1. Introduction
Psoriasis is a chronic, inflammatory, immune-mediated skin disease with a prominent TNFα-IL23/IL17 immune axis [
1]. Given that TNFα plays the key role in the pathogenesis of immunoinflammatory diseases, there are several FDA-approved anti-TNFα drugs for the treatment of psoriasis: infliximab, adalimumab, certolizumab pegol, golimumab, and etanercept [
2]. Nowadays, the use of targeted therapies, especially TNFα and IL17 inhibitors, has become common practise in moderate-to-severe cases of psoriasis and has led to its effective management [
3,
4]. However, the existing data show that despite the TNFα inhibitors are the drugs of choice in the treatment of immune-mediated inflammatory diseases, approximately 30-40% of patients experience “primary therapeutic failure” or loss of response over time [
4,
5,
6,
7]. In this regard, great efforts are being made to identify biomarkers that can be used as predictive tools in patients suitable for treatment with TNFα inhibitors [
2]. It has been shown that low baseline levels of S100A12 and prealbumin together with high platelet factor 4 may predict the response to anti-TNFα drugs as adalimumab, infliximab, and etanercept in patients with RA [
8]. A systematic review of patients with psoriatic arthritis and psoriasis reported that serum levels of IL12 and SNPs in the IL12B gene show promise as biomarkers of response to anti-TNFα treatment [
9]. In a study by Andersen et al., it was shown that in patients who responded to treatment with adalimumab, the initial level of IL6 in the blood was lower than in those who did not respond (0.99 (0.42–1.4) vs 1.62 (0.96–2.41) pg/ml; p = 0.02). Therefore, the authors concluded that IL6 can serve as a perspective marker to predict the response to TNFα inhibitors in psoriasis [
10]. Meanwhile, the authors noted that IL17A, IL1β and IFNγ concentrations were below the quantification limits in most cases [
10]. This fact intricates the use of cytokines as prognostic markers. Currently, there are no unambiguous diagnostic indicators that can be used to predict response to therapy with TNF inhibitors, since the data of numerous studies are very contradictory [
2].
Given that, determination of factors associated with the failure or success of therapy with TNFα inhibitors is one of the most difficult aspects in the treatment of psoriasis [
11], further research is needed to find immunological predictors of treatment effectiveness. Therefore, this study aimed to assess plasma cytokine levels and identify possible predictors of treatment effectiveness with TNFα inhibitors in patients with moderate-to-severe psoriasis.
2. Materials and Methods
The study included 81 patients with moderate-to-severe severity psoriasis vulgaris (L40.0, ICD-10) aged 19 to 76 years (mean age was 46.56±13.69 years), including 63 (78%) men and 18 (22%) women, treated with either etanercept (Enbrel®, Pfizer, Belgium), adalimumab (Humira®, Vetter Pharma-Fertigung, Germany), or infliximab (Remicade®, MSD International GmbH, Singapure). The study was approved by the Local Ethics Committee (protocol №04, 27/04/2018) and meets the standards of good clinical practice and evidence-based medicine. All patients included in the study provided written informed consent.
To assess the severity of psoriasis and the effectiveness of the therapy PASI (Psoriasis Area and Severity Index), BSA (Body Surface Area), and sPGA (static Physician Global Assessment) scores were used. The severity of psoriasis was defined as moderate at 10≤PASI<20, and severe at PASI≥20. Treatment efficacy was assessed at week 16 by PASI score and patients were classified into the positive effect group (PASI≥75, good and satisfactory effect) and no effect group (PASI≤50, bad effect). Exclusion criteria were the presence of any symptom suggestive of tuberculosis, viral hepatitis B and C, HIV infection, syphilis, and standard contraindications to the use of etanercept, infliximab, adalimumab. An additional inclusion criterion was the failure or intolerance to previous therapy (phototherapy, therapy with methotrexate or acitretin).
Physical examination of patients with moderate-to-severe psoriasis and plasma sampling were performed by employees of the State Research Center of Dermatovenereology and Cosmetology. Assessment of the baseline and week 16 plasma cytokine profile was performed on a BioPlex200 (Bio-Rad, USA) using Procarta immunossay Human Mag 9 plex One PL (PC1009M) kit (Thermo Scientific, USA), Bio-Plex Pro Human Cytokine ICAM-1 (171В6009М) Plex (Bio-Rad, USA), Bio-Plex Pro Human Th17 Cytokine Panel 15-Plex (Bio-Rad, USA) kit with the corresponding calibration kit sand expressed in pg/ml. All stages were carried out according to the manufacturer’s instructions, under the same conditions for all samples. Cytokines whose concentrations were below assay working range were assigned zero value.
Analysis and visualization of data were carried out using RStudio for MacOS and the R programming language (version 4.2.2) [
12]. Data distribution was evaluated using the Shapiro-Wilk test. Data were presented as mean±standard deviation and medians and quartiles (Me [Q1; Q3]) depends on the data distribution. Difference between three or more groups characterized by Gaussian distribution was assessed using one-way ANOVA followed by a Tukey post hoc test for multiple comparisons. To compare two groups with non-Gaussian distribution the two-sample Wilcoxon test for independent samples was used; the Kruskal-Wallis test followed by a pairwise Wilcoxon test with Bonferroni correction for multiple testing was used for comparing three or more groups. Pairwise comparisons before/after treatment were performed using paired samples Wilcoxon test. We used the “nparLD” package to perform a non-parametric within–between ANOVA for the nonparametric analysis of longitudinal data [
13] to evaluate the effect of time (before/after treatment), type of drug used, and their interaction on cytokine levels (data from Supplementary
Tables S1–S3). If any differences were found to be significant, the Wilcoxon signed rank test was used as a post hoc test. Changes in cytokine levels before/after the treatment defined as delta cytokine were calculated by subtracting the baseline levels from the levels at week 16; to assess difference the resulted delta were transformed to remove negative values. Differences were considered significant at p<0.05.
The search for relationships between the baseline levels of cytokines and the effectiveness of therapy included a preliminary analysis of the data to exclude multicollinearity (by computing a Spearman correlation matrix) and variables related by linear dependencies (using the findLinearCombos function of the “caret” package). The development of the model included: 1) description of the available data by CART (Classification and Regression Trees) algorithm based on recursive breakdown of the entire amount of data, followed by pruning of branches to minimize the error; 2) random forest (RF) model with data splitting into training and test samples in the ratio of 80% and 20% using the factor as an argument of the createDataPartition function [
14].The RF model was trained using the randomForest function of the “randomForest” package, with the parameters ntree=500, mtry = 18 (equal to the number of predictors) [
15]. After fitting the model on the training sample with the specified parameters, the response was predicted on the test sample with the evaluation of the model metrics (accuracy, specificity, sensitivity, area under the ROC curve) and the evaluation of the importance of predictors.
3. Results
General characteristic of patients
All patients were divided into three groups and received different TNFα inhibitors (etanercept, infliximab and adalimumab). Clinical data of patients are shown in
Table 1. Moderate and severe psoriasis was diagnosed in 39 (48%) and 42 (52%) patients, respectively. There were differences in age between patients treated with infliximab and etanercept (p = 0.010), whereas no differences were found in BMI. Baseline PASI, BSA, and sPGA values were significantly different between the studied groups. The values of PASI scores recorded during the initial examination ranged from 10.3 to 64.2, the BSA score was from 11 to 90, sPGA from 2 to 4 (
Table 1). PASI in the group treated with infliximab was 47.5% higher than that in the etanercept group (p<0.001) and 84.8% higher than that in the adalimumab group (p=0.001). BSA in the infliximab group was 2.25 and almost 2-fold higher than that in the etanercept (p<0.001) and adalimumab group (p<0.001), respectively. The same differences were observed in sPGA score: it was 1,4 and 1,33-fold higher than that in the etanercept (p<0.001) and adalimumab group (p=0.005), respectively. This fact is explained by taking into account the route of administration of the drug, the possible side effects, and the effectiveness of therapy when prescribing drugs by a dermatologist.
As can be seen from the
Table 2, 11 patients (41%) treated with etanercept, 14 patients (52%) treated with adalimumab and 26 patients (96%) treated with infliximab reached PASI≥75. In general, a positive effect of treatment was recorded in 51 patients (63%) and no effect – in 30 patients (37%).
Comparative analysis of cytokine levels depending on the effectiveness of therapy with TNFα inhibitors
Statistical analysis of the cytokine levels before the therapy depending on the treatment effectiveness did not reveal significant differences between the study groups (
Table 3). At the end of week 16, there were no significant differences between the groups except TNFα levels (p = 0.045), which was more than 50 times higher in the group with no effect compared with patients with a positive effect of therapy.
Pairwise comparison before/after the treatment revealed significant changes in IL1b both in the total group (p<0.001) and ingroups with no effect and positive effect of therapy (p=0.002), as well as IL4 (p<0.001, p=0.005, p=0.005), IL17F (p<0.001, p<0.001, p<0.001), IL25 (p<0.001, p=0.003, p=0.017), IL31 (p<0.0016 p<0.001, p<0.001 ), sCD40L (p<0.001, p=0.006, p=0.022), VEGF (p<0.001, p=0.004, p<0.001) and TNFα (p<0.001, p<0.001, p<0.001), respectively (
Table 3).
Significant differences before/after therapy in the levels of IL20, ICAM1, IL22, IL23 were observed only in no effect group (p=0.010; p=0.011; p=0.016; p<0.001, respectively) (
Table 3).
A search for relationships between baseline levels of cytokines and the effectiveness of therapy
The development of a model to identify the relationship between baseline levels of cytokines and the effectiveness of therapy was performed on the next stage of the study. To search for multicollinearity, a preliminary analysis of correlations was carried out. There were 3 pairs of predictors with strong correlations (r>0.75): IL33 and IL4 (r=0.958, p<0.001), IL10 and IL4 (r=0.944, <0.001), IL33 and IL10 (r=0.889, p<0.001) which were excluded from subsequent analysis. The search for uninformative predictors that exhibited linear dependencies (carrying duplicate information) did not find any.
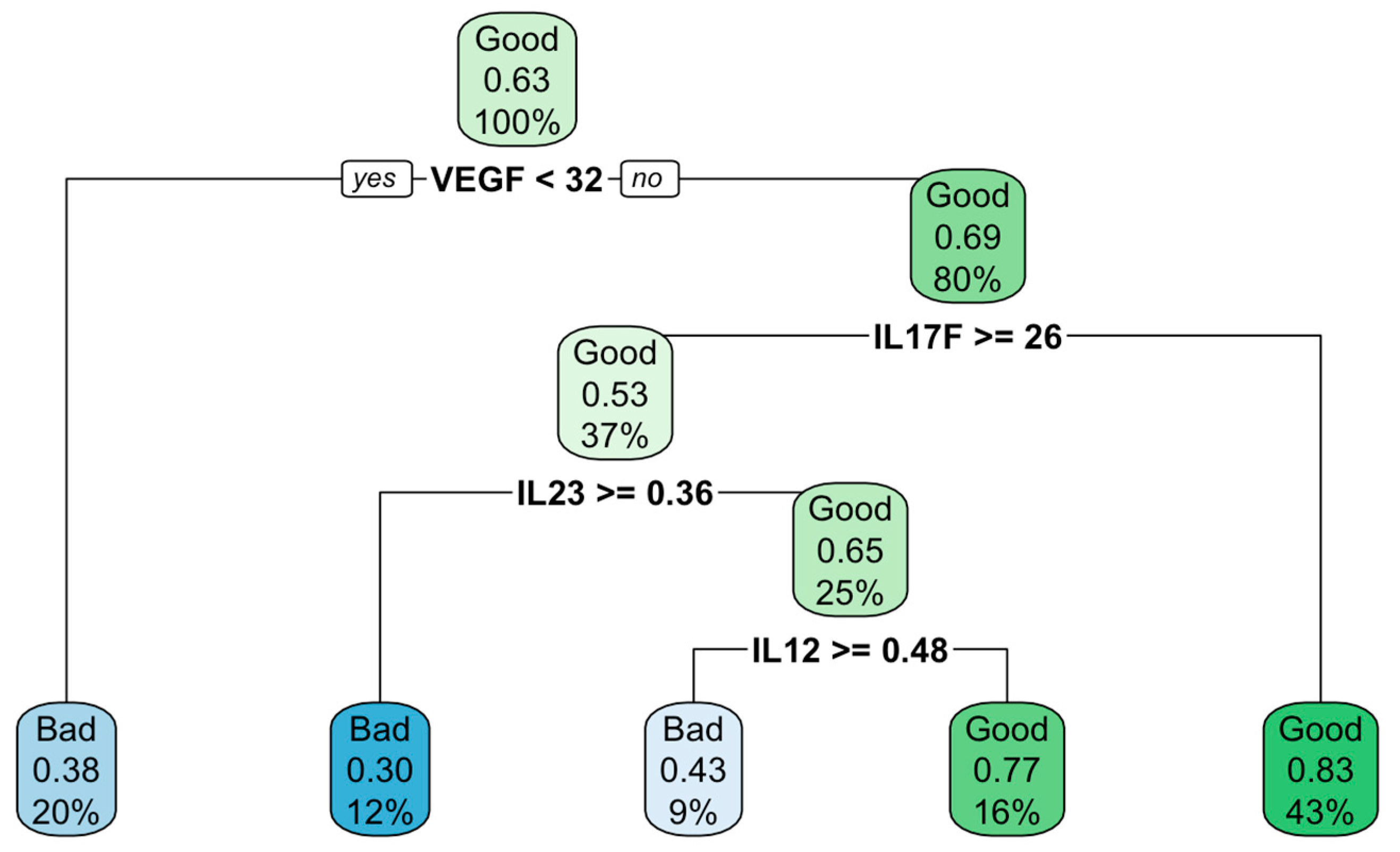
To search for predictors of treatment efficacy, a description of the available data was carried out using the classification and regression tree (CART) building based on a recursive breakdown of the entire amount of data. The result presented in
Figure 1 indicate that at the baseline level of VEGF ≥ 32 pg/ml in combination with IL17F<26pg/ml there was an 83% probability of the positive effect of treatment, whereas at VEGF < 32 pg/ml ml there was a 38% probability of the bad results.
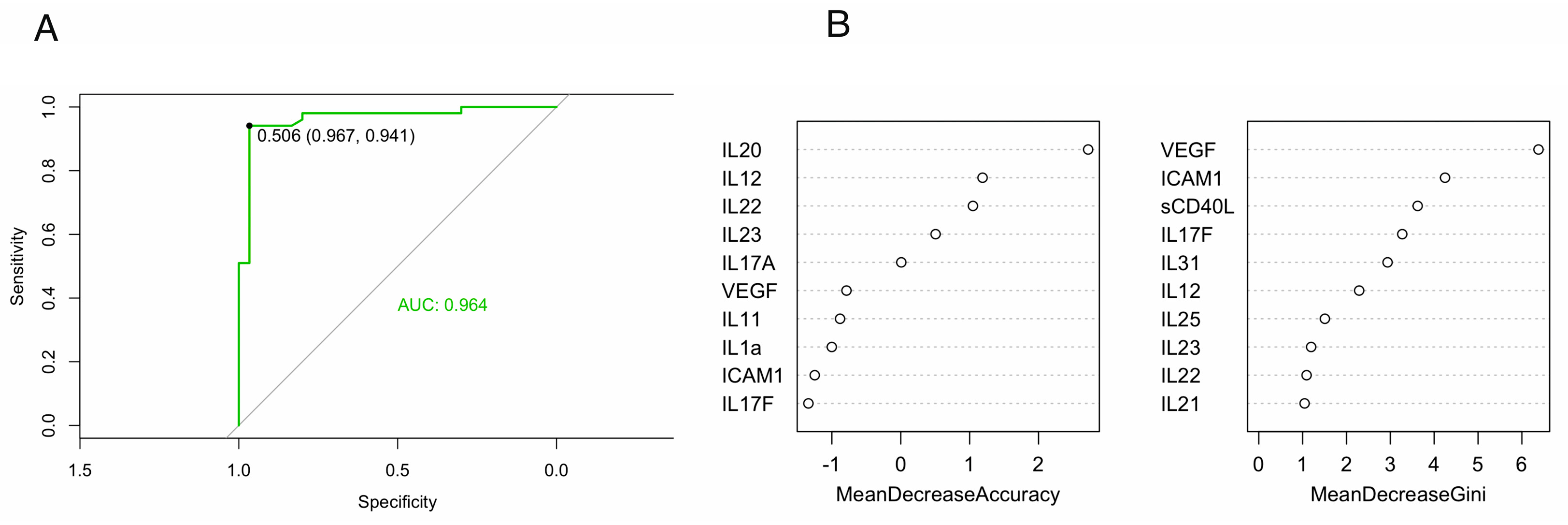
It should be noted that decision trees are sensitive to noise in the input data, subject to overfitting when used for prediction, and therefore, in order to more accurately predict the effectiveness of treatment based on baseline cytokines levels a more progressive random forests (RF) model was chosen. After data preprocessing, the entire dataset was divided into training (80%) and testing (20%) samples and the RF model was built. The metrics of the final model were: accuracy=0.750, specificity=0.700, sensitivity=0.833. The area under the ROC curve (AUC) was 0.964, which was considered as a good result (
Figure 2A) [
16].
Based on the results of RF model10 significant variables were obtained (
Figure 2B), where the most important predictors of treatment effectiveness are located at the top of the graph. As can be seen from
Figure 2B, the most important variables were IL20, IL12, IL22, IL23, IL17A based on Mean Decrease Accuracy and VEGF, ICAM1, sCD40L, IL17F, IL31 based on Mean Decrease Gini. Considering the fact that the repeated building the model revealed the variability of predictors estimated by Mean Decrease Accuracy, while maintaining constant those of variables estimated by Mean Decrease Gini, we considered plasma levels of VEGF, ICAM1, sCD40L, IL17F and IL31 to be truly important.
Comparative analysis of cytokine levels depending on the treatment effectiveness with etanercept, infliximab and adalimumab
On the next stage of the study a comparative analysis of cytokine levels based on the treatment effectiveness was performed for etanercept, infliximab and adalimumab separately. A summary of descriptive statistics by drug used and treatment effectiveness is presented in Supplementtary
Tables S1–S3. It should be pointed out that there was only one patient in infliximab treated group with no effect of therapy, which predetermined the data analysis being carried out only in positive effect group (n=26).
Comparison of baseline cytokine levels between patients treated with different TNFα inhibitors revealed differences in IL20 levels between patients received infliximab and etanercept (p=0.028) and in IL22 between infliximab and etanercept (p=0.036), infliximab and adalimumab (p=0.003) groups.
There was no effect of drug used, timepoint (before/after) and their interaction on the levels of IL10, IL17A, IL21, IL12 and IFNg. However, the drug type was found to be associated with changes in IL11 (p=0.034) and IL23 (p<0.001) despite no changes in these cytokines were found in subsequent before/after pairwise comparisons.
Changes in (↑)IL1b (p=0.012, p=0.036), (↓)IL4, (↓)IL17F (p=0.003, p=0.024), (↑)IL20, (↓) IL25 in total group and in no effect group were found in patients treated with etanercept (Supp.
Table 1). Pairwise comparisons showed a significant decrease in IL4 (p=0.004, p=0.045, respectively) to zero values from baseline to week 16; at the same time, IL20 levels significantly increased from zero to 7.64 pg/ml in the total group (p=0.014) and in no effect group (p=0.020). Comparison of IL25 levels revealed a significant decrease in the total group (p=0.003) and in no effect group (p=0.014). ICAM1 levels significantly increased by 30 and 33.3% in the total group (p=0.030) and in positive effect group (p=0.011) to week 16, respectively. Pairwise comparison before/after treatment revealed significant changes in IL31 and VEGF levels in all studied groups, respectively (6.1, 5.6, 16.2-fold decrease in IL31 (p<0.001, p=0.004, p=0.023) and 2.6, 2.8, 2.8-fold decrease in VEGF (p<0.001, p=0.046, p=0.003)). TNFα levels increased after treatment in all study groups from zero to 8.47, 9.2 and 8.19 pg/ml, respectively (p<0.001, p=0.001, p=0.005).
Treatment with adalimumab (Supp.
Table 2) resulted in a decrease in IL4 levels in positive effect group (p=0.014), and IL33 in the total group (p=0.003) and in positive effect group (p=0.022) from baseline levels to zero values. The levels of IL25 and VEGF after treatment significantly decreased to zero and by 9.7% only in the total group (p=0.017 and p=0.009, respectively). Pairwise comparison of IL31 and IL17F levels before/after the treatment revealed differences in all study groups (p<0.001, p=0.003, p=0.003 and p=0.001, p=0.014 and p=0.035, respectively). Differences before/after the treatment in TNFα, ICAM1 and sCD40L levels were shown for total group and no effect group: increase in TNFα (p=0.017 and p=0.025, respectively), 26.5 (p=0.009) and 48.1% increase in ICAM1 (p=0.014), and 2.36 (p=0.004) and 4.77-fold (p=0.012) decrease in sCD40L were revealed.
Finally, treatment with infliximab (Supp.
Table 3) resulted in significant changes in IL1b (p=0.008), IL17F (p=0.028), IL22 (p=0.001) IL31 (p=0.004), TNFα (p=0.012) and VEGF (p<0.001) in positive effect group. To week 16, the level of IL31 decreased by a factor of 3.6, level of VEGF by 42.5%, and IL22 dropped to zero. TNFα slightly increased after treatment; it was also significantly different from that in the etanercept-treated group at week 16 (p<0.001). The changes in IL4, IL20, IL25, IL33, ICAM1, sCD40L in the infliximab-treated group were not found.
Analysis of cytokine dynamics depending on the effectiveness of treatment
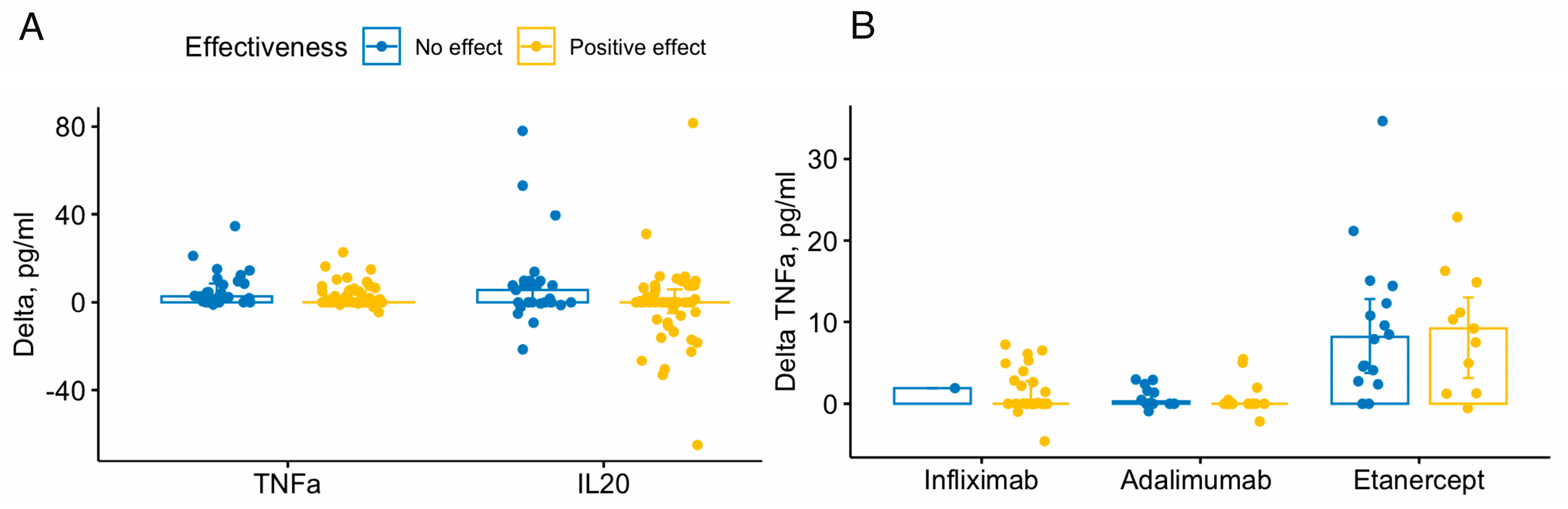
Analysis of cytokine dynamics revealed significant differences between the no effect and positive effect groups in delta TNFα (p=0.034) and IL20 (p=0.046) – the median delta values in no effect group were higher than those in positive effect group– 2.73 versus 0.45 for TNFα and 5.64 versus 0 for IL20 (
Figure 3A).
A comparative analysis of cytokine dynamics taking into account the drug used and the treatment effectiveness has revealed differences in TNFα delta between etanercept and adalimumab groups (p=0.002), etanercept and infliximab (p=0.002) in positive effect group, and between etanercept and adalimumab (p=0.002) in no effect group (
Figure 3B).
4. Discussion
The emergence of biological therapies has led to effective control of psoriasis [
4]. Our study involved 81 patients with moderate-to-severe psoriasis received targeted therapy with TNFα inhibitors for 16 weeks. Results of this study demonstrated positive effect of treatment in 51 (63%) and no effect in 30 patients (37%). The treatment with etanercept, adalimumab and infliximab showed a difference in the therapeutic efficacy, which is consistent with the literature data, since each of these TNFα inhibitors has a different structure and mechanism of action [
17,
18]. Etanercept is a fusion protein engineered from human receptors: it consists of the extracellular domain of human p75 TNF receptor linked to the Fc portion of human IgG1; it is structurally different from adalimumab and infliximab which are monoclonal antibodies against human TNFα. Scallon et al. state that etanercept is less immunogenic than infliximab due to formation of the unstable complexes with TNFα and, as a result, exhibits a less pronounced therapeutic effect [
19]. This fact was confirmed in our study: the effectiveness of etanercept among the three TNFα inhibitors studied was minimal and the 16-days incidence of therapeutic failure was 59%. It should be noted that etanercept is safest when used in elderly patients with severe psoriasis [
20].
Analysis of the cytokine levels and dynamics during treatment with TNFα inhibitors showed rather difficult to interpret results. The levels of TNFα were increased in the all studied groups to the end of week 16, especially in the no effect group – it was more than 50 times higher than that in positive effect group. This finding was also revealed when comparing deltas TNFα. Comparison of delta TNFα and the TNFα levels at week 16 revealed that the highest TNFα levels and delta were observed in patients treated with etanercept. This fact is consistent with the worst efficacy of etanercept in the present study and with existing data on the instability of its complexes with TNFα and lower efficacy compared to other drugs [
19]. There was also higher delta IL20 in patients with no effect compared with patients with a positive effect of therapy. In the current concept of psoriasis immunopathogenesis, immune cells (keratinocytes, pDCs, macrophages, NKT cells) under the action of triggers start to produce INFγ, TNFα, IL1β and IL6, which activate mDC [
21]. Those, in turn, stimulate the differentiation and proliferation of different types of T1, T17 and T22 lymphocytes. The pathogenetic TNFα - IL23/IL17 axis leads to increased production of IL22 and IL17A/F, affects keratinocytes proliferation and differentiation, cause characteristic psoriatic lesions [
21,
22]. The IL20 belongs to the IL-10 family with IL22 and possess proinflammatory properties. It was shown that its expression increased in lesional psoriatic skin [
23]. Consequently, higher delta IL20 levels in patients with no effect of therapy do not contradict this concept.
An analysis of the baseline level of cytokines using the CART method showed that that at the baseline level of VEGF≥32pg/ml and IL17F<26pg/ml there was an 83% probability of the positive effect of TNFα inhibitors therapy. VEGF is a key factor in angiogenesis and its overexpression and an increase in blood in psoriasis has been described in a lot of studies [
24,
25]. Angiogenesis disorders play an important role in the pathogenesis of psoriasis in addition to disturbances in keratinocyte function and recruitment of immune cells – they are characterised by expansion, tortuosity, and increased permeability of capillaries in the dermal papilla [
26]. The VEGFA gene located in the PSORS1 locus is characterised by high polymorphism and is associated with the risk of psoriasis development [
27,
28] and its early manifestation up to 40 years [
29]. Moreover, VEGFA inhibitors are indicated as one of the new promising drugs for the treatment of psoriasis [
30]. The revealed decrease in VEGF after the therapy is consistent with the available data on the improvement in the condition of the vascular network after treatment with etanercept [
31] and infliximab [
32]. It should be noted that together with a wide heterogeneity of psoriasis pathogenesis, vascular growth and VEGF activity are may be the most universal mechanism; in present study, the non-zero levels of VEGF were determined in 100% of plasma samples, which also confirms its diagnostic value. VEGF is associated with other cytokines and factors playing role in the pathogenesis of psoriasis. It is assumed that it is able to increase the expression of ICAM-1, in the K14-VEGF transgenic mouse model the use of a potent VEGF antagonists caused normalisation of increased ICAM1expression in basal keratinocytes and the vasculature [
33]. In a study by Wen et al., the use of gambogic acid (which is an inhibitor of angiogenesis) on the K14-VEGF transgenic model resulted in a significant decrease in both the expression of VEGF receptor 2 and it signalling pathway [
34]. In our opinion, the observed increase in ICAM-1 levels in no effect group (
Table 3) may reflect the lower rate of ICAM-1 decline compared with positive effect group –TNFα inhibitors can simultaneously reduce ICAM-1 levels while reducing VEGF. Moreover, ICAM-1 did not differ from the baseline in positive effect group which indicates an effective inhibition of the VEGF and ICAM-1 production in these patients.
The mentioned data were also confirmed by significant variables selected in the RF model for prediction of the effectiveness of TNFα inhibitors therapy. According to the literature data, there is no clear understanding in the selection of important predictors when building an RF model, and each of the methods has its drawbacks. Many biological studies analyse rather small datasets, so it is often advised to use the mean decrease Gini, since too detailed results will be obtained with small samples when calculating the mean decrease accuracy using “out-of-bag (OOB) samples” [
35]. For example, Yu et al. used the mean decrease Gini to evaluate imaging biomarkers in predicting the stage of non-small cell lung cancer [
36]. In a study by Lloyd et al., this index was also used to predict effective biomarkers of recent and remote infections with
Mycobacterium tuberculosis [
37]. At the same time, in the metabolomic analysis of the dogs’ sera with type I diabetes mellitus, the authors used a mean decrease accuracy, despite the small sample size [
38]. In the present study, an analysis of the RF model data showed differences in the features selection for predictors of treatment effectiveness depending on whether the mean decrease accuracy or mean decrease Gini was used for ranking. However, as noted in the Results section, we found variability in the predictors examined by mean decrease accuracy. Therefore, we defined VEGF, ICAM1, sCD40L, IL17F and IL31 as important predictors of treatment efficiency. Interestingly, despite the differences in delta IL20, this cytokine was not included in the top 10 predictors.
Also, the levels of CD40L, IL17F and IL31 which were selected as important predictors decreased from baseline after the treatment independently of treatment effectiveness and did not differ between the drugs used. In a study by Venerito et al., a significant decrease in sCD40L levels was observed in patients with psoriatic arthritis treated with ampremilast; authors concluded that it can predict clinical response to ampremilast therapy [
39]. In our previous study, the levels of sCD40L and IL31 (as well as IL23 and IL25) in lesional skin were significantly reduced by weeks 14 and 26 of treatment with ampremilast, while other cytokines first decreased at week 14 and then inexplicably increased [
40]. IL17F is undoubtedly an important link in the pathogenesis of psoriasis as part of the TNFα - IL23/IL17 axis [
17], and its decrease during therapy with TNFα inhibitors is a logical consequence of the suppression of TNFα action.
The limitation of this study was the limited number of patients, which did not allow to identify possible differences when comparing the drugs used. Also, the analysis was complicated by a large number of patients with multiply cytokines below the detection limits.
5. Conclusions
Finally, following conclusions can be drawn from this study:
The treatment with TNFα inhibitors significantly changes almost all plasma cytokines, where the analysis of the cytokine dynamics showed rather difficult to interpret results.
Pairwise comparisons of cytokine levels before/after treatment showed significant changes in IL1b, IL4, IL17F, IL25, IL31, sCD40L, VEGF and TNFα levels appears to be independent of the effect of therapy. However, a change in the levels of IL20, ICAM1, IL22, IL23 was shown only in patients with no effect of therapy; moreover, when comparing cytokine deltas, differences were revealed only for delta IL20 and TNFα.
At the end of week 16, there were no significant differences between the groups except TNFα levels which was more than 50 times higher in the no effect group compared with the positive effect group; this was also confirmed when comparing deltas.
Analysis of the data using the CART method revealed that at the baseline level of VEGF ≥ 32 pg/ml and IL17F < 26 pg/ml, there was an 83% probability of the positive effect.
Prediction of treatment response using RF model showed the importance of baseline VEGF, ICAM1, sCD40L, IL17F and IL31 levels. This fact allows us to consider these cytokines as possible predictors of treatment efficiency with TNFα inhibitors. However, more data and further studies are required to clarify and confirm the results obtained.6.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org., Table S1, Plasma cytokines in patients with moderate-to-severe psoriasis treated with etanercept (Enbrel); Table S2, Plasma cytokines in patients with moderate-to-severe psoriasis treated with adalimumab (Humira); Table S3, Plasma cytokines in patients with moderate-to-severe psoriasis treated with infliximab (Remicade).
Author Contributions
A.A.K. and A.E.K. conceptualized the study; A.E.K. and D.G.D. developed the methodology, provided resources, and investigated the study; A.A.N and E.R.N. did formal analysis, provided software, and visualized the study; A.A.N. and O.A.O. validated the study; A.E.K., A.A.V. and L.F. Z. curated the data; A.A.N. and E.R.N. wrote the original draft; A.E.K. and D.G.D. reviewed and edited the manuscript; A.A.K. supervised the study; A.E.K. did project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of State Research Center of Dermatovenereology and Cosmetology (protocol №04, 27/04/2018).” for studies involving humans. OR “The animal study protocol was approved by the Institutional Review Board (or Ethics Committee) of NAME OF INSTITUTE (protocol code XXX and date of approval).” for studies involving animals. OR “Ethical review and approval were waived for this study due to REASON (please provide a detailed justification).” OR “Not applicable” for studies not involving humans or animals.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The dataset analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors wish to express their sincere gratitude to the patients participating in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ten Bergen LL, Petrovic A, Krogh Aarebrot A, Appel S. The TNF/IL-23/IL-17 axis-Head-to-head trials comparing different biologics in psoriasis treatment. Scand J Immunol. 2020, 92, e12946. [Google Scholar] [CrossRef] [PubMed]
- Leone GM, Mangano K, Petralia MC, Nicoletti F, Fagone P. Past, Present and (Foreseeable) Future of Biological Anti-TNF Alpha Therapy. Journal of Clinical Medicine. 2023, 12, 1630. [Google Scholar] [CrossRef] [PubMed]
- Jiraskova Zakostelska Z, Reiss Z, Tlaskalova-Hogenova H. et al. Paradoxical Reactions to Anti-TNFα and Anti-IL-17 Treatment in Psoriasis Patients: Are Skin and/or Gut Microbiota Involved? Dermatol Ther (Heidelb). 2023, 13, 911–933. [Google Scholar] [CrossRef] [PubMed]
- Warren RB, Gooderham M, Burge R. et al. Comparison of cumulative clinical benefits of biologics for the treatment of psoriasis over 16 weeks: Results from a network meta-analysis. J Am Acad Dermatol. 2020, 82, 1138–1149. [Google Scholar] [CrossRef]
- Wong U, Cross RK. Primary and Secondary Nonresponse to Infliximab: Mechanisms and Countermeasures. Expert Opin. Drug Metab. Toxicol. 2017, 13, 1039–1046. [Google Scholar] [CrossRef]
- Fine S, Papamichael K, Cheifetz AS. Etiology and Management of Lack or Loss of Response to Anti-Tumor Necrosis Factor Therapy in Patients With Inflammatory Bowel Disease. Gastroenterol Hepatol. 2019, 15, 656–665. [Google Scholar]
- 7. Lauffer F, Eyerich K. Eczematized psoriasis – a frequent but often neglected variant of plaque psoriasis. JDDG: Journal der Deutschen Dermatologischen Gesellschaft. 2023; 1–9. [CrossRef]
- Bystrom J, Clanchy FI, Taher TE. et al. Response to Treatment with TNFα Inhibitors in Rheumatoid Arthritis Is Associated with High Levels of GM-CSF and GM-CSF+ T Lymphocytes. Clin Rev Allergy Immunol. 2017, 53, 265–276. [Google Scholar] [CrossRef]
- Magee C, Jethwa H, FitzGerald OM, Jadon DR. Biomarkers Predictive of Treatment Response in Psoriasis and Psoriatic Arthritis: A Systematic Review. Ther Adv Musculoskelet Dis. 2021, 13, 1759720X2110140. [Google Scholar] [CrossRef]
- Andersen CSB, Kvist-Hansen A, Siewertsen M. et al. Blood Cell Biomarkers of Inflammation and Cytokine Levels as Predictors of Response to Biologics in Patients with Psoriasis. Int J Mol Sci. 2023, 24, 6111. [Google Scholar] [CrossRef]
- Romero IB, Puchades AM, Pibernat MR. et al. Criteria used to define tumor necrosis factor-alpha inhibitors failure in patients with moderate-to-severe psoriasis: A systematic literature review. Annals of Medicine. 2023, 55, 1335–1345. [Google Scholar] [CrossRef]
- R Core Team. R version 4.2. 2 (2022–10-31 ucrt) --” Innocent and Trusting”: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, 2022. URL https://www. R-project. org.
- Noguchi K, Gel YR, Brunner E & Konietschke F. nparLD: An R software package for the nonparametric analysis of longitudinal data in factorial experiments. Journal of Statistical Software. 2012, 50, 1–23. [Google Scholar]
- Shitikov VK & Mastitsky, SE. Classification, regression, Data Mining algorithms using R. 2017. https://github.com/ranalytics/data-mining [in Russian].
- Strobl C, Boulesteix AL, Kneib T, Augustin T & Zeileis A. Conditional variable importance for random forests. BMC bioinformatics. 2008, 9, 1–11. [Google Scholar]
- Ling CX, Huang J, & Zhang H. AUC: A better measure than accuracy in comparing learning algorithms. In Advances in Artificial Intelligence: 16th Conference of the Canadian Society for Computational Studies of Intelligence, AI 2003, Halifax, Canada, –13, 2003, Proceedings 16 (pp. 329-341). SpringerBerlinHeidelberg. 11 June.
- Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: Clinical features and possible immunopathogenesis. Semin Arthritis Rheum. 2010, 40, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Fiorino G, Danese S, Pariente B, Allez M. Paradoxical immune-mediated inflammation in inflammatory bowel disease patients receiving anti-TNF-alpha agents. Autoimmun Rev. 2014, 13, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, T.; Mitoma, H.; Harashima, S.-i.; Tsukamoto, H.; Shimoda, T. Transmembrane TNF-: Structure, Function and Interaction with Anti-TNF Agents. Rheumatology 2010, 49, 1215–1228. [Google Scholar] [CrossRef]
- Brownstone ND, Hong J, Mosca M et al. Biologic treatments of psoriasis: An update for the clinician. Biologics. 2021, 15, 39–51. [Google Scholar]
- Vičić M, Kaštelan M, Brajac I, Sotošek V, Massari LP. Current Concepts of Psoriasis Immunopathogenesis. Int J Mol Sci. 2021, 22, 11574. [Google Scholar] [CrossRef]
- Chiricozzi A, Romanelli P, Volpe E, Borsellino G, Romanelli M. Scanning the immunopathogenesis of psoriasis. Int J Mol Sci. 2018, 19, 179. [Google Scholar] [CrossRef]
- Wei, C. C.; Chen, W. Y.; Wang, Y. C.; Chen, P. J.; Lee, J. Y. Y.; Won Wong, T. W. . & Lin, Y. C. Detection of IL-20 and its receptors on psoriatic skin. Clinical Immunology 2005, 117, 65–72. [Google Scholar]
- Pritulo OA, Petrov AA, & Maraqa MYN. Clinical significance of plasma markers of angiogenesis in patients with psoriasis. Russian Journal of Skin and Venereal Diseases. 2022, 25, 409–418. [Google Scholar]
- Marina ME, Roman II, Constantin AM, Mihu CM & Tătaru AD. VEGF involvement in psoriasis. Clujul medical. 2015, 88, 247. [Google Scholar]
- Barton SP, Abdullah MS. & Marks R. Quantification of microvascular changes in the skin in patients with psoriasis. British journal of Dermatology. 1992, 126, 569–574. [Google Scholar]
- Sudhesan A, Rajappa M, Chandrashekar L. et al. Vascular endothelial growth factor (VEGF) gene polymorphisms (rs699947, rs833061, and rs2010963) and psoriatic risk in South Indian Tamils. Human immunology. 2017, 78, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Bozduman T, Evans SE, Karahan S. et al. Genetic risk factors for psoriasis in Turkish population:-1540 C/A,-1512 Ins18, and+ 405 C/G polymorphisms within the vascular endothelial growth factor gene. Annals of Dermatology. 2016, 28, 30–39. [Google Scholar] [CrossRef]
- Young HS, Bhushan M, Griffiths CE, Summers AM. & Brenchley PE. Single-nucleotide polymorphisms of vascular endothelial growth factor in psoriasis of early onset. Journal of investigative dermatology. 2004, 122, 209–215. [Google Scholar]
- Luengas-Martinez A, Paus R. & Young HS. A novel personalized treatment approach for psoriasis: Anti-vascular endothelial growth factor-A (VEGF-A) therapy. British Journal of Dermatology. 2022, 186, 782–791. [Google Scholar]
- Campanati A, Goteri G, Simonetti O. et al. Angiogenesis in psoriatic skin and its modifications after administration of etanercept: Videocapillaroscopic, histological and immunohistochemical evaluation. International Journal of Immunopathology and Pharmacology. 2009, 22, 371–377. [Google Scholar] [CrossRef]
- Cordiali-Fei P, Trento E, D’Agosto G. et al. Effective therapy with anti-TNF-alpha in patients with psoriatic arthritis is associated with decreased levels of metalloproteinases and angiogenic cytokines in the sera and skin lesions. Ann N Y Acad Sci. 2007, 1110, 578–589. [Google Scholar] [CrossRef]
- Xia YP, Li B, Hylton D. et al. Transgenic delivery of VEGF to mouse skin leads to an inflammatory condition resembling human psoriasis. Blood. 2003, 102, 161–168. [Google Scholar] [CrossRef]
- Wen J, Pei H, Wang X et al. Gambogic acid exhibits anti-psoriatic efficacy through inhibition of angiogenesis and inflammation. Journal of Dermatological Science. 2014, 74, 242–250. [Google Scholar] [CrossRef]
- Archer KJ & Kimes, RV. Empirical characterization of random forest variable importance measures. Computational statistics & data analysis. 2008, 52, 2249–2260.
- Yu L, Tao G, Zhu L. et al. Prediction of pathologic stage in non-small cell lung cancer using machine learning algorithm based on CT image feature analysis. BMC cancer. 2019, 19, 1–12. [Google Scholar]
- Lloyd T, Steigler P, Mpande CA. et al. Multidimensional analysis of immune responses identified biomarkers of recent Mycobacterium tuberculosis infection. PLoS Computational Biology. 2021, 17, e1009197. [Google Scholar]
- O’Kell AL, Garrett TJ, Wasserfall C & Atkinson MA. Untargeted metabolomic analysis in naturally occurring canine diabetes mellitus identifies similarities to human Type 1 Diabetes. Scientific Reports. 2017, 7, 9467. [Google Scholar] [CrossRef] [PubMed]
- Venerito V, Natuzzi D, Bizzoca R. et al. Serum sCD40L levels are increased in patients with psoriatic arthritis and are associated with clinical response to apremilast. Clinical and Experimental Immunology. 2020, 201, 200–204. [Google Scholar] [CrossRef]
- Kubanov AA, Solomka VS, Karamova AE. et al. The effect of apremilast therapy on skin cytokine levels in patients with psoriasis. Russian Open Medical Journal. 2020, 9, 310–310. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).