, H.; Cao, G.; Cai, B.C. Simultaneous determination of 10 active components in Baizhu Shaoyao San and its single herbs by high-performance liquid chromatography coupled with diode array detection. J. Chromatogr. Sci. 2015, 53, 633–40. [Google Scholar] [CrossRef] [PubMed]
- Hetrick, E.M.; Kramer, T.T.; Risley, D.S. Evaluation of a hydrophilic interaction liquid chromatography design space for sugars and sugar alcohols. J. Chromatogr. A. 2017, 1489, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Xue, X.Y.; Guo, Z.M.; Xu, Q.; Zhang, F.F.; Liang, X.M. Novel two-dimensional reversed-phase liquid chromatography/hydrophilic interaction chromatography, an excellent orthogonal system for practical analysis. J. Chromatogr. A. 2008, 1208, 133–140. [Google Scholar] [CrossRef] [PubMed]
- D’Attoma, A.; Heinisch, S. On-line comprehensive two dimensional separations of charged compounds using reversed-phase high performance liquid chromatography and hydrophilic interaction chromatography. Part II: Application to the separation of peptides. J. Chromatogr. A 2013, 1306, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; Tredoux, A.G.J.; Villiers, A. Application of kinetically optimised online HILIC×RP-LC methods hyphenated to high resolution MS for the analysis of natural phenolics. Chromatographia 2019, 82, 181–196. [Google Scholar] [CrossRef]
- Rampler, E.; Schoeny, H.; Mitic, B.M.; Abiead, Y.E.; Schwaiger, M.; Koellensperger, G. Simultaneous non-polar and polar lipid analysis by on-line combination of HILIC, RP and high resolution MS. Analyst 2018, 143, 1250–1258. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, X.; Xu, G.W. Simultaneous separation of hydrophilic and hydrophobic compounds by using an online HILIC-RPLC system with two detectors. J. Sep. Sci. 2008, 31, 1564–1572. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.B.; Xu, N.B.; Feng, J.Y.; Qian, F.Z.; Chen, Z.Q. Simultaneous determination of acrylamide, caprolactam, benzidine and aniline by ultra performance liquid chromatography tandem mass spectrometry with two coupled columns. Chinese J. Anal. Chem. 2013, 41, 594–597. [Google Scholar] [CrossRef]
- Granafei, S.; Azzone, P.; Spinelli, V.A.; Losito, I.; Palmisano, F.; Cataldi, T.R.I. Hydrophilic interaction and reversed phase mixed-mode liquid chromatography coupled to high resolution tandem mass spectrometry for polar lipids analysis. J. Chromatogr. A. 2016, 1477, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Falasca, S.; Petruzziello, F.; Kretz, R.; Rainer, G.; Zhang, X.Z. Analysis of multiple quaternary ammonium compounds in the brain using tandem capillary column separation and high resolution mass spectrometric detection. J. Chromatogr. A. 2012, 1241, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Greco, G.; Grosse, S.; Letzel, T. Serial coupling of reversed-phase and zwitterionic hydrophilic interaction LC/MS for the analysis of polar and nonpolar phenols in wine. J. Sep. Sci. 2013, 36, 1379–1388. [Google Scholar] [CrossRef]
- Chalcraft, K.R.; McCarry, B.E. Tandem LC columns for the simultaneous retention of polar and nonpolar molecules in comprehensive metabolomics analysis. J. Sep. Sci. 2013, 36, 3478–3485. [Google Scholar] [CrossRef] [PubMed]
- Louw, S.; Pereira, A.S.; Lynen, F.; Hanna-Brown, M.; Sandra, P. Serial coupling of reversed-phase and hydrophilic interaction liquid chromatography to broaden the elution window for the analysis of pharmaceutical compounds. J. Chromatogr. A. 2008, 1208, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Wang, F.; Zhang, Y.H.; Zheng, X.H. Kinetics and mechanism studies on oxidizing reaction of atractylon in essential oil from Atractylodes Macrocephala Koidz. Chinese J. Appl. Chem. 2007, 24, 301–305. [Google Scholar]
- Li, W.; Wen, H.M.; Cui, X.B.; Zhang, K.W. Process mechanism of Atractylodes macrocephala and conversion of sesquiterpenes. J. Chin. Tradit. Med. 2006, 31, 1600–1603. [Google Scholar]
- Zeng, X.X.; Chen, B.; Gu, Z.X.; Liu, J.J.; Wang, J.F.; Ma, N.; Ruan, X.N. Identification of raw and processed atractylodes by DCBI-MS. China Med. Herald 2018, 15, 32–36. [Google Scholar]
- Zhang, Y.W.; Li, Z.M.; Wu, L.Y. Anti-diabetic effect of extract from Baizhu on db/db mice and its mechanism. Pharmocol. Clin. Chin. Mater. Med. 2022, 38, 120–125. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).