1. Introduction
Neurological impairments such as stroke, multiple sclerosis and cerebral palsy, represent a major public health issue worldwide (WHO report 2007). Motor impairments associated with these pathologies, and more specifically spasticity, are associated with loss of mobility and social participation [
2]. Spasticity is defined as a motor disorder characterized by a velocity-dependent increase in the tonic stretch reflexes (muscle tone) with exaggerated tendon jerks, resulting from hyperexcitability of the stretch reflexes as one component of the upper motor neuron (UMN) syndrome (Lance, 1980). Intramuscular botulinum toxin injection is a first-line treatment guidelines for the management of spasticity in adult patients [
3]. Toxin injections help to reduce disturbing muscle hypertonia, and consequently improve functional capacities, relieve pain related to spasticity, enhance hygiene gesture capabilities, and in fine, improve quality of life [
3,
4]. While the literature provides strong evidence on the clinical benefits of botulinum toxin, the injection procedure is associated with procedural pain and anxiety [
5,
6,
7].
Several factors influence procedural pain, such as the use of an anesthetic or not at the injection site [
8], or method of injection site location (ultrasound or electrostimulation) [
6,
9]. Regardless of the type of procedures, pain is still reported by patients [
9], and repetitions of injection exacerbate pain symptoms [
10]. Ultimately, pain could lead to discontinuation of the injection process, representing a loss of chance for the patient [
11]. In this context, pain should be managed in view of improving treatment adherence and optimizing therapy.
In first intention, pharmacological approaches, such as local anesthetic cream of Lidocaine/Prilocaine (EMLA®) or systemic therapeutics like MEOPA and midazolam, showed clinical efficacy in relieving pain and discomfort during toxin botulinum injection [
3,
11,
12,
13,
14,
15,
16]. However, these drugs have been associated with adverse effects including sleepiness, nausea and dizziness [
14,
17].
As a non-pharmacological complementary, innovative technology such as Virtual Reality (VR) has recently been introduced in different departments to manage pain (in acute, chronic and experimental settings) [
1,
18,
19,
20,
21]. VR is defined by a computerized system that creates a virtual environment where a person undergoes an immersive sensory experience with an enriched environment involving augmented multiple sensory feedbacks (auditory, visual, tactile VR enriched environment) [
22]. VR has the advantage of being easy to use, quick to set up, accessible with very short training, not requiring any supplementary staff and having few non-serious undesirable effects (0 to 8% of nausea and dizziness)[
23]. The immersive environment is reinforced by combining audio guidance with display of an appeasing visual scene. VR has proven its effectiveness in management of procedural pain[
24], particularly pain associated with venipuncture [
23,
25]. In a retrospective chart review, Chau et al. [
26] showed the feasibility of using VR during botulinum toxin injections in 14 pediatric patients, and reported benefits in management of procedural pain [
26]. In adults, VR also seems to offer advantages in some hospital settings, even in other types of injections, regarding pain, anxiety and anger management, related to the distraction provided by this technology[
27,
28,
29]. However, the effects and feasibility of VR during intramuscular injection of botulinum toxin in adults presenting with spasticity have yet to be determined.
The main objective of our study was to assess VR efficacy in management of procedural pain during intramuscular injections of botulinum toxin in adult patients presenting with spasticity. Based on the literature, we hypothesized that in comparison with non-VR, VR would induce a decrease in procedural pain. The secondary objectives were to determine the potential efficacy of VR to alleviate anxiety and to assess level of patient satisfaction.
2. Materials and Methods
2.1. Study Design
This is a retrospective study conducted in the Physical Medicine and Rehabilitation Department of the University Hospital of Poitiers between February and August 2022. Data collection was conducted according to the guidelines of the Declaration of Helsinki and the French Data Protection Authority (CNIL, MR-004). All data collection was declared to Health Data Hub (number F20220719114034). All participants received a non-opposition form and thus agreed that their data was used for research purposes.
2.2. Inclusion and Exclusion Criteria
To be included, patients had to be over 18 years old, to have undergone botulinum toxin injection in their care pathway, to present with focal spastic hypertonia in at least 1 muscle of the upper or lower limbs justifying the use of botulinum toxin, and to be able to provide answers with no cognitive disease for evaluating pain intensity and level of anxiety. All contraindications to toxin botulinum injection (myasthenia, pregnancy, breast-feeding, hypersensitivity to one of the substances in the product, infection at the injection site) and any pathological condition not allowing optimal use of the virtual reality helmet (blindness, major visual acuity disorders, deafness) were not included. Patients who were unable to retain the virtual reality headset during the procedure (appearance of adverse effects, patient wishing to stop during the procedure) were excluded from the study.
2.3. Procedure
The patient was informed about the procedure of VR utilization and consented to wear the device. The patient was comfortably seated on the examination table. The VR device (HYPNOVR,
https://hypnovr.io/fr/produits/hypnovr/) combined with headset (TaoTronic, model TT-BH085, reference 6972103466158) was set on the patient as comfortably as possible, and a movie showing calm visual environments (walking on the beach, diving among colored fishes, or traveling in space) combined with relaxing music was displayed. The VR program consisted of a 2-minutef induction phase (before injection), 10-20 minutes of the main VR pathway (during injection), and a 2-minute exit phase. Data were collected before and after the procedures.
2.4. Outcomes
Pain intensity, considered as the primary endpoint, was determined using a numerical rating scale (NRS) ranging from 0 (no pain) to 10 (maximum imaginable pain)[
30,
31]. The patient reported the pain experienced during previous injections without VR before injection and the pain experienced in the current procedure with VR after the end of the procedure. In addition, perceived improvement was determined by the patient as a percentage of added value of VR on pain intensity compared to procedure without VR.
Level of anxiety, related to previous injection without VR and current injection with VR, was determined with a numerical anxiety rating scale from 0 (no anxiety) to 10 (maximum imaginable anxiety)[
32]. In addition, perceived improvement was determined by the patient as a percentage of added value of VR on level of anxiety compared to procedure without VR.
Level of satisfaction was determined using an 11-point scale ranging from 0 (not satisfied at all) to 10 (very satisfied) and asking if they agreed to reuse VR for the subsequent toxin botulinum injection, and if they would recommend VR to other patients.
The muscles targeted for injection, the method of localizing injection sites (ultrasound or electrostimulation), analgesic medication intake, use of additive analgesic for toxin injection, time of the day, and occurrence of adverse events were collected.
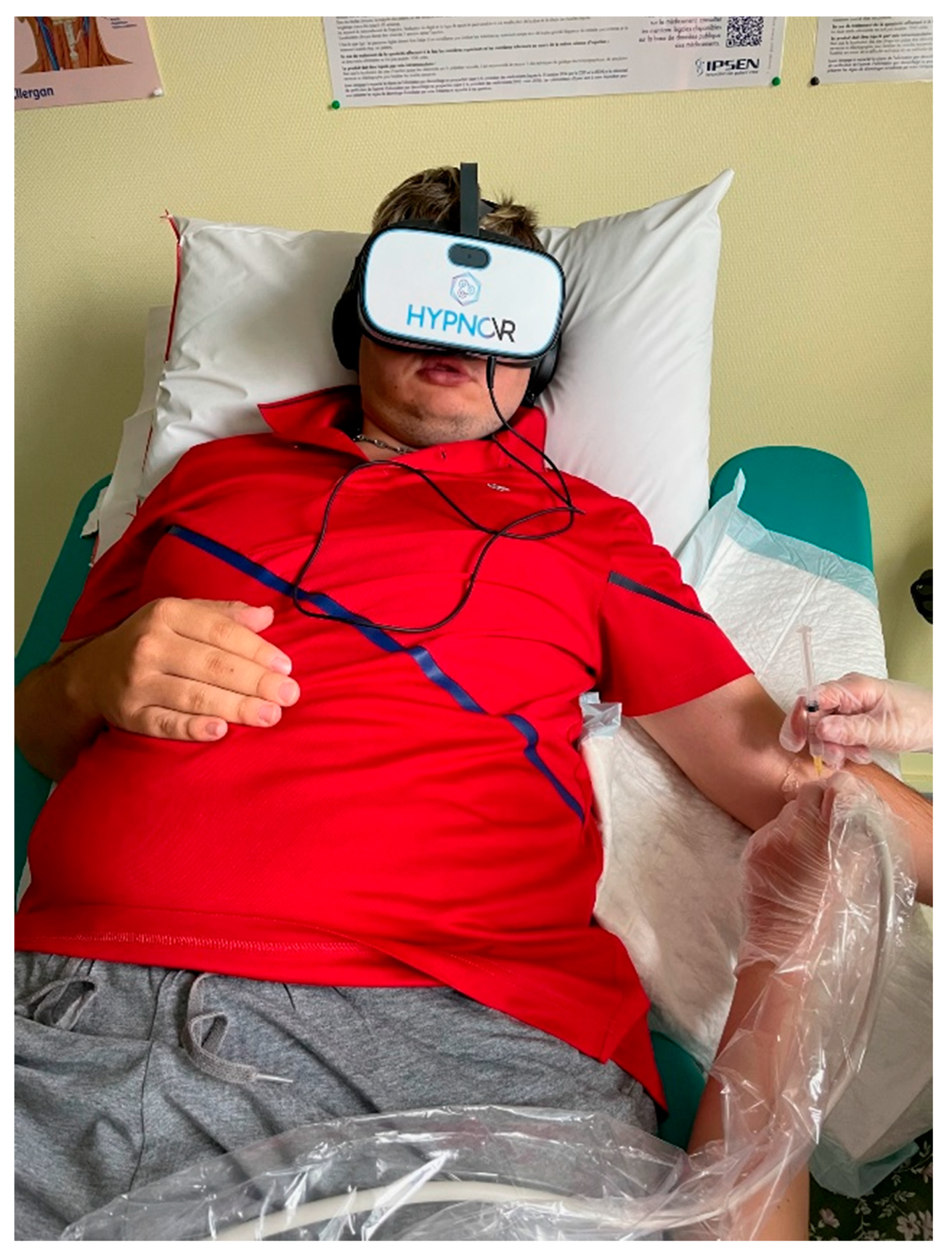
Figure 2.
Patient with virtual reality headset.
Figure 2.
Patient with virtual reality headset.
2.5. Statistical Analysis
The study population was described by age, sex, disease, and baseline pain intensity. Quantitative variables were described by either mean and standard deviation or median and interquartile range depending on data normality. Categorical variables were described by numbers and percentages. Normality was verified using the Shapiro-Wilk test.
Pain intensity NRS and level of anxiety during the procedure with and without VR were compared using a Wilcoxon signed-rank test (paired test) since the variable was not normally distributed.
Mean and standard deviation of perceived improvement using VR and satisfaction with VR were also reported. R software version 4.2.0 was used for the analyses. All statistical tests were two-sided and the significance threshold was fixed at 0.05.
3. Results
3.1. Participants
Twenty-one patients were identified over the 7-month inclusion period. Four patients were excluded, three due to incomplete data collection and one due to cybersickness occurrence with VR[
33]. All in all, 17 patients were included and analyzed.
Patient characteristics are presented in
Table 1. The mean participant age was 49.9 ± 10.6 years, with 9 females (53%). Spasticity was subsequent to stroke for 9 (52.9%) patients, cerebral palsy for 3 (17.6%), multiple sclerosis for 2 (11.8%), cervico-arthrosic myelopathy for 1 (5.9%), hereditary spastic paraplegia for 1 (5.9%), and meningitis for 1 (5.9%). One patient received MEOPA and 1 patient EMLA®. One patient was treated with long-term TRAMADOL.
On average, 5.4 muscles were targeted per person, and the median of injected muscles was 5. The different injection sites are presented in
Table 2 and
Table 3.
3.2. Primary and secondary outcomes
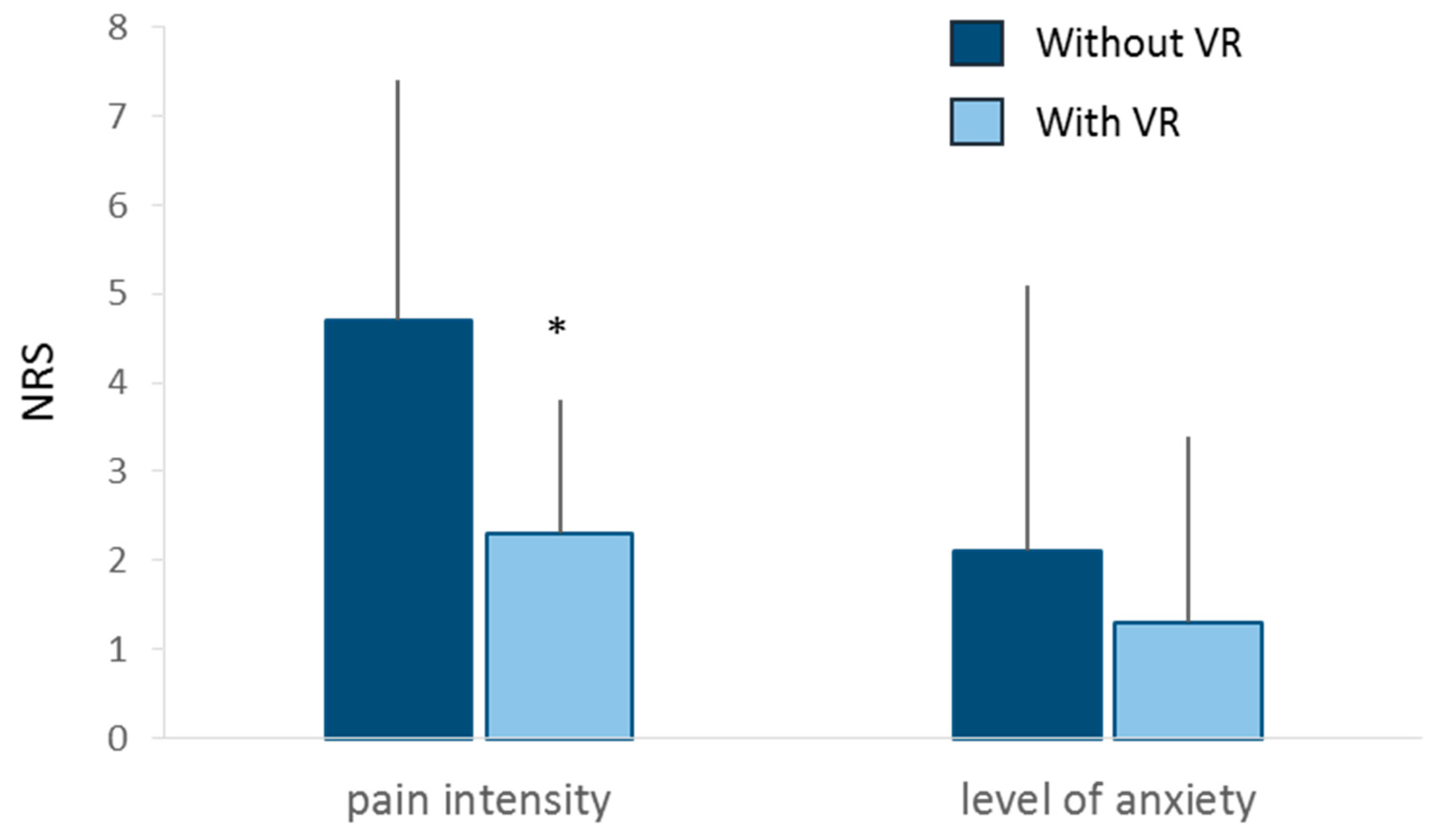
Pain intensity was significantly lower during injection procedure with VR (2.3 ± 1.5) than without VR (4.3 ± 2.7, p=0.014) (
Figure 4 and
Table 4). The proportion of responding patients was 76%
Level of anxiety NRS was not significantly different between injection with (1.3 ± 2.1) and without VR (2.1 ± 3.0, p=0.054) (
Figure 4 and
Table 4). The proportion of responding patients was 29%.
Patients reported a mean subjective impression of improvement of 39.7 ± 30.9% for pain and 21.5 ± 25.0% for anxiety during the procedure with VR. Patients’ mean overall satisfaction was 7.9 ± 1.6 out of 10 (
Table 2).
Regarding adverse events, only 1 patient (5.9%) experienced cyberkinetosis.
Fifteen patients (88.2%) agreed to reuse VR for the subsequent toxin botulinum injection, and all patients (100%) would recommend VR to other patients (
Table 2).
4. Discussion
The objective of this study was to assess VR efficacy in reducing procedural pain and anxiety during toxin botulinum injection in adults presenting with spasticity. We showed that VR was able to significantly decrease procedural pain. Patients were very satisfied with use of VR during the injection and agreed to reuse the VR helmet and recommend this approach for other patients.
In a systematic review including 18 studies, Smith et al. [
23] reported that 12 studies demonstrated that VR led to significant pain reduction during painful procedure for burns, wounds, or injection . Similarly, in a systematic review with meta-analysis, Mallari et al. [
34] showed that VR could reduce acute procedural pain in adults. More specifically for botulinum injection procedure, using the Face, Legs, Activity, Cry, Consolability (FLACC) test for pain assessment, Chau et al. [
26] reported a median score of 2.5 in 14 children treated for spasticity. In an adult population, we reported a pain intensity score of 2.3 with VR. Although previous research did not focus on procedural pain for Botulinum toxin in adults, our study suggests that a non-invasive VR device can easily improve procedural injection, lasting every 3 months and may ultimately enhance therapeutic adherence.
The main principle of VR is to provide strong and sufficient distraction to redirect attention initially focused on pain to a calm environment[
35]. Thereby, VR can effectively modify sensory perceptions such as pain by monopolizing a high amount of attentional resources [
39]. By having attention compete between VR environment and pain, Rutter et al. [
40] reported that VR led to maintenance of an increased pain threshold and pain tolerance over 8 weeks (8 testing sessions) in 28 healthy participants during the cold-pressor task. In the current study, VR was applied with an immersive device providing high degree of immersion in a specific peaceful environment during one session of Botulinum toxin injection [
41,
42,
43]. While VR successfully managed chronic pain [
44], the long-term and sustainable effect of VR in Botulinum toxin injection in adults remains to be determined. In addition, combining hypnosis with VR could potentiate the effect of VR in managing procedural pain [
45,
46,
47,
48,
49], and should be investigated in the future.
Although a positive effect of VR on anxiety has been reported in the literature [
35,
50], our results showed only a trend toward decrease. That said, while we failed to observe a strong effect on anxiety, it safe to assume that the level of anxiety at baseline (2.1) was too low for it to be significantly reduced in a population having already undergone several botulinum toxin injection procedures. Investigation of a population of patients receiving their very first injection would probably yield significantly decreased procedural anxiety in Botulinum toxin injection in adult populations.
Patients in the current study were very satisfied with VR (7.9 out of 10) and would agree to reuse VR for their next injection (88%) and to recommend VR for other patients (100%). Smith et al. [
23] highlighted rare adverse events (8-10%) which are consistent with the single case of cybersickness observed in our study. The side effects observed during VR are considered as transient and reversible, making VR a safe approach to manage procedural pain in neurologic populations[
33]. In addition, VR might be considered as a valuable alternative to medical therapies insofar as, in comparison with pharmacological management, it does not necessitate additional practitioners and cost other than the VR device itself. A medico-economic study should be conducted to validate this hypothesis.
This pilot study is associated with limitations. While the retrospective design in clinical routine provides real world, data it entails potential bias connected with declarative assessment; what is more, the results were not compared with those of a parallel control group. The long-term added value of VR in Botulinum toxin injection remains to be determined in a randomized controlled trial.
5. Conclusions
Our study showed that VR was useful for management of pain related to botulinum toxin injection in adults, as patients were very satisfied with the device. In addition, they agreed to reuse VR for their next injection and to recommend this approach to other patients presenting with spasticity. While VR should be considered as an alternative treatment option to pharmacological approaches in Botulinum toxin injection a prospective randomized controlled trial with long-term follow-up and cost-effectiveness analysis is still required.
Author Contributions
Conceptualization, Romain DAVID, Maxime BILLOT, Solène DUVAL, Alexis DUMAS; methodology, Romain DAVID, Maxime BILLOT and Alexis DUMAS; formal analysis, Amine OUNAJIM; investigation, Romain DAVID, Alexis DUMAS, Valentine GILQUIN, Laura MAININI, Rémi CABIROL, Emmanuel AMAUGER, Anne JOSSART; data curation, Alexis DUMAS and Amine OUNAJIM; writing—original draft preparation, Alexis DUMAS, Romain DAVID and Maxime BILLOT; writing—review and editing, Etienne OJARDIAS, Solène DUVAL, Valentine GILQUIN, Laura MAININI, Rémi CABIROL, Emmanuel AMAUGER, Anne JOSSART, Amine OUNAJIM, Anaick PERROCHON, Carlos LUQUE-MORENO, Frédéric VISEUX, Maarten MOENS, Lisa GOUDMAN, Philippe RIGOARD; visualization, Romain DAVID, Alexis DUMAS and Maxime BILLOT; supervision, Romain David and Maxime BILLOT; project administration, Romain DAVID. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Health Data Hub (protocol code XXX and date of approval).” for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical consideration.
Acknowledgments
We thank Jeffrey Arsham for his proofreading of the manuscript and his suggestions regarding medical writing. The authors would like to thank HypnoVR and Merz pharma for their financial support for the publication and submission of the present work, without any involvement in the conduct of the study.
Conflicts of Interest
Romain David reports speaker fees from Abbvie, Merz Pharma, Ipsen Pharma and Medtronic, outside the submitted work. Philippe Rigoard reports grants and personal fees from Medtronic, Abbott, Boston Scientific, Abbvie and Merz pharma outside the submitted work. Maarten Moens reports speaker fees from Medtronic and Nevro, outside the submitted work. Maxime Billot reports speaker fees from Abbvie outside the submitted work. All other authors have nothing to disclose. Etienne Ojardias received meeting sponsorship and compensation for consulting from Abbvie, Ipsen, and Merz. Etienne Ojardias received through his institution a research support grant from Merz.
References
- Goudman L, Jansen J, De Smedt A, Billot M, Roulaud M, Rigoard P, et al. Virtual Reality during Intrathecal Pump Refills in Children: A Case Series. J Clin Med 2022;11:5877. [CrossRef]
- Chau JPC, Lo SHS, Choi KC, Butt L, Zhao J, Thompson DR. Participation self-efficacy plays a mediation role in the association between mobility and social participation among stroke survivors. Heart Lung J Crit Care 2021;50:857–62. [CrossRef]
- Afssaps. Recommandations de bonne pratique : Traitements médicamenteux de la spasticité. 2009.
- Cameron MH, Bethoux F, Davis N, Frederick M. Botulinum Toxin for Symptomatic Therapy in Multiple Sclerosis. Curr Neurol Neurosci Rep 2014;14:463. [CrossRef]
- Chaléat-Valayer E, Parratte B, Colin C, Denis A, Oudin S, Bérard C, et al. A French observational study of botulinum toxin use in the management of children with cerebral palsy: BOTULOSCOPE. Eur J Paediatr Neurol EJPN Off J Eur Paediatr Neurol Soc 2011;15:439–48. [CrossRef]
- Brochard S, Blajan V, Lempereur M, Garlantezec R, Houx L, Le Moine P, et al. Determining the technical and clinical factors associated with pain for children undergoing botulinum toxin injections under nitrous oxide and anesthetic cream. Eur J Paediatr Neurol EJPN Off J Eur Paediatr Neurol Soc 2011;15:310–5. [CrossRef]
- Mathevon L, Davoine P, Tardy M, Bouchet N, Gornushkina I, Pérennou D. Pain during botulinum toxin injections in spastic adults: Influence of patients’ clinical characteristics and of the procedure. Ann Phys Rehabil Med 2018;61:e359. [CrossRef]
- Fisher MT, Zigler CK, Houtrow AJ. Factors affecting procedural pain in children during and immediately after intramuscular botulinum toxin injections for spasticity. J Pediatr Rehabil Med 2018;11:193–7. [CrossRef]
- Bayon-Mottu M, Gambart G, Deries X, Tessiot C, Richard I, Dinomais M. Pain during injections of botulinum toxin in children: Influence of the localization technique. Ann Phys Rehabil Med 2014;57:578–86. [CrossRef]
- JOINDREAU-GAUDE V, NOËL N. Douleur et soins. In: Fondation Garches, Institut fédératif de recherche sur le handicap, editors. Handicap Douleur Actes 24es Entret. Fond. Garches Nanterre 24-25 Novembre 2011, Neuilly-Sur-Seine: Éd. GM Santé; 2011, p. 101–9.
- Francisco GE, Balbert A, Bavikatte G, Bensmail D, Carda S, Deltombe T, et al. A practical guide to optimizing the benefits of post-stroke spasticity interventions with botulinum toxin A: An international group consensus. J Rehabil Med 2021;53:jrm00134. [CrossRef]
- Nugud A, Alhoot S, Agabna M, Babiker MOE, El Bashir H. Analgesia and sedation modalities used with botulinum toxin injections in children with cerebral palsy: a literature review. Sudan J Paediatr 2021;21:6–12. [CrossRef]
- Fung S, Phadke CP, Kam A, Ismail F, Boulias C. Effect of topical anesthetics on needle insertion pain during botulinum toxin type A injections for limb spasticity. Arch Phys Med Rehabil 2012;93:1643–7. [CrossRef]
- Gubbay A, Langdon K. “Effectiveness of sedation using nitrous oxide compared with enteral midazolam for botulinum toxin A injections in children.” Dev Med Child Neurol 2009;51:491–2; author reply 492. [CrossRef]
- Nilsson S, Brunsson I, Askljung B, Påhlman M, Himmelmann K. A rectally administered combination of midazolam and ketamine was easy, effective and feasible for procedural pain in children with cerebral palsy. Acta Paediatr Oslo Nor 1992 2017;106:458–62. [CrossRef]
- Weiss RA, Lavin PT. Reduction of pain and anxiety prior to botulinum toxin injections with a new topical anesthetic method. Ophthal Plast Reconstr Surg 2009;25:173–7. [CrossRef]
- Gambart G, Mette F, Pellot A-S, Richard I. [Evaluation of analgesic protocol with nitrous oxide and EMLA cream during botulinum toxin injections in children]. Ann Readaptation Med Phys Rev Sci Soc Francaise Reeducation Fonct Readaptation Med Phys 2007;50:275–9. [CrossRef]
- Jones T, Moore T, Choo J. The Impact of Virtual Reality on Chronic Pain. PloS One 2016;11:e0167523. [CrossRef]
- Trost Z, France C, Anam M, Shum C. Virtual reality approaches to pain: toward a state of the science. Pain 2021;162:325–31. [CrossRef]
- Luque-Moreno C, Kiper P, Solís-Marcos I, Agostini M, Polli A, Turolla A, et al. Virtual Reality and Physiotherapy in Post-Stroke Functional Re-Education of the Lower Extremity: A Controlled Clinical Trial on a New Approach. J Pers Med 2021;11:1210. [CrossRef]
- Lambert V, Boylan P, Boran L, Hicks P, Kirubakaran R, Devane D, et al. Virtual reality distraction for acute pain in children. Cochrane Database Syst Rev 2020;10:CD010686. [CrossRef]
- Alemanno F, Houdayer E, Emedoli D, Locatelli M, Mortini P, Mandelli C, et al. Efficacy of virtual reality to reduce chronic low back pain: Proof-of-concept of a non-pharmacological approach on pain, quality of life, neuropsychological and functional outcome. PLOS ONE 2019;14:e0216858. [CrossRef]
- Smith V, Warty RR, Sursas JA, Payne O, Nair A, Krishnan S, et al. The Effectiveness of Virtual Reality in Managing Acute Pain and Anxiety for Medical Inpatients: Systematic Review. J Med Internet Res 2020;22:e17980. [CrossRef]
- Perrochon A, Borel B, Istrate D, Compagnat M, Daviet J-C. Exercise-based games interventions at home in individuals with a neurological disease: A systematic review and meta-analysis. Ann Phys Rehabil Med 2019;62:366–78. [CrossRef]
- Gold JI, Mahrer NE. Is Virtual Reality Ready for Prime Time in the Medical Space? A Randomized Control Trial of Pediatric Virtual Reality for Acute Procedural Pain Management. J Pediatr Psychol 2018;43:266–75. [CrossRef]
- Chau B, Chi B, Wilson T. Decreasing pediatric pain and agitation during botulinum toxin injections for spasticity with virtual reality: Lessons learned from clinical use. J Pediatr Rehabil Med 2018;11:199–204. [CrossRef]
- Sikka N, Shu L, Ritchie B, Amdur RL, Pourmand A. Virtual Reality-Assisted Pain, Anxiety, and Anger Management in the Emergency Department. Telemed J E-Health Off J Am Telemed Assoc 2019;25:1207–15. [CrossRef]
- Almugait M, AbuMostafa A. Author Correction: Comparison between the analgesic effectiveness and patients’ preference for virtual reality vs. topical anesthesia gel during the administration of local anesthesia in adult dental patients: a randomized clinical study. Sci Rep 2022;12:805. [CrossRef]
- Basak T, Demirtas A, Yorubulut SM. Virtual reality and distraction cards to reduce pain during intramuscular benzathine penicillin injection procedure in adults: A randomized controlled trial. J Adv Nurs 2021;77:2511–8. [CrossRef]
- Trompetto C, Marinelli L, Mori L, Puce L, Avanti C, Saretti E, et al. Effectiveness of Botulinum Toxin on Pain in Stroke Patients Suffering from Upper Limb Spastic Dystonia. Toxins 2022;14:39. [CrossRef]
- Baricich A, Battaglia M, Cuneo D, Cosenza L, Millevolte M, Cosma M, et al. Clinical efficacy of botulinum toxin type A in patients with traumatic brain injury, spinal cord injury, or multiple sclerosis: An observational longitudinal study. Front Neurol 2023;14:1133390. [CrossRef]
- Anwar K, Barnes MP. A pilot study of a comparison between a patient scored numeric rating scale and clinician scored measures of spasticity in multiple sclerosis. NeuroRehabilitation 2009;24:333–40. [CrossRef]
- Freitas L, de Araújo Val S, Magalhães F, Marinho V, Ayres C, Teixeira S, et al. Virtual reality exposure therapy for neuro-psychomotor recovery in adults: a systematic review. Disabil Rehabil Assist Technol 2021;16:646–52. [CrossRef]
- Mallari B, Spaeth EK, Goh H, Boyd BS. Virtual reality as an analgesic for acute and chronic pain in adults: a systematic review and meta-analysis. J Pain Res 2019;Volume 12:2053–85. [CrossRef]
- Eijlers R, Utens EMWJ, Staals LM, de Nijs PFA, Berghmans JM, Wijnen RMH, et al. Systematic Review and Meta-analysis of Virtual Reality in Pediatrics: Effects on Pain and Anxiety. Anesth Analg 2019;129:1344–53. [CrossRef]
- Johnson, MH. How does distraction work in the management of pain? Curr Pain Headache Rep 2005;9:90–5. [CrossRef]
- Indovina P, Barone D, Gallo L, Chirico A, De Pietro G, Giordano A. Virtual Reality as a Distraction Intervention to Relieve Pain and Distress During Medical Procedures: A Comprehensive Literature Review. Clin J Pain 2018;34:858–77. [CrossRef]
- Abiodun AO, Adesina MA. Virtual reality: a breakthrough in pain management? World News Nat Sci 2019:24–33.
- Wismeijer AAJ, Vingerhoets AJJM. The use of virtual reality and audiovisual eyeglass systems as adjunct analgesic techniques: a review of the literature. Ann Behav Med Publ Soc Behav Med 2005;30:268–78. [CrossRef]
- Rutter CE, Dahlquist LM, Weiss KE. Sustained efficacy of virtual reality distraction. J Pain 2009;10:391–7. [CrossRef]
- Birnie KA, Noel M, Parker JA, Chambers CT, Uman LS, Kisely SR, et al. Systematic review and meta-analysis of distraction and hypnosis for needle-related pain and distress in children and adolescents. J Pediatr Psychol 2014;39:783–808. [CrossRef]
- Koller D, Goldman RD. Distraction techniques for children undergoing procedures: a critical review of pediatric research. J Pediatr Nurs 2012;27:652–81. [CrossRef]
- Ventura S, Brivio E, Riva G, Baños RM. Immersive Versus Non-immersive Experience: Exploring the Feasibility of Memory Assessment Through 360° Technology. Front Psychol 2019;10:2509. [CrossRef]
- Goudman L, Jansen J, Billot M, Vets N, De Smedt A, Roulaud M, et al. Virtual Reality Applications in Chronic Pain Management: Systematic Review and Meta-analysis. JMIR Serious Games 2022;10:e34402. [CrossRef]
- Patterson DR, Hoffman HG, Chambers G, Bennetts D, Hunner HH, Wiechman SA, et al. Hypnotic Enhancement of Virtual Reality Distraction Analgesia during Thermal Pain: A Randomized Trial. Int J Clin Exp Hypn 2021;69:225–45. [CrossRef]
- Rousseaux F, Bicego A, Ledoux D, Massion P, Nyssen A-S, Faymonville M-E, et al. Hypnosis Associated with 3D Immersive Virtual Reality Technology in the Management of Pain: A Review of the Literature. J Pain Res 2020;13:1129–38. [CrossRef]
- Rousseaux F, Dardenne N, Massion PB, Ledoux D, Bicego A, Donneau A-F, et al. Virtual reality and hypnosis for anxiety and pain management in intensive care units: A prospective randomised trial among cardiac surgery patients. Eur J Anaesthesiol 2022;39:58–66. [CrossRef]
- Rousseaux F, Panda R, Toussaint C, Bicego A, Niimi M, Faymonville M-E, et al. Virtual reality hypnosis in the management of pain: Self-reported and neurophysiological measures in healthy subjects. Eur J Pain Lond Engl 2023;27:148–62. [CrossRef]
- Langlois P, Perrochon A, David R, Rainville P, Wood C, Vanhaudenhuyse A, et al. Hypnosis to manage musculoskeletal and neuropathic chronic pain: A systematic review and meta-analysis. Neurosci Biobehav Rev 2022;135:104591. [CrossRef]
- Bani Mohammad E, Ahmad M. Virtual reality as a distraction technique for pain and anxiety among patients with breast cancer: A randomized control trial. Palliat Support Care 2019;17:29–34. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).